Abstract
Purpose
This trial was conducted to determine the maximum tolerated dose (MTD) and preliminary efficacy of buparlisib, an oral pan-class I PI3K inhibitor, plus fulvestrant in postmenopausal women with metastatic estrogen receptor positive breast cancer (ER+BC).
Experimental Design
Phase IA employed a 3+3 design to determine the MTD of buparlisib daily plus fulvestrant. Subsequent cohorts evaluated intermittent (5 of 7 day dosing) and continuous buparlisib (100mg daily). No more than 3 prior systemic treatments in the metastatic setting were allowed in Phase IB and Cohort C.
Results
Thirty one patients were enrolled. MTD was defined as buparlisib 100mg daily plus fulvestrant. Common adverse events (AEs) included fatigue (38.7 %), transaminases elevation (35.5 %), rash (29%), and diarrhea (19.4%). C-peptide was significantly increased during treatment, consistent with on-target effect of buparlisib. Compared to intermittent dosing, daily buparlisib was associated with more frequent early onset AEs and higher buparlisib plasma concentrations. Among the 29 evaluable patients, the clinical benefit rate was 58.6% (95% CI 40.7–74.5%). Response was not associated with PIK3CA mutation or treatment cohort, however loss of PTEN, progesterone receptor (PgR) expression, or mutation in TP53 was commoner in resistant cases and mutations in AKT1 and ESR1 did not exclude treatment response.
Conclusion
Buparlisib plus fulvestrant is clinically active with manageable AEs in patients with metastatic ER+BC. Weekend breaks in buparlisib dosing reduced toxicity. Patients with PgR negative and TP53 mutation did poorly, suggesting buparlisib plus fulvestrant may not be adequately effective against tumors with these poor prognostic molecular features.
Keywords: BKM120 (Buparlisib), fulvestrant, estrogen receptor positive breast cancer, Phase I study, phosphatidylinositol-3-kinase (PI3K)
Introduction
The class I phosphatidylinositol 3-kinase (PI3K) pathway plays a key role in mediating cell growth, proliferation, survival, migration, and angiogenesis (1). Genetic alterations in components of the PI3K pathway, including mutations in PIK3CA (25 – 40%) (2–4), PIK3R1 (0.4% – 2%), AKT1 (2–4%), and PTEN (4%), are frequently observed in estrogen receptor positive (ER+) breast cancer (4). In preclinical studies up-regulation of PI3K pathway signaling promotes estrogen independent tumor growth, and inhibition of PI3K, either by RNAi or pharmaceutical approaches, induces tumor cell apoptosis particularly when combined with simultaneous ER targeting (5–8).
Buparlisib is an oral selective pan-class I PI3K inhibitor (9) that inhibits all four PI3K isoforms (p110α, -β, -δ and -γ) as well as somatically mutated p110α (PIK3CA) (9). In preclinical studies, buparlisib plus fulvestrant, an ER down regulator, produced synergistic anti-tumor effects in ER+ breast cancer (6, 10). We therefore conducted a phase I study of buparlisib in combination with fulvestrant in postmenopausal women with metastatic ER+ breast cancer (NCT01339442).
The primary objectives of the study were to determine the maximum tolerated dose (MTD) of buparlisib plus fulvestrant and to evaluate the toxicity profile of this combination. Secondary objectives included: 1) determination of the steady state blood levels of buparlisib and C-peptide, and 2) evaluation of the anti-tumor effect in metastatic ER+ breast cancer. Exploratory objectives included examining archival tumor specimens for mutations in PIK3CA and other genes recurrently mutated in ER+ breast cancer, and expressions of PTEN and progesterone receptor (PgR) by immunohistochemistry (IHC), and to correlate with response.
Patient Population and Methods
Eligibility
Eligible patients included postmenopausal women with metastatic ER+ breast cancer (≥ 1% tumor cell staining or an Allred Score of ≥ 3) with measurable disease per RECIST 1.1. Any number of prior therapies was acceptable in Phase IA. No more than 3 prior lines of systemic therapy were allowed in Phase IB and Cohort C in order to focus on a population with less treatment-related resistant mechanisms. Prior fulvestrant without immediate disease progression was allowed, in addition to central nervous system metastasis if at least 4 weeks from completion of radiation and/or surgery, stable and not receiving corticosteroid. Additional eligibility criteria included: Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) 0–2, fasting glucose ≤120 mg/dL, and adequate organ function. Exclusion criteria included prior PI3K inhibitor, untreated brain metastasis, pancreatitis, history of or active cardiac disease, major depressive episode, bipolar disorder (I or II), obsessive-compulsive disorder, schizophrenia, suicidal or homicidal attempt or ideation, greater than grade 2 anxiety, known HIV positivity, and uncontrolled intercurrent illnesses, greater than grade 1 diarrhea, consumption of fruits or herbal medications that inhibit CYP3A4 within 7 days, chemotherapy or monoclonal antibody within 4 weeks, small molecule inhibitor within 5 half-lives, wide field radiotherapy within 4 weeks or limited field radiation within 2 weeks, or major surgery within 2 weeks before starting buparlisib, and medications that prolong QT interval, chronic steroid or immunosuppressive agents, moderate or strong inhibitors or inducers of CYP3A4, therapeutic doses of warfarin or other coumadin-derivative. The study was approved by the Washington University Institutional Review Board and followed the Declaration of Helsinki and Good Clinical Practice guidelines. Written informed consent was required for enrollment.
Study Design and Treatment
This study was composed of a dose escalation cohort (Phase IA) and 2 subsequent expansion cohorts (Phase 1B and Cohort C). In Phase IA, a standard 3 +3 phase I design was used to define the MTD of buparlisib PO daily (80 mg, 100 mg) when combined with fulvestrant 500 mg IM on days 1 and 15 of cycle 1, followed by day 1 of each subsequent cycles. The MTD was defined as the highest dose level at which no more than 1 in 6 patients developed a Dose Limiting Toxicity (DLT) during cycle 1 (each cycle is 28 days). The expansion cohorts were to assess the tolerability of long-term treatment (at least 3 cycles) with buparlisib 100mg administered intermittently (5 of 7 days) (Phase IB, n=10) or daily (Cohort C, n=10). Only patients who completed at least 3 cycles or discontinued due to toxicity were considered evaluable. Treatment continued until disease progression, unacceptable toxicity, or withdrawal of consent.
