Abstract
The recent approval of two PD-1 inhibitors for the treatment of non–small cell lung cancer (NSCLC) has rapidly led to the widespread use of these agents in oncology practices. Pneumonitis has been recognized as a potentially life-threatening adverse event among NSCLC patients treated with PD-1 inhibitors; however, the detailed clinical and radiographic manifestations of this entity remain to be described. We report two cases of anti–PD-1 pneumonitis in advanced NSCLC patients treated with nivolumab after its FDA approval. Both cases presented with ground-glass and reticular opacities and consolidations in a peripheral distribution on CT, demonstrating a radiographic pattern of cryptogenic organizing pneumonia (COP). Consolidations were extensive and rapidly developed within 8 weeks of therapy in both cases. Both patients were treated with corticosteroids with subsequent improvement of respiratory symptoms and radiographic findings. One patient experienced recurrent pneumonitis after completing corticosteroid taper, or a “pneumonitis flare”, in the absence of nivolumab retreatment, with subsequent improvement upon corticosteroid re-administration. With the increasing use of immune checkpoint inhibitors in a growing number of tumor types, awareness of the radiographic and clinical manifestations of PD-1 inhibitor–related pneumonitis will be critical for the prompt diagnosis and management of this potentially serious adverse event.
Keywords: pneumonitis, PD-1 inhibitor, immunotherapy, lung cancer, computed tomography
INTRODUCTION
Immune checkpoint blockade with PD-1 inhibitors has revolutionized the treatment of an increasing number of tumor types, including melanoma and non–small cell lung cancer (NSCLC).(1–7) Nivolumab has demonstrated a survival benefit over docetaxel in both squamous (8) and nonsquamous (9) NSCLC, and was granted FDA approval for squamous NSCLC in March, 2015, and for nonsquamous NSCLC in October, 2015. Another PD-1 inhibitor, pembrolizumab, has also shown marked antitumor activity in previously-treated NSCLC (10), and was granted accelerated FDA approval for PD-L1+ NSCLCs in October, 2015.
With more widespread prescribing of PD-1 inhibitors, prompt recognition of serious toxicities is necessary for the safe use of these agents. Among immune-related adverse events (irAEs) noted during trials of PD-1 inhibitors, pneumonitis has been recognized as an “event of special interest,” occurring at a rate of 3% (9/296) and resulting in three treatment-related deaths (two patients with NSCLC and one patient with colorectal cancer) in a phase 1 trial of nivolumab (5). The long-term safety in the NSCLC cohort from this phase 1 trial was updated and pneumonitis was reported in 7% (9/129), with three pneumonitis-associated deaths (1). In a phase 2 trial of nivolumab in squamous NSCLC, pneumonitis was one of the most common irAEs, occurring in 5% of patients (6/117), including four patients with grade 3 pneumonitis (3).
In response to the increasing awareness of pneumonitis as a serious irAE, our group has described clinical and radiographic features of anti–PD-1 pneumonitis in melanoma patients treated in trials of nivolumab (11). However, this entity has not been previously reported specifically in the NSCLC population. Given the large number of advanced lung patients diagnosed in the U.S. every year who could potentially be treated with immune checkpoint blockade, and the fact that many symptoms of PD-1 inhibitor-related pneumonitis overlap with common symptoms of lung cancer patients, clinical and radiographic descriptions of this potentially life-threatening, but treatable, entity are urgently needed.
We report two cases of anti–PD-1 pneumonitis in advanced NSCLC patients treated with nivolumab after its FDA approval. Improving our understanding of PD-1 inhibitor-related pneumonitis will enable radiologists and oncologists to accurately recognize this entity and promptly provide appropriate treatment.
MATERIALS AND METHODS
Among the advanced NSCLC patients treated with nivolumab after its FDA approval as a part of clinical care at our institution, two cases of anti–PD-1-related pneumonitis were identified based on the review of the medical records. The imaging studies of these patients were retrospectively reviewed with an institutional review board–approved clinical research protocol. Chest computed tomography (CT) scans at baseline, during therapy, and at follow-up were reviewed by a consensus of three radiologists with expertise in thoracic and oncologic imaging (M.N., N.H.R., H.H.) for findings of pneumonitis, as described (11, 12). CT findings of pneumonitis were assessed for 1) extent in upper, middle, and lower lungs (none, < 5%, 5–25%,25–50%, > 50%), 2) distributions in terms of (a) peripheral, diffuse, central or mixed; and (b) upper, lower, diffuse, multifocal or focal, 3) lobar involvement, and 4) specific CT findings including traction bronchiectasis, consolidation, reticular opacities, ground glass opacities (GGO), centrilobular nodularity, honeycombing. In each case, radiographic patterns of pneumonitis were classified according to ATS/ERS international multidisciplinary classification of interstitial pneumonias and the related conditions, as 1) usual interstitial pneumonia (UIP) pattern, 2) non-specific interstitial pneumonia (NSIP) pattern, 3) cryptogenic organizing pneumonia (COP) pattern, 4) acute interstitial pneumonia (AIP)/acute respiratory distress syndrome (ARDS) pattern, 5) hypersensitivity pneumonitis (HP) pattern, and 6) not applicable, as described (11–13). Clinical presentation and treatment course of pneumonitis were obtained from the medical record review.
