Abstract
Introduction
This study characterized the relationship of patient-reported functional limitations, gait speed, and mortality risk among cancer survivors.
Materials and Methods
This study included cancer survivors from the Third National Health and Nutrition Survey. Patient-reported functional limitations were quantified by asking participants to assess their ability to complete five tasks: (1) walking ¼ mile, (2) walking up 10-steps, (3) stooping, crouching, kneeling, (4) lifting or carrying an object of 10 pounds, and (5) standing up from an armless chair. Gait speed was quantified using a 2.4-meter walk. Vital status was obtained through the United States National Center for Health Statistics.
Results
The study sample included 428 cancer survivors who averaged 72.1 years of age. The average number of patient-reported functional limitations was 1.8 (out of 5) and 66% of participants reported ≥1 functional limitation. Patient-reported functional limitations and gait speed were related, such that each functional limitation associated with a −0.08 meter/second slower gait speed (95% Confidence Interval: −0.10 to −0.06; P<0.001). During a median follow-up of 11-years, 329 (77%) participants died. In multivariable-adjusted analysis, patient-reported functional limitations and survival were related, such that each additional reported functional limitation was associated with a 19% increase in the risk of death (95% Confidence Interval: 9 to 29%; P<0.001).
Discussion
Patient-reported functional limitations are prevalent among cancer survivors, and associate with slower gait speeds and shorter survival. These data may provide increased insight on long-term prognosis and inform clinical decision-making by identifying subgroups of cancer survivors who may benefit from rehabilitative intervention.
Keywords: physical function, aging, oncology, disability, physical activity, exercise
INTRODUCTION
The assessment of functional limitations is an important component for evaluating the overall health and physiologic reserve of cancer survivors (1-3). After a diagnosis of cancer, patient-reported physical function deteriorates at an accelerated rate compared to that of age-matched cancer-free persons (4, 5). This may be a result of cancer treatment, which impairs multiple physiologic systems such as the cardiopulmonary (6, 7), neurologic (8), and musculoskeletal systems (9, 10), that are necessary to enable physical function. Treatment-related physiologic impairments may explain why cancer survivors are up to nine-fold more likely to report a functional limitation compared to similar-aged persons without a history of cancer (11, 12).
Clinicians that appropriately characterize functional limitations may have unique insight into their patients’ risk of progression in the disablement pathway (13). Options to measure functional limitations include validated objective metrics of physical function such as gait speed, also known as walking speed, which predicts survival among older adults and cancer survivors (14, 15). Gait speed is also associated with cognitive impairment, cardiopulmonary disease, hospitalization, and nursing home placement (16). Alternatively, implementing patient-reported outcomes of physical function may be more feasible in clinical practice, but studies to date have not confirmed that patient-reported functional limitations correlate with objectively-measured physical function, such as gait speed, among cancer survivors.
Identifying clinical assessments that accurately risk-stratify patients who have survived cancer will benefit care providers and scientists in targeting therapies to the most vulnerable cancer survivors. Therefore, the goal of this study was to characterize the association between patient-reported functional limitations and objectively-measured physical function (i.e., gait speed), and describe the relationship between patient-reported functional limitations and mortality risk among a population-based sample of cancer survivors.
METHODS
Study Design
The Third National Health and Nutrition Examination Survey, 1988–1994 (NHANES III) was a stratified multistage study designed to provide health information on a nationally-representative sample of U.S. civilians (17). A stratified multistage sampling design was used to select participants that were representative of the U.S. population. The four sampling stages included: 1) counties within states; 2) city blocks within each county; 3) households within each city block and; 4) individuals within each household. The study protocol for NHANES III was approved by the National Center for Health Statistics of the Centers for Disease Control and Prevention Institutional Review Board. All participants provided written informed consent prior to participating in any study-related activities.
Study Participants
Participants aged ≥60 years were invited to complete an evaluation that included patient-reported measures of functional limitations and objective measures of physical function (18). We identified 4,881 participants who completed the requisite study measures, 428 (9%) of whom reported a prior diagnosis of non-skin-related cancer.
Patient-Reported Functional Limitations
Functional limitations were assessed by asking participants to report the level of difficulty for five common tasks that included: (1) walking for a ¼ quarter of a mile, (2) walking up 10-steps, (3) stooping, crouching, or kneeling, (4) carrying something as heavy as 10 pounds, and (5) standing up from an armless chair. For each question, participants were provided answers of: (1) no difficulty, (2) some difficulty, (3) much difficulty, and (4) unable to do. Participants who reported at least some difficulty were considered to have functional limitation in that task (19-21). Participants who reported limitations in three or more tasks were classified as disabled (19).
Objectively-Measured Physical Function
Gait speed is an objective measure that quantifies overall health and functional ability (15, 22), and has been shown to predict survival among cancer survivors (14). Gait speed was assessed using a 2.4-meter walk on a straight and level surface (18). Time required to complete the 2.4-meter course was recorded to the nearest tenth of a second using a stopwatch. Gait speed was quantified in units of meters per second (m/s), by dividing 2.4-meters into the number of seconds required to complete the walk.
Mortality Outcome
The primary outcome of this study was death from any cause. Vital status was identified using the National Death Index (NDI) database through December 31, 2006. Participants were linked to the NDI database using a probabilistic matching algorithm that included 12 identifiers including Social Security Number, sex, date of birth, race, state of residence, and marital status (23). The United States National Center for Health Statistics found that 96.1% of deceased participants and 99.4% of living participants were correctly classified using the probabilistic matching algorithm (24).
