Abstract
There is substantial recent interest in the role of oxytocin in social and affiliative behaviors— animal models of depression have suggested a link between oxytocin and mood. We reviewed literature to date for evidence of a potential relationship between peripheral oxytocin concentration and depressive symptoms in humans. Pubmed® and PsychINFO® were searched for biomedical and social sciences literature from 1960 – May 19, 2015 for empirical articles in English involving human subjects focused on the relationship between peripheral oxytocin concentration and depressive symptoms, excluding articles on the oxytocin receptor gene, or involving exogenous (i.e. intranasal) administration of oxytocin. Eight studies meeting criteria were identified and formally reviewed. Studies of pregnant women suggested an inverse relationship between oxytocin level and depressive symptom severity. Findings in non-pregnant women were broadly consistent with the role of oxytocin release in response to stress supported by animal studies. The relationship between oxytocin and depression in men appeared to be in the opposite direction, possibly reflecting the influence of gonadal hormones on oxytocinergic functioning found in other mammalian species. Overall, small sample sizes, heterogeneity in study designs, and other methodological limitations may account for inconsistent findings. Future research utilizing reliable oxytocin measurement protocols including measurements across time, larger sample sizes, and sample homogeneity with respect to multiple possible confounders (age, gender, race and ethnicity, ovarian status among women, and psychosocial context) are needed to elucidate the role of oxytocin in the pathogenesis of depression, and could guide the design of novel pharmacologic agents.
Keywords: Biological markers, Neuropeptides, Depressive Disorder, Humans, Pregnancy
Introduction
Depression is a common and debilitating illness that adversely affects functioning, quality of life, and the course of other medical illnesses.1 Nearly 8% of persons 12 years of age or older (6% of males and 10% of females) report current depression.2 Despite the increasing number of pharmacologic therapies for depression, only 60 – 70% of patients will respond to first-line monotherapy, and more than one third are considered treatment-resistant.3 One reason for treatment resistance may be the inherent heterogeneity that exists in the clinical presentation of depression.4 For example, different subtypes of depression (melancholic, atypical, hostile, and anxious depression, double depression, bipolar depression, and depression with comorbid personality disorders) may actually involve different biological substrates.5 While the development of most pharmacologic approaches to depression have primarily centered on the monoamine hypothesis of depression,6 hypothalamic-pituitary-adrenocortical (HPA) axis dysregulation represents another important area of inquiry, 7 particularly as a substantial proportion of individuals do not respond to currently available antidepressants.3
The neuropeptide hormone, oxytocin, is produced in the hypothalamus and released from the posterior pituitary gland in response to cervical and uterine distension during childbirth, stimulation of nipples during lactation, and a variety of types of social interactions.8-10 Animal studies first raised the possibility of a relationship between oxytocin and mood when immobility in mice in the forced swim test could be diminished through intracerebrovascular administration of oxytocin, and systemic administration of oxytocin was as effective as the antidepressant, imipramine, in reducing immobility11. Demonstration of the role of oxytocin in social and affiliative behaviors in animals and humans led to further interest in the role of oxytocinergic function in the pathogenesis of depression.12-15
In light of the problem of treatment resistance, and the apparent influence of oxytocin in animal studies, elucidating the role oxytocinergic functioning in the pathogenesis of depression in humans can guide the design of novel pharmacologic and behavioral treatments.15 As such, this review was guided by the question, what is the relationship between oxytocin concentration and depressive symptoms in humans?
