Abstract
Objective
To clarify the characteristics of cryptogenic stroke in patients with active cancer.
Methods
Patients with or without cancer diagnosed with acute ischemic stroke between January 2006 and February 2015 were extracted from a prospectively collected stroke database of Osaka University Hospital. Patients were categorized according to the presence of active cancer and known stroke mechanisms.
Results
Among 1191 patients with acute ischemic stroke, 145 (12%) had active cancer. Patients with active cancer were diagnosed more often with cryptogenic stroke than were patients without cancer (47% vs. 12%, P < 0.001). Compared with cryptogenic stroke patients without cancer, cryptogenic stroke patients with active cancer had fewer atherosclerotic risk factors, lower nutrition status, higher plasma D‐dimer levels, and multiple vascular lesions. In a multivariate logistic analysis, plasma D‐dimer level (odds ratio [OR] per 1 standard deviation increase: 6.30; 95% confidence interval [CI]: 2.94–15.69; P < 0.001), and the presence of multiple vascular lesions (OR: 6.40; 95% CI: 2.35–18.35; P < 0.001) were independent predictors of active cancer. When comparing active cancer patients who had known stroke mechanisms with those who had cryptogenic stroke, high plasma D‐dimer levels, multiple vascular lesions, and receiving chemotherapy and/or radiation therapy were associated with cryptogenic stroke etiology.
Interpretation
In cryptogenic stroke, patients with active cancer has a unique pathology characterized by high plasma D‐dimer levels and multiple vascular lesions. The hypercoagulable state and malnutrition due to cancer and its treatments potentially influence the development of cryptogenic stroke in cancer patients.
Introduction
Thromboembolism is a common complication in cancer.1 Approximately 7% of cancer patients have symptomatic cerebrovascular diseases during the clinical course, and 15% have cerebrovascular lesions at autopsy.2 The incidence of stroke is approximately 1.5 times higher in cancer patients than in the general population.3
Stroke complicated by cancer has distinct mechanisms characterized by hypercoagulability in cancer, manifesting as, e.g., nonbacterial thrombotic endocarditis, intravascular coagulation, and tumor embolism.4, 5 However, these stroke mechanisms are often difficult to diagnose except in an autopsy2; as a result, they can be diagnosed as cryptogenic stroke in clinical practice. In fact, in patients with cancer, the incidence of cryptogenic stroke is reported to account for approximately 40–50% of stroke mechanisms6, 7, 8, 9 and is more prevalent in patients without cancer.10, 11 Previous studies have demonstrated that cancer patients with cryptogenic stroke often have high plasma D‐dimer levels and multiple vascular lesion patterns6, 7 and are associated with reduced survival,8 suggesting that cryptogenic stroke in these patients needs specific consideration. However, the characteristics of cryptogenic stroke in cancer patients are less well understood. Little is available on the comparative data of cryptogenic stroke in both cancer and noncancer patients within the same population. In our experience, cancer patients with stroke often seem to be suffering from malnutrition. Therefore, we hypothesized that nutritional status might be associated with the development of ischemic stroke in cancer patients.
The purpose of this study was to clarify the characteristics of ischemic stroke, especially of cryptogenic etiology, in cancer patients by using a prospectively collected stroke database that included both cancer and noncancer patients.
Methods
Standard protocol approvals, registrations, and patient consent
The ethics committee for clinical research at Osaka University Hospital approved this study. The need for informed consent was waived owing to the retrospective nature of the study.
Patients
We reviewed 2620 consecutive stroke patients admitted to the stroke unit of Osaka University Hospital between January 2006 and February 2015 from the hospital's prospectively collected stroke database. Osaka University Hospital is a large academic urban hospital, which has a role as a regional comprehensive cancer center. Patients diagnosed with acute ischemic stroke within 7 days after symptom onset (n = 1191) were extracted from the database and categorized into 2 groups according to the presence of cancer: the cancer group (n = 265), and the noncancer group (n = 926). In the cancer group, patients with active cancer were selected. Active cancer was defined as a diagnosis of cancer either treated in the last 6 months before admission or untreated. The diagnosis of cancer was identified by reviewing each medical record. Patients who had an inactive cancer (n = 114), a primary brain tumor (n = 5), or an undiagnosed tumor (n = 1) were excluded. We finally identified 145 patients with acute ischemic stroke and active cancer. Further inclusion and exclusion criteria are shown in Figure 1.
