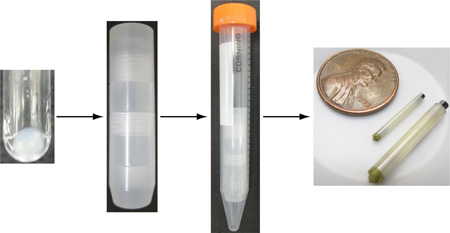
Abstract
The study of mass-limited biological samples by magic angle spinning (MAS) solid-state NMR spectroscopy critically relies upon the high-yield transfer of material from a biological preparation into the MAS rotor. This issue is particularly important for maintaining biological activity and hydration of semi-solid samples such as membrane proteins in lipid bilayers, pharmaceutical formulations, microcrystalline proteins and protein fibrils. Here we present protocols and designs for rotor-packing devices specifically suited for packing hydrated samples into Pencil-style 1.6 mm, 3.2 mm standard, and 3.2 mm limited speed MAS rotors. The devices are modular and therefore readily adaptable to other rotor and/or ultracentrifugation tube geometries.
Keywords: Solid-State NMR, Magic Angle Spinning, MAS Rotor-packing, Gel phase samples
Graphical abstract
Introduction
Magic angle spinning (MAS) solid-state NMR spectroscopy (SSNMR) is now a well-established analytical technique that has been used widely in the field of biomolecular research to study the structure and dynamics of many important biological molecules [1,2]. With all of these studies, sample preparation is a vital component, which often includes development of approaches to label molecules with isotopes, at substantial cost of materials and labor. The culmination of the sample preparation is an often very delicate transfer of the precious sample into the MAS rotor. At this stage, the integrity of the sample and yield of transfer are key design criteria. In many biological SSNMR samples, the hydration of the analyte has been shown to be an important factor in maximizing the resolution of spectra [3–5]. Thus, proper hydration is a critical consideration when preparing and packing samples into SSNMR rotors.
The optimal method for packing a sample depends upon the sample type. Some samples can tolerate lyophilization and rehydration, in which case standard methods for packing dry powders can be utilized, and then the sample rehydrated by adding water or buffer directly into the rotor prior to sealing. However, this approach often fails to hydrate samples uniformly, and the spectral quality is typically not as good with lyophilized proteins as with microcrystals [5,6]. To avoid complications of rehydrating samples in the rotor, it would be preferred to pack the wet, semi-solid sample directly into the rotor. However, many approaches to manipulate such samples are complicated by the high viscosity and adhesive properties of the pellets.
Specifically, hydrated semi-solid materials behave like pastes or gels that are difficult to scoop with tools designed for dry powders. The high viscosity also prohibits pipetting operations. To address these challenges, customized packing funnels have been developed [7], enabling samples to be centrifuged directly into the rotors to reduce sample loss. Nevertheless, sample loss can still occur upon transfer of the material from the penultimate preparation step into the funnel. An alternative method [8] achieves packing by transferring material into rotors by centrifugation directly from centrifuge tubes, using micropipette tips as funnels. Though this method works efficiently for the transfer of samples into larger rotors (e.g. 3.2 mm), it again requires multiple transfer steps and is difficult to scale down to smaller rotors, given the limitations on the physical robustness of small micropipette tips.
In this contribution, we present a series of rotor-packing devices suitable for packing soft, gel phase samples into Pencil style 1.6 mm, 3.2 mm standard, and 3.2 mm limited speed (thin wall) MAS rotors with minimal sample loss. Samples are transferred directly from 200 µL ultracentrifuge tubes into rotors using moderate speed centrifugation for the final transfer. The device is robust, enabling nearly quantitative transfer into the rotor, and material not transferred can be recovered since the parts are inexpensive and chemically resistant. Moreover, the modularity of the device allows it to be adapted to different sized rotors and centrifuge tubes with minimal modification.
