Abstract
Background
Cotrimoxazole (CTX) prophylaxis is among the key interventions provided to HIV-infected individuals in resource-limited settings. We conducted a systematic review of the available evidence.
Methods
MEDLINE, Embase, Global Health, CINAHL, SOCA, and African Index Medicus (AIM) were used to identify articles relevant to the CTX prophylaxis intervention from 1995 to 2014. Included articles addressed impact of CTX prophylaxis on the outcomes of mortality, morbidity, retention in care, quality of life, and/or prevention of ongoing HIV transmission. We rated the quality of evidence in individual articles and assessed the overall quality of the body of evidence, the expected impact, and the cost effectiveness (CE) for each outcome.
Results
Of the initial 1418 identified articles, 42 met all inclusion criteria. These included 9 randomized controlled trials, 26 observational studies, 2 systematic reviews with meta-analysis, 1 other systematic review, and 4 CE studies. The overall quality of evidence was rated as “good” and the expected impact “high” for both mortality and morbidity. The overall quality of evidence from the 4 studies addressing retention in care was rated as “poor,” and the expected impact on retention was rated as “uncertain.” The 4 assessed CE studies showed that provision of CTX prophylaxis is cost effective and sometimes cost saving. No studies addressed impact on quality of life or HIV transmission.
Conclusions
CTX prophylaxis is a cost-effective intervention with expected high impact on morbidity and mortality reduction in HIV-infected adults in resource-limited settings. Benefits are seen in both pre-antiretroviral therapy and antiretroviral therapy populations.
Keywords: cotrimoxazole, trimethoprim–sulfamethoxazole, HIV, resource-limited settings
INTRODUCTION
Cotrimoxazole (CTX) is a widely used fixed-dose combination of 2 antibiotics, sulfamethoxazole and trimetho-prim. CTX was mainly prescribed in the United States and in Europe, since the early days of the HIV/AIDS epidemic, to prevent Pneumocystis carinii pneumonia. CTX is an affordable medicine, including in resource-constrained contexts (commonly costing less than a few US cents per day of treatment).1,2
Although the World Health Organization (WHO) and the Joint United Nations Program on HIV/AIDS (UNAIDS) issued the “provisional statement on the use of CTX prophylaxis in sub-Saharan Africa in 2000 (Provisional WHO/UNAIDS secretariat recommendations),”3 until the middle part of the next decade, most countries had not implemented this intervention widely. A cross-sectional survey of WHO HIV/AIDS program officers conducted in 2007 showed that CTX prophylaxis–related national guidance and policy documents were available in 93% of the 41 countries that responded, but only 66% of these countries responded that CTX was widely implemented.4 Efforts to implement CTX prophylaxis programs were hindered by concerns about the efficacy of CTX prophylaxis and by emergence of high levels of anti-sulfonamide resistance.1,5 Evidence on the efficacy of CTX prophylaxis and experience with its widespread use have accumulated since 2007.
In 2006, WHO issued guidance addressing CTX prophylaxis for people living with HIV (PLHIV) in resource-limited settings (RLS).1 These guidelines addressed CTX prophylaxis initiation and discontinuation by age, CD4 count, and WHO clinical stage. WHO recommended that, “When the CD4 count is not available, CTX prophylaxis should be initiated for adults or adolescents with WHO clinical stage 2, 3, or 4 diseases.” If the CD4 cell count is available, the guidance recommended “initiation of CTX in PLHIV with CD4 ≤350 cells per microliter regardless of WHO clinical stage and for those with WHO clinical stage 3 or 4 irrespective of CD4 count.”1 The WHO also provided a universal option for CTX initiation in areas with high HIV prevalence and weak health systems. The June 2013 WHO consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection stated that “CTX prophylaxis should be implemented as an integral component of a package of HIV-related services.”6 Recommendations covered use of CTX in adults, adolescents, pregnant women, and children for prevention of Pneumocystis pneumonia, toxoplasmosis, bacterial infections, and malaria.6
On World AIDS Day 2014, WHO released an update to the 2013 consolidated guidelines, stating that, “in settings where malaria and/or severe bacterial infections (SBIs) are highly prevalent, adults and pregnant/breastfeeding women should be initiated with CTX prophylaxis, regardless of CD4 cell count or WHO stage.”2 Regarding CTX discontinuation, WHO recommended that “CTX prophylaxis may be discontinued in adults (including pregnant women) with HIV infection who are clinically stable on ART, with evidence of immune recovery and virologic suppression” and that “in settings where malaria and/or SBIs are highly prevalent, CTX prophylaxis should be continued regardless of CD4 cell count or WHO clinical stage.”2
This review aims at assessing the quality of evidence and the expected impact of CTX prophylaxis in HIV-infected adults. We addressed the following outcomes: (1) mortality, (2) morbidity, (3) retention in HIV care, (4) quality of life, and (5) prevention of ongoing HIV transmission. Cost effectiveness (CE) of CTX prophylaxis was also assessed.