Figure 1.
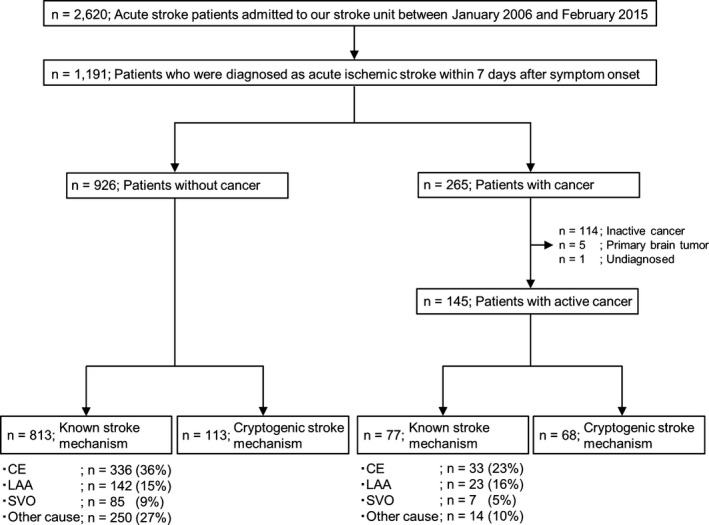
Patient selection flow chart. Numbers were rounded to the nearest whole number. CE, cardioembolism; LAA, large‐artery atherosclerosis; SVO, small‐vessel occlusion.
For the purpose of exploring the complication rate of ischemic stroke in each cancer type, we referred to the total number of inpatients and outpatients with each cancer type during the study period from the Osaka University Hospital database. We subsequently divided the number of ischemic stroke patients by the total number of patients with each cancer type, thereby obtaining the complication rate for ischemic stroke.
Clinical variables
The following clinical data were obtained from the clinical records of all the patients: age, sex, body mass index (BMI), and the presence of atherosclerotic risk factors, including hypertension, diabetes mellitus, hyperlipidemia, current smoking, and past history of stroke. Cancer‐related data, including type of cancer, cancer treatment, and the presence of systemic metastases, were also obtained. All the patients had baseline blood samples drawn at admission, and the samples were analyzed for the following: white blood cell count, total lymphocyte count, platelet count, and levels of hemoglobin, D‐dimer, high sensitivity C‐reactive protein (hsCRP), albumin, and total cholesterol. Nutritional status was assessed using controlling nutritional status (CONUT) score. The CONUT is an automated malnutrition screening system calculated by using serum albumin, total cholesterol, and total lymphocyte counts, and scoring (maximum 12 points) is as follows: 0–4 points, low risk; 5–8 points, moderate risk; and 9–12 points, high risk.12 .
Definition of vascular risk factors
Hypertension was defined as the history of hypertension or the use of antihypertensive medications. Hyperlipidemia was defined as the use of cholesterol‐lowering therapy, a fasting total serum cholesterol level ≥220 mg/dL, a triglyceride level ≥150 mg/dL, or a low‐density lipoprotein cholesterol level ≥140 mg/dL. Diabetes mellitus was defined as a fasting blood glucose level ≥126 mg/dL, a glycosylated hemoglobin A1c concentration ≥6.5% according to the National Glycohemoglobin Standardization Program, or the use of glucose‐lowering agents. BMI was calculated as weight in kilograms divided by the square of height in meters (kg/m2). Smoking was defined as current smoking.
Imaging
Three‐tesla magnetic resonance imaging (MRI) was performed at admission. Diffusion‐weighted images, T1‐ and T2‐weighted images, fluid‐attenuated inversion recovery images, and 3‐dimensional time‐of‐flight magnetic resonance angiography were obtained. If MRI was not possible for individual reasons, brain computed tomography (CT) was used. Multiple vascular lesions were defined as the multiple infarct lesions involving multiple arterial territories.
