Abstract
Purpose
Lactoferrin (LF) is a multifunctional protein known to provide innate defense due to its antimicrobial and anti-inflammatory properties. In the eye, LF has been identified in the tears and vitreous humor. Its presence in other ocular tissues has not been determined. Our aim is to assess the presence of LF in the cornea, iris, retina and retinal pigment epithelium (RPE) of humans and mice.
Methods
To test for the endogenous production of LF, reverse transcription polymerase chain reaction was performed in cultured human cells from the cornea and RPE and in murine tissues. To confirm LF localization in specific ocular tissue, immunohistochemistry was performed on flat mounts of cornea, retina and RPE in human donor eyes. The presence of LF was assessed by western blotting in human and mouse ocular tissue and human culture cells (cornea and RPE). To verify antibody specificity, purified human LF and transferrin (TF) were used on 1D and 2D western blots.
Results
LF gene expression was confirmed in the cornea and RPE cell cultures from humans, suggesting that LF is an endogenously produced protein. PCR results from mouse ocular tissue showed LF expression in cornea, iris, RPE, but not in retina. These results were also consistent with immunohistochemical localization of LF in human donor tissue. Antibody reaction for human LF was specific and western blotting showed its presence in the cornea, iris and RPE tissues. A faint reaction for the retina was observed but was likely due to contamination from other ocular tissues. Multiple commercially available antibodies for murine LF cross-reacted with TF, so no reliable results were obtained for murine western blot.
Conclusion
LF is expressed in multiple eye tissues of humans and mice. This widespread expression and multifunctional activity of LF suggests that it may play an important role in protecting eye tissues from inflammation-associated diseases.
Keywords: Cornea, iris, lactoferrin, retina, retinal pigment epithelium, transferrin
Introduction
Lactoferrin (LF) is an 80-kDa glycoprotein that shares sequence homology (~65%) and structural similarity with transferrin (TF), an iron-binding protein that transfers iron within and across tissues.1,2 LF is present in several human fluids including saliva, tears, and milk,3 and expressed in various organs, including the mammary gland, uterus,4,5 kidney6 and brain.7 Both LF and TF are members of the TF family, which is crucial for maintenance of iron homeostasis; however, more evidence now suggest that they are essential for multiple cellular processes in addition to the iron binding and transport.
LF plays an important part in the host defense mechanisms in various tissues, with demonstrated potent anti-bacterial, anti-viral, anti-fungal and anti-parasitic effects.2,8–10 In addition, LF has been shown to have anti-angiogenic and anti-tumor properties, and most recently, essential roles in the neuronal differentiation.2,11 While the mechanism for LF’s multipotency awaits further investigation, there is evidence suggesting that its anti-bacterial effect is conveyed in more than one way. LF confers its bacteriostatic activity on certain iron-dependent bacteria, including Escherichia coli, by sequestering available iron.12,13 LF can also bind to lipopolysaccharide (LPS) on the surface of Gram-negative enterobacteria, resulting in destabilization of bacterial membrane and increasing bacterial permeability. Furthermore, LF neutralizes LPS by keeping it from making complexes that activate Toll-Like Receptor 4 (TLR-4). When over-activated, TLR-4 signaling can lead a dysregulated production of pro-inflammatory mediators including tumor necrosis factor.14 Additional protective properties attributed to LF include its antioxidant activity, which has been demonstrated in human breast milk,15 and as part of immune surveillance. In macrophages, LF inhibits prostaglandin synthesis and activates nonspecific immune responses by stimulating phagocytosis and complement activation.16
In addition to its proven anti-bacterial and anti-viral effects in the ocular surface,17 LF also displays remarkable protective properties toward injury in the cornea and retina. Topical application of bovine LF has been shown to reduce UVB irradiation-induced corneal epithelial damage in rats.18 LF promotes corneal wound healing after alkali-burn injury in mice19 and epithelial regrowth in the in vitro studies of monolayers of corneal epithelial cells.20,21 Using a laser-induced choroidal neovascularization murine model, we were able to demonstrated that the endogenous LF provides noticeable protection from choroidal neovascularization lesions in WT mice compared to LF knockout (LFKO) mice. Furthermore, treatment of LFKO mice with exogenous LF reduced choroidal neovascularization lesions.22 Taken together, these results establish the protective roles of LF during ocular wound healing and suggest therapeutic potentials of LF for treating ocular injuries.
While LF has been found in human vitreous23–26 and tear film,10 its expression in ocular tissues has only been confirmed in human lachrymal gland and bovine corneal epithelia.17 Given the multifunctional properties described above for LF, it is important to identify where LF is expressed as a first step toward understanding its potential role in retinal health.
Methods
Mice
Three-month-old C57/BL6 mice weighing approximately 25 g were purchased from The Jackson Laboratory (Bar Harbor, ME). These mice were negative for the rd8 mutation based on genotyping.27 Mice were maintained on a diet of standard rodent chow and water supplied ad libitum, with a 12-h light/12-h dark cycle. All animal experiments adhered to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. The University of Minnesota Institutional Animal Care and Use Committee approved protocols.
Mice were euthanized by carbon dioxide inhalation and perfused with PBS through the heart to remove the blood, which could carry LF to the eyes. The eyes were enucleated. The cornea, iris, retina and RPE were dissected under 10× magnification and snap frozen in liquid nitrogen.
Preparation of mouse retina, iris and cornea for western blot
The retinas were processed in a homogenization buffer (135 µL/retina) containing 20 mM Tris (pH 7.4), 20% (wt/vol) sucrose, 2 mM MgCl2, 10 mM glucose and 2% (wt/vol) 3-[(3-cholamidopropyl)dimethylamino]-1-propanesulfonate(CHAPS).