A DLT was defined by the following: neutropenia (≥grade 3 for >7 days), febrile neutropenia, thrombocytopenia (grade 3 for 7 days or grade 4), creatinine elevation (≥grade 3 or ≥2.0 × upper limit of normal (ULN) to ≤3.0 × ULN for >7 days), bilirubin elevation (≥grade 3 or ≥2×ULN to ≤3.0 × ULN for >7 days); aspartate aminotransferase (AST), alanine aminotransferase (ALT) (grade 3 for >7 days or grade 4), hyperglycemia (not resolved in 14 days on hypoglycemics, or ≥grade 3), asymptomatic amylase and/or lipase (grade 3 >7 days, or grade 4), pancreatitis (≥grade 2), cardiac (≥grade 3 or symptomatic), neuro (≥1 grade increase), mood alteration (grade 2 >14 days or ≥grade 3), phototoxicity ≥grade 2, or skin toxicity (rash) resulting in interruption of buparlisib for >21 days, ≥grade 3 vomiting, nausea, diarrhea despite supportive care, fatigue (grade 3 for >7 days or grade 4), and all other adverse events (AEs) ≥grade 3 (excluding ≥grade 3 elevations in alkaline phosphatase). The NCI CTCAE 4.0 was used to record severity and attribution of toxicities.
Dose adjustments and interruptions were allowed for buparlisib according to protocol guidelines. Buparlisib was reduced by 20 mg per day with each dose reduction, with the lowest dose being 60mg 5 of 7 days. Patients required dose interruption >3 weeks were discontinued from study therapy. Treatment continued if AEs were ≤grade 1. Grade 2 transaminase elevations required buparlisib interruption until resolution to ≤grade 1. If resolution time was ≤7 days the current dose level was maintained. However, if resolution time was >7 days, or if the transaminase elevation was grade 3 or 4, the dose was reduced by 1 dose level. Grade 1 or 2 rash was managed by maintaining the current dose level, while initiating/intensifying therapy with antihistimines or topical corticosteroids. If rash was grade 3, buparlisib was omitted until resolution to ≤grade 1. If resolution time was ≤7 days, the dose was reduced by 1 level. The dose was permanently discontinued if resolution time was >7 days or if grade 4. Maintain the current buparlisib dose if fasting plasma glucose (FPG) was grade 1 or the first grade 2 occurrence while initiating/intensifying hypoglycemics treatment. Grade 2 FPG required a FPG re-check within 24 hours and resolution to grade 1 within 14 days. If not resolved to ≤grade 1 within 14 days, buparlisib dose was reduced by 1 level. For a second grade 2 FPG occurrence, buparlisib was omitted until resolution to grade 1 then reduced by one dose level. Grade 3 FPG resulted in immediate omission of buparlisib, twice weekly FPG check and reducing buparlisib by 1 dose level upon resolution to ≤grade 1.
Physical exam, 12-lead electrocardiogram (EKG), and fasting C-peptide levels were performed on day 1 of each cycle. Vital signs, neuro-psychiatric assessment with PHQ-9 and GAD-7 questionnaires, complete blood cell count, serum chemistry, and fasting glucose, were performed on days 1 and 15 of the first 2 cycles, followed by day 1 in subsequent cycles. Cardiac evaluation with either Multi Gated Acquisition Scan (MUGA) or echocardiogram was performed every 3 cycles. Radiologic tumor measurement using CT or MRI was performed every 12 weeks. Treatment response was evaluated according to RECIST 1.1.
Archival tumor DNA sequencing
Tumor DNA, extracted from archival formalin fixed paraffin embedded (FFPE) tumor specimens using QIAamp DNA FFPE Tissue Kit (Qiagen, cat# 56404), and matched leukocyte germ-line DNA were subjected to targeted Illumina next generation sequencing by 2×100 paired end reads of an 83-gene panel as previously described (11). All sequence data were processed using the Genome Modeling System (GMS) (12, 13) reference alignment, somatic variation, and clinical sequencing pipelines. Briefly, raw sequence data were aligned to the human reference genome (build 37) with bwa v0.5.9 (14) using default parameters except for ‘-t 4 -q 5’. The resulting bam files were sorted and duplicate reads marked with Picard v1.46 (15). Single nucleotide variants (SNVs) were detected by taking the union of three variant callers: Somatic Sniper v1.0.2 (16) run with default parameters except ‘-F vcf -q 1 -Q 15’, Varscan v2.2.6 (17) using default parameters, and Strelka v0.4.6.2 (18) using default parameter except for ‘isSkipDepthFilters = 1’. All resulting SNVs were filtered with custom filtering modules available in the GMS. Small insertions and deletions (indels) were detected by taking the union of four variant callers: GATK somatic indel (15), Pindel v0.5, Varscan v2.2.6, and Strelka v0.4.6.2 all using default parameters except for ‘isSkipDepthFilters = 1’ for Strelka. All variants were manually reviewed using the Integrated Genome Browser (IGV) (19) as previously described (13). All SNVs and indels passing manual review were annotated to determine their predicted effect on amino acid sequences using the GMS transcript annotator and known transcript models from Ensembl (version 74) (20).
Pharmacokinetic analysis
Plasma samples from peripheral blood collected in EDTA tube prior to the first dose of buparlisib and on day 1 of Cycles 2 (C2D1) and 3 (C3D1) were kept at −70°C until analysis. Solid phase extraction of plasma samples followed by evaporation of the extract to dryness and analysis of the reconstituted samples for quantitative determination of buparlisib was performed using a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) in multiple reaction monitoring (MRM) positive mode using electrospray ionization (ESI) as the ionization technique according to the analytical study protocol and standard operation procedure (SOP) predefined by WuXi AppTec.
Serum C-peptide Level
Peripheral blood was collected at baseline and day 1 of each cycle for C-peptide measurement at the Core Laboratory for Clinical Studies at Washington University School of Medicine and was measured using the solid-phase, two-site chemiluminescent immunometric assay on the Immulite 1000 System.
Immunohitochemistry for PTEN and PgR
The antibody and IHC methodology for PTEN has been previously described in our previous publication (21). Stromal PTEN staining was used as the internal control. Loss of PTEN (or negative PTEN) was defined as no PTEN staining in the tumor cells, with strong or intermediate intensity staining in the internal control stromal cells. PgR status was per pathology report. PgR positive is defined as at least 10% tumor cell staining or Allred score >3.