Classification of stroke mechanisms
All patients underwent 24‐hour electrocardiographic monitoring at least 7 days after admission, carotid ultrasonography, transthoracic echocardiography, and brain imaging (MRI or CT scan). Transesophageal echocardiography was performed as necessary. Two neurologists classified the mechanisms of ischemic stroke according to the Trial of Org 10172 in Acute Stroke Treatment classification13 and the mechanisms were finalized by consensus. Patients were classified into 2 subgroups according to stroke mechanisms: the known stroke mechanism group (large‐artery atherosclerosis, small‐vessel occlusion, cardioembolism, or other causes) and the cryptogenic stroke group.
Statistical analysis
All data are expressed as median and interquartile ranges or counts and percentages. Because the distributions of plasma D‐dimer levels were left‐skewed, they were normalized using logarithmic transformation. Values were compared using the Mann–Whitney U‐test for continuous variables and the chi‐square test for categorical variables. Among cryptogenic stroke patients, multivariate logistic regression analysis was performed to determine the independent predictors of active cancer, including age, sex, number of atherosclerotic risk factors, plasma D‐dimer levels, CONUT score, and the presence of multiple vascular lesions, because these factors are known to be related to cancer‐associated stroke etiology.6, 7 Similarly, among active cancer patients, multivariate logistic regression analysis was performed to determine the independent risk factors for cryptogenic stroke using the same variables and adding the variables for the presence of systemic metastases and chemotherapy and/or radiation therapy. All analyses were performed using JMP Pro software version 10.0.2 (SAS Institute Inc., Cary, NC). A P value <0.05 was considered statistically significant.
Results
Among 1191 patients with acute ischemic stroke, 926 (78%) had no cancer, and 145 (12%) had active cancer. Known stroke mechanisms were identified in 813 of 926 (88%) patients without cancer but in only 77 of 145 (53%) patients with active cancer. Therefore, patients with active cancer were diagnosed more often as having cryptogenic stroke than were those without cancer (47% vs. 12%, P < 0.001). In 145 ischemic stroke patients with cancer (38% women; median age, 71 years; interquartile range, 63–78), 75 developed ischemic stroke during hospitalization for cancer treatment. Twenty patients presented with ischemic stroke before the diagnosis of cancer. Transesophageal echocardiography was performed in 33 patients (18 in the cryptogenic stroke group). Table 1 shows the specific stroke mechanisms in 145 active cancer patients.
Table 1.
Index stroke mechanisms with active cancer patients
| Specific mechanisms | n (%)a |
|---|---|
| Cryptogenic | 68 (47) |
| Large artery atherosclerosis | 23 (16) |
| Atrial fibrillation | 21 (14) |
| Small vessel occlusion | 7 (5) |
| Nonbacterial endocarditis | 6 (4) |
| Catheter induced embolism | 4 (3) |
| Branch atheromatous disease | 3 (2) |
| Infective endocarditis | 3 (2) |
| Disseminated intravascular coagulation | 2 (1) |
| Cardiomyopathy | 2 (1) |
| Myocardial infarction | 1 (1) |
| Aortagenic embolism | 1 (1) |
| Arterial dissection | 1 (1) |
| Cerebral venous thrombosis | 1 (1) |
| Anti‐phospholipid antibody syndrome | 1 (1) |
| Air embolism | 1 (1) |
Percentages have been rounded up for simplicity of presentation and may not equal 100% in all cases.
Table 2 compares baseline characteristics and laboratory findings of cryptogenic stroke patients both with and without cancer. Compared with patients without cancer, patients with active cancer had lower BMI, fewer atherosclerotic risk factors, lower nutrition status, and higher plasma D‐dimer and hsCRP levels. Multiple vascular lesions were found more often in patients with active cancer than in those without cancer. In a multivariate logistic regression analysis (Table 3), plasma D‐dimer level (OR per 1‐SD increase, 6.30; 95% CI: 2.94–15.69; P < 0.001), and the presence of multiple vascular lesions (OR: 6.40; 95% CI: 2.35–18.35; P < 0.001) were independently associated with the presence of active cancer in cryptogenic stroke patients.
Table 2.