CASE REPORTS
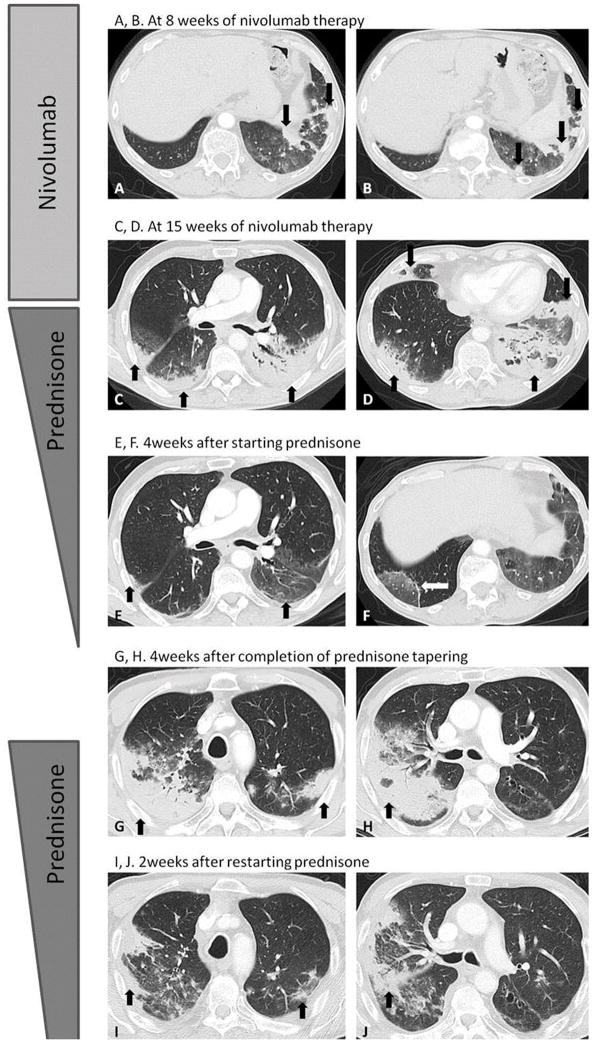
Case 1 is a 72-year-old man with stage IV squamous NSCLC who had disease progression after four cycles of carboplatin and paclitaxel. The patient was then treated with second-line nivolumab monotherapy at a standard dose of 3 mg/kg every two weeks. After four doses of nivolumab, the patient reported only mild fatigue but no respiratory symptoms. Chest CT scan at 8 weeks of therapy demonstrated new ground glass opacities (GGOs), reticular opacities, and consolidation in lower lobes, with a peripheral and lower distribution, representing a cryptogenic organizing pneumonia (COP) pattern based on the radiographic appearance (Fig. 1A and B). On the same CT, the dominant right upper lobe tumor had markedly decreased in size compared to baseline, indicating response to therapy.
Fig. 1. Chest CT images for Case 1.
A, B. Chest CT at 8 weeks of nivolumab therapy demonstrated new GGO, reticular opacities, and consolidation in lower lobes predominantly on the left, with a peripheral and lower distribution, radiographically representing a COP pattern (arrows). C–D. On chest CT at 15 weeks of therapy, the findings significantly increased and involved all lobes, with multifocal areas of GGO, reticular opacities, and consolidation (arrows), as well as centrilobular nodularity and traction bronchiectasis in predominantly peripheral distribution. The overall features demonstrated a COP pattern, while the progressive nature was also indicative of developing ARDS.
E–F. Further follow-up CT after 4 weeks of prednisone treatment showed a significant decrease of the CT findings with residual GGOs, demonstrating a “reversed halo” sign with central GGO surrounded by dense air-space consolidation of crescentic shape (F, arrows), which has been reported as a radiographic manifestation of COP.
