Abstract
Animal studies provide strong evidence that general anesthetics (GAs), administered during the early postnatal period, induce long-term cognitive and neurological abnormalities. Because the brain growth spurt in rodents is delayed compared to that in humans, a fundamental question is whether the postnatal human brain is similarly vulnerable. Sevoflurane and propofol, GAs that share positive modulation of the gamma-aminobutyric acid type A receptor (GABAAR) function cause marked increase in corticosterone levels and induce long-term developmental alterations in synaptic activity in rodents. If synaptogenesis is affected, investigation of mechanisms of the synaptic effects of GAs is of high interest because synaptogenesis in humans continues for several years after birth. Here, we compared long-term synaptic effects of etomidate with those of propofol. Etomidate and propofol both positively modulate GABAAR activity, but in contrast to propofol, etomidate inhibits the adrenal synthesis of corticosterone.
Postnatal day (P) 4, 5, or 6 rats received five injections of etomidate, propofol, or vehicle control during 5 h of maternal separation. Endocrine effects of the anesthetics were evaluated by measuring serum levels of corticosterone immediately after anesthesia or maternal separation. The frequency and amplitude of miniature inhibitory postsynaptic currents (mIPSCs) in hippocampal CA1 pyramidal neurons were measured at P24-40 and P≥80.
Only propofol caused a significant increase in serum corticosterone levels (F(4,26) = 17.739, P < 0.001). In contrast to increased frequency of mIPSCs in the propofol group (F(4,23) = 8.731, p < 0.001), mIPSC activity in the etomidate group was not different from that in the vehicle groups.
The results of this study together with previously published data suggest that anesthetic-caused increase in corticosterone levels is required for GABAergic GAs to induce synaptic effects in the form of a long-term increase in the frequency of hippocampal mIPSCs.
Keywords: GABA, synapse, corticosterone, anesthetic, neonatal, brain
Introduction
Numerous animal studies, especially in rodents, provide strong evidence that general anesthesia administered during the early postnatal period induces long-term neurocognitive abnormalities [1]. Rodents become largely resistant to this effect of general anesthetics by the end of the second postnatal week [2]. Given the difference between rodent and human ontogeny, a central question in the field is whether humans are vulnerable to the developmental effects of general anesthetics in a way similar to that seen in rodents. Historically, a 1-week-old rat brain is considered equivalent in development to the human brain at birth based on the measurements of post-mortem brain tissue weights in Dobbing and Sands’ studies during the 1970s, which compared among species the timing of the brain growth spurt, defined as the total brain weight gain as a percentage of its adult weight [3,4]. Therefore, considering comparable stages of the brain growth spurt in rodents and humans, human susceptibility to the developmental effects of general anesthetics may wane by the time of birth. However, subsequent studies, especially those of neurogenesis and synaptogenesis, revealed more complex chronological relationships between comparable stages of brain maturation in rodents and humans. Based on rates of neurogenesis, current thinking holds that the rat brain during this period of life resembles the human brain during the gestation period. Based on the intensity of cortical synaptogenesis, a 1-week-old rat can be compared to a 2- to 3-year-old human [5-7]. Synapse formation in the human prefrontal cortex reaches its peak at about 5 years of age [8]. The few rodent studies on the synaptic developmental effects of neonatal anesthetic exposure report prominent alterations of synaptic structure and activity later in life [9-13]. This suggests that humans may remain vulnerable to the detrimental effects of general anesthetics at least for several years after birth, the period of intense synaptic maturation in humans. Thus, understanding the long-term synaptic effects of general anesthetics and their underlying mechanisms in rodents may advance a better understanding of the issue of human vulnerability to the developmental effects of general anesthetic exposure.