Results and Discussion
Design of device
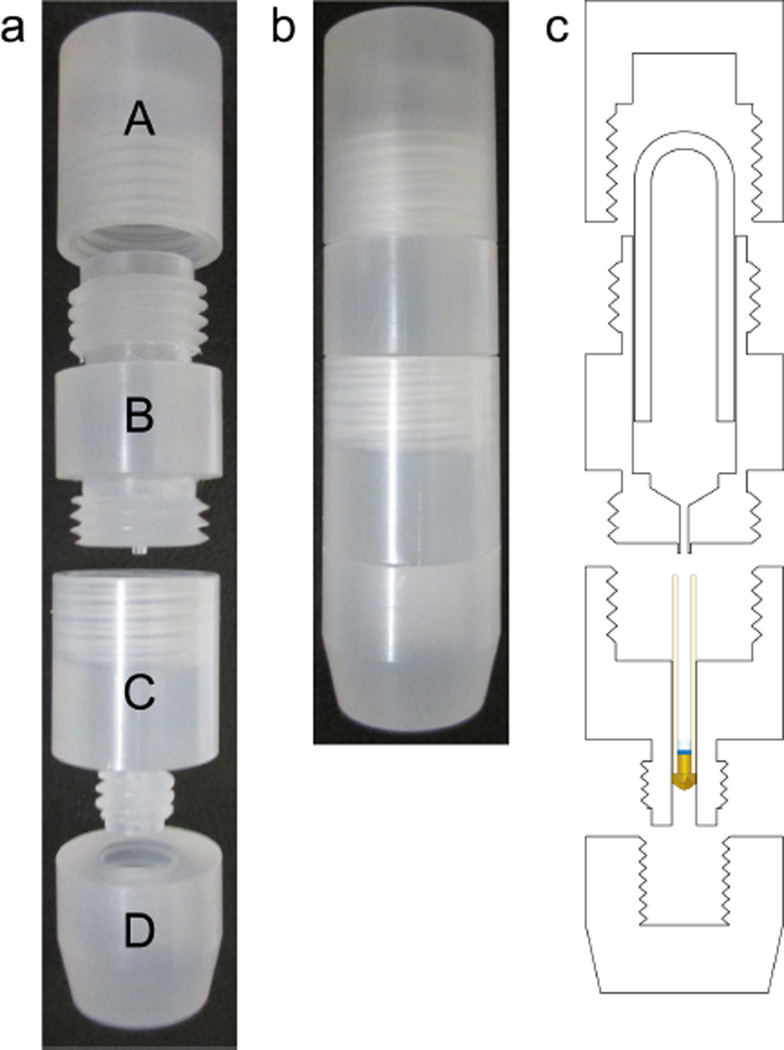
Three rotor-packing devices (RPDs) were designed to pack hydrated, gel phase samples into 1.6 mm, 3.2 mm standard wall, and 3.2 mm limited speed (thin wall) Pencil-style MAS rotors directly from 200 µL ultracentrifuge tubes. As shown in Fig. 1, these devices are comprised of 4 parts (A, B, C, D) that machined out of polychlorotrifluoroethylene (PCTFE, also known as Kel-F) and screwed together as an assembly.
Fig. 1.
The rotor-packing device is made of four threaded parts (a) and are screwed together (b). The four parts of the top piece, funnel piece, rotor chamber, and bottom piece. The device is designed to pack samples directly from an inverted 200 µL ultracentrifuge tube into a Pencil-style MAS rotor (c).