Covariates
Demographic information including date of birth and sex were patient-reported using a standardized questionnaire. Clinical information including type of cancer, date of cancer diagnosis, smoking history, alcohol consumption, hospitalizations in the prior year, patient-reported health status, and frequency of physical activity were assessed using standardized questionnaires. Bouts of walking in the past week were patient-reported and included any bout of walking that was estimated to be ≥1 mile in duration, and of moderate or vigorous intensity. The presence of comorbid health conditions were determined by asking participants if a doctor had ever told them that they had any of the following: hypertension, diabetes, hyperlipidemia, asthma, arthritis, myocardial infarction, stroke, or congestive heart failure.
Height in meters and weight in kilograms were measured by study technicians. Body mass index was calculated as weight divided by the square of height (kg/m2). The healthy eating index (HEI) was calculated from 24-hour food recalls to form a score than ranges from 0 to 100 to quantify aspects of a healthy diet (25). Hemoglobin was quantified using a Coulter S-Plus Jr electronic counter (Coulter Electronics, Hialeah, FL) with a coefficient of variation <3.0%. Albumin was quantified using a Hitachi 737 multichannel analyzer (Boehringer Mannheim Diagnostics, Indianapolis, IN, USA) with a coefficient of variation <2.8%. C-reactive protein was quantified using latex-enhanced nephelometry immunoassay (Behring Diagnostics, Somerville, NJ) with a coefficient of variation <6.3%. Detailed blood collection procedures and laboratory assay methods used in NHANES III are described elsewhere (26).
Statistical Analysis
Descriptive variables are presented as means and 95% confidence intervals (95% CI) for continuous variables and percentages for categorical variables. We used unadjusted linear regression models to estimate the mean difference in gait speed by varying levels of patient-reported functional limitation. We used Cox proportional hazards regression models to estimate the hazard ratio (HR), and 95% CI for all-cause mortality by varying levels of patient-reported functional limitation. Models were estimated adjusted for sex and age (model 1) and fully-adjusted for demographic, behavioral, and clinical characteristics (model 2). We confirmed the assumption of proportional hazards for all analyses using log-log plots. To determine if the observed relationships varied between younger (<75 years) and older (≥75 years) cancer survivors we incorporated a statistical interaction between functional limitation and age category when modeling gait speed and survival. No statistically significant interactions were identified; consequently all analyses are presented for the overall cohort. We incorporated sample weights into all statistical analyses to account for nonresponse bias, multistage sampling probabilities, and the subpopulation of participants that were included in this analysis (27). Stata/SE v.13.1 statistical software was used for all analyses.
RESULTS
Participant Characteristics
Among 428 cancer survivors, the average age was 72.1 (95% CI: 71.3-73.0). Participant characteristics stratified by age category (Table 1) demonstrate that those who were older were less likely to be smokers, consumed fewer alcoholic drinks, less likely to report hyperlipidemia, and less likely to participate in regular physical activity, but were more likely to report a history of myocardial infarction and stroke, and had a slower gait speed.
Table 1.
Demographic and Clinical Characteristics, Stratified by Age Categorya
| Age |
||||
|---|---|---|---|---|
| Characteristic | Overall (n=428) [mean or (%)] |
<75 years (n=204) |
≥75 years (n=224) |
P-Value |
| Age — yr. | 72.1 (71.3-73.0) | 67.2 (66.5-68.0) | 80.4 (79.8-81.1) | <0.001 |
| Sex | ||||
| Male | 38.9% | 37.4% | 41.5% | 0.483 |
| Female | 61.1% | 62.6% | 58.5% | |
| Type of Cancer | ||||
| Breast | 26.8% | 28.7% | 23.5% | 0.201 |
| Genitourinary | 21.0% | 15.5% | 24.1% | |
| Gastrointestinal | 18.7% | 19.6% | 23.5% | |
| Gynecologic | 14.2% | 17.3% | 9.0% | |
| Lung | 2.9% | 3.5% | 1.8% | |
| Hematologic | 2.3% | 2.4% | 2.4% | |
| Other, missing, or can’t remember | 14.1% | 13.1% | 15.7% | |
| Time Since Cancer Diagnosis — yr. | ||||
| Mean (continuous) | 10.9 (9.7-12.1) | 10.8 (9.1-12.5) | 11.1 (9.5-12.7) | 0.792 |
| <5 | 33.9% | 33.8% | 34.0% | 0.610 |
| 5-10 | 21.4% | 23.1% | 18.6% | |
| ≥10 | 44.7% | 43.1% | 47.4% | |
| Body Mass Index — kg/m2 | ||||