We recently reported that adverse developmental effects of two different general anesthetics, sevoflurane, a gaseous anesthetic whose polyvalent actions include enhancement of gamma-aminobutyric acid type A receptor (GABAAR) activity, and propofol, an intravenous anesthetic with a selective GABAAR-mediated action, included long-term alterations in synaptic activity that could be detected in the form of impaired long-term potentiation [9] and increased frequencies of GABAAR-mediated miniature postsynaptic currents (mPSCs) in the hippocampus later in life [12,13]. In general, changes in mPSC frequency are linked to presynaptic events such as a change in the probability of transmitter release and/or a change in the number of functional synapses, whereas changes in amplitude are explained by a change in postsynaptic receptor number or conductance [14]. Sevoflurane and propofol also caused an acute multifold increase in levels of the stress hormone, corticosterone [13,15]. These findings suggest that the anesthetic-caused increase in corticosteroid levels may contribute to the observed long-term propofol- and sevoflurane-induced alteration of synaptic activity. To test this possibility, we studied hippocampal synaptic activity in rats that were anesthetized with etomidate or propofol at P4, P5, or P6. Etomidate is an intravenous anesthetic that selectively enhances GABAAR activity similar to propofol [16,17], but in contrast to propofol, disrupts the adrenal synthesis of corticosterone [18].
Materials and Methods
Animals
All experimental procedures were approved by the University of Florida Institutional Animal Care and Use Committee (Gainesville, FL). Sprague-Dawley rats of both genders were studied. Animals were housed under controlled illumination (12-h light/dark cycle, lights on at 07:00) and temperature (23–24°C) with free access to food and water. Within 24 h of delivery, litters were culled to 12 pups. At the age of 21 days, pups were weaned and housed in sex-matched groups of two for the rest of the study. To control for litter variability, we used several pups from each litter for different treatment conditions.
Anesthesia protocols and treatment groups
P4, P5, or P6 rat pups of both genders were kept in a thermostated chamber (+37°C) with a continuous supply of oxygen (1.5 l/min) during anesthesia and/or maternal separation. Postnatal day (P) 4, 5, or 6 rats received five injections of etomidate, propofol, or vehicle control during 5 h of maternal separation. The animals received 8 mg/kg (IP) of etomidate or 40 mg/kg (IP) of propofol for the first hour. Every following hour for four hours etomidate was administered at a dose of 4 mg/kg (IP) and propofol was given at 20 mg/kg (IP). Based on measurement of the time to loss and return of righting, the presence or absence of a response to tail clamp, the degree of respiratory depression, and EEG activity, these doses of etomidate and propofol induced a depth of anesthesia that was in a similar range. This anesthesia protocol with propofol, which was adopted with some modifications from Briner et al. [10], was used in our previous studies [12,15]. Neither Briner et al. [10], whose protocol consisted of six injections of propofol at the same doses and lasting for 6 h, nor the other authors using a single injection of propofol at 75 mg/kg IP detected significant changes in blood gasses or glucose in neonatal rats [19]. The control animals were separated from the dams for 5 h in a temperature-controlled chamber with a continuous supply of oxygen and received five injections of equal volumes of propylene glycol (solvent for etomidate), intralipid (solvent for propofol), or saline (maternal separation only). Hippocampal electrophysiology at ≥P24 and serum levels of corticosterone immediately after anesthesia at P4, P5, or P6 were studied in separate groups of rats because both were terminal procedures.
Slice electrophysiology
Brain hippocampal slices were prepared from ≥P24 rats. The brain was removed after decapitation and was placed into ice-cold sucrose buffer containing (in mM): 254 sucrose,10 D-glucose, 26 NaHCO3, 2 CaCl2, 2 MgSO4, 3 KCl, and 1.25 NaH2PO4, saturated with 95% O2/5% CO2, at pH 7.4, 300 mOsm. Transverse hippocampal slices (300 μM thick) were cut with a VT 1000S microtome (Leica, Deerfield, IL). Slices were transferred immediately into a holding chamber and were incubated at 32 to 33°C for a 30-min recovery period in a mixture of 50% sucrose saline and 50% artificial cerebrospinal fluid (aCSF) containing (in mM): 128 NaCl, 10 D-glucose, 26 NaHCO3, 2 CaCl2, 2 MgSO4, 3 KCl, and 1.25 NaH2PO4. Slices were then placed on a nylon mesh, submerged in normal aCSF bubbled continuously with 95% O2/5% CO2, and maintained at room temperature (~21–24°C) until whole-cell patch-clamp recording, typically within 0.5 to 5 h.