Retina homogenates were centrifuged at 4 °C, the supernatant was collected, the pellet was re-homogenized and supernatants containing the soluble retinal proteins were combined and stored at −80 °C.28 Iris were processed in a similar fashion (50 µL/iris, with retinal homogenization buffer). The corneas were dissected into 1 mm × 1 mm tissue blocks and homogenized in RIPA buffer (Sigma-Aldrich, St. Louis, MO) (50 µL/cornea). Centrifugation and storage conditions were as stated above. Protein concentrations were determined with the bicinchoninic acid (BCA) assay (Pierce, Rockford, IL), with bovine serum albumin as the standard.
Preparation of mouse RPE for western blot
Following removal of the retina, the RPE cells were harvested from the eyecups by gentle disruption of the cell layer with a soft bristled paintbrush. Cells were collected in PBS solution containing 0.3 M sucrose, 20 mM Tris-acetate (pH 7.2) and pelleted at 1200g for 15 min at 4 °C. The cell pellet was resuspended in 50 mM Tris (pH 7.8), 2% CHAPS, then transferred to cryotubes and incubated on ice for 45 min. Each sample was then subjected to two cycles of freeze/thaw with liquid nitrogen followed by homogenization with 10–20 passes in an insulin syringe. Cellular debris and unlysed cells were collected by centrifugation at 600g for 15 min at 4 °C. The supernatant was retained and the protein concentration determined using the BCA protein assay.
Preparation of mouse retina and RPE for reverse transcription-polymerase chain reaction
Tissues were dissected, and immediately snap frozen in liquid nitrogen. Tissues were homogenized in lysis buffer (RNeasy Mini Kit, QIAGEN, Venlo, Netherlands) and any remaining intact tissues were pelleted. Total RNA in the supernatant was collected using an RNeasy Mini Kit (QIAGEN), and genomic DNA was digested with RNase-free DNase I (QIAGEN).
Analysis of LF expression by RT-PCR of mouse tissue and human RPE and cornea Cells
Total RNA (5 µg) was reverse transcribed using SuperScript III First Strand Synthesis System (Invitrogen, Grand Island, NY). Reverse transcription polymerase chain reaction (RT-PCR) was performed using a RoboCycler Gradient 96 thermocycler (Stratagene, Santa Clara, CA) with Choice-Taq DNA polymerase and PCR buffer (Denville Scientific, Holleston, MA) and primers for human LF (F, 5′-AAA CTT GTC TTC CTC GTC CT-3′; R, 5′-CAC CAG AGT AGC TGA AGT AC-3′) or murine LF (F, 5′-GCT GGA GAT GTG GCT TTT AC-3′; R, 5′-CAG AGA TTG GAT TTG GGG TC-3′), respectively. LF message was amplified over 33 cycles consisting of 45 s at 95 °C, 30 s at annealing temperature and 30 s at 72 °C; annealing temperature for murine primers was 55 °C, and for human primers, 58 °C. RT-PCR products were separated by electrophoresis on 1.2% agarose gels containing ethidium bromide and visualized under UV light. Bands of expected product size were excised from gels, and RT-PCR products were purified using a QIAquick Gel Extraction Kit (QIAGEN). The identity of the PCR products was confirmed by the standard automated sequencing methods provided by the BioMedical Genomics Center at the University of Minnesota (Minneapolis, MN).
Western blotting of human and murine ocular proteins
Human and murine ocular proteins were electrophoretically separated on 10% SDS–polyacrylamide gels and transferred to polyvinylidene difluoride (PVDF) membranes. Membranes were incubated for 24 h at 4 °C with the primary antibody (Table 1). The appropriate secondary antibody conjugated to horseradish peroxidase (HRP; Pierce) was applied to the membrane. Reactions were developed with chemiluminescence substrate (SuperSignal West Dura Extended Duration substrate; Pierce). Images were taken with a gel documentation system (ChemiDoc XRS; Bio-Rad, Hercules, CA).29
Table 1.
Application and specificity of antibodies used in this study.
| Antibody | Dilution | Type | Product numbera |
Cross Reacts with TF |
Application |
|---|---|---|---|---|---|
| Anti-LF | 1:5000 | Rp | ab77705 | + | 1D, 2D |
| Anti-LF | 1:1000 | Rp | ab77780 | + | 2D |
| Anti-LF | 1:1000 | M | ab10110 | + | 2D |
| Anti-LF | 1:1000 | M | ab38155 | − | 1D |
| Anti-LF | 1:100 | M | ab166803 | − | IHC |
| Anti-TF | 1:10,000 | Rp | ab82411 | + | 1D, 2D |
M, monoclonal, host species mouse; RP, IgG polyclonal, host species rabbit; 1D, 2D, one or two dimensional western blots; IHC, immunohistochemistry.
All products were purchased from Abcam (Cambridge, MA).
Preparation of human retina, RPE, Iris and cornea for western blot and immunohistochemistry
Preparation of human tissue for 1D and 2D western blot
Human retina, RPE, iris and cornea were isolated from donor eyes (n = 3) obtained from the Minnesota Lions Eye Bank (Table 2) (Minneapolis, MN), in accordance with the criteria outlined in the Declaration of Helsinki. The Minnesota Grading System (MGS) was used to evaluate the donor eyes for the presence of retinal disease.30 The human RPE tissue for this study was obtained from donors graded MGS-1, which are without age-related macular degeneration or other clinically obvious retinal diseases. Proteins from the neural retina, RPE cells (300 µL buffer volume) and iris (100 µL buffer volume) were prepared as described previously.31,32 Human corneal epithelial cells were scraped from the cornea, and corneal endothelium cells were isolated under a dissection microscope at 4× magnification. Cells were pelleted and then incubated in RIPA buffer (Sigma-Aldrich) (+Protease inhibitors II and III) for 1 h for protein extraction. Protein concentration was determined using the BCA protein Assay kit (Pierce). Human iris was processed in a similar fashion using a buffer volume of 100 µL buffer/iris.
Table 2.
