Abstract
In addition to the neuromodulatory role of cholinergic systems, brief, temporally discrete cholinergic release events, or “transients”, have been associated with the detection of cues in attention tasks. Here we review four main findings about cholinergic transients during cognitive processing. Cholinergic transients are: 1) associated with the detection of a cue and influenced by cognitive state; 2) not dependent on reward outcome, although the timing of the transient peak co-varies with the temporal relationship between detection and reward delivery; 3) correlated with the mobilization of the cue-evoked response; 4) causal mediators of shifts from monitoring to cue detection. We next discuss some of the key questions concerning the timing and occurrence of transients within the framework of available evidence including: 1) Why does the shift from monitoring to cue detection require a transient? 2) What determines whether a cholinergic transient will be generated? 3) How can cognitive state influence transient occurrence? 4) Why do cholinergic transients peak at around the time of reward delivery? 5) Is there evidence of cholinergic transients in humans? We conclude by outlining future research studies necessary to more fully understand the role of cholinergic transients in mediating cue detection.
Keywords: Acetylcholine, Cortex, Attention, Cognition
1. Introduction
Cholinergic neurons originating in the nucleus basalis of Meynert, substantia innominata and the diagonal band (henceforth termed basal forebrain; BF) project to virtually all cortical areas and layers. In the last decade, anatomical research has greatly revised traditional views about the organization of this projection system. Long-held notions of a “diffuse” or “reticular” projection system have been replaced by descriptions of BF cholinergic cell clusters, cluster-specific dendritic organization, and a highly topographic organization of BF cholinergic projections (Zaborszky, van den Pol, & Gyengesi, 2012; Zaborszky, Csordas, et al., 2015; Zaborszky, Duque, et al., 2015). Important anatomical aspects of this projection system remain undetermined and even disputed, such as the organization of inputs to individual BF cell clusters, the synaptic space of individual BF neurons, and the ultrastructural characteristics of cholinergic synapses and the identity of their neuronal targets. However, future research is expected to reveal the circuit-specificity of the organization of individual BF cells, which would reject notions of redundancy, overlap and diffuseness in the organization of the BF cholinergic projection system. The anatomical descriptions of other putatively “diffusely” organized ascending projection systems originating in brain stem have the potential to follow suit and undergo a similar revision (e.g., Helboe, Egebjerg, & de Jong, 2015; Schwarz & Luo, 2015).
A similar evolution is taking place in the description and conceptualization of presynaptic cholinergic signaling. The traditional focus on slow and regionally non-specific changes in extracellular and extrasynaptic (or “volume-transmitted”) “ambient” basal acetylcholine (ACh) levels (for review see Sarter, Parikh, & Howe, 2009) has been challenged by our more recent demonstration of regionally-specific phasic cholinergic signaling in cortex (“cholinergic transients”; below). The present review focuses on those transients, though it should be noted that the larger body of evidence supports a multi-modal, multi-timescale view of cholinergic function. That is, in addition to the transients, cholinergic terminals also support a more canonical neuromodulatory component of cholinergic neurotransmission, varying perhaps at the scale of tens of seconds to minutes and being particularly active in association with demands on attentional control (e.g., St Peters, Demeter, Lustig, Bruno, & Sarter, 2011). Interactions between cholinergic neuromodulation and transients are discussed in Sarter, Lustig, Howe, Gritton, & Berry (2014) and Sarter (2015). Importantly, the neuromodulatory and transient components of cholinergic neurotransmission are dissociable. The modulatory component, measured by microdialysis, can be relatively high while cholinergic transient frequencies are relatively low, rejecting the possibility that methodological (i.e., analytical) limitations have confounded the conclusion that a cholinergic neuromodulatory component is present. In other words, ACh levels in minute-based dialysate collections are unlikely to represent integrated transients (for more discussion of measurement issues see Sarter & Kim, 2015). Below we will focus on the functions of cholinergic transients.
2. Cholinergic transients: technical and conceptual origins
The measurement scheme underlying choline-sensitive amperometric biosensors and their potential usefulness for the neurosciences has long been proposed (e.g., Kawagoe, Niehaus, & Wightman, 1991; Garguilo & Michael, 1994, 1996). However, not until the work of Gerhardt and colleagues were sensors available with adequate sensitivity and responsivity, as well as ceramic bases equipped with multiple recording sites that afford important analytical control measurements (Burmeister, Moxon, & Gerhardt, 2000; Parikh et al., 2004). Our original interest in searching for phasic cholinergic responses was largely based on the observation that acetylcholinesterase (AChE) has one of the highest catalytic powers ever reported for an enzyme (Quinn, 1987). Thus, contrary to the traditional slow neuromodulatory conceptualization of cholinergic function, cholinergic synapses appear to be specifically suitable for rapid, highly phasic and spatially selective synaptic signaling. Although the regulation of AChE remains poorly understood (e.g., Dobbertin et al., 2009), results from our experiments using sensors with choline oxidase and AChE co-immobilized onto recording sites suggest that even after large and likely non-physiological ACh release events in vivo, endogenous AChE hydrolyzes all detectable ACh so rapidly that the process cannot be detected (Giuliano, Parikh, Ward, Chiamulera, & Sarter, 2008). For this reason, choline currents, measured with amperometry and biosensors, have been interpreted as indicating newly released ACh, although it is important to remain mindful that new insights into the regulation of AChE may complicate the interpretation of brain choline currents.
We originally hypothesized that phasic ACh release events (henceforth termed “transients”) are associated with the detection of cues. “Detection” here concerns a cognitive process as defined by Posner and colleagues. It is worth quoting their full definition because of the important distinction made between detection and orienting: “By detection, we will mean the entry of information concerning the presence of a signal into a system that allows the subject to report the existence of the signal by an arbitrary response indicated by the experimenter. We mean to distinguish detection in this sense from more limited automatic responses that may occur to the event. Orienting, as we will use the term, involves the more limited process of aligning sensory (e.g., eyes) or central systems with the input channel over which the signal is to occur. Thus it is possible to entertain the hypothesis that subjects may orient toward a signal without having first detected it. This would mean simply that the signal was capable of eliciting certain kinds of responses (e.g., eye movements or shifts of attention) but has not yet reached systems capable of generating responses not habitual for that type of signal” (Posner, Snyder, & Davidson, 1980, p. 162). Thus, detection involves execution of a previously acquired response to a cue (or signal). For example, monitoring traffic lights and orienting towards the switch to green per se does not constitute detection. However, using this signal (the switch to green) to activate the signal-associated response rule (“go”) and executing it fulfills this definition.