METHODS
We conducted a search of 6 medical literature databases—MEDLINE, Embase, Global Health, CINAHL, SOCA, and African Index Medicus (AIM)—to identify articles relevant to the CTX prophylaxis intervention published from January 1995 to May 2014. Articles eligible for inclusion (1) studied adult PLHIV, (2) were conducted in RLS, and (3) reported at least 1 of the 5 outcomes of interest (mortality, morbidity, retention in HIV care, quality of life, or prevention of HIV transmission) or costing or CE. A detailed description of the search terms applied and the geographic filters used in the literature search can be found in the introductory article of this supplement.7 Search terms related specifically to CTX prophylaxis included “CTX,” “cotrimoxazole,” “trimethoprim–sulfamethoxazole,” and “trimethoprim sulfamethoxazole combination.”
Search outputs (titles and abstracts) were reviewed by the authors to identify potentially relevant studies. Articles that seemed to contain data relevant to the intervention and to at least one of the outcomes of interest (“eligible” studies) were read in their entirety; those that in fact satisfied criteria for inclusion (“included” studies) were abstracted and summarized as to study design (eg, randomized control trial, cohort study), population studied, comparison group(s), number of participants, and assessment of impact on the outcome(s) of interest [expressed as hazard ratios (HR), odds ratios, relative risk (RR), or incidence rate ratios (IRR) and the respective 95% confidence intervals (CI) if available].
The quality of evidence from each of the included studies for each outcome of interest was rated based on the type of study and other factors, such as the number of study participants and internal and external validity of the study data. The overall quality of evidence for each study was rated as “strong,” “medium,” or “weak.” Further information on the rating of quality of evidence can be found in the abovementioned introductory article.7
Because of the nature of the review and the heterogeneity of study populations, study methods, settings, and outcomes, we did not attempt quantitative synthesis of study results overall. Rather, the authors grouped the studies by the outcome(s) addressed and rated the overall quality of the body of evidence for each outcome as good, fair, or poor.
The expected impact of the intervention by outcome was then assessed based on the magnitude of effect demonstrated in individual studies, the quality of the body of evidence, and consistency across the studies. Expected impact was rated as high, moderate, low, or uncertain based on criteria agreed on by the reviewers a priori. At least 2 members of the review team participated in assigning expected impact ratings for each outcome. Further details about the ratings can be found in the introductory article of this supplement.7
CE studies were also assessed. Articles that reported CE were rated separately by a health economist and rated as level 1—full economic evaluation (includes CE analysis, cost-utility analysis, or cost–benefit analysis); level 2—partial economic evaluations (ie, cost analyses, cost-description studies, cost-outcome descriptions); or level 3—randomized trials and studies (reporting more limited information, such as estimates of resource use or costs associated with the intervention(s) and comparator(s)).7
RESULTS
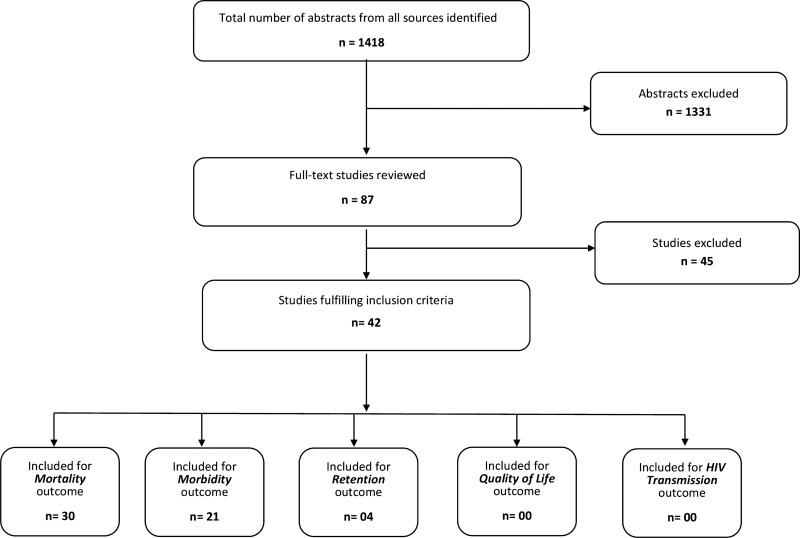
Of 1418 citations identified using the search terms, 87 contained information on CTX prophylaxis that seemed to address at least 1 of the 5 outcomes of interest and/or CE (“eligible” studies; Fig. 1). Of these 87 articles, 42 contained information that satisfied the criteria for inclusion (“included” studies). Of these 42 studies, 14 individual studies addressed exclusively mortality-related outcomes,8–21 7 individual studies focused on morbidity,22–28 and 10 individual studies addressed both morbidity and mortality.29–38 Each of the 3 included systematic review (SR) and systematic reviews with meta-analysis (SRM) addressed mortality,39 morbidity,40 or both outcomes.41 Four studies addressed retention in care,42–45 and 4 studies addressed costing and/or CE46–49; none of the 42 studies assessed quality of life or HIV transmission as outcome measures. Table S1 (see Supplemental Digital Content, http://links.lww.com/QAI/A650) summarizes the study design, sample size, key findings, and quality of evidence rating of the 42 included studies.
FIGURE 1.
Study flow diagram.