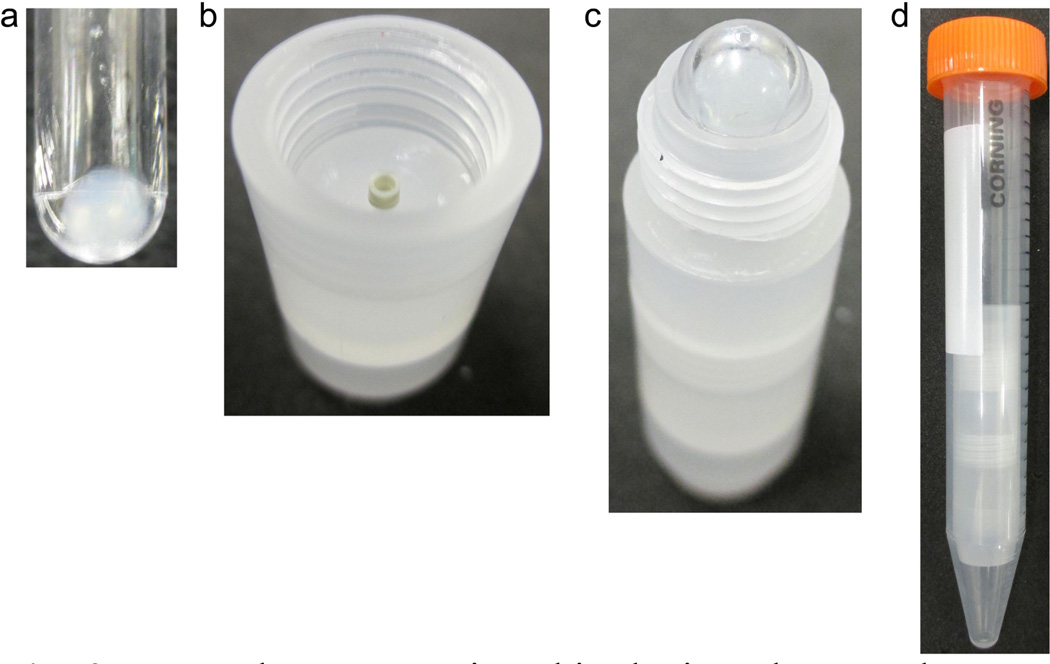
The final step of the sample preparation prior to packing typically is a precipitation or pelleting step by ultracentrifugation. Thus samples are pelleted in 200 µL ultracentrifugation tubes (Fig. 2a). Then the first step of assembly is to secure the rotor with pieces C and D (Fig. 2b), which make up the rotor chamber. The rotor chamber consists of two separate pieces in order to minimize the material loss and readily enable cleaning of all surfaces that might encounter overflow of sample.
Fig. 2.
To pack a rotor using this device, the sample must first be pelleted in a 200 µL ultracentrifuge tube (a). Pieces C and D create a chamber where the rotor is secured (b) and piece B is screwed on. The inverted ultracentrifuge tube is placed directly into piece B (c) before it is capped and placed directly into a 15 mL conical centrifuge tube (d). The entire apparatus is then spun in a swinging bucket centrifuge at approximately 3,000 × g.
The second step of the assembly is to screw Piece B into the rotor chamber such that the tip of the funnel is securely inserted into the rotor. The third step is to place the ultracentrifuge tube upside down into the funnel piece (Fig 2c) and to secure the tube with Piece A. The whole assembled apparatus—consisting of Pieces A, B, C and D, the NMR rotor and the ultracentrifuge tube—is then placed in a 15 mL conical centrifuge tube (Fig 2d) and is spun in a swinging bucket centrifuge at approximately 3,000 g, allowing the material to flow from the centrifuge tube down through the funnel into the rotor. Detailed schematics with dimensions can be found in Fig. S1.
