Abstract
Developing strategies to enhance cancer prevention is a paramount goal, particularly given recent concerns about surgical treatment of pre-invasive states such as ductal carcinoma in situ. Promoting effective immunosurveillance by leukocytes that scan for nascent neoplastic transformations represents a potential means to achieve this goal. Since most breast cancers arise within the ductal epithelium, enhancing protective immunosurveillance will likely necessitate targeting one or more of the distinctive lymphocyte types found in these sites under normal conditions. Here, we have characterized the intraepithelial lymphocyte compartment of non-cancerous human breast tissue and identified a subset of T lymphocytes that can be pharmacologically targeted to enhance their responses to breast cancer cells. Specifically, Vδ2+ γδ T cells were consistently present in preparations of mammary ductal epithelial organoids and they proliferated in response to zoledronic acid, an aminobisphosphonate drug. Vδ2+ T cells from breast ductal organoids produced the anti-tumor cytokine IFN-γ and efficiently killed bisphosphonate-pulsed breast carcinoma cells. These findings demonstrate the potential for exploiting the ability of Vδ2+ γδ T cells to respond to FDA-approved bisphosphonate drugs as a novel immunotherapeutic approach to inhibit the outgrowth of breast cancers.
Introduction
Identifying genetic loci associated with reduced risk of breast cancer may provide novel targets for cancer prevention (1). Such loci may operate directly within mammary epithelial cells or may be mediated by the activities of non-mammary cells. We have recently reported that the rat Mcs5a locus acts via the immune system and that the resistant allele of Mcs5a is associated with increased frequency and functional activity of γδ T cells within spleen and mammary epithelium (2). These findings suggest that engaging key immune cell types to phenocopy the effects of the resistant Mcs5a allele may represent an effective breast cancer prevention strategy. However, given the substantial differences between humans and muroid rodents in the molecular specificities of innate immune cells that mediate defense against incipient threats, an essential prerequisite to such an effort is identifying the immune subsets typically present in human mammary ductal epithelial tissues and determining how these can be medically targeted.
Cancerous cells are culled from tissues through the process of immunosurveillance, whereby several different types of leukocytes continuously scan for neoplastically transformed cells and eliminate them (3). Since breast cancers typically originate from the epithelial cells lining the mammary ducts and lobules (4), the immune cells responsible for immunosurveillance of transformed breast cells are likely to be those that patrol the ductal epithelium. Although recent studies have illustrated the presence of leukocytes in the human breast (5–8) and even in the epithelium (6, 7), the specific leukocyte subsets within this specialized tissue niche have remained poorly characterized. Moreover, a key unanswered question is whether immune cells are present that can be targeted to promote enhanced immunosurveillance of pre-cancerous or cancerous cells.
Conserved T lymphocyte populations are particularly attractive for this type of approach because they recognize non-polymorphic antigen presenting molecules and thus are present in all individuals regardless of human leukocyte antigen (HLA) type, and they can selectively be activated based on features of the T cell receptor (TCR). Some examples of conserved T lymphocytes are γδ T cells, mucosal-associated invariant T (MAIT) cells, and invariant natural killer T (iNKT) cells. Based on their characteristic TCR chain usages, these types of T cells can be specifically targeted using monoclonal antibodies (mAbs), or in some cases by synthetic compounds.
For example, human Vδ2+ T cells are selectively activated by FDA approved aminobisphosphonate (BP) drugs. These compounds act on Vδ2+ T cells because they block the mevalonate biosynthetic pathway within target cells, which leads to the accumulation of a particular metabolic intermediate called isopentenylpyrophosphate (IPP). IPP associates with the cytoplasmic tail of a cell surface protein called butyrophilin 3A1 (BTN3A1), causing a recognizable change in molecular features of the extracellular domain of BTN3A1 (9, 10). Target cells that express BTN3A1 and that have undergone an intracellular accumulation of IPP trigger TCR-dependent activation of Vδ2+ T cells (11–13), causing them to proliferate, secrete cytokines such as interferon-γ (IFN-γ), and to kill the target cells (14). Thus, BPs may promote the anti-tumor functions of human γδ T cells in several ways, including: i) by expanding the numbers of Vδ2+ T cells; ii) by promoting their production of the anti-tumor cytokine IFN-γ; and iii) by promoting their killing of tumor cells. Indeed, administration of BPs to human cancer patients who had bone metastases (3 females with breast cancer and 6 males with prostate cancer) was associated with the expansion of an effector population of Vδ2+ T cells in the blood, and with enhanced IFN-γ production (15). These findings suggest that BP treatment may also provide an effective means to enhance the immunosurveillance functions of human Vδ2+ T cells that play a critical role in eliminating nascent neoplastic cells before they can develop into tumors.
Administration of BP is likely to be particularly effective for cancers located in tissues patrolled by Vδ2+ T cells (e.g. highly vascularized sites, such as bone). However, it is less clear whether this strategy represents a viable option for promoting the elimination of cancer cells located in epithelial tissues, since the γδ T cells in mucosal tissues mainly belong to other subtypes that do not respond to BPs. Some studies have shown lower prevalence of postmenopausal breast cancer in certain populations who have received BP treatment (16–20), although other studies found no evidence of decreased breast cancer risk in women who have taken BPs (21). Thus, the potential utility of BP administration for promoting immunological control of incipient breast cancer remains unclear. Here, we have addressed this question by investigating the presence and functionality of targetable T lymphocyte effector populations from primary human breast epithelial tissues.
Materials and Methods
Breast tissue acquisition and preparation
Non-cancerous breast tissue was obtained from the Cooperative Human Tissue Network (CHTN, funded by the National Cancer Institute) or provided by the University of Wisconsin’s Translational Science BioCore-BioBank from reduction mammoplasties or contralateral prophylactic mastectomies (Table 1). Acquisition and analysis of the breast tissue was approved by the University of Wisconsin Health Services Institutional Review Board.
Table 1.
Human breast tissue donor information
| Sample ID | Age, sex, race | Elective Procedure | Pathology Report |
|---|---|---|---|
| L625 A1 | 47 yrs, female, N/A | prophylactic mastectomy | N/A |
| L625 B1 | 56 yrs, female, N/A | prophylactic mastectomy | N/A |
| L625 C1 | 36 yrs, female, N/A | reduction mammoplasty | N/A |
| L625 D1 | 28 yrs, female, N/A | reduction mammoplasty | N/A |
| L625 G1 | 37 yrs, female, N/A | prophylactic mastectomy | N/A |
| L625 I1 | 36 yrs, female, N/A | reduction mammoplasty | N/A |
| L625 J1 | 18 yrs, female, BL | reduction mammoplasty (macromastia) | Normal |
| L625 K1 | 41 yrs female, WH | reduction mammoplasty (macromastia) | FPCI |
| L625 L1 | 23 yrs, female, BL | reduction mammoplasty (macromastia) | Normal |
| L625 M1 | 36 yrs, female, WH | reduction mammoplasty (macromastia) | Fibrosis; CLI; PASH |
| L625 N1 | 38 yrs, female, BL | reduction mammoplasty (macromastia) | Normal |
| L625 O1 | 46 yrs, female, WH | reduction mammoplasty (macromastia) | Normal |
| L625 P1 | 42 yrs, female; WH | reduction mammoplasty (macromastia) | Fibrosis |
| L625 Q1 | 37 yr old female, BL | reduction mammoplasty (macromastia) | Normal |
| L625 S1 | 50 yr old female; BL | reduction mammoplasty (macromastia) | Normal |
| L625 T1 | 24 yrs, female, WH | reduction mammoplasty (macromastia) | Normal |
| L625 U1 | 51 yrs, female, BL | reduction mammoplasty (macromastia) | Normal |
| L625 V1 | 27 yrs, female, WH | reduction mammoplasty (macromastia) | PASH |
| L625 W1 | 19 yrs, female, BL | reduction mammoplasty (macromastia) | Fibrosis |
| L625 X1 | 33 yrs, female, WH | reduction mammoplasty (macromastia) | PASH |
| L625 Y1 | 21 yrs, female, BL | reduction mammoplasty (macromastia) | Normal |
| L625 Z1 | 44 yrs, female, WH | reduction mammoplasty (macromastia) | Normal |
| L625 A2 | 36 yrs, female; WH | reduction mammoplasty (macromastia) | Normal |
Pathology Report Key
FPCI – focal periductal chronic inflammation
CLI = chronic lobular inflammation
PASH - Pseudoangiomatous stromal hyperplasia
Human breast organoids were isolated as previously published (22, 23). Briefly, breast tissue was minced and digested overnight in a 37°C shaker with 1X collagenase/hyaluronidase in Complete EpiCult B Human Media (Stem Cell Technologies) supplemented with 5% fetal bovine serum (FBS; Hyclone). After incubation, the digested tissue was spun for 1 minute or less at 80–100 × g to form a visible cell pellet enriched for epithelial ductal organoids. This cell pellet was washed and the breast organoids were collected on a 40 µm filter. In addition, a cell pellet containing stromal cells, red blood cells and small ductal epithelial fragments was also collected from the supernatant of the digested tissue. Organoids and stromal cell fractions were cryopreserved in 50% FBS/6% dimethyl sulfoxide, and stored in liquid nitrogen until needed. Single cell suspensions from the organoids were prepared for all flow cytometric analyses and used for in vitro experiments by trypsinizing the organoids using 2 mLs of ethylenediaminetetraacetic acid (EDTA)/trypsin solution for 1–2 minutes. EDTA (Thermo Fisher Scientific)/trypsin (Worthington Biochemical Corporation) solutions were made by adding 50 mg EDTA to 25 mLs warm HBSS (Life Technologies) or PBS (Corning) without Ca2+/Mg2+; subsequently 5 mg trypsin was then added to 2 mLs of EDTA solution and diluted 1:100 for usage.
