Abstract
Aim
Mood disorders are associated with low levels of the long-chain omega-3 (LCn-3) fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). This study investigated LCn-3 fatty acid biostatus in youth with or at varying risk for developing mania to assess its utility as a prodromal risk biomarker.
Method
Erythrocyte fatty acid composition was determined in healthy adolescents (n=28, HC), asymptomatic adolescents with a biological parent with bipolar I disorder (n=30; ‘high-risk’, HR), adolescents with a biological parent with bipolar I disorder and major depressive disorder (MDD) or depressive disorder not-otherwise-specified (n=36; ‘ultra high-risk’, UHR), and first-episode adolescent bipolar manic patients (n=35, BP).
Results
Group differences were observed for DHA (p≤0.0001) and EPA (p=0.03). Compared with HC, erythrocyte EPA+DHA (‘omega-3 index’) was significantly lower in BP (−24%, p≤0.0001) and UHR (−19%, p=0.0006) groups, and there was a trend in the HR group (−11%, p=0.06). Compared with HC (61%), a greater percentage of HR (77%, p=0.02), UHR (80%, p=0.005), and BP (97%, p=0.001) subjects exhibited EPA+DHA levels of ≤4.0%. Among all subjects (n=130) EPA+DHA was inversely correlated with manic (r = −0.29, p=0.0008) and depressive (r = −0.28, p=0.003) symptom severity. The AA/EPA+DHA ratio was significantly greater in BP (+22%, p=0.0002) and UHR (+16%, p=0.001) groups.
Conclusions
Low EPA+DHA levels coincide with the initial onset of mania, and increasing risk for developing bipolar disorder is associated with graded erythrocyte EPA+DHA deficits. Low erythrocyte EPA+DHA biostatus may represent a promising prodromal risk biomarker warranting additional evaluation in future prospective studies.
Keywords: Bipolar disorder, Mania, Ultra high-risk, Erythrocyte, Arachidonic acid (AA), Docosahexaenoic acid (DHA), Eicosapentaenoic acid (EPA), Omega-3 Index
INTRODUCTION
The initial onset of mania, and by definition bipolar I disorder, typically occurs during adolescence and is associated with significant psychosocial morbidity and increased risk for suicide.1,2 Heritability estimates for bipolar disorder range from 60–87%,3 and family studies demonstrate that having a first-degree relative with bipolar I disorder substantially increases risk for developing bipolar disorder.4–6 Retrospective and prospective longitudinal studies additionally suggest that mood symptoms, including episodic subsyndromal depressive symptoms and major depressive disorder (MDD), frequently precede the initial onset of mania by several years.7–9 While emerging retrospective and prospective evidence suggests that adolescents with a positive family history and prodromal mood symptoms are at increased risk for developing bipolar disorder,10,11 identifying prodromal biomarkers may serve to augment prognostic accuracy as well as inform novel early interventions.12,13
One candidate risk factor that may be relevant to the pathoetiology of mood disorders is long-chain omega-3 (LCn-3) fatty acid deficiency. LCn-3 fatty acids, including eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3), have anti-inflammatory, neurotrophic, and neuroprotective properties and are required for healthy brain development.14 LCn-3 fatty acids are primarily obtained from the diet and cross-national epidemiological studies suggest that lower habitual dietary intake of fish/seafood, primary dietary sources of preformed EPA and DHA, is associated with increased prevalence rates of MDD and bipolar disorder.15–17 A large percentage of adolescents residing in western countries consume very low quantities of LCn-3 fatty acids in their diet,18–21 which is associated with depressive symptoms.22–24 Meta-analyses of controlled trials further support the antidepressant effects of dietary fish oil supplementation in patients with MDD25 and bipolar disorder,26 and preliminary trials suggest that fish oil supplementation is effective for youth with MDD27,28 or bipolar disorder.29,30 Together, this body of evidence suggests that dietary LCn-3 fatty acid deficiency may represent a modifiable risk factor for mood disorders.
Erythrocyte (red blood cell) membrane EPA and DHA composition correlates with habitual fish or fish oil intake and represents a valid biomarker of LCn-3 fatty acid biostatus.31–33 Erythrocyte EPA+DHA composition (‘omega-3 index’) has been characterized as a risk biomarker in the context of coronary heart disease, with erythrocyte EPA+DHA composition of ≤4% of total fatty acid composition considered to be high risk whereas >8% is protective.34 Cardiovascular disease is a leading cause of excess premature mortality among subjects with mood disorders,35,36 and case-control studies have consistently observed lower erythrocyte EPA and/or DHA levels in adolescents and adults with MDD27,37–38 or bipolar disorder.39–43 However, to our knowledge no studies have investigated erythrocyte EPA+DHA in youth with or at varying risk for developing mood disorders.
AIM
The aim of the present cross-sectional study was to compare erythrocyte EPA+DHA biostatus in youth with or at varying risk for developing bipolar disorder to evaluate its potential utility as a prodromal risk biomarker.
METHOD
Study participants
Study participants were recruited from inpatient units and outpatient clinics at Cincinnati Children’s Hospital Medical Center and University of Cincinnati Medical Center. Demographically-matched healthy comparison subjects were recruited from the communities in which the other participants resided. The cohort consisted of 4 groups of adolescents (9–20 years of age): 1) healthy comparison (HC) subjects without a personal or family history of a DSM-IV Axis I disorder (n=28), 2) subjects with no personal DSM-IV Axis I diagnosis and at least one biological parent with bipolar I disorder (high-risk; HR, n=30), 3) adolescents with at least one biological parent with bipolar I disorder and a DSM-IV Axis I diagnosis of MDD or depressive disorder not-otherwise-specified (‘ultra high-risk’; UHR, n=36), and 4) first-episode adolescent patient exhibiting mixed or manic symptoms who received a DSM-IV diagnosis of bipolar I disorder (BP, n=35). DSM-IV diagnoses were determined using the Washington University in St. Louis Kiddie-Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS).44 Parental diagnoses were determined using the Structured Clinical Interview for DSM-IV (SCID)45 and confirmed using the Family Interview for Genetic Studies (FIGS).46 All participants were assessed by psychiatrists with established inter-rater reliabilities (kappa >0.9). IQ was estimated using the Wechsler Abbreviated Scale of Intelligence (WASI).47 Manic and depressive symptom severity ratings were obtained using the Young Mania Rating Scale (YMRS)48 and the 28-item Hamilton Depression Rating Scale (HDRS),49 respectively. Pubertal development was measured using the self-measured Tanner Scale.50 Subjects were excluded by a diagnosis of substance dependence within the previous 3 months, major medical or neurological illness (e.g., head trauma resulting in loss of consciousness), an IQ<70, and if female a positive urine pregnancy test. Cigarette use in the past 30 days was documented and was not exclusionary. All patients were medication-free at the time of the blood draw. Written informed consent was obtained from each parent or adult subject and written assent was obtained for patients younger than 18 years of age. This study was approved by the Institutional Review Boards of University of Cincinnati Medical Center and Cincinnati Children’s Hospital Medical Center.
