Abstract
We hypothesized that changes in the expression of Kv4.3 contribute to the mechanical hyperalgesia induced by vibration injury, a rodent model for hand-arm vibration syndrome in humans. Here we show that the exposure of the gastrocnemius muscle to vibration injury induces muscle hyperalgesia that is accompanied by a significant down-regulation of Kv4.3 in affected sensory nerve fibers in dorsal root ganglia (DRG). We additionally demonstrate that the intrathecal administration of antisense oligonucleotides for Kv4.3 mRNA itself induces muscle hyperalgesia in the rat. Our results suggest that attenuation in the expression of Kv4.3 may contribute to neuropathic pain in people affected by hand-arm vibration syndrome.
Keywords: Hand-arm vibration syndrome, neuropathic pain, Kv channels, muscle hyperalgesia, IB4-binding nociceptors
Introduction
Hand-arm vibration syndrome is an occupational disorder that is often observed after prolonged exposure to vibrating tools such as chainsaws or jackhammers 27,28. Typical symptoms include ongoing pain, mechanical hyperalgesia, paresthesia and dysethesia, thought to be due to neurovascular trauma and ischemia in the affected extremities 27. Although this disorder was first described almost a century ago, little is known about its underlying pathophysiology.
Our laboratory developed a rodent model for hand-arm vibration syndrome in which the hind limb of an anesthetized rat is vibrated at frequencies comparable to those produced by hand-held power tools 15,21. The electrophysiological changes observed in this model suggest that they are caused by a traumatic injury of the tibial nerve; many nociceptors innervating the vibration-exposed gastrocnemius muscle become hyperexcitable, displaying enhanced responses to noxious mechanical stimuli and a reduced mechanical threshold 15. Moreover, nociceptors exposed to vibration-injury are primed and therefore demonstrate enhanced and prolonged hyperalgesia in response to a subsequent pro-inflammatory mediator, even months after the initial injury 21,44.
Nociceptors express a number of different voltage-gated potassium channels (Kv) whose expression is crucial for setting their threshold and excitability 16,32,33,43,45,57. One of the best characterized Kv channels is Kv 4.3, which is exclusively expressed by Isolectin B4 (IB4)-binding C-fiber nociceptors in the dorsal root ganglia (DRG) 16. The expression of Kv 4.3, which is down-regulated in several animal models of neuropathic pain 16,32 lies under regulatory control of neuron-restrictive silencing factor (NRSF) 56. Of note, the down-regulation of Kv 4.3 in the setting of nerve trauma is accompanied by neuronal hyperexcitability and a decrease in mechanical nociceptive threshold 16,32.
In the present study, we tested the hypothesis that Kv 4.3 is involved in vibration-induced muscle pain and explored whether changes in Kv 4.3 expression in nociceptors contributes to the mechanical hyperalgesia in a model of hand-arm vibration syndrome in the rat.
Methods
Animals
Adult male Sprague-Dawley rats (250–300 g; Charles River Laboratories, Hollister, CA) were used in experiments. Animals were housed in the animal care facility of the University of California, San Francisco, under environmentally controlled conditions (21-23°C; 12 hour alternating light–dark cycle; food and water ad libitum). Animal care and use adhered to the guidelines set by the National Institutes of Health and the Committee for Research and Ethical Issues of the International Association for the Study of Pain. The Institutional Animal Care and Use Committee (IACUC) at the University of California, San Francisco approved all experimental protocols, in which a concerted effort has been made to minimize the number of animals used and their suffering.
Mechanical vibration
To induce muscle hyperalgesia, the hind limbs of rats were subjected to mechanical vibration with a laboratory vortex mixer (Digital Vortex Genie II; Thermo Fisher Scientific, Waltham, MA), as previously described 3,15,21. These rats were anesthetized with 3% isoflurane in oxygen before one hind leg was affixed to the platform of the vortex mixer with Micropore® surgical tape (Thermo Fisher Scientific) so that the knee and ankle joint were both at 90°, without rotational torque on the leg. Each hind leg was vibrated at a frequency of 60–80 Hz with 5-mm peak-to-peak displacement amplitude for 15 minutes. These vibration frequencies are within the range produced by hand-held power tools (35–150 Hz) 41.
For rats that were used to analyze whether Kv 4.3 or NRSF (Fig. 2, 3) may contribute to the mechanical hyperalgesia in rats exposed to vibration-injury, both legs were sequentially vibrated. For rats that were used to demonstrate that vibration-injury causes muscle hyperalgesia (Fig. 1) or to evaluate the effect of intrathecal antisense for Kv4.3 (Fig. 4) only one leg was exposed to vibration-injury.