Peripheral blood mononuclear cell isolation
Peripheral blood mononuclear cells (PBMCs) were isolated from healthy donors according to protocols approved by the UW Health Sciences and Minimal Risk IRBs. Written informed consent was obtained from all donors. Blood was processed using Ficoll-paque PLUS (GE Healthcare) and spun for 40 minutes without brake or acceleration at 400 RCF. The buffy coat was removed and washed with PBS for 15 minutes at 400 RCF. The supernatant was discarded and pellet resuspended in PBS and washed for 10 minutes at 300 RCF.
Flow cytometry and intracellular staining
For surface stains, cells were harvested, washed with PBS, blocked with 20% human AB serum (Fc block) for 15 minutes, stained with antibodies for 30 minutes at 4°C, washed, resuspended in PBS, and analyzed on a LSRII (BD Biosciences) with FlowJo analysis software (Version 9.3.1; Tree Star Inc.). Intracellular (IFN)-γ and IL-17A staining was performed according to manufacturer’s recommendations using the BD Cytofix/Cytoperm kit (BD Biosciences) in the presence of BD GolgiStop™ protein transport inhibitor (BD Biosciences). Intracellular FoxP3 staining was performed according to manufacturer’s instructions using the True-Nuclear™ transcription factor buffer set (BioLegend).
Directly conjugated fluorescent antibodies used for flow cytometry included: CD3 (clone OKT3; BioLegend), CD45 (HI30; BioLegend), CD4 (RPA-T4; BioLegend), CD8α (RPA-T8; BioLegend), CD8β (SIDI8BEE; eBioscience), Vδ2 (B6; BioLegend), Vα7.2 (3C10; BioLegend), NKT cell receptor Vα24Jα18 (6B11; BioLegend), Vδ1 (REA173; Miltenyi Biotec), CD103 (Ber-ACT8; BioLegend), EpCAM (9C4; BioLegend), CD49f (GoH3; BioLegend), CD24 (ML5; BioLegend), CD29 (TS2/16; BioLegend), CD10 (HI10a; BioLegend), Muc-1 (16A; BioLegend), CD31 (WM59; BioLegend), CD27 (O323; BioLegend), CD45RO (UCHL-1; BD Biosciences), LAMP-1 (H4A3; BioLegend), IFN-γ (4S.B3; BioLegend), IL-17A (BL168; BioLegend), FoxP3 (206D; BioLegend), NKG2D (1D11; BioLegend), Butryophilin 3A1 (BT3.1; BioLegend), MICA/B (6D4; BioLegend), ULBP1 (170818; R&D Systems), ULBP2/5/6 (165903; R&D Systems), ULBP3 ((166510; R&D Systems), ULBP4 ((709116; R&D Systems).
Bisphosphonate stimulation and Vδ2 T cell expansion
PBMCs or single cells prepared from the breast organoids were exposed to 2.5 µM Zoledronate in combination with irradiated feeder PBMCs (exposed to 7×103-8×103 Rads) to stimulate and expand Vδ2 γδ T cells. Specifically, 2×105 isolated PBMCs were cultured with 5×105 irradiated feeders in an end volume of 0.2 mLs T cell media in 96-well plates. Single cell preparations from organoids were plated with 5×105 total irradiated feeders in an end volume of 0.2 mLs T cell media in 96-well plates. Lymphocyte cultures were maintained in RPMI 1640 with L-glutamine (Corning) supplemented with 15% heat inactivated bovine calf serum (HI-BCS; Thermo Fisher Scientific), 3% human AB serum (Atlanta Biologicals), 1% penicillin/streptomycin (P/S; Mediatech) and 200 units/mL IL-2 (Peprotech) referred to as T cell media. Percentages of Vδ2 T cells were obtained by flow cytometry 2–3 weeks post-stimulation and compared to media only controls; Vδ2 frequencies above 1% were considered positive for expansion. Alternately, freshly isolated PBMCs were also stimulated with 2.5 µM Zoledronate in T cell media at 1×106 cells/well in 24-well plates and expanded for seven days. Bisphosphonate (BP) expanded cells were used in cytotoxicity assays. BP dose response curves were generated using fresh PBMCs with either Zoledronate or Alendronate for seven days in T cell media. Fresh media was provided when needed. Zoledronate (Novation LLC and Novartis) and Alendronate (Teva Pharmaceuticals USA) BP were obtained from UW Health Pharmacy Services.
Analysis of Vδ2+ T cell responses to tumor cells
MDA-MB-468 breast carcinoma cells were obtained from ATCC as an authenticated cell line, and maintained in DMEM/F12 (Corning) supplemented with 10% HI-BCS (Thermo Fisher Scientific) and 1% P/S (Mediatech) The MDA-MB-468 cells were pulsed with or without 5 µM Zoledronate overnight, then washed and resuspended at 3×106 cells/1.0 mL media. Cells from primary organoids and cell suspensions containing BP expanded organoid- or blood-derived Vδ2 T cells were each incubated with MDA-MB-468 cells for 4–6 hours at 37°C in a total of 0.2 mLs in 96-well plates. After the incubation, Vδ2 T cells were analyzed for cell surface LAMP-1 and/or intracellular IFN-γ expression by flow cytometry.
To assess γδ T cell killing of target cells, MDA-MB-468 cells were used as targets and day seven BP expanded PBMC-derived Vδ2 T cells were used as effectors. Briefly, targets were pulsed with or without 10 µM Zoledronate overnight. At the same time, day seven stimulated Vδ2 T cells were cultured with IL-2 overnight. The following day, targets were trypsinized, washed and prepared at a concentration of 1–2×105 cells/0.1 mLs T cell media without IL-2. Effectors were prepared without IL-2 and co-incubated with targets at different effector-to-target ratios in 96-well plates at 37°C for 4.5 hours. Additionally, 0.1 mLs of 0.1% trypsin was used for several minutes to recover adherent cells. The cells were washed with Fc block and stained with CD45 for 30 minutes at 4°C to delineate effectors from targets. Samples were washed with PBS, spun, and resuspended in Annexin V binding buffer (BioLegend). Annexin V (5 µLs/tube; BioLegend) and propidium iodide (10 µLs/tube; BioLegend) were added to samples and incubated for 15 minutes in the dark at room temperature. Additional Annexin V binding buffer was added before flow cytometry analysis.
Statistics
Graphpad Prism versions 4.0 and 4.0c software (GraphPad Software, La Jolla, CA) were used to construct data graphs and determine statistical significance using the Wilcoxon test for paired samples or the Student’s unpaired two-tailed t test or the Mann-Whitney. The p value cutoffs and notation were used as follows: * p <0.05, **p < 0.01, ***p ≤ 0.0001.