Erythrocyte fatty acid composition
Whole venous blood (4 ml) was collected into EDTA-coated BD Vacutainer tubes, and immediately centrifuged at 4°C for 20 min (1,500 ×g). Plasma and buffy coat were removed and erythrocytes washed 3 times with 0.9% NaCl and stored at −80°C. Total erythrocyte membrane fatty acid composition was determined with a Shimadzu GC-2010 equipped with an auto-injector (Shimadzu Scientific Instruments Inc., Columbia MD), using the direct saponification method described previously.27,41,43 The column was a DB-23 (123-2332): 30m (length), I.D. (mm) 0.32 wide bore, film thickness of 0.25 µM (J&W Scientific, Folsom CA). The carrier gas was helium with a column flow rate of 2.5 ml/min. Fatty acid identification was determined using retention times of authenticated fatty acid methyl ester standards (Matreya LLC Inc., Pleasant Gap PA). Analysis of fatty acid methyl esters was based on areas calculated with Shimadzu Class VP 4.3 software. Fatty acid data are expressed as weight percent of total fatty acids (mg fatty acid/100 mg fatty acids) which we have found to be highly correlated with fatty acid total mass (µmol/g). Our primary measures of interest were the LCn-3 fatty acids EPA (20:5n-3), docosapentaenoic acid (DPA, 22:5n-3), and DHA (22:6n-3), EPA+DHA (‘omega-3 index’), and the omega-6 fatty acid arachidonic acid (20:4n-6). Samples were processed by a technician blinded to group assignment.
Statistical analysis
Statistical analyses were performed using the Statistical Analysis System (SAS Institute, Cary, NC, USA). Prior to data analysis the distribution of dependent variables was assessed for normality (α=0.05), and each dependent variable was found to be normally distributed within each group. Group differences in demographic variables were identified using a one-way ANOVA for continuous variables and Chi-square tests for dichotomous variables. For fatty acids and fatty acid ratio selected a priori (n=6), an overall group effect was evaluated with a one-way ANOVA and individual group differences evaluated with t-tests adjusted for multiple comparisons (α=0.05/6=0.008). Categorical assessments were used to determine the percentage of subjects with an ‘omega-3 index’ (EPA+DHA) of ≤4.0 percent or a AA/EPA+DHA ratio of 4.6 (median-split)(Chi-square test). Interactions with selected demographic variables were evaluated with a two-way ANOVA. Pearson correlation coefficients were used to evaluate relationships between fatty acid levels and manic (YMRS) and depression (HDRS) symptom severity scores as well as selected demographic variables. For the primary outcome measure (EPA+DHA), interquartile range (IQR) and effect size (Cohen’s d) were calculated.
RESULTS
Subject characteristics
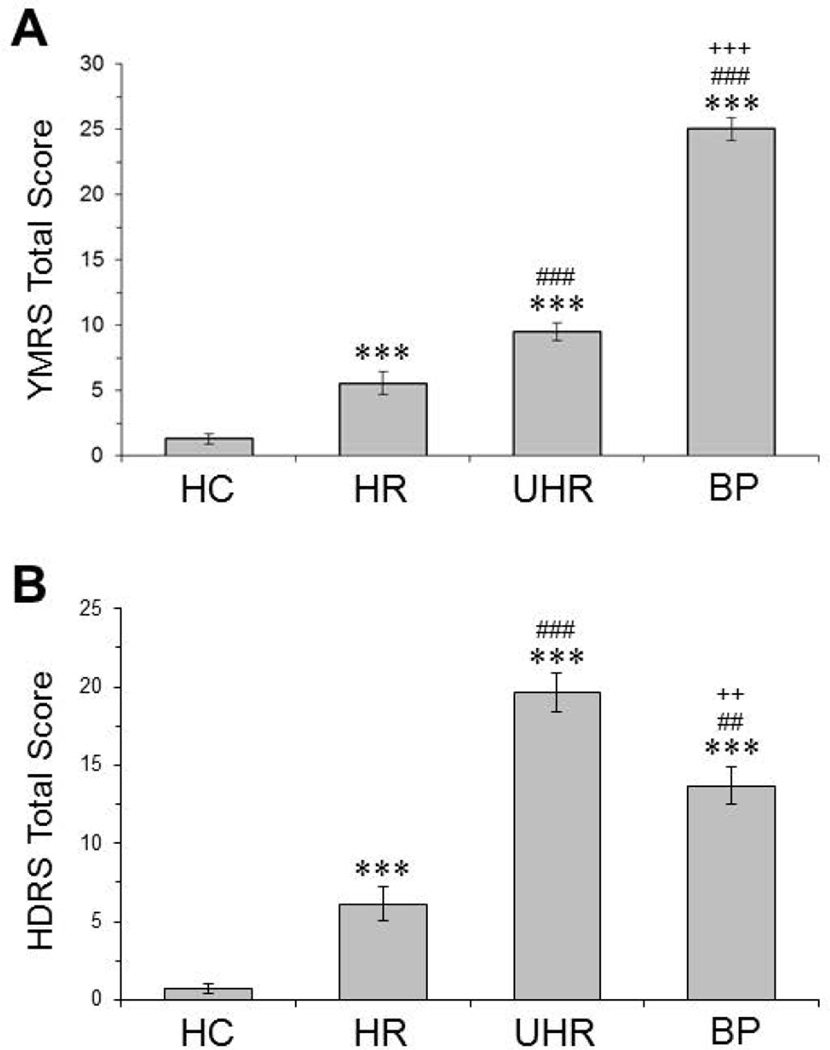
Demographic characteristics of study participants are presented in Table 1. The four groups were similar in age, BMI, and race. There were significantly more girls in the UHR group (72%) compared with HC (54%, p=0.01) and HR (48%, p=0.001) but not BP (63%, p=0.87) groups. Compared with HC (7%), significantly more UHR (22%, p=0.004) and BP (29%, p=0.0001) participants, but not HR participants (7%, p=1.0), reported cigarette use in the past 30 days. Compared with HC (0%), significantly more HR (33%, p=0.001), UHR (36%, p=0.001) and BP (34%, p=0.001) subjects had a lifetime history of attention deficit hyperactivity disorder (ADHD). In the UHR group, n=30 (83%) adolescents were diagnosed with MDD and n=6 (17%) diagnosed with depressive disorder not-otherwise-specified. In the BP group, n=13 had a positive family history for bipolar disorder and n=22 had unknown or no family history. A significant main effect of group was observed for YMRS total score (p≤0.0001, Fig. 1A) and HDRS total score (p≤0.0001, Fig. 1B).
Table 1.