Slices were transferred to a submersion-type recording chamber (Warner Instruments, Hamden, CT) on a Burleigh Gibraltar fixed-stage system (Burleigh Instruments, Fisher, NY) secured beneath a nylon harp, and perfused with aCSF heated to 30 to 33°C with an inline heater (SC-20, Warner) at a rate of 2 to 3 ml/min. CA1 pyramidal cells and interneurons were identified visually by using a microscope (Leica DM LFS, Leica Microsystems, Wetzlar, Germany) equipped with a 40× water-immersion objective coupled with an infrared differential interference contrast camera system. Whole-cell patch-clamp recordings from pyramidal CA1 neurons were established using an Axopatch 200B amplifier (Axon Instruments, Union City, CA). Membrane current and potential signals were digitized and analyzed with Digidata 1322A and pClamp 10.0 systems (Molecular Devices, Sunnyvale, CA). Patch pipettes of ≈5 MΩ were pulled with a P-1000 puller (Sutter Instruments, Novato, CA). The pipette solution had the following composition (in mM unless otherwise stated): 140 KCl, 0.1 CaCl2, 5 EGTA, 10 HEPES, 4 ATP-Mg2+, 0.4 GTP-2Na+, 1 QX314 (lidocaine N-ethyl bromide), pH 7.2, 290 mOsm. The diffusion potential (liquid junction potential) was 4 mV, calculated by Clampex software. QX314 was added to the pipette solution to block the GABABR-mediated currents and to prevent the generation of Na+-dependent action potentials. Under these conditions, total mPSCs were acquired in aCSF containing tetrodotoxin (1 μM) at a holding potential of −70 mV. To record miniature inhibitory postsynaptic currents (mIPSCs), glutamate receptor antagonists 6,7-dinitroquinoxaline-2,3-dione (20 μM) and DL-2-amino-5-phosphonovaleric acid (20 μM) were added to the aCSF. The changes in excitatory mPSCs (mEPSCs) were studied by subtracting mIPSCs from the total mPSCs. Drugs were administered by bath application. Synaptic currents were collected for 5 min for each experimental condition. Access resistance (<25 MΩ) was regularly monitored during recordings, and cells were rejected if resistance changed >15% during the experiment. If the access resistance increased during the course of the experiment and caused significant reductions in the synaptic current amplitudes, efforts were made to improve access (such as applying additional suction or slight positive pressure); if this failed, the experiment was discontinued. Spontaneously occurring synaptic currents were filtered at 2 kHz and were digitized at 10 kHz using Digidata 1322A. Offline data analysis was performed using the MiniAnalysis software (version 6.0.7; Synaptosoft, Decatur, GA). Synaptic currents were screened automatically using an amplitude threshold of 10 pA. Events were then visually screened to ensure that the analysis was not distorted by changes in the noise level or by membrane fluctuations. If the background noise increased during the recording, the data from that cell were discarded.
Measurement of serum corticosterone and aldosterone
Serum corticosterone and aldosterone were measured using commercial ELISA kits (Cayman Chemical Company, Ann Arbor, MI). The trunk blood samples were collected from P4, P5, or P6 rats after sacrifice by decapitation immediately after completion of 5 h of etomidate or propofol anesthesia or after 5 h of maternal separation in rats that received equal volumes of propylene glycol, intralipid, or saline. Anesthesia exposure started at 9 am. Blood samples were collected at the same day time from all experimental groups.