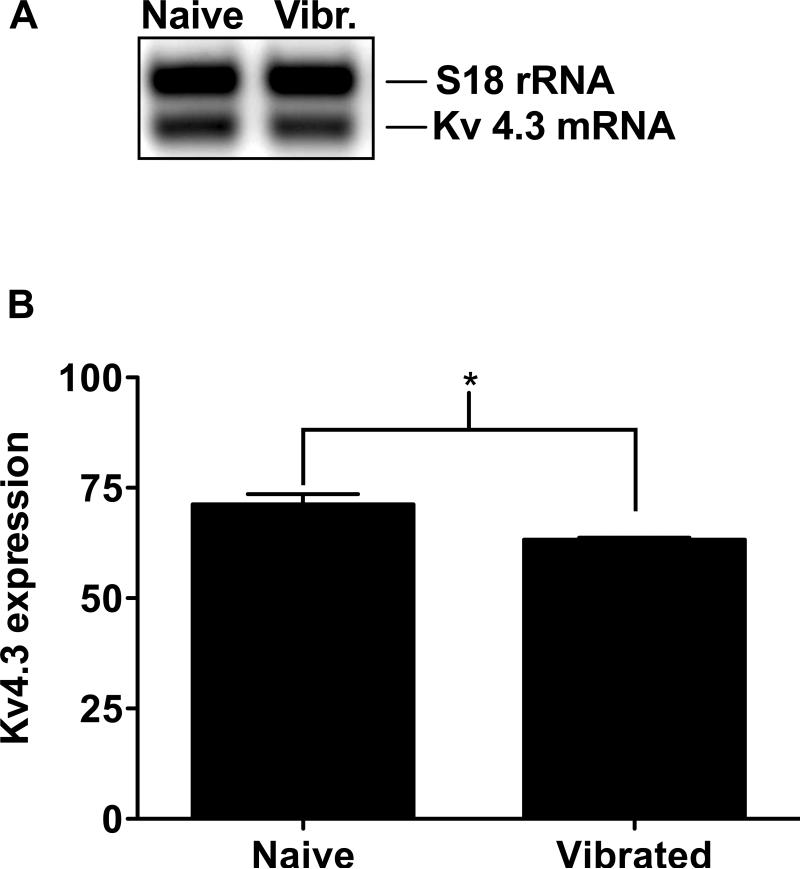
Figure 2. Vibration induced changes in Kv 4.3 mRNA in DRG.
Analysis of Kv 4.3 mRNA expression in L4 and L5 DRG 24 hours after vibration injury to the hind limb gastrocnemius muscles: (A) A semi-quantitative RT-PCR analysis was performed comparing injured and uninjured animals. The density of the PCR product of the Kv 4.3 gene at 301 bp was normalized to the density of the PCR product of the S18 rRNA standard at 324 bp. (B) A significant down-regulation of Kv 4.3 mRNA (8.03 ± 2.3%, n=3 per group) was observed after vibration injury, in comparison to the non-vibrated control. * P < 0.01.
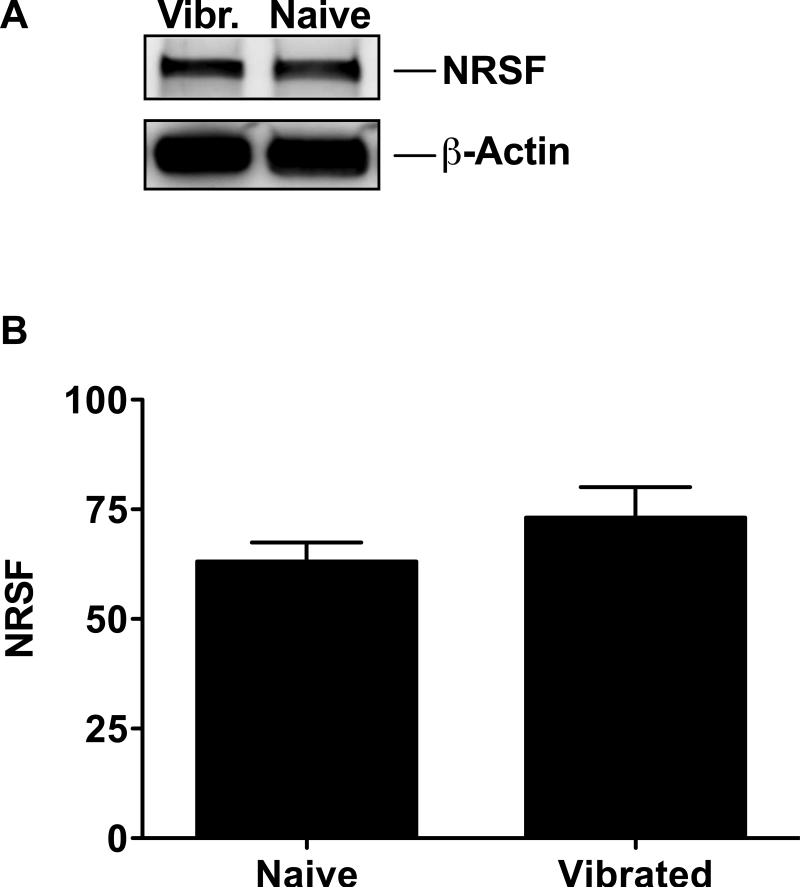
Figure 3. Vibration induced changes in NRSF protein expression.
Western blot analysis of NRSF protein levels in L4 and L5 bilateral DRG neurons. (A) β-actin, used as a housekeeping gene in this analysis, has the calculated molecular weight of ~42 kDa; the calculated molecular weight of NRSF is ~117 kDa. (B) A detectable but non-significant increase in NRSF 10.0 ± 8.2 % (n=9 per group) was present after vibration injury. Values are represented as mean percent of NRSF expression in comparison to β-actin band intensity.
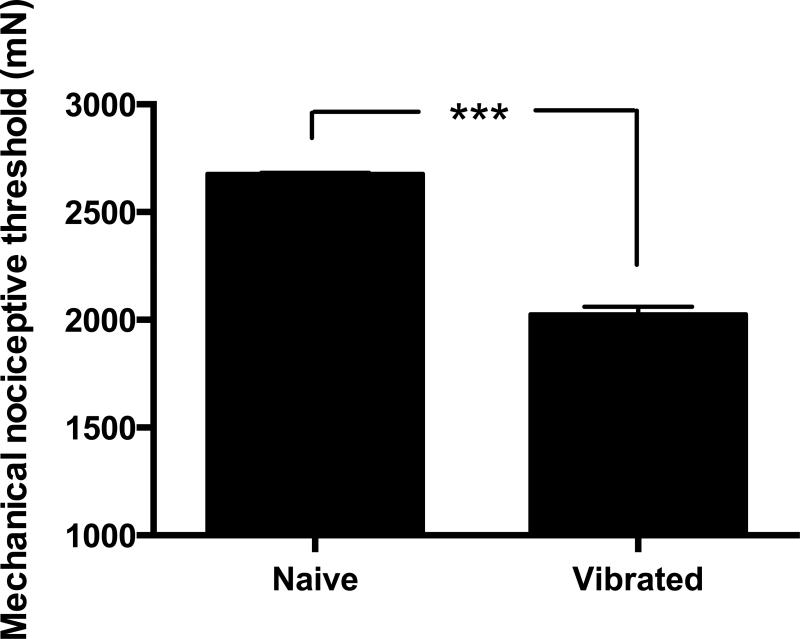
Figure 1. Muscle vibration induces mechanical hyperalgesia in skeletal muscle.