Demographic and clinical characteristics of study participants
| Variable1 | HC (n=28) |
HR (n=30) |
UHR (n=36) |
BP (n=35) |
P- Value2 |
|---|---|---|---|---|---|
| Age (years) | 15.9 ± 2.5 | 15.1 ± 2.8 | 15.1 ± 2.2 | 15.8 ± 1.4 | 0.17 |
| Gender (% female) | 54 | 48 | 72 | 63 | 0.003 |
| Race (n) | □ | □ | |||
| Caucacian | 18 | 22 | 27 | 26 | 0.29 |
| African American | 5 | 8 | 6 | 4 | □ |
| Other | 5 | 0 | 3 | 5 | |
| BMI (kg/m2) | 23.3 ± 4.6 | 26.2 ± 9.9 | 25.2 ± 8.2 | 26.0 ± 7.8 | 0.69 |
| Smoking status (current) (n) | 2 | 2 | 8 | 10 | 0.001 |
| ADHD (lifetime)(n) | 0 | 9 | 13 | 12 | 0.001 |
Values are group mean ± S.D., group percentage, or number of subjects (n).
One-way ANOVA or Chi-square test
Figure 1.
YMRS (A) and HDRS (B) total scores for healthy comparison subjects (HC, n=28), asymptomatic adolescents with a biological parent with bipolar I disorder (‘high-risk’, HR; n=31), adolescents with a biological parent with bipolar I disorder and MDD or depressive disorder-NOS (‘ultra high-risk’, UHR; n=36), and first-episode bipolar patients (BP, n=35). Values are group mean ± S.E.M. ***P≤0.0001 vs. HC, ##P≤0.01, ###P≤0.001 vs. HR, ++P≤0.01, +++P≤0.001 vs. UHR.
Fatty acid analyses
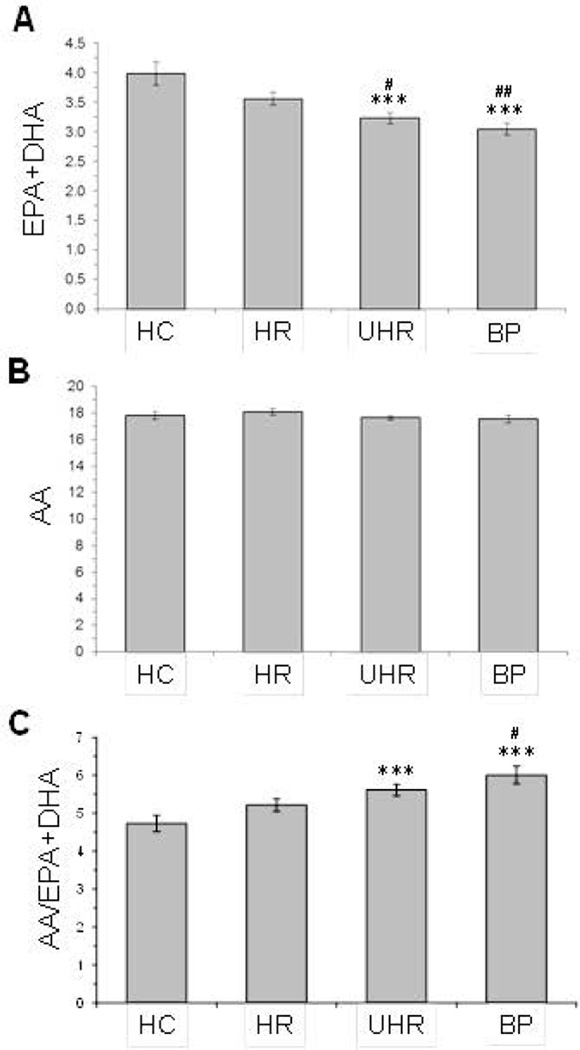
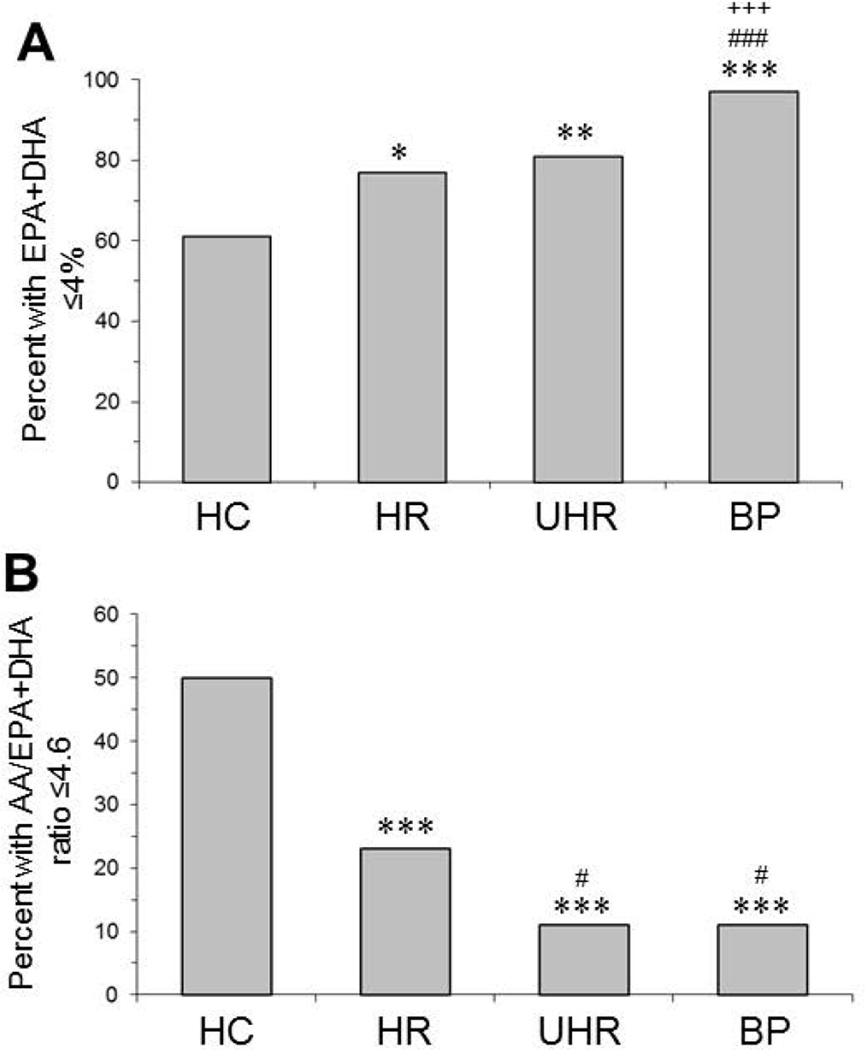
Significant group effects were observed for DHA (p≤0.0001), EPA (p=0.03), but not docosapentaenoic acid (22:5n-3)(p=0.69). A significant group effect was observed for EPA+DHA (‘omega-3 index’)(p≤0.0001). Compared with HC (mean±S.D.: 4.0±1.0, IQR: 1.2), erythrocyte EPA+DHA composition was significantly lower in BP (3.0±0.6, IQR: 0.93, p≤0.0001, d = 1.15) and UHR (3.2±0.6, IQR: 0.76, p=0.0006, d = 0.9) groups, and there was a trend with medium effect size in the HR group (3.6±0.6, IQR: 0.84, p=0.06, d = 0.5)(Fig. 2A). No group effect was observed for arachidonic acid (AA, 20:4n-6)(p=0.44)(Fig. 2B), and a significant group differences was found for the AA/EPA+DHA ratio (p=0.0001). Compared with HC, the AA/EPA+DHA ratio was significantly greater in BP (+22%, p=0.0002) and UHR (+16%, p=0.001) groups, and there was a trend in the HR group (+10%, p=0.08)(Fig. 2C). Compared with HC (61%), a significantly greater percentage of HR (77%, p=0.02), UHR (81%, p=0.005), and BP (97%, p=0.001) subjects exhibited an ‘omega-3 index’ (EPA+DHA) of ≤4.0 percent (Fig. 3A). Following a median-split of the AA/EPA+DHA ratio (4.6), a significantly smaller percentage of HR (23%, p≤0.0001), UHR (11%, p≤0.0001), and BP (11%, p≤0.0001) subjects exhibited a AA/EPA+DHA ratio of ≤4.6 compared with HC (50%)(Fig. 3B).
