Abstract
Green eco-friendly housing includes approaches to reduce indoor air pollutant sources and to increase energy efficiency. Although sealing/tightening buildings can save energy and reduce the penetration of outdoor pollutants, an adverse outcome can be increased buildup of pollutants with indoor sources. The objective of this study was to determine the differences in the indoor air quality (IAQ) between green and non-green homes in low-income housing complexes. In one housing complex, apartments were renovated using green principles (n=28). Home visits were conducted immediately after the renovation, and subsequently at 6 months and at 12 months following the renovation. Of these homes, eight homes had pre-renovation home visits; this allowed pre- and post-renovation comparisons within the same homes. Parallel visits were conducted in non-green (control) apartments (n=14) in a nearby low-income housing complex. The IAQ assessments included PM2.5, black carbon, ultrafine particles, sulfur, total volatile organic compounds (VOCs), formaldehyde, and air exchange rate. Data were analyzed using linear mixed-effects models. None of the indoor pollutant concentrations were significantly different between green and non-green homes. However, we found differences when comparing the concentrations before and after renovation. Measured immediately after renovation, indoor black carbon concentrations were significantly lower averaging 682 ng/m3 in post-renovation vs. 2,364 ng/m3 in pre-renovation home visits (p=0.01). In contrast, formaldehyde concentrations were significantly higher in post-renovated (0.03 ppm) than in pre-renovated homes (0.01 ppm) (p=0.004). Questionnaire data showed that opening of windows occurred less frequently in homes immediately post-renovation compared to pre-renovation; this factor likely affected the levels of indoor black carbon (from outdoor sources) and formaldehyde (from indoor sources) more than the renovation status itself. To reduce IAQ problems and potentially improve health, careful selection of indoor building materials and ensuring sufficient ventilation are important for green building designs.
Keywords: Green renovation, PM 2.5, VOC, Black carbon, Formaldehyde
Graphical abstract
1. Introduction
Environmental concerns for improved energy consumption and reduced carbon emissions have motivated increased adoption of green principles in new construction and remodeling practices. “Green” housing is designed by utilizing building materials with low-emissions, increasing energy efficiency and improving the health of occupants. As buildings become more “green”, there have been rising concerns about the long-term effects of changes in building materials as well as operations and construction practices. Concerns about indoor air quality (IAQ) in energy-efficient buildings started as early as the 1970’s. By the mid-1980’s, it was reported that up to 30% of new or remodeled energy-efficient buildings might have an unusually high rate of complaints of sick building syndrome (Akimento et al., 1986). It has been suggested that “green” housing solutions may be detrimental to residents’ health if factors affecting the IAQ are not considered. Improper selection and implementation of retrofits such as continuous and adequate outdoor air flow and HVAC operational parameters can directly affect indoor environmental quality and may be detrimental to resident’s health (Mudarri et al., 2006).
Additionally, Americans may have increased exposure to indoor contaminants as they spend increasing amount of time indoors. It has been estimated that adults spend 90% of their time indoors, whereas children younger than 3 years spend up to 100% of their time in indoor environments. Additionally between the ages of 7–12 years, which is the age group of interest in the overarching study, children can spend up to 87% of their time indoors (Moya et al., 2011). Indoor environment in homes can present significant health risks (Samet et al., 1993; Weisel et al., 2005; Logue et al., 2011), with some of the most vulnerable populations affected being children and those with existing respiratory diseases (Peat et al., 1998; Emenius et al., 2004; Breysse et al., 2010). Furthermore, poor indoor air quality has increased health implications in low-income communities (Krieger et al., 2002; Perlin et al., 2001). It has been suggested that multilevel interventions are necessary to properly assess and improve the indoor environment of low-income residents (Brugge et al., 2003; Sandel et al., 2004). Previous studies have emphasized the potential for IAQ improvements through retrofit measures that also make houses more energy-efficient (Noris et al., 2013). However, even with lower emission materials, tighter homes still have the potential of poorer IAQ due to reduced air exchange. It is important to assess the extent to which green-built, low-income housing actually improves indoor air quality when compared to standard-built, low-income housing.
Green building studies that have focused on the indoor environment have been mostly qualitative and based on data collected from questionnaires rather than quantitative indoor sampling and analysis (Jacobs et al., 2010; Jacobs et al., 2011). Among few efforts that aimed at quantitatively assessing the overall indoor environment within green buildings, little has been reported regarding the impact of aerosol particles on the IAQ of low-income green homes (Colton et al., 2014; Frey et al., 2014; Xiong et al., 2015)
This research is a subset of the Green Housing Study (GHS), a multi-site study designed by the Centers for Disease Control and Prevention (CDC) and the Department of Housing and Urban Development (HUD). A main objective of the GHS is to investigate how green housing factors are associated with IAQ and children’s respiratory health. For the current manuscript, we compare and quantitatively evaluate the indoor air quality between green and non-green low-income homes in one of the GHS sites, Cincinnati, Ohio.
2. Methods
2.1 Study Design
The study site in Cincinnati was a low-income multi-family housing complex of approximately 800 apartments. The housing complex renovations were subsidized by a federal housing program. The occupants of these residences were primarily English-speaking African Americans. The corresponding control homes, located about 6 miles from the test site, belonged to a Cincinnati Metropolitan Housing Authority (CHMA) housing community built in the 1940s that is populated by low-income (mostly English-speaking African American) residents.
Subject recruitment in Cincinnati was initiated at a town hall meeting in October 2011 in order to reach a sample size goal of 64 children (established for each study site of the GHS). All residents were invited to the meeting by mailing fliers to their units. Following the town hall meeting, recruitment proceeded mainly by door-to-door home visits which likely resulted in a convenience sample of eligible households. The main inclusion criterion was having a child (ages 7–12 years) with a report of doctor-diagnosed asthma residing in the unit. Table 1 describes the elements of renovation in the “green” housing complex.