Statistical analysis
Clinical benefit rate (CBR) was calculated as the proportion of complete responses (CR), partial responses (PR) or stable disease events (SD) lasting for at least 24 weeks accompanied with 95% Wilcox confidence intervals (CIs). Progression free survival (PFS) was defined as the time interval between the date on treatment to the date off treatment (due to disease progression recorded as events or adverse events as censored) or to the date of 05/03/2015 for the two patients that treatment is ongoing. Empirical PFS probabilities were estimated by the Kaplan-Meier (KM) product limit method and visualized by the KM curves while the log rank test was used to compare survival difference. Hazard ratio (HR) was reported with 95% CI from fitting Cox proportional hazard model. Wilcoxon rank sum test compared drug concentrations between cohorts of patients while paired drug concentrations at C2D1 and C3D1 were compared by Wilcoxon signed rank test.
A linear mixed effects model was fitted on C-peptide levels (in log scale) across treatment cycles with the fixed effects of baseline C-peptide level (in log scale), treatment cycle at measurement, average daily drug administered in the previous cycle, and treatment cohort (phase IB or cohort C), while accounting for the repeated measures on patients. Various longitudinal correlation structures including compound symmetry and auto correlation by lag 1 were considered and the results from the compound symmetry structure were reported as leading to smaller Alkain's information criteria. SAS 9.3 was used for the linear mixed effect model fitting.
Results
Patients Characteristics
Between November 28, 2011 and April 17, 2014, 31 patients were enrolled (Table 1). These included 9 patients in Phase IA who were treated at two dose levels of buparlisib, 11 patients in Phase IB and 11 patients in Cohort C (Table 2). All patients had HER2 negative disease except 1 patient in Cohort C, who had HER2 positive disease. The median age was 57 (range 35–71) years and 84% of the patients had visceral metastasis. Majority of patients (58.1%) went off study due to disease progression, few (12.9%) due to AEs. Treatment is ongoing in 2 patients as of May 3, 2015.
Table 1.
Patient characteristics
| Total | Phase IA | Phase IB | Cohort C | |
|---|---|---|---|---|
| N=31 | N=9 | N=11 | N=11 | |
| Age, years | ||||
| Median | 57 | 60 | 61 | 50 |
| Range | 35–71 | 48–71 | 50–71 | 35–65 |
| Prior adjuvant endocrine | ||||
| Yes | 15 (48%) | 4 | 5 | 6 |
| No | 16 (52%) | 5 | 6 | 5 |
| # prior met endocrine regimen | ||||
| Median | 1 | 2 | 1 | 1 |
| Range | 0 – 9 | 0–8 | 0–3* | 0 to 2 |
| # prior met chemo regimen | ||||
| Median | 0 | 0 | 0 | 0 |
| Range | 0 – 2 | 0–2 | 0–2 | 0–2 |
| Visceral involvement | ||||
| Yes | 26 (84%) | 9 | 8 | 9 |
| No | 5 (16%) | 0 | 3 | 2 |
| Reason off study | ||||
| Adverse event | 4 (12.9%) | 1 | 3 | 0 |
| Withdrew consent | 4 (12.9%) | 1 | 1 | 2 |
| Physician decision | 3 (9.7%) | 0 | 3 | 0 |
| Progressive disease | 18 (58.1%) | 7 | 4 | 7 |
| Treatment ongoing | 2 (6.4%) | 0 | 2 |
This range did not include 1 patient who had 9 prior endocrine therapy in the metastatic setting.
Table 2.
Dose level, treatment cohorts and number of patients with buparlisib dose interruption or reduction
| Cohort | Buparlisib | N | Median #cycles received (range) |
N (%) patients with buparlisib interrupted/reduced (first cycle) |
N (%) patients with buparlisib interrupted/reduced (first 3 cycles) |
N (%) patients with buparlisib interrupted/reduced (all cycles) |
|
|---|---|---|---|---|---|---|---|
| Phase 1A | DL 1 | 80 mg daily | 3 | 3.2 (3 – 22.3) | 0 | 2 (66.7%) | 2 (66.7%) |
| DL 2 | 100 mg daily | 6 | 5.3 (1.4 – 12.4) | 0 | 2 (33.3%) | 3 (50.0%) | |
| Phase IB | 100 mg 5 of 7 days | 11 | 6.0 (1.8 – 26) | 0 | 4 (36.4%) | 6 (54.5%) | |
| Cohort C | 100 mg daily | 11 | 4.3 (3 – 16) | 2 (18.2%)† | 6 (54.5%) | 8 (72.7%) | |
| Total | 31 | 6.0 (1.4 – 26) | 2 (6.5%) | 14 (45.2%) | 19 (61.3%) | ||
1 patient with grade 3 diarrhea (DLT) and 1 patient with grade 3 rash that resolved within 10 days (did not meet criteria for DLT).
Dose Escalation and Adverse Events
Phase IA started at dose level 1 with buparlisib 80mg PO daily (Table 2). No DLT was observed in cycle 1 at the two dose levels of buparlisib, 80mg (n=3) and 100mg (n=6). Therefore, MTD was defined as buparlisib 100mg daily in combination with fulvestrant. Since 5 of the 9 patients in Phase IA required buparlisib reduction in cycle 2 and beyond, two expansion cohorts, including an intermittent schedule (phase IB) and a continuous schedule of buparlisib 100mg daily (Cohort C), were tested for side effects profiles with treatment over 3 cycles. Eleven patients each were enrolled in Phase IB and Cohort C, respectively, 1 patient in phase IB had more than 3 prior metastatic therapies (replaced) and 1 patient in Cohort C went off study within 3 cycles due to disease progression (replaced).