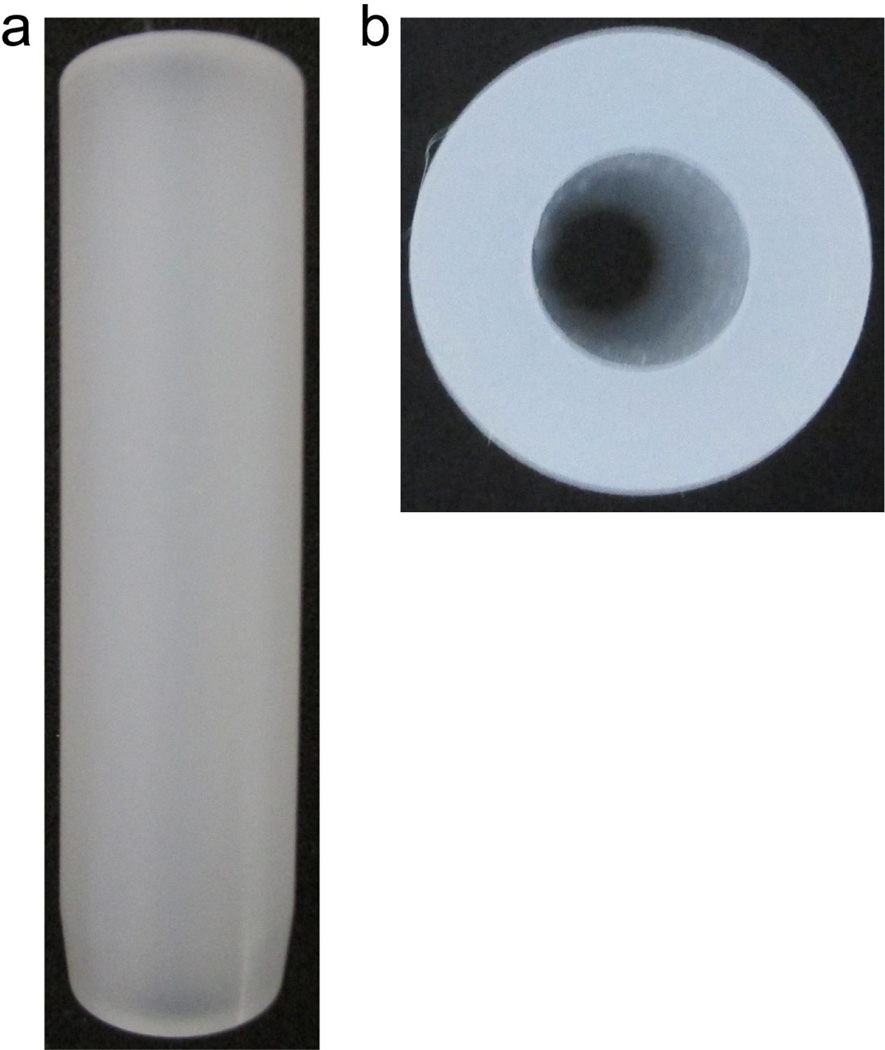
Since the RPDs are made of PCTFE, which has a density [9] of approximately 2.1 g/cm3, balancing the RPD with water in the centrifugation step of packing is suboptimal, as the shape of the RPD and the difference in densities between PCTFE and water can cause strain on the centrifuge rotor. Thus, a balance piece (Fig. 3) was made. This piece is simply a rod of PCTFE of similar shape and size as the RPD with a hollow center. The mass of the piece is slightly less than the mass of the 1.6 mm RPD and is supplemented with small amount of water to achieve final balance of the two tubes. A single balance piece is suitable for all three RPD models. The schematic of the balance piece is in Fig S2.
Fig. 3.
Side view (a) and top view (b) of the balance piece used to balance the centrifuge. The piece is geometrically similar to RPD and fits into a 15 mL conical centrifuge tube, where a small amount of water is added to balance the RPD.
The RPD design allows for the packing of samples directly from centrifuge tubes into MAS rotors efficiently. Since the tip of the funnel piece fits directly into the rotor, the majority of the sample will flow directly into the rotor, and the excess will be contained within the device. The use of PCTFE is a critical aspect of the RPD. The low surface tension of the material [10] limits the amount of material that can stick on the tools. The chemical stability of PCTFE [11] also allows the device to be compatible with many types of samples. Furthermore, one can easily recover samples from the device by rinsing it with a solvent of choice.