Table 1.
Comparison of building features between the green-renovated homes and non-green homes.
| Renovation features implemented in the “Green” Homes | Present in Non-Green(Control) Homes |
|---|---|
| Integrated pest management (“green” feature) | No |
| Low VOC countertops, doors, and paint (“green” feature) | No |
| Energy efficient windows and doors (“green” feature) | No |
| Energy efficient lighting and bulbs (“green” feature) | No |
| Low flow toilets (“green” feature) | No |
| New roofing (not a “green” feature) | No |
| Whole house insulation (“green” feature) | No |
| Energy efficient central heating/cooling systems with programmable thermostats (“green” feature) | No |
| Bathroom exhaust fans | Yes |
| Combination smoke/carbon monoxide alarms | No |
| Designated parking for low emission vehicles (“green” feature) | No |
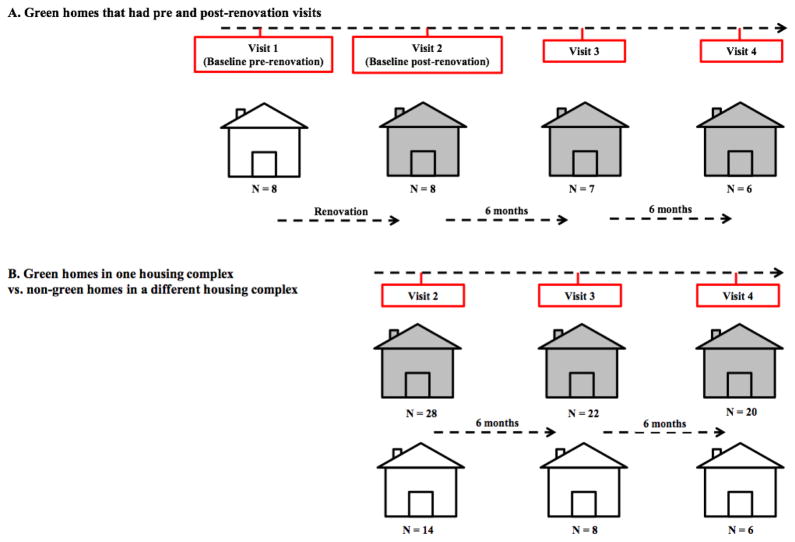
Figure 1A & B depicts the timeline of the study in 42 homes, of which 14 were considered non-green units, and 28 were green units. Figure 1A depicts eight homes for which we were able to also conduct assessments before (i.e, baseline pre-renovation visit = Visit 1) Baseline (post-renovation) data on green-renovated homes was collected within four months of renovation (Visit 2). Thereafter, data were collected from these homes every six months for a period of one year (Visits 3 and 4). Parallel assessments were simultaneously conducted in the non-green homes at a different low-income housing complex located within 5 km of the first housing complex. For the non-green homes, no Visit 1 assessments occurred because there was no “pre-renovation” measurement.
Figure 1.
Schematic of an overall timeline for the Cincinnati Green Housing Study (CGHS).
(A) Green homes (n=8) in the housing complex that had visits before and after renovation. White home indicates the pre-renovation visit.
(B) Green homes in one housing complex (n=28) vs. non-green homes in a different housing complex (n=14). Gray shading indicates green homes.
2.2 Indoor Air Sampling and Analysis
We measured six pollutants indoors: particulate matter less than 2.5 μm (PM2.5), black carbon (BC), sulfur (S), ultrafine particles (UFP), total volatile organic compounds (TVOCs) and formaldehyde. Sulfur concentrations were calculated to determine the indoor/outdoor (I/O) ratio, which has been used as a surrogate for air exchange rates. Each air sample was collected in the child’s bedroom to determine the level of indoor pollution in the space where the asthmatic child was likely to spend most of his/her time indoors. In cases where the child spent the majority of his/her time in the parent’s bedroom, the sampling event was conducted in the parent’s bedroom instead. Additionally, questionnaires were administered to families to collect data on household and occupant characteristics. The following questionnaire data were analyzed to determine if other occupant activities had an impact on indoor concentrations pre- and post-renovation:
frequency of opening of windows,
use of paint thinner, brush cleaner, furniture stripper,
use of varnish, lacquer, wood stain, wet paint,
use of toilet bowl deodorant,
use of air freshener, room deodorizer,
use of mothballs, and
use of fingernail polish.
The majority of the pollutants, except for ultrafine particles, were measured over a five-day period. Temperature and relative humidity measurements were made using a HOBO® data logger (Onset Computer Corporation, Bourne, MA) and were continuously recorded every five minutes throughout the five-day sampling duration. The data were downloaded to a computer for further analysis. Integrated PM2.5 samples were collected at a height of 1 m above the floor for five days. The PM2.5 sample collection was performed onto 37 mm diameter, 2.0 μm pore-size polytetrafluoroethylene (PTFE) membrane filters using single-stage Personal Modular Impactors (SKC, Inc., Eighty Four, PA) connected to AirChek 2000 (Model 200–2002; SKC, Inc., Eighty Four, PA) pumps. These pumps are portable and designed for quiet indoor operation. The pump was calibrated to a flow rate of 3 (± 10%) L/min prior to the start of sampling and checked immediately after sampling with a BIOS DryCal DC-2 flow meter (SKC, Inc., Eighty Four, PA).