Human donor information.
| Donor | Gender | Age | Cause of death | Time to freezing | Application |
|---|---|---|---|---|---|
| 1 | F | 73 | ESRD | 3’40” | 1D |
| 2 | F | 84 | CVA | 4’43” | 1D |
| 3 | M | 92 | Heart Failure | 7’30” | 1D |
| 4 | F | 40 | BSH | 4’40” | IHC |
| 5 | M | 84 | Pneumonia | 2’55” | IHC |
| 6 | F | 52 | Colon cancer | 5’20” | PCR from culture |
| 7 | M | 59 | Cancer | 4’10” | PCR from culture |
ESRD, end stage renal disease, CVA: cerebral vascular accident; BSH: brain stem hemorrhage; 1D, one dimensional western blot; IHC: immunohistochemestry.
Immunohistochemistry
Two pair of human donor eyes (Table 2) obtained from the Minnesota Lions Eye Bank were received less than 5 hours postmortem. For one eye, the anterior segment, lens and vitreous was removed and the eyecup was photographed and processed for evaluation of ocular disease. The fellow eye was fixed at 4 °C in 4% paraformaldehyde for 16 h, the retina and RPE layer were removed from the eye cups and together with the cornea processed for immunohistochemistry as free-floating whole mounts. Prior to antibody labeling, the tissues were washed in PBS and blocked for 2 h with 10% normal donkey serum (Jackson Immuno Research) at room temperature. The whole mounts were incubated with anti-LF (ab166803, Table 1) overnight at 4 °C. After three rinses in PBS, a corresponding antibody (Alexa Fluor 488, 1:800, Molecular Probes, Carlesbad, CA) was applied and the tissues were incubated for 4 h at room temperature. To confirm specificity, the primary antibody was omitted. The tissues were mounted on Superfrost Plus Microscope Slides and counterstained with DAPI (Vector Laboratories, Inc., Burlingame, CA). Fluorescence images were obtained using a LEICA DM 4000B microscope (Leica Microscopy, Wetzler, Germany).
RPE cell culture
RPE cells were isolated according to a well described procedure.33 The human RPE cells were cultured with Minimum Essential Medium Eagle (Alpha modification) supplemented with 5% heat inactivated fetal bovine serum, 100 mM sodium pyruvate, 1% nonessential amino acids, 50 U/mL Penicillin and 50 µg/mL Streptomycin. After removal of the media, cells were lysed in 20 mM Tris–HCl, 150 mM NaCl and 2% Triton X-100 (pH 7.6) for western Blot assay or were scrapped from the dishes with Dulbecco’s phosphate buffered saline for PCR assay. The lysate was centrifuged for 10 min at 3000g and supernatants were collected and frozen −80 °C.
2D gel electrophoresis and western immunoblot of 2D and 1D gels
Conditions for 2D gel electrophoresis were as described.29 2D gels were loaded with 5 µg of purified Human LF from milk and Human TF protein from plasma (Sigma-Aldrich). For the first dimension, a pH 3–10 linear gradient (Bio-Rad) was used, and for the second dimension a 12% SDS–PAGE. The proteins were separated by charge and size, transferred to PVDF membranes and probed with one or more of the primary monoclonal or polyclonal antibodies listed in Table 1. Goat anti-rabbit and goat anti-mouse phosphatase-conjugated secondary antibodies were used in conjunction with SuperSignal West Dura Extended Duration Substrate, enhanced with chemiluminescence HRP to visualize immunoreaction. Membranes were imaged with a gel documentation system (ChemiDoc XRS; Bio-Rad).
Human corneal epithelial cell preparation for RT-PCR
Human corneal epithelial cells were isolated from donor corneas obtained from the Minnesota Lions Eye Bank (Minneapolis, MN). For RT-PCR experiments, central corneas were dissected from donor corneal buttons with endothelium removed and then treated with dispase II (2 mg/mL) at 37 °C for 1 h (Roche Applied Science, Indianapolis, IN) to dissociate the epithelial sheets. After trypsin digestion, epithelial cells were cultured in keratinocyte growth medium-2 (Lonza) for one passage followed by RNA extraction.34
Results
LF expression
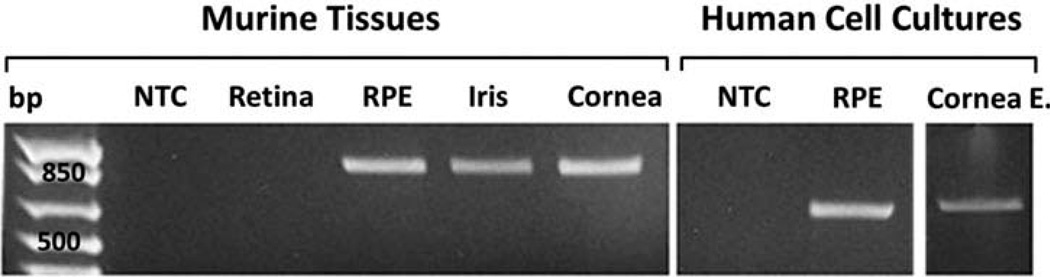
Murine Retina, RPE, iris and corneal tissues, as well as human RPE and corneal epithelial cells cultivated from donor eyes (Table 2), were analyzed for the expression of LF by RT-PCR. A PCR product with the expected size of 879 base pair was amplified from cDNAs of murine RPE, iris and corneal tissues but not retina (Figure 1). Cultured human RPE and corneal epithelial cells also expressed LF according to our RT-PCR results showing an amplicon of 600 base pairs (Figure 1). Sequencing results validated the identity of both amplicons to be LF. These results confirm that the LF message is being expressed in all tested ocular cells and tissues except the retina. Due to the inherent postmortem instability of mRNA,35 RT-PCR experiment of human donor tissues for LF expression was not performed.
Figure 1.