Test packing using liposomes
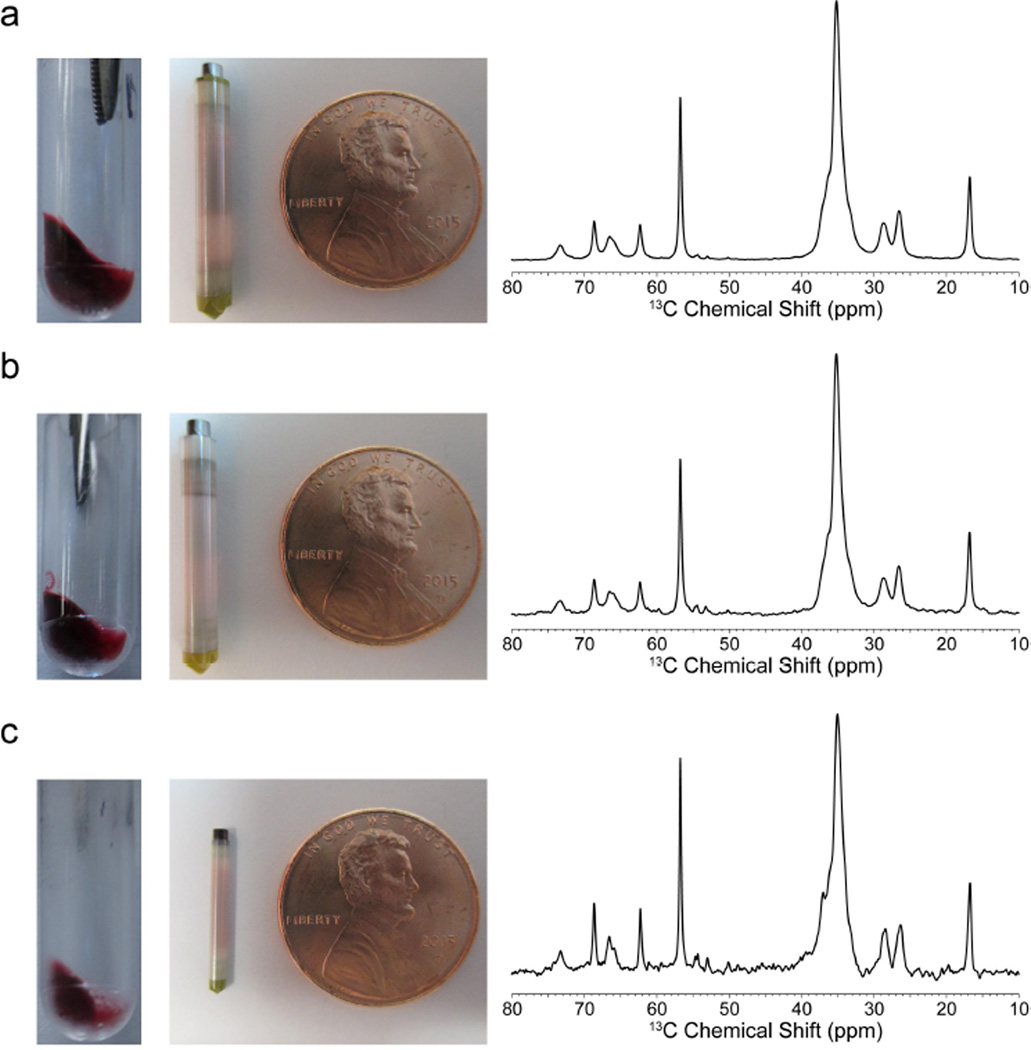
To test the efficacy of the RPD and packing method, samples of natural abundance 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) liposomes containing oil red O dye (ORO) were prepared (see Experimental Section). A 30.7 mg pellet, 19.9 mg pellet, and a 8.4 mg pellet (gravimetric mass) were made to pack a 3.2 mm thin wall rotor, 3.2 mm standard wall rotor, and a 1.6 mm rotor fast MAS rotor respectively (Fig. 4).
Fig. 4.
Image of the pellet and rotor of as well as the one-dimensional direct polarization 13C NMR spectra of the DMPC:ORO samples: (a) 3.2 mm thin wall; (b) 3.2 mm standard wall; (c) 1.6 mm fast MAS. Data collection of the 3.2 mm rotor samples was done at 600 MHz 1H frequency, 10 kHz MAS. Data collected on the 1.6 mm rotor sample was done at 750 MHz 1H frequency, 33 kHz MAS. 4,096 scans were taken for each spectrum with a short refocusing echo and 6 s pulse delay.
Table I summarizes the amount of material transferred in each rotor as well as the mass of lipids in each sample, determined through quantitative 13C NMR (Fig. 4) and the approximate hydration levels that were determined by one-dimensional 1H spectra (Fig. S3). This confirms that the rotor-packing was successful in transferring hydrated liposomes into each type of rotor.
Table I.