Results
Preparation of human breast tissue yields highly enriched ductal epithelial organoids
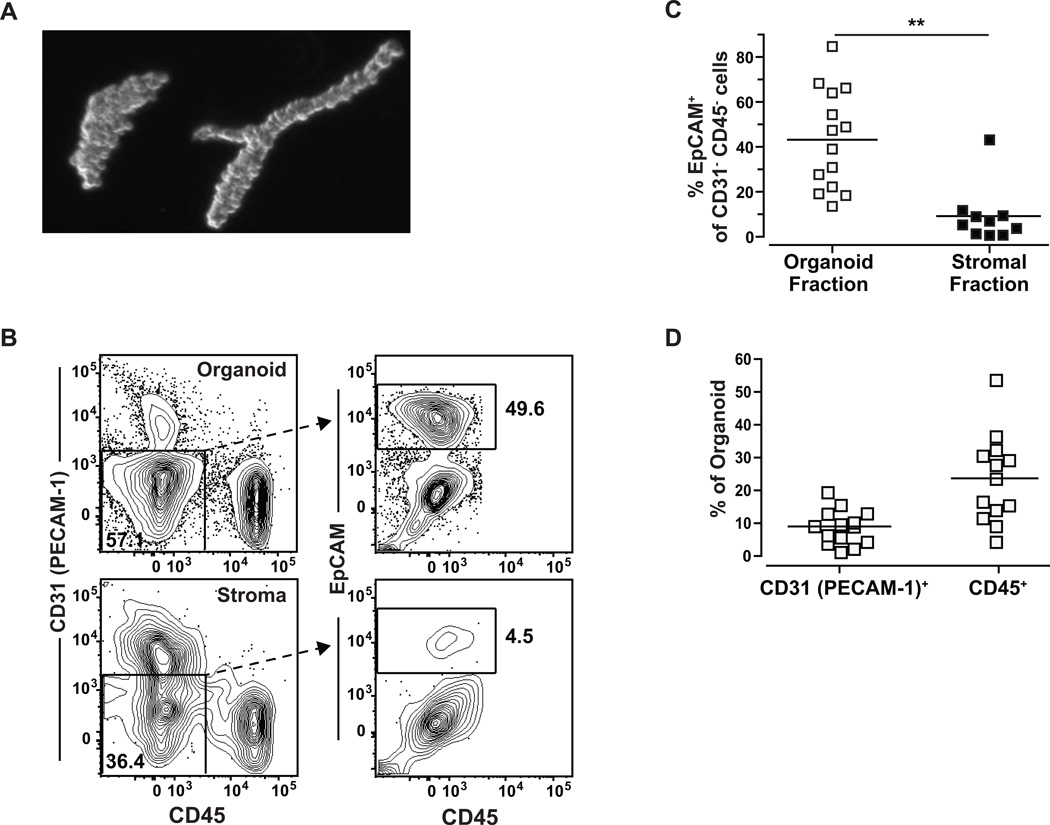
Samples of breast tissue from human subjects who had undergone reduction mammoplasty or prophylactic mastectomy were collected for analysis (see Table 1). The samples were prepared using a protocol designed to separate tissue fragments representing ductal organoids from stromal layers. A representative image of the resulting tissue fragments showing ductal and alveolar structures is shown in Fig. 1A. To confirm that the fragment preparations were enriched for epithelial cells compared to the stromal fraction, we utilized multi-parameter flow cytometry to assess relative frequencies of cells expressing epithelial, endothelial, or hematopoietic markers (Fig. 1B). Cells that were double negative for both the hematopoietic linage marker CD45 and the endothelial marker platelet/endothelial cell adhesion molecule-1 (PECAM-1; CD31) (24), were assessed for expression of the epithelial cell adhesion molecule (EpCAM) (25, 26) to delineate epithelial content (Fig. 1B). Analysis of organoid and stromal fractions from at least 10 different breast tissue samples demonstrated that the organoids were significantly enriched for EpCAM+ cells compared to the stromal cell fractions, 43% and 9% respectively (Fig. 1C). EpCAM expressing cells within the organoid preparations also expressed other markers associated with mammary gland-derived epithelial cells, including CD49flow/+(25, 26), CD10low (25, 26), CD24+ (24), CD29+ (24), and Muc-1int/high (25) (Supplementary Fig. 1A–E). Organoid preparations contained an average of 24% CD45+ cells (leukocytes), and 9% CD31+ cells (endothelial cells) (Fig. 1D). These results indicated that our breast tissue preparation method successfully enriches for ductal epithelial organoids, and thus the associated leukocytes are likely to be highly enriched for cells from the ductal epithelial tissue.
Figure 1.
Purified organoid fragments demonstrate epithelial enrichment compared to stromal fraction. A, light microscopic image of representative organoid fragments purified from human breast reduction tissue. B, flow cytometry analysis of the CD31 (non-endothelial) and CD45 (non-hematopoietic) cells shows that a higher percentage of cells express EpCAM (an epithelial marker) in the organoid fraction compared to the stromal fraction. C, quantification of ≥10 different patient reduction samples. ** p=.0003 (Mann Whitney). D, quantification of the CD31+ or CD45+ cells from the organoids from ≥13 different patient reductions. Each symbol represents a different donor’s tissue sample.
Leukocyte populations associated with human breast organoids differ from those in peripheral blood
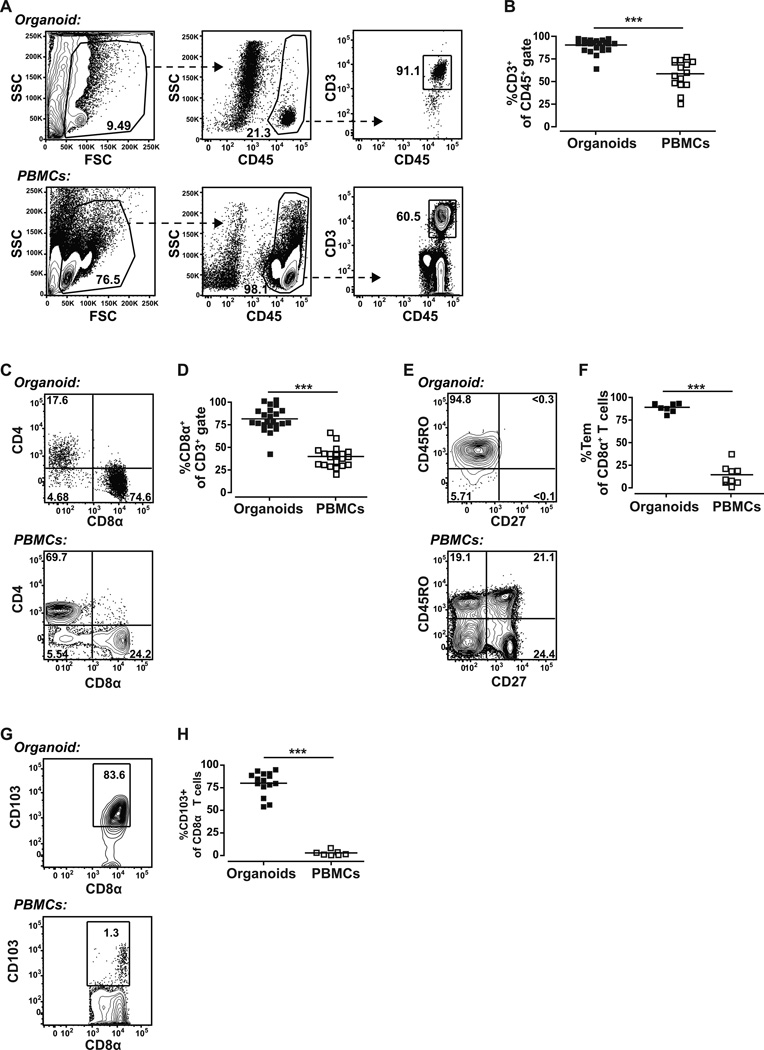
We next examined the immune cell subsets within the CD45+ population of the breast organoid preparations. Typically, at least 90% (90.63±7.51%) of the CD45+ cells were CD3+ indicating that they are T lymphocytes (Fig. 2A and 2B). In contrast, CD3+ cells made up on average about 60% of the CD45+ cells in the peripheral blood (Fig. 2A and 2B). Of the CD3+ cells in the organoid preparations about 75% (75.67±13.32%) were typically CD8+, whereas CD3+ cells in the blood typically contained less than 34% CD8+ cells (Fig. 2C and 2D). Essentially all of the organoid-derived CD8α+ T cells co-expressed CD8β, indicating that they are likely distinct from the CD8αα+ intra-epithelial lymphocytes that have been identified within intestinal epithelium (27) (Supplementary Fig. S2A). Further analysis of the organoid CD8+ T cell population demonstrated that it consists almost exclusively of CD45RO+CD27− cells (Fig. 2E and 2F), a phenotype that is characteristic of effector memory T cells (28). In contrast, CD8+ T cells in the blood are comprised of almost equal proportions of naive T cells (CD27+CD45RO−) (28), effector T cells (CD27−CD45RO−) (29), central memory T cells (CD27+CD45RO+) (28), and effector memory T cells (CD27−CD45RO+) (28) (Fig. 2E and 2F). Moreover, typically 80% (80±12.94%) of the organoid preparation CD8+ T cells expressed the integrin αE (CD103), which is a marker of intraepithelial lymphocytes (30), whereas less than 3% of the peripheral blood CD8+ T cells expressed this marker (Fig. 2G and 2H). Most of the CD8− organoid T cells expressed intermediate levels of CD4 (Figure 2C) and were essentially all CD45RO+CD27− (Supplementary Fig. S2B). On average about 4% of the organoid CD4+ cells expressed FoxP3 (Supplementary Fig. S2C & S2D), which is characteristic of a regulatory T cell phenotype. Notably, in contrast to the CD8+ T cells, only a small fraction of organoid-derived CD4+ T cells expressed CD103 (Supplementary Fig. S2E). Together, these data demonstrate that the immune cell populations associated with our organoid preparations are clearly distinct from those of the blood, and are highly enriched for T cells with characteristics of IELs.