This definition appears almost hopelessly complex as it encompasses steps ranging from perception to working memory operations, response preparation and response execution. However, signal - response relationships need to be established, and outcomes need to be integrated into this associative framework in order to increase the efficacy of subsequent detection operations and facilitate the revision of response selection based on the results of previous choices. To extend Posner’s definition to encompass the entirety of processes described above: By detection, we mean the entirety of information concerning the presence of a signal into a system that allows the subject to report the existence of the signal (or cue) by an arbitrary response specified by the experimenter, and that provides feedback about the adequacy/accuracy of the response based on response outcome.
Our original hypothesis that cholinergic transients mediate signal detection was derived from the effects of selective lesions of the cortical cholinergic input system on detection performance. In this research in rodents (McGaughy & Sarter, 1995; St Peters, Cherian, Bradshaw, & Sarter, 2011), and later also in humans (Demeter, Sarter, & Lustig, 2008), we have used a task, originally designed as a sustained attention task (SAT), that consists of a random sequence of signal (with variable salience) and nonsignal trials, each of which requires the reporting of the presence or absence of the signal via separate response keys. In signal trials, reporting the signal is a “hit” and leads to reward while reporting that there was no signal (“miss”) leads to no reward and triggers the intertrial interval (ITI). In nonsignal trials, operating the no-signal response key is counted as a “correct rejection” and rewarded, while claiming that a signal was present (“false alarm”) is not. Importantly, the SAT rewards both signal- and nonsignal-linked responses. As we discuss below, this eliminates the possibility that cholinergic transients encode reward per se. The cognitive and perceptual demands of SAT are optimized by successive (as opposed to simultaneous) discrimination, event asynchrony, and variable event rate and signal saliency (Davies & Parasuraman, 1982).
Following immunotoxin-induced selective lesions of BF cholinergic cell groups projecting to cortex, rats permanently missed the majority of signals, with only ~30% residual hits regardless of signal duration. In contrast, their correct rejection rate remained high (~80%) and unaffected (McGaughy, Kaiser, & Sarter, 1996). This finding indicates the necessity of cholinergic activity for signal detection but it does not identify the essential component of cholinergic neurotransmission (neuromodulatory or transient). Halorhodopsin photoactivationinduced silencing of cholinergic activity specifically during signal presentation reproduced the effects of cholinergic lesions (Gritton et al., 2016). This suggests that the primary cause of signal detection impairments in lesioned animals was the absence of cholinergic transients.
3. Cholinergic transients during signal detection performance
Because amperometric recordings of choline currents are in the low pA- range, our initial experiments designed to record currents during signal detection necessitated the use of a simplified cued appetitive response task that could be performed in an environment devoid of devices that generate electrostatic energy (Parikh, Kozak, Martinez, & Sarter, 2007). Rats were trained to respond to a signal by approaching two response ports for retrieval of the reward. Detection was defined as orienting towards the signal and approaching the ports. If signals failed to elicit food port approach these trials were counted as a miss. Trials were separated by 90±30 s. During misses, brief orienting responses, triggered by signal onset, were frequently observed but they did not disrupt the animal’s ongoing behavior, typically grooming. Separate groups of rats were trained in versions of the task in which reward was delivered 2±1 s or 6±2 s following the signal, to test the hypothesis that the shorter signal-reward interval required engagement of the signal detection process closer in time relative to signal presentation. Recording in the medial prefrontal cortex (middle layers of the prelimbic region; mPFC), choline currents were recorded exclusively in trials in which the signal was detected, not those in which the signal was missed. Choline currents increased with signal-onset and peaked early into the reward delivery period, at ~1.8 and 5.0 s post-signal, respectively (Fig. 1A). We also found that the time at which choline currents exhibited a significant increase from baseline (by 25%) correlated highly with the onset of animals’ response. Furthermore, choline current amplitudes correlated with the animals’ speed of retrieving reward, with a 1-µM increase in choline concentration predicting a 1.75 s shorter time from signal to reward retrieval. Additional experiments indicated that catch-trials without reward delivery did not affect cholinergic transients, transients were not found in motor cortex and, during the acquisition of this task, transients emerged as signal-evoked behavioral responses began to form (see also Supplemental Materials in Parikh et al., 2007).
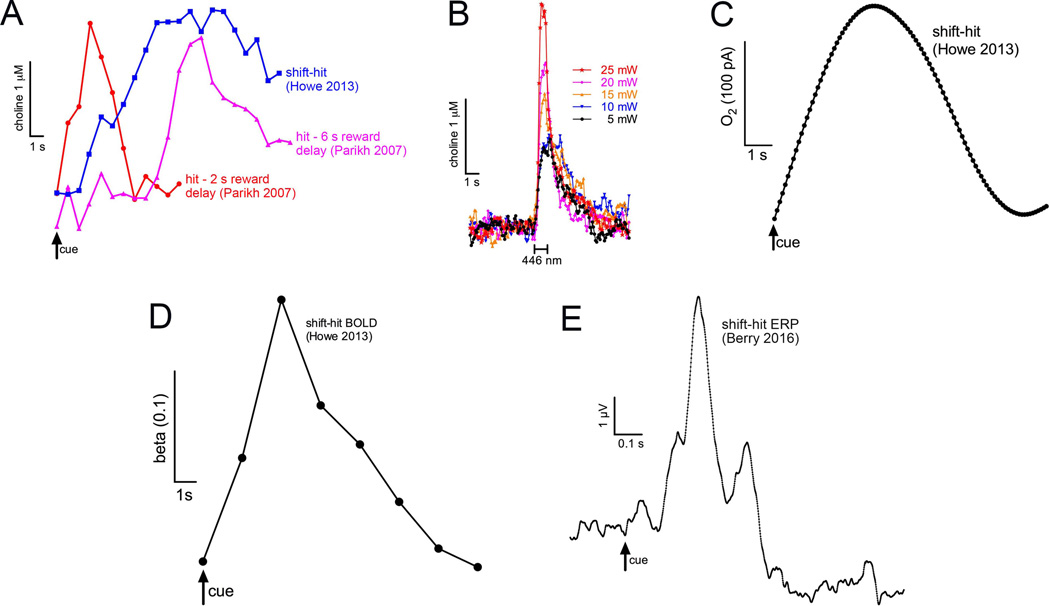
Figure 1.