The 42 “included” studies used different study designs. Nine were randomized control trials (RCTs),18,19,23,24,27,29,33,35,38 26 were observational studies (OS),8–17,20–22,25,26,28,30–32,34,36,37,42–45 2 were SRM,39,41 1 was another SR,40 and 4 addressed CE.46–49
Participants included in the studies varied with respect to receipt of antiretroviral therapy (ART), WHO clinical stage, history of AIDS-defining illnesses, and CD4 cell count. Of the 35 individual studies (excluding the SRs and CE studies), 20 (including 4 of the 9 RCTs and 16 of the 26 OS) included participants either already on or initiating ART.8,9,11,13,15,16,20,23–28,31,32,35,36,42,43,45 Fourteen studies (including 5 RCTs and 9 OS) included persons who had not received ART (“non-ART” participants).10,12,14,17–19,21,29,30,33,34,37,38,44 One study did not report whether patients received ART. Of these 35 individual studies, 25 included patients from the general population, whereas 10 included patients from specific groups [6 studies included only patients diagnosed with tuberculosis (TB)10,12,17,18,21,38 and 4 studies included only pregnant women].24,26–28
Of the 4 RCTs that enrolled ART patients, 2 addressed CTX discontinuation23,35 and the other 2 compared CTX prophylaxis with intermittent preventive treatment of malaria with either sulfadoxine–pyrimethamine (SP) or mefloquine (MQ) in HIV-infected pregnant women.24,27
Five studies included both HIV-infected and HIV-uninfected individuals, and analysis by HIV status was conducted in only 2 of these.17,34 The other 3 studies, despite not differentiating between HIV-infected and HIV-uninfected persons, contained some relevant information. Less than 10% of the enrollees in the study by Grimwade et al12 had a known HIV status, and outcome by HIV status was not analyzed. Newman et al28 assessed the effect of CTX and intermittent preventive therapy with pyrimethamine–sulfadoxine (IPT-SP) in HIV-infected and HIV-uninfected women (SP being the antimalarial that is most widely used for malaria prevention in pregnant women50,51), and Bulabula et al22 compared pregnant women receiving CTX with an HIV-negative comparator group.
Most studies evaluated a CTX dose of 960 mg daily. Three studies evaluated lower doses.10,30,33 In the study by Badri et al30 in South Africa, a dose of 960 mg 3 days per week was used initially; thereafter, a dose of 480 mg per day was used. Boeree et al10 in Malawi used both 480 and 960 mg doses of CTX (for the latter, mortality outcomes were compared with those in 2 historical cohorts). Maynart et al33 compared patients who received 480 mg of CTX vs. a placebo.
Summary of Results of Studies in Persons Who Did Not Receive ART
Mortality
Fourteen of the 35 individual studies (CE-related studies, SRM, and SR studies excluded) enrolled only patients not on ART; 13 of these, including 5 RCTs18,19,29,33,38 and 8 OS,10,12,14,17,21,30,34,37 addressed mortality (the 14th study addressed the retention outcome).
Evidence From the RCTs (n = 5)
The study by Wiktor et al38 among patients with TB/HIV in Côte d'Ivoire found a 46% reduction in mortality rate in the group that received CTX prophylaxis (95% CI: 23% to 62%; P < 0.001). The study by Anglaret et al29 (also in Côte d’Ivoire) showed no decrease in mortality risk, likely as a result of lack of statistical power.28
Another RCT by Nunn et al18 (the LUCOT study), which was conducted in Zambia and enrolled patients initiating TB treatment, showed a 21% reduction in all-cause mortality in persons receiving CTX (HR: 0.79; 95% CI: 0.63 to 0.99; P = 0.04). The mortality benefit was most consistent between 6 and 18 months (45% reduction in mortality in participants receiving CTX); no benefit was found beyond 18 months. CD4 cell count was not found to have an impact on the benefit of CTX prophylaxis. Of note, another RCT by Nunn et al19 (the TOPAZ study) did not show a significant effect of CTX prophylaxis on mortality reduction in HIV-infected postpartum women in Zambia. Investigators suggested that the absence of CTX effect, in contrast to the LUCOT study,18 may have been due to antibacterial resistance, high loss to follow-up (40%), and the relatively healthy status of the women in the study.19
One RCT by Maynart et al,33 conducted in Senegal, did not show any benefit of the 480 mg dose of CTX prophylaxis on mortality or incidence of opportunistic or nonopportunistic infections in persons with HIV-1 infection.
Evidence From Observational Studies (n = 8)
Eight of the 13 studies addressing mortality in patients not on ART were observational. They addressed mortality either alone or in combination with morbidity-related outcomes. All 8 OS showed a statistically significant benefit of CTX prophylaxis on survival. Of note, 6 of the 8 OS addressed mortality exclusively in patients with TB.
Watera et al37 assessed mortality before and after CTX introduction and found a 24% decrease in mortality after adjusting for age and CD4 count. Both the studies by Badri et al30 and Mermin et al34 showed that CTX prophylaxis significantly reduced mortality—an effect that was limited to enrollees with CD4 cell counts below the 200 per mictoliter threshold or with WHO stage 3 or 4 conditions.