Drugs
Etomidate and propofol were purchased from APP Pharmaceuticals, LLC (Schaumburg, IL) and Hospira, Inc. (Lake Forest, IL), respectively. Propylene glycol, intralipid, tetrodotoxin, and QX314 were acquired from Sigma-Aldrich (St. Louis, MO). DL-2-amino-5-phosphonovaleric acid and 6,7-dinitroquinoxaline-2,3-dione were purchased from Tocris Cookson, Inc. (Ellisville, MO).
Statistical analysis
Values are reported as mean ± SEM. SigmaPlot 13.0 software (Systat Software, Inc., Point Richmond, CA) was used for statistical analyses. Single comparisons were tested using the t test, whereas multiple comparisons among groups were analyzed using ANOVA, followed by Holm-Sidak tests. All comparisons were run as two-tailed tests. P < 0.05 was considered significant.
Results
Differential acute endocrine and long-term hippocampal synaptic effects of etomidate and propofol
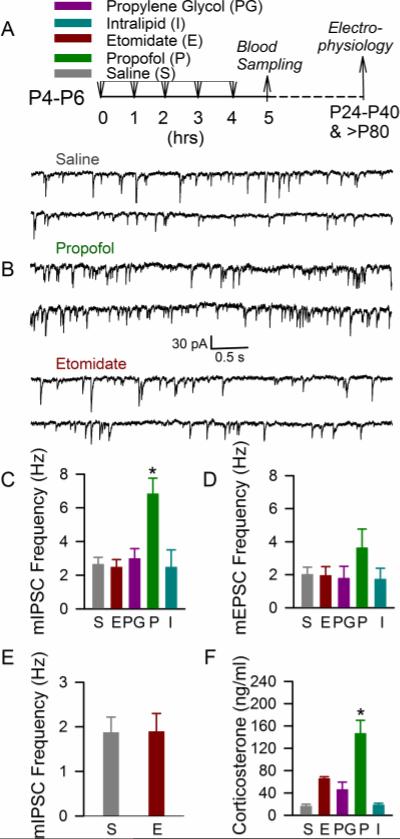
One-way ANOVA analysis revealed that the frequency of mIPSCs in hippocampal CA1 pyramidal neurons of P24-P40 rats that received five injections of etomidate during 5 h of maternal separation at P4, P5, or P6 was similar to the mIPSC frequency in rats that were neonatally treated with an equal number of injections of saline (p = 0.996), propylene glycol (p = 0.994), or intralipid (p = 1.0) during maternal separation for 5 h. In contrast, P24-P40 rats that were anesthetized with propofol at P4, P5, or P6 had increased mIPSC frequencies when compared to all other treatment groups) (F(4,23=8.731, p < 0.001; Fig 1, A-C). Pairwise comparisons using the Holm-Sidak test revealed significant effects of propofol versus etomidate (P<0.001), saline (P<0.001), intralipid (P=0.006) and propylene glycol (P=0.004). The mIPSC amplitude remained similar in all treatment groups of P24-40 rats, including the propofol group (p = 0.788). The frequency of mEPSCs in the propofol group of the P24-40 rats was higher when compared to all other treatment groups, but the difference was not big enough to achieve statistical significance (p = 0.357; Fig 1, A and D). We have previously shown that neonatal exposure to propofol or sevoflurane resulted in an increased frequency of GABAAR-mediated mIPSCs at >P80 [12]. To test whether neonatal exposure to etomidate may have an effect on hippocampal synaptic activity at an older age, activity of GABAAR-mediated mIPSCs was studied in a separate group of rats at >P80. Similar to the younger cohort of animals, etomidate did not induce any detectable alterations in the frequencies of mIPSCs when compared to non-anesthetized counterparts that received equal numbers of saline injections at P4-P6 (t(15) = −0.0366; p = 0.971; Fig 1E).
Figure 1.