Figure 2.
Erythrocyte membrane EPA+DHA (omega-3 index)(A), arachidonic acid (AA)(B), and the AA/EPA+DHA ratio (C) in healthy comparison (HC), high-risk (HR), ultra high-risk (UHR) and first-episode bipolar (BP) groups. Fatty acids are expressed as weight percent of total fatty acids (mg fatty acid/100 mg fatty acids) or ratio. Values are group mean ± S.E.M. ***P≤0.0001 vs. HC, #P≤0.05, ##P≤0.01 vs. HR.
Figure 3.
Percentage of subjects in the healthy comparison (HC), high-risk (HR), ultra high-risk (UHR), and first-episode bipolar (BP) groups with an omega-3 index (EPA+DHA) of ≤4.0% (A) or a AA/EPA+DHA ratio ≤4.6 (median-split)(B). *P≤0.05, **P≤0.01, ***P≤0.0001 vs. HC, #P≤0.05, ###P≤0.001 vs. HR, +++P≤0.001 vs. UHR (Chi-Square test).
Among all subjects (n=130), EPA+DHA was not significantly correlated with BMI (r = −0.01, p=0.89) or age (r = −0.02, p=0.79), and EPA+DHA was not significantly correlated with BMI or age within each group. For EPA+DHA, the interaction term for group by smoking status (p=0.57) and group by gender (p=0.27) were not significant. The group by ADHD interaction term was significant (p=0.03). EPA+DHA levels were significantly lower in all subjects with ADHD (n=34) compared to healthy subjects (−18.5%, p=0.001, d = 0.85). Within the URH group, EPA+DHA did not differ between those with a DSM-IV Axis I diagnosis of MDD compared with a diagnosis of depressive disorder NOS (p=0.70). Within the BP group, EPA+DHA did not differ from those with a positive family history for bipolar I disorder and those with unknown (p=0.99) or no family history (p=0.16). Although EPA+DHA was not significantly correlated with either YMRS or HDRS total scores within each group, among all subjects (n=130) EPA+DHA was inversely correlated with YMRS (r = −0.29, p=0.0008) and HDRS (r = −0.28, p=0.003) total scores.
DISCUSSION
The present cross-sectional study investigated erythrocyte LCn-3 fatty acid biostatus in adolescents with and at varying risk for bipolar disorder. We found that erythrocyte EPA+DHA composition was significantly lower in first-episode BP and UHR groups compared with healthy subjects, and a significantly greater percentage of subjects in the HR, UHR, and BP groups exhibited EPA+DHA levels of ≤4.0% compared with healthy subjects. Group differences in erythrocyte EPA+DHA levels could not be attributed to differences in age, BMI, race, smoking status, or comorbid ADHD. The significant group differences in YMRS and HDRS total scores were inversely correlated with erythrocyte EPA+DHA among all subjects only. These data suggest that low EPA+DHA levels coincide with the initial onset of bipolar disorder and that increasing risk level for bipolar disorder onset is associated with graded decreases in erythrocyte EPA+DHA. Together these data suggest that low erythrocyte EPA+DHA may represent a promising prodromal risk biomarker warranting additional evaluation in future prospective studies.
The mean erythrocyte EPA+DHA composition exhibited by healthy adolescents (4.0%) in the present sample is similar to that observed in a separate cohort of healthy adolescents residing in the United States (4.3%).27 The observation that UHR patients (i.e., adolescents with MDD or depressive disorder NOS) exhibit EPA+DHA deficits compared with healthy subjects is consistent with previous case-control studies employing adolescent and adult MDD patients.27,37,38 Similarly, the observed EPA+DHA deficit observed in adolescent BP patients is consistent with previous case-control studies in pediatric and adult BP patients.39–42 We did not observe erythrocyte AA deficits in any group which is consistent with the majority of previous case-control studies of MDD27,37,38 and bipolar disorder.39–42 It is notable that prior case-control studies did observe significant erythrocyte AA deficits in addition to DHA deficits in medication-naïve first-episode psychotic patients,51 suggesting that AA deficits may distinguish risk for psychosis versus MDD or bipolar disorder. In general, the present cross-sectional evidence is very consistent with prior studies observing significant EPA+DHA deficits in patients with bipolar disorder and MDD, and further suggest that EPA+DHA deficits coincide with, and may precede, the initial onset of manic symptoms in high-risk youth.
The EPA+DHA deficits observed in youth at familial risk for bipolar disorder may be attributable in part to heritable genetic factors. For example, heritable polymorphisms in delta-6 and/or delta-5 desaturase enzymes, which mediate EPA and DHA biosynthesis from the short-chain fatty acid precursor alpha-linolenic acid (ALA), have been identified and may be associated with lower erythrocyte EPA and/or DHA levels.52–54 While extant evidence does not support an association between polymorphisms in delta-6 and delta-5 desaturase genes and bipolar disorder,55 epigenetic alterations (i.e., DNA methylation) may also contribute to alterations in desaturase activity.56 Nevertheless, ALA→DHA biosynthesis is negligible even in healthy subjects,57 and we found that other long-chain fatty acids which also require delta-6 and delta-5 desaturases, including docosapentaenoic (22:5n-3) and arachidonic acid (20:4n-6), did not differ between groups. Alternatively, the final biosynthesis of DHA requires peroxisomal β-oxidation,58 and several functional polymorphisms in peroxisome assembly genes have been identified59 and are associated with low DHA biostatus.60 However, in a separate study we recently found that subjects with or at risk for bipolar disorder do not exhibit other lipid abnormalities characteristic of peroxisome defects (i.e., elevated plasma very long-chain fatty acids).61 Additional studies are warranted to directly investigate potential interactions between these and other heritable genetic factors and LCn-3 fatty acid biostatus in individuals with familial risk for bipolar disorder.