Characteristics of cryptogenic stroke patients with and without cancer
| Cryptogenic stroke patient characteristics | With cancer (n = 68) | Without cancer (n = 113) | P Value |
|---|---|---|---|
| Age, years | 67 (60–75) | 68 (57–74) | 0.76 |
| Women, % | 46 | 39 | 0.38 |
| Body mass index, kg/m2 | 19.7 (16.9–22.4) | 22.7 (20.3–25.2) | <0.001 |
| Atherosclerotic risk factors, n | 1 (0–2) | 2 (1–3) | <0.001 |
| Hypertension, % | 35 | 58 | 0.005 |
| Hyperlipidemia, % | 37 | 57 | 0.001 |
| Diabetes mellitus, % | 19 | 31 | 0.08 |
| Current smoking, % | 15 | 20 | 0.34 |
| Past stroke, % | 18 | 22 | 0.47 |
| Laboratory findings | |||
| White blood cells, /μL | 7,565 (4,985–10,738) | 6,750 (5,110–8,620) | 0.36 |
| Total lymphocytes, /μL | 904 (585–1270) | 1,492 (1,068–1,892) | <0.001 |
| Hemoglobin, g/dL | 10.6 (8.4–12.1) | 12.9 (11.4–14.7) | <0.001 |
| Platelets × 104/μL | 14.4 (8.3 – 22.1) | 21.5 (17.0–25.7) | <0.001 |
| Total cholesterol, mg/dL | 169 (135–205) | 192 (156–215) | 0.02 |
| Albumin, g/dL | 3.2 (2.6–3.7) | 4.0 (3.6–4.2) | <0.001 |
| D‐dimer, μg/mL | 5.1 (2.6–16.2) | 0.5 (0.3–1.0) | <0.001 |
| hsCRP, mg/dL | 1.33 (0.35‐6.80) | 0.14 (0.04‐0.34) | <0.001 |
| CONUT score | 5 (3–8) | 1 (0–3) | <0.001 |
| Imaging findings | |||
| Multiple vascular lesions, % | 71 | 16 | <0.001 |
CONUT, controlling nutritional status; hsCRP, high sensitivity C‐reactive protein.
Table 3.
Variables independently associated with the presence of active cancer in cryptogenic stroke mechanisms (multivariate analysis)
| Patient characteristics | OR | 95% CI | P Value |
|---|---|---|---|
| Age | 0.67a | 0.37–1.19 | 0.18 |
| Women | 0.77 | 0.23–2.05 | 0.52 |
| Atherosclerotic risk factors, n | 0.73b | 0.46–1.14 | 0.17 |
| Plasma D‐dimer level, μg/mL | 6.30a | 2.94–15.69 | <0.001 |
| CONUT score | 1.18b | 0.96–1.47 | 0.11 |
| Multiple vascular lesions | 6.40 | 2.35–18.35 | <0.001 |
CI, confidence interval; CONUT, controlling nutritional status; OR, odds ratio.
OR per 1 standard deviation increase.
OR per 1 unit increase.
When comparing active cancer patients with known stroke mechanisms and those with cryptogenic stroke (Table 4), the patients with cryptogenic stroke were younger, had lower BMI, fewer atherosclerotic risk factors, lower nutrition status, and higher plasma D‐dimer levels. Multiple vascular lesions were more common in the patients with cryptogenic stroke than in those with known stroke mechanisms. The frequency of systemic metastases and chemotherapy and/or radiation therapy was significantly higher in patients with cryptogenic stroke than in those with known stroke mechanisms. Multivariate logistic analysis revealed that plasma D‐dimer levels (OR per 1‐SD increase, 1.77; 95% CI: 1.07–3.08; P = 0.02), the presence of multiple vascular lesions (OR: 3.06; 95% CI: 1.25–7.75; P = 0.01), and undergoing chemotherapy and/or radiation therapy (OR: 7.48; 95% CI: 2.53–25.74; P < 0.001) were independently associated with cryptogenic stroke etiology (Table 5).
Table 4.