Anesthesia of P4-P6 rats with etomidate, in contrast to anesthesia with propofol, did not cause a significant acute increase in systemic levels of corticosterone and long-term changes in hippocampal synaptic activity. (A) Illustration of the experimental protocols. The P4, P5, or P6 rats of both genders received five injections of etomidate or propofol or their vehicles during 5 h separation from their mothers. Blood samples were collected immediately after 5 h of maternal separation to measure serum levels of corticosterone. Electrophysiological measurements were performed in two age groups: P24-P40 and >P80. (B) Examples of mIPSC recordings in hippocampal CA1 pyramidal neurons of the P24-P40 rats that were treated at P4, P5, or P6 with saline, propofol, or etomidate. (C and D) Histograms showing the frequencies of mIPSCs and mEPSCs in hippocampal CA1 pyramidal neurons of the P24-P40 rats. Number of recorded cells for each treatment group: saline (7), etomidate (7), propofol (6), propylene glycol (5), and intralipid (3). *P < 0.05 vs. all other treatment groups. (E) Histograms showing frequencies of mIPSCs in hippocampal CA1 neurons of the >P80 rats. Number of recorded cells for each treatment group: etomidate (9) and saline (8). (F) Histograms showing serum levels of corticosterone in the P4-P6 rats. Number of animals for each treatment group: saline (6), etomidate (6), propofol (6), propylene glycol (7), and intralipid (6). *P < 0.001 vs. all other treatment groups.
Measurements of serum levels of corticosterone immediately after 5 h of anesthesia showed that the etomidate group had an increased level of corticosterone, though the difference was not big enough to achieve statistical significance when compared to the propylene glycol (p = 0.466), intralipid (p = 0.067), or saline (p = 0.062) groups. Propofol, on the other hand, significantly increased serum levels of corticosterone when compared with all other treatment groups (F(4,26) = 17.739, p < 0.001; Fig 1F). Post-hoc pairwise comparisons using the Holm-Sidak test revealed significant effects of propofol versus etomidate (P<0.001), saline (P<0.001), intralipid (P<0.001) and propylene glycol (P<0.001).
Discussion
This study demonstrates that neonatal anesthesia with two similar GABA-ergic anesthetics, i.e., etomidate or propofol, leads to different alterations in synaptic activity in juvenile and adult rats, i.e., propofol, but not etomidate, increased the frequency of mIPSCs in hippocampal CA1 pyramidal neurons. Likewise, etomidate, when compared to propofol, was much weaker at enhancing the secretion of corticosterone in neonatal rats. We previously reported that sevoflurane, a general anesthetic whose mechanism of action includes enhancement of GABAAR activity, caused an acute increase in serum levels of corticosterone and long-term alterations in hippocampal synaptic activity similar to propofol [12,13,15]. In addition, exogenous corticosterone, administered to non-anesthetized rat pups, induced long-term alterations in synaptic activity similar to propofol [12]. These findings, in combination with previously published data showing that etomidate failed to cause excitatory electroencephalogram effects comparable to those of propofol or sevoflurane, but significantly enhanced the seizure-like electroencephalogram activity caused by exogenous corticosterone [15,20,21], suggest that the anesthetic-caused increase of corticosterone levels are required for at least some of the developmental effects of the anesthetics in neonatal rats.