Figure 2.
Characterization of immune cells from breast-derived organoids yields unique lymphocyte percentages compared to blood. A, gating strategy to delineate CD3+CD45+ T lymphocytes. B, quantification of CD3+CD45+ T lymphocytes. C, expression of the co-receptors CD8α+ and CD4+ by CD3+ T cells. D, quantification of CD8α+ expression by the CD3+ T cells. E, CD45RO−/+ and CD27−/+ (markers of T cell activation and differentiation) staining of CD8α+ T cells. F, quantification of CD8α+ T effector memory cells (CD45RO+CD27). G, expression of CD103+ (an intraepithelial cell associated integrin) by cells of the CD8α+ gate. H, quantification of CD103+ CD8α+ T cells. *** p<0.0001 (Students t test).
Conserved T lymphocyte subsets are present in organoids and expand in response to BP
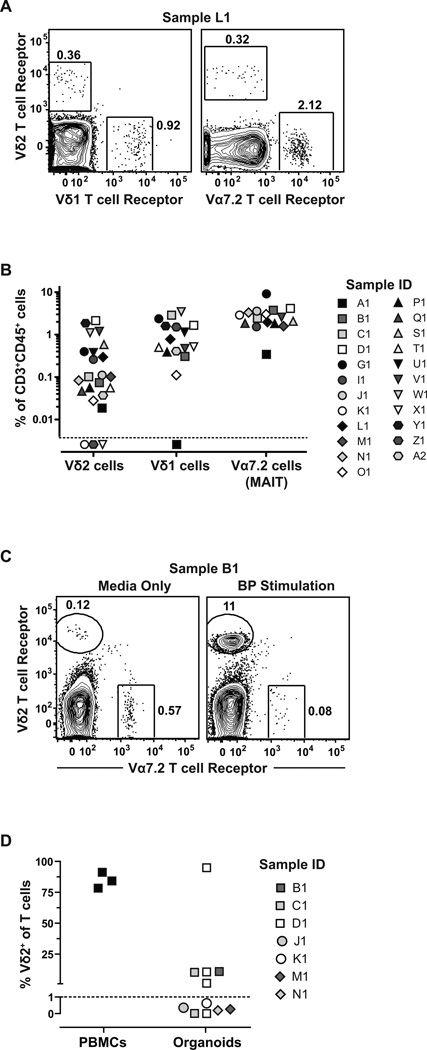
We next wanted to determine whether we could detect lymphocyte subsets with conserved T cell receptors within the breast organoids that might be targetable in a chemoprevention strategy against breast cancer. We screened for two different subpopulations of γδ T cells (Vδ1 and Vδ2), MAIT cells, and iNKT cells. Both subsets of γδ T cells were clearly detectable in almost all of the samples analyzed (Fig. 3A and 3B), although they made up comparatively small percentages of the CD3+ cells (Vδ1 mean=1.27%; Vδ2 mean=0.42%). MAIT cells, which were identified using the Vα7.2 T cell receptor, were detectable as >3.1% of the CD3+ cells in organoids (Fig. 3A and 3B). iNKT cells were detected in some of the samples, but did not appear to be markedly enriched (data not shown). Thus, although Vδ2 cells are predominantly found within the blood, these results indicated that these potentially targetable T lymphocytes are typically present in the breast ductal epithelium.
Figure 3.
Breast organoids contain specifically targetable lymphocytes that respond to an FDA-approved BP drug. A, flow cytometry analysis of an organoid preparation from tissue sample L1 (see Table 1) for γδ T cells and MAIT cells (Vα7.2 T cell receptor). B, quantification of T cell subsets from organoid preparations from the indicated tissue samples (see Table 1 for tissue donor information). Symbols under the dashed line were below the limit of detection. C, example of flow cytometry results showing Vδ2+ T cell expansion in the presence of 2.5 µM BP compared to culture medium alone. Vα7.2+ MAIT cells were used as a control for non-responsiveness to BP. D, quantification of Vδ2 T cell frequency from organoids compared to PBMCs as a positive control after 2–3 weeks of culture in the presence of BP. Five of 11 samples (45.5%) displayed Vδ2+ T cell expansion from the purified organoid fraction. Each symbol represents an independent expansion attempt using the indicated tissue samples. Dashed line indicates the threshold used to delineate expansion.
We next investigated whether breast epithelial organoid Vδ2+ T cells are able to respond functionally to an FDA approved BP. Preliminary studies confirmed that Zoledronate more potently stimulated blood-derived Vδ2+ T cells compared to Alendronate, (Supplementary Fig. S3), consistent with prior studies by other groups (15, 31). Therefore, we cultured the total cells from organoid preparations with Zoledronate or with medium alone for 2–3 weeks, and performed flow cytometric analysis to determine the relative frequencies within the culture of Vδ2+ T cells or MAIT cells as a control. Exposure to BP resulted in marked increases in the frequency of Vδ2+ cells, but not of MAIT cells, within the cultures (Fig. 3C). Increases in total Vδ2+ cell number were also observed, suggesting that the Vδ2+ T cells had proliferated (data not shown). Overall, exposure to BP induced clearly detectable Vδ2+ T cell expansion in approximately 45% of the organoid samples we tested (Fig. 3D). These data demonstrate that organoid-derived Vδ2+ T cells can respond to an FDA approved BP drug.
BP treatment facilitates IFN-γ production by organoid-derived Vδ2+ T cells in response to triple negative breast carcinoma cells
Whereas γδ T cells typically have a T helper 1 (TH1) cytokine production phenotype that is characterized by the production of high levels of IFN-γ, it has recently become clear that lymphoid cells in and around epithelial tissues are also responsible for the production of interleukin (IL-) 17, a cytokine that promotes epithelial integrity but that may also play a pathogenic role in tumorigenesis (32). Therefore, we tested cytokine production by primary T cells in organoid preparations by stimulating them with PMA and ionomycin, then performing intracellular cytokine staining for IFN-γ and IL-17. Detectable populations of cells expressing IL-17 were observed in the non-Vδ2 CD8+ and CD8− T cell subsets, however, the Vδ2+ T cell population appeared heavily biased towards production of IFN-γ with little or no evidence of IL-17 producing cells (Supplementary Fig. S4). Based on these results, we focused our further analyses on IFN-γ production.