Zachariah et al21 reported significant decreases in deaths in TB/HIV patients receiving CTX prophylaxis. Compared with the “non-CTX group,” the “CTX group” showed a 22% reduction in mortality (by the end of the anti-TB treatment). A mortality difference was observed in the subgroup with smear-negative TB; however, for smear-positive patients, neither mortality nor treatment success were significantly different between those receiving and those not receiving CTX. It is noteworthy that the study by Zachariah et al,21 is in conflict with the study by Wiktor et al,38 from Côte d’Ivoire, in which all participants had sputum smear-positive TB at enrollment and in which the association between CTX prophylaxis and the reduction in mortality risk was found to be statistically significant [46% risk reduction (95% CI: 23 to 62), P < 0.001]. A number of reasons were suggested to explain this finding: relatively few patients with smear-positive TB received CTX, the smear-positive patients may have been less immunosuppressed than other patients with TB (in other studies, CTX had the most significant effect on mortality in those most immunosuppressed30), differences in the “timing of administration” of CTX, and possibly “differences in patterns of HIV-related disease and rates of CTX resistance.”
Mwaungulu et al17 compared end-of-TB-treatment outcomes, including survival in patients with TB registered in 1999 vs. those registered in 2000; 70% of enrollees were HIV infected. Patients with either TB or HIV were given CTX prophylaxis in 2000 but not in 1999. Mortality rates did not differ between the 2 study periods for HIV-negative patients but fell in HIV-positive patients from 43% to 24% (the effect was strongest in those who were TB smear positive).
Boeree et al10 described an RCT in which 480 or 960 mg of CTX was prescribed to HIV-infected clients starting TB treatment. Enrollees in this study were followed until the completion of their TB treatment. The study aimed at assessing mortality in enrollees receiving the 480 mg dose vs. the 960 mg dose. The study also compared mortality with that in 2 historical cohorts (which were not on CTX). At TB treatment completion, mortality rates were estimated at 15.4% in enrollees who received the 480 mg dose and 14.0% in those who received the 960 mg dose, respectively. Mortality rates were lower than in the 2 historical cohorts (19.2%; P = 0.10 and 21.0%; P < 0.001, respectively). Grimwade et al12 found a 29% lower mortality at 6 months in patients with TB (and a 78% HIV prevalence) receiving CTX prophylaxis compared with a historical control group (TB patients not receiving CTX). Finally, Khoza et al,14 in a study of 234 patients of whom 19% received CTX prophylaxis, found that CTX prophylaxis significantly reduced mortality (P = 0.0017).
Morbidity
Of the 13 studies (excluding SRM/SR and those addressing CE) that exclusively enrolled non-ART patients, 6 studies addressed morbidity; 3 of these were RCTs29,33,38 and 3 were OS.30,34,37
Evidence From the RCTs (n = 3)
Two RCTs (Anglaret et al29 and Wiktor et al38) showed a significant reduction in morbidity in the CTX group. Anglaret et al reported 43% fewer severe events (95% CI: 0.25 to 0.57) in patients on CTX vs. placebo; the benefits were seen mainly for bacterial pneumonia, malaria, and isosporosis and were observed at all levels of baseline CD4 count. Wiktor et al observed a 43% reduction in hospitalizations (95% CI: 0.10 to 0.64) in sputum smear-positive TB patients receiving CTX vs. those not receiving CTX. In the third RCT, Maynart et al33 showed no benefit of CTX prophylaxis on the occurrence of opportunistic or nonopportunistic infections in patients in Senegal. Possible explanations for the lack of benefit included the lower dose of CTX used in the Senegal study, lower rates of malaria than in Côte d'Ivoire, and differences in bacterial resistance in the 2 countries.33
Evidence From Observational Studies (n = 3)
Three OS enrolling patients not on ART showed that CTX prophylaxis had a protective effect on morbidity-related events. In the study by Mermin et al,34 CTX was found to decrease the incidence of malaria by 72%; the effect was seen irrespective of age and CD4 cell count; CTX was also associated with a reduced rate of diarrhea [0.65 (95% CI), P < 0.0001], also irrespective of age and CD4 cell count. In the study by Badri et al,30 enrollees with WHO clinical stages 3 and 4, or a CD4 cell count below 200 per microliter on CTX demonstrated a 48% reduction of “severe HIV-related illnesses,” compared with those not on CTX. Watera et al37 reported no change in the frequencies of febrile and other morbidity events after CTX prophylaxis was introduced. They, however, showed a reduction in malaria incidence (estimated at 0.31; 95% CI: 0.13 to 0.72).
Summary of Results of Studies in Persons Who Received ART
Mortality
Eleven articles that addressed mortality in patients on ART were assessed, including 1 RCT,35 9 OS,9,11,13,15,16,20,31,32,36 and 1 SRM.39 Patients enrolled in these studies initiated CTX prophylaxis before, at the same time as, or after ART initiation.
Evidence From the RCT (n = 1)
The single RCT addressing CTX prophylaxis and mortality among persons on ART did so in the context of discontinuing CTX in persons stable on ART. Polyak et al35 assessed a “composite” primary end-point of morbidity and mortality among ART patients who discontinued vs. those who continued CTX in Kenya. This combined morbidity/ mortality end-point was significantly more frequent in the CTX discontinuation arm than in the continuation arm (IRR = 2.27; 95% CI: 1.52 to 3.38; P < 0.001) and was mainly driven by malaria morbidity.