| Mean (continuous) | 26.0 (25.3-26.7) | 26.5 (25.5-27.4) | 25.2 (24.5-26.0) | 0.060 |
| <18.5 | 3.4% | 1.8% | 6.1% | 0.136 |
| 18.5-24.9 | 43.4% | 42.1% | 45.5% | |
| 25.0-29.9 | 34.5% | 34.6% | 34.4% | |
| ≥30.0 | 18.7% | 21.5% | 14.0% | |
| Smoking Status — n (%) | ||||
| Never | 41.7% | 36.6% | 50.3% | 0.025 |
| Former | 45.3% | 47.2% | 42.1% | |
| Current | 13.0% | 16.2% | 7.6% | |
| Healthy Eating Index | 69.1 (67.4-70.8) | 69.3 (67.1-71.6) | 68.6 (66.2-71.1) | 0.675 |
| No. of alcoholic drinks consumed in past wk. | 1.1 (0.8-1.4) | 1.3 (0.9-1.7) | 0.8 (0.5-1.1) | 0.052 |
| Comorbid health conditions | ||||
| Hypertension | 48.1% | 51.1% | 43.1% | 0.189 |
| Diabetes | 11.3% | 9.9% | 13.7% | 0.342 |
| Hyperlipidemia | 32.4% | 38.2% | 22.7% | 0.008 |
| Asthma | 7.1% | 7.7% | 6.1% | 0.596 |
| Arthritis | 46.9% | 46.0% | 48.5% | 0.683 |
| Myocardial infarction | 14.5% | 10.8% | 20.8% | 0.014 |
| Stroke | 8.2% | 4.7% | 14.1% | 0.004 |
| Congestive heart failure | 7.8% | 7.0% | 9.3% | 0.495 |
| Hospitalization(s) in past yr. — n (%) | 28.9% | 30.6% | 26.1% | 0.382 |
| Hemoglobin (g/dL) | 13.5 (13.4-13.7) | 13.6 (13.4-13.8) | 13.5 (13.3-13.7) | 0.648 |
| Albumin (g/dL) | 4.0 (3.9-4.1) | 4.0 (4.0-4.1) | 3.9 (3.9-4.0) | 0.064 |
| C-reactive protein (mg/L) | 0.6 (0.5-0.7) | 0.5 (0.4-0.6) | 0.7 (0.5-0.8) | 0.153 |
| No. of bouts of walking in past wk. | ||||
| 0 | 76.0% | 69.7% | 86.6% | 0.003 |
| 1-3 | 6.7% | 8.3% | 3.9% | |
| ≥3 | 17.3% | 22.0% | 9.5% | |
| Gait Speed — meters/second | ||||
| Mean (continuous) | 0.67 (0.64-0.71) | 0.75 (0.71-0.79) | 0.55 (0.51-0.59) | <0.001 |
| <0.6 | 31.2% | 16.9% | 55.3% | <0.001 |
| ≥0.6 | 68.8% | 83.1% | 44.7% | |
Continuous variables are mean (95% confidence interval) and categorical variables are column percentages
Association between Patient-Reported Functional Limitations and Objective Physical Function
The prevalence of patient-reported functional limitations ranged from 27.3% for standing up from an armless chair to 53.3% for stooping, crouching, and kneeling (Table 2). The average number of functional limitations was 1.8 (95% CI: 1.6-2.0) and 66% of participants reported ≥1 functional limitation. The average gait speed was 0.67 m/s (95% CI: 0.64-0.71). A cane and walker were used by 25 (5.8%) and 10 (2.3%) cancer survivors during the 2.4-meter walk, respectively. Participants with functional limitation in any of the five tasks had slower gait speed compared to those without functional limitation (all P<0.001). As the total number of functional limitations increased, gait speed decreased linearly, such that each additional functional limitation associated with a −0.08 m/s [(95% CI: −0.10 to −0.06); P<0.001] decrease in gait speed. The prevalence of disability was 31.1%, and disabled participants had slower gait speeds compared to participants who were non-disabled [−0.28 m/s (95% CI: −0.35 to −0.21); P<0.001].
Table 2.
Prevalence of Patient-Reported Functional Limitation and Association with Objectively Measured Physical Function
| Patient-Reported Functional Limitation |
Proportion (%) |
Gait Speed, Mean (95% CI) |
Mean Difference (95% CI) |
P |
|---|---|---|---|---|
| Walking ¼ Mile | ||||
| Limited | 33.4% | 0.50 (0.44–0.56) | −0.27 (−0.33 to −0.20) | <0.001 |
| Not Limited | 66.6% | 0.77 (0.74–0.80) | ||
| Walking Up 10-Steps | ||||
| Limited | 30.8% | 0.49 (0.42–0.55) | −0.27 (−0.34 to −0.20) | <0.001 |
| Not Limited | 69.2% | 0.76 (0.73–0.79) | ||
| Stooping, Crouching, Kneeling | ||||
| Limited | 53.3% | 0.60 (0.55–0.66) | −0.15 (−0.22 to −0.09) | <0.001 |
| Not Limited | 46.7% | 0.76 (0.73–0.79) | ||
| Lifting or Carrying Object of 10 lb. | ||||
| Limited | 32.0% | 0.53 (0.46–0.60) | −0.22 (−0.29 to −0.14) | <0.001 |
| Not Limited | 68.0% | 0.75 (0.72–0.78) | ||
| Standing Up From Armless Chair | ||||
| Limited | 27.3% | 0.47 (0.40–0.54) | −0.28 (−0.36 to −0.21) | <0.001 |
| Not Limited | 72.7% | 0.75 (0.72–0.78) | ||
|
| ||||
| Total Number of Limitations | ||||
| 0 | 33.8% | 0.78 (0.74–0.82) | 0.00 — Referentb | |
| 1 | 24.4% | 0.75 (0.69–0.81) | −0.03 (−0.10 to 0.04) | 0.346 |
| 2 | 10.7% | 0.74 (0.68–0.80) | −0.05 (−0.12 to 0.02) | 0.202 |
| 3 | 8.1% | 0.67 (0.63–0.73) | −0.10 (−0.17 to −0.04) | 0.001 |
| 4 | 8.0% | 0.61 (0.51–0.72) | −0.17 (−0.28 to −0.06) | 0.004 |
| 5 | 15.0% | 0.32 (0.24–0.39) | −0.47 (−0.55 to −0.38) | <0.001 |
| Composite Disability Outcomea | ||||
| Disabled | 31.1% | 0.49 (0.42–0.54) | −0.28 (−0.35 to −0.21) | <0.001 |
| Non-Disabled | 68.9% | 0.76 (0.73–0.79) | ||
Defined as self-reporting limitations in ≥3 of 5 functional limitations.