Expression of LF in murine tissue and human cultured cells. Image is an agarose gel resolving PCR products corresponding to LF that were amplified from the RPE, iris and cornea of C57/BL6J mice (879 bp). Human cultured cells from the RPE and corneal epithelium also produced a PCR product for LF (600 bp). Cornea E., corneal epithelium, NTC, no template control; bp, 1 kb base pair ladder.
LF localization
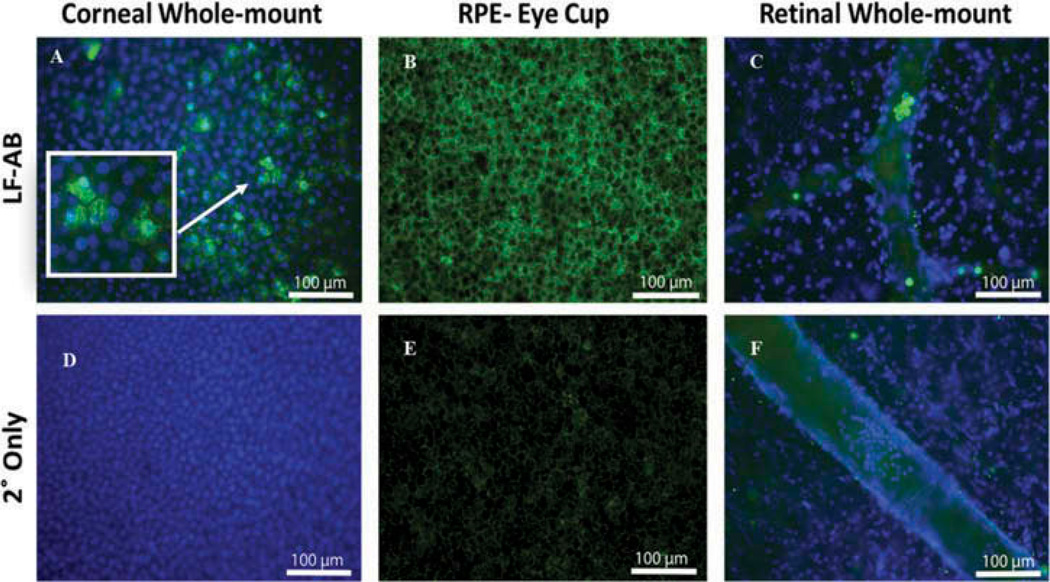
Immunostaining experiments were performed on donor eyes to investigate the presence of LF in human ocular tissues. An anti-LF antibody (ab166803, Table 1) that is suitable for immunohistochemistry applications in humans was used. Strong signals of punctate staining for LF were observed all over the corneal epithelium, and appeared to be located in the nuclei and cytoplasm of the epithelial cells (Figure 2A). Staining with secondary antibody only (Figure 2D) produced no signals in the corneal epithelium. RPE displayed a more uniform distribution of LF staining (Figure 2B) with their nuclei devoid of staining signals. We did observe faint background fluorescence when RPE were stained with secondary antibody only (Figure 2E), which is likely caused by the autoflourescence of lipofuscin. No staining signals were observed in the retina (Figure 2C). However, cells in the blood vessels stained positive for LF. Staining with secondary antibody only (Figure 2F) showed no signals either in the retina or cells in the blood vessels. The immunohistochemical staining corroborates our PCR findings that LF expression was not detected in the retina.
Figure 2.
Immunohistochemistry of flat mounts of cornea, RPE and retina from a human donor eye. (A) LF (ab166803) staining (green) in human cornea with DAPI-stained nuclei (blue). Arrows point to cells with nuclear and cytoplasmic staining. Box shows enlarged image of cells. (B) LF staining in human RPE. (C) LF staining in human retinal tissue counterstained with DAPI to mark nuclei. There is a positive LF staining present only in cells within blood vessels. (D) Secondary only staining of corneal epithelium cells. (E) Secondary only staining of RPE cells. Background fluorescence is caused by the lipofuscin autofluorescence. (F) Secondary only staining of retina containing a retinal blood vessel. *Green, LF; blue, DAPI; arrow, blood cells in C and F.
LF protein content
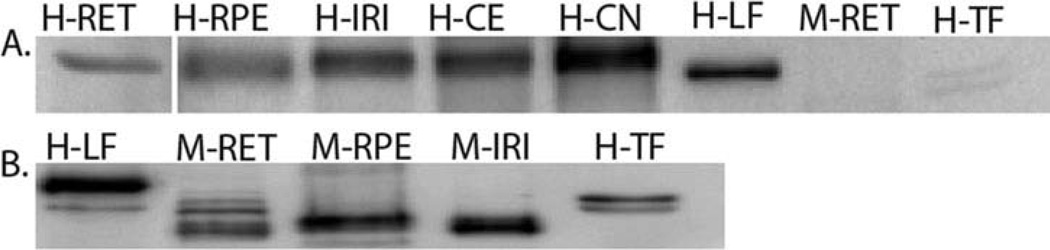
In light of our results from RT-PCR and immunostaining, we further investigated if LF protein was present in ocular tissues by western blotting (Figure 3). Prominent bands with molecular weight close to 80 kDa were detected in human RPE, Iris, cornea endothelium and epithelium by the antihuman LF antibody ab38155 (Table 1). In contrast, much weaker bands were present in the retina. Note that the purified human LF displayed slightly lower molecular weight than the endogenous proteins. This variation of gel mobility can be caused by the commercial methods of separation/purification of LF. The antibody (ab38155) used for western blots specifically recognizes human LF, and does not cross-react with purified human TF or murine LF (either in the retina, Figure 3A, or iris, data not shown).
Figure 3.