Evidence From Observational Studies (n = 9)
In a study by Alemu and Sebastian,8 multivariate analysis showed that CTX prophylaxis begun at or before ART initiation was significantly associated with 2-year survival. The studies by Amuron et al,9 Fairall et al,11 and Lowrance et al15 all showed that not being on CTX prophylaxis was independently associated with increased mortality. In the study by Van Oosterhout et al,20 not receiving CTX prophylaxis was an independent risk factor for mortality at 14 and 26 weeks in the logistic regression analysis. Walker et al36 reported a 35% reduction in mortality associated with CTX prophylaxis. The benefit was highest in the first 12 weeks of follow-up (59%), maintained from 12 to 72 weeks (44%), and waned beyond the 72 weeks of follow-up 0.96, 0.63–1.45; heterogeneity P = 0.02 no statistically significant association was found between mortality and the CD4 cell count. Madec et al found that the protective effect of CTX was limited to patients with CD4 cell count <200 cells per microliter.33
Hoffmann et al13 found a 36% reduction in mortality (HR: 0.64; 95 CI: 0.57 to 0.72; P < 0.001) associated with CTX. In a later study, Hoffman et al31 found a 52% reduction in mortality (effect size: 0.48; 95% CI: 0.21 to 1.1; P = 0.09). The limited number of deaths reported in the latter study contributed to the non-statistically significant mortality reduction.
Lim et al32 examined the effect of “Pneumocystis Pneumonia (PCP) prophylaxis” on the occurrence PCP and on survival among the patients enrolled in the “TREAT Asia HIV Observational Database (TAHOD).” Enrollees not on prophylaxis were significantly less likely to survive compared with those who were on prophylaxis (incident rate ratio for death estimated at 10.8, P < 0.001).
Evidence From Systematic Reviews (n = 1)
A SRM conducted by Suthar et al39 evaluated the effect of CTX prophylaxis on mortality among participants on ART and reported a decreased death rate in persons on CTX as compared with those not on CTX (“summary estimate”: 0.42; 95% CI: 0.29 to 0.61). Of note, only 2 of the 8 studies included in this SR followed participants for more than an average of 13 months. Examining the association between CTX prophylaxis and baseline CD4 cell count was not possible as, in most of the studies included in the meta-analysis, enrollees were started on ART based on a CD4 cell count below 200 cells per microliter. However, Hoffmann et al13 (cited above and included in the meta-analysis) demonstrated that enrollees were more likely to survive when they received CTX prophylaxis at ART initiation (a 36% reduction in mortality). No statistically significant association was found between CTX prophylaxis and survival in the subgroup of persons with both CD4 cell count above 200 cells per microliter and WHO stage 1 or 2.13
Morbidity
Of the 10 studies that addressed morbidity among patients on ART, 4 were RCTs (including 2 studies that enrolled pregnant women and 2 that addressed CTX discontinuation).23,24,27,35 The remaining 6 studies were observational.
RCTs Enrolling Pregnant Women (n = 2)
The study by Denoeud-Ndam et al24 was conducted in Benin where Plasmodium falciparum transmission is considered to be “intense and perennial,” with recrudescence during the rainy season. The authors enrolled HIV-infected pregnant women with CD4 counts of <350 per microliter who were randomized to receive CTX alone or in combination with MQ-IPTp. CTX alone was non-inferior to the combination in preventing malaria. However, polymerase chain reaction–detected placental parasitemia was reduced in the CTX + MQ group (0/105 vs. 5/103; P = 0.03).24 A second RCT by Klement et al27 examined the effect of CTX prophylaxis, as compared with that of IPTp-SP, on malaria prevention in HIV-infected pregnant women in a P. falciparum–endemic West African country (Togo).27 In this study, 75.4% of women who received CTX prophylaxis did not develop malaria as compared with 84.7% of women who received IPTp-SP—a difference of 9.3% (95% CI: 20.53 to 19.1), not meeting the predefined “non-inferiority” criterion. The authors did not conclude that CTX was inferior to IPTp-SP regarding malaria-free survival (the study was designed to assess “noninferiority”), but the difference in point estimates of malaria-free rates suggests that CTX could potentially be inferior to IPTp-SP. However, HIV-infected pregnant women on CTX prophylaxis are more likely to remain malaria-free than those who are not receiving any antimalarial drug for prophylaxis. Daily CTX was also demonstrated to be safe and at least similarly effective at reducing parasitemia or placental malaria and adverse birth outcomes.27
RCTs Addressing CTX Discontinuation (n = 2)
Campbell et al23 showed, in an evaluation of CTX discontinuation among patients receiving ART, that discontinuation of CTX prophylaxis, as compared with its continuation, resulted in an increased risk for malaria (RR = 32.5; 95% CI: 8.6 to 275.0; P < 0.001) and diarrhea (RR = 1.8; 95% CI: 1.3 to 2.4; P < 0.001). This study was stopped at the recommendation of the Data Safety Monitoring Board after just 4 months, which prevented investigators from evaluating the duration of the increased risk for malaria. A longer follow-up duration would have helped determine if the increased incidence of malaria was due to a short-lived rebound effect after discontinuation CTX.23 Of note, “rebound effects” have been suggested to reflect a short-term impairment of protective immunity as a result of the suppressive effect of malaria medications,52–54 including “persisting asymptomatic and polyclonal P. falciparum infections” that occur in the absence of prophylaxis.54–56
The recent RCT by Polyak et al,35 a non-blinded noninferiority randomized clinical trial of CTX prophylaxis cessation vs. continuation among HIV-infected adults who had been on ART for >18 months and had CD4 >350 per microliter, found that patients who discontinued CTX had an increased incidence of clinical malaria but not pneumonia or diarrhea compared with those who continued CTX.35 There were 34 cases of malaria, of which 33 occurred in the CTX discontinuation arm (IRR = 33.02; 95% CI: 4.52 to 241.02; P = 0.001). The significantly higher combined morbidity/mortality in the CTX discontinuation arm (IRR = 2.27; 95% CI: 1.52 to 3.38; P < 0.001) was driven by malaria morbidity.