G–H. Chest CT scan 4 weeks after the completion of prednisone treatment showed a development of dense consolidations with GGOs and reticular opacities (arrows) in peripheral and multifocal distributions, involving both upper and lower lobes, again demonstrating COP pattern as noted during the first episode of PD-1 pneumonitis. Given the similarity of radiographic and clinical manifestations with the 1st episode, the patient restarted prednisone for treatment of a “pneumonitis flare”.
I–J. Follow-up chest CT taken 2 weeks after starting the 2nd course of prednisone therapy demonstrated decrease of consolidation and GGOs (arrows), indicating improving pneumonitis in response to corticosteroid therapy.
Over the subsequent weeks while on continued nivolumab therapy, the patient developed progressive dyspnea with cough and wheezing, but no fever. A follow-up chest CT at 15 weeks of therapy showed a significant increase in the radiographic findings which involved all lung lobes (Fig. 1C and D). The distribution of the findings was peripheral and multifocal, representing a COP pattern, and the progressive nature was also indicative of acute respiratory distress syndrome (ARDS). Nivolumab was held, and the patient was treated with prednisone 60 mg by mouth daily as an outpatient, with rapid clinical improvement. He was also placed on trimethoprim-sulfamethoxazole for pneumocystis prophylaxis. Four weeks later, while on prednisone taper, a repeat scan of the chest showed a marked decrease of consolidations (Fig. 1E and F), and residual GGOs demonstrated a “reversed halo” sign, with central GGO surrounded by dense consolidation of crescentic shape (Fig. 1F) (14). The patient had been on a corticosteroid taper for a total of 8 weeks before prednisone was discontinued altogether, with near-complete resolution of his pneumonitis-related symptoms at that time.
However, within 4 weeks after discontinuation of prednisone, without restarting nivolumab or initiation of any other systemic cancer therapy, the patient again developed respiratory symptoms including dyspnea, cough, and wheezing, but no fever. Chest CT scan demonstrated bilateral dense consolidations with GGOs and reticular opacities in peripheral and multifocal distributions, again representing a COP pattern (Fig. 1G and H) as noted at the initial onset of PD-1 pneumonitis. Given the similarities in clinical and radiographic manifestations with the first episode, the findings were most indicative of recurrent pneumonitis after discontinuation of prednisone, or a “pneumonitis flare” in the absence of nivolumab retreatment. The patient restarted prednisone at a dose of 60 mg daily with levofloxacin at a dose of 750 mg by mouth daily, again with improvement in cough, wheezing, and dyspnea. A follow-up CT at 2 weeks on the second course of prednisone treatment showed resolving consolidations and GGOs (Fig. 1I and J).
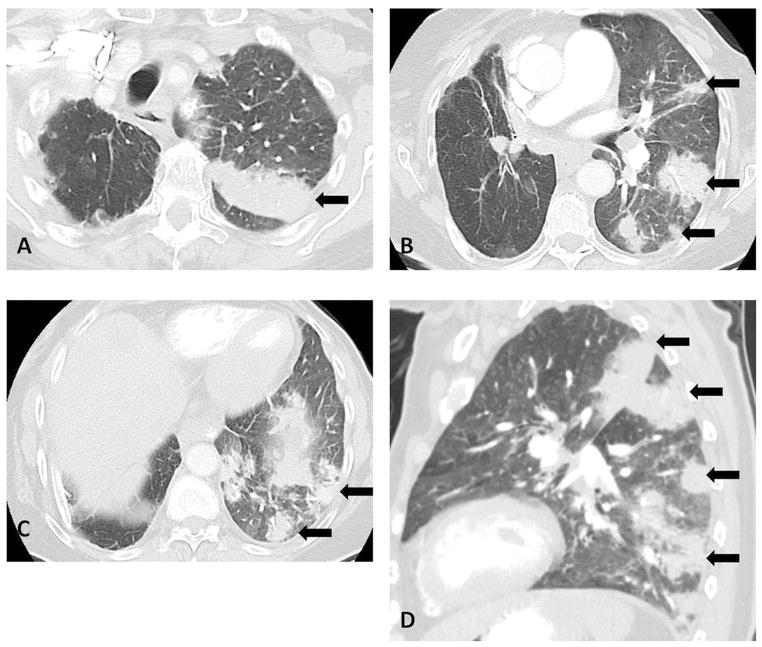
Case 2 is an 83-year-old woman who originally had a stage IIA NSCLC with neuroendocrine features. She underwent right upper lobectomy and had declined adjuvant chemotherapy. She was subsequently found to have liver metastases and was felt to be a poor candidate for cytotoxic chemotherapy. She was treated with nivolumab and after four weeks of therapy, the patient was admitted to the hospital with an increasing dry cough, dyspnea, and hypoxemia. A CT scan on admission demonstrated new multifocal areas of GGO, reticular opacities, and consolidation in the left lung with a peripheral distribution (Fig. 2A–D), demonstrating a COP pattern based on the radiographic appearance. The liver metastasis decreased in size, in response to nivolumab. She was treated with a prednisone taper, starting at a dose of 60 mg daily, along with sulfamethoxazole-trimethoprim. A chest CT scan taken two weeks later showed marked decrease of the multifocal lung opacities. The patient has completed a prednisone taper over the past several weeks. Due to the severity of this patient’s pneumonitis, nivolumab was not restarted and she was started on another systemic therapy.