Methods
Literature Search and Study Selection
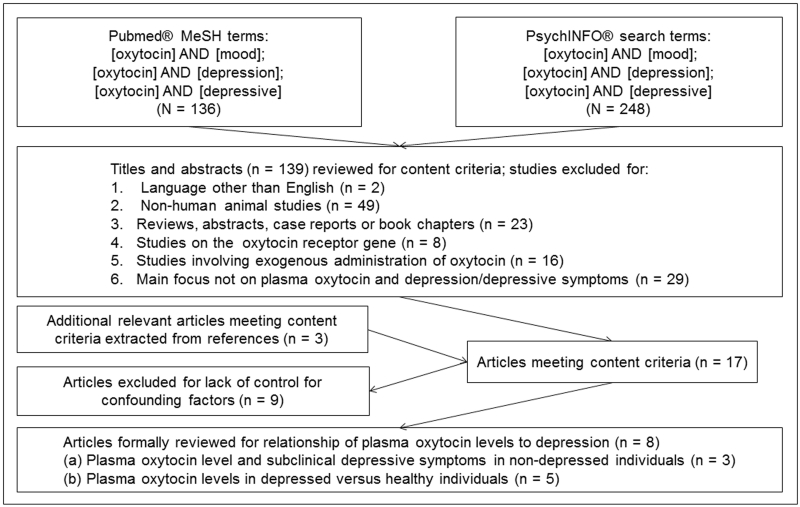
To search the literature, we utilized PubMed® and PsychINFO® using the key word ‘oxytocin,’ combined with each of the following terms: ‘depression,’ ‘mood,’ and ‘depressive.’ We restricted the search to articles written or translated into English from December 1960 to May 19, 2015 with a main focus on the relationship between endogenous oxytocin levels and depressive symptoms. While studies involving the exogenous (i.e. intranasal) administration of oxytocin are of substantial importance, the focus of this review was on endogenous oxytocin release, as reflected by peripheral measurements. We refer readers to the excellent review by McQuaid et al., 2014 for a summary of studies on exogenously administered oxytocin.15 The search was initiated on January 6, 2015, and completed on May 19, 2015. Titles and abstracts from the 139 potentially-relevant citations were examined and limited to original journal articles in English involving human subjects (figure). Studies on the oxytocin receptor (OXTR) gene, intranasal or other exogenous administration of oxytocin, and studies that did not measure depressive symptoms, or were not primarily focused on the relationship between oxytocin and depression or depressive symptoms were excluded. Fourteen remaining articles were ascertained in full and reviewed in detail. References were hand-searched for additional relevant articles (n = 3). The seventeen articles remaining were then examined for the use of multivariable analysis to control for confounding factors. Nine studies were excluded, resulting in a remaining 8 studies constituting the formal review. As shown in the table, we further detail other parameters of study quality that may affect findings including: (a) the method used for diagnosing depression (self-report questionnaires versus interview-based assessments); and (b) the method of assaying oxytocin levels, including whether assays were performed after extraction.
Figure.
Flow chart illustrating review of literature on plasma oxytocin and depression or depressive symptoms (1960 – May 19, 2015).
Table.
Summary of articles reviewed on plasma oxytocin and depression or depressive symptoms
| Plasma oxytocin levels and subclinical depressive symptoms in non-depressed individuals | |||||
|---|---|---|---|---|---|
| Authors | Sample | Analyses of Interest | Measures of depression & oxytocin |
Confounders controlled |
Findings |
| Eapen et al., 2014 | Pregnant women ≥ 18 years old (range 17 – 47) < 38 weeks gestation with singleton pregnancy attending hospital antenatal clinic in Sydney, Australia recruited for larger study on separation anxiety (N = 127) |
Cross-sectional and longitudinal relationship between plasma oxytocin level and depressive symptoms in pregnancy and oxytocin level, separation anxiety, and disturbances in maternal infant attachment at 3 months postpartum |
Self-report - Edinburgh Postpartum Depression Scale (EPDS) |
Regression analysis used, but covariates not reported. |
Lower postpartum oxytocin level associated with greater postpartum depressive symptoms |
| Enzyme immunoassay (EIA) of extracted plasma samples | |||||
| Seay et al., 2014 | Ethnic minority (African-American or Caribbean) HIV-positive women recruited for a larger study on the influence of a cognitive behavioral stress intervention on health among women living with HIV in Miami, FL, USA (N = 70) |
Linear and curvilinear relationships between plasma oxytocin levels and depressive symptoms over a 10-week interval. |
Self-report – Beck Depression Inventory (BDI) |
Age, ethnicity, employment, income, relationship status |
Lower baseline oxytocin levels marginally predicted greater depressive symptoms 10 weeks later (p = .06). |
| EIA of extracted plasma samples | |||||
| Zelkowitz et al., 2014 | Community sample of pregnant women 12-14 weeks gestation with singleton pregnancy recruited from prenatal clinics in Montreal, Quebec, Canada (N = 287) |
Relationship between plasma oxytocin in pregnancy and 8 weeks postpartum to stress, depressive symptoms, maternal interactive behavior with infant. |
Self-report – EPDS | Age, education, marital status |
Among women reporting high hpsychosocial stress, higher oxytocin levels were associated with fewer depressive symptoms and more sensitive maternal behavior. |
| EIA of unextracted plasma samples | |||||