Evidence From Observational Studies (n = 6)
In the study by Walker et al,36 CTX prophylaxis reduced the frequency of malaria (odds ratio: 0.74; 95% CI: 0.63 to 0.88; P = 0.0005); the effect was maintained beyond 72 weeks. It is noteworthy that trials in ART-naive participants have typically had little follow-up beyond 72 weeks.
In the study by Dow et al,25 protection against malaria was similar in HIV-infected pregnant women who received CTX prophylaxis compared with those who received IPTp-SP. In the study by Kapito-Tembo et al,26 CTX prophylaxis led to a higher protection against malaria parasitemia compared with IPTp-SP.
Newman et al28 reported that, when compared with HIV-uninfected women on IPTp-SP, CTX prophylaxis was not found to have an effect on the risk for placental malaria in HIV-infected women.
Because of some evidence of in vitro activity of CTX against Mycobacterium tuberculosis, Hoffman et al31 addressed the effect of CTX on TB incidence and “TB diagnostic yield” in a cohort of HIV-infected adults living in a high TB prevalence region. Enrollees who received CTX prophylaxis were found to be at an increased risk for TB (HR estimated at 1.7; 95% CI: 1.2 to 2.2). However, this association was believed to be due to confounding; no effect of CTX prophylaxis was found when analysis was based on data exclusively from laboratory-confirmed TB cases (HR estimated at: 0.97; 95% CI: 0.39 to 2.4).31 Finally, Lim et al32 showed no statistically significant association between CTX prophylaxis and the risk for PCP.
Retention in Care (n = 4)
All 4 studies that met criteria for inclusion in our review for this outcome were observational. All showed, with a variable level of quality of evidence, that CTX prophylaxis is either associated with a higher retention in care or a higher likelihood of being started on ART within 1 year of the initial CD4 testing.
Kohler et al44 found a statistically significant association (P < 0.001) between CTX prophylaxis and 12-month retention in care among patients not yet eligible for ART (84%) as compared with those who did not receive CTX prophylaxis (63%). Enhanced patient follow-up, including the use of phone calls when CTX was not picked up on time, was a potentially confounding factor.44 In a study by Auld et al42 to evaluate outcomes of patients who initiated ART between 2004 and 2007 in Mozambique, “lack of CTX prescription” was a “predictor of attrition” (adjusted HR = 1.4; 95% CI: 1.0 to 1.8).
Clouse et al43 reported that the proportions of those who went on to initiate ART within 1 year of initial CD4 testing were 96.4% and 9.1% for those who initiated CTX prophylaxis and those who did not, respectively.43 Finally, Msellati et al45 has shown, in a multivariate analysis, that not being enrolled in the “Drug Access Initiative” and not being on ART were both related, among several factors, to not being on CTX prophylaxis.45
Quality of Evidence and the Expected Impact on Mortality, Morbidity, and Retention in Care
Of the 26 studies (excluding the 4 CE studies) that addressed mortality, the quality of evidence was rated as “strong” for 3 studies,29,35,38 “medium” for 22 studies,8,10–21,30–34,36,37,39,41 and “weak” for 1 study (Table 1; see also Table S1, Supplemental Digital Content, http://links.lww.com/QAI/A650).9 The overall quality of evidence in these studies for the mortality outcome was rated as “good.” The expected impact of CTX prophylaxis on mortality was rated as “high.” Of the 21 studies that addressed morbidity (excluding the 2 CE studies), the quality of evidence was rated as “strong” for 6 studies,23,24,27,29,35,38 “medium” for 12 studies,25,26,28,30–34,36,37,40,41 and “weak” for 1 study.22 The overall quality of evidence for the morbidity outcome was rated as “good.” The expected impact of CTX prophylaxis on morbidity was rated as “high.” For the retention in care outcome, the quality of evidence was rated as “medium” for 2 studies42,44 and “weak” for 2 studies43,45. The overall quality of evidence for this outcome was rated as “poor.” The expected impact of CTX prophylaxis on retention in care was rated as “uncertain.”