A significant decrease of 24.4 ± 1.3% (n=9 per group) in nociceptive threshold in the gastrocnemius muscle, hyperalgesia, was observed 24 hours after vibration injury. Nociceptive threshold was defined as the force in milliNewtons (mN) at which the rat withdrew its hind leg from the force transducer applied to the belly of the muscle. *** P < 0.0001.
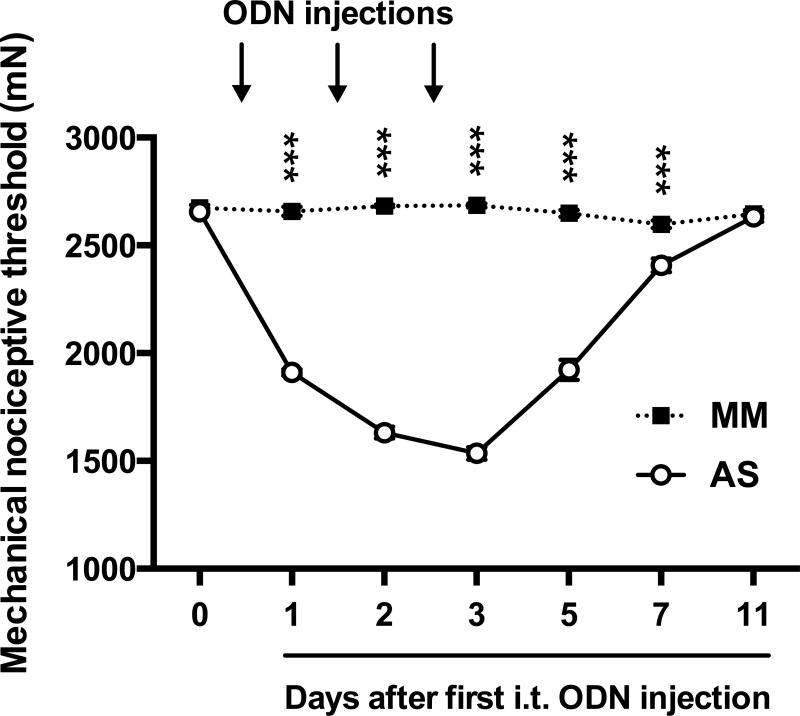
Figure 4. Effect of intrathecal AS/MM ODN directed against Kv4.3 mRNA on muscle mechanical nociceptive threshold.
Attenuation of Kv 4.3 mRNA with three consecutive intrathecal injections of AS-ODN (open symbols, n=6) resulted in a 42.2 ± 1.1% reduction in nociceptive threshold, with no significant changes in the MM-ODN group (black symbols, n=6). This finding supports the suggestion that the down-regulation of Kv 4.3 contributes to the mechanical hyperalgesia observed in our rodent model for hand-arm vibration syndrome. Black arrows indicate time points of ODN injections. ***P<0.0001.
Measurement of mechanical nociceptive threshold
Mechanical nociceptive threshold in the gastrocnemius muscle was quantified using a Chatillon digital force transducer (model DFI2, Amtek Inc., Largo, FL), as previously described 6,20,22. Approximately 24 hours post vibration injury; rats were lightly restrained in a cylindrical acrylic holder that allows for easy access to the hind limb for mechanical nociceptive threshold testing. A 7-mm diameter probe was used to stimulate the belly of the gastrocnemius muscle with a gradually increasing compression force. The nociceptive threshold was defined as the force in milliNewtons (mN) at which the rat withdrew its hind leg. Rats were placed in restrainers where they were trained with the force transducer for four consecutive days prior to measuring baseline mechanical nociceptive threshold, with application of a force of 2.0 to 2.6 N (increased by 0.2 N daily), 3-5 times per hind leg. Baseline nociceptive withdrawal threshold was defined as the mean of three readings taken at 5-min intervals and the magnitude of hyperalgesia was calculated as the percentage decrease from the baseline withdrawal threshold. The individual that was performing the behavioral experiments presented in this manuscript was not blinded to treatment group.
Surgical excision of DRG neurons
Rats were euthanized by exsanguination, while under isoflurane anesthesia, 24 hours after receiving the vibration injury. This was previously shown to be the time point for peak hyperalgesia in the vibration injury model 21. Lumbar DRGs were quickly dissected out bilaterally, immediately placed on dry ice, then transferred to storage at −80 °C until use for further analyses.