| Comparison of plasma oxytocin levels in depressed versus non-depressed individuals | |||||
|---|---|---|---|---|---|
| Authors | Sample | Analyses of Interest | Measures of depression and oxytocin |
Control for confounders |
Findings |
| Cyranowski et al., 2008 | Currently depressed (n = 17) and never depressed (n = 17) women aged 20 – 40 years free of antidepressant medication for 2 or more weeks recruited from a mood disorders clinic in Pittsburgh, PA, United States (N = 34) |
Compared repeated plasma oxytocin levels and patterns between currently depressed and never depressed women before, during and after completion of an affiliation- focused imagery task and a stress-focused task |
Clinician rated Hamilton Depression Rating Scale and self- reported BDI |
Age | Depressed women had higher oxytocin levels during affiliation task compared to controls. Depressed women displayed more variability in pulsatile oxytocin release in general. |
| Radioimmunoassay of extracted plasma samples | |||||
| Ozsoy et al., 2009 | Psychiatric inpatients (30 women, 10 men) with major depressive disorder (n = 29) or bipolar depression (n = 11) hospitalized at an academic medical hospital in Kayseri, Turkey and 32 (20 women, 12 men) healthy controls recruited from hospital staff (N = 72) |
Comparison of plasma oxytocin levels in (a) depressed versus controls; unipolar vs. bipolar depression; (b) male versus female; (c) pharmacotherapy versus ECT treatment |
Clinical interview by two independent psychiatrists based on DSM-IV criteria |
Age, body mass index (BMI), number of children |
|
| Radioimmunoassay of unextracted serum samples | |||||
| Skrundz et al., 2011 | Community sample of German-speaking healthy pregnant women 21 to 32 weeks gestation in Basel, Switzerland with singleton pregnancy, pre-pregnancy BMI < 32, without major depressive episode in past 2 years (N = 73) |
Relationship between pregnancy plasma oxytocin concentration and risk-for postpartum depression (PPD) group versus no-risk for PPD using EPDS cutoff of 10 |
Self-report EPDS at 2 weeks postpartum |
Prenatal EPDS score, length of gestation |
Lower oxytocin level in pregnancy was associated with greater risk of postpartum depression at 2 weeks postpartum |
| EIA of unextracted plasma samples | |||||
| Turan et al., 2013 | Sixty-seven (39 male, 28 female) psychiatric outpatients with bipolar disorder (22 with acute mania, 21 with acute depression, 24 in remission) aged 18 – 65 years seen at an academic medical center clinic and 24 healthy controls recruited from hospital staff in Kayseri, Turkey (N = 91) |
Compared plasma oxytocin levels among bipolar mania, bipolar depression, and bipolar remission and control groups |
Self-report – Hamilton Depression Rating Scale |
Age, gender, BMI, cigarettes/day |
Oxytocin levels were higher in bipolar patients in any phase compared to controls. No statistically significant difference in oxytocin levels among bipolar groups. |
| EIA of unextracted serum samples | |||||
| Yuen et al., 2014 | Adults with major depressive disorder with psychosis (10 female, 4 male) and without psychosis (12 female, 5 male), and 19 healthy controls recruited from a large academic medical center in California, USA participating in a larger study on HPA axis physiology and depressive disorders (N = 50) |
Comparison of plasma oxytocin levels among psychotic depressed, non-psychotic depressed, and healthy individuals; gender differences in oxytocin levels in depressed versus non-depressed individuals |
Self-report – Hamilton Depression Rating Scale |
Gender, cortisol concentration |
Oxytocin concentration differed between female and male depressed individuals, and were decreased in depressed females versus controls, while marginally increased in depressed males versus controls. |
| EIA of extracted plasma samples | |||||
Results
The 8 studies formally reviewed were conducted in 5 countries worldwide: the United States (n = 3), Canada (n = 1), Switzerland (n = 1), Turkey (n = 2), and Australia (n = 1), and varied substantially with regard to sample size (N = 34 to 287 subjects), racial and ethnic composition of participants, and measures used to diagnose depression or measure depressive symptoms (table). Five studies included exclusively women,16-20 three of which involved only pregnant women,16,17,19 and three studies involved mixed-gendered samples.21-23 The table summarizes study characteristics and findings from these reviewed studies.