The filters were analyzed for PM2.5 and BC content at the Lamont-Doherty Earth Observatory following standard protocols for PTFE filters (Grass et al 2010; Yan et al 2011). Briefly, after equilibration for at least 24 h in a temperature- and humidity-controlled chamber, filters were statically discharged via polonium sources and weighed on a microbalance located inside the chamber. Two reference weights and one internal lab filter were measured every weighing session. A minor correction was made to all filters for differences in filter buoyancy due to atmospheric pressure difference pre- and post-measurement. The mass concentration of black carbon on the PM2.5 filters was measured using a multi-wavelength integrating sphere method (Yan et al. 2011) that deployed a balanced deuterium tungsten halogen light source (DH-2000-BAL), an integrating sphere (ISP-50-8-R), a lab-made filter holder, and an Ocean Optics USB4000-VIS-NIR miniature fiber-optic spectrometer. Furthermore, sulfur concentrations of the PM2.5 filters were quantified utilizing energy dispersive X-ray fluorescence (XRF) spectrometry (EPA, 1999).
Real-time concentrations of ultrafine particles were measured in the child’s bedroom with a P-Trak (TSI, Inc., Shoreview, MN) for 45–60 minutes on day one of the five-day sampling. The P-Trak Ultrafine Particle Counter is a portable handheld device used to detect and count particles between the size range of 0.02 – 1 μm.
Simultaneously with the five-day PM2.5 sampling, continuous air sampling was conducted using passive diffusion dosimeters for VOCs; solvents were measured with organic vapor monitors (3520 Badge, 3M, Saint Paul, MN), and aldehydes were sampled with passive sampling badges (UMEx 100, SKC Inc., Eighty Four, PA). VOCs and aldehyde samples were sent to the Wisconsin Occupational Health Laboratory (WOHL) for analysis. VOCs were desorbed from the passive sampling media with carbon disulfide and quantified using gas chromatography coupled with flame ionization (GC-FID) and mass spectrometric (GC/MS) detection (Agilent, Santa Clara, CA) (WOHL 2011). Aldehydes were desorbed from passive 2,4-dinitrophenylhydrazine (DNPH) treated media with acetonitrile, and the derivatized aldehydes were quantified using ultra high-performance liquid chromatography (UPLC) with UV detection (Acquity, Waters, Milford, MA) (WOHL 2010).
Air exchange rates (AER) were assessed using perfluorocarbon (PFT) technique (Dietz et al, 1986). Briefly, sources of perfluoromethyl cyclohexane gas (PMCH) were placed on external walls of the investigated apartment and a capillary absorption tube (CAT) was placed in the center of the home to sample the gas by diffusion. The PFT was quantified by gas chromatography and AER was calculated by assuming a well-mixed interior (Dietz et al., 1986). Due to a batch-specific PMCH contamination problem, our results were deemed reliable for only 40 total of 119 AER assessments. Negligible levels of tracer gas on lab and field blanks were used as the criteria to confirm the removal of the source of contamination. However, due to a batch-specific PMCH contamination problem, our results were deemed reliable for only 40 total of 119 AER assessments. Negligible levels of tracer gas on lab and field blanks were used as the criteria to confirm the removal of the source of contamination.
2.3 Outdoor Air Sampling
To obtain an estimate of outdoor PM2.5, black carbon and VOC concentrations, outdoor sampling was conducted on the roof of a building located at a central site that was within a 5 km radius of both housing complexes. The outdoor sampling was performed during one week per each season using the same methods and protocols as for indoor sampling. These measurements were repeated throughout the duration of the study to yield an estimate of the average outdoor PM2.5 and VOC concentrations. To compare the outdoor-to-indoor data, an average was calculated from the total indoor-to-outdoor ratios determined for individual homes. For this calculation, indoor measurements were taken from homes where sampling corresponded with the same dates as outdoor sampling events.
2.4 Statistical Analysis
Part 1: “Green vs. Non-Green”
Differences in concentrations of all six pollutants between green or non-green homes (Figure 1B) were assessed using mixed effects linear models. Only observations from visits 2, 3, and 4 were used in this portion of the analysis. To take into account autocorrelation among repeated measurements in a home, home ID was treated as a random effect in the models. (i.e., each home had its own intercept). The models were adjusted for indoor relative humidity, season and number of occupants. The number of occupants were used instead of occupant density given that square footage of green (average = 987 ft2) and non-green (average = 970 ft2) homes in this study were comparable. Seasons were categorized as heating (October – March) vs. non-heating (April – September), based on personal communication with representatives from a local energy company in Cincinnati. Each model only used observations with complete information on all relevant variables. Models were fit using the Nonlinear Mixed Effects Model (NLME) package in the R Project for Statistical Computing software (RStudio, Boston, MA) and MATLAB (Mathworks, Natick, MA).
Part 2: “Before and after renovation”
Differences between pre- and post-renovation data for homes within the same housing complex were assessed in a separate analysis (Figure 1A). Only homes with visit 1 were used in this portion of the analysis. Visit 1 (Baseline pre-renovation) was considered non-green and visits 2, 3, and 4(all of which were post-renovation) were considered as green for these homes. Differences in concentrations of all six pollutants between visit 1 compared to visits 2, 3, and 4 were assessed using a Wilcoxon signed rank test. Questionnaire responses about home characteristics were dichotomized (e.g, Windows opened on average more than 3 hours during the week of air sampling (Yes/No)), then a McNemar’s test was used to identify changes in occupant behavior that could potentially affect ventilation and have an influence on the indoor air pollution, based on questionnaire data, from pre-renovation to post-renovation. .
3. Results and Discussion
Household demographics (Table 2) showed that more than 95% of the population in both green and non-green homes identified as African-American, with the entire study population earning <$25,000 per annum. While it appears that many of the primary caregivers (mainly mothers) of the green homes had attained a high school diploma or GED (test of high school ability), many of the primary caregivers in the non-green homes did not finish high school. Additionally, the majority of the primary caregivers in green homes reported an annual household income <$5000, whereas a higher number of primary caregivers in non-green homes reported an annual household income between $10,000 and $25,000. It is not clear if these differences could influence household characteristics that could subsequently affect environmental exposure or asthma outcomes of their children. However socioeconomic status will be investigated further in future Green Housing study sites. Furthermore, green and non-green homes had an equally high number of smokers. The presence of pets was considerably low in both green (18%) and non-green (29%) homes.