The erythrocyte EPA+DHA deficits observed in youth at familial risk for bipolar disorder may also be attributable to non-heritable factors including dietary deficiency. While not directly evaluated in the present study, dietary fish or fish oil intake is linearly correlated with erythrocyte EPA+DHA levels,31,33 and fish oil supplementation is sufficient to increase erythrocyte EPA+DHA levels in pediatric and adolescent patients with bipolar disorder.29,30 It is relevant therefore that less frequent dietary fish intake is associated with erythrocyte EPA+DHA deficits and depressive symptoms in adolescents.22–24 While it is not known whether bipolar families consume fish less frequently than the general population, emerging evidence suggests that patients with mood disorders have poorer nutritional habits62–64 and have lower intake of EPA+DHA.65 While these data suggest that the erythrocyte EPA+DHA deficits observed in youth at familial risk for bipolar disorder may reflect dietary deficits in preformed EPA+DHA, additional studies will be required to confirm this association.
Based on prospective longitudinal studies erythrocyte EPA+DHA (‘omega-3 index’) composition of ≤4% of total fatty acid composition may be considered a risk factor for coronary heart disease.34 In the present study, a large majority of adolescents with (97%) or at high- (77%) or ultra-high risk (80%) for bipolar disorder exhibited erythrocyte EPA+DHA composition of ≤4%. It is relevant, therefore, that cardiovascular disease is a leading cause of excess premature mortality among subjects with bipolar disorder.35,36 Indeed, the mean erythrocyte EPA+DHA composition exhibited by adolescents with (3.0%) or ultra-high risk (3.2%) for bipolar disorder resemble that observed in adult patients with acute coronary syndrome (3.4%).66 It is also notable that the majority of healthy adolescents (61%) exhibited erythrocyte EPA+DHA composition at ≤4%, which would also be anticipated to increase their risk for cardiovascular disease. Overall, these data are consistent with extant evidence indicating that a large percentage of adolescents consume very low quantities of LCn-3 fatty acids,18–21 and highlight a need to increase awareness of the importance of consuming sufficient quantities of EPA+DHA in habitual diets.
The present findings may take on additional significance in view of evidence that DHA is the principal LCn-3 fatty acid found in cortical gray matter, and erythrocyte DHA is correlated with cortical gray matter DHA composition.67,68 Moreover, the adolescent period is associated with a sharp increase in frontal cortex DHA concentrations,67 and significant DHA deficits have been observed in the postmortem prefrontal cortex of adult patients with bipolar disorder and MDD.69,70 It is relevant therefore that adolescence is a developmental period associated with the maturation of corticolimbic structural and functional connectivity,71–73 and emerging neuroimaging evidence suggests that blood DHA status is correlated with corticolimbic structural and functional integrity.74–77 Neuroimaging studies are warranted to investigate associations between erythrocyte DHA biostatus and corticolimbic structural and functional maturation in youth at high-risk for bipolar disorder.
A limitation of this study is the small number of individuals in each group, and the data obtained may not be representative of all individuals in each risk category. Second, we did not administer a diet questionnaire to determine the contribution of habitual dietary patterns to the observed findings. Third, the cross-sectional design precludes evaluation of causality, and prospective longitudinal studies will be required to clarify the role of EPA+DHA biostatus in bipolar risk progression.
In summary, the present cross-sectional data suggest that adolescents with and at elevated risk for developing bipolar disorder exhibit graded erythrocyte EPA+DHA deficits, and very low EPA+DHA levels accompany MDD in bipolar offspring and coincide with the initial onset of bipolar disorder. Taken collectively, these and previous data suggest that erythrocyte EPA+DHA represents a candidate prodromal risk biomarker that may serve to augment demographic and clinical criteria to help identify individuals at increased risk for bipolar disorder. Additionally, because low erythrocyte EPA+DHA biostatus can be corrected by increasing dietary fish or fish oil intake, prospective supplementation studies are warranted to determine whether increasing EPA+DHA intake can mitigate risk progression in high-risk youth as has been observed in youth at high-risk for psychosis.78
Acknowledgements
This study was supported in part by NIH/NIMH grant P50 MH077138 to S.M.S., R34 NIH/NIMH grant MH083924 to R.K.M and M.P.D (Co-PIs), NIH/NIMH grant MH080973 to M.P.D, and NIH/NIDDK grant DK59630 to P.T.; the NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
R.K.M. has received research support from Martek Biosciences Inc, Inflammation Research Foundation, Ortho-McNeil Janssen, AstraZeneca, Eli Lilly, NARSAD, and national institutes of health (NIH), and served on the scientific advisory board of the Inflammation Research Foundation. J.R.S. has received research support from Eli Lilly, Shire, the American Academy of Child and Adolescent Psychiatry, and NIH. S.M.S. has received research grant support from Eli Lilly, Janssen, AstraZeneca, Nutrition 21, Repligen, NIDA, NIAAA, NARSAD, NIH, Thrasher Foundation, and has served as a consultant for Pfizer. M.P.D. has received research support from, Johnson & Johnson, Shire, Ortho-McNeil Janssen, Pfizer, Bristol Myers Squibb, Repligen, Somerset, Sumitomo, Thrasher Foundation, GlaxoSmithKline, and NIH, and has served as a consultant for GlaxoSmithKline, Eli Lilly, France Foundation, Kappa Clinical, Pfizer, Medical Communications Media, Shering-Plough.
Footnotes
Disclosures
The remaining authors do not have disclosures.