Plasma oxytocin level and depressive symptoms in pregnancy and the postpartum period
An inverse relationship between plasma oxytocin concentration and depressive symptoms was found in all 3 studies of perinatal women. Skrundz and colleagues (2011) were the first to explore the link between oxytocin and risk for postpartum depression in a sample of 73 middle-class German-speaking Swiss, non-smoking, non-depressed, non-obese, healthy women with singleton pregnancies.19 Lower plasma oxytocin concentration measured in the 30th to 34th weeks of pregnancy predicted a positive screen for depression (score of 10 or higher) at two weeks postpartum using the German version of the Edinburgh Postpartum Depression Scale (EPDS).24 The other two studies of perinatal women found similar inverse relationships between plasma oxytocin concentration and depressive symptoms. While Eapen and colleagues (2014) did not show a longitudinal relationship between pregnancy oxytocin and postpartum depressive symptoms in a fairly educated sample of 127 racially and ethnically diverse pregnant women (52% Caucasian, 23% Asian, 10% Indian, 8% Arabic) in Australia, lower oxytocin concentration at 3 months postpartum was associated with greater depressive symptoms at that time, measured by the EPDS.25 Zelkowitz et al (2014) found that under conditions of high psychosocial stress as measured by the Antenatal Risk Questionnaire,26 higher oxytocin levels in pregnancy were correlated with lower depressive symptoms in pregnancy, and at 8 weeks postpartum. In summary, the 3 reviewed studies of pregnant and postpartum women suggested inverse relationships between plasma oxytocin concentration and depressive symptoms.
Oxytocin and depressive symptoms in non-pregnant women
Studies of non-pregnant women suggested non-linear relationships. In a study of plasma oxytocin release following an affiliation-focused task and a stress-focused task in 17 depressed non-medicated women and 17 non-depressed women, Cyranowski and colleagues (2008) found that, compared to controls, depressed women displayed greater variability in pulsatile oxytocin release during both tasks, and higher oxytocin levels during the affiliation task. Moreover, oxytocin levels in depressed women were directly associated with severity of depressive symptoms measured by the Beck Depression Inventory.27 The other study of oxytocin and depression in non-pregnant women examined the relationship between oxytocin levels and depressive symptoms over a 10-week period in an ethnic minority (African-American or Caribbean) sample of Human Immunodeficiency Virus (HIV) -positive women participating in a larger study on the influence of stress on health outcomes in women living with HIV.20 Interestingly, a curvilinear relationship was found; very high and very low plasma oxytocin levels were predictive of an increase in depressive symptoms measured by the BDI over the 10 week period. Taken together, in non-pregnant women, both extremes of oxytocin levels (high and low), in addition to greater variability in oxytocin levels, were associated with greater depressive symptoms.