Table 2.
Occupant and household characteristics of low-income green and non-green homes.
| Characteristics | Green Homes (N=28) | Non -Green Homes (N=14) | p-value |
|---|---|---|---|
| N (%) | N (%) | ||
|
| |||
| Race/Ethnicity | |||
| African American | 27 (96%) | 14 (100%) | 0.53 |
| White | 1 (4%) | 0 | 0.53 |
| Number of Occupants Per Household(median) | 4 | 4 | 0.29 a |
| Adults | 3 | 3 | 0.09 a |
| Children | 1 | 1 | 0.23 a |
| Presence of Pets | 5 (18%) | 4 (29%) | 0.41 |
| Smoking | |||
| Household member | 20 (71%) | 8 (57%) | 0.3 |
| Visitors in house | 19 (68%) | 9 (64%) | 0.84 |
| Level of Education | |||
| < High school diploma or GED | 3 (11%) | 6 (43%) | 0.02 |
| High school diploma or GED | 11 (39%) | 2 (14%) | 0.09 |
| Some college but no degree | 12 (43%) | 3 (21%) | 0.18 |
| Associate degree | 1 (4%) | 3 (21%) | 0.06 |
| Bachelor's degree | 1 (4%) | 0 | 0.49 |
| Annual Household Income | |||
| < $5000 | 18 (64%) | 5 (36%) | 0.08 |
| $5000 – $9,999 | 7 (25%) | 0 | 0.04 |
| $10,000 – $25,000 | 3 (11%) | 9 (64%) | <0.001 |
Two-sample t-test used to test differences for continuous variables. A chi-square test was used to test differences for categorical variables.
Table 3 summarizes the overall averages of indoor and outdoor pollutant concentrations. To understand whether these concentrations were high or low, we compared to other studies. We found that indoor PM2.5 levels measured in our study were much higher than those reported in other studies. Xiong et al. (2015) reported ranges between 1 – 6 μg/m3 in the green buildings; Colton et al. (2014) reported medians of 8.9 μg/m3 and 15.1 μg/m3 in green and non-green homes, respectively; Frey et al. (2014) reported medians of 13 μg/m3 in non-renovated and 20 μg/m3 in post-renovated buildings. Additionally, Martuzevicius et al. (2008) reported indoor PM2.5 ranges between 9.1 and 46 μg/m3 within traditional non-green homes in Cincinnati, which is much lower than the indoor PM2.5 concentrations that we observed in this investigation. Indoor black carbon measurements, used as a surrogate for elemental carbon, however, were similar to those reported by Martuzevicius et al. (2008). Compared to the levels of indoor sulfur found by Martuzevicius et al. (2008), 0.01 – 4 μg/m3, our measurements were lower. The ultrafine particle concentrations observed in this study were somewhat lower than those reported by Hochstetler et al. (2011). They found concentrations ranging from 2,000 to 56,000 count/cm3 for indoor air in schools of the Cincinnati metropolitan area. Indoor total VOC levels measured in this investigation were fairly low compared to other studies conducted in the north-eastern US [210 – 6,000 μg/m3 (Pickett et al., 2011)]. The indoor formaldehyde measurements also revealed somewhat lower levels than those reported by other investigators; Frey et al. (2014) reported a median of 0.04 ppm in non-renovated homes and 0.05 ppm in renovated homes and Xiong et al. (2015) reported medians ranging from <0.01 ppm – 0.04 ppm in green homes.
Table 3.
Medians and interquartile ranges of pollutant concentrations from all repeated home visits and outdoor sampling sessions.
| Indoor Pollutants | n | Median | Interquartile range |
|---|---|---|---|
| PM2.5 (μg/m3) | 94 | 41 | 25 – 62 |
| Black Carbon (ng/m3) | 93 | 988 | 720 – 1,400 |
| Sulfur (μg/m3) | 93 | 0.51 | 0.42 – 0.61 |
| Ultrafine Particles (count/cm3) | 102 | 19,000 | 10,000 – 32,000 |
| Total VOCs (ppm) | 96 | 0.32 | 0.24 – 0.61 |
| Total VOCs (μg/m3) | 96 | 1,283 | 830 – 2,200 |
| Formaldehyde (ppm) | 96 | 0.02 | 0.014 – 0.033 |
|
| |||
| Outdoor Pollutants | n | Median | Interquartile range |
|
| |||
| PM2.5 (μg/m3) | 34 | 28 | 27 – 29 |
| Black Carbon (ng/m3) | 40 | 939 | 670 – 1,170 |
| Sulfur (μg/m3) | 41 | 0.6 | 0.4 – 1 |
| Total VOCs (ppm) | 7 | 0.1 | 0.09 – 0.2 |
| Total VOCs (μg/m3) | 7 | 429 | 350 – 710 |
| Formaldehyde (ppm) | 7 | 0.0053 | 0.004 – 0.006 |
n = number of observations for all homes that were sampled or outdoor sampling sessions
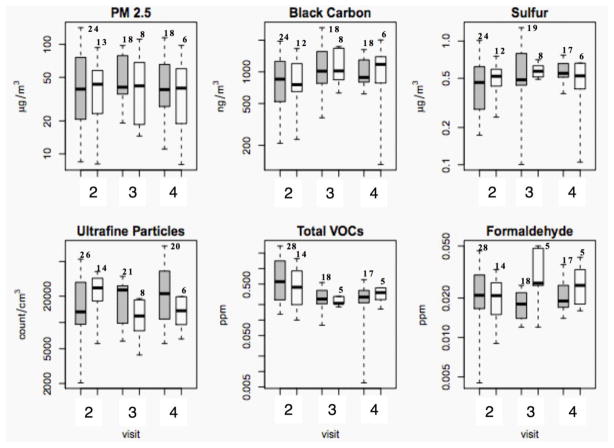
Figure 2 presents the indoor concentrations of all the six variables in green and non-green homes. There were no significant differences between green and non-green homes in the concentrations for any of the pollutants, in either the unadjusted mixed effects model or in the model adjusted for humidity, occupants and season. Unlike the findings of recent studies conducted by Frey et al. (2014) and Colton et al. (2014), where the investigators studied the impact of green new construction on the indoor air quality and noticed PM2.5 levels decreasing significantly over time, we did not find significant difference between either green or non-green homes nor between sampling visits (2, 3 and 4) in green homes. Indoor PM2.5 and BC followed similar trends in green homes, where concentrations rose slightly from Visit 2 (i.e., Baseline Post-renovation visit) to Visit 3 (i.e., 6-month follow-up visit), then declined so that during visit 4 (i.e., 12-month follow-up visit), the concentrations were similar to those observed during the Visit 2 (i.e., Baseline Post-renovation visit).