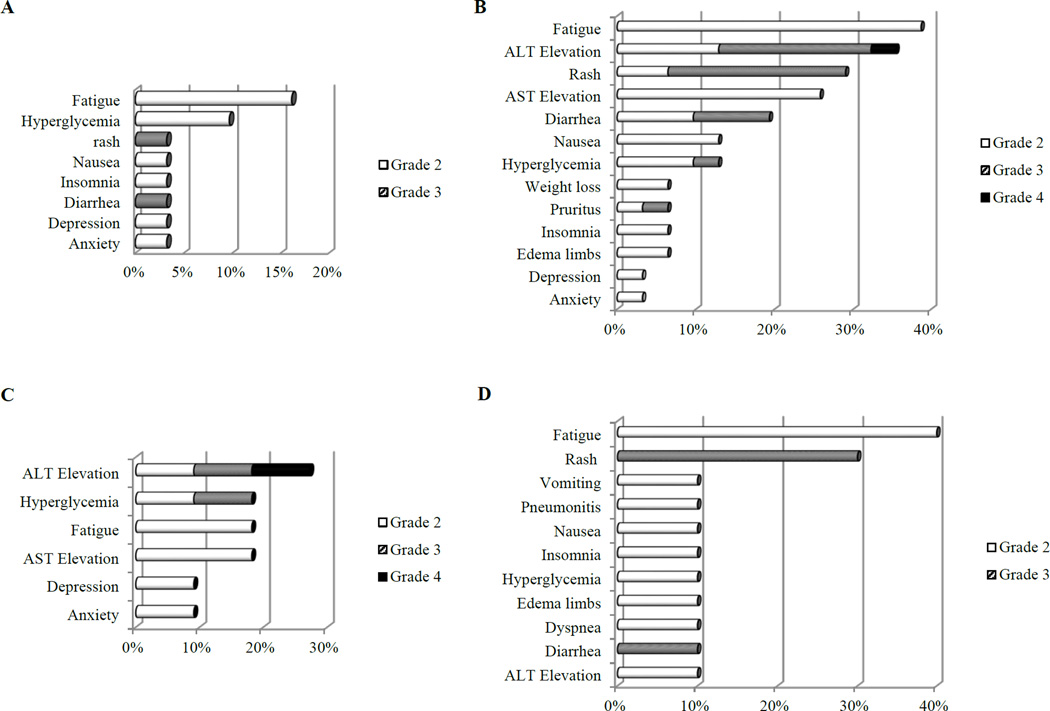
All patients were evaluable for AE assessments. Fig. 1 summarized grade 2 and above treatment related AEs. During cycle 1, treatment was well tolerated (Fig. 1A) and dose interruption was rare (Table 2). One DLT (grade 3 diarrhea) occurred in cycle 1 in Cohort C. The incidence and severity of AEs, including fatigue, AST/ALT elevation, rash, diarrhea and hyperglycemia, increased in subsequent cycles (Fig. 1B), leading to dose interruption/reduction of buparlisib in 61.3% of patients within a median of 6 cycles (range 1.4–26 cycles) (Table 2). The most common AE leading to buparlisib dose interruption/reduction was asymptomatic ALT elevation (33.3%), rash (33.3%) and diarrhea (7.4%) (Supp. Table S1). Compared to the intermittent schedule (Phase IB), daily buparlisib (Cohort C) was associated with a higher incidence of grade 2 and above fatigue (40% in Cohort C vs 18% in Phase IB) and rash (30% in Cohort C vs 0% in Phase IB) during the first 3 cycles of therapy (Fig. 1C and 1D). More frequent dose interruption/reduction was observed in Cohort C than Phase IB during the first 3 and all cycles (Table 2, Supp. Fig. S1). The chronic doses of buparlisib for cycle 6 and beyond were 80mg to 100mg 5 of 7 days and 60mg to 80mg daily in patients in Phase IB and Cohort C, respectively (Supp. Fig. S1).
Fig. 1. Grade 2 and above adverse events (AE) at least possibly related to study treatment.
A. Cycle 1 AE. B. All-cycle AE. C. 3-cycle AE in Phase IB. D. 3-cycle AE in Cohort C.
AST/ALT elevations were asymptomatic without increased bilirubin and commonly resolved to grade 1 within 2–4 weeks with dose interruption. Most patients were able to resume buparlisib with reduced doses. Rashes were typically maculopapular in appearance and resolved quickly within 1–2 weeks with antihistamine and occasional topical or systemic steroids. Patients could be re-challenged at a lower dose of buparlisib without subsequent occurrence of rash. Psychiatric AEs, including grade 1 and 2 anxiety, confusion, and depression occurred in 7 patients (22.6%) (Suppl. Table S2), resolved within 1–2 weeks with or without dose interruption. There were no grade 3 or 4 psychiatric AEs. Four patients discontinued study drug due to AEs, including grade 4 ALT (n=1), grade 2 confusion in cycle 2 (n=1), grade 3 diarrhea in cycle 24 (n=1), and grade 3 reduction in left ventricular ejection fraction (LVEF) in cycle 24 (n=1). All were reversed with drug discontinuation, suggesting possible relationship with buparlisib.
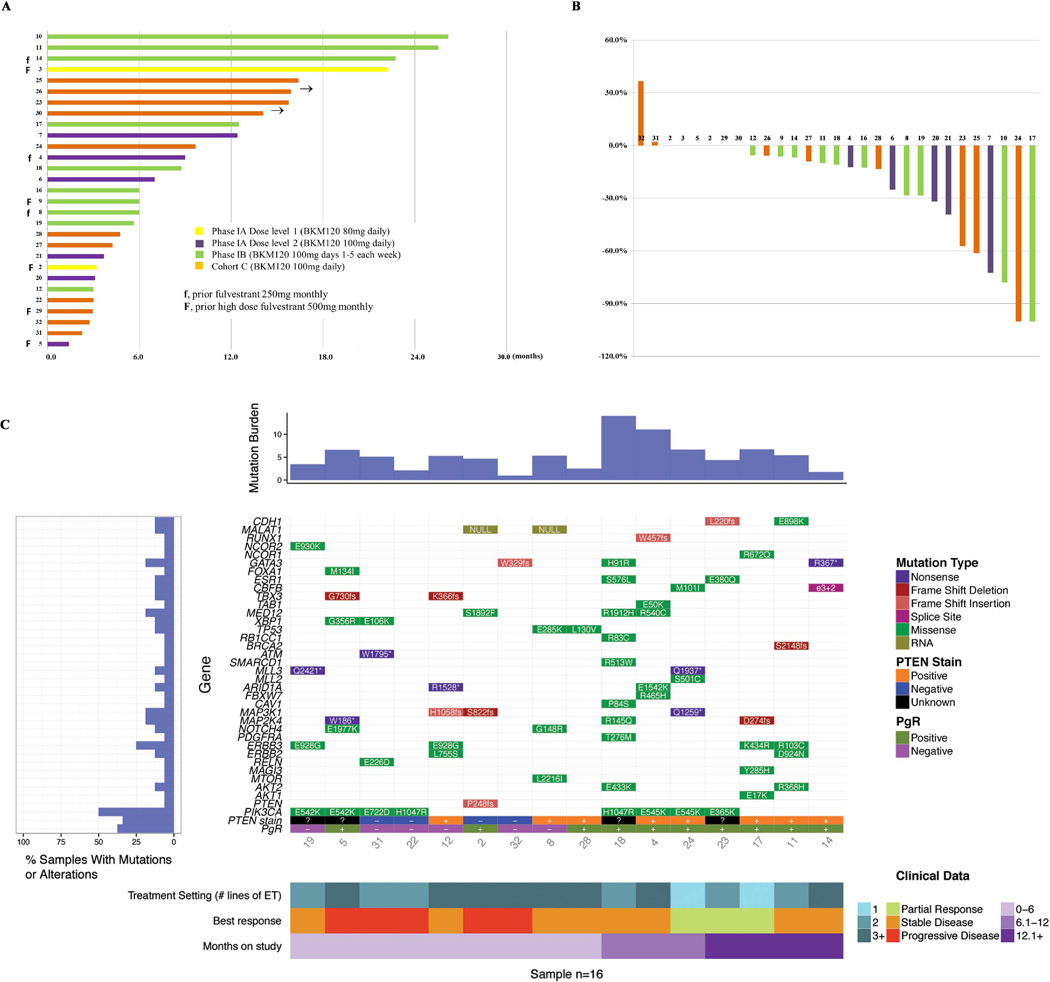
Anti-tumor Activity
Two patients (1 in phase IA, and 1 in Phase IB) discontinued study drug in cycle 2 due to AE, therefore not evaluable for response. Among the 29 evaluable patients, the CBR was 58.6% (95% CI: 40.7–74.5%) (Table 3), including 7 (24.1%) with PR and 10 (34.5%) with SD for at least 24 weeks. There were no difference in CBRs between Phase 1B (70%, 95% CI: 39.7–89.2%) and Cohort C (45. 5%, 95% CI: 21.3–72.0%) (Fisher's exact test p=0.39). The median PFS was 12.4 months for all 29 evaluable patients. There is a trend toward longer PFS in Phase IB compared to Cohort C, but did not reach statistical significance (log rank p=0.098212).
Table 3.
Response by Cohort per RECIST 1.1
| Best Response |
All patients N (%) |
Phase IAN (%) |
Phase IB N (%) |
Cohort C N (%) |
1st line ET |
2nd line ET |
3rd line ET and above |
|---|---|---|---|---|---|---|---|
| PR | 7 (24.1%) | 2 (25.0%) | 2 (20.0%) | 3 (27.3%) | 4 (80%) | 2 (20%) | 1 (7%) |
| SD | 15 (51.7%) | 3 (37.5%) | 8 (80%) | 4 (36.4%) | 1 (20%) | 5 (50%) | 9 (64%) |
| PD | 7 (24.1%) | 3 (37.5%) | 0 | 4 (36.4%) | 0 | 3 (30%) | 4 (29%) |
| CB | 17 (58.6%) | 5 (62.5%) | 7 (70.0%) | 5 (45.5%) | 5 (100%) | 6 (60%) | 6 (43%) |
| N | 29 | 8 | 10 | 11 | 5 | 10 | 14 |