Characteristics of active cancer patients diagnosed with cryptogenic stroke and known stroke mechanisms
| Patient characteristics | Cryptogenic stroke (n = 68) | Known stroke mechanisms (n = 77) | P Value |
|---|---|---|---|
| Age, years | 67 (60–75) | 74 (67–79) | <0.001 |
| Women, % | 46 | 30 | 0.06 |
| Body mass index, kg/m2 | 19.7 (16.9–22.4) | 22.1 (19.6–24.1) | 0.002 |
| Atherosclerotic risk factors, n | 1 (0–2) | 2 (1–3) | <0.001 |
| Hypertension, % | 35 | 74 | <0.001 |
| Hyperlipidemia, % | 37 | 42 | 0.56 |
| Diabetes mellitus, % | 19 | 34 | 0.04 |
| Current smoking, % | 15 | 21 | 0.34 |
| Past stroke, % | 18 | 25 | 0.30 |
| Laboratory findings | |||
| White blood cells, /μL | 7,565 (4,985–10,738) | 6,750 (5,110–9,305) | 0.56 |
| Total lymphocytes, /μL | 904 (585–1270) | 1,149 (777–1,547) | 0.02 |
| Hemoglobin, g/dL | 10.6 (8.4–12.1) | 11.4 (10.1–13.1) | 0.008 |
| Platelets × 104/μL | 14.4 (8.3–22.1) | 19.7 (13.4–25.6) | 0.02 |
| Total cholesterol, mg/dL | 169 (135–205) | 164 (144–189) | 0.78 |
| Albumin, g/dL | 3.2 (2.6–3.6) | 3.4 (2.9–3.8) | 0.07 |
| D‐dimer, μg/mL | 5.1 (2.6–16.2) | 2.6 (0.9–7.6) | <0.001 |
| hsCRP, mg/dL | 1.33 (0.35‐6.80) | 0.72 (0.13‐4.11) | 0.06 |
| CONUT score | 5 (3–8) | 4 (2–7) | 0.02 |
| Imaging findings | |||
| Multiple vascular lesions, % | 71 | 40 | <0.001 |
| Cancer status | |||
| Adenocarcinoma, % | 68 | 59 | 0.29 |
| Systemic metastases, % | 54 | 31 | 0.005 |
| Chemotherapy and/or radiation therapy, % | 40 | 9 | <0.001 |
CONUT, controlling nutritional status; hsCRP, high sensitivity C‐reactive protein.
Table 5.
Variables independently associated with cryptogenic stroke mechanisms in active cancer patients (multivariate analysis)
| Patient characteristics | OR | 95% CI | P Value |
|---|---|---|---|
| Age | 0.69a | 0.42–1.10 | 0.12 |
| Women | 1.17 | 0.46–2.97 | 0.74 |
| Atherosclerotic risk factors, n | 0.76b | 0.52–1.11 | 0.16 |
| Plasma D‐dimer level, μg/mL | 1.77a | 1.07–3.08 | 0.02 |
| CONUT score | 1.04b | 0.88–1.21 | 0.67 |
| Multiple vascular lesions | 3.06 | 1.25–7.75 | 0.01 |
| Systemic metastases | 1.15 | 0.44–2.89 | 0.77 |
| Chemotherapy and/or radiation therapy | 7.48 | 2.53–25.74 | <0.001 |
CI, confidence interval; CONUT, controlling nutritional status; OR, odds ratio.
OR per 1 SD increase.
OR per 1 unit increase.
The number of patients with each cancer type is presented in Table 6, and the frequency of ischemic stroke in each cancer type is shown in Figure 2. Lung cancer was associated with the highest number of ischemic stroke patients (n = 24), followed by colorectal cancer (n = 17), prostate cancer (n = 13), gastric cancer (n = 11), and pancreatic cancer (n = 10). With regard to the complication rates for ischemic stroke in each cancer type, lung cancer was also associated with the highest frequency of ischemic stroke (8.0 per 1000 patients), followed by pancreatic cancer (7.5/1000), colorectal cancer (7.4/1000), renal cancer (6.6/1000), and ovarian cancer (5.6/1000).
Table 6.