Ptrend<0.001; the mean difference and 95% CI for total number of functional difficulties as a continuous variable was −0.08 (−0.10 to −0.06).
Patient-Reported Functional Limitations and Survival
Limitations reported in walking ¼ mile, walking up 10-steps, lifting or carrying an object of 10 pounds, and standing up from an armless chair were associated with survival in multivariable-adjusted regression models (Table 3). Limitations in stooping, crouching, and keeling were not associated with survival in the multivariable-adjusted regression model.
Table 3.
Association of Patient-Reported Functional Limitation and Association with All-Cause Mortality
| Patient-Reported Functional Limitation |
Death Rate, Per 100 Person-Years (95% CI) |
Median Survival, Years [Interquartile Range] |
Hazard Ratio (95% CI) |
|||
|---|---|---|---|---|---|---|
| Model 1a | P | Model 2b | P | |||
| Walking ¼ Mile | ||||||
| Limited | 12.3 (9.9–15.2) | 6.3 [2.5–11.2] | 1.88 (1.40–2.52) | <0.001 | 1.57 (1.12–2.21) | 0.009 |
| Not Limited | 6.1 (5.2–7.2) | 13.1 [6.5–NR]f | 1.00 — Referent | — | 1.00 — Referent | — |
| Walking Up 10-Steps | ||||||
| Limited | 12.5 (10.1–15.4) | 6.3 [2.6–11.8] | 2.01 (1.52–2.66) | <0.001 | 1.78 (1.33–2.39) | <0.001 |
| Not Limited | 6.1 (5.2–7.3) | 12.1 [6.3–NR]f | 1.00 — Referent | — | 1.00 — Referent | — |
| Stooping, Crouching, Kneeling | ||||||
| Limited | 8.9 (7.5–10.7) | 8.7 [3.2–15.0] | 1.31 (1.01–1.70) | 0.046 | 1.27 (0.94–1.71) | 0.117 |
| Not Limited | 6.3 (5.1–7.8) | 12.1 [6.5–NR]f | 1.00 — Referent | — | 1.00 — Referent | — |
| Lifting or Carrying Object of 10 lb. | ||||||
| Limited | 10.2 (8.1–12.8) | 7.8 [2.6–13.1] | 1.57 (1.16–2.11) | 0.003 | 1.64 (1.16–2.32) | 0.005 |
| Not Limited | 6.7 (5.6–7.9) | 12.1 [5.5–16.7] | 1.00 — Referent | — | 1.00 — Referent | — |
| Standing Up From Armless Chair | ||||||
| Limited | 11.9 (9.0–15.4) | 6.7 [2.5–11.2] | 1.66 (1.21–2.29) | 0.002 | 1.73 (1.25–2.41) | 0.001 |
| Not Limited | 6.5 (5.6–7.6) | 12.0 [5.7–NR]f | 1.00 — Referent | — | 1.00 — Referent | — |
|
| ||||||
| Total Number of Limitations | ||||||
| 0 | 5.2 (4.0–6.7) | 13.7 [7.1–NR]f | 1.00 — Referentd | — | 1.00 — Referente | — |
| 1 | 7.5 (5.7–9.8) | 11.3 [4.2–16.7] | 1.32 (0.92–1.90 | 0.129 | 1.39 (0.87–2.23) | 0.166 |
| 2 | 8.0 (5.5–11.5) | 11.2 [4.1–15.6] | 1.37 (0.87–2.16 | 0.171 | 1.35 (0.80–2.26) | 0.256 |
| 3 | 8.7 (5.9–12.8) | 8.6 [5.5–13.9] | 1.70 (1.06–2.72 | 0.027 | 1.86 (1.03–3.37) | 0.039 |
| 4 | 10.3 (7.3–14.4) | 8.0 [4.7–11.7] | 1.71 (1.12–2.61 | 0.013 | 1.71 (1.04–2.79) | 0.033 |
| 5 | 15.2 (9.9–22.6) | 4.9 [1.6–8.8] | 2.84 (1.77–4.56) | <0.001 | 2.71 (1.67–4.41) | <0.001 |
| Composite Disability Outcomec | ||||||
| Disabled | 11.7 (9.3–15.6) | 6.7 [2.6–11.3] | 1.80 (1.34–2.42) | <0.001 | 1.76 (1.26–2.44) | 0.001 |
| Non-Disabled | 6.3 (5.4–7.5) | 12.5 [6.2–NR]f | 1.00 — Referent | — | 1.00 — Referent | — |
Adjusted for age and sex.