Semi-quantitative multiplex RT-PCR
Total RNA was extracted from left and right L4 and L5 DRG using Trizol reagent (Invitrogen, Carlsbad, CA) with the PureLink™ RNA mini kit (Ambion @ Invitrogen), according to the manufacturer's instruction. The amount of RNA was measured by UV absorbance at 260 nm (Shimadzu UV-160 Spectrophotometer), followed by cDNA synthesis (1 μg total RNA per sample) using the SuperScript III Platinum One-Step quantitative RT-PCR System (Invitrogen). The PCR-primers used for the amplification of rat Kv 4.3 were: F1: 5’-CCACGAGTTTATTGATGAGCAGAT-3’ and B1: 5’-TGTTTTGCAGTTTGGTCTCAGTC-3’ 34, which were matched against all three variants (NCBI database-entries NM_001270962, NM_001270963, NM_031739). To compensate for variations in the quality or quantity of the samples, a multiplex RT-PCR was performed with S18 rRNA as an endogenous standard (Quantum RNA Classic II 18S Internal Standards, Ambion @ Invitrogen). The amplification product of the Kv 4.3 gene (301 bp) was normalized to the PCR product of the S18 rRNA (324 bp). Pilot experiments were performed to optimize for: 1) annealing temperature (61.3°C); 2) number of PCR-cycles (35); and 3) ratio of S18 rRNA primer to competimer™ (Ambion; 2:8). The PCR-products were separated on 2% agarose gels and visualized by ethidium bromide intercalation. Images of the gels were acquired with the ChemiImager system and analyzed with AlphaEaseFC Software (Alpha Innotech Corporation, San Leandro, CA).
NRSF protein expression
To determine whether the change in Kv4.3 expression induced by vibration is related to an increase in NRSF, a Western blot analysis was performed, following the methods previously described 9,53. Bilateral L4 and L5 DRGs were harvested and transferred into cold homogenization buffer (150 mM NaCl, 10 mM EDTA, 2% SDS, 50 mM Tris-HCl, pH 7.4) supplemented with a 2× protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). Four DRGs per sample were homogenized manually with a plastic pestle and proteins were solubilized by a 2 hour incubation in an Eppendorf Thermomixer at 23°C and 1400 rpm (Eppendorf AG, Hamburg, Germany). Proteins were extracted by a 15 minute centrifugation in a table top centrifuge and the protein concentration of the samples were determined using the micro BCA Protein Assay Kit (Pierce, Rockford, IL) with bovine serum albumin (BSA) as the standard.
Mixtures of 40 μg of protein per sample were denatured at 90°C for 10 minutes and electrophoresed on a 4 to 15% pre-cast polyacrylamide gel (Biorad, Hercules, CA) in 25 mM Tris containing 192 mM glycine and 0.1% SDS. Proteins were transferred to a nitrocellulose membrane using the semi-dry method (transfer time 1 hour at 10 V). The nitrocellulose membranes were saturated by shaking in antibody dilution buffer (5% BSA in Tris-buffered saline containing 0.1% Tween20 (TBST)) for 1 hour at room temperature (RT) and probed with rabbit anti-NRSF (ab21635, 1:500, Santa Cruz Biotechnology, Santa Cruz, CA), or rabbit anti-ß-actin (ab8227, 1:1000; Abcam, Cambridge, MA) antibodies in antibody dilution buffer, at 4°C overnight. After washing with TBST (3 times at RT, 15 min each), the NRSF blot was probed with a biotinylated goat anti-rabbit antibody (1:2500 in antibody dilution buffer, Jackson Immunoresearch, West Grove, PA) for 2 hours at RT, while the ß-actin blot was probed with a horseradish peroxidase (HRP) conjugated anti-rabbit antibody (NA934V, 1:2500 in antibody dilution buffer, GE Healthcare, Piscataway, NJ) for 2 hours at RT. Blots were washed with TBST (3 times at RT, 15 min each) and the NRSF blot was then probed with streptavidinperoxidase polymer (S2438, 1:5000 in antibody dilution buffer, Sigma-Aldrich, Saint Louis, MO) for 1.5 hours while shaking at RT. After washing the NRSF Western blot with TBST (3 times at RT, 15 min each), immunoreactivity was visualized using the sensitive femto chemiluminescence detection system (Pierce, Rockford, IL). Results were analyzed by computer-assisted densitometry and levels of NRSF immunoreactivity were normalized with respect to the ß-actin control levels in each sample.
Intrathecal injection of antisense oligodeoxynucleotides
To evaluate the relationship between Kv 4.3 and mechanical nociceptive threshold in the gastrocnemius muscle, we attenuated its expression by the intrathecal injection of antisense oligodeoxynucleotides (AS-ODNs) directed against Kv 4.3 mRNA. The 24-mer AS-ODN sequence [5’-TCATCTTGCCGCTTGTTCTTGTCG-3’] (GenBank accession number L48619) used in this study was previously shown to produce a knockdown of Kv 4.3 protein in nociceptive DRG neurons 16. The mismatch (MM) ODN sequence [5’-TCATGTTCGCGGTTCTTGTTCTCG-3’] corresponds to the AS sequence with seven base changes, as indicated in bold. The AS and MM ODNs were synthesized by Invitrogen (Carlsbad, CA).
Three consecutive daily intrathecal injections of AS or MM ODN (40 μg) were performed under brief anesthesia with 2.5% isoflurane in oxygen. To inject ODNs, a 29-gauge hypodermic needle was inserted into the subarachnoid space on the midline between the fourth and fifth lumbar vertebrae, and 20 μL of either AS or MM ODN dissolved in physiological NaCl solution was slowly administered. Observation of a tail flick was used to determine proper placement of the needle in the subarachnoid space during intrathecal injection 36. Animals regained consciousness approximately one minute post-injection.
Statistics
Group data are expressed as mean ± SEM of n distinct observations. Statistical comparisons were made by a two-tailed Student's t-test (for paired and independent samples) or by two-way repeated measures ANOVA for comparing treatments over time, using Prism statistical software. Statistical significance was considered as P < 0.05.
Results
Vibration-induced mechanical hyperalgesia
As previously established 21, our protocol of hind limb vibration at 60-80 Hz for 15 minutes resulted in marked mechanical hyperalgesia in the gastrocnemius muscle 21. A significant decrease of 24.4 ± 1.3% (P < 0.0001, n=9 per group) in mechanical nociceptive threshold was observed 24 hours after vibration injury to the hind limb (Fig. 1).