Shift-hit associated neurochemical (A,C), imaging- (D), and EEG-based (E) signals in rodents (A–C) and humans (D,E). A: Cholinergic transients recorded in rats performing a cued appetitive response task (Parikh et al., 2007) or a Sustained Attention Task (SAT; Howe et al., 2013). For the former, separate groups of rats were trained to retrieve reward 2 or 6 s (means) following the detection of the signal (red and pink trace, respectively). In the SAT reward was delivered as soon as the levers were extended (2 s after the signal) and the animals scored a hit (+640 ms) or latest at ~ 6.5 s (as levers remained available for 4 s). In all three cases, choline signal amplitudes peaked at around the learned reward delivery periods. As discussed in the main text, currents recorded during shift-hits in SAT performing rats were multiplied by 10 to scale with currents recorded in rats scoring hits in the cued appetitive response task. B: Cholinergic transients evoked by ChR2 stimulation using stimulation parameters that increased hits if stimulation occurred during cued trials, and false alarms if stimulation occurred during nonsignal trials (Gritton et al., 2016). Photoactivation stimulates ACh release in isolation which contrasts with endogenous release events that reflect the product of heterogeneous mechanisms and the interactions across multiple neuronal networks. This could explain why stimulation in isolation results in transients with faster rise and decay times and lower variability then endogenous transients (see main text). C: Oxygen levels in the right mPFC of rats performing shift-hits paralleled choline currents (Howe et al., 2013). As tissue oxygen levels serve as a proxy for fmri BOLD measures in rats, shift-hit associated BOLD signals in humans (D) are hypothesized to reflect in part cholinergic activity. D: Contrast between the right frontal (Brodmann area 10) fmri BOLD response during a shift-hit and a consecutive hit (Howe et al., 2013) E: Evoked response potential (ERP) for shift-hits. The trace depicts the group grand average waveform for frontal electrodes (Fz, AFz, FPz). The P300a peak approximately 290 ms following signal onset (arrow) was significantly larger for shift-hits relative to consecutive hits (Berry et al., 2016).
In later experiments, we were able to modify operant equipment to minimize electrostatic interference, allowing us to record choline currents in SAT performing rats. However, recording choline currents in SAT required normalizing individual trials to pre-trial currents to control for remaining electrostatic noise and residual shifts in current from the previous trial due to the relatively fast-paced SAT (ITI: 9±3 s) (Howe et al., 2013). As a result, SAT trial-based currents were smaller that in our initial study (above; in Fig. 1A these currents were multiplied by ×10 to scale these currents with those measured by Parikh et al. 2007). Given our prior findings, we expected to record choline currents in signal trials that resulted in a hit, but not during misses and not in non-signal trials, regardless of outcome. Results were consistent with this prediction except that we did not observe cholinergic transients in about 40% of hits. Hits not associated with cholinergic transients turned out to be preceded by hits, whereas hits associated with cholinergic transients were preceded by non-signal trials (correct rejections) or perceived nonsignal trials (misses; henceforth hits following either actual or perceived non-signal trials are termed “shift-hits”, as they involve a shift from monitoring for cues to signal detection, as opposed to consecutive hits). False alarms were rare and were inconsistently associated with cholinergic transients (see below for more discussion in the context of optogenetically-evoked false alarms). We also observed that, relative to baseline, choline currents increased significantly by the time the levers were extended (2 s following an event) and they peaked at 6.5 s after the signal (Fig. 1A), with the peak approximately coinciding with the maximum time period during which reward was delivered. Furthermore, transient amplitudes did not differ by signal saliency and, during correct rejections, there was a non-significant trend for a decrease in choline currents.
Because of the relatively long ITI (90±30 s) in our initial experiments that used the simpler cued appetitive-response task (Parikh et al., 2007), rats returned to task-unrelated behavior between signals, such as grooming. Thus, in this initial experiment, all signal-hit trials may be considered shift-hits, explaining why they were associated with cholinergic transients. This assumption implies that longer ITIs in the SAT likewise would reduce or even abolish hits not associated with transients.
The collective findings from choline current recordings in performing rats can be summarized as follows: 1. Cholinergic transients are associated exclusively with the detection of a signal (except for consecutive detections that occur close in time). 2. Transients do not depend on reward outcome but the timing of the transient peak coincides with the (learned) timing of reward delivery. 3. Transient onset and peak amplitudes correlate with the onset and speed, respectively, of the signal-evoked response. Before turning to the main question about the functions of these transients we will establish, beyond the lesion data discussed above, that they are causal mediators for signal detection.
4. Cholinergic transients cause signal detection
The hypothesis that cholinergic transients are sufficient for signal detection predicts that the reliable presence of such transients will benefit signal detection rates. As an extension of this hypothesis, cholinergic transients may have the capacity to cause signal detection behavior even in the absence of signals. To test this possibility, we expressed channelrhodopsin (ChR2) in cholinergic neurons of (ChAT-Cre) mice and photostimulated these neurons at the level of the soma in the BF as well as the cholinergic terminals in the mPFC in SAT performing mice. Specifically, photostimulation coincided with signal or non-signal onset (for details see Gritton et al., 2016).