Western blot of murine and human ocular tissue. (A) Human eye tissue (retina, RPE, iris, corneal epithelium and endothelium) were probed with anti-LF antibody (ab38155). Mouse retina was included to check cross reactivity with this antibody. HLF and HTF were positive and negative controls, respectively. *H, human, M, mouse; RET, retina; RPE, Retinal pigmented epithelium, IRI, iris; COE, corneal epithelium; CON, corneal endothelium; LF, lactoferrin; TF, transferrin. (B) C57/BL6J mice eye tissue processed for protein and analyzed using anti-Lactoferrin antibody (ab77705). Human purified lactoferrin (HLF) and transferrin (HTF) were included as positive and negative controls, respectively.
In contrast to the positive outcomes from human tissue, the western blot results from murine ocular tissues were inconclusive. Anti-LF antibodies that work well in human ocular tissues either lack specificity toward murine LF or reacted nonspecifically toward TF and other proteins. We have extensively tested an array of commercially available anti-LF antibodies (Table 1) and have yet to identify a suitable antibody for murine study. For example, Figure 3(B) shows the western blot results using an anti-LF antibody (ab77705). While the particular antibody recognizes purified human LF, and detects protein bands corresponding to a protein at ~80 kDa in mouse retina, RPE and iris, it also cross-reacts with human TF (Figure 3B) and produced nonspecific reactions in ocular tissues from LF-knockout mice (data not shown).
Evaluation of Anti-LF antibodies by 2D gel and western blots
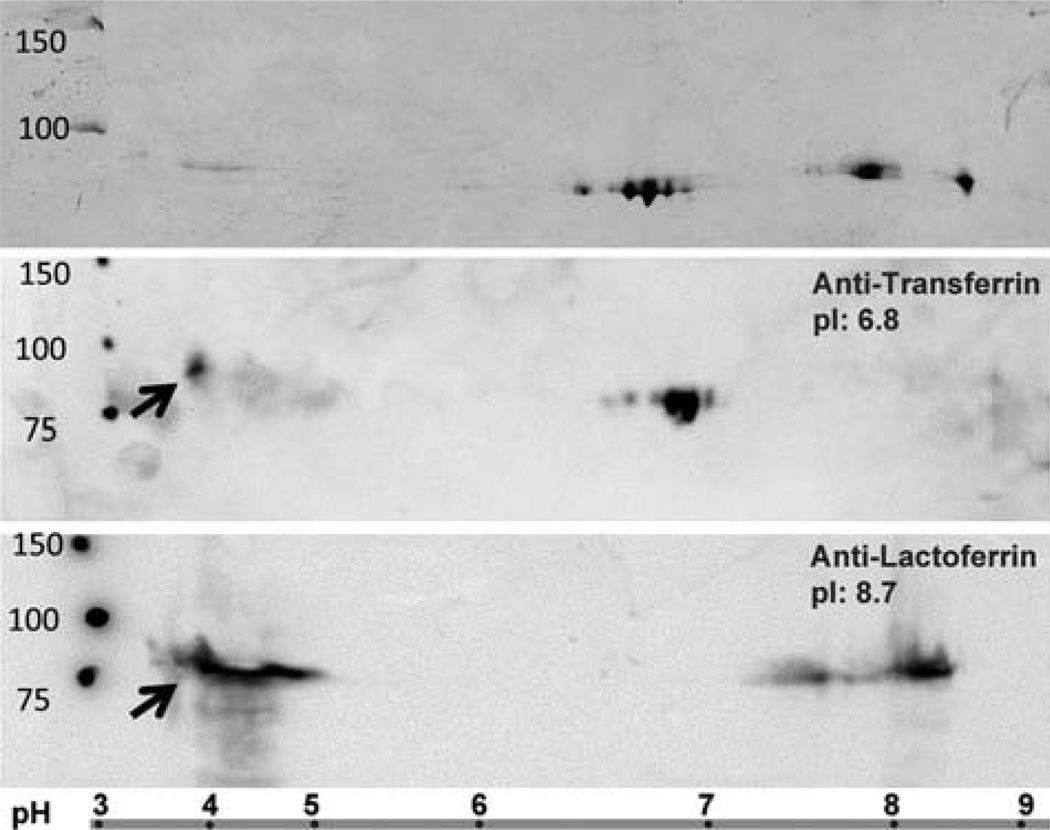
LF and TF share ~65% amino acid sequence homology,3 so it is possible that antibodies developed for LF could cross-react with TF. Although both proteins have approximately the same molecular weight (~80 KDa) and migrate similarly by SDS–PAGE, LF and TF differ substantially in their isoelectric points (pI); the pI for LF ranges from 8.4 to 9.036 and pI for TF is 6.8.37 Therefore, 2D gel electrophoresis was performed to separate human LF and TF for testing antibody specificity (Figure 4). The Coomassie Blue stained gel showed clear separation of three groups of protein spots (Figure 4, top). Two groups clustered at the expected pI for LF and TF, and a third group of faint stainings was located in the acidic range. Probing with an anti-TF antibody (ab82411) revealed that TF runs as a “charge train” with the main reaction at predicted pI ~6.8 (Figure 4, middle). The multiple protein spots may be due to the varied phosphorylation status. (Note that there are 39 predicted phosphorylation sites for TF, and 42 for LF.)38 No reaction on the TF blot was observed at the expected pI for LF (pI ~8.7), but faint spots were noted within the low pH range (3.5–5).
Figure 4.
2D gel separations of purified LF and TF. Purified proteins (LF and TF) were resolved by isoelectric focusing on an immobilized strip with a nonlinear pH gradient (3–10) in the first dimension, followed by SDS–PAGE (12%) in the second dimension. Top: Image is the Coomasie blue stained gel. Middle: PVDF membrane stained with the anti-TF antibody (ab82411) reacts with the TF protein (pI 6.8) but not LF. Bottom: PVDF membrane stained with anti-LF antibody (ab77705) reacts with LF protein (pI 8.7) but not TF. Arrows show a cross reaction with both anti-TF (B) and anti-LF (C) for an acidic protein. Ladder at the bottom of the figure shows the nonlinear pH gradient for proteins separation in the first dimensions. MW, Molecular weight marker. Numbers indicate protein size.