Multivariable-adjusted for age, sex, type of cancer, time since cancer diagnosis (continuous), body mass index (continuous), smoking status, healthy eating index, weekly drinking, hypertension, diabetes, asthma, arthritis, myocardial infarction, stroke, congestive heart failure, hospitalization in the prior year, hemoglobin, albumin, and C-reactive protein, and weekly walking (ordinal).
Defined as self-reporting limitations in ≥3 of 5 functional limitations.
Ptrend=0.001; the age and sex-adjusted hazard ratio and 95% CI for total number of functional difficulties as a continuous variable was 1.20 (1.10-1.30).
Ptrend<0.001; the multivariable-adjusted hazard ratio and 95% CI for total number of functional difficulties as a continuous variable was 1.19 (1.09-1.29).
NR: not reached
When the five functional limitations were simultaneously entered into a multivariable-adjusted regression model, patient-reported difficulty walking up 10 steps was associated with survival [HR=1.49 (95 % CI: 1.03-2.15); P=0.035]. Patient-reported difficulty walking ¼ mile [HR: 1.05 (95% CI: 0.62-1.78); P=0.853], stooping, crouching, kneeling [HR: 0.97 (95% CI: 0.68-1.36); P=0.844], lifting or carrying an object of 10 pounds [HR: 1.30 (95% CI: 0.85-2.01); P=0.228], and standing up from an armless chair [HR: 1.22 (95% CI: 0.76-1.95); P=0.415] were not independently associated with survival.
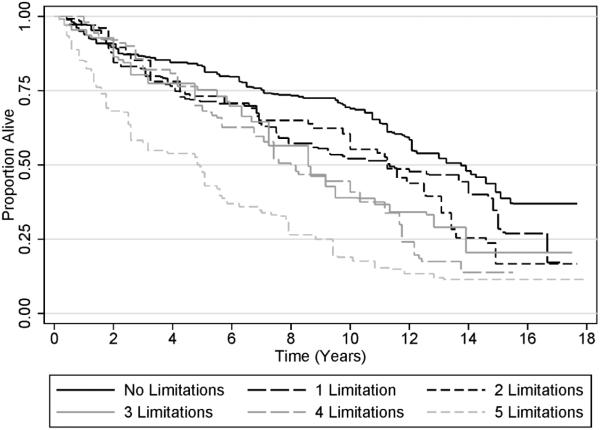
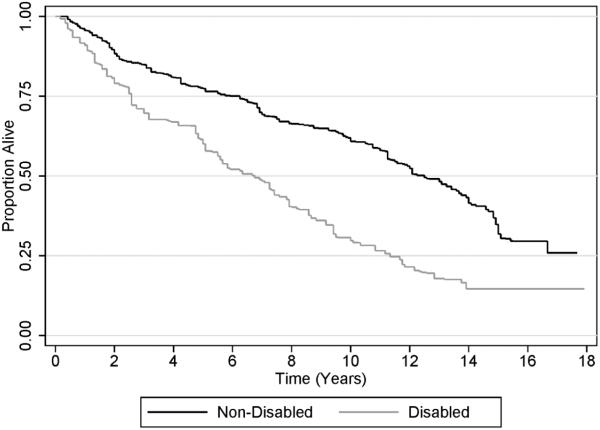
Figure 1 shows that as the total number of functional limitations increased, the risk of death increased [HR: 1.19 per additional limitation (95% CI: 1.09-1.29); P<0.001]. Disabled cancer survivors had a 76% greater risk of death than non-disabled cancer survivors [HR: 1.76 (95% CI: 1.26-2.44); P=0.001; (Figure 2)].
Figure 1.
Kaplan-Meier Plot of Survival, Stratified by Number of Patient-Reported Functional Limitations
Figure 2.
Kaplan-Meier Plot of Survival, Stratified by Composite Disability Outcome
Given that more functional limitations could reflect impending death, in exploratory analyses we excluded participants who died within one to five years of completing the functional limitations questionnaire and our results remained unchanged (data not shown).
DISCUSSION
Cancer survivors are a heterogeneous population representing an array of functional capacities and physiologic reserves (28). In this nationally-representative cohort of cancer survivors, patient-reported functional limitations were highly prevalent, 66% of participants patient-reported one or more functional limitation and 31% of participants reported three or more functional limitations (i.e., functionally disabled). Cancer survivors with functional limitations were more likely to die than those without functional limitations, and patient-reported limitations strongly correlated with the degree of objective limitations as measured by gait speed, a known predictor of mortality among cancer survivors (14). These data suggest that integrating queries of patient-reported physical function may provide increased insight on long-term prognosis of cancer survivors and aid in clinical decision making.
Our findings support prior reports that functional limitations are prevalent among cancer survivors (11, 12), and provide new evidence that patient-reported functional limitations are associated with slower measured gait speed. Cancer survivors are significantly more likely to report poor physical function compared to non-cancer controls (11, 12). However, interpretation of these prior reports has been challenging because certain subgroups of adults may be unable to accurately estimate their physical capacity (29). Gait speed is considered a gold-standard measure of physical function (30), and is associated with overall health, survival, cognitive impairment, cardiopulmonary disease, hospitalization, and nursing home placement among older adults (16). However, in settings in which implementing objective metrics of physical function are not as feasible as administering patient-reported metrics, our data demonstrate that patient-reported functional limitations are consistently associated with slower gait speeds among cancer survivors, as well as with mortality risk. For example, as an ordinal measure, each patient-reported functional limitation was associated with a −0.08 m/s slower gait speed (P<0.001). This is clinically meaningful because declines in gait speed are a predictor of disability (31) and conversely, improvements in gait speed are a predictor of improved survival (32). The observed relationship between patient-reported functional limitations and objectively-measured gait speed suggests that using patient-reported functional limitations to inform patient prognosis is a potentially valid strategy in cancer survivors. Our findings also highlight the gravity of prior studies that have documented that patient-reported functional limitations are highly prevalent among cancer survivors.