Oxytocin and depressive symptoms in mixed gender samples
Ozsoy and colleagues (2009) found lower oxytocin levels in 40 depressed patients (30 women, 10 men) relative to controls, though the difference appeared to be driven by lower oxytocin in females only; levels among depressed and non-depressed males did not differ. The two remaining mixed-gendered studies examined bipolar depression and psychotic depression, respectively. Turan and colleagues (2013) compared plasma oxytocin levels among 21 patients with bipolar depression, 22 patients with acute mania, 24 bipolar patients in remission, and 24 healthy controls. Bipolar patients in any phase of illness exhibited higher oxytocin levels than controls, and there was no difference in oxytocin levels among any of the bipolar groups, leading authors to postulate that oxytocin may be a marker of bipolar illness. Yuen and colleagues (2014) compared plasma oxytocin levels among adults with psychotic depression (10 women, 4 men), non-psychotic depression (12 women, 4 men), and 19 healthy controls healthy controls (11 women, 8 men). Oxytocin levels did not differ between psychotic and non-psychotic depression groups when both genders were compared together. However, depressed females had lower oxytocin levels than healthy females, while depressed men showed a trend toward higher oxytocin levels compared to healthy men (difference not statistically significant).
Discussion
While animal models have suggested that oxytocin attenuates depression-like behavior 10, 28-31 raising the possibility of an oxytocinergic pharmacologic intervention in humans, the relationship between plasma oxytocin concentration and depressive symptoms in humans appears more complicated and more difficult to study directly, as suggested by the studies reviewed. We discuss findings in the context of hypothesized actions of oxytocin based on literature to date, and suggest directions for future work.
Oxytocin and stress
Elevations in peripheral oxytocin have been previously proposed to represent a biomarker of stress and social separation in females.29 Grippo et al., (2007) showed that female prairie voles exposed to extended periods of social isolation displayed an increased number or oxytocin-immuno-reactive cells in the paraventircular nucleus of the hypothalamus. 30 Moreover, in several mammalian species, oxytocin appears to exert a tonic inhibitory influence over the HPA axis, thereby attenuating rises in cortisol in response to stress.30-32 Interpreted within the context of these animal studies, the inverse relationships observed between oxytocin levels and depressive symptoms among pregnant16,17,19 and non-pregnant women20,21 could reflect a failure of the oxytocin system to effectively adjust to stress. However, it is also possible that both high and low extremes of oxytocin levels could be observed in women experiencing depressive symptoms, such as documented in the Cyranowski (2008) study. Thus, it is unclear whether depressive symptoms precedes a change in oxytocin levels, or whether oxytocin levels reflect adaptations made in response to depression.
What could account for this inter-individual variability in oxytocinergic function? There is evidence to suggest that common variations in the OXTR gene are associated with different degrees of stress reactivity,33 or interact with social support to reduce stress in humans.34 Future research that combines the study of oxytocin responses longitudinally with genotyping at OXTR could elucidate how variations in OXTR may mediate differences in oxytocinergic functioning in women.
Gender differences in oxytocinergic functioning
Yuen and colleagues (2014) showed that depressed females had lower oxytocin levels than healthy females, but depressed males showed a trend in the opposite direction. This could reflect the complex interplay between oxytocin and gonadal hormones documented in other mammalian species—oxytocin synthesis is partially estrogen dependent in mice 35 and oxytocin release and the oxytocin receptor respond to changes in ovarian status across the female menstrual cycle. 36 Future studies that examine men and women separately and take ovarian status into account is advisable.