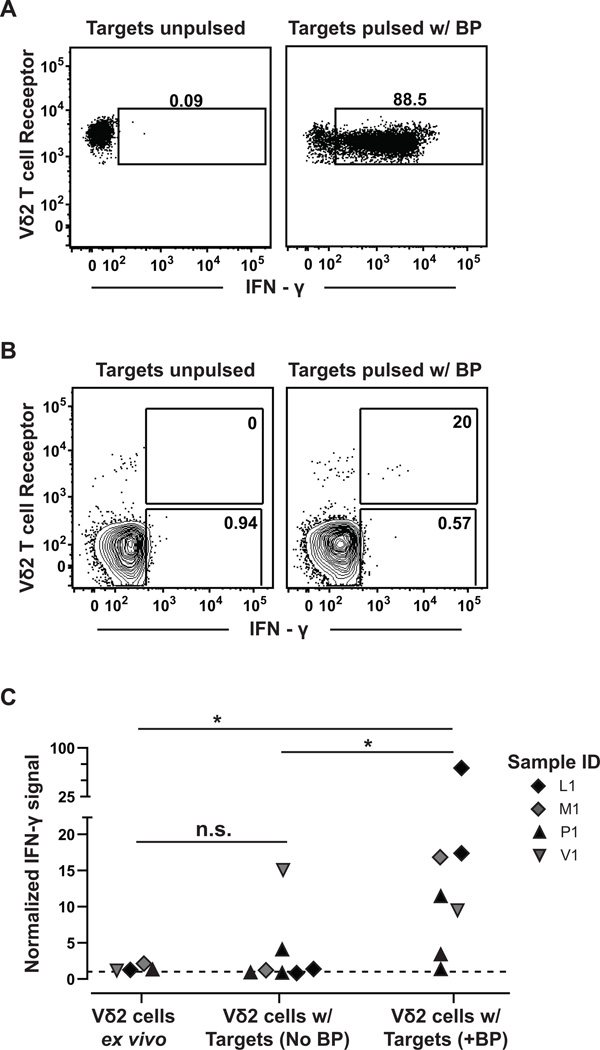
We determined whether the organoid-derived Vδ2+ T cells produce cytokines in response to a human breast carcinoma cell line, MDA-MB-468, which is triple-negative for estrogen receptor, progesterone receptor, and HER2/neu. Organoid cells were cultured with BP for 3–4 weeks, then exposed to MDA-MB-468 cells that were either pulsed with BP or mock-treated. Intracellular cytokine staining was performed to detect the frequency of γδ T cells expressing IFN-γ. We observed robust IFN-γ responses to BP pulsed target cells, but little or no detectable IFN-γ staining in response to mock-treated target cells (Fig. 4A). These results demonstrated that organoid-derived Vδ2+ T cells that expand in response to BP treatment also produce cytokines in response to BP-pulsed breast cancer cells, but did not clarify whether a significant frequency of the primary γδ T cells within breast organoids can mount similar responses to transformed cells.
Figure 4.
Breast organoid-derived Vδ2 γδ T cells produce IFN-γ in response to a triple negative breast carcinoma cell line pulsed with BP. A, Vδ2+ T cells were expanded in vitro from organoid preparations by exposure to BP. The T cells were co-incubated with MDA-MB-468 breast carcinoma cells that were pulsed (right) or not pulsed (left) with BP, and intracellular IFN-γ production was assessed by flow cytometry. Results shown are representative of two independent experiments. B, primary organoid-derived cells were co-incubated with MDA-MB-468 cells that were pulsed (right column) or not pulsed (left column) with BP, and intracellular IFN-γ production by Vδ2+ and Vδ2 T cells was assessed by flow cytometric analysis. Numbers shown in the gates are the percentage of IFN-γ expressing cells from the Vδ2+ or Vδ2 T cell populations. C, plot showing aggregated results for IFN-γ production by primary Vδ2+ T cells from the indicated breast tissue samples. Normalized mean fluorescence intensity (MFI) for IFN-γ was determined by dividing the IFN-γ MFI of the Vδ2+ cells by the IFN-γ MFI of the corresponding Vδ2 T cells in the same sample. Each symbol represents an independent experiment to assess IFN-γ production by T cells from the indicated breast tissue samples. The dashed line represents a normalization ratio of 1. n.s. = not significant; * p=0.0121 (Mann Whitney test; Plus BP to Ex vivo); * p=0.0262 (Mann Whitney test; Plus BP to No BP).
Therefore, we tested the ability of primary Vδ2+ T cells within organoid cell preparations to produce cytokines in response to tumor cells directly ex vivo. Indeed, when we exposed organoid-derived cells to BP-pulsed or mock treated MDA-MB-468 breast carcinoma cells, we found that a detectable fraction of the Vδ2+ subset showed intracellular IFN-γ staining (Fig. 4B). Analysis of multiple different primary tissue samples revealed that the normalized IFN-γ mean fluorescence intensity of the Vδ2+ cells was consistently higher in response to BP-pulsed MDA-MB-468 breast carcinoma cells than to mock-treated carcinoma cells (Fig. 4C). These results demonstrate that primary γδ T cells from breast ductal epithelia are able to respond functionally to breast carcinoma cells that have been exposed to BP.
Organoid-derived Vδ2+ T cells demonstrate cytotoxicity to BP-treated triple negative breast carcinomas
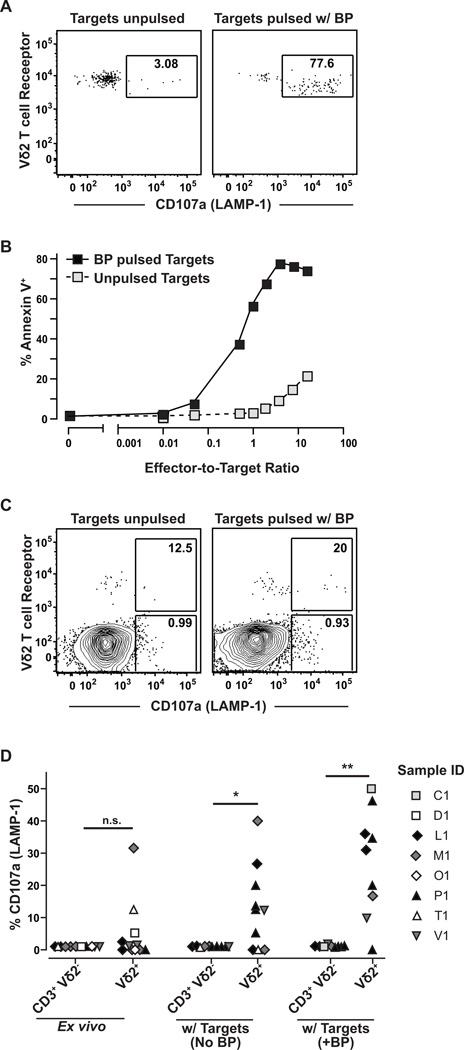
To further investigate, we tested the cytotoxic responses of Vδ2+ T cells to MDA-MB-468 breast carcinoma cells. We first analyzed surface expression of the LAMP-1 protein (CD107a), which becomes expressed at the cell surface as a result of vesicle fusion with the plasma membrane during a killing response. Vδ2+ T cells within BP-expanded breast tissue cultures showed robust cell surface LAMP-1 expression after exposure to BP pulsed MDA-MB-468 target cells, and a small percentage of the Vδ2+ cells typically also showed cell surface LAMP-1 expression in response to mock-treated target cells (Fig. 5A). To confirm that Vδ2+ T cell surface LAMP-1 expression was correlated with target cell killing, we assessed the viability of the MDA-MB-468 carcinoma cells by Annexin V staining. BP-pulsed MDA-MB-468 carcinoma cells were efficiently killed upon exposure to in vitro-expanded Vδ2+ T cell cultures (greater than 75% target cell death within 4 hours) (Fig. 5B). Notably, there was also significant killing of mock-treated MDA-MB-468 breast carcinoma cells, although this required much higher effector-to-target cell ratios (Fig. 5B). Analysis of T cells within primary organoid cell preparations revealed that a fraction of the Vδ2+ cells typically showed cell surface LAMP-1 expression after exposure to MDA-MB-468 cells (Fig. 5C). Exposure to BP-pulsed target cells elicited detectable cell surface LAMP-1 expression on Vδ2+ T cells nearly in 100% of the samples tested, while exposure to mock-treated MDA-MB-468 cells resulted in cell surface LAMP-1 expression by Vδ2 cells in about 50% of the samples (Fig. 5D). These results demonstrate that BP treatment of breast carcinoma cells promotes highly efficient cytotoxic responses by Vδ2+ T cells, and indicate that Vδ2+ T cells also mediate less efficient cytotoxicity that is independent of BP exposure of the breast carcinoma cells.