REFERENCES
- 1.Perlis RH, Miyahara S, Marangell LB, Wisniewski SR, Ostacher M, DelBello MP, Bowden CL, Sachs GS, Nierenberg AA. STEP-BD Investigators. Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD) Biol Psychiatry. 2004;55:875–881. doi: 10.1016/j.biopsych.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Perlis RH, Dennehy EB, Miklowitz DJ, Delbello MP, Ostacher M, Calabrese JR, Ametrano RM, Wisniewski SR, Bowden CL, Thase ME, Nierenberg AA, Sachs G. Retrospective age at onset of bipolar disorder and outcome during two-year follow-up: results from the STEP-BD study. Bipolar Disord. 2009;11:391–400. doi: 10.1111/j.1399-5618.2009.00686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smoller JW, Finn CT. Family, twin, and adoption studies of bipolar disorder. Am J Med Genet C Semin Med Genet. 2003;123C:48–58. doi: 10.1002/ajmg.c.20013. [DOI] [PubMed] [Google Scholar]
- 4.DelBello MP, Geller B. Review of studies of child and adolescent offspring of bipolar parents. Bipolar Disord. 2001;3:325–334. doi: 10.1034/j.1399-5618.2001.30607.x. [DOI] [PubMed] [Google Scholar]
- 5.Goodwin FK, Jamison KR. Manic-Depressive Illness: Bipolar Disorders and Recurrent Depression. 2nd Edition. New York: Oxford University Press; 2007. [Google Scholar]
- 6.Mortensen PB, Pedersen CB, Melbye M, Mors O, Ewald H. Individual and familial risk factors for bipolar affective disorders in Denmark. Arch Gen Psychiatry. 2003;60:1209–1215. doi: 10.1001/archpsyc.60.12.1209. [DOI] [PubMed] [Google Scholar]
- 7.Egeland JA, Hostetter AM, Pauls DL, Sussex JN. Prodromal symptoms before onset of manic-depressive disorder suggested by first hospital admission histories. J Am Acad Child Adolesc Psychiatry. 2000;39:1245–1252. doi: 10.1097/00004583-200010000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Howes OD, Lim S, Theologos G, Yung AR, Goodwin GM, McGuire P. A comprehensive review and model of putative prodromal features of bipolar affective disorder. Psychol Med. 2011;41:1567–1577. doi: 10.1017/S0033291710001790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skjelstad DV, Malt UF, Holte A. Symptoms and signs of the initial prodrome of bipolar disorder: a systematic review. J Affect Disord. 2010;126:1–13. doi: 10.1016/j.jad.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Bechdolf A, Nelson B, Cotton SM, Chanen A, Thompson A, Kettle J, Conus P, Amminger GP, Yung AR, Berk M, McGorry PD. A preliminary evaluation of the validity of at-risk criteria for bipolar disorders in help-seeking adolescents and young adults. J Affect Disord. 2010;127:316–320. doi: 10.1016/j.jad.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 11.Bechdolf A, Ratheesh A, Cotton SM, Nelson B, Chanen AM, Betts J, Bingmann T, Yung AR, Berk M, McGorry PD. The predictive validity of bipolar at-risk (prodromal) criteria in help-seeking adolescents and young adults: a prospective study. Bipolar Disord. 2014;16:493–504. doi: 10.1111/bdi.12205. [DOI] [PubMed] [Google Scholar]
- 12.McGorry P, Keshavan M, Goldstone S, Amminger P, Allott K, Berk M, Lavoie S, Pantelis C, Yung A, Wood S, Hickie I. Biomarkers and clinical staging in psychiatry. World Psychiatry. 2014;13:211–223. doi: 10.1002/wps.20144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNamara RK, Nandagopal JJ, Strakowski SM, DelBello MP. Preventative strategies for early-onset bipolar disorder: Towards a clinical staging model. CNS Drugs. 2010;24:983–996. doi: 10.2165/11539700-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.McNamara RK, Vannest JJ, Valentine CJ. Role of perinatal long-chain omega-3 fatty acids in cortical circuit maturation: Mechanisms and implications for psychopathology. World J Psychiatr. 2015;5:15–34. doi: 10.5498/wjp.v5.i1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hibbeln JR. Fish consumption and major depression. Lancet. 1998;351:1213. doi: 10.1016/S0140-6736(05)79168-6. [DOI] [PubMed] [Google Scholar]
- 16.Noaghiul S, Hibbeln JR. Cross-national comparisons of seafood consumption and rates of bipolar disorders. Am J Psychiatry. 2003;160:2222–2227. doi: 10.1176/appi.ajp.160.12.2222. [DOI] [PubMed] [Google Scholar]
- 17.Peet M. International variations in the outcome of schizophrenia and the prevalence of depression in relation to national dietary practices: an ecological analysis. Br J Psychiatry. 2004;184:404–408. doi: 10.1192/bjp.184.5.404. [DOI] [PubMed] [Google Scholar]
- 18.Clayton EH, Hanstock TL, Watson JF. Estimated intakes of meat and fish by children and adolescents in Australia and comparison with recommendations. Br J Nutr. 2009;101:1731–1735. doi: 10.1017/S0007114508135887. [DOI] [PubMed] [Google Scholar]
- 19.Harel Z, Riggs S, Vaz R, White L, Menzies G. Omega-3 polyunsaturated fatty acids in adolescents: knowledge and consumption. J Adolesc Health. 2001;28:10–15. doi: 10.1016/s1054-139x(00)00179-8. [DOI] [PubMed] [Google Scholar]
- 20.Lauritzen L, Harsløf LB, Hellgren LI, Pedersen MH, Mølgaard C, Michaelsen KF. Fish intake, erythrocyte n-3 fatty acid status and metabolic health in Danish adolescent girls and boys. Br J Nutr. 2012;107:697–704. doi: 10.1017/S0007114511002418. [DOI] [PubMed] [Google Scholar]
- 21.Sichert-Hellert W, Wicher M, Kersting M. Age and time trends in fish consumption pattern of children and adolescents, and consequences for the intake of long-chain n-3 polyunsaturated fatty acids. Eur J Clin Nutr. 2009;63:1071–1075. doi: 10.1038/ejcn.2009.40. [DOI] [PubMed] [Google Scholar]
- 22.Allen KL, Mori TA, Beilin L, Byrne SM, Hickling S, Oddy WH. Dietary intake in population-based adolescents: support for a relationship between eating disorder symptoms, low fatty acid intake and depressive symptoms. J Hum Nutr Diet. 2013;26:459–469. doi: 10.1111/jhn.12024. [DOI] [PubMed] [Google Scholar]
- 23.Oddy WH, Hickling S, Smith MA, O'Sullivan TA, Robinson M, de Klerk NH, Beilin LJ, Mori TA, Syrette J, Zubrick SR, Silburn SR. Dietary intake of omega-3 fatty acids and risk of depressive symptoms in adolescents. Depress Anxiety. 2011;28:582–588. doi: 10.1002/da.20822. [DOI] [PubMed] [Google Scholar]
- 24.Swenne I, Rosling A, Tengblad S, Vessby B. Omega-3 polyunsaturated essential fatty acids are associated with depression in adolescents with eating disorders and weight loss. Acta Paediatr. 2011;100:1610–1615. doi: 10.1111/j.1651-2227.2011.02400.x. [DOI] [PubMed] [Google Scholar]
- 25.Grosso G, Pajak A, Marventano S, Castellano S, Galvano F, Bucolo C, Drago F, Caraci F. Role of omega-3 fatty acids in the treatment of depressive disorders: a comprehensive meta-analysis of randomized clinical trials. PLoS One. 2014;9:e96905. doi: 10.1371/journal.pone.0096905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarris J, Mischoulon D, Schweitzer I. Omega-3 for bipolar disorder: meta-analyses of use in mania and bipolar depression. J Clin Psychiatry. 2012;73:81–86. doi: 10.4088/JCP.10r06710. [DOI] [PubMed] [Google Scholar]