Oxytocin in unipolar versus bipolar depression
Turan and colleagues (2013) found that plasma oxytocin concentration was higher among bipolar disorder patients in any phase (depressed, euthymic, manic) relative to healthy controls, while most studies of unipolar depressed patients suggested an inverse relationship between oxytocin levels and depressive symptoms. In individuals with bipolar disorder, abnormal sensitivity in reward-related neural circuitry involving dopamine may account for observed excesses in motivation during life events involving rewards and goal attainment. On the other hand, individuals with unipolar depression show decreased reward responsivity and reward-related neural activation.37 Strong reciprocal interactions likely exist between oxytocin and dopamine systems as oxytocin receptor density has been found to be high in the mesocorticolimbic dopamine pathway including the prefrontal cortex and the nucleus accumbens among prairie voles.38 Thus, mood dysregulation marked by either excessive or diminished social-affiliative behaviors may be future targets of pharmacologic agents designed to alter oxytocin levels.
Methodological challenges in the study of oxytocin
Results need to be considered in the context of several methodological issues. First, the relationship between peripherally measured oxytocin (plasma, salivary) and central levels of oxytocin (cerebrospinal fluid) is unclear.39 Next, diurnal variations in plasma oxytocin concentrations40 and the possibility of variation in oxytocin function by ethnicity41 complicates comparisons across studies. Finally, measurements of oxytocin made on unextracted samples could result in the tagging and detection of other molecules in addition to oxytocin42 leading to an overestimation of oxytocin levels. As there is increased attention paid to oxytocin in the lay press,43 clinicians and healthcare providers might be prepared to address questions from patients and inform them about the preliminary nature of findings to date, based on information from this review.
Conclusion
While studies employing animal models of depression have supported a clear role of oxytocin in relation to depressive symptoms, studies in humans are inadequate to support translation to the clinical setting at this time. Future investigations should utilize samples large enough to test for gender differences (or separate the study of men and women), employ serial measurements of oxytocin, consider ovarian status among women (premenopausal versus menopausal and phase of menstrual cycle), use reliable and reproducible techniques for assaying oxytocin, utilize consistent socio-environmental contexts and racially and ethnically homogeneous samples, and examine homogenous subtypes of depression (chronic versus acute, unipolar versus bipolar) to most accurately clarify the role of oxytocin in the pathogenesis of depression in humans.
Acknowledgments
This work was supported by a grant from the Evergreen Invitational Women’s Health Grants Initiative of the Northwestern Memorial Foundation (PI: Massey, 9/12/13 Agreement Date) and grant K23 DA037913 from the National Institute on Drug Abuse (NIDA) of the National Institutes of Health (PI: Massey). The Evergreen Invitational, Northwestern Memorial Foundation, and NIDA had no role in the study design, collection, analysis or interpretation of data, writing the manuscript, or the decision to submit the paper for publication.
Footnotes
Disclosures: Authors Massey, Backes, and Schuette have no conflicts of interest or financial disclosures to report.
References
- 1.Fawcett J. The morbidity and mortality of clinical depression. Int Clin Psychopharmacol. 1993 doi: 10.1097/00004850-199300840-00002. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention Prevalence of current depression* among persons aged ≥12 years, by age group and sex — united states, national health and nutrition examination survey, 2007–2010. 2012:1747. [Google Scholar]
- 3.Souery D, Papakostas GI, Trivedi MH. Treatment-resistant depression. Journal of Clinical Psychiatry. 2006;67:16. [PubMed] [Google Scholar]
- 4.Fava M, Uebelacker LA, Alpert JE, Nierenberg AA, Pava JA, Rosenbaum JF. Major depressive subtypes and treatment response. Biological psychiatry. 1997;42(7):568–576. doi: 10.1016/S0006-3223(96)00440-4. [DOI] [PubMed] [Google Scholar]