The interpretation of results from these experiments necessitates an important technical comment. To select photostimulation parameters we measured optogenetically-evoked transients in mice that were – necessarily – anesthetized because the weight of the headstage did not allow conducting these experiments in freely moving mice. These transients (Fig. 1B) approach the characteristics, in terms of amplitude and peak amplitude timing, of the transients recorded in trials with the shortest signal-reward interval among our recording experiments and thus with the earliest transient peak time (see “hit-3 s reward” trace in Fig. 1A). However, optogenetically-evoked transients differ from endogenous transients by rising and decaying faster, primarily reflecting that optogenetically-evoked transients essentially are artifacts of stimulating one particular population of neurons in isolation, whereas endogenous transients reflect the cholinergic component of the activation of distributed and heterogeneous neuronal networks (see also Melchior, Ferris, Stuber, Riddle, & Jones, 2015; Millard, Whitmire, Gollnick, Rozell, & Stanley, 2015). With the rising popularity of optogenetic methods, it is important to note this limitation which optogenetic methods share with many, if not all, more traditional methods used to evoke brain function, such as electrical stimulation or pharmacological receptor stimulation. In our context, the test of defined hypotheses, predicting specific behavioral effects, and testing the behavioral effects of a wide range of laser stimulation power are important in assuring the validity of results of stimulating just one particular neuronal system.
When coinciding with signals, photostimulation of cholinergic neurons increased hit rates, specifically to less salient signals. Moreover, when generating cholinergic transients that coincided with non-signal events, we found a significant and indeed rather dramatic increase in false alarms, (e.g., false claims for signals, or false hits). All effects scaled with stimulation power. Importantly, the results of several analyses assured that effects were specific for the trials stimulated and did not reflect stimulation-induced, trial-independent response biases. As already mentioned, we also demonstrated that inhibiting cholinergic neurons, by photoactivation of halorhodopsin expressed by cholinergic neurons, decreased hit rates, specifically in trials with more salient signals while, similar to cholinergic lesions, not affecting correct rejection rates. Thus, we add to our summary of findings (above) an essential fourth point: Cholinergic transients are causal mediators of shifts from monitoring to signal detection.
As already mentioned, endogenous transients were inconsistently recorded during false alarms. In rats and mice, less than 20% of non-cued trials typically yield false alarms. A proportion of these responses may reflect random lever (or nose poker) selection, as may be the case for all four response types. False alarms not associated with endogenous cholinergic transients may have been due to random responses and thus did not constitute true false alarms. Clearly, a separate behavioral index that discriminates false alarms from random responses would be needed to test this possibility and avoid using circular logic (“all false alarms associated with transients are truly false alarms”). Next we will address key questions about the functions of cholinergic transients.
5. Main questions about the timing and occurrence of cholinergic transients
Question #1: The “brain already knows” that a signal is there – so why does the signal need to coincide with a transient to be detected?
As described above, if a signal does not evoke a transient, or if the transient is experimentally suppressed, the signal is likely to be missed. However it is likely that the signal may have been already inserted into the cortical circuit mediating signal detection, primarily via signal-evoked thalamic glutamatergic activity (Hasselmo & Bower, 1992; Parikh, Ji, Decker, & Sarter, 2010). Indeed, signal-evoked glutamatergic transients in mPFC were found to precede signal-evoked cholinergic transients, and the glutamatergic transients are present in all signal trials, irrespective of whether the outcome is a hit or a miss. Moreover, and in contrast to cholinergic transients, longer cues were associated with greater glutamatergic amplitudes (Howe, Gritton, Lusk, Berke, & Sarter, 2012), further supporting the hypothesis that thalamic or other glutamate “imports” cue information into cortical circuitry.
Moreover, in the analyses of local field potentials, we found that shift-hits were associated with greater gamma power than consecutive hits, and that blockade of M1 mAChRs attenuated shift-hit-associated high frequency oscillations (Howe, Gritton, Berke, & Sarter, 2011; Howe et al., 2012). Signal-evoked gamma oscillations have been proposed to facilitate the processing of signal-related information across larger networks and cortical regions (Gregoriou, Gotts, Zhou, & Desimone, 2009). Thus, cholinergic transients may be essential for synchronizing cortical neuronal output driven by salient signals (Rodriguez, Kallenbach, Singer, & Munk, 2004; Bauer et al., 2012), thereby supporting the mediation of the complex steps involved in signal detection (as defined above). Thus, the signal “is already there” but cholinergic transient-evoked high frequency oscillations are required to synchronize signal-bound actions, so that the detection process can be followed through. If a transient is not generated (below) so that gamma power will be relatively low, orientation responses toward the signal may still be observed (see above) but the detection process will not succeed.
Question #2: What determines whether a cholinergic transient will be generated?
Most obviously, more salient signals are more likely to generate cholinergic transients as they are more likely to be detected. The observation that the amplitudes of signal-evoked glutamatergic transients in the mPFC, likely reflecting thalamic inputs, vary by signal duration (above), establishes this glutamate signal as a primary determinant of cholinergic transient generation. It should be noted that glutamatergic signaling from other cortical regions extends to the mPFC and may convey information about the stimulus in conjunction with thalamic input. The finding that cholinergic photostimulation during cued trials predominantly enhanced hits to shorter signals is consistent with this view. Furthermore, higher levels of cholinergic neuromodulation, such as the levels seen in the presence of distractors (St Peters, Demeter, Lustig, Bruno, & Sarter, 2011) or in rats with relatively high levels of attentional control as a trait (Paolone, Angelakos, Meyer, Robinson, & Sarter, 2013), recover or maintain, respectively, high detection rates by upregulating glutamatergic transients (Sarter, Lustig, Howe, Gritton, & Berry, 2014). Thus, higher levels of cholinergic neuromodulation or, cognitively, attentional control, are a secondary determinant of cholinergic transient generation.
Question # 3: Why are there no transients during consecutive hits?