This study also found that patient-reported functional limitations were associated with survival, complementing the known relationship between objectively-measured physical function and survival among cancer survivors (14). The ability to perform functional activities without difficulty is critical to maintaining a high quality-of-life and avoiding premature morbidity and mortality. In a prior study of 7,417 patients representing 11 different types of cancer, higher patient-reported global physical functioning was associated with a reduced likelihood of death (33). Our study complements these findings by adding knowledge of the relationship of specific functional limitations and survival. For example, difficulty stooping, crouching or kneeling was not associated with survival (HR: 1.27, P=0.117). Conversely, difficulty walking up 10-steps emerged as the most important prognostic factor for mortality in a fully adjusted model (HR: 1.49, P=0.035). These data deepen our understanding between specific patient-reported functional limitations and survival among cancer survivors, and may inform prognosis of cancer survivorship beyond that of traditional demographic, behavioral, and clinical variables.
As a majority of participants in this study were older and long-term survivors of cancer, the results of this study may inform geriatric oncology practice. The National Comprehensive Cancer Network (NCCN) and International Society of Geriatric Oncology (SIOG) recommend that a geriatric assessment be performed to guide healthcare providers with selecting the best cancer treatments for older adults (2, 34). The geriatric assessment has been emphasized at time points prior to the initiation of cancer treatment (3). While less focus has been made to the potential importance of the geriatric assessment after the completion of cancer treatment, this focus is necessary to promptly identify and intervene on functional impairments, before they contribute to a trajectory of worsening health (35). Therefore the geriatric assessment may be a clinically important tool at multiple points along the cancer care continuum, from pretreatment decision making to the monitoring of health and wellness in long-term survivorship.
Identifying cancer survivors who report functional limitations may help to demarcate a population that may benefit from rehabilitative intervention (36). Physical activity is a promising intervention that may provide numerous benefits for cancer survivors (37). For example, among 1,600 adults with lower-extremity functional limitations, a structured physical activity program focused on walking resulted in an 18% reduction in the development of major mobility disability, defined as the inability to walk 400-meters, over 2.6 years (38). A program of home-based diet and exercise significantly attenuated the loss of physical function among 641 older, overweight survivors of breast, colorectal, and prostate cancer over one year (39). Among breast cancer survivors, slowly progressive weight lifting slowed the rate of deterioration of physical function over one year (40). For certain cancer sites, observational studies suggest physical activity may be associated with a reduction in cancer-specific mortality (41, 42), though this relationship has not yet been confirmed in a randomized controlled trial setting. Collectively, these data demonstrate the potential utility for physical activity, and provide evidence for the continued investigation of physical activity as an adjuvant intervention in oncology practice.
The results of this study must be considered alongside its strengths and limitations. First, this analysis was not able to identify incident cases of patient-reported functional limitation. We cannot completely exclude the possibility that patient-reported functional limitation may be reflective of occult disease (i.e., reverse causality). In our exploratory analyses, excluding participants who died within one to five years of completing the functional limitations questionnaire did not alter our results. This study used a non-standardized measure of functional limitation that was designed to briefly cover a wide range of functional activities as derived from the work of Nagi, Rosow, Breslau, and Lawton (43). We did not have information regarding stage of cancer or specific cancer treatments received. Comorbid health conditions were self-reported, which may underestimate the burden of comorbidity in our sample. It is likely that these aforementioned variables may have attenuated the association between patient-reported functional limitation and survival by accounting for variation associated with cancer stage, specific cancer treatments received, and comorbid health conditions. However, this limitation is unlikely to impact the clinical importance of our findings to identify and rehabilitate cancer survivors with patient-reported functional limitations. Our analysis did not include cancer survivors who were unable to safely complete the 2.4-meter walk used to quantify gait speed (n=17). Therefore our analyses are applicable to cancer survivors who are able to safely walk without assistance from another person. We relied solely on gait speed as an objective measure of physical function. Gait speed does not quantify upper-extremity function such as that of the arm or hand.
This study also possesses several strengths. As a result of the sampling framework of NHANES, our study cohort is a nationally-representative sample of community-dwelling cancer survivors in the United States (17). Our study had an extensive median length of follow up of 11.0-years, which allowed us to observe a high proportion of deaths. We accounted for a variety of covariates that may influence the association of patient-reported functional limitation and survival such as demographic, behavioral, and clinical characteristics, including blood chemistry measures.
In conclusion, patient-reported functional limitations are highly prevalent, are associated with slower gait speeds, and are associated with survival among cancer survivors. These data inform clinicians seeking to improve their insight on long-term prognosis and identify subgroups of cancer survivors in need of rehabilitative intervention. The findings demonstrate the need for additional research in the area of geriatric oncology, to find efficacious interventions to prevent or treat functional limitations among cancer survivors. Physical activity is a promising intervention with many possible benefits for cancer survivors. Randomized studies are necessary to determine if physical activity can prevent, delay, or rehabilitate functional limitations in this population, and improve cancer survivorship.