Figure 5.
Cytotoxic functions by breast-derived Vδ2 γδ T cells in response to a triple negative carcinoma cell line. A, Vδ2+ T cells were expanded in vitro from breast tissue preparations by exposure to BP. The T cells were co-incubated with MDA-MB-468 breast carcinoma cells that were pulsed (right) or not pulsed (left) with BP, and cell surface expression of CD107a (LAMP-1, a marker of recent cytotoxic activity) was assessed by flow cytometry after 4 hours. B, MDA-MB-468 cells were pulsed (black squares) or not pulsed (grey squares) with BP, and co-incubated with in vitro expanded Vδ2+ T cells at the indicated effector:target ratios. The plot shows cytotoxicity of the target cells as assessed by cell-surface up-regulation of Annexin V. Similar results were obtained in five independent experiments. C, primary organoid cells were co-incubated with MDA-MB-468 cells that were pulsed (right) or not pulsed (left) with BP, and CD107a expression by T cells was assessed by flow cytometry. Numbers in the gates indicate the percentage of CD107a expressing cells from the Vδ2+ or Vδ2 T cell populations. The figure shows representative results from one out of seven independent experiments. D, plot showing aggregated results for cell surface CD107a expression by primary T cells from the indicated breast tissue samples after exposure to MDA-MB-468 cells that were pulsed or not pulsed with BP, or by primary organoid-derived T cells that were not exposed to tumor cells (“Ex vivo”). Each symbol represents an independent experiment to assess CD107a cell surface expression by T cells from the indicated breast tissue samples. n.s. = not significant; * p=0.0273; ** p=0.0078 (Wilcoxon test).
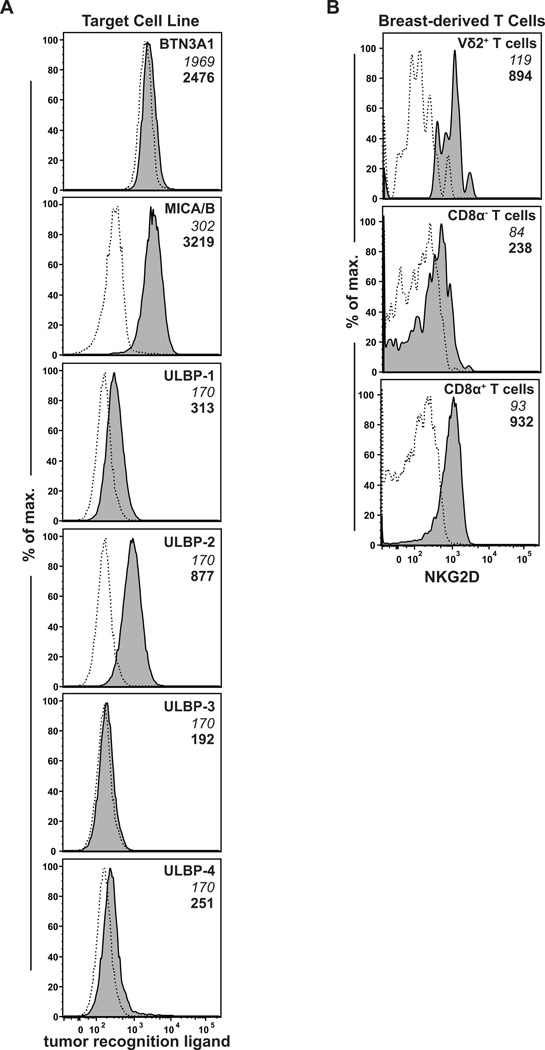
The functional responses by breast Vδ2+ T cells may result from TCR-mediated recognition of BTN3A1 that has associated with IPP (33). Additionally, these responses could be mediated by the binding of NKG2D receptors on the γδ T cells to a family of ligands, called MICA/B and ULBP1–6, that are up-regulated during cellular stress and that are often expressed by tumor cells (34). We therefore investigated the expression of these key tumor control molecules. Flow cytometric analysis of the MDA-MB-468 cells revealed weakly positive staining for BTN3A1 (Fig. 6A). Staining for expression of NKG2D ligands showed clearly positive staining for MICA/B and at least one member of the ULBP family (Fig. 6A). We also analyzed the organoid-derived lymphocytes for expression of the NKG2D receptor, and found that nearly all Vδ2+ and CD8+ T cells, and some of the CD8− T cells were NKG2D positive (Fig 6B). Thus, most primary breast epithelial organoid associated T cells express receptors for recognition of stressed or neoplastic cells.
Figure 6.
Expression of tumor-associated ligands and receptors. A, flow cytometric analysis of MDA-MB-468 cells using antibodies specific for BTN3A1 (Vδ2+ T cell receptor ligand), or for the following NKG2D–ligands: MICA and MICB, ULBP-1, ULBP-2 (antibody also cross-reacts with ULBP-5 and −6), ULBP-3, or ULBP-4. Filled histograms show staining with the specific mAb; dashed histograms show staining by an isotype control mAb. B, Expression of NKG2D (an activating receptor that recognizes MICA/B) by Vδ2+ T cells, CD8α T cells, and CD8α+ T cells from organoid preparations. Median fluorescence intensity (MFI) values for isotype control mAb staining are shown in italics, and specific mAb MFI values are shown in bold.
Discussion
It has recently become clear that the role of γδ T cells in cancer can include both anti- and pro-tumorigenic functions. Anti-neoplastic functions of γδ T cells include their critical role in tumor immunosurveillance and as early responders to nascent transformations (35–37). Additionally, γδ T cells can mediate rejection (killing) of established tumors (38, 39). Paradoxically, however, studies from murine models have indicated that γδ T cells can also promote the outgrowth of carcinomas as a result of the expression of factors that promote epithelial cell growth (40). Moreover, a recent analysis of human breast cancer patients showed that the presence of regulatory γδ T cells within the tumor was correlated with poor survival and high risks of relapse (41). These contrasting roles of γδ T cells underscore that it is critical to understand the characteristics of the immune cells in ductal epithelial tissues in order to design effective immuno-preventative strategies.
Prior analyses of immune populations associated with human breast have utilized unfractionated breast tissue that was not enriched specifically for the epithelial compartment. For example, Ruffell et al. analyzed tissue samples from prophylactic mastectomies and found that 60–70% of CD45+ cells were CD3+ T lymphocytes of several different varieties (5). The results presented here contrast with earlier studies of breast tissue, and demonstrate that the human breast epithelial organoid represents a specialized immunological niche that is comprised mainly of T cells (>90% of the CD45+ cells). In contrast to the composition of T cells from blood, the breast epithelial T cell population was markedly skewed towards CD8+ cells, which is consistent with a recent study demonstrating that CD8 T cells are directly integrated within the breast epithelium (7). Moreover, our observation that both the CD4+ and CD8+ T cells from the breast organoids had a CD27− effector memory phenotype contrasts sharply with the dominant phenotypes of T cells from blood. Thus, our analysis clearly establishes that a distinct make-up of leukocyte subsets is associated with the breast epithelial organoids, and that blood contamination of the samples is not a major factor.