Although etomidate and propofol may interact with different binding sites on GABAARs to modulate their activity, GABAAR-mediated inhibition, enhanced by the anesthetics, is the most plausible mechanism whereby the two anesthetics induce a state of general anesthesia. Thus, Li and colleagues [22] found that isoflurane at millimolar concentrations competitively inhibited binding of [(3)H]azietomidate to GABAARs, suggesting a common binding site for etomidate and isoflurane, whereas inhibition by propofol was only partial, consistent with an allosteric interaction. Despite the difference in interaction with GABAARs, the same mutation involving a single amino acid in the β3 subunit of GABAARs reduced the ability of both etomidate and propofol to induce loss of the righting reflex and eliminated their ability to prevent the response to a painful stimulus [16,17]. Similar anesthetic actions involving enhancement of GABAAR activity and differential long-term alterations in synaptic function, induced by etomidate and propofol, suggest that additional mechanisms, other than anesthetic-caused acute enhancement of GABAAR activity, are involved in mediation of the long-term developmental synaptic effects. Propofol caused an increase in systemic levels of corticosterone in neonatal rats [15], whereas etomidate is known to inhibit the adrenal synthesis of corticosteroids by down-regulating the activity of 11β-hydroxylase, a limiting factor in synthesis of the corticosteroids [23-25]. The significantly smaller increase in serum corticosterone levels in neonatal rats anesthetized with etomidate or with the vehicle for etomidate, propylene glycol, when compared to corticosterone levels in rats anesthetized with propofol, is a plausible reason why etomidate did not induce an increase in the frequency of mIPSCs. In support of this possibility, propofol and sevoflurane, two otherwise different anesthetic agents that both enhance GABAAR activity and that similarly increased systemic levels of corticosterone, induced similar developmental alterations in hippocampal synaptic function [13,15]. In addition, exogenous corticosterone induced similar long-term alterations in synaptic activity [12].
Based on etomidate's acute effects on systemic levels of corticosterone and electroencephalographic activity [15] and its long-term synaptic effects (this study), etomidate seems to have a preferable neuro-safety profile in neonatal rats compared to propofol and sevoflurane [13,15,20]. However, despite the fact that etomidate is significantly less potent at inducing the production of corticosterone, etomidate still has a profound effect on the limbic-hypothalamic-pituitary-adrenal (LHPA) axis. Thus, etomidate, by disrupting the synthesis of corticosterone from deoxycorticosterone, is known to increase levels of endogenous neuroactive steroids in rodents [26] and humans [23-25]. In the mature brain, neurosteroid-enhanced GABAAR-mediated inhibition of LHPA axis activity is considered a crucial mechanism for adaption to stress [27,28]. Because GABAAR-mediated signaling can cause excitation in immature neurons [29,30], it is plausible that in neonatal rats, etomidate and its associated increase in neuroactive steroid levels may lead to abnormal LHPA axis activity. Additional studies of etomidate's safety profile in early postnatal subjects will be needed.
In summary, the results of this study and our previously published data demonstrate that neonatal exposure to etomidate, in contrast to exposure to propofol or sevoflurane, does not lead to abnormal, increased frequency of mIPSCs in hippocampal CA1 pyramidal neurons of ≥P24 rats. Available data suggest that etomidate's comparatively weak stimulation of adrenal corticosterone secretion contributes to its less prominent adverse neurodevelopmental effects. The known acute endocrine and electroencephalographic [15] and delayed synaptic effects (this study) suggest that etomidate may have a preferable safety profile during the vulnerable period in neonatal rodents compared to propofol and sevoflurane.
Highlights.