Mass of material in rotors
| Rotor Type | Pellet Mass |
Total Mass Transferred to Rotor |
Transfer Yield (%) |
Hydration Level |
|---|---|---|---|---|
| 3.2 mm Thin Wall rotor | 30.7 mg | 29.4 mg | 96% | 80 % |
| 3.2 mm Standard Wall rotor | 19.9 mg | 17.2 mg | 86% | 90 % |
| 1.6 mm mm | 8.4 mg | 8.0 mg | 95% | 85 % |
Conclusion
The packing of hydrated, semi solid samples into MAS rotors for solid-state NMR has been greatly simplified using a newly developed rotor-packing device. The device allows for the efficient packing of samples into the rotor directly from a 200 µL ultracentrifuge tube. Three distinct designs have been made for different sizes of Pencil-style rotors and can serve as a prototype for RPDs of other types of MAS rotors.
Experimental
DMPC liposome preparation
DMPC (Avanti Polar Lipids, Inc., Alabaster, AL) was dissolved in chloroform at 20 mg/mL in a glass vial. ORO in 1:1 chloroform:ethanol was added to the DMPC mixture at a molar ratio of 125:1 DMPC:ORO. The bulk of the solvent was removed using a flow of argon and the vial was kept under vacuum overnight to pull off any remaining solvent.
HBS-100 buffer (20 mM HEPES, 100 mM NaCl, 0.02 % sodium azide, pH 7.5) heated to ~ 42 °C was added to the dried lipid film such that the final concentration of lipids were approximately 1.76 mg DMPC/mL. The solution was then shaken for 1 hour at 37 °C, 200 rpm to homogenize the solution. The cloudy suspension was then bath sonicated for at least 30 min., until the solution turned transparent. The solution was then stored at 4 °C until the sample was pelleted.
The liposomes were initially pelleted in 3 mL ultracentrifuge tubes in a TLA 100.3 centrifuge rotor (Beckman Coulter Item No. 349481) at 240,000 × g for 4 hrs, 8 °C (Beckman TL-100). The supernatant was removed immediately and the pellets were resuspended in a small volume of HBS-100 and transferred to 200 µL ultracentrifuge tubes and were spun down in a TLA-100 centrifuge rotor (Beckman Coulter Item No. 343840) at 220,000 × g for 2 hrs, 8 °C right before packing.
NMR data collection
Data collection of the 1.6 mm fast MAS sample was done on a 750 MHz 1H frequency Agilent/Varian VNMRS spectrometer equipped with a triple resonance 1H/13C/15N 1.6 mm fast MAS probe configured in double resonance 1H/13C mode. Pulse widths for 1H and 13C were 1.75 µs and 1.6 µs respectively and SPINAL-64 decoupling [12] was used at ~ 97 kHz. MAS was set to 33 kHz and the variable temperature was set at 0 °C. A pulse delay of 6 s was used and 4,096 scans were collected.
Data collection of the samples in 3.2 mm rotors were done on a 600 MHz 1H frequency Agilent/Varian Infinity Plus spectrometer equipped with a triple resonance 1H/13C/15N 3.2 mm Balun probe. Pulse widths for 1H and 13C were 2.3 µs and 2.6 µs respectively and SPINAL-64 decoupling [12] was used at ~ 80 kHz. MAS was set to 10 kHz and the variable temperature was set at 0 °C. A pulse delay of 6 s was used and 4,096 scans were collected.
In all cases, chemical shifts were referenced externally to adamantane (downfield peak set to 40.48 ppm) [13]. Spectra were processed using NMRPipe [14] or VnmrJ 3.2.
Supplementary Material
Highlights.
Development of a reusable, modular packing device for Pencil-style SSNMR rotors
Results presented for three rotor geometries (3.2 mm, 3.2 mm thin wall, 1.6 mm).
Routinely achieves 50% to 95% transfer from centrifugation tube to rotor.
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01-GM112845, R01-GM073770 and R01-HL103999), the University of Illinois Centennial Scholars Award (to C.M.R.), and the J & M Witt Fellowship (to G.S.H.) The authors would also like to thank Dr. John Stringer and Dr. R. Andrew Byrd for comments on the manuscript, Dr. Kristin M. Nuzzio for discussions in the initial portion of this study, and Prof. Patrick Tranel (University of Illinois at Urbana-Champaign, Department of Crop Sciences) for loaning the Beckman TL-100 ultracentrifuge. The authors would like to dedicate this paper to the memory of our coauthor, Mr. Robert A. Brown, who passed away during the course of this investigation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Conflict of Interest
The authors declare no conflict of interest.