Figure 2.
Indoor concentrations in green versus non-green homes. Panel of boxplots for the concentrations of the various pollutants separated by visit number and green/non-green status of homes in Figure 1B. Green homes are represented by the shaded gray bars and non-green homes by the white bars. The y-axis is plotted on the log scale and the number of observations during each visit is stated on top of each boxplot. The boxes represent the interquartile range, the dark line represents the median and the whiskers represent the most extreme data point, which is no more than one and a half interquartile range away from the box. Points beyond the whiskers have been omitted.
It has been shown in previous studies that both indoor (tobacco smoke, etc.) and outdoor sources (automobile exhaust, etc.) contribute to PM2.5 concentrations in the indoor environment (Abt et al., 2000; Na and Cocker, 2005; Götschi et al., 2002). The median of indoor PM2.5 concentrations was higher than that of the outdoor concentrations (41 μg/m3 vs. 28 μg/m3) (Table 3). Additionally, the I/O ratio for PM2.5 in this study ranged from 1 to 11, with a median of 5.3 (data not shown) suggesting that PM2.5 was dominated by indoor sources. This may, at least partially, be associated with a high percentage of smokers in the majority of our study homes (Table 2). Na and Cocker (2005) have shown that 24-hour average concentration of PM2.5 was over 200 μg/m3 in homes with a frequent smoker whereas only about 30 μg/m3in similar homes without smokers. Another study using real-time monitoring in low-income multifamily housing showed that transient PM2.5 concentration can be up to 1000 μg/m3 indoors during smoking (Russo et al., 2015).
The median of indoor BC concentrations was close to that observed outdoors (988 ng/m3 and 939 ng/m3, respectively) (Table 3). Additionally, the I/O ratio for BC ranged from 0.3 to 5.1, with a median of 0.7 (data not shown) suggesting that indoor concentrations mostly followed outdoor concentration trends. Therefore, our data on BC corroborated previously reported findings that indoor BC concentration depends predominantly on the outdoor concentration (Gotschi et al., 2002).
Data on the I/O ratio for sulfur also showed no difference between green apartments (average = 3.1) at and the non-green (average = 3.3) apartments at the control housing complex. This could be due to the influence of other environmental and emission parameters such as smoking indoors and the opening of windows, which could affect the indoor concentrations of sulfur. The median calculated from indoor sulfur data was similar to that calculated from the outdoor data (0.5 μg/m3 and 0.6 μg/m3, respectively). Overall, the I/O ratio for sulfur ranged from 0.1 to 4.5, with a median of 1.1 (data not shown); this, again, suggests that indoor concentration followed the outdoor trends. Additionally, the I/O ratio for sulfur in green homes ranged from 0.1 to 3.9 with a median of 1.3, whereas in non-green homes it ranged from 0.4 to 4.5 with a median of 1.4. As I/O ratio of sulfur is a surrogate for air exchange (Wallace et al., 2005), our sulfur data indicate that air exchange rates were similar in green and non-green homes.
Sulfur I/O data were supported by the data from AER measurements as there was no significant difference in air exchange rate per hour between green (median = 0.5) and non-green (median = 0.6) home types (p=0.3; data not shown). This did not follow the trend observed by Colton et al. (2014) who showed that there was marginally lower AER in green homes. The difference could potentially be attributed to the smaller sample size of the present study.
Real-time ultrafine particle measurements did not follow the trends identified for either black carbon or PM2.5. One of the potential reasons for this can be explained by the different sampling schedule, as ultrafine particles were measured for only 45–60 minutes on the days of equipment deployment instead of a continuous 5 day sampling regimen used for PM2.5 and BC. Since resident behaviors can vary widely throughout the day, this likely affected variability in ultra-fine measurements.,
There was no significant difference in the levels of formaldehyde and total VOC levels between green and non-green homes (Figure 2). This could be explained by the high levels of indoor smoking in both types of homes (Table 2). It was established that indoor formaldehyde and total VOC concentrations were dependent mainly on indoor sources as the outdoor concentrations were much lower than the indoor concentrations (Table 3). Similar trends have been established for formaldehyde in previous studies (Salthammer et al., 2010).