- 27.McNamara RK, Strimpfel J, Jandacek R, Rider T, Tso P, Welge JA, Strawn JR, DelBello MP. Detection and treatment of long-chain omega-3 fatty acid deficiency in adolescents with SSRI-resistant major depressive disorder. PharmaNutrition. 2014;2:38–46. doi: 10.1016/j.phanu.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nemets H, Nemets B, Apter A, Bracha Z, Belmaker RH. Omega-3 treatment of childhood depression: a controlled, double-blind pilot study. Am J Psychiatry. 2006;163:1098–1100. doi: 10.1176/ajp.2006.163.6.1098. [DOI] [PubMed] [Google Scholar]
- 29.Clayton EH, Hanstock TL, Hirneth SJ, Kable CJ, Garg ML, Hazell PL. Reduced mania and depression in juvenile bipolar disorder associated with long-chain omega-3 polyunsaturated fatty acid supplementation. Eur J Clin Nutr. 2009;63:1037–1040. doi: 10.1038/ejcn.2008.81. [DOI] [PubMed] [Google Scholar]
- 30.Wozniak J, Biederman J, Mick E, Waxmonsky J, Hantsoo L, Best C, Cluette-Brown JE, Laposata M. Omega-3 fatty acid monotherapy for pediatric bipolar disorder: a prospective open-label trial. Eur Neuropsychopharmacol. 2007;17:440–447. doi: 10.1016/j.euroneuro.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Flock MR, Skulas-Ray AC, Harris WS, Etherton TD, Fleming JA, Kris-Etherton PM. Determinants of erythrocyte omega-3 fatty acid content in response to fish oil supplementation: a dose-response randomized controlled trial. J Am Heart Assoc. 2013;2:e000513. doi: 10.1161/JAHA.113.000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris WS, von Schacky C, Park Y. Standardizing methods for assessing omega-3 fatty acid biostatus. In: McNamara RK, editor. The Omega-3 Fatty Acid Deficiency Syndrome: Opportunities for Disease Prevention. U.S.A.: Nova Science Publishers, Inc.; 2013. pp. 385–398. [Google Scholar]
- 33.Sands SA, Reid KJ, Windsor SL, Harris WS. The impact of age, body mass index, and fish intake on the EPA and DHA content of human erythrocytes. Lipids. 2005;40:343–347. doi: 10.1007/s11745-006-1392-2. [DOI] [PubMed] [Google Scholar]
- 34.Harris WS, Von Schacky C. The Omeg-3 Index: a new risk factor for death from coronary heart disease? Prev Med. 2004;39:212–220. doi: 10.1016/j.ypmed.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 35.Angst F, Stassen HH, Clayton PJ, Angst J. Mortality of patients with mood disorders: follow-up over 34–38 years. J Affect Disord. 2002;68:167–181. doi: 10.1016/s0165-0327(01)00377-9. [DOI] [PubMed] [Google Scholar]
- 36.Osby U, Brandt L, Correia N, Ekbom A, Sparén P. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry. 2001;58:844–850. doi: 10.1001/archpsyc.58.9.844. [DOI] [PubMed] [Google Scholar]
- 37.Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry. 2010;68:140–147. doi: 10.1016/j.biopsych.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 38.Pottala JV, Talley JA, Churchill SW, Lynch DA, von Schacky C, Harris WS. Red blood cell fatty acids are associated with depression in a case-control study of adolescents. Prostaglandins Leukot Essent Fatty Acids. 2012;86:161–165. doi: 10.1016/j.plefa.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Chiu CC, Huang SY, Su KP, Lu ML, Huang MC, Chen CC, Shen WW. Polyunsaturated fatty acid deficit in patients with bipolar mania. Eur Neuropsychopharmacol. 2003;13:99–103. doi: 10.1016/s0924-977x(02)00130-x. [DOI] [PubMed] [Google Scholar]
- 40.Clayton EH, Hanstock TL, Hirneth SJ, Kable CJ, Garg ML, Hazell PL. Long-chain omega-3 polyunsaturated fatty acids in the blood of children and adolescents with juvenile bipolar disorder. Lipids. 2008;43:1031–1038. doi: 10.1007/s11745-008-3224-z. [DOI] [PubMed] [Google Scholar]
- 41.McNamara RK, Jandacek R, Rider T, Tso P, Dwivedi Y, Pandey GN. Selective deficits in erythrocyte docosahexaenoic acid composition in adult patients with bipolar disorder and major depressive disorder. J Affect Disord. 2010;126:303–311. doi: 10.1016/j.jad.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ranjekar PK, Hinge A, Hegde MV, Ghate M, Kale A, Sitasawad S, Wagh UV, Debsikdar VB, Mahadik SP. Decreased antioxidant enzymes and membrane essential polyunsaturated fatty acids in schizophrenic and bipolar mood disorder patients. Psychiatry Res. 2003;121:109–122. doi: 10.1016/s0165-1781(03)00220-8. [DOI] [PubMed] [Google Scholar]
- 43.McNamara RK, Jandacek RJ, Tso P, Blom TJ, Welge JA, Strawn JR, Adler CM, DelBello MP, Strakowski SM. First-episode bipolar disorder is associated with erythrocyte membrane docosahexaenoic acid deficits: Dissociation from clinical response to lithium or quetiapine. Psychiatry Res. 2015 doi: 10.1016/j.psychres.2015.09.035. [in press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geller B, Zimerman B, Williams M, Bolhofner K, Craney JL, DelBello MP, Soutullo C. Reliability of the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) mania and rapid cycling sections. J Am Acad Child Adolesc Psychiatry. 2001;40:450–455. doi: 10.1097/00004583-200104000-00014. [DOI] [PubMed] [Google Scholar]
- 45.First MB, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research Department. 722 West 168th Street, New York, NY 10032: New York State Psychiatric institute; 1995. Structured Clinical Interview for DSM-IV Axis I Disorders- Patient Edition (SCID-I/P) [Google Scholar]
- 46.Maxwell E. Family Interview for Genetics Studies. Washington,DC: National Institutes of Mental Health; 1999. Feb, [Google Scholar]
- 47.Wechsler D. Wechsler Adult Intelligence Scale-third edition: administration and scoring manual. San Antonio, TX: Psychological Corportation; 1997. [Google Scholar]
- 48.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 49.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;25:56–61. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duke P, Litt I, Gross R. Adolescents' self-assessment of sexual maturation. Pediatrics. 1980;66:918–920. [PubMed] [Google Scholar]
- 51.Hoen WP, Lijmer JG, Duran M, Wanders RJ, van Beveren NJ, de Haan L. Red blood cell polyunsaturated fatty acids measured in red blood cells and schizophrenia: A meta-analysis. Psychiatry Res. 2013;207:1–12. doi: 10.1016/j.psychres.2012.09.041. [DOI] [PubMed] [Google Scholar]
- 52.Harsløf LB, Larsen LH, Ritz C, Hellgren LI, Michaelsen KF, Vogel U, Lauritzen L. FADS genotype and diet are important determinants of DHA status: a cross-sectional study in Danish infants. Am J Clin Nutr. 2013;97:1403–1410. doi: 10.3945/ajcn.113.058685. [DOI] [PubMed] [Google Scholar]
- 53.Koletzko B, Lattka E, Zeilinger S, Illig T, Steer C. Genetic variants of the fatty acid desaturase gene cluster predict amounts of red blood cell docosahexaenoic and other polyunsaturated fatty acids in pregnant women: findings from the Avon Longitudinal Study of Parents and Children. Am J Clin Nutr. 2011;93:211–219. doi: 10.3945/ajcn.110.006189. [DOI] [PubMed] [Google Scholar]