Cancer types and metastases incidence in patients with ischemic stroke
| Cancer type | Number of patients with ischemic stroke | Metastases incidence in patients with ischemic stroke | Incidence of ischemic stroke per 1,000 patients | ||||
|---|---|---|---|---|---|---|---|
| Total | Cryptogenic stroke | Known stroke mechanisms | Total | Cryptogenic stroke | Known stroke mechanisms | ||
| Lung cancer | 24 | 12 | 12 | 9 | 7 | 2 | 8.0 |
| Pancreatic cancer | 11 | 9 | 2 | 8 | 7 | 1 | 7.5 |
| Colorectal cancer | 17 | 7 | 10 | 3 | 1 | 2 | 7.4 |
| Renal cancer | 5 | 3 | 2 | 2 | 1 | 1 | 6.6 |
| Ovarian cancer | 7 | 1 | 6 | 4 | 1 | 3 | 5.6 |
| Prostate cancer | 13 | 3 | 10 | 5 | 0 | 5 | 5.6 |
| Malignant lymphoma | 7 | 4 | 3 | 4 | 2 | 2 | 4.7 |
| Bladder cancer | 4 | 0 | 4 | 1 | 0 | 1 | 4.7 |
| Esophageal cancer | 7 | 3 | 4 | 4 | 2 | 2 | 4.1 |
| Gastric cancer | 11 | 3 | 8 | 6 | 3 | 3 | 4.0 |
| Leukemia | 3 | 2 | 1 | 1 | 0 | 1 | 3.5 |
| Biliary tract cancer | 3 | 2 | 1 | 3 | 2 | 1 | 2.6 |
| Liver cancer | 5 | 1 | 4 | 1 | 1 | 0 | 2.5 |
| Uterine cancer | 3 | 3 | 0 | 1 | 1 | 0 | 2.4 |
| Breast cancer | 7 | 5 | 2 | 3 | 3 | 0 | 1.8 |
| Othersa | 18 | 6 | 12 | 4 | 3 | 1 | |
The top 15 cancers associated with ischemic stroke are shown in this table. The proportion of systemic metastases was similar when comparing the top 3 cancers (lung cancer, pancreatic cancer, and colorectal cancer) with other cancers (38% vs. 42%).
Includes hematologic malignancy in 4, parotid cancer in 3, pharyngeal cancer in 2, sarcoma in 2, skin cancer in 2, oral cancer in 2, cervical cancer in 1, nasal cancer in 1, and malignant mesothelioma in 1.
Figure 2.
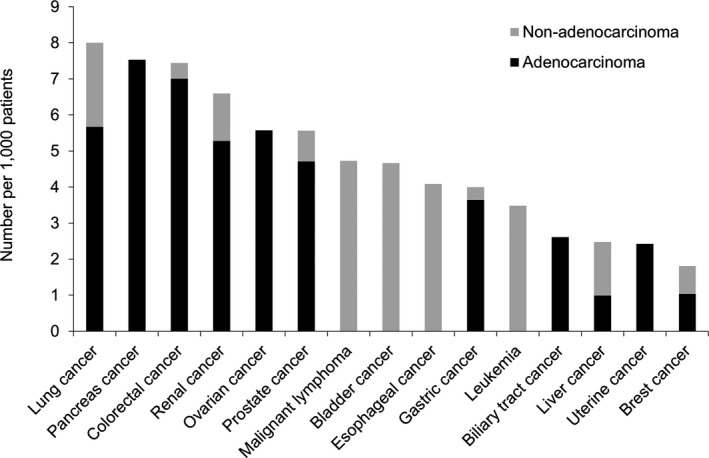
The frequency of ischemic stroke in patients with various types of cancer. The 15 leading types of cancer associated with ischemic stroke are shown. Lung cancer was associated with the highest frequency of ischemic stroke (8.0/1,000 patients), followed by pancreatic cancer (7.5/1,000), colorectal cancer (7.4/1,000), renal cancer (6.6/1,000), and ovarian cancer (5.6/1,000). The dark gray bar shows adenocarcinoma and the light gray bar shows non‐adenocarcinoma.
Discussion
This study revealed the following findings: (1) the cryptogenic stroke rate in cancer patients was approximately half of cancer patients with ischemic stroke, which was much higher than in noncancer patients; (2) the clinical features of cryptogenic stroke in cancer patients differed considerably from those in noncancer patients; and (3) high plasma D‐dimer levels, the presence of multiple vascular lesions, and receiving chemotherapy and/or radiation therapy were independently associated with cryptogenic stroke in cancer patients.