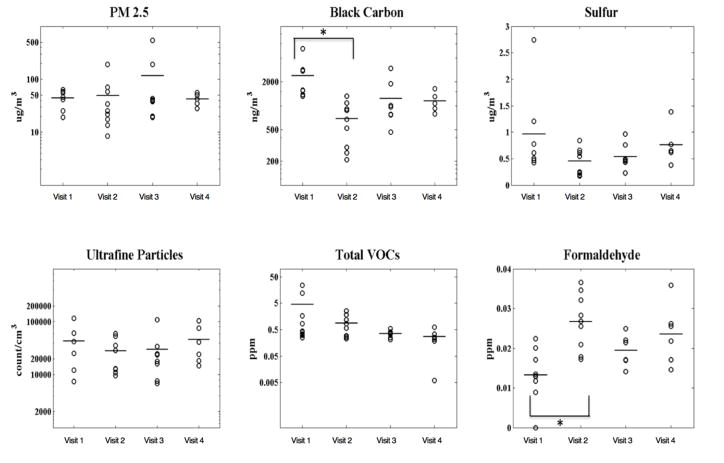
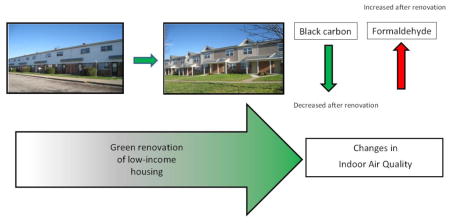
Although there were no significant differences in any of the six pollutants between the green units at the test site and non-green units at the control site, significant differences were found in BC and formaldehyde levels measured before and after green renovations in the units at the test site (Figure 3). BC observed immediately post renovations at visit 1 (average = 682 ng/m3) was significantly lower (p=0.01) than the pre- renovation level found at visit 0 (average = 2,364 ng/m3). Formaldehyde concentrations were found to be significantly higher (p=0.004) in homes immediately post renovation as compared to pre-renovation (0.03 vs. 0.01 ppm). The significant increase in the levels of formaldehyde, however, was not consistent with the findings of previous studies which either reported significant decrease of the formaldehyde level (Frey et al., 2015) or failed to find its increase in the green buildings post-renovation. In the current study, an increase in indoor formaldehyde levels post-renovation can be explained by indoor smoking (WHO 2010) amongst other parameters such as the presence of new building material consisting of particleboard and plywood, which could potentially contain formaldehyde-based resins (WHO 2010).
Figure 3.
Indoor concentrations of environmental pollutants in pre- and post-renovated homes presented in Figure 1A. Each symbol in the figure represents a single measurement. The data represent measurements conducted only in homes of families that stayed within the community pre- and post-renovation. The lines in the figure represent the medians. * represents statistically sigificant difference (p<0.05)
Based on questionnaire data from all pre- and post-renovated homes, frequency of opening of windows tended to be lower trend immediately post renovation. Five homes tended to have opened windows for at least 3 hours per day during the sampling period prior to renovation (Visit 1, N=8) compared to two homes post renovation (Visit 2, N=8) (p=0.1). These results were likely not affected by seasonal variation as sampling was spread throughout all seasons. A decrease in the opening of windows post-renovation potentially reduced the transport of BC from outdoors to indoors. In contrast, this behavior may have increased the accumulation of formaldehyde from indoor sources. Additionally, although AERs were not obtained from all the homes in this study, lower sulfur levels in homes post-renovation implied increased air tightness in renovated homes. This could lead to a decrease in the infiltration of outdoor air pollutants and an increase in accumulation of pollutants from indoor sources. This in turn would explain a higher indoor formaldehyde concentration from indoor sources and lower BC concentration, which originated primarily from outdoor sources. Besides the opening of windows, no statistical significance/trend was identified for any of the other questionnaire data.
All of the pollutants evaluated in this paper have been reported to generate detrimental effects on human health. Although no limits have been established for indoor PM2.5, the World Health Organization (WHO) retains the existing 24-hr fine particle standard for outdoor air (issued in 2005) at 25 μg/m3 (WHO, 2010). In our study, 71%, 89% and 78% of green homes were higher than the WHO PM2.5 standard during visits 1, 2 and 3, respectively. Among non-green homes, the respective percentages were 69%, 62% and 67%. Carbonyls, especially formaldehyde is ubiquitous in the indoor environment. The levels of formaldehyde measured in all green and control homes exceeded the recommended exposure limit (REL) of 7 ppb, established by the California EPA (2008). However, none of the measured formaldehyde levels were higher than the 0.1 mg/m3 (i.e., 81 ppb) WHO guideline (WHO, 2010). No guideline values are available for black carbon, sulfur, ultrafine particles or TVOCs.
4. Study Limitations
There were several limitations in the study, starting with the small sample size. Small sample size combined with high variability in pollutant concentrations decreased our ability to detect significant differences in IAQ between green and non-green homes. The ultrafine particles were measured for shorter time than the PM2.5, which limits the comparability of results between ultrafine and PM2.5 particles. However, it is notable that significant differences were seen only in the comparison that had the smallest sample size: pre- vs. post-renovation visits of the eight homes in the green housing complex. There were also several homes that were lost to follow-up after visit 1, which further reduced the sample size. Selection bias could be an issue if households with perceived poor IAQ preferentially enrolled from the green or from the non-green housing complexes. However, this is unlikely since we had almost equal smoking prevalence, one of the strongest indicators of poor IAQ (IOM, 2011), in both types of home. Additionally, air exchange data was available only for a fraction of homes, which limited our ability to appropriately determine the tightness of the building envelopes and consequently narrowed our options in examining factors affecting the transport of air pollutants. The limited air exchange data, however, agreed well with the I/O ratio of sulfur. Smoking could potentially be an effect modifier in this study. Both green and non-green homes had over 50% of household members smoking indoors. Therefore, even if the green homes were tighter and prevented more outdoor particles from getting in, indoor smoking would still make the levels in both green and non-green homes appear to be the same. Lastly, the building materials in the non-renovated homes were older than the newly renovated homes and would therefore generate fewer indoor emissions. This could have affected the formaldehyde concentrations indoors. With our small sample size of n=28 green homes and n=14 non-green homes, we were not able to test for effect modification in our models, but future studies might be able to investigate this further.
5. Conclusions
There was no significant difference in the levels of PM2.5, black carbon, sulfur, ultrafine particles, total VOCs or formaldehyde between green homes at the test site and non-green homes at the control site. Differences were observed only when the homes located in the same community were compared before and after the “green” renovation. Black carbon levels were significantly lower immediately post-renovation whereas formaldehyde was significantly higher immediately post-renovation. Both of these trends appear to be attributable to opening of windows that decreased after renovation. Overall, the results indicate that occupants’ activities appear to affect the indoor air quality more than the renovation status.