TABLE 1.
Summary of the Body of the Evidence From All Studies by Outcome
| Overall Quality of Evidence |
Impact of the Intervention |
Evidence From Economic Evaluation |
||||
|---|---|---|---|---|---|---|
| Outcome | Studies (No. Studies Addressing Each Outcome) | Overall Quality of the Body of Evidence (“Good, Fair, Poor”) | Expected Impact of the Intervention* (“High, Moderate, Low, Uncertain”) | Studies (No. Studies With CE Data Addressing Each Outcome) | Quality of Evidence From Economic Evaluation | Comments |
| Mortality | 30 (including 4 CE studies) | Good | High | 4 | Four level 1 “good-quality” studies have shown that CTX prophylaxis intervention is CE compared with “no-intervention.” Furthermore, it is a CE intervention when added to ART, compared with CTX prophylaxis alone46-49 | Of the 7 assessed RCTs, 6 included non-ART participants.10,18,19,29,33,38 One study included patients on ART35 |
| Of the 7 assessed RCTs, 2 enrolled exclusively TB participants10,38 | ||||||
| Most of participants included in the 7 RCTs had available baseline CD4 counts | ||||||
| Of the 17 assessed OS, 10 included participants on ART8,9,11,13,15,16,20,31,32,36 | ||||||
| Of the 3 assessed SRM/SR, 1 included studies with participants on ART only,39 1 included both studies with ART and non-ART participants,40 and 1 included studies with patients not on ART41 | ||||||
| Morbidity | 21 (including 2 CE studies) | Good | High | 2 | Two level 1 (full economic evaluations: CE analysis) studies46,48 reported significant cost savings and CE with CTX compared no-CTX (Pitter's study showed however a modest gain in DALYS with the CTX prophylaxis without clinical or immunological screening algorithm, as compared with 3 other algorithms involving initial screening | Of the 7 assessed RCTs, 4 included participants on ART23,24,27,35 |
| Of the 10 assessed OS, 3 included patients not on ART30,34,37, and 1 did not report the ART status of the participants22 | ||||||
| Only 1 SRM included exclusively participants not on ART41; All 3 studies included in the SRM were RCTs29,33,38 | ||||||
| Of the 21 assessed, 6 studies included exclusively TB patients10,12,17,18,21,38 | ||||||
| Retention in care | 4 | Poor | Uncertain | 0 | NA | Of the 4 assessed studies 3 included participants on or initiating ART.42,43,45 |
| Of the 4 assessed studies 1 enrolled patients not on ART44 | ||||||
Assessment of the expected impact of the intervention was based on published evidence. Additional considerations that would inform implementation decisions would have to take into account the CE information and country-specific contextual considerations.
The expected impact of the intervention was rated as high = intervention expected to have a high impact on the outcome; moderate = likely to have a moderate impact on the outcome; low = intervention expected to have a low impact on the outcome; or uncertain = available information is not adequate to assess estimated impact on the outcome.
ART, antiretroviral therapy; CE, cost-effectiveness; CTX, cotrimoxazole; DALY, disability adjusted life years; OS, observational study; TB, tuberculosis; RCT, randomized controlled trial; SR, systematic review; SRM, systematic review meta-analysis.
Cost Effectiveness (n = 4)
Four studies that addressed the CE of CTX prophylaxis were evaluated; all were rated level 1 (full economic evaluation: cost-effectiveness analysis).
Using a “simulation model-based study” of HIV disease, based on country-specific data from Côte d’Ivoire, Yazdanpanah et al49 assessed the CE of alternative strategies for initiation of CTX. The investigators concluded that CTX prophylaxis is “most effective” and “reasonably cost-effective” when initiated at WHO stage 2 (vs. stage 3 or 4). For instance, the “incremental CE” of CTX initiation at stage 2 disease was US $150 per year of life gained, and “lifetime costs” were increased by US $60, when compared with the “no-prophylaxis” option. Strategies including CD4 cell count testing were found to be more costly and less effective.49
Using a computer-based simulation model, Goldie et al47 assessed the potential long-term “clinical and economic impact” associated with each of 4 different strategies—ART, ART and CTX prophylaxis, CTX prophylaxis, or “no-treatment.” Strategies that included CTX prophylaxis were always found to be more cost-effective than those that used ART alone. The authors concluded that ART and CTX is a more attractive strategy, particularly in resource-constrained settings, regardless of whether ART initiation is determined using clinical or immuno-logical criteria. Findings included an “incremental cost per year of life gained” estimated at $240 for the “CTX-alone” strategy.
In a study that modeled the CE of daily CTX prophylaxis from a prospective cohort study in a rural area in Uganda in the pre-ART era, Pitter et al48 showed that providing “universal CTX prophylaxis” (CTX initiated regardless of CD4 cell count and WHO clinical stage), as compared with the “non-CTX prophylaxis” option, resulted in 7.3 additional life-years and 7.6 additional disability adjusted life-years per 100 person-years, respectively, at a “gross cost” (before deducting “medical care-related costs”) of $11.88 per person-year and “a net saving” estimated at $2.50 per person-year.