ET, endocrine therapy; 1st line ET, no prior ET in the metastatic setting and at least 12 months after completion of adjuvant endocrine therapy; 2nd line ET, progression on 1st line ET or adjuvant ET; PR, partial response; SD, stable disease; PD, progressive disease; CB, clinical benefit (PR+ SD ≥ 6 months); N, number of evaluable patients
To investigate the effect of prior endocrine therapy on treatment efficacy, we categorized patients into groups that receive the study drug in the setting of either 1st line (no prior endocrine therapy in the metastatic setting and at least 12 months since the completion of adjuvant endocrine therapy), 2nd line (post progression on 1st line endocrine therapy for metastatic disease or adjuvant endocrine therapy), or 3rd line and above endocrine therapy (post 2nd line or above) (Table 3). All 5 patients (100%) who received the study drug as 1st line endocrine therapy derived clinical benefit, including 4 PR and 1 SD over 1 year (Table 3). Among the 10 patients who received study treatment as 2nd line endocrine therapy, the CBR was 60%, including 2 PR and 4 SD for at least 24 weeks (Table 3). The CBR rate in the 1st line setting (100%, 95% CI 56.6% ~100%) was significantly higher than that in the 3rd line or beyond (42.9%, 95% CI 21.4% ~ 67.4%, Fisher's exact test p=0.045). The median PFS of patients on 1st, 2nd and 3rd line or beyond were 14.4, 15.8 and 6 months, respectively (log rank test P=0.12, not significant due to small sample size). The median PFS of patients on 1st or 2nd line endocrine treatment versus patients on 3rd line or beyond were 15.8 and 6 months, respectively (HR=2.69, 95% CI: 1.02~7.10, log rank test p=0.039).
Correlative studies based on archival tumor specimens
Archival tumor specimens were available from 16 patients who were evaluable for response (primary site, n=10; metastatic site, n=6) for next generation sequencing analysis of an 83-gene panel and PTEN IHC. Identified mutations are indicated in Fig. 2C, Suppl. Table S3 and Suppl. Fig. S2. PIK3CA mutation was most common (n=8, 50%), but did not associate with treatment response or duration on therapy. However, 6 of the 8 patients with PIK3CA wild type tumors were treated in the setting of 3rd line or above endocrine therapy, which could potentially confound the analysis. Majority of the patients (5 of 8) who did not have a PIK3CA mutation were found to have mutations in other PI3K pathway genes, including PTEN truncation (n=1), AKT1 E17K (n=1), AKT2 R368H (n=1), mTOR L2216I (n=1), or mutations in receptor tyrosine kinases, including ERBB2 L755S (n=1). Loss of PTEN expression was observed in 4 patients, including the patient with PTEN truncation identified by sequencing. All 4 patients had PD (Fig. 2C). The patient with tumor AKT1 E17K mutation had a PR and was on therapy for 12 months. The patient with ERBB2 L755S mutation, however, did not derive benefit. In addition, mutations in ER pathway genes were common, including RUNX1 (n=1), GATA3 (n=3), NCOR2 (n=1), NCOR1 (n=1), FOXA1 (n=1) and ESR1 (n=2, 1 each of E380Q and S576L). Each of the ESR1 mutations was identified concurrently with a PIK3CA mutation and both patients derived clinical benefit, including one with PR. Although the sample size was small, the benefit of the combination observed in tumors with AKT1 mutation or ESR1 mutation indicated the potential efficacy of PI3K inhibitor and fulvestrant in these patient populations. Two patients had mutations in TP53 and neither derived benefit. An exploratory analysis of PgR status per pathology report in relation to PFS was also performed. Discordant PgR status between primary and metastatic sites was observed in 7 of 27 (26%) patients (Suppl. Table S4). There was a trend toward longer PFS in patients with positive PgR of the primary (hazard ratio: 0.38, 95% CI:0.12~1.2, Log rank P=0.094) or of the metastatic sites (hazard ratio: 0.47, 95% CI:0.18~1.6, Log rank P=0.128) compared to those with negative PgR tumors, but this analysis did not reach statistical significance (Suppl. Fig. S4 and Fig. 2c).