Kv 4.3 mRNA levels
To determine whether vibration injury to skeletal muscle afferents induces Kv 4.3 down-regulation, we measured Kv 4.3 mRNA expression in the DRG using semi-quantitative RT-PCR. A significant reduction in Kv 4.3 mRNA in the vibration injury group was observed in comparison to the level in naïve animals (8.03 ± 2.3%, P ≤ 0.01, n=3 per group) (Fig. 2).
NRSF protein levels
To determine if the change in Kv 4.3 could be secondary to changes in NRSF protein level, which blocks the transcription of the Kv 4.3 gene 56, we measured NRSF protein expression in the lumbar DRG using Western blot analysis. Protein extracts derived from DRG of vibration-injured animals showed higher levels of NRSF protein, compared to that of control animals, although the difference did not reach statistical significance (10.0 ± 8.2 %, P > 0.05, n=9 per group) (Fig. 3).
Effect of antisense against Kv 4.3
Finally, to determine the contribution of Kv 4.3 to nociception in the gastrocnemius muscle, AS and MM ODNs designed to target the rat Kv 4.3 mRNA sequence were injected intrathecally in naïve rats for three consecutive days. Following ODN treatment, the nociceptive threshold was markedly reduced, 42.2 ± 1.1%, in the Kv 4.3 AS-ODN-treated group, compared to the MM-ODN treated group (P < 0.0001; n=6 per group) (Fig. 4).
Discussion
In the present study we tested the hypothesis that the down-regulation of Kv 4.3 in the peripheral terminals of nociceptive muscle afferents contributes to the mechanical hyperalgesia observed in rats exposed to muscle vibration. Our hypothesis was based on the assumption that: a) the changes in the phenotype of muscle nociceptors are caused by a traumatic injury of the tibial nerve, and b) this injury leads to changes in the activity or expression of Kv 4.3, which could explain the increased excitability and decreased mechanical threshold observed in our rodent model for hand-arm vibration syndrome 15.
Neuropathic pain can be the consequence of a traumatic nerve injury, a metabolic disease such as diabetes, or exposure to neurotoxins such as the drugs used to fight cancer 19. Clinical symptoms include allodynia, hyperalgesia, and spontaneous (stimulus-independent) pain. Each of these symptoms can be caused by a number of different pathophysiological mechanisms, which explains why neuropathic pain is so difficult to treat 17,18,19,29. We have previously demonstrated that nociceptive C-fiber afferents innervating the vibration-exposed gastrocnemius muscle become hyper-responsive to a prolonged noxious mechanical stimulus 15. Mechanically hyper-responsive C-fiber nociceptors have also been observed in animal models for nerve injury 50,51, diabetic neuropathy 1, and chemotherapy-induced neuropathy 55.
We have also demonstrated that rats exposed to vibration injury develop a latent and long-lasting hypersensitivity and hyperresponsiveness to a subsequent inflammatory stimulus 4,21, a phenomenon known as hyperalgesic priming 2,9,39,44.
One of the hallmarks of hand-arm vibration syndrome in humans and corresponding animal models is the demyelination and orthograde degeneration of axons that have been separated from the neuronal cell body in the DRG 31,35,42,54, a process known as Wallerian degeneration 52. Wallerian degeneration is accompanied by an inflammatory response during which injured axons release molecules that enhance vascular permeability and activate glial cells, which in turn produce and release molecules that ensure the survival of injured neurons and attract leukocytes to the site of injury 49. Many of the molecules that are produced and released by injured axons, glial cells, and leukocytes - such as adenosine triphosphate (ATP), interleukin 6 (IL-6), tumor necrosis factor alpha (TNFα), monocyte chemoattractant protein 1 (MCP-1), nerve growth factor (NGF), and glial-derived neurotrophic factor (GDNF) - orchestrate the tissue response in order to clear cellular debris, restore tissue homeostasis, and initiate neuronal regeneration, and are known to directly sensitize nociceptors 11,12,37,46,47,48,53. Many of these have also been shown to induce hyperalgesic priming 5,20,25,38. It is therefore not surprising that a knockdown of cellular receptors for these molecules in nociceptors attenuates both mechanical hyperalgesia 15,21 and hyperalgesic priming 21 after exposure to vibration.
Nociceptors are a heterogeneous group of sensory neurons that have been differentiated according to somal size, axonal diameter, conduction velocity, activation threshold, stimulus response, peripheral innervation area, termination area in the spinal cord, dependence on specific neurotrophins, and expression of cell markers 8,24. One subset of small-diameter nociceptors, the GDNF-dependent IB4-binding C-fibers 10, appears to be of particular importance in our rodent model of vibration-induced muscle pain since their ablation with the selective neurotoxin IB4-saporin, produced a marked attenuation of mechanical hyperalgesia in rats with vibration injury 4.
GDNF-dependent IB4-binding C-fibers express at least three different A-type voltage-gated potassium channels (Kv 1.4, Kv 3.4, and Kv 4.3). However, only Kv 4.3 is exclusively expressed by this population of nociceptors within the DRG 23,57. Functional suppression or down-regulation of these channels is known to increase the excitability of axotomized neurons both in vivo 16 and in vitro 57, in addition to inducing mechanical hypersensitivity in rodent models of neuropathic pain 14,45. Given that vibration-induced muscle pain depends on GDNF-dependent IB4-binding C-fiber nociceptors and that Kv 4.3 is the only A-type potassium channel exclusively expressed by this subset of nociceptive afferents, we sought to analyze its contribution to vibration-induced muscle pain. Here we demonstrate that the vibration-induced mechanical hyperalgesia in the gastrocnemius muscle is indeed accompanied by a decrease in the mRNA for Kv 4.3.