Consecutive hits in the SAT combine two characteristics that have yet to be disambiguated. First, they constitute trial repeats. Second, they occur with a relatively short time period (thus far observed at ~12 s as opposed to 90 s in the cued-appetitive response task) from each other. As already discussed, increasing the ITI would be expected to restore transients for consecutive hits, converting all hits into shift-hits (ignoring other impacts of such a manipulation on SAT performance). Thus, the absence of transients is speculated to reflect that cortical detection circuitry remains in an active mode and thus no additional transient is needed to allow the next signal to be detected. In this context, it is worth noting that the proportion of hits that were not associated with cholinergic transients in our recording experiments (Howe et al., 2013) was roughly similar to the residual hit rates in rats with large cholinergic lesions (McGaughy et al., 1996). This suggests that, in rats with cholinergic lesions, most residual hits were from a series of signal trials that followed the occasional hit (likely to longest signals).
Thus far, a candidate neuronal mechanism that could sustain such a “signal detection up-state” across associational and sensory cortex has not been identified in vivo. However, clues can be gleaned from in vitro studies, where it has been shown that consecutive stimuli could induce a persistent firing pattern that required stimulation of mAChRs and lasted for minutes (Egorov, Hamam, Fransen, Hasselmo, & Alonso, 2002; see also Schon et al., 2005). Such persistent, cholinergically-dependent firing may contribute to the relatively enduring, signal-evoked cholinergic transients (Fig. 1A) and also to the associated generation of gamma oscillations. Both of these phenomena last for several seconds and are thus capable of potentially bridging the temporal gap between trials (see above for a discussion of the implications of enduring transients; see also Rodriguez et al., 2004). Indeed, we speculate that as a result of a (perceived or actual) non-signal trial, an active mechanism yields a “signal-detection down-state” shift, as indicated by the strong trend towards decreased choline currents in trials where animals correctly indicate the absence of a signal in a non-signal trial that was preceded by a hit (Howe et al., 2013).
Suppressing cholinergic transients during consecutive hits may support the subject’s ability to respond flexibly to changing and – in the SAT – unpredictable signal/non-signal sequences. If consecutive hits were also associated with cholinergic transients, enhanced high-frequency oscillations, and outcome feedback (below), this could result in an overwhelming bias toward detection responses. As a result, shifting from signal-oriented to non-signal-oriented performance would be impaired. In tasks involving relatively high event rates, allowing transients to exclusively shift to the “signal detection up-state”, but not to further enhance that up-state, therefore may be crucial for maintaining flexible performance. If so, transients that are ill-timed may be as maladaptive as the loss of transients.
Question # 4: Why do cholinergic transients peak at around the time of reward delivery?
Above we discussed mechanisms that could allow transients to last for several seconds and to bridge trials, and the finding that transients peak approximately at the time of reward delivery. Our evidence indicates that the onset of the cholinergic transient correlates with the onset of behavior (indicating detection). Therefore, improved recording and stimulation techniques may demonstrate that the cholinergic mediation of detection is completed within a small fraction of a second, perhaps on the scale of electroencephalgraphy signals associated with shift hits (Fig. 1E; below). However, the observation that the peak (rather than the onset) of cholinergic transients roughly coincides with reward delivery (Fig. 1A) suggests additional functions of cholinergic activity. Critically, reward per se is not associated with cholinergic activity, as transients are absent during rewarded correct rejections.
However, other studies have demonstrated that BF cholinergic neurons respond, with high precision, to reward and punishment, and they are also compute reinforcement errors (Danielmeier et al., 2015; Hangya, Ranade, Lorenc, & Kepecs, 2015). In our studies, where highly trained rodents received, invariantly, reward for hits and correct rejections over tens of thousands of trials by the time of our recordings or of photostimulation, cholinergic neurons may no longer be involved in coding reinforcement signals that are entirely predictable. However, prediction errors may still be coded by these neurons. Following a shift to the “signal detection up-state” (above), feedback concerning the accuracy of the up-shift may be instrumental for generating and shaping transients in future trials. Although not delivering reward following hits did not affect cholinergic transients (Parikh et al., 2007), there are no systematic data concerning the effects of such non-reward trials on choline currents acutely, or on subsequent performance and transient generation. Thus, it is not clear how reward omission during shift -hit trials might alter subsequent choline currents. One possibility is that such omissions could return cholinergic neurons to coding reward in a more discrete manner, as may be the case during the learning of the task, i.e., before the animal has fully acquired the response-reward association. More insights into the exact peak timing relative to the (learned) timing of reward delivery, and in the effects of non-reward on performance and cholinergic transient generation, may also enhance our understanding of the mesolimbic influences on cholinergic function (Botvinick, Huffstetler, & McGuire, 2009; St Peters, Demeter, et al., 2011).
Question #5: Is there evidence of cholinergic transients in humans?
We trained humans in the SAT and examined the fMRI BOLD signal contrasts between shift-hits and consecutive hits in a manner consistent with observations in rodents (Howe et al., 2013). Relative to consecutive hits, shift-hits increased activation in right rostrolateral/orbitofrontal cortex, approximately in Brodmann Area 10 (Fig. 1D), as well as a smaller activation in right basal forebrain. In addition, greater activation in the rostrolateral/orbitofrontal region during shift-hits correlated with faster response times, suggesting functional significance. This latter finding parallels the result that larger choline current amplitudes were correlated with faster response times for hits in the cued appetitive response task (above; Parikh et al., 2007).
Of course, the neuronal mechanisms underlying a particular BOLD signal cannot be known and may be rather heterogeneous and potentially even unrelated to cholinergic activity. We began bridging this translational gap by measuring prefrontal oxygen levels in performing rats, as a proxy for BOLD in animals. Oxygen levels closely paralleled choline currents, with shift-hit trials being associated with an increase in oxygen levels (Fig. 1C), while oxygen levels decreased significantly during correct rejections. Furthermore, direct infusion of cholinergic receptor agonists increased oxygen levels, indicating that cholinergic receptor stimulation reproduces the metabolic correlate of shift-hits. By extension, these findings suggest that a component of the human BOLD response reflected cholinergic activity (Howe et al., 2013).