ACKNOWLEDGEMENT
Justin C. Brown had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Research reported in this publication was supported by the National Cancer Institute (F31-CA192560, R21-CA182726), National Heart, Lung, and Blood Institute (F31-HL127947) and the National Institute of Diabetes and Digestive and Kidney Diseases (F32-DK096758) of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES AND CONFLICT OF INTEREST STATEMENTS
The authors declare they have no conflicts of interest to disclose in relation to this work.
AUTHOR CONTRIBUTIONS
Study Concepts: JC Brown, MO Harhay, MN Harhay
Study Design: JC Brown, MO Harhay, MN Harhay
Data Acquisition: JC Brown, MO Harhay, MN Harhay
Quality Control of Data and Algorithms: JC Brown, MO Harhay, MN Harhay
Data Analysis and Interpretation: JC Brown, MO Harhay, MN Harhay
Statistical Analysis: JC Brown, MO Harhay
Manuscript Preparation: JC Brown (initial draft)
Manuscript Editing: JC Brown, MO Harhay, MN Harhay
Manuscript Review: JC Brown, MO Harhay, MN Harhay
REFERENCES
- 1.Rowland JH, Bellizzi KM. Cancer survivorship issues: life after treatment and implications for an aging population. J Clin Oncol. 2014;32:2662–2668. doi: 10.1200/JCO.2014.55.8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wildiers H, Heeren P, Puts M, et al. International society of geriatric oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32:2595–2603. doi: 10.1200/JCO.2013.54.8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Puts MT, Hardt J, Monette J, Girre V, Springall E, Alibhai SM. Use of geriatric assessment for older adults in the oncology setting: a systematic review. J Natl Cancer Inst. 2012;104:1133–1163. doi: 10.1093/jnci/djs285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrick JL, Reeve BB, Kucharska-Newton AM, et al. Functional status declines among cancer survivors: Trajectory and contributing factors. Journal of geriatric oncology. 2014 doi: 10.1016/j.jgo.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petrick JL, Foraker RE, Kucharska-Newton AM, et al. Trajectory of overall health from self-report and factors contributing to health declines among cancer survivors. Cancer Causes & Control. 2014;25:1179–1186. doi: 10.1007/s10552-014-0421-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53:2231–2247. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 7.Jones LW, Eves ND, Haykowsky M, Freedland SJ, Mackey JR. Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. The lancet oncology. 2009;10:598–605. doi: 10.1016/S1470-2045(09)70031-2. [DOI] [PubMed] [Google Scholar]
- 8.Soussain C, Ricard D, Fike JR, Mazeron J, Psimaras D, Delattre J. CNS complications of radiotherapy and chemotherapy. The Lancet. 2009;374:1639–1651. doi: 10.1016/S0140-6736(09)61299-X. [DOI] [PubMed] [Google Scholar]
- 9.Tisdale MJ. Loss of skeletal muscle in cancer: biochemical mechanisms. Front Biosci. 2001;6:D164–74. doi: 10.2741/tisdale. [DOI] [PubMed] [Google Scholar]
- 10.Stava CJ, Jimenez C, Hu MI, Vassilopoulou-Sellin R. Skeletal sequelae of cancer and cancer treatment. J Cancer Surviv. 2009;3:75–88. doi: 10.1007/s11764-009-0083-4. [DOI] [PubMed] [Google Scholar]
- 11.Schootman M, Aft R, Jeffe DB. An evaluation of lower-body functional limitations among long-term survivors of 11 different types of cancers. Cancer. 2009;115:5329–5338. doi: 10.1002/cncr.24606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hewitt M, Rowland JH, Yancik R. Cancer survivors in the United States: age, health, and disability. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2003;58:M82–M91. doi: 10.1093/gerona/58.1.m82. [DOI] [PubMed] [Google Scholar]
- 13.Verbrugge LM, Jette AM. The disablement process. Soc Sci Med. 1994;38:1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- 14.Brown J, Harhay M, Harhay M. Physical function as a prognostic biomarker among cancer survivors. Br J Cancer. 2014 doi: 10.1038/bjc.2014.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA: the journal of the American Medical Association. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Kan GA, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13:881–889. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- 17.Plan and operation of the Third National Health and Nutrition Examination Survey, 1988-94. Series 1: programs and collection procedures. Vital Health Stat 1. 1994;(32):1–407. [PubMed] [Google Scholar]
- 18.OSTCHEGA Y, HARRIS TB, HIRSCH R, PARSONS VL, KINGTON R, KATZOFF M. Reliability and prevalence of physical performance examination assessing mobility and balance in older persons in the US: data from the Third National Health and Nutrition Examination Survey. J Am Geriatr Soc. 2000;48:1136–1141. doi: 10.1111/j.1532-5415.2000.tb04792.x. [DOI] [PubMed] [Google Scholar]
- 19.Tom SE, Cooper R, Patel KV, Guralnik JM. Menopausal characteristics and physical functioning in older adulthood in the National Health and Nutrition Examination Survey III. Menopause. 2012;19:283–289. doi: 10.1097/gme.0b013e3182292b06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davison KK, Ford ES, Cogswell ME, Dietz WH. Percentage of body fat and body mass index are associated with mobility limitations in people aged 70 and older from NHANES III. J Am Geriatr Soc. 2002;50:1802–1809. doi: 10.1046/j.1532-5415.2002.50508.x. [DOI] [PubMed] [Google Scholar]
- 21.Seeman TE, Merkin SS, Crimmins EM, Karlamangla AS. Disability trends among older Americans: National Health And Nutrition Examination Surveys, 1988-1994 and 1999-2004. Am J Public Health. 2010;100:100–107. doi: 10.2105/AJPH.2008.157388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 23.Rogot E, Sorlie P, Johnson NJ. Probabilistic methods in matching census samples to the National Death Index. J Chronic Dis. 1986;39:719–734. doi: 10.1016/0021-9681(86)90155-4. [DOI] [PubMed] [Google Scholar]
- 24.National Center for Health Statistics. The Third National Nutrition and Health Survey Linked Mortality File: Matching Methodology Available at: http://www.cdc.gov/nchs/data/datalinkage/mort_calibration_study.pdf.2014.