Contributor Information
Grant S. Hisao, Email: hisao2@illinois.edu.
Michael A. Harland, Email: mharland@illinois.edu.
Deborah A. Berthold, Email: berthold@illinois.edu.
Thomas E. Wilson, Email: thomew@illinois.edu.
Chad M. Rienstra, Email: rienstra@illinois.edu.
References
- 1.Comellas G, Rienstra CM. Protein structure determination by magic-angle spinning solid-state NMR, and insights into the formation, structure, and stability of amyloid fibrils. Annu Rev Biophys. 2013;42:515–536. doi: 10.1146/annurev-biophys-083012-130356. [DOI] [PubMed] [Google Scholar]
- 2.Tang M, Comellas G, Rienstra CM. Advanced solid-state NMR approaches for structure determination of membrane proteins and amyloid fibrils. Acc Chem Res. 2013;46:2080–2088. doi: 10.1021/ar4000168. [DOI] [PubMed] [Google Scholar]
- 3.Kloepper KD, Hartman KL, Ladror DT, Rienstra CM. Solid-state NMR spectroscopy reveals that water is nonessential to the core structure of alpha-synuclein fibrils. J Phys Chem B. 2007;111:13353–13356. doi: 10.1021/jp077036z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olsen GL, Bardaro MF, Echodu DC, Drobny GP, Varani G. Hydration dependent dynamics in RNA. J Biomol NMR. 2009;45:133–142. doi: 10.1007/s10858-009-9355-6. [DOI] [PubMed] [Google Scholar]
- 5.Pauli J, van Rossum B, Forster H, de Groot HJM, Oschkinat H. Sample optimization and identification of signal patterns of amino acid side chains in 2D RFDR spectra of the alpha-spectrin SH3 domain. J Magn Reson. 2000;143:411–416. doi: 10.1006/jmre.2000.2029. [DOI] [PubMed] [Google Scholar]
- 6.Martin RW, Zilm KW. Preparation of protein nanocrystals and their characterization by solid state NMR. J Magn Reson. 2003;165:162–174. doi: 10.1016/s1090-7807(03)00253-2. [DOI] [PubMed] [Google Scholar]
- 7.Bockmann A, Gardiennet C, Verel R, et al. Characterization of different water pools in solid-state NMR protein samples. J Biomol NMR. 2009;45:319–327. doi: 10.1007/s10858-009-9374-3. [DOI] [PubMed] [Google Scholar]
- 8.Das N, Murray DT, Cross TA. Lipid bilayer preparations of membrane proteins for oriented and magic-angle spinning solid-state NMR samples. Nat Protoc. 2013;8:2256–2270. doi: 10.1038/nprot.2013.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murthy NS, Khanna YP, Signorelli AJ. Crystallinities of Poly(Chlorotrifluoroethylene) and Its Copolymers by Differential Scanning Calorimetry, X-Ray-Diffraction, and Density-Measurements. Polym Eng Sci. 1994;34:1254–1259. [Google Scholar]
- 10.Gardiner J. Fluoropolymers: Origin, Production, and Industrial and Commercial Applications. Aust J Chem. 2015;68:13–22. [Google Scholar]
- 11.Millet GH, Kosmala JL. Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Inc.; 2000. Fluorine-Containing Polymers, Polychlorotrifluoroethylene. [Google Scholar]
- 12.Comellas G, Lopez JJ, Nieuwkoop AJ, Lemkau LR, Rienstra CM. Straightforward, effective calibration of SPINAL-64 decoupling results in the enhancement of sensitivity and resolution of biomolecular solid-state NMR. J Magn Reson. 2011;209:131–135. doi: 10.1016/j.jmr.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morcombe CR, Zilm KW. Chemical shift referencing in MAS solid state NMR. J Magn Reson. 2003;162:479–486. doi: 10.1016/s1090-7807(03)00082-x. [DOI] [PubMed] [Google Scholar]
- 14.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRpipe - a Multidimensional Spectral Processing System Based on Unix Pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.