Finally, we employed electroencephalography and contrasted event related potentials associated with signal-evoked responses for shift-hits versus consecutive hits (Berry, Sarter, Gehring, & Lustig, 2016). We found enhanced signal-evoked frontal P300a amplitude for shift-hits relative to consecutive hits (Fig. 1E), with the strongest effects over right orbitofrontal regions, comparable to the fMRI results. Furthermore, functional connectivity analysis demonstrated increased gamma phase locking for shift-hits between right frontal cortex and parietal cortex, followed by parietal P300b modulation. Cholinergic involvement in the P300a wave is not well understood, owing in part to the complex effects of current drugs available for probing the cholinergic system in humans (Hasselmo & Sarter, 2011). However, the psychopharmacological evidence that does exist points to a significant cholinergic contribution to the generation of the P300 (e.g., Curran, Pooviboonsuk, Dalton, & Lader, 1998; Kenemans & Kahkonen, 2011; Brown, van der Wee, van Noorden, Giltay, & Nieuwenhuis, 2015). This suggests that the right-lateralized P300a seen in our study (Fig. 1E) may be a good index of the cholinergic mediation of shift-hits in humans. If we were able to record cholinergic transients at the temporal resolution afforded by EEG, we would expect to see an early cholinergic peak at 300 ms post-signal. The potential EEG correlate(s) of the lasting increases in cholinergic activity, for several seconds post-signal (above), remain unclear but may be found in lower-frequency oscillation bands (e.g., Sacchet et al., 2015). Taken together, although the evidence remains largely based on parallels between human brain responses and neurochemical responses in animals, cholinergic transients are hypothesized to significantly mediate signal detection operations in humans as in rodents.
Conclusions
The detection of signals (as defined above) is a fundamental cognitive and behavioral process. Degeneration of the cholinergic system therefore is expected to disrupt both the learning of associations between signals and the response rules needed to interact with the outside world and the use of (learned) signals to guide the retrieval of associative frameworks guiding action selection (e.g., Mesulam, 2004; Sarter, Albin, Kucinski, & Lustig, 2014). Furthermore, ill-timed cholinergic transients, due to abnormalities in the organization of corticosubcortical circuitry, may cause false detections or inappropriate attention and response to behaviorally-insignificant signals and therefore support psychopathological states (Lustig & Sarter, 2016).
Important questions remain about the role of cholinergic transients. Because of limitations in the temporal resolution of electrochemical recording techniques, the precise temporal dynamics of these transients, specifically the timing of onset with relation to the signal cue and the arrival of glutamatergic input are still unsettled. Our combined findings from electrophysiology data in humans and rodents suggests that cholinergic release events promote changes in gamma oscillations that occur as early as 100–300 ms post-signal. Although the importance of the timing of cholinergic input relative to glutamate is unknown in cortex, it has been reported that altering cholinergic input timing in the hippocampus can promote both LTP and LTD depending on when the input arrives and the receptor pathway activated (Gu & Yakel, 2011). Whether similar mechanisms are in play in the cortex, or if the timing of this input is adjusted with learning and expectation, are currently unknown. Furthermore, better temporal measures will allow us to dissociate if data from previously recorded traces, using relatively low sampling rates, may represent the sum of multiple cholinergic spikes that mediate independent steps of the (complex) signal detection process. It also not clear whether our current understanding of the cholinergic control of the signal detection process in right-lateralized mPFC generalizes to the functions of cholinergic inputs to other cortical, sensory and motor regions (Goard & Dan, 2009; Pinto et al., 2013), or how cholinergic transients contribute to performance in other tasks. It is likely that some or all of these findings will apply to other association areas involved in the cue-detection process. This is supported in part by our findings that suppression of cholinergic transients in mPFC alone using halorhodopsin was insufficient to reduce cuedetection to the same level as cortex-wide inactivation of ACh release. This is consistent with the premise that cholinergic activity in fronto-parietal networks also contribute to the cue processing (Bucci, Holland, & Gallagher, 1998; Broussard, Karelina, Sarter, & Givens, 2009), while also accounting for our own human EEG and BOLD signal results revealing orbitofrontal and parietal network contributions. Finally, the nature of longer-timescale cholinergic neuromodulation and the interactions between this component and the generation of transients are only prematurely understood. Studies combining electrochemical recordings with manipulations of transient generation in performing animals, which currently remains extremely technically challenging, are needed to begin answering these questions.
Highlights.
Phasic or “transient” acetylcholine release events mediate cue detection.
Transients act to synchronize cue-bound actions.
Variables that determine the presence and absence of transients are discussed.
Important gaps in our knowledge about cholinergic transients are pointed out.