Previous studies have reported that the cryptogenic stroke rate in cancer patients is relatively high6, 7, 8, 9; however, there has been no study of the cryptogenic stroke rate according to the presence of active cancer within the same study population. It has been suggested that paroxysmal atrial fibrillation is the latent common cause of cryptogenic stroke in the general population.14 Because we conducted a thorough investigation to determine stroke etiology, including 24‐hour electrocardiographic monitoring throughout hospitalization, only 12% of acute stroke patients without cancer were diagnosed as having cryptogenic stroke. Meanwhile, it is notable that approximately half of acute stroke patients with active cancer who were still classified as having cryptogenic stroke. This result suggests that the mechanisms underlying cryptogenic stroke in cancer patients need special consideration.
Our data demonstrated that patients with cryptogenic stroke and active cancer had unique clinical characteristics. These patients had lower BMI, fewer atherosclerotic risk factors, lower nutrition status, higher plasma D‐dimer levels, and more frequent multiple vascular lesions than those in the noncancer group and the active cancer group. High plasma D‐dimer levels and multiple vascular lesions were independently associated with cryptogenic stroke in the setting of active cancer. These results are highly consistent with previous studies.6, 7 These data suggest that cancer‐related conditions, such as coagulation abnormalities and malnutrition caused by malignancy or the cachectic state, potentially affect the development of ischemic stroke in cancer patients. In other words, if patients with cryptogenic stroke display such characteristics, then a detailed examination should be considered check for the presence of subclinical active cancer.
In addition to these clinical characteristics, when comparing active cancer patients with known stroke mechanisms and cryptogenic stroke, it was remarkable that chemotherapy and/or radiation therapy was also independently associated with cryptogenic stroke. Cancer treatment, such as chemotherapy and/or radiation therapy, possibly causes the hypercoagulable state through the direct oncolytic reaction and the treatment itself.15 Cancer treatments are also a surrogate for the degree of cancer activity and therefore this finding might actually be due to confounding effect of indication bias. However, these findings indicate that active cancer patients undergoing treatment, especially those with high D‐dimer levels, face a high risk of ischemic stroke.
To date, only a few studies have investigated the relationship between the prevalence of ischemic stroke and active cancer.6, 7, 16 In this study, we presented the complication rates for ischemic stroke in each type of cancer during the study period. These results accord well with those of a previous study showing that incident cancer is associated with an increased short‐term risk of stroke, especially in lung, pancreatic, and colorectal cancers.16 The authors speculated that patients with those cancers often had advanced‐stage disease.16 In this regard, the rate of systemic metastases in our series was similar in patients with lung, pancreatic, and colorectal cancer versus those who did not. Further discussion is warranted on this problem; however, potential factors such as dehydration, infectious conditions, and cancerous invasion to the surrounding tissues could have affected the development of ischemic stroke in these patients.
Our study had several limitations. First, we showed the differences in cryptogenic stroke between cancer patients and noncancer patients, but it is possible that these differences derived from the cachectic state of cancer patients in general and were not restricted to cryptogenic stroke patients. To precisely distinguish between these factors is difficult; even so, we found similar differences between cryptogenic stroke and known stroke mechanisms in patients with active cancer. Therefore, we believe that our data reflect the unique clinical characteristics of cryptogenic stroke in the setting of cancer. Second, only a few cancer patients underwent TEE due to poor clinical condition or gastrointestinal complications. Because TEE is superior to TTE for the evaluation of cardiac embolic sources such as nonbacterial thrombotic endocarditis,17 this might influence the classification of stroke etiology. Similarly, lack of prolonged Holter monitoring might overestimate the rate of cryptogenic stroke in cancer patients.18 Third, although this study demonstrated the complication rates of ischemic stroke for each type of cancer during the study period, it did not rigorously measure the complication rate of ischemic stroke in all such cancer patients. That is, not all patients who developed ischemic stroke during a routine visit to our hospital were admitted there. Fourth, our cohort was limited to Japanese patients of single‐center series and therefore, our finding may not generalize to other stroke populations. Finally, many of association variables in the multivariate analyses had wide CIs suggesting low statistical power and poor reliability. Further researches are needed to elucidate the optimal predictors of active cancer and cryptogenic stroke.