We could not detect significant differences between green and non-green homes, since this study was underpowered. However with future study sites and additional homes in different locations, it might be able to detect differences in green and non-green homes
Highlights.
We examined the indoor air quality (IAQ) of low-income green and non-green homes in Cincinnati, Ohio, USA.
Black carbon decreased and formaldehyde increased immediately post-renovation.
We found that occupants’ activities affect the IAQ more than the renovation status.
Acknowledgments
This study was funded by the Centers for Disease Control and Prevention (CDC); grant #5UO1EH000990. We would also like to acknowledge NIEHS grant # P30 ES009089 for the PM2.5 and black carbon data analysis. The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the CDC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abt E, Suh HH, Catalano P, Koutrakis P. Relative contribution of outdoor and indoor particle sources to indoor concentrations. Environmental Science & Technology. 2000;34(17):3579–3587. [Google Scholar]
- Akimenko VV, Andersen I, Lebowitz MD, Lindvall T. The sick building syndrome. Indoor air. 1986;6:87–97. [Google Scholar]
- Breysse PN, Diette GB, Matsui EC, Butz AM, Hansel NN, McCormack MC. Indoor air pollution and asthma in children. Proceedings of the American Thoracic Society. 2010;7(2):102–106. doi: 10.1513/pats.200908-083RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge D, Melly S, Russell M, Perez R, Heeren T, Snell J, Helms D, Hynes HP. A community-based participatory survey of public housing conditions and associations between renovations and possible building related symptoms. Epidemiology. 2004;15(4):S132. [Google Scholar]
- Colton MD, MacNaughton P, Vallarino J, Kane J, Bennett-Fripp M, Spengler JD, Adamkiewicz G. Indoor Air Quality in Green Vs Conventional Multifamily Low-Income Housing. Environmental Science & Technology. 2014;48(14):7833–7841. doi: 10.1021/es501489u. [DOI] [PubMed] [Google Scholar]
- Dietz RN, Goodrich RW, Cote EA, Wieser RF. Detailed description and performance of a passive perfluorocarbon tracer system for building ventilation and air exchange measurements. Measured air leakage of buildings, ASTM STP. 1986;904:203–264. [Google Scholar]
- Emenius G, Svartengren M, Korsgaard J, Nordvall L, Pershagen G, Wickman M. Building characteristics, indoor air quality and recurrent wheezing in very young children (BAMSE) Indoor Air. 2004;14(1):34–42. doi: 10.1046/j.1600-0668.2003.00207.x. [DOI] [PubMed] [Google Scholar]
- EPA (U.S. Environmental Protection Agency) Determination of Metals in Ambient Particulate Matter Using X-ray Fluorescence (XRF) Spectroscopy -Compendium of Methods for the Determination of Inorganic Compounds in Ambient Air Compendium Method IO-3.3.; EPA/625/R-96/010a. U.S. Environmental Protection Agency; Cincinnati, OH: 1999. http://www.epa.gov/ttnamti1/files/Ambient/inorganic/mthd-3-3.pdf. [Google Scholar]
- Frey SE, Destaillats H, Cohn S, Ahrentzen S, Fraser MP. The effects of an energy efficiency retrofit on indoor air quality. Indoor Air. 2015;25(2):210–219. doi: 10.1111/ina.12134. [DOI] [PubMed] [Google Scholar]
- Fromme H, Lahrz T, Hainsch A, Oddoy A, Piloty M, Rüden H. Elemental carbon and respirable particulate matter in the indoor air of apartments and nursery schools and ambient air in Berlin (Germany) Indoor Air. 2005;15(5):335–341. doi: 10.1111/j.1600-0668.2005.00377.x. [DOI] [PubMed] [Google Scholar]
- Grass DS, Ross JM, Family F, Barbour J, Simpson HJ, Coulibaly D, Hernandez J, Chen Y, Slavkovich V, Li Y, Santella RM, Brandt-Rauf P, Chillrud SN. Airborne particulate metals in the New York City subway: a pilot study to assess the potential for health impacts. Environmental Research. 2010;110(1):1–11. doi: 10.1016/j.envres.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götschi T, Oglesby L, Mathys P, Monn C, Manalis N, Koistinen K, Jantunen M, Hänninen O, Polanska L, Künzli N. Comparison of black smoke and PM2. 5 levels in indoor and outdoor environments of four European cities. Environmental Science & Technology. 2002;36(6):1191–1197. doi: 10.1021/es010079n. [DOI] [PubMed] [Google Scholar]
- Hochstetler HA, Yermakov M, Reponen T, Ryan PH, Grinshpun SA. Aerosol particles generated by diesel-powered school buses at urban schools as a source of children’s exposure. Atmospheric Environment. 2011;45(7):1444–1453. doi: 10.1016/j.atmosenv.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IOM (Institute of Medicine) Climate Change, the Indoor Environment, and Health. Washington, DC: The National Academies Press; 2011. [Google Scholar]
- Jacobs DE. Environmental health disparities in housing. American Journal of Public Health. 2011;101(S1):S115–S122. doi: 10.2105/AJPH.2010.300058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs DE, Brown MJ, Baeder A, Sucosky MS, Margolis S, Hershovitz J, Kolb L, Morley RL. A systematic review of housing interventions and health: introduction, methods, and summary findings. Journal of Public Health Management and Practice. 2010;16(5):S5–S10. doi: 10.1097/PHH.0b013e3181e31d09. [DOI] [PubMed] [Google Scholar]