Acknowledgments
The authors’ research was supported by PHS grants MH086530, DA031656, and NS091856
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bauer M, Kluge C, Bach D, Bradbury D, Heinze HJ, Dolan RJ, Driver J. Cholinergic enhancement of visual attention and neural oscillations in the human brain. Curr Biol. 2012;22(5):397–402. doi: 10.1016/j.cub.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AS, Sarter M, Gehring WJ, Lustig C. What happens after perception: a right frontal signature of shifts from perceptual to reflective attention. 2016 In preparation. [Google Scholar]
- Botvinick MM, Huffstetler S, McGuire JT. Effort discounting in human nucleus accumbens. Cogn Affect Behav Neurosci. 2009;9(1):16–27. doi: 10.3758/CABN.9.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard JI, Karelina K, Sarter M, Givens B. Cholinergic optimization of cue-evoked parietal activity during challenged attentional performance. Eur J Neurosci. 2009;29(8):1711–1722. doi: 10.1111/j.1460-9568.2009.06713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SB, van der Wee NJ, van Noorden MS, Giltay EJ, Nieuwenhuis S. Noradrenergic and cholinergic modulation of late ERP responses to deviant stimuli. Psychophysiology. 2015;52(12):1620–1631. doi: 10.1111/psyp.12544. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Holland PC, Gallagher M. Removal of cholinergic input to rat posterior parietal cortex disrupts incremental processing of conditioned stimuli. J Neurosci. 1998;18(19):8038–8046. doi: 10.1523/JNEUROSCI.18-19-08038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister JJ, Moxon K, Gerhardt GA. Ceramic-based multisite microelectrodes for electrochemical recordings. Anal Chem. 2000;72(1):187–192. doi: 10.1021/ac9907991. [DOI] [PubMed] [Google Scholar]
- Curran HV, Pooviboonsuk P, Dalton JA, Lader MH. Differentiating the effects of centrally acting drugs on arousal and memory: an event-related potential study of scopolamine, lorazepam and diphenhydramine. Psychopharmacology (Berl) 1998;135(1):27–36. doi: 10.1007/s002130050482. [DOI] [PubMed] [Google Scholar]
- Danielmeier C, Allen EA, Jocham G, Onur OA, Eichele T, Ullsperger M. Acetylcholine Mediates Behavioral and Neural Post-Error Control. Curr Biol. 2015 doi: 10.1016/j.cub.2015.04.022. [DOI] [PubMed] [Google Scholar]
- Davies DR, Parasuraman R. The psychology of vigilance. London; New York: Academic Press; 1982. [Google Scholar]
- Demeter E, Sarter M, Lustig C. Rats and humans paying attention: cross-species task development for translational research. Neuropsychology. 2008;22(6):787–799. doi: 10.1037/a0013712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbertin A, Hrabovska A, Dembele K, Camp S, Taylor P, Krejci E, Bernard V. Targeting of acetylcholinesterase in neurons in vivo: a dual processing function for the proline-rich membrane anchor subunit and the attachment domain on the catalytic subunit. J Neurosci. 2009;29(14):4519–4530. doi: 10.1523/JNEUROSCI.3863-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorov AV, Hamam BN, Fransen E, Hasselmo ME, Alonso AA. Graded persistent activity in entorhinal cortex neurons. Nature. 2002;420(6912):173–178. doi: 10.1038/nature01171. [DOI] [PubMed] [Google Scholar]
- Garguilo MG, Michael AC. Quantitation of choline in the extracellular fluid of brain tissue with amperometric microsensors. Anal Chem. 1994;66(17):2621–2629. doi: 10.1021/ac00089a006. [DOI] [PubMed] [Google Scholar]
- Garguilo MG, Michael AC. Amperometric microsensors for monitoring choline in the extracellular fluid of brain. J Neurosci Methods. 1996;70(1):73–82. doi: 10.1016/S0165-0270(96)00105-7. [DOI] [PubMed] [Google Scholar]
- Giuliano C, Parikh V, Ward JR, Chiamulera C, Sarter M. Increases in cholinergic neurotransmission measured by using choline-sensitive microelectrodes: enhanced detection by hydrolysis of acetylcholine on recording sites? Neurochem Int. 2008;52(7):1343–1350. doi: 10.1016/j.neuint.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goard M, Dan Y. Basal forebrain activation enhances cortical coding of natural scenes. Nat Neurosci. 2009;12(11):1444–1449. doi: 10.1038/nn.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriou GG, Gotts SJ, Zhou H, Desimone R. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science. 2009;324(5931):1207–1210. doi: 10.1126/science.1171402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritton HJ, Howe WM, Mallory CS, Hetrick VL, Berke JD, Sarter M. Cortical cholinergic signaling controls the detection of cues. Proc Natl Acad Sci USA. 2016 doi: 10.1073/pnas.1516134113. Epub published on 1/19/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Yakel JL. Timing-dependent septal cholinergic induction of dynamic hippocampal synaptic plasticity. Neuron. 2011;71(1):155–165. doi: 10.1016/j.neuron.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangya B, Ranade SP, Lorenc M, Kepecs A. Central cholinergic neurons are rapidly recruited by reinforcement feedback. Cell. 2015;162(5):1155–1168. doi: 10.1016/j.cell.2015.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Bower JM. Cholinergic suppression specific to intrinsic not afferent fiber synapses in rat piriform (olfactory) cortex. J Neurophysiol. 1992;67(5):1222–1229. doi: 10.1152/jn.1992.67.5.1222. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology. 2011;36(1):52–73. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helboe L, Egebjerg J, de Jong IE. Distribution of serotonin receptor 5-HT6 mRNA in rat neuronal subpopulations: A double in situ hybridization study. Neuroscience. 2015;310:442–454. doi: 10.1016/j.neuroscience.2015.09.064. [DOI] [PubMed] [Google Scholar]
- Howe WM, Berry AS, Francois J, Gilmour G, Carp JM, Tricklebank M, Sarter M. Prefrontal cholinergic mechanisms instigating shifts from monitoring for cues to cue-guided performance: converging electrochemical and fMRI evidence from rats and humans. J Neurosci. 2013;33(20):8742–8752. doi: 10.1523/JNEUROSCI.5809-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe WM, Gritton H, Berke J, Sarter M. Attention-demanding cues evoke prefrontal gamma oscillations and are differentially modulated by prefrontal muscarinic and nicotinic receptors. Society for Neuroscience Abstracts. 2011 abstract # 197.109. [Google Scholar]
- Howe WM, Gritton H, Lusk N, Berke JD, Sarter M. Distinct behavioral and neurophysiological correlates of prefrontal acetylcholine and glutamate transients during attentional task performance. Society for Neuroscience Abstracts. 2012 abstract # 913.906. [Google Scholar]
- Kawagoe JL, Niehaus DE, Wightman RM. Enzyme-modified organic conducting salt microelectrode. Anal Chem. 1991;63(24):2961–2965. doi: 10.1021/ac00024a029. [DOI] [PubMed] [Google Scholar]