It has been suggested that the down-regulation of Kv 4.3 in the setting of neuropathic pain is due to the binding of NRSF to regulatory DNA-sequences within the Kv 4.3 gene, which suppresses its transcription 56. It also has been shown that an ischemic insult – a characteristic feature of hand-arm vibration syndrome 27 – triggers an up-regulation of NRSF 13,26. We therefore analyzed whether the down-regulation of Kv 4.3 could be due to an up-regulation of NRSF expression. While the expression level of NRSF in protein extracts derived from DRG of animals exposed to vibration injury is higher than that of protein extracts derived from naïve, non-vibrated animals, the increase in the NRSF protein level did not reach statistical significance. However, this result might be expected, since the number of GDNF-dependent IB4-binding C-fiber afferents in the L4 and L5 DRGs that innervate the gastrocnemius muscle is relatively low 7,40.
If Kv 4.3 contributes to the mechanical hyperalgesia of rats exposed to vibration-injury, one would expect that a functional suppression or decrease in Kv 4.3 protein expression level itself would induce mechanical hyperalgesia. To analyze whether Kv 4.3 down-regulation affects the mechanical nociceptive threshold of muscle afferents innervating the gastrocnemius muscle, a group of rats were treated with an antisense oligonucleotide against Kv 4.3 mRNA for three consecutive days. Rats treated with antisense displayed a reduced mechanical nociceptive threshold compared to those treated with mismatch oligonucleotides. This finding supports our hypothesis that Kv 4.3 down-regulation contributes to the mechanical hyperalgesia observed in our rodent model for hand-arm vibration syndrome.
Taken together, our results support our initial hypothesis that the mechanical hyperalgesia in our rodent model for hand-arm syndrome is caused by a traumatic injury of the tibial nerve, which leads to a down-regulation of Kv 4.3 in the peripheral terminals of GDNF-dependent IB4-binding C-fiber nociceptors innervating the gastrocnemius muscle. Given that vibration injury induces hyperalgesic priming, which depends on GDNF-dependent IB4-binding nociceptors 25,30, it would be interesting to analyze whether the down-regulation of Kv 4.3 also contributes to the phenotypic changes and long-term consequences that are associated with this subset of nociceptors in the setting of priming.
Highlights.
- Exposure to vibration-injury produces muscle hyperalgesia in the rat
- Vibration-injury also produces a decrease in the expression of Kv4.3 in nociceptors
- Intrathecal treatment with antisense for Kv4.3 mRNA produces muscle hyperalgesia
Perspective.
Our findings establish Kv4.3 as a potential molecular target for the treatment of hand-arm vibration syndrome.
Acknowledgments
This work was financially supported by the NIH (Grant AR063312).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no conflict of interest to declare.
References
- 1.Ahlgren SC, White DM, Levine JD. Increased responsiveness of sensory neurons in the saphenous nerve of the streptozotocin-diabetic rat. J Neurophysiol. 1992;68:2077–2085. doi: 10.1152/jn.1992.68.6.2077. [DOI] [PubMed] [Google Scholar]
- 2.Aley KO, Messing RO, Mochly-Rosen D, Levine JD. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J Neurosci. 2000;20:4680–4685. doi: 10.1523/JNEUROSCI.20-12-04680.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez P, Chen X, Bogen O, Green PG, Levine JD. IB4(+) nociceptors mediate persistent muscle pain induced by GDNF. J Neurophysiol. 2012;108:2545–2553. doi: 10.1152/jn.00576.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez P, Gear RW, Green PG, Levine JD. IB4-saporin attenuates acute and eliminates chronic muscle pain in the rat. Exp Neurol. 2012;233:859–865. doi: 10.1016/j.expneurol.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvarez P, Green PG, Levine JD. Role for monocyte chemoattractant protein-1 in the induction of chronic muscle pain in the rat. Pain. 2014;155:1161–1167. doi: 10.1016/j.pain.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alvarez P, Levine JD, Green PG. Eccentric exercise induces chronic alterations in musculoskeletal nociception in the rat. Eur J Neurosci. 2010;32:819–825. doi: 10.1111/j.1460-9568.2010.07359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ambalavanar R, Moritani M, Haines A, Hilton T, Dessem D. Chemical phenotypes of muscle and cutaneous afferent neurons in the rat trigeminal ganglion. J Comp Neurol. 2003;460:167–179. doi: 10.1002/cne.10655. [DOI] [PubMed] [Google Scholar]
- 8.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bogen O, Alessandri-Haber N, Chu C, Gear RW, Levine JD. Generation of a pain memory in the primary afferent nociceptor triggered by PKCε activation of CPEB. J Neurosci. 2012;32:2018–2026. doi: 10.1523/JNEUROSCI.5138-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bogen O, Bender O, Löwe J, Blenau W, Thevis B, Schröder W, Margolis RU, Levine JD, Hucho F. Neuronally produced versican V2 renders C-fiber nociceptors IB4 -positive. J Neurochem. 2015;134:147–155. doi: 10.1111/jnc.13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bogen O, Dina OA, Gear RW, Levine JD. Dependence of monocyte chemoattractant protein 1 induced hyperalgesia on the isolectin B4-binding protein versican. Neuroscience. 2009;159:780–786. doi: 10.1016/j.neuroscience.2008.