- Krieger JK, Takaro TK, Allen C, Song L, Weaver M, Chai S, Dickey P. The Seattle-King County Healthy Homes Project: implementation of a comprehensive approach to improving indoor environmental quality for low-income children with asthma. Environmental Health Perspectives. 2002;110(Suppl 2):311. doi: 10.1289/ehp.02110s2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue JM, Price PN, Sherman MH, Singer BC. A method to estimate the chronic health impact of air pollutants in US residences. Environmental Health Perspectives. 2012;120(2):216. doi: 10.1289/ehp.1104035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martuzevicius D, Grinshpun SA, Lee T, Hu S, Biswas P, Reponen T, LeMasters G. Traffic-related PM 2.5 aerosol in residential houses located near major highways: indoor versus outdoor concentrations. Atmospheric Environment. 2008;42(27):6575–6585. [Google Scholar]
- Moya J, Phillips L, Schuda L, Wood P, Diaz A, Lee R, Clickner R, Birch R, Adjei N, Blood P. Exposure Factors Handbook: 2011 edition. US Environmental Protection Agency; Washington: 2011. [Google Scholar]
- Mudarri DH. The economics of enhanced environmental services in buildings. In: Clements-Croome D, editor. Creating the Productive Workplace. 2. London and New York: Taylor & Francis; 2006. pp. 99–112. [Google Scholar]
- Na K, Cocker DR. Organic and elemental carbon concentrations in fine particulate matter in residences, schoolrooms, and outdoor air in Mira Loma, California. Atmospheric Environment. 2005;39(18):3325–3333. [Google Scholar]
- Noris F, Adamkiewicz G, Delp WW, Hotchi T, Russell M, Singer BC, Spears M, Vermeer K, Fisk WJ. Indoor environmental quality benefits of apartment energy retrofits. Building and Environment. 2013;68:170–178. [Google Scholar]
- Office of Environmental Health Hazard Assessment (OEEHA) Acute, 8-hour and Chronic Reference Exposure Level (REL)s.OEEHA. California Environmental Protection Agency; Sacramento, CA: [accessed Jan 2015]. Available at http://www.oehha.ca.gov/air/allrels.html. [Google Scholar]
- Peat JK, Dickerson J, Li J. Effects of damp and mould in the home on respiratory health: a review of the literature. Allergy. 1998;53(2):120–128. doi: 10.1111/j.1398-9995.1998.tb03859.x. [DOI] [PubMed] [Google Scholar]
- Perlin SA, Wong D, Sexton K. Residential proximity to industrial sources of air pollution: interrelationships among race, poverty, and age. Journal of the Air & Waste Management Association. 2001;51(3):406–421. doi: 10.1080/10473289.2001.10464271. [DOI] [PubMed] [Google Scholar]
- Pickett AR, Bell ML. Assessment of indoor air pollution in homes with infants. InternationalJjournal of Environmental Research and Public Health. 2011;8(12):4502–4520. doi: 10.3390/ijerph8124502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo ET, Hulse TE, Adamkiewicz G, Levy DE, Bethune L, Kane J, Reid M, Shah SN. Comparison of indoor air quality in smoke-permitted and smoke-free multiunit housing: Findings from the Boston Housing Authority. Nicotine and Tobacco Research. 2015;17(3):316–322. doi: 10.1093/ntr/ntu146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthammer T, Mentese S, Marutzky R. Formaldehyde in the indoor environment. Chemical Reviews. 2010;110(4):2536–2572. doi: 10.1021/cr800399g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JM. Indoor air pollution: a public health perspective. Indoor Air. 1993;3(4):219–226. [Google Scholar]
- Sandel M, Phelan K, Wright R, Hynes HP, Lanphear BP. The effects of housing interventions on child health. Pediatric annals. 2004;33(7):474–481. doi: 10.3928/0090-4481-20040701-14. [DOI] [PubMed] [Google Scholar]
- Wallace L, Williams R. Use of personal-indoor-outdoor sulfur concentrations to estimate the infiltration factor and outdoor exposure factor for individual homes and persons. Environmental Science & Technology. 2005;39(6):1707–1714. doi: 10.1021/es049547u. [DOI] [PubMed] [Google Scholar]
- Weisel CP, Zhang J, Turpin BJ, Morandi MT, Colome S, Stock TH, Spektor DM, Korn L, Winer AM, Meng QY, Zhang L, Harrington R, Liu W, Reff A, Lee JH, Alimokhtari S, Mohan K, Shendell D, Jones J, Farrar L, Fan T. Relationships of Indoor, Outdoor, and Personal Air (RIOPA). Part I. Collection methods and descriptive analyses. Research Report (Health Effects Institute) 2005;(130 Pt 1):1–107. [PubMed] [Google Scholar]
- Wisconsin Occupational Health Laboratory (WOHL) Method WL051.13 Aldehydes collected on DNPH treated tubes, Sep PACs, and badges, analysis by HPLC using UV detection 2010 [Google Scholar]
- Wisconsin Occupational Health Laboratory (WOHL) Method WG030.4 Analytes on 3M Badges in CS2 2011 [Google Scholar]
- World Health Organization (WHO) Selected pollutants. WHO Regional Office for Europe; Copenhagen: 2010. WHO guidelines for indoor air quality. [PubMed] [Google Scholar]
- Xiong Y, Krogmann U, Mainelis G, Rodenburg LA, Andrews CJ. Indoor air quality in green buildings: A case-study in a residential high-rise building in the northeastern United States. Journal of Environmental Science and Health, Part A. 2015;50(3):225–242. doi: 10.1080/10934529.2015.981101. [DOI] [PubMed] [Google Scholar]
- Yan B, Kennedy D, Miller RL, Cowin JP, Jung KH, Perzanowski M, Balletta M, Perera FP, Kinney PL, Chillrud SN. Validating a nondestructive optical method for apportioning colored particulate matter into black carbon and additional components. Atmospheric Environment. 2011;45(39):7478–7486. doi: 10.1016/j.atmosenv.2011.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]