In conclusion, cryptogenic stroke is highly prevalent in patients with active cancer and has a unique pathology characterized by high plasma D‐dimer levels and multiple vascular lesions. The hypercoagulable state and malnutrition due to cancer and its treatment potentially influence the development of cryptogenic stroke in cancer patients.
Author Contributions
Y.G. and S.O. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The authors contributed to this work as follows. Study concept and design: Y.G., S.O., M.S., T.Y. Acquisition of data: Y.G., M.S., Y.T., T.S. Analysis and interpretation of data: Y.G., S.O. Drafting of the manuscript: Y.G. Critical revision of the manuscript for intellectual content: S.O., M.S., H.M. Study supervision: S.O., M.S., T.Y., and H.M.
Conflict of Interest
None Declared.
Acknowledgment
The authors thank Ayumi Shintani for her statistical advice.
References
- 1. Bick RL. Cancer‐associated thrombosis. N Engl J Med 2003;349:109–111. [DOI] [PubMed] [Google Scholar]
- 2. Graus F, Rogers LR, Posner JM. Cerebrovascular complication in patients with cancer. Medicine (Baltimore) 1985;64:16–35. [DOI] [PubMed] [Google Scholar]
- 3. Chen PC, Muo CH, Lee YT, et al. Lung cancer and incidence of stroke: a population‐based cohort study. Stroke 2011;42:3034–3039. [DOI] [PubMed] [Google Scholar]
- 4. Cestari DM, Weine DM, Panageas MD, et al. Stroke in patients with cancer: incidence and etiology. Neurology 2004;62:2025–2030. [DOI] [PubMed] [Google Scholar]
- 5. Khasraw M, Posner JB. Neurological complication of systemic cancer. Lancet Neurol 2010;9:1214–1227. [DOI] [PubMed] [Google Scholar]
- 6. Kim SG, Hong JM, Kim HY, et al. Ischemic stroke in cancer patients with and without conventional mechanisms: a multicenter study in Korea. Stroke 2010;41:798–801. [DOI] [PubMed] [Google Scholar]
- 7. Schwarzbach CJ, Schaefer A, Ebert A, et al. Stroke and cancer: the importance of cancer‐associated hypercoagulation as a possible stroke etiology. Stroke 2012;43:3029–3034. [DOI] [PubMed] [Google Scholar]
- 8. Navi BB, Singer S, Merkler AE, et al. Cryptogenic subtype predicts reduced survival among cancer patients with ischemic stroke. Stroke 2014;45:2292–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shen B, Fong MK, Chu YP, et al. Stroke and cancer: misfortunes never come singularly. Int J Stroke 2013;8:E30. [DOI] [PubMed] [Google Scholar]
- 10. Kolominsky‐Rabes PL, Wever M, Gefeller O, et al. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long‐term survival in ischemic stroke subtypes: a population‐based study. Stroke 2001;32:2735–2740. [DOI] [PubMed] [Google Scholar]
- 11. Schulz UG, Rothwell PM. Differences in vascular risk factors between etiological subtypes of ischemic stroke: importance of population‐based studies. Stroke 2003;34:2050–2059. [DOI] [PubMed] [Google Scholar]
- 12. de Ignacio Ulíbarri J, González‐Madroño A, de Villar NG, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp 2005;20:38–45. [PubMed] [Google Scholar]
- 13. Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtypes of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 14. Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014;370:2478–2486. [DOI] [PubMed] [Google Scholar]
- 15. Varki A. Trousseau's syndrome: multiple definitions and multiple mechanisms. Blood 2007;110:1723–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Navi BB, Reiner AS, Kamel H, et al. Association between incident cancer and subsequent stroke. Ann Neurol 2015;77:291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Merkler AE, Navi BB, Singer S, et al. Diagnostic yield of echocardiography in cancer patients with ischemic stroke. J Neurooncol 2015;123:115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O'Neal WT, Lakoski SG, Qureshi W, et al. Relation between cancer and atrial fibrillation (from the REasons for Geographic And Racial Differences in Stroke Study). Am J Cardiol 2015;15:1090–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]