- 5.Schneider B, Prvulovic D. Biomarkers for Depression. General Methods in Biomarker Research and their Applications. 2015:957–978. [Google Scholar]
- 6.Hirschfeld R. History and evolution of the monoamine hypothesis of depression. Journal of Clinical Psychiatry. 2000 [PubMed] [Google Scholar]
- 7.Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends in neurosciences. 2008;31(9):464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiological reviews. 2001;81(2):629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 9.Winslow JT, Shapiro L, Carter CSInsel TR. Oxytocin and complex social behavior: Species comparisons. Psychopharmacol Bull. 1993 [PubMed] [Google Scholar]
- 10.Carter CS. Developmental consequences of oxytocin. Physiol Behav. 2003;79:383–397. doi: 10.1016/s0031-9384(03)00151-3. [DOI] [PubMed] [Google Scholar]
- 11.Arletti RBertolini A. Oxytocin acts as an antidepressant in two animal models of depression. Life Sci. 1987;41:1725–1730. doi: 10.1016/0024-3205(87)90600-x. [DOI] [PubMed] [Google Scholar]
- 12.Anacker AM, Beery AK. Life in groups: the roles of oxytocin in mammalian sociality. Frontiers in behavioral neuroscience. 2013;7 doi: 10.3389/fnbeh.2013.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Insel TR, Young LJ. The neurobiology of attachment. Nature Reviews Neuroscience. 2001;2:129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- 14.Neumann ID. Brain oxytocin: A key regulator of emotional and social behaviours in both females and males. J Neuroendocrinol. 2008;20:858–865. doi: 10.1111/j.1365-2826.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- 15.McQuaid RJ, McInnis OA, Abizaid A, Anisman H. Making room for oxytocin in understanding depression. Neuroscience & Biobehavioral Reviews. 2014;45:305–322. doi: 10.1016/j.neubiorev.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Eapen V, Dadds M, Barnett B, Kohlhoff J, et al. Separation anxiety, attachment and inter-personal representations: Disentangling the role of oxytocin in the perinatal period. PLoS One. 2014;9:e107745. doi: 10.1371/journal.pone.0107745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zelkowitz P, Gold I, Feeley N, Hayton B, et al. Psychosocial stress moderates the relationships between oxytocin, perinatal depression, and maternal behavior. Horm Behav. 2014;66:351–360. doi: 10.1016/j.yhbeh.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 18.Cyranowski JM, Hofkens TL, Frank E, Seltman H, et al. Evidence of dysregulated peripheral oxytocin release among depressed women. Psychosom Med. 2008;70:967–975. doi: 10.1097/PSY.0b013e318188ade4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skrundz M, Bolten M, Nast I, Hellhammer DH, et al. Plasma oxytocin concentration during pregnancy is associated with development of postpartum depression. Neuropsychopharmacology. 2011;36:1886–1893. doi: 10.1038/npp.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seay JS, Lattie E, Schneiderman N, Antoni MH, et al. Linear and quadratic associations of plasma oxytocin with depressive symptoms in ethnic minority women living with hiv. Journal of Applied Biobehavioral Research. 2014;19:70–78. [Google Scholar]
- 21.Ozsoy S, Esel EKula M. Serum oxytocin levels in patients with depression and the effects of gender and antidepressant treatment. Psychiatry Res. 2009;169:249–252. doi: 10.1016/j.psychres.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 22.Yuen KW, Garner JP, Carson DS, Keller J, et al. Plasma oxytocin concentrations are lower in depressed vs. Healthy control women and are independent of cortisol. J Psychiatr Res. 2014;51:30–36. doi: 10.1016/j.jpsychires.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turan T, Uysal C, Asdemir AKılıç E. May oxytocin be a trait marker for bipolar disorder? Psychoneuroendocrinology. 2013;38:2890–2896. doi: 10.1016/j.psyneuen.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 24.Bergant A, Nguyen T, Heim K, Ulmer H, et al. german language version and validation of the edinburgh postnatal depression scale. Deutsche medizinische Wochenschrift (1946) 1998;123:35–40. doi: 10.1055/s-2007-1023895. [DOI] [PubMed] [Google Scholar]