Fig. 2. Anti-tumor activity.
A. Duration on study B. Percentage change in target lesion at best response compared to baseline C. Mutation landscape, PTEN status by IHC, and treatment setting based on lines of endocrine therapy in relation to tumor response and duration on therapy
Mutation burden (number of genes with mutations identified), genes and mutation type, PTEN IHC results, PgR of metastatic site, clinical response and duration on therapy are annotated for each patient. % of samples (patients) with mutations in specific genes are also presented on the left panel of Fig. 2C.
Pharmacokinetics
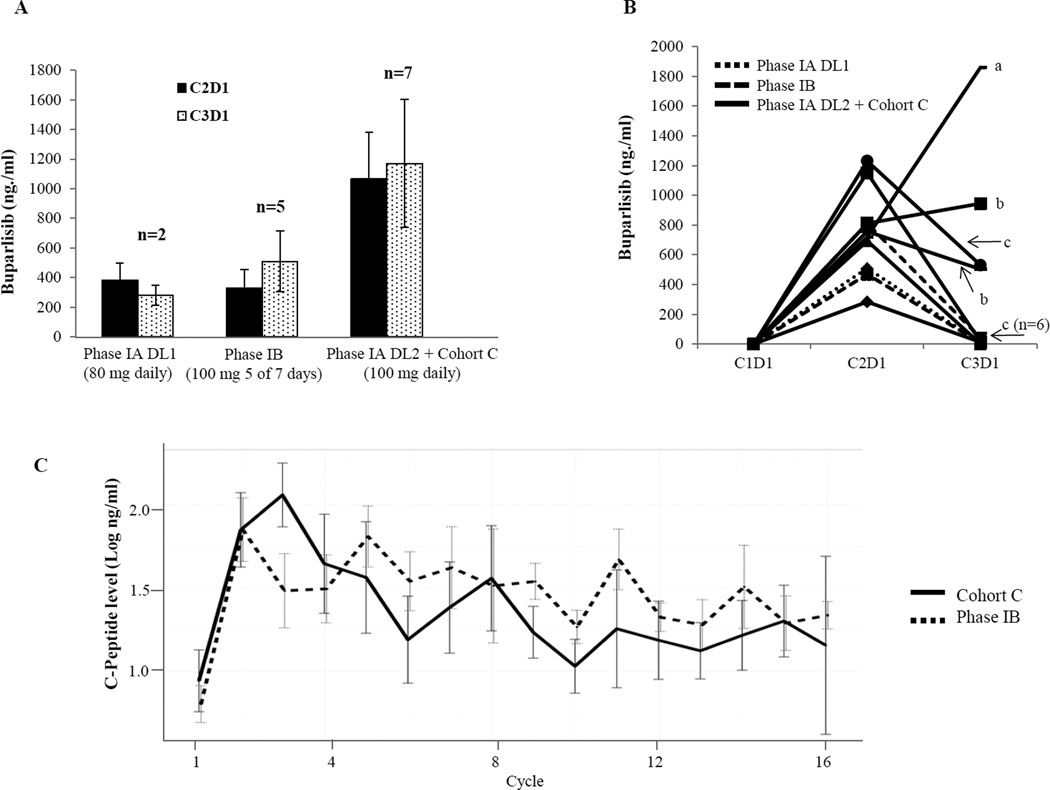
Plasma concentrations of buparlisib at baseline and prior to drug administration on day 1 of cycles 2 (C2D1) (n=27) and 3 (C3D1) (n=26) were assessed by LC-MS/MS for 27 patients. In patients without dose interruption prior to the blood draw at the corresponding time point, buparlisib 100 mg daily was associated with a significantly higher drug concentration compared to 80 mg daily or 100 mg 5 of 7 days on C2D1 (the median buparlisib levels were 873 ng/ml (n=14, buparlisib100mg daily), 464 ng/ml (n=3, buparlisib 80mg daily), and 412 ng/ml (n=10, buparlisib 100mg 5 of 7 days); Wilcoxon rank sum test p=0.049 and 0.0065, respectively) and on C3D1 (median 1,100 ng/ml (n=7, buparlisib100mg daily), 279.5 ng/ml (n=2, buparlisib 80mg daily) and 502 ng/ml (n=5, buparlisib 100mg 5 of 7 days), Wilcoxon rank sum test p=0.071 and 0.0043, respectively). These results are consistent with the higher incidence of AEs associated with daily schedule of buparlisib. No statistical difference (Wilcoxon signed rank test p=0.6698) in buparlisib concentration was observed between paired samples collected on C2D1 and C3D1 at each dose level in patients who did not have dose interruptions prior to the blood draw (n=14) (Fig. 3A), suggesting no obvious drug accumulation between C2D1 and C3D1. Buparlisib plasma concentrations decreased to minimal levels after 2 weeks of dose interruption in majority of patients (Fig. 3B). In few cases, however, accumulation of buparlisib was observed (Fig. 3B), indicating existence of individual variations in buparlisib metabolism and clearance. Interestingly, the trough buparlisib plasma concentration was 414 ng/ml on C2D1, a level comparable to others, in the patient who experienced grade 4 ALT elevation.
Fig. 3. Quantification of plasma buparlisib concentration (A, B), and serum C-peptide (C).
A. Average trough plasma buparlisib levels on cycle 2 day 1 (C2D1) and cycle 3 day 1 (C3D1) by dose level and cohorts in patients who did not have drug interruption during the first 2 cycles.
B. Trough plasma levels of buparlisib in patients who had dose interruptions prior to C3D1 blood draw. A, Buparlisib was held between C1D8 to C1D15 and C2D1 to C2D8. B, Buparlisib was held for 1 week prior to C3D1 blood draw. C, Buparlisib was held for 2 weeks prior to C3D1 blood draw.