Fig. 2. Chest CT images for Case 2.
A–D. Axial (A–C) and sagittal (D) images of chest CT scan at 4 weeks of therapy demonstrated multifocal areas of GGO, reticular opacities, and extensive consolidation in the left lung with a peripheral distribution, demonstrating a COP pattern (arrows).
DISCUSSION
The present report provides the first detailed description of the clinical and radiographic characteristics of PD-1 inhibitor–related pneumonitis in NSCLC patients treated with nivolumab, which was administered as part of standard clinical care and not in the trial setting. The onset of pneumonitis in both cases was rather early, developing within 8 weeks after treatment initiation. Both patients were symptomatic with cough and shortness of breath, and one patient experienced hypoxemia. Imaging findings were similar in the two cases, with GGOs and consolidations in a peripheral distribution, which is radiographically indicative of a COP pattern based on the radiographic appearance on CT. Both cases were successfully treated with oral corticosteroids, with marked improvement of clinical symptoms and radiographic findings; however, one patient experienced a “pneumonitis flare” after completing a prednisone taper, which was responsive to reinitiation of corticosteroids.
Multifocal consolidations were the major radiographic findings of pneumonitis in the two cases; the consolidations on CT were extensive and developed rapidly within two months, which may be a notable feature of the entity. Because of this feature, CT scans of both cases at the onset of pneumonitis were initially interpreted as “infectious pneumonia versus tumor progression”. While these differential diagnoses are reasonable, treatment-related pneumonitis should also be strongly considered in patients receiving PD-1 inhibitors presenting with new pulmonary findings on imaging (11). The respiratory symptoms of pneumonitis, including cough, shortness of breath, and hypoxemia, overlap with the symptoms of advanced lung cancer itself, providing additional challenges for early identification of anti–PD-1 pneumonitis specifically among NSCLC patients. The increased awareness of the entity among cancer care providers, and the multidisciplinary approach including oncologists, radiologists, and pulmonologists are necessary for prompt diagnosis and management of this potentially serious adverse event.
There has been one previously published report of the radiographic appearance of anti–PD-1 pneumonitis, which included 3 melanoma patients (11). The report included a spectrum of radiographic and clinical manifestations, with 2 patients demonstrating an AIP/ARDS pattern requiring admission to intensive care units, and one patient with a NSIP pattern who was treated with oral corticosteroids as an outpatient and was able to restart nivolumab therapy. While similarities and differences of the radiographic and clinical features of anti–PD-1 pneumonitis across different tumor types need to be further studied in larger cohorts, the present report describes a COP pattern consistently noted in both patients, indicating it may be one of the leading radiographic patterns of this entity among NSCLC population, which was not described in the previous report of melanoma patients.
Both patients were successfully treated by oral prednisone taper with clinical and radiographic improvement. However, the patient described in Case 1 experienced recurrent pneumonitis after discontinuation of prednisone, with a very similar radiographic COP pattern as seen in the first episode, indicating a “pneumonitis flare” after a completing steroid taper. Notably, a pneumonitis flare occurred without restarting nivolumab, which may be a unique feature of immune-related pneumonitis. Although the detailed mechanisms of this unusual phenomenon remain to be investigated, it may be at least in part related to the durable nature of the effect of immune-checkpoint inhibitors which have been shown to be associated with durable and persisted response after drug discontinuation.(6) The observation, although limited to one case in our initial experience, raises an important question as to when to discontinue corticosteroids in those who have experienced PD-1 pneumonitis. It may also indicate the needs for additional caution when discussing nivolumab retreatment in these patients.