Given this, it was particularly noteworthy that we detected Vδ2+ T cells in 87% of organoids. This γδ T cell subset is thought to localize mainly to the blood, while other types of γδ T cells (e.g. Vδ1+ T cells) are thought to be characteristic of peripheral tissues (42). Since the organoid-associated Vδ2+ T cells were not uniformly positive for the epithelial-residency marker CD103 (data not shown), some of the Vδ2+ T cells we detected may have been in the process of transiently trafficking through the tissue rather than permanently residing there. Nevertheless, the presence of Vδ2+ cells as part of the steady-state surveillance population of breast epithelial organoids has important implications for immunotherapeutic enhancement of cancer prevention, since this population is highly targetable.
Vδ2+ T cells recognize the accumulation of specific molecules associated with the biosynthesis of isoprenoid lipids, and thus they detect changes to cellular metabolism that are associated with hyper-proliferation and neoplastic transformation (43). As a result of this, Vδ2+ T cells become activated by administration of FDA-approved BP drugs since these drugs block the farnesyl pyrophosphate synthase enzyme and cause accumulation of IPP within cells. Interestingly, it has recently been shown that human breast cancer cell lines vary in their endogenous accumulation of IPP after exposure to BP, and that the amount of IPP produced in response to BP treatment correlated with the ability of Vδ2+ T cells to infiltrate and control the growth of transplanted tumor cells in vivo (34). We found that breast-derived Vδ2+ T cells showed efficient cytotoxicity towards the MDA-MB-486 triple negative breast carcinoma cell line after BP exposure, despite the comparatively low cell surface expression of BTN3A1 on the tumor cells. Thus, these tumor cells may be particularly rich in their accumulation of IPP after BP exposure. Alternatively, they may elicit strong cytotoxic responses by γδ T cells as a result of their high cell surface expression of NKG2D ligands such as MICA/B. These results suggest that administering BPs or related compounds to breast epithelial tissues may be an efficient way to enhance the killing of nascent neoplastic cells by Vδ2+ T cells within the breast tissue. However, since we also found that the Vδ2+ T cells showed a less potent, but still significant cytotoxic response to the tumor cells that were not treated with BP (an effect that might be due to endogenous accumulation of IPP within the tumor cells, or simply to their up-regulation of MICA/B molecules), it may be sufficient to use BP administration as a means to induce the proliferation of Vδ2+ T cells and thus to increase their frequency in vivo, without actually targeting breast epithelial cells for BP uptake.
In addition to Vδ2+ cells, we also detected Vδ1+ T cells in the breast epithelial organoid fraction. Vδ1 cells have been shown to be highly anti-tumorigenic to different tumors such as multiple myeloma (44), acute myeloid leukemia (45), and acute lymphoblastic leukemia (45). As a result of recent advances in understanding molecular interactions involved in activating Vδ1+ T cells, such as CD1d-mediated presentation of the cellular lipid sulfatide (46), it may soon be feasible to specifically target this subset. We also detected a subset of Vα7.2 TCR expressing lymphocytes that have been broadly characterized as MAIT cells (47). MAIT cells recognize vitamin metabolites that are presented by the non-classical antigen-presenting molecule MR-1 (47). Since many different microbes produce the chemical compounds recognized by MAIT cells, including Staphylococcus aureus and epidermidis, which are the major causative agents for human mastitis, it is likely that there is an important interplay between the resident microbiota and the immune cells in the ductal networks of human breast.
Together, our findings demonstrate that the lymphocyte compartment associated with human breast ductal epithelial organoids contains several conserved T cell populations that could be targeted, or in some cases possibly inhibited, to promote the immune-mediated clearance of nascent neoplastic cells. Such an approach may be particularly important for prophylactically treating women thought to be at high risk for breast cancer, and may also provide a novel non-invasive means to treat ductal carcinoma in situ.
Supplementary Material
Acknowledgments
Grant Support: Major funding for this project was from a Collaborative Health Sciences Program grant from the Wisconsin Partnership Project to M.N. Gould, J.E. Gumperz, and L.G. Wilke. N.A. Zumwalde was supported by NIH T32 CA157322. Additional funding was provided by NIH U01 ES019466 to M.N. Gould.
Footnotes
Conflicts of interest: None
References
- 1.Gould MN. The utility of comparative genetics to inform breast cancer prevention strategies. Genetics. 2009;183:409–412. doi: 10.1534/genetics.109.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smits BM, Sharma D, Samuelson DJ, Woditschka S, Mau B, Haag JD, et al. The non-protein coding breast cancer susceptibility locus Mcs5a acts in a non-mammary cell-autonomous fashion through the immune system and modulates T-cell homeostasis and functions. Breast cancer research : BCR. 2011;13:R81. doi: 10.1186/bcr2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006;90:1–50. doi: 10.1016/S0065-2776(06)90001-7. [DOI] [PubMed] [Google Scholar]
- 4.Visvader JE. Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Genes & development. 2009;23:2563–2577. doi: 10.1101/gad.1849509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruffell B, Au A, Rugo HS, Esserman LJ, Hwang ES, Coussens LM. Leukocyte composition of human breast cancer. Proc Natl Acad Sci U S A. 2012;109:2796–2801. doi: 10.1073/pnas.1104303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huo CW, Chew G, Hill P, Huang D, Ingman W, Hodson L, et al. High mammographic density is associated with an increase in stromal collagen and immune cells within the mammary epithelium. Breast cancer research : BCR. 2015;17:79. doi: 10.1186/s13058-015-0592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Degnim AC, Brahmbhatt RD, Radisky DC, Hoskin TL, Stallings-Mann M, Laudenschlager M, et al. Immune cell quantitation in normal breast tissue lobules with and without lobulitis. Breast cancer research and treatment. 2014;144:539–549. doi: 10.1007/s10549-014-2896-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hussein MR, Hassan HI. Analysis of the mononuclear inflammatory cell infiltrate in the normal breast, benign proliferative breast disease, in situ and infiltrating ductal breast carcinomas: preliminary observations. Journal of clinical pathology. 2006;59:972–977. doi: 10.1136/jcp.2005.031252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandstrom A, Peigne CM, Leger A, Crooks JE, Konczak F, Gesnel MC, et al. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vgamma9Vdelta2 T cells. Immunity. 2014;40:490–500. doi: 10.1016/j.immuni.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H, Henry O, Distefano MD, Wang YC, Raikkonen J, Monkkonen J, et al. Butyrophilin 3A1 plays an essential role in prenyl pyrophosphate stimulation of human Vgamma2 Vdelta2 T cells. J Immunol. 2013;191:1029–1042. doi: 10.4049/jimmunol.1300658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. The Journal of experimental medicine. 2003;197:163–168. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bukowski JF, Morita CT, Tanaka Y, Bloom BR, Brenner MB, Band H. V gamma 2V delta 2 TCR-dependent recognition of non-peptide antigens and Daudi cells analyzed by TCR gene transfer. J Immunol. 1995;154:998–1006. [PubMed] [Google Scholar]
- 13.Bukowski JF, Morita CT, Band H, Brenner MB. Crucial role of TCR gamma chain junctional region in prenyl pyrophosphate antigen recognition by gamma delta T cells. J Immunol. 1998;161:286–293. [PubMed] [Google Scholar]
- 14.Morita CT, Jin C, Sarikonda G, Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vgamma2Vdelta2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunological reviews. 2007;215:59–76. doi: 10.1111/j.1600-065X.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- 15.Dieli F, Gebbia N, Poccia F, Caccamo N, Montesano C, Fulfaro F, et al. Induction of gammadelta T-lymphocyte effector functions by bisphosphonate zoledronic acid in cancer patients in vivo. Blood. 2003;102:2310–2311. doi: 10.1182/blood-2003-05-1655. [DOI] [PubMed] [Google Scholar]