C. C-peptide levels by treatment cycle and cohorts
Serum C-peptide level
C-peptide levels increased significantly following 1 cycle of treatment and maintained throughout the treatment cycles (Fig 3C). C-peptide levels (in log scale) at current cycle correlated with baseline level (coefficient estimate=0.30, p<0.0001) and also daily drug dosing at the previous cycle (the coefficient was estimated to be 0.008, p<0.0001). Treatment cycle (p=0.49) and cohort (p=0.2944 between cohort C and phase 1B) show no significant effect on C-peptide levels. This data suggested that the intermittent schedule of buparlisib (5 of 7 days) achieved similar on-target effect compared to continuous daily dosing.
Discussion
This phase I trial defined the MTD of buparlisib (100mg PO daily) in combination with fulvestrant (500mg IM on days 1 and 15 in cycle 1, followed by 500mg IM on day 1 of each subsequent 28-day cycles) in patients with metastatic ER+ breast cancer. The treatment was well tolerated. During cycle 1, AEs were uncommon, with DLT (grade 3 diarrhea) occurring in only 1 of 31 patients. In subsequent cycles, we observed an increased incidence of grade 2 and 3 AEs, including fatigue, transaminitis, and rash, leading to buparlisib dose interruption/reduction in 19 of the 31 patients. However majority of patients were able to resume treatment, often with reduced dose of buparlisib, after 1–3 weeks of dose interruption. Intermittent dosing schedule (5 of 7 days) of buparlisib was better tolerated compared to the daily dosing. Grade 2 and above fatigue and rash were more common during the first 3 cycles of therapy with daily dosing. The AEs observed in this trial were similar to previous studies of buparlisib (22, 23). Rash and hyperglycemia were consistent with on-target effect from PI3K inhibition. Patients with a history of significant psychiatric illess were not eligible for this study due to concerns of mood alteration reported in trials of buparlisib. Twenty percent patients experienced mood alterations in this trial, although mild and rarely required psychiatric intervention. Routine screening and monitoring is therefore important for appropriate and timely management of these side effects. There has been less experience with long term buparlisib administration. In this trial, 2 patients went off study at 24 months due to AEs, including colitis and LVEF reduction. Both resolved after drug discontinuation, suggesting possible relationship with buparlisib.
Pharmacokinetic quantification of plasma buparlisib at C2D1 and C3D1 indicated a much higher drug level with daily administration of buparlisib compared to intermittent dosing schedule. No obvious accumulation in buparlisib was observed between C3D1 and C2D1. Consistent with the quick resolution of treatment related AEs, buparlisib levels were minimally detected after 2 weeks of dose interruption in majority of patients. Buparlisib levels observed in this study were similar to that in the initial phase I study of single agent buparlisib in patients with advanced solid tumor malignancies (22). The use of intermittent schedule is also supported by the C-peptide data, which indicated a significant increase following study drug therapy comparable to that in the daily dosing schedule.
Buparlisib plus fulvestrant achieved CBR of 58.6% in this trial, including 7 with PR and 10 with SD ≥ 24 weeks. The clinical benefit was particularly significant in patients who receive the study drug as 1st or 2nd line endocrine treatment, in which an ORR of 80% (1st line) and 20% (2nd line), and a CBR of 100% (1st line) and 60% (2nd line) were observed. Since fulvestrant alone led to an ORR of 23% (1st line) and 10% (2nd line), and CBR of 72.5% (1st line) and 45% (2nd line) in previous studies (24, 25), the efficacy data from this trial warrants further evaluation.
In this small study, no obvious interaction between PIK3CA mutation and anti-tumor response was observed, which is consistent with previous studies of buparlisib, other pan-PI3K inhibitors and everolimus (23), although our analysis could be biased by the higher number of patients with PIK3CA wild type tumors treated in the 3rd line or higher endocrine therapy setting and the use of the primary rather than metastatic samples in 10 of the 16 patients tested. We also observed that majority of patients had mutations in other PI3K pathway genes which potentially influence the activity of buparlisb. Interestingly, prolonged disease stabilization was observed in a patient with ESR1 mutation and in a patient with AKT1 mutation, demonstrating potential efficacy of this regimen in these patients. Interestingly, markers for poor prognosis breast cancers, such as PgR negativity, TP53 mutation and PTEN loss, were associated with less responsive tumors. Further studies in larger clinical trials are needed for definitive conclusion in regards to response predictors for the combination of buparlisib and fulvestrant. It is, however, already clear the development of a mutation-based predictor for PI3K pathway inhibition will not be straightforward, and next generation sequencing reports that list PIK3CA as a marker for sensitivity for PI3K pathway inhibitor treatment are incorrect.
In conclusion, this study provided the safety and preliminary efficacy information of buparlisib in combination with fulvestrant in patients with ER+ metastatic breast cancer. Phase III trials comparing fulvestrant with fulvestrant in combination with Buparlisib (BELLE-2 and BELLE-3) are being evaluated aromatase inhibitor (AI) resistant metastatic ER+ breast cancer. While pending the phase III data to confirm the therapeutic efficacy of buparlisib and fulvestrant in AI resistant ER+ breast cancer, the promising activity observed in the 1st line setting in this study warrant further investigation. Our data also provided the justification for the the use of the intermittent dosing schedule of buparlisib for future studies.
Supplementary Material
Statement of Translational Relevance.
Buparlisib, an oral pan-class I phosphatidylinositol-3-kinase (PI3K) inhibitor, was evaluated in a Phase I trial in combination with fulvestrant using daily or intermittent schedules (days 1–5 each week). Common adverse events (AE) included transaminitis and rash. Compared to daily dosing, intermittent buparlisib was associated with less frequent early onset AEs. On-target effect of buparlisib was demonstrated by a significant increase in C-peptide. The clinical benefit rate was 58.6%, defined as no disease progression within 24 weeks. PIK3CA mutation did not predict tumor response but loss of PTEN or progesterone receptor expression, or mutation in TP53 was associated with poor outcomes suggesting that buparlisib may be less effective in tumors with these molecular features. Mutations in AKT1 and ESR1 did not exclude tumor response. This study established the dosing strategy and suggested clinical activity for buparlisib plus fulvestrant in ER+ breast cancer that need to be further evaluated in randomized trials.
Acknowledgments
This work is funded in part by the Siteman Cancer Center Protocol Specific Research Support, Siteman Cancer Center, Novartis, and NCI Cancer Clinical Investigator Team Leadership Award (to C. Ma) and Susan G. Komen Foudation grant PG12220321 (to M. J. Ellis).