Figure 4 bottom shows the western blot results using anti-LF antibody (ab77705) to detect human LF in the 2D gels. In addition to the protein spots corresponding to LF (pI ~8.7), a strong reaction to the acidic proteins from pH 3.5 to 5 was noted as well. These acidic proteins may contribute to the cross-reactivity of anti-LF and anti-TF antibodies. Interestingly, one of the anti-LF antibodies we tested reacted only to proteins within this low pH range (ab77780, data not shown).
Discussion
In the current study, we investigated LF expression and presence of LF protein in various human and murine ocular tissues. Our RT-PCR results (Figure 1) support the expression of LF in mouse iris, RPE, cornea, and in cultured human epithelial cells from the cornea and RPE. In human donor tissues, the presence of LF in the cornea, iris and RPE was further confirmed based on western blot and immunohistochemistry results (Figures 2 and 3A). Although western blots of human retinal lysates showed a faint band detected by the LF antibody (Figure 3A), absence of LF mRNA in the murine retina (Figure 1) and the negative staining signals in human retinal whole mounts (Figure 2C) indicate that this reaction may be due to LF-positive cells circulating in the blood vessels or to contamination from other retinal tissues, such as the RPE and vitreous, during dissection. Due to the poor specificity of commercially available antibodies against murine LF, we were unable to obtain a definitive conclusion on the LF distribution in murine ocular tissues by either immunohistochemistry (data not shown) or western blot experiments. To the best of our knowledge, this is the first report that extensively investigates the expression of this multifunctional protein in human and murine ocular tissues. The results presented in the current study may help us understand the physiological roles of LF and identify its therapeutic potential for a variety of eye disorders.
LF is known to have a protective effect against tissue injuries. In the cornea, Pattamatta et al. demonstrated that topical application of bovine LF accelerated the healing of corneal alkali injury in BALB/c mice and this was associated with suppression of IL-1 and reduced infiltrating cells.19 Fujihara et al. also showed that pretreatment of topical LF suppressed development of a corneal epithelial defect induced by UV-B irradiation in rats.18 These studies suggest promising potential for LF treatment in the healing process of different corneal injury models. Although present in the tear film, LF’s content can be significantly reduced under pathological conditions such as dry eye10 or the corneal injuries mentioned above, therefore providing less protection. Further study to investigate the expression of LF under injury or insults may provide insights for the protective roles played by LF.
The TF family plays an important role in iron homeostasis, so it is possible that different family members can assume this important function. In the current study we observed LF expression in the RPE but not retina. Other researchers have reported TF is expressed in the retina.9 Chowers et al. has shown that TF expression was higher in retinas of patients with age-related macular degeneration (AMD) compared to control retinas from healthy individuals.39 Their results suggest that altered iron homeostasis is associated with AMD, and thus the metabolism of iron may serve as a target for therapeutic interventions.40 Our previous study of a laser-induced choroidal neovascularization murine model indicates that LF protects ocular tissues from pathologic neovascularization as LFKO mice displayed significantly severe laser-induced choroidal lesions when compared to WT. Administration of additional exogenous bovine LF can further decrease the chorioretinal damage in the laser induced CNV model in mice.22 While the mechanism of action remains unclear, it may involve LF’s role in iron binding and subsequent reduction in the Fenton Chemistry reaction that produce damaging hydroxyl radicals by laser insult. Knowing that LF is expressed in the RPE tissue is the first step in identifying the potential mechanism to explain the larger lesion volumes of LFKO versus WT mice. This new information could guide us to a potential targeted treatment in retinal diseases complicated by choroidal neovascularization and offer a novel therapy for patients at risk for blindness secondary to neovascularization.
Prior papers had identified the expression of LF in the vitreous,23–26 thus, we did not confirm these reported findings. However, we did notice a faint LF reaction in the retina that we interpreted as possible contamination from retina circulation. Contamination from the vitreous or RPE cells could also be a source of the low levels of LF observed in retinal preparations. Alternatively, it is possible that the LF identified previously in the vitreous, is due to leakage from retina blood circulation and/or RPE secretion. These possibilities will need to be experimentally tested in order to definitively know the exact sources of LF in the retina.
Our current study has identified suitable antibodies that are highly specific to human LF for immunohistochemistry and western blot applications. This information is essential for further research to understand the function of these iron-binding proteins in human ocular tissues, including the change of these proteins with aging between normal and disease states. Even with our extensive efforts, we did not find any commercially available anti-LF antibodies that demonstrated specificity for LF in murine tissues. In the process to identify potential cause for cross-reactivity or low specificity, we have confirmed the heterogeneous compositions of purified TF and LF proteins, which are likely due to multiple post-translational modifications or preparation methods.41 The reaction of several anti-LF antibodies to certain acidic proteins (Figure 4) may contribute to the observed cross-reactivity and necessitate better antigen preparation for anti-LF antibody production.
In summary, LF is expressed in the cornea, iris and RPE of human and mouse tissues, but not in the retina. Future studies are warranted for clinical applications.
Acknowledgments
Declaration of interest
This research was supported by funds from the University of Minnesota Foundation, Minnesota Lions Vision Foundation, an anonymous donor to the Department of Ophthalmology and Visual Neurosciences for AMD research, and an unrestricted grant to the Department of Ophthalmology and Visual Neurosciences from the Research to Prevent Blindness (RPB), New York, NY, USA. The NIH provides support for the Histology Core for Vision Research (P30-EY00374).