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bogen O, Joseph EK, Chen X, Levine JD. GDNF hyperalgesia is mediated by PLCgamma, MAPK/ERK, PI3K, CDK5 and Src family kinase signaling and dependent on the IB4-binding protein versican. Eur J Neurosci. 2008;28:12–19. doi: 10.1111/j.1460-9568.2008.06308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calderone A, Jover T, Noh KM, Tanaka H, Yokota H, Lin Y, Grooms SY, Regis R, Bennett MV, Zukin RS. Ischemic insults derepress the gene silencer REST in neurons destined to die. J Neurosci. 2003;23:2112–2121. doi: 10.1523/JNEUROSCI.23-06-02112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao XH, Byun HS, Chen SR, Cai YQ, Pan HL. Reduction in voltage-gated K+ channel activity in primary sensory neurons in painful diabetic neuropathy: role of brain-derived neurotrophic factor. J Neurochem. 2010;114:1460–1475. doi: 10.1111/j.1471-4159.2010.06863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Green PG, Levine JD. Neuropathic pain-like alterations in muscle nociceptor function associated with vibration-induced muscle pain. Pain. 2010;151:460–466. doi: 10.1016/j.pain.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chien LY, Cheng JK, Chu D, Cheng CF, Tsaur ML. Reduced expression of A-type potassium channels in primary sensory neurons induces mechanical hypersensitivity. J Neurosci. 2007;27:9855–9865. doi: 10.1523/JNEUROSCI.0604-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen SP, Mao J. Neuropathic pain: mechanisms and their clinical implications. BMJ. 2014;348:f7656. doi: 10.1136/bmj.f7656. [DOI] [PubMed] [Google Scholar]
- 18.Colvin LA, Dougherty PM. Peripheral neuropathic pain: signs, symptoms, mechanisms, and causes: are they linked. Br J Anaesth. 2015;114:361–363. doi: 10.1093/bja/aeu323. [DOI] [PubMed] [Google Scholar]
- 19.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dina OA, Green PG, Levine JD. Role of interleukin-6 in chronic muscle hyperalgesic priming. Neuroscience. 2008;152:521–525. doi: 10.1016/j.neuroscience.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dina OA, Joseph EK, Levine JD, Green PG. Mechanisms mediating vibration-induced chronic musculoskeletal pain analyzed in the rat. J Pain. 2010;11:369–377. doi: 10.1016/j.jpain.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dina OA, Levine JD, Green PG. Muscle inflammation induces a protein kinase Cepsilon-dependent chronic-latent muscle pain. J Pain. 2008;9:457–462. doi: 10.1016/j.jpain.2008.01.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duan KZ, Xu Q, Zhang XM, Zhao ZQ, Mei YA, Zhang YQ. Targeting A-type K(+) channels in primary sensory neurons for bone cancer pain in a rat model. Pain. 2012;153:562–574. doi: 10.1016/j.pain.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 24.Dubin AE, Patapoutian A. Nociceptors: the sensors of the pain pathway. J Clin Invest. 2010;120:3760–3772. doi: 10.1172/JCI42843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrari LF, Bogen O, Levine JD. Nociceptor subpopulations involved in hyperalgesic priming. Neuroscience. 2010;165:896–901. doi: 10.1016/j.neuroscience.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Formisano L, Noh KM, Miyawaki T, Mashiko T, Bennett MV, Zukin RS. Ischemic insults promote epigenetic reprogramming of mu opioid receptor expression in hippocampal neurons. Proc Natl Acad Sci U S A. 2007;104:4170–4175. doi: 10.1073/pnas.0611704104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fridén J. Vibration damage to the hand: clinical presentation, prognosis and length and severity of vibration required. J Hand Surg Br. 2001;26:471–474. doi: 10.1054/jhsb.2001.0633. [DOI] [PubMed] [Google Scholar]
- 28.House R, Wills M, Liss G, Switzer-McIntyre S, Lander L, Jiang D. The effect of hand-arm vibration syndrome on quality of life. Occup Med (Lond) 2014;64:133–135. doi: 10.1093/occmed/kqt167. [DOI] [PubMed] [Google Scholar]
- 29.Jensen TS, Finnerup NB. Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol. 2014;13:924–935. doi: 10.1016/S1474-4422(14)70102-4. [DOI] [PubMed] [Google Scholar]
- 30.Joseph EK, Levine JD. Hyperalgesic priming is restricted to isolectin B4-positive nociceptors. Neuroscience. 2010;169:431–435. doi: 10.1016/j.neuroscience.2010.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Juntunen J, Taskinen H. Pathogenic and clinical aspects of polyneuropathies, with reference to the hand-arm vibration syndrome. Scand J Work Environ Health. 1987;13:363–366. doi: 10.5271/sjweh.2043. [DOI] [PubMed] [Google Scholar]
- 32.Kim DS, Choi JO, Rim HD, Cho HJ. Downregulation of voltage-gated potassium channel alpha gene expression in dorsal root ganglia following chronic constriction injury of the rat sciatic nerve. Brain Res Mol Brain Res. 2002;105:146–152. doi: 10.1016/s0169-328x(02)00388-1. [DOI] [PubMed] [Google Scholar]
- 33.King CH, Lancaster E, Salomon D, Peles E, Scherer SS. Kv7.2 regulates the function of peripheral sensory neurons. J Comp Neurol. 2014;522:3262–3280. doi: 10.1002/cne.23595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu W, Deng J, Xu J, Wang H, Yuan M, Liu N, Jiang Y, Liu J. High-mobility group box 1 (HMGB1) downregulates cardiac transient outward potassium current (Ito) through downregulation of Kv4.2 and Kv4.3 channel transcripts and proteins. J Mol Cell Cardiol. 2010;49:438–448. doi: 10.1016/j.yjmcc.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Loffredo MA, Yan JG, Kao D, Zhang LL, Matloub HS, Riley DA. Persistent reduction of conduction velocity and myelinated axon damage in vibrated rat tail nerves. Muscle Nerve. 2009;39:770–775. doi: 10.1002/mus.21235. [DOI] [PubMed] [Google Scholar]