C. Ma received research funding from Novartis for preclinical and clinical investigation of buparlisib in the treatment of breast cancer. C. Lockhart received research funding from Novartis. M. Naughton is on Novartis Speakers Bureau.
The study is funded in part by the Siteman Cancer Center Protocol Specific Research Support and Novartis Pharmaceutical Company. We wish to thank the patients and their families for participation in this study. We also thank the nurses, clinical research and regulatory coordinators at Washington University Siteman Cancer Center, and Stephanie Myles for protocol development.
Footnotes
Conflict of Interest Statement: Other authors disclosed no conflict of interest.
Presented in part at the 2013 and 2014 San Antonio Breast Cancer Symposium, San Antonio, Texas.
References
- 1.Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nature reviews Drug discovery. 2014;13:140–156. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellis MJ, Lin L, Crowder R, Tao Y, Hoog J, Snider J, et al. Phosphatidyl-inositol-3-kinase alpha catalytic subunit mutation and response to neoadjuvant endocrine therapy for estrogen receptor positive breast cancer. Breast Cancer Res Treat. 2010;119:379–390. doi: 10.1007/s10549-009-0575-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellis MJ, Ding L, Shen D, Luo J, Suman VJ, Wallis JW, et al. Whole-genome analysis informs breast cancer response to aromatase inhibition. Nature. 2012;486:353–360. doi: 10.1038/nature11143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Network TCGA. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crowder RJ, Phommaly C, Tao Y, Hoog J, Luo J, Perou CM, et al. PIK3CA and PIK3CB inhibition produce synthetic lethality when combined with estrogen deprivation in estrogen receptor-positive breast cancer. Cancer research. 2009;69:3955–3962. doi: 10.1158/0008-5472.CAN-08-4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanchez CG, Ma CX, Crowder RJ, Guintoli T, Phommaly C, Gao F, et al. Preclinical modeling of combined phosphatidylinositol-3-kinase inhibition with endocrine therapy for estrogen receptor-positive breast cancer. Breast cancer research : BCR. 2011;13:R21. doi: 10.1186/bcr2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma CX, Crowder RJ, Ellis MJ. Importance of PI3-kinase pathway in response/resistance to aromatase inhibitors. Steroids. 2011;76:750–752. doi: 10.1016/j.steroids.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 8.Miller TW, Hennessy BT, Gonzalez-Angulo AM, Fox EM, Mills GB, Chen H, et al. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J Clin Invest. 2010;120:2406–2413. doi: 10.1172/JCI41680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maira SM, Pecchi S, Huang A, Burger M, Knapp M, Sterker D, et al. Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. Molecular cancer therapeutics. 2012;11:317–328. doi: 10.1158/1535-7163.MCT-11-0474. [DOI] [PubMed] [Google Scholar]
- 10.Miller TW, Balko JM, Fox EM, Ghazoui Z, Dunbier A, Anderson H, et al. ERalpha-dependent E2F transcription can mediate resistance to estrogen deprivation in human breast cancer. Cancer Discov. 2011;1:338–351. doi: 10.1158/2159-8290.CD-11-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffith OL, Griffith M, Luo J, Hundall J, Miller CA, Larson DE, et al. Prognostic effects of gene mutation in estrogen receptor positive breast cancer; San Antonio Breast Cancer Symposium; 2014. abstract S1-02. [Google Scholar]
- 12.Griffith M, Griffith OL, Smith SM, Ramu A, Callaway MB, Brummett AM, et al. Genome Modeling System: A Knowledge Management Platform for Genomics. PLoS Comput Biol. 2015;11:e1004274. doi: 10.1371/journal.pcbi.1004274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffith M, Griffith OL, Smith SM, Ramu A, Callaway M, Brummett AM, et al. Genome Modeling System: A Knowledge Management Platform for Genomics. PLOS Computational Biology. 2015 doi: 10.1371/journal.pcbi.1004274. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome research. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larson DE, Harris CC, Chen K, Koboldt DC, Abbott TE, Dooling DJ, et al. SomaticSniper: identification of somatic point mutations in whole genome sequencing data. Bioinformatics. 2012;28:311–317. doi: 10.1093/bioinformatics/btr665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koboldt DC, Zhang Q, Larson DE, Shen D, McLellan MD, Lin L, et al. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome research. 2012;22:568–576. doi: 10.1101/gr.129684.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saunders CT, Wong WS, Swamy S, Becq J, Murray LJ, Cheetham RK. Strelka: accurate somatic small-variant calling from sequenced tumor-normal sample pairs. Bioinformatics. 2012;28:1811–1817. doi: 10.1093/bioinformatics/bts271. [DOI] [PubMed] [Google Scholar]
- 19.Nicol JW, Helt GA, Blanchard SG, Jr, Raja A, Loraine AE. The Integrated Genome Browser: free software for distribution and exploration of genome-scale datasets. Bioinformatics. 2009;25:2730–2731. doi: 10.1093/bioinformatics/btp472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flicek P, Amode MR, Barrell D, Beal K, Billis K, Brent S, et al. Ensembl 2014. Nucleic acids research. 2014;42:D749–D755. doi: 10.1093/nar/gkt1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu S, Li S, Guo Z, Luo J, Ellis MJ, Ma CX. Combined targeting of mTOR and AKT is an effective strategy for basal-like breast cancer in patient-derived xenograft models. Molecular cancer therapeutics. 2013;12:1665–1675. doi: 10.1158/1535-7163.MCT-13-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bendell JC, Rodon J, Burris HA, de Jonge M, Verweij J, Birle D, et al. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:282–290. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 23.Mayer IA, Abramson VG, Isakoff SJ, Forero A, Balko JM, Kuba MG, et al. Stand up to cancer phase ib study of pan-phosphoinositide-3-kinase inhibitor buparlisib with letrozole in estrogen receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:1202–1209. doi: 10.1200/JCO.2013.54.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robertson JF, Lindemann JP, Llombart-Cussac A, Rolski J, Feltl D, Dewar J, et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: follow-up analysis from the randomized 'FIRST' study. Breast Cancer Res Treat. 2012;136:503–511. doi: 10.1007/s10549-012-2192-4. [DOI] [PubMed] [Google Scholar]
- 25.Di Leo A, Jerusalem G, Petruzelka L, Torres R, Bondarenko IN, Khasanov R, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:4594–4600. doi: 10.1200/JCO.2010.28.8415. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.