References
- 1.Wally J, Buchanan SK. A structural comparison of human serum transferrin and human lactoferrin. Biometals. 2007;20:249–262. doi: 10.1007/s10534-006-9062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gifford JL, Hunter HN, Vogel HJ. Lactoferricin: a lactoferrin derived peptide with antimicrobial, antiviral, antitumor and immunological properties. Cell Mol Life Sci. 2005;62:2588–2598. doi: 10.1007/s00018-005-5373-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker EN, Baker HM. Molecular structure, binding properties and dynamics of lactoferrin. Cell Mol Life Sci. 2005;62:2531–2539. doi: 10.1007/s00018-005-5368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teng CT, Pentecost BT, Chen YH, Newbold RR, Eddy EM, McLachlan JA. Lactotransferrin gene expression in the mouse uterus and mammary gland. Endocrinology. 1989;124:992–999. doi: 10.1210/endo-124-2-992. [DOI] [PubMed] [Google Scholar]
- 5.Teng CT, Beard C, Gladwell W. Differential expression and estrogen response of lactoferrin gene in the female reproductive tract of mouse, rat, and hamster. Biol Reprod. 2002;67:1439–1449. doi: 10.1095/biolreprod.101.002089. [DOI] [PubMed] [Google Scholar]
- 6.Abrink M, Larsson E, Gobl A, Hellman L. Expression of lactoferrin in the kidney: implications for innate immunity and iron metabolism. Kidney Int. 2000;57:2004–2010. doi: 10.1046/j.1523-1755.2000.00050.x. [DOI] [PubMed] [Google Scholar]
- 7.Fillebeen C, Mitchell V, Dexter D, Benaissa M, Beauvillain JC, Spik G, et al. Lactoferrin is synthesized by mouse brain tissue and its expression is enhanced after MPTP treatment. Brain Res Mol Brain Res. 1999;72:183–194. doi: 10.1016/s0169-328x(99)00221-1. [DOI] [PubMed] [Google Scholar]
- 8.Isamida T, Tanaka T, Omata Y, Yamauchi K, Shimazaki K, Saito A. Protective effect of lactoferricin against Toxoplasma gondii infection in mice. J Vet Med Sci. 1998;60:241–244. doi: 10.1292/jvms.60.241. [DOI] [PubMed] [Google Scholar]
- 9.Chowers I, Gunatilaka T, Farkas R, Qian J, Hackam AS, Duh E, et al. Identification of novel genes preferentially expressed in the retina using a custom human retina cDNA microarray. Invest Ophthalmol Vis Sci. 2003;44:3732–3741. doi: 10.1167/iovs.02-1080. [DOI] [PubMed] [Google Scholar]
- 10.Flanagan JL, Willcox MD. Role of lactoferrin in the tear film. Biochimie. 2009;91:35–43. doi: 10.1016/j.biochi.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Sriramoju B, Kanwar RK, Kanwar JR. Lactoferrin induced neuronal differentiation: a boon for brain tumours. Int J Dev Neurosci. 2014;41C:28–36. doi: 10.1016/j.ijdevneu.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Arnold RR, Russell JE, Champion WJ, Brewer M, Gauthier JJ. Bactericidal activity of human lactoferrin: differentiation from the stasis of iron deprivation. Infect Immun. 1982;35:792–799. doi: 10.1128/iai.35.3.792-799.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnold RR, Cole MF, McGhee JR. A bactericidal effect for human lactoferrin. Science. 1977;197:263–265. doi: 10.1126/science.327545. [DOI] [PubMed] [Google Scholar]
- 14.Drago-Serrano M, Garza Amaya M, Serrano Luna J, Campos-Rodriguez R. Lactoferrin–lipopolysaccharide (LPS) binding as key to antibacterial and antiendotoxic effects. Int Immunopharmacol. 2012;12:1–9. doi: 10.1016/j.intimp.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Friel JK, Martin SM, Langdon M, Herzberg GR, Buettner GR. Milk from mothers of both premature and full-term infants provides better antioxidant protection than does infant formula. Pediatr Res. 2002;51:612–618. doi: 10.1203/00006450-200205000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Bezault J, Bhimani R, Wiprovnick J, Furmanski P. Human lactoferrin inhibits growth of solid tumors and development of experimental metastases in mice. Cancer Res. 1994;54:2310–2312. [PubMed] [Google Scholar]
- 17.Santagati MG, La Terra Mule S, Amico C, Pistone M, Rusciano D, Enea V. Lactoferrin expression by bovine ocular surface epithelia: a primary cell culture model to study lactoferrin gene promoter activity. Ophthal Res. 2005;37:270–278. doi: 10.1159/000087372. [DOI] [PubMed] [Google Scholar]
- 18.Fujihara T, Nagano T, Endo K, Nakamura M, Nakata K. Lactoferrin protects against UV-B irradiation-induced corneal epithelial damage in rats. Cornea. 2000;19:207–211. doi: 10.1097/00003226-200003000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Pattamatta U, Willcox M, Stapelton F, Garrett Q. Bovine lactoferrin promotes corneal wound healing and supresses IL-1 expression in alkali wounded mouse cornea. Curr Eye Res. 2013;38:1110–1117. doi: 10.3109/02713683.2013.811259. [DOI] [PubMed] [Google Scholar]
- 20.Pattamatta U, Willcox M, Stapleton F, Cole N, Garrett Q. Bovine lactoferrin stimulates human corneal epithelial alkali wound healing in vitro. Investig Ophthalmol Vis Sci. 2009;50:1636–1643. doi: 10.1167/iovs.08-1882. [DOI] [PubMed] [Google Scholar]
- 21.Ashby B, Garrett Q, Willcox M. Bovine lactoferrin structures promoting corneal epithelial wound healing in vitro. Invest Ophthalmol Vis Sci. 2011;52:2719–2726. doi: 10.1167/iovs.10-6352. [DOI] [PubMed] [Google Scholar]