Anesthesia of neonatal rats with propofol was accompanied by a significantly greater increase in serum corticosterone levels when compared to anesthesia with etomidate, both selective enhancers of GABAAR activity
Anesthesia with propofol, but not with etomidate, resulted in long-term increase in frequency of hippocampal miniature postsynaptic currents
Anesthetic-caused increase in corticosterone level may be required for the long-term synaptic effects of GABAergic anesthetics
Acknowledgment
This work was supported by the National Institutes of Health (R01GM93036 and R01NS091542 to A.E.M.) and by the Jerome H. Modell, M.D., F.A.H.A. Endowed Professorship to N.G. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding sources were not involved in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
We would like to acknowledge the technical contribution of Wanting Zhu and Sijie Tan Department of Anesthesiology, University of Florida College of Medicine, Gainesville, FL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liu J, Rossaint R, Sanders RD, Coburn M. Toxic and protective effects of inhaled anaesthetics on the developing animal brain: systematic review and update of recent experimental work. Eur. J. Anaesthesiol. 2014;31:669–677. doi: 10.1097/EJA.0000000000000073. [DOI] [PubMed] [Google Scholar]
- 2.Stratmann G. Review article: Neurotoxicity of anesthetic drugs in the developing brain. Anesth. Analg. 2011;113:1170–1179. doi: 10.1213/ANE.0b013e318232066c. [DOI] [PubMed] [Google Scholar]
- 3.Dobbing J, Sands J. Quantitative growth and development of human brain. Arch. Dis. Child. 1973;48:757–767. doi: 10.1136/adc.48.10.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dobbing J, Sands J. Comparative aspects of brain growth spurt. Early Human Dev. 1979;311:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- 5.Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013:106–107. 1–16. doi: 10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ. Health Perspective. 2000;108:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Babikian T, Prins ML, Cai Y, Barkhoudarian G, Hartonian I, Hovda DA, Giza CC. Molecular and physiological responses to juvenile traumatic brain injury: focus on growth and metabolism. Dev. Neurosci. 2010;32:431–441. doi: 10.1159/000320667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X, Somel M, Tang L, Yan Z, Jiang X, Guo S, Yuan Y, He L, Oleksiak A, Zhang Y, Li N, Hu Y, Chen W, Qiu Z, Pääbo S, Khaitovich P. Extension of cortical synaptic development distinguishes humans from chimpanzees and macaques. Genome Res. 2012;22:611–622. doi: 10.1101/gr.127324.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edwards DA, Shah HP, Cao W, Gravenstein N, Seubert CN, Martynyuk AE. Bumetanide alleviates epileptogenic and neurotoxic effects of sevoflurane in neonatal rat brain. Anesthesiology. 2010;112:567–575. doi: 10.1097/ALN.0b013e3181cf9138. [DOI] [PubMed] [Google Scholar]
- 10.Briner A, Nikonenko I, De Roo M, Dayer A, Muller D, Vutskits L. Developmental stage-dependent persistent impact of propofol anesthesia on dendritic spines in the rat medial prefrontal cortex. Anesthesiology. 2011;115:282–293. doi: 10.1097/ALN.0b013e318221fbbd. [DOI] [PubMed] [Google Scholar]
- 11.Amrock LG, Starner ML, Murphy KL, Baxter MG. Long-term effects of single or multiple neonatal sevoflurane exposures on rat hippocampal ultrastructure. Anesthesiology. 2015;122:87–95. doi: 10.1097/ALN.0000000000000477. [DOI] [PubMed] [Google Scholar]