- Kenemans JL, Kahkonen S. How human electrophysiology informs psychopharmacology: from bottom-up driven processing to top-down control. Neuropsychopharmacology. 2011;36(1):26–51. doi: 10.1038/npp.2010.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig C, Sarter M. Attention and the cholinergic system: relevance to schizophrenia. Current Topics in Behavioral Neuroscience. 2016 doi: 10.1007/7854_2015_5009. in press. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Kaiser T, Sarter M. Behavioral vigilance following infusions of 192 IgG-saporin into the basal forebrain: selectivity of the behavioral impairment and relation to cortical AChE-positive fiber density. Behav Neurosci. 1996;110(2):247–265. doi: 10.1037//0735-7044.110.2.247. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Sarter M. Behavioral vigilance in rats: task validation and effects of age, amphetamine, and benzodiazepine receptor ligands. Psychopharmacology (Berl) 1995;117(3):340–357. doi: 10.1007/BF02246109. [DOI] [PubMed] [Google Scholar]
- Melchior JR, Ferris MJ, Stuber GD, Riddle DR, Jones SR. Optogenetic versus electrical stimulation of dopamine terminals in the nucleus accumbens reveals local modulation of presynaptic release. J Neurochem. 2015;134(5):833–844. doi: 10.1111/jnc.13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M. The cholinergic lesion of Alzheimer's disease: pivotal factor or side show? Learn Mem. 2004;11(1):43–49. doi: 10.1101/lm.69204. [DOI] [PubMed] [Google Scholar]
- Millard DC, Whitmire CJ, Gollnick CA, Rozell CJ, Stanley GB. Electrical and optical activation of mesoscale neural circuits with implications for coding. J Neurosci. 2015;35(47):15702–15715. doi: 10.1523/JNEUROSCI.5045-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolone G, Angelakos CC, Meyer PJ, Robinson TE, Sarter M. Cholinergic control over attention in rats prone to attribute incentive salience to reward cues. J Neurosci. 2013;33(19):8321–8335. doi: 10.1523/JNEUROSCI.0709-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Ji J, Decker MW, Sarter M. Prefrontal beta2 subunit-containing and alpha7 nicotinic acetylcholine receptors differentially control glutamatergic and cholinergic signaling. J Neurosci. 2010;30(9):3518–3530. doi: 10.1523/JNEUROSCI.5712-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron. 2007;56(1):141–154. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Pomerleau F, Huettl P, Gerhardt GA, Sarter M, Bruno JP. Rapid assessment of in vivo cholinergic transmission by amperometric detection of changes in extracellular choline levels. Eur J Neurosci. 2004;20(6):1545–1554. doi: 10.1111/j.1460-9568.2004.03614.x. [DOI] [PubMed] [Google Scholar]
- Pinto L, Goard MJ, Estandian D, Xu M, Kwan AC, Lee S-H, Dan Y. Fast modulation of visual perception by basal forebrain cholinergic neurons. Nature Neurosci. 2013;16:1857–1863. doi: 10.1038/nn.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Snyder CR, Davidson BJ. Attention and the detection of signals. J Exp Psychol. 1980;109(2):160–174. [PubMed] [Google Scholar]
- Quinn DM. Acetylcholinesterase: enzyme structure, reaction dynamics, and virtual transition states. Chemical Reviews. 1987;87(5):955–979. [Google Scholar]
- Rodriguez R, Kallenbach U, Singer W, Munk MHJ. Short- and long-term effects of cholinergic modulation on gamma oscillations and response synchronization in the visual cortex. J Neurosci. 2004;24(46):10369–10378. doi: 10.1523/JNEUROSCI.1839-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchet MD, LaPlante RA, Wan Q, Pritchett DL, Lee AK, Hamalainen M, Jones SR. Attention drives synchronization of alpha and beta rhythms between right inferior frontal and primary sensory neocortex. J Neurosci. 2015;35(5):2074–2082. doi: 10.1523/JNEUROSCI.1292-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Albin RL, Kucinski A, Lustig C. Where attention falls: Increased risk of falls from the converging impact of cortical cholinergic and midbrain dopamine loss on striatal function. Exp Neurol. 2014;257:120–129. doi: 10.1016/j.expneurol.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Kim Y. Interpreting chemical neurotransmission in vivo: techniques, time scales, and theories. ACS Chem Neurosci. 2015;6(1):8–10. doi: 10.1021/cn500319m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Lustig C, Howe WM, Gritton H, Berry AS. Deterministic functions of cortical acetylcholine. Eur J Neurosci. 2014;39(11):1912–1920. doi: 10.1111/ejn.12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Parikh V, Howe WM. Phasic acetylcholine release and the volume transmission hypothesis: time to move on. Nat Rev Neurosci. 2009;10(5):383–390. doi: 10.1038/nm2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon K, Atri A, Hasselmo ME, Tricarico MD, LoPresti ML, Stern CE. Scopolamine reduces persistent activity related to long-term encoding in the parahippocampal gyrus during delayed matching in humans. J Neurosci. 2005;25(40):9112–9123. doi: 10.1523/JNEUROSCI.1982-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz LA, Luo L. Organization of the locus coeruleus-norepinephrine system. Curr Biol. 2015;25(21):R1051–R1056. doi: 10.1016/j.cub.2015.09.039. [DOI] [PubMed] [Google Scholar]
- St Peters M, Cherian AK, Bradshaw M, Sarter M. Sustained attention in mice: Expanding the translational utility of the SAT by incorporating the Michigan Controlled Access Response Port (MICARP) Behav Brain Res. 2011;225(2):574–583. doi: 10.1016/j.bbr.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Peters M, Demeter E, Lustig C, Bruno JP, Sarter M. Enhanced control of attention by stimulating mesolimbic-corticopetal cholinergic circuitry. J Neurosci. 2011;31(26):9760–9771. doi: 10.1523/JNEUROSCI.1902-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, Csordas A, Mosca K, Kim J, Gielow MR, Vadasz C, Nadasdy Z. Neurons in the basal forebrain project to the cortex in a complex topographic organization that reflects corticocortical connectivity patterns: an experimental study based on retrograde tracing and 3D reconstruction. Cereb Cortex. 2015;25(1):118–137. doi: 10.1093/cercor/bht210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, Duque A, Gielow M, Gombkoto P, Nadasdy Z, Somogyi J. Organization of the basal forebrain cholinergic projection system: Specific or diffuse? In: Paxinos G, editor. The rat nervous system. San Diego: Academic Press; 2015. pp. 491–507. [Google Scholar]
- Zaborszky L, van den Pol A, Gyengesi E. The basal forebrain cholinergic projection system in mice. In: Watson C, Paxinos G, Puelles L, editors. The Mouse Nervous System. Elsevier; 2012. pp. 684–718. [Google Scholar]