- 25.Cox JL, Holden JMSagovsky R. Detection of postnatal depression. Development of the 10-item edinburgh postnatal depression scale. The British journal of psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 26.Austin MP, Hadzi-Pavlovic D, Saint KParker G. Antenatal screening for the prediction of postnatal depression: Validation of a psychosocial pregnancy risk questionnaire. Acta Psychiatr Scand. 2005;112:310–317. doi: 10.1111/j.1600-0447.2005.00594.x. [DOI] [PubMed] [Google Scholar]
- 27.Beck AT, Steer RACarbin MG. Psychometric properties of the beck depression inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 28.Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats 1. Endocrinology. 1997;138(7):2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- 29.Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RA, Updegraff JA. Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychological review. 2000;107(3):411. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- 30.Grippo AJ, Cushing BS, Carter CS. Depression-like behavior and stressor-induced neuroendocrine activation in female prairie voles exposed to chronic social isolation. Psychosomatic medicine. 2007;69(2):149. doi: 10.1097/PSY.0b013e31802f054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neumann ID, Krömer SA, Toschi N, Ebner K. Brain oxytocin inhibits the (re) activity of the hypothalamo–pituitary–adrenal axis in male rats: involvement of hypothalamic and limbic brain regions. Regulatory peptides. 2000;96(1):31–38. doi: 10.1016/s0167-0115(00)00197-x. [DOI] [PubMed] [Google Scholar]
- 32.Rotzinger S, Lovejoy DA, Tan LA. Behavioral effects of neuropeptides in rodent models of depression and anxiety. Peptides. 2010;31(4):736–756. doi: 10.1016/j.peptides.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 33.Rodrigues SM, Saslow LR, Garcia N, John OP, Keltner D. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proceedings of the National Academy of Sciences. 2009;106(50):21437–21441. doi: 10.1073/pnas.0909579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen FS, Kumsta R, von Dawans B, Monakhov M, Ebstein RP, Heinrichs M. Common oxytocin receptor gene (OXTR) polymorphism and social support interact to reduce stress in humans. Proceedings of the National Academy of Sciences. 2011;108(50):19937–19942. doi: 10.1073/pnas.1113079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nomura M, McKenna E, Korach KS, Pfaff DW, Ogawa S. Estrogen receptor-beta regulates transcript levels for oxytocin and arginine vasopressin in the hypothalamic paraventricular nucleus of male mice. Brain Res Mol Brain Res. 2002;109:84–94. doi: 10.1016/s0169-328x(02)00525-9. [DOI] [PubMed] [Google Scholar]
- 36.Fuchs AR. Oxytocin and ovarian function. Journal of reproduction and fertility. 1987;36(Supplement):39–47. [PubMed] [Google Scholar]
- 37.Nusslock R, Young CBDamme KS. Elevated reward-related neural activation as a unique biological marker of bipolar disorder: Assessment and treatment implications. Behav Res Ther. 2014;62:74–87. doi: 10.1016/j.brat.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proceedings of the National Academy of Sciences. 1992;89:5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCullough ME, Churchland PSMendez AJ. Problems with measuring peripheral oxytocin: Can the data on oxytocin and human behavior be trusted? Neurosci Biobehav Rev. 2013;37:1485–1492. doi: 10.1016/j.neubiorev.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 40.Forsling ML. Diurnal rhythms in neurohypophysial function. Exp Physiol. 2000;85:179S–186S. doi: 10.1111/j.1469-445x.2000.tb00022.x. [DOI] [PubMed] [Google Scholar]
- 41.Grewen KM, Light KC, Mechlin B, Girdler SS. Ethnicity is associated with alterations in oxytocin relationships to pain sensitivity in women. Ethnicity and Health. 2008;13(3):219–241. doi: 10.1080/13557850701837310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szeto A, McCabe PM, Nation DA, Tabak BA, Rossetti MA, McCullough ME, Schneiderman N, Mendez AJ. Evaluation of enzyme immunoassay and radioimmunoassay methods for the measurement of plasma oxytocin. Psychosom. Med. 2011;73(5):393. doi: 10.1097/PSY.0b013e31821df0c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stix G. [July 18, 2015];Fact of Fiction?: Oxytocin is the Love Hormone. Scientific American. 2014 Septmebr; http://www.scientificamerican.com/article/fact-or-fiction-oxytocin-is-the-love-hormone/