- 54.Steer CD, Hibbeln JR, Golding J, Davey Smith G. Polyunsaturated fatty acid levels in blood during pregnancy, at birth and at 7 years: their associations with two common FADS2 polymorphisms. Hum Mol Genet. 2012;21:1504–1512. doi: 10.1093/hmg/ddr588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seifuddin F, Mahon PB, Judy J, Pirooznia M, Jancic D, Taylor J, Goes FS, Potash JB, Zandi PP. Meta-analysis of genetic association studies on bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:508–518. doi: 10.1002/ajmg.b.32057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Howard TD, Mathias RA, Seeds MC, Herrington DM, Hixson JE, Shimmin LC, Hawkins GA, Sellers M, Ainsworth HC, Sergeant S, Miller LR, Chilton FH. DNA methylation in an enhancer region of the FADS cluster is associated with FADS activity in human liver. PLoS One. 2014;9:e97510. doi: 10.1371/journal.pone.0097510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brenna JT, Salem N, Jr, Sinclair AJ, Cunnane SC. International Society for the Study of Fatty Acids and Lipids, ISSFAL. alpha-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot Essent Fatty Acids. 2009;80:85–91. doi: 10.1016/j.plefa.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 58.Wanders RJA. Peroxisomal biosynthesis of omega-3 fatty acids and human peroxisomal diseases. In: McNamara RK, editor. The Omega-3 Fatty Acid Deficiency Syndrome: Opportunities for Disease Prevention. U.S.A.: Nova Science Publishers, Inc.; 2013. pp. 19–30. [Google Scholar]
- 59.Steinberg S, Chen L, Wei L, Moser A, Moser H, Cutting G, Braverman N. The PEX Gene Screen: molecular diagnosis of peroxisome biogenesis disorders in the Zellweger syndrome spectrum. Mol Genet Metab. 2004;83:252–263. doi: 10.1016/j.ymgme.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 60.Moser AB, Jones DS, Raymond GV, Moser HW. Plasma and red blood cell fatty acids in peroxisomal disorders. Neurochem Res. 1999;24:187–197. doi: 10.1023/a:1022549618333. [DOI] [PubMed] [Google Scholar]
- 61.McNamara RK, Strawn JR, Stahl L, Jandacek R, Tso P, Rider T, DelBello MP. Long-chain omega-3 fatty acid deficits in youth with or at high or ultra-high risk for bipolar disorder are not due to impaired peroxisomal function. Biol Psychiatry. 2014;75:S319. [Google Scholar]
- 62.Davison KM, Kaplan BJ. Food intake and blood cholesterol levels of community-based adults with mood disorders. BMC Psychiatry. 2012;12:10. doi: 10.1186/1471-244X-12-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jacka FN, Pasco JA, Mykletun A, Williams LJ, Nicholson GC, Kotowicz MA, Berk M. Diet quality in bipolar disorder in a population based sample of women. J Affect Disord. 2011;129:332–337. doi: 10.1016/j.jad.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 64.Kilbourne AM, Rofey DL, McCarthy JF, Post EP, Welsh D, Blow FC. Nutrition and exercise behavior among patients with bipolar disorder. Bipolar Disord. 2007;9:443–452. doi: 10.1111/j.1399-5618.2007.00386.x. [DOI] [PubMed] [Google Scholar]
- 65.Evans SJ, Ringrose RN, Harrington GJ, Mancuso P, Burant CF, McInnis MG. Dietary intake and plasma metabolomic analysis of polyunsaturated fatty acids in bipolar subjects reveal dysregulation of linoleic acid metabolism. J Psychiatr Res. 2014;57:58–64. doi: 10.1016/j.jpsychires.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Block RC, Harris WS, Reid KJ, Sands SA, Spertus JA. EPA and DHA in blood cell membranes from acute coronary syndrome patients and controls. Atherosclerosis. 2008;197:821–828. doi: 10.1016/j.atherosclerosis.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 67.Carver JD, Benford VJ, Han B, Cantor AB. The relationship between age and the fatty acid composition of cerebral cortex and erythrocytes in human subjects. Brain Res Bull. 2001;56:79–85. doi: 10.1016/s0361-9230(01)00551-2. [DOI] [PubMed] [Google Scholar]
- 68.Connor WE, Neuringer M, Lin DS. Dietary effects on brain fatty acid composition: the reversibility of n-3 fatty acid deficiency and turnover of docosahexaenoic acid in the brain, erythrocytes, and plasma of rhesus monkeys. J Lipid Res. 1990;31:237–247. [PubMed] [Google Scholar]
- 69.McNamara RK, Hahn CG, Jandacek R, Rider T, Tso P, Stanford K, Richtand NM. Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol Psychiatry. 2007;62:17–24. doi: 10.1016/j.biopsych.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 70.McNamara RK, Jandacek R, Rider T, Tso P, Stanford K, Hahn C-G, Richtand NM. Deficits in docosahexaenoic acid and associated elevations in the metabolism of arachidonic acid and saturated fatty acids in the postmortem orbitofrontal cortex of patients with bipolar disorder. Psychiatric Res. 2008;160:285–299. doi: 10.1016/j.psychres.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Giedd JN, Lalonde FM, Celano MJ, White SL, Wallace GL, Lee NR, Lenroot RK. Anatomical brain magnetic resonance imaging of typically developing children and adolescents. J Am Acad Child Adolesc Psychiatry. 2009;48:465–470. doi: 10.1097/CHI.0b013e31819f2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- 73.Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. Neuroimage. 1999;9:587–597. doi: 10.1006/nimg.1999.0436. [DOI] [PubMed] [Google Scholar]
- 74.Conklin SM, Gianaros PJ, Brown SM, Yao JK, Hariri AR, Manuck SB, Muldoon MF. Long-chain omega-3 fatty acid intake is associated positively with corticolimbic gray matter volume in healthy adults. Neurosci Lett. 2007;421:209–212. doi: 10.1016/j.neulet.2007.04.086. [DOI] [PubMed] [Google Scholar]
- 75.McNamara RK, Able JA, Jandacek R, Rider T, Tso P, Eliassen J, Alfieri D, Weber W, Jarvis K, DelBello MP, Strakowski SM, Adler CM. Docosahexaenoic acid supplementation increases prefrontal cortex activation during sustained attention in healthy boys: A placebo-controlled, dose-ranging, functional magnetic resonance imaging study. Am J Clin Nutr. 2010;91:1060–1067. doi: 10.3945/ajcn.2009.28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sublette ME, Milak MS, Hibbeln JR, Freed PJ, Oquendo MA, Malone KM, Parsey RV, Mann JJ. Plasma polyunsaturated fatty acids and regional cerebral glucose metabolism in major depression. Prostaglandins Leukot Essent Fatty Acids. 2009;80:57–64. doi: 10.1016/j.plefa.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grayson DS, Kroenke CD, Neuringer M, Fair DA. Dietary omega-3 fatty acids modulate large-scale systems organization in the rhesus macaque brain. J Neurosci. 2014;34:2065–2074. doi: 10.1523/JNEUROSCI.3038-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Amminger GP, Schäfer MR, Papageorgiou K, Klier CM, Cotton SM, Harrigan SM, Mackinnon A, McGorry PD, Berger GE. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010;67:146–154. doi: 10.1001/archgenpsychiatry.2009.192. [DOI] [PubMed] [Google Scholar]