- 36.Mestre C, Pélissier T, Fialip J, Wilcox G, Eschalier A. A method to perform direct transcutaneous intrathecal injection in rats. J Pharmacol Toxicol Methods. 1994;32:197–200. doi: 10.1016/1056-8719(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 37.Oliveira MC, Pelegrini-da-Silva A, Tambeli CH, Parada CA. Peripheral mechanisms underlying the essential role of P2X3,2/3 receptors in the development of inflammatory hyperalgesia. Pain. 2009;141:127–134. doi: 10.1016/j.pain.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 38.Parada CA, Yeh JJ, Joseph EK, Levine JD. Tumor necrosis factor receptor type-1 in sensory neurons contributes to induction of chronic enhancement of inflammatory hyperalgesia in rat. Eur J Neurosci. 2003;17:1847–1852. doi: 10.1046/j.1460-9568.2003.02626.x. [DOI] [PubMed] [Google Scholar]
- 39.Parada CA, Yeh JJ, Reichling DB, Levine JD. Transient attenuation of protein kinase Cepsilon can terminate a chronic hyperalgesic state in the rat. Neuroscience. 2003;120:219–226. doi: 10.1016/s0306-4522(03)00267-7. [DOI] [PubMed] [Google Scholar]
- 40.Plenderleith MB, Snow PJ. The plant lectin Bandeiraea simplicifolia I-B4 identifies a subpopulation of small diameter primary sensory neurones which innervate the skin in the rat. Neurosci Lett. 1993;159:17–20. doi: 10.1016/0304-3940(93)90787-l. [DOI] [PubMed] [Google Scholar]
- 41.Radwin RG, Armstrong TJ, Vanbergeijk E. Vibration exposure for selected power hand tools used in automobile assembly. Am Ind Hyg Assoc J. 1990;51:510–518. doi: 10.1080/15298669091370013. [DOI] [PubMed] [Google Scholar]
- 42.Raju SG, Rogness O, Persson M, Bain J, Riley D. Vibration from a riveting hammer causes severe nerve damage in the rat tail model. Muscle Nerve. 2011;44:795–804. doi: 10.1002/mus.22206. [DOI] [PubMed] [Google Scholar]
- 43.Rasband MN, Park EW, Vanderah TW, Lai J, Porreca F, Trimmer JS. Distinct potassium channels on pain-sensing neurons. Proc Natl Acad Sci U S A. 2001;98:13373–13378. doi: 10.1073/pnas.231376298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009;32:611–618. doi: 10.1016/j.tins.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ritter DM, Zemel BM, Hala TJ, O'Leary ME, Lepore AC, Covarrubias M. Dysregulation of Kv3.4 channels in dorsal root ganglia following spinal cord injury. J Neurosci. 2015;35:1260–1273. doi: 10.1523/JNEUROSCI.1594-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rukwied R, Mayer A, Kluschina O, Obreja O, Schley M, Schmelz M. NGF induces non-inflammatory localized and lasting mechanical and thermal hypersensitivity in human skin. Pain. 2010;148:407–413. doi: 10.1016/j.pain.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 47.Schäfers M, Sorkin LS, Sommer C. Intramuscular injection of tumor necrosis factor-alpha induces muscle hyperalgesia in rats. Pain. 2003;104:579–588. doi: 10.1016/S0304-3959(03)00115-5. [DOI] [PubMed] [Google Scholar]
- 48.Schiavuzzo JG, Teixeira JM, Melo B, da Silva dos Santos DF, Jorge CO, Oliveira-Fusaro MC, Parada CA. Muscle hyperalgesia induced by peripheral P2X3 receptors is modulated by inflammatory mediators. Neuroscience. 2015;285:24–33. doi: 10.1016/j.neuroscience.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 49.Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 50.Shim B, Kim DW, Kim BH, Nam TS, Leem JW, Chung JM. Mechanical and heat sensitization of cutaneous nociceptors in rats with experimental peripheral neuropathy. Neuroscience. 2005;132:193–201. doi: 10.1016/j.neuroscience.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 51.Smith AK, O'Hara CL, Stucky CL. Mechanical sensitization of cutaneous sensory fibers in the spared nerve injury mouse model. Mol Pain. 2013;9:61. doi: 10.1186/1744-8069-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stoll G, Jander S, Myers RR. Degeneration and regeneration of the peripheral nervous system: from Augustus Waller's observations to neuroinflammation. J Peripher Nerv Syst. 2002;7:13–27. doi: 10.1046/j.1529-8027.2002.02002.x. [DOI] [PubMed] [Google Scholar]
- 53.Summer GJ, Romero-Sandoval EA, Bogen O, Dina OA, Khasar SG, Levine JD. Proinflammatory cytokines mediating burn-injury pain. Pain. 2008;135:98–107. doi: 10.1016/j.pain.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 54.Takeuchi T, Futatsuka M, Imanishi H, Yamada S. Pathological changes observed in the finger biopsy of patients with vibration-induced white finger. Scand J Work Environ Health. 1986;12:280–283. doi: 10.5271/sjweh.2140. [DOI] [PubMed] [Google Scholar]
- 55.Tanner KD, Reichling DB, Levine JD. Nociceptor hyper-responsiveness during vincristine-induced painful peripheral neuropathy in the rat. J Neurosci. 1998;18:6480–6491. doi: 10.1523/JNEUROSCI.18-16-06480.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uchida H, Sasaki K, Ma L, Ueda H. Neuron-restrictive silencer factor causes epigenetic silencing of Kv4.3 gene after peripheral nerve injury. Neuroscience. 2010;166:1–4. doi: 10.1016/j.neuroscience.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 57.Vydyanathan A, Wu ZZ, Chen SR, Pan HL. A-type voltage-gated K+ currents influence firing properties of isolectin B4-positive but not isolectin B4-negative primary sensory neurons. J Neurophysiol. 2005;93:3401–3409. doi: 10.1152/jn.01267.2004. [DOI] [PubMed] [Google Scholar]