- 12.Tan S, Xu C, Zhu W, Willis J, Seubert CN, Gravenstein N, Sumners C, Martynyuk AE. Endocrine and neurobehavioral abnormalities induced by propofol administered to neonatal rats. Anesthesiology. 2014;121:1010–1017. doi: 10.1097/ALN.0000000000000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu C, Tan S, Zhang J, Seubert CN, Gravenstein N, Sumners C, Vasilopoulos T, Martynyuk AE. Anesthesia with sevoflurane in neonatal rats: developmental neuroendocrine abnormalities and alleviating effects of the corticosteroid and Cl- importer antagonists. Psychoneuroendocrinology. 2015;60:173–181. doi: 10.1016/j.psyneuen.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat. Rev. Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- 15.Willis J, Zhu W, Perez-Downes J, Tan S, Xu C, Seubert C, Gravenstein N, Martynyuk A. Propofol-induced electroencephalographic seizures in neonatal rats: the role of corticosteroids and γ-aminobutyric acid type a receptor-mediated excitation. Anesth. Analg. 2015;120:433–439. doi: 10.1213/ANE.0000000000000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jurd R, Arras M, Lambert S, Drexler B, Siegwart R, Crestani F, Zaugg M, Vogt KE, Ledermann B, Antkowiak B, Rudolph U. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta 3 subunit. FASEB J. 2003;17:250–252. doi: 10.1096/fj.02-0611fje. [DOI] [PubMed] [Google Scholar]
- 17.Zeller A, Arras M, Lazaris A, Jurd R, Rudolph U. Distinct molecular targets for the central respiratory and cardiac actions of the general anesthetics etomidate and propofol. FASEB J. 2005;19:1677–1679. doi: 10.1096/fj.04-3443fje. [DOI] [PubMed] [Google Scholar]
- 18.Vanlersberghe C, Camu F. Etomidate and other non-barbiturates. Handb. Exp. Pharmacol. 2008;182:267–282. doi: 10.1007/978-3-540-74806-9_13. [DOI] [PubMed] [Google Scholar]
- 19.Yu D, Jiang Y, Gao J, Liu B, Chen P. Repeated exposure to propofol potentiates neuroapoptosis and long-term behavioral deficits in neonatal rats. Neurosci. Lett. 2013;534:41–46. doi: 10.1016/j.neulet.2012.12.033. [DOI] [PubMed] [Google Scholar]
- 20.Cao W, C Pavlinec N, Gravenstein CN, Seubert AE. Martynyuk, Roles of aldosterone and oxytocin in abnormalities caused by sevoflurane anesthesia in neonatal rats. Anesthesiology. 2012;117:791–800. doi: 10.1097/ALN.0b013e318266c62d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seubert CN, Zhu W, Pavlinec C, Gravenstein N, Martynyuk AE. Developmental effects of neonatal isoflurane and sevoflurane exposure in rats. Anesthesiology. 2013;119:358–364. doi: 10.1097/ALN.0b013e318291c04e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li GD, Chiara DC, Cohen JB, Olsen RW. Numerous classes of general anesthetics inhibit etomidate binding to gamma-aminobutyric acid type A (GABAA) receptors. J. Biol. Chem. 2010;285:8615–8620. doi: 10.1074/jbc.M109.074708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Coster R, Helmers JH, Noorduin H. Effect of etomidate on cortisol biosynthesis: site of action after induction of anaesthesia. Acta Endocrinol. (Copenh) 1985;110:526–531. doi: 10.1530/acta.0.1100526. [DOI] [PubMed] [Google Scholar]
- 24.Dörr HG, Kuhnle U, Holthausen H, Bidlingmaier F, Knorr D. Etomidate: a selective adrenocortical 11 beta-hydroxylase inhibitor. Klin Wochenschr. 1984;62:1011–1013. doi: 10.1007/BF01711722. [DOI] [PubMed] [Google Scholar]
- 25.Duthie DJ, Fraser R, Nimmo WS. Effect of induction of anaesthesia with etomidate on corticosteroid synthesis in man. Br. J. Anaesth. 1985;57:156–159. doi: 10.1093/bja/57.2.156. [DOI] [PubMed] [Google Scholar]
- 26.Kaminski RM, Rogawski MA. 11β-Hydroxylase inhibitors protect against seizures in mice by increasing endogenous neurosteroid synthesis. Neuropharmacology. 2011;61:133–137. doi: 10.1016/j.neuropharm.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mody I, Maguire J. The reciprocal regulation of stress hormones and GABA(A) receptors. Front. Cell. Neurosci. 2012;6:4. doi: 10.3389/fncel.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crowley SK, Girdler SS. Neurosteroid, GABAergic and hypothalamic pituitary adrenal (HPA) axis regulation: what is the current state of knowledge in humans? Psychopharmacology (Berl) 2014;231:3619–3634. doi: 10.1007/s00213-014-3572-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ben-Ari Y, Khalilov I, Kahle KT, Cherubini E. The GABA excitatory/inhibitory shift in brain maturation and neurological disorders. Neuroscientist. 2012;18:467–486. doi: 10.1177/1073858412438697. [DOI] [PubMed] [Google Scholar]
- 30.Gao XB, van den Pol AN. GABA, not glutamate, a primary transmitter driving action potentials in developing hypothalamic neurons. J. Neurophysiol. 2001;85:425–434. doi: 10.1152/jn.2001.85.1.425. [DOI] [PubMed] [Google Scholar]