- 22.Montezuma SR, Dolezal L, Rageh A, Mar K, Jordan M, Ferrington DA. Lactoferrin reduces chorioretinal damage in the murine laser model of choroidal neovascularization. Curr Eye Res. 2015;40:946–953. doi: 10.3109/02713683.2014.969808. [DOI] [PubMed] [Google Scholar]
- 23.Clausen R, Weller M, Wiedemann P, Heimann K, Hilgers RD, Zilles K. An immunochemical quantitative analysis of the protein pattern in physiologic and pathologic vitreous. Graefe’s Arch Clin Exp Ophthalmol. 1991;229:186–190. doi: 10.1007/BF00170555. [DOI] [PubMed] [Google Scholar]
- 24.Moter H, Weller M, Heimann K, Wiedemann P. Lactoferrin and transferrin–iron-binding proteins in physiological and pathological vitreous bodies. Fortsch Ophthalmol Zeit Deuts Ophthalmol Gesellschaft. 1990;87:336–339. [PubMed] [Google Scholar]
- 25.Van Bockxmeer FM, Martin CE, Constable IJ. Iron-binding proteins in vitreous humour. Biochim Biophys Acta. 1983;758:17–23. doi: 10.1016/0304-4165(83)90004-1. [DOI] [PubMed] [Google Scholar]
- 26.Weller M, Clausen R, Heimann K, Wiedemann P. Iron-binding proteins in the human vitreous: lactoferrin and transferrin in health and in proliferative intraocular disorders. Ophthal Res. 1990;22:194–200. doi: 10.1159/000267023. [DOI] [PubMed] [Google Scholar]
- 27.Mattapallil MJ, Wawrousek EF, Chan CC, Zhao H, Roychouhury J, Ferguson TA, Caspi RR. The Rd8 mutation of the Crb1 gene is present in vendor lines of C57BL/6N mice and embryonic stem cells, and confounds ocular induced mutant phenotypes. Invest Ophthalmol Vis Sci. 2012;53:2921–2927. doi: 10.1167/iovs.12-9662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrington DA, Hussong SA, Roehrich H, Kapphahn RJ, Kavanaugh SM, Heuss ND, Gregerson DS. Immunoproteasome responds to injury in the retina and brain. J Neurochem. 2008;106:158–169. doi: 10.1111/j.1471-4159.2008.05345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapphahn RJ, Giwa BM, Berg KM, Roehrich H, Feng X, Olsen TW, et al. Retinal proteins modified by 4-hydroxynonenal: identification of molecular targets. Exp Eye Res. 2006;83:165–175. doi: 10.1016/j.exer.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 30.Olsen TW, Feng X. The Minnesota Grading System of eye bank eyes for age-related macular degeneration. Invest Ophthalmol Vis Sci. 2004;45:4484–4490. doi: 10.1167/iovs.04-0342. [DOI] [PubMed] [Google Scholar]
- 31.Nordgaard CL, Berg KM, Kapphahn RJ, Reilly C, Feng X, Olsen TW, et al. Proteomics of the retinal pigment epithelium reveals altered protein expression at progressive stages of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2006;47:815–822. doi: 10.1167/iovs.05-0976. [DOI] [PubMed] [Google Scholar]
- 32.Ethen CM, Feng X, Olsen TW, Ferrington DA. Declines in arrestin and rhodopsin in the macula with progression of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2005;46:769–775. doi: 10.1167/iovs.04-0810. [DOI] [PubMed] [Google Scholar]
- 33.Sonoda S, Spee C, Barron E, Ryan SJ, Kannan R, Hinton DR. A protocol for the culture and differentiation of highly polarized human retinal pigment epithelial cells. Nat Protoc. 2009;4:662–673. doi: 10.1038/nprot.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amirjamshidi H, Milani BY, Sagha HM, Movahedan A, Shafig MA, Lavker RM, et al. Limbal fibroblast conditioned media: a non-invasive treatment for limbal stem cell deficiency. Mol Vis. 2011;17:658–666. [PMC free article] [PubMed] [Google Scholar]
- 35.Malik KJ, Chen CD, Olsen TW. Stability of RNA from the retina and retinal pigment epithelium in a porcine model of simulating eye bank conditions. Invest Ophthalmol Vis Sci. 2003;44:2730–2735. doi: 10.1167/iovs.02-1120. [DOI] [PubMed] [Google Scholar]
- 36.Farnaud S, Evans RW. Lactoferrin – a multifunctional protein with antimicrobial properties. Mol Immunol. 2003;40:395–405. doi: 10.1016/s0161-5890(03)00152-4. [DOI] [PubMed] [Google Scholar]
- 37.Luo LZ, Jin HW, Huang HQ. Application of capillary isoelectric focusing and peptide mass fingerprinting in carbohydrate-deficient transferrin detection. Rapid Commun Mass Spectrom. 2011;25:1391–1398. doi: 10.1002/rcm.4993. [DOI] [PubMed] [Google Scholar]
- 38.Blom N, Gammeltoft S, Brunak S. Sequence- and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol. 1999;294:1351–1362. doi: 10.1006/jmbi.1999.3310. [DOI] [PubMed] [Google Scholar]
- 39.Chowers I, Wong R, Dentchev T, Farkas RH, Lacovelli J, Gunatilaka TL, et al. The iron carrier transferrin is upregulated in retinas from patients with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2006;47:2135–2140. doi: 10.1167/iovs.05-1135. [DOI] [PubMed] [Google Scholar]
- 40.Yefimova MG, Jeanny JC, Guillonneau X, Keller N, Nguyen-Legros J, Sergeant C, et al. Iron, ferritin, transferrin, and transferrin receptor in the adult rat retina. Invest Ophthalmol Vis Sci. 2000;41:2343–2351. [PubMed] [Google Scholar]
- 41.Steijns JM, Van Hooijdonk ACM. Occurrence, structure, biochemical properties and technological characteristics of lactoferrin. Br J Nutr. 2007;84:S11–S17. doi: 10.1017/s0007114500002191. [DOI] [PubMed] [Google Scholar]