Prolonged use of corticosteroids raises a concern for a possible negative impact on the antitumor efficacy of immune checkpoint inhibitors. In a recent retrospective review of 298 melanoma patients treated with ipilimumab, 254 (85%) experienced an irAE of any grade, and 103 patients (35%) required systemic corticosteroids to treat an irAE; however, overall survival and time to treatment failure were not affected by the occurrence of irAEs or by the need for systemic corticosteroids (15). Additional studies will be needed to determine if these findings will also extend to patients requiring corticosteroids for the treatment of irAEs after PD-1 inhibitor administration.
Neither patient underwent bronchoscopy or biopsy for pneumonitis. The precise role of bronchoscopy and bronchoalveolar lavage (BAL) in the management of PD-1 pneumonitis is currently unclear, but BAL may help identify an underlying pulmonary infection. Without biopsy, both patients in the present series lack histologic assessments of pneumonitis, which is often the case with patients who have known advanced malignancy receiving systemic therapy (12, 16). A COP pattern of pneumonitis in the present cases was characterized by the radiographic appearance of the lung abnormalities of pneumonitis, based on the assessment of extent, distribution, and specific CT findings, as described previously (11, 12).
In conclusion, in our initial experience with anti–PD-1-related pneumonitis in NSCLC, both cases demonstrated a radiographic COP pattern with extensive consolidation in peripheral distribution. One patient experienced a pneumonitis flare after completing a prednisone taper, which responsive to a second course of corticosteroids. Further investigations in larger cohorts are needed to characterize the full spectrum of this entity. Given the rapidly growing indications for immune checkpoint inhibitors in a variety of tumor types, knowledge of the clinical and radiographic manifestations of drug-related pneumonitis is essential to maximizing the therapeutic benefit of these agents.
Acknowledgments
The investigator, M.N., was supported by 1K23CA157631 (NCI).
Footnotes
Conflict of Interest:
Nishino: Consultant, Bristol-Myers Squibb
Hodi: Consultant to Merck, Genentech, and Novartis.
Awad: Consultant to Merck, Genentech, and AstraZeneca
References
- 1.Gettinger SN, Horn L, Gandhi L, et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33(18):2004–12. doi: 10.1200/JCO.2014.58.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. The Lancet. Oncology. 2015;16(8):908–18. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. The Lancet. Oncology. 2015;16(3):257–65. doi: 10.1016/s1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384(9948):1109–17. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 5.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine. 2012;366(26):2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(10):1020–30. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishino M, Tirumani SH, Ramaiya NH, Hodi FS. Cancer immunotherapy and immune-related response assessment: The role of radiologists in the new arena of cancer treatment. European journal of radiology. 2015;84(7):1259–68. doi: 10.1016/j.ejrad.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. The New England journal of medicine. 2015;373(2):123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. The New England journal of medicine. 2015;373(17):1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. The New England journal of medicine. 2015;372(21):2018–28. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 11.Nishino M, Sholl LM, Hodi FS, Hatabu H, Ramaiya NH. Anti-PD-1-Related Pneumonitis during Cancer Immunotherapy. The New England journal of medicine. 2015;373(3):288–90. doi: 10.1056/NEJMc1505197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishino M, Boswell EN, Hatabu H, Ghobrial IM, Ramaiya NH. Drug-Related Pneumonitis During Mammalian Target of Rapamycin Inhibitor Therapy: Radiographic Pattern-Based Approach in Waldenstrom Macroglobulinemia as a Paradigm. Oncologist. 2015;20(9):1077–83. doi: 10.1634/theoncologist.2015-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. American journal of respiratory and critical care medicine. 2013;188(6):733–48. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim SJ, Lee KS, Ryu YH, et al. Reversed halo sign on high-resolution CT of cryptogenic organizing pneumonia: diagnostic implications. AJR. American journal of roentgenology. 2003;180(5):1251–4. doi: 10.2214/ajr.180.5.1801251. [DOI] [PubMed] [Google Scholar]
- 15.Horvat TZ, Adel NG, Dang TO, et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33(28):3193–8. doi: 10.1200/jco.2015.60.8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishino M, Cardarella S, Dahlberg SE, et al. Interstitial lung abnormalities in treatment-naive advanced non-small-cell lung cancer patients are associated with shorter survival. European journal of radiology. 2015;84(5):998–1004. doi: 10.1016/j.ejrad.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]