- 25.Kennedy ET, Ohls J, Carlson S, Fleming K. The healthy eating index: design and applications. J Am Diet Assoc. 1995;95:1103–1108. doi: 10.1016/S0002-8223(95)00300-2. [DOI] [PubMed] [Google Scholar]
- 26.National Center for Health Statistics. Laboratory Procedures Used for the Third National Health and Nutrition Exam Survey (NHANES III), 1988-1994 Available at: http://wonder.cdc.gov/wonder/sci_data/surveys/hanes/hanes3/type_txt/lab.asp.
- 27.Korn EL, Graubard BI. Epidemiologic studies utilizing surveys: accounting for the sampling design. Am J Public Health. 1991;81:1166–1173. doi: 10.2105/ajph.81.9.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohile SG, Xian Y, Dale W, et al. Association of a cancer diagnosis with vulnerability and frailty in older Medicare beneficiaries. J Natl Cancer Inst. 2009;101:1206–1215. doi: 10.1093/jnci/djp239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reuben DB, Seeman TE, Keeler E, et al. Refining the categorization of physical functional status: the added value of combining self-reported and performance-based measures. J Gerontol A Biol Sci Med Sci. 2004;59:1056–1061. doi: 10.1093/gerona/59.10.m1056. [DOI] [PubMed] [Google Scholar]
- 30.Studenski S. Bradypedia: is gait speed ready for clinical use? J Nutr Health Aging. 2009;13:878–880. doi: 10.1007/s12603-009-0245-0. [DOI] [PubMed] [Google Scholar]
- 31.Artaud F, Singh-Manoux A, Dugravot A, Tzourio C, Elbaz A. Decline in fast gait speed as a predictor of disability in older adults. J Am Geriatr Soc. 2015;63:1129–1136. doi: 10.1111/jgs.13442. [DOI] [PubMed] [Google Scholar]
- 32.Hardy SE, Perera S, Roumani YF, Chandler JM, Studenski SA. Improvement in usual gait speed predicts better survival in older adults. J Am Geriatr Soc. 2007;55:1727–1734. doi: 10.1111/j.1532-5415.2007.01413.x. [DOI] [PubMed] [Google Scholar]
- 33.Quinten C, Coens C, Mauer M, et al. Baseline quality of life as a prognostic indicator of survival: a meta-analysis of individual patient data from EORTC clinical trials. The lancet oncology. 2009;10:865–871. doi: 10.1016/S1470-2045(09)70200-1. [DOI] [PubMed] [Google Scholar]
- 34.Balducci L, Cohen HJ, Engstrom PF, et al. Senior adult oncology clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2005;3:572–590. doi: 10.6004/jnccn.2005.0032. [DOI] [PubMed] [Google Scholar]
- 35.Fried LP, Bandeen-Roche K, Chaves P, Johnson BA. Preclinical mobility disability predicts incident mobility disability in older women. Journals of Gerontology-Biological Sciences and Medical Sciences. 2000;55:M43. doi: 10.1093/gerona/55.1.m43. [DOI] [PubMed] [Google Scholar]
- 36.Brown CJ, Flood KL. Mobility limitation in the older patient: a clinical review. JAMA. 2013;310:1168–1177. doi: 10.1001/jama.2013.276566. [DOI] [PubMed] [Google Scholar]
- 37.Klepin HD, Mohile SG, Mihalko S. Exercise for Older Cancer Patients: Feasible and Helpful? Cancer and Aging: From Bench to Clinics. 2013:38. doi: 10.1159/000343597. [DOI] [PubMed] [Google Scholar]
- 38.Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: The life study randomized clinical trial. JAMA. 2014 doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morey MC, Snyder DC, Sloane R, et al. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors. JAMA: the journal of the American Medical Association. 2009;301:1883–1891. doi: 10.1001/jama.2009.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown JC, Schmitz KH. Weight Lifting and Physical Function Among Survivors of Breast Cancer: A Post Hoc Analysis of a Randomized Controlled Trial. J Clin Oncol. 2015 doi: 10.1200/JCO.2014.57.7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104:815–840. doi: 10.1093/jnci/djs207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmitz KH, Courneya KS, Matthews C, et al. American college of sports medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 43.Ostchega Y, Harris TB, Hirsch R, Parsons VL, Kington R. The prevalence of functional limitations and disability in older persons in the US: data from the National Health and Nutrition Examination Survey III. J Am Geriatr Soc. 2000;48:1132–1135. doi: 10.1111/j.1532-5415.2000.tb04791.x. [DOI] [PubMed] [Google Scholar]