- 16.Winter MC, Holen I, Coleman RE. Exploring the anti-tumour activity of bisphosphonates in early breast cancer. Cancer treatment reviews. 2008;34:453–475. doi: 10.1016/j.ctrv.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Postlberger S, Menzel C, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. The New England journal of medicine. 2009;360:679–691. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]
- 18.Eidtmann H, de Boer R, Bundred N, Llombart-Cussac A, Davidson N, Neven P, et al. Efficacy of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: 36-month results of the ZO-FAST Study. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2010;21:2188–2194. doi: 10.1093/annonc/mdq217. [DOI] [PubMed] [Google Scholar]
- 19.Chlebowski RT, Col N. Bisphosphonates and breast cancer incidence and recurrence. Breast disease. 2011;33:93–101. doi: 10.3233/BD-2010-0324. [DOI] [PubMed] [Google Scholar]
- 20.Coleman R, Powles T, Paterson A, Gnant M, Anderson S, Diel I, et al. Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015;386:1353–1361. doi: 10.1016/S0140-6736(15)60908-4. [DOI] [PubMed] [Google Scholar]
- 21.Hue TF, Cummings SR, Cauley JA, Bauer DC, Ensrud KE, Barrett-Connor E, et al. Effect of bisphosphonate use on risk of postmenopausal breast cancer: results from the randomized clinical trials of alendronate and zoledronic acid. JAMA internal medicine. 2014;174:1550–1557. doi: 10.1001/jamainternmed.2014.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Proia DA, Kuperwasser C. Reconstruction of human mammary tissues in a mouse model. Nature protocols. 2006;1:206–214. doi: 10.1038/nprot.2006.31. [DOI] [PubMed] [Google Scholar]
- 23.Labarge MA, Garbe JC, Stampfer MR. Processing of human reduction mammoplasty and mastectomy tissues for cell culture. Journal of visualized experiments : JoVE. 2013 doi: 10.3791/50011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma D, Smits BM, Eichelberg MR, Meilahn AL, Muelbl MJ, Haag JD, et al. Quantification of epithelial cell differentiation in mammary glands and carcinomas from DMBA- and MNU-exposed rats. PloS one. 2011;6:e26145. doi: 10.1371/journal.pone.0026145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keller PJ, Arendt LM, Skibinski A, Logvinenko T, Klebba I, Dong S, et al. Defining the cellular precursors to human breast cancer. Proc Natl Acad Sci U S A. 2012;109:2772–2777. doi: 10.1073/pnas.1017626108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arendt LM, Keller PJ, Skibinski A, Goncalves K, Naber SP, Buchsbaum RJ, et al. Anatomical localization of progenitor cells in human breast tissue reveals enrichment of uncommitted cells within immature lobules. Breast cancer research : BCR. 2014;16:453. doi: 10.1186/s13058-014-0453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishikawa H, Naito T, Iwanaga T, Takahashi-Iwanaga H, Suematsu M, Hibi T, et al. Curriculum vitae of intestinal intraepithelial T cells: their developmental and behavioral characteristics. Immunological reviews. 2007;215:154–165. doi: 10.1111/j.1600-065X.2006.00473.x. [DOI] [PubMed] [Google Scholar]
- 28.Ochsenbein AF, Riddell SR, Brown M, Corey L, Baerlocher GM, Lansdorp PM, et al. CD27 expression promotes long-term survival of functional effector-memory CD8+ cytotoxic T lymphocytes in HIV-infected patients. The Journal of experimental medicine. 2004;200:1407–1417. doi: 10.1084/jem.20040717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuss I, Donnenberg AD, Gooding W, Whiteside TL. Effector CD8+CD45RO-CD27-T cells have signalling defects in patients with squamous cell carcinoma of the head and neck. British journal of cancer. 2003;88:223–230. doi: 10.1038/sj.bjc.6600694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, et al. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature. 1994;372:190–193. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- 31.Thompson K, Roelofs AJ, Jauhiainen M, Monkkonen H, Monkkonen J, Rogers MJ. Activation of gammadelta T cells by bisphosphonates. Advances in experimental medicine and biology. 2010;658:11–20. doi: 10.1007/978-1-4419-1050-9_2. [DOI] [PubMed] [Google Scholar]
- 32.Benevides L, da Fonseca DM, Donate PB, Tiezzi DG, De Carvalho DD, de Andrade JM, et al. IL17 Promotes Mammary Tumor Progression by Changing the Behavior of Tumor Cells and Eliciting Tumorigenic Neutrophils Recruitment. Cancer research. 2015 doi: 10.1158/0008-5472.CAN-15-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wrobel P, Shojaei H, Schittek B, Gieseler F, Wollenberg B, Kalthoff H, et al. Lysis of a broad range of epithelial tumour cells by human gamma delta T cells: involvement of NKG2D ligands and T-cell receptor- versus NKG2D–dependent recognition. Scandinavian journal of immunology. 2007;66:320–328. doi: 10.1111/j.1365-3083.2007.01963.x. [DOI] [PubMed] [Google Scholar]
- 34.Benzaid I, Monkkonen H, Stresing V, Bonnelye E, Green J, Monkkonen J, et al. High phosphoantigen levels in bisphosphonate-treated human breast tumors promote Vgamma9Vdelta2 T-cell chemotaxis and cytotoxicity in vivo. Cancer research. 2011;71:4562–4572. doi: 10.1158/0008-5472.CAN-10-3862. [DOI] [PubMed] [Google Scholar]
- 35.Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, et al. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294:605–609. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]
- 36.Liu Z, Eltoum IE, Guo B, Beck BH, Cloud GA, Lopez RD. Protective immunosurveillance and therapeutic antitumor activity of gammadelta T cells demonstrated in a mouse model of prostate cancer. J Immunol. 2008;180:6044–6053. doi: 10.4049/jimmunol.180.9.6044. [DOI] [PubMed] [Google Scholar]
- 37.Strid J, Roberts SJ, Filler RB, Lewis JM, Kwong BY, Schpero W, et al. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat Immunol. 2008;9:146–154. doi: 10.1038/ni1556. [DOI] [PubMed] [Google Scholar]
- 38.Gertner-Dardenne J, Castellano R, Mamessier E, Garbit S, Kochbati E, Etienne A, et al. Human Vgamma9Vdelta2 T cells specifically recognize and kill acute myeloid leukemic blasts. J Immunol. 2012;188:4701–4708. doi: 10.4049/jimmunol.1103710. [DOI] [PubMed] [Google Scholar]
- 39.D’Asaro M, La Mendola C, Di Liberto D, Orlando V, Todaro M, Spina M, et al. V gamma 9V delta 2 T lymphocytes efficiently recognize and kill zoledronate-sensitized, imatinib-sensitive, and imatinib-resistant chronic myelogenous leukemia cells. J Immunol. 2010;184:3260–3268. doi: 10.4049/jimmunol.0903454. [DOI] [PubMed] [Google Scholar]
- 40.Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522:345–348. doi: 10.1038/nature14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma C, Zhang Q, Ye J, Wang F, Zhang Y, Wevers E, et al. Tumor-infiltrating gammadelta T lymphocytes predict clinical outcome in human breast cancer. J Immunol. 2012;189:5029–5036. doi: 10.4049/jimmunol.1201892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Girardi M. Immunosurveillance and immunoregulation by gammadelta T cells. The Journal of investigative dermatology. 2006;126:25–31. doi: 10.1038/sj.jid.5700003. [DOI] [PubMed] [Google Scholar]
- 43.Wang H, Sarikonda G, Puan KJ, Tanaka Y, Feng J, Giner JL, et al. Indirect stimulation of human Vgamma2Vdelta2 T cells through alterations in isoprenoid metabolism. J Immunol. 2011;187:5099–5113. doi: 10.4049/jimmunol.1002697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knight A, Mackinnon S, Lowdell MW. Human Vdelta1 gamma-delta T cells exert potent specific cytotoxicity against primary multiple myeloma cells. Cytotherapy. 2012;14:1110–1118. doi: 10.3109/14653249.2012.700766. [DOI] [PubMed] [Google Scholar]
- 45.Meeh PF, King M, O’Brien RL, Muga S, Buckhalts P, Neuberg R, et al. Characterization of the gammadelta T cell response to acute leukemia. Cancer immunology, immunotherapy : CII. 2006;55:1072–1080. doi: 10.1007/s00262-005-0094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luoma AM, Castro CD, Mayassi T, Bembinster LA, Bai L, Picard D, et al. Crystal structure of Vdelta1 T cell receptor in complex with CD1d–sulfatide shows MHC-like recognition of a self-lipid by human gammadelta T cells. Immunity. 2013;39:1032–1042. doi: 10.1016/j.immuni.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gapin L. Check MAIT. J Immunol. 2014;192:4475–4480. doi: 10.4049/jimmunol.1400119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.