Finally, Abimbola and Marston46 used a “decision analytic model” to estimate the incremental cost, deaths averted, and incremental CE ratio of CTX prophylaxis in improving survival during the first 6 months of ART. “Full coverage” of the CTX scenario, compared with the “base case” scenario, resulted in an estimated “incremental cost” of $3.29 per patient ($163.65 vs. $160.36). “Full coverage” was also found to be associated with a reduction in the number of deaths, particularly during the first 6 months, compared with the “base case” of ART alone (22 deaths averted). The study concluded that CTX prophylaxis was a cost-effective strategy to improve survival among severely immunocompromised newly registered HIV-infected persons starting on ART.
DISCUSSION
Our review revealed significant heterogeneity in studies in terms of design, participant numbers, study population, duration of follow-up, implementation setting, timing of CTX initiation, and end-point definitions (Table 2; see also Table S1, Supplemental Digital Content, http://links.lww.com/QAI/A650). Despite this heterogeneity, the impact of CTX prophylaxis on mortality, AIDS-related illnesses, and malaria was remarkably consistent in both persons receiving and not receiving ART. Evidence for impact is strongest for patients with CD4 <350 cells per microliter or with WHO clinical stage 3 or 4 disease. In fact, data showing a mortality benefit are scarce for patients with higher CD4 counts. However, morbidity benefits, especially in preventing malaria, are consistently seen in persons with CD4 counts >350 cells per microliter.
TABLE 2.
FU Durations, Morbidity Definitions, and Enrollment in Study Criteria
| Citation | FU Duration | Morbidity Definition (When Applicable) | Criteria for Enrollment in Study |
|---|---|---|---|
| Abimbola and Marston46 | CE study | CE study | CE study |
| Alemu and Sebastian8 | Mean = 2 yrs | NA | Aged ≥15 yrs and receiving ART on at least 2 clinic visit |
| Amuron et al9 | FU duration: 3.5 yrs | NA | Aged 18 yrs or older |
| Anglaret et al29 | Mean = 0.9 yrs | Morbidity-related events that could be prevented by CTX, including bacterial infections and malaria | Aged 18 yrs or older with HIV-1 or (HIV-1 and HIV-2) infection. WHO stages 2 or 3 |
| Auld et al42 | Median = 1.3 yrs | NA | WHO stage 4, stage 3 disease and CD4 counts <350/μL or stage 1 or 2 and CD4 cell counts <200/μL |
| Badri et al30 | Median = 1.15 yrs | “Newly diagnosed severe HIV-related illnesses”: “AIDS-defining illnesses,” or WHO stage 4 bacterial infections and PTB | HIV-infected patients in South Africa (WHO clinical stages 2–4 or CD4 count <500 cells/μL or total lymphocyte count equivalent). Patients using ART were excluded |
| Boeree et al10 | FU duration = 0.7 yrs | NA | HIV-positive new smear-positive PTB patients |
| Bulabula et al22 | CS study (duration of recruitment: 0.3 yrs) | Malaria prevalence (parasitemia): positive smear for plasmodia | HIV-infected and HIV-noninfected individuals |
| Campbell et al23 | FU duration: 0.3 yrs | Malaria: “smear-positive episode of fever.” Diarrhea: “3 or more loose or watery stools reported by the enrollee, in a given 24-hr duration since the previous visit” | Enrollees through their fourth year of follow-up, with CD4 cell counts >200 cells/μL: “continue” or “discontinue” CTX |
| Clouse et al43 | FU duration: 1 yr | NA | Newly enrolled in the program, no history of previous ART uptake, pregnancy excluded, aged 18 yrs or older, with first CD4 count test performed in 2010; initial CD4 cell count <200 cells/μL |
| Denoeud-Ndam et al24 | FU duration: from 16 to 28 wk gestation until birth | For malaria diagnosis: blood smear (thin and thick) | HIV-infection, pregnancy, women aged 18 yrs or older Pregnancy: 16-28 wk |
| Dow et al25 | FU duration: 0.54 yrs, since study second antenatal visit, to assess probability of malaria-free survival | Malaria was defined as “the first episode after the second prenatal visit” and was diagnosed by a positive blood smear from a woman presenting with malaria symptoms (including fever >38°C, sweats, chills, malaise, headache, or pallor) | HIV-infection and pregnancy No history of ART uptake Aged 14 yrs or older 30 wk of gestation or less Hemoglobin >7 g/dL, CD4 cell count ≥250 cells/μL |
| Fairall et al11 | Median = 0.3 | NA | Aged 16 yrs or older who had been in contact with the program at least twice |
| Goldie et al47 | CE study | CE study | CE study |
| Grimwade and Swingler41 | SRM | NA | SRM |
| Grimwade et al12 | FU duration: 0.66 yrs | NA | Active TB, irrespective of HIV status |
| Hoffmann et al31 | Person-years of FU: 4875 | Routine symptom-based assessment for TB with laboratory investigations as indicated | Aged 18 yrs and older with CD4 counts < 350 cells/μL |
| Hoffmann et al13 | Mean = 0.8 | NA | Patients starting ART |
| Kapito-Tembo et al26 | CSS: duration of recruitment: 4 yrs | “Microscopic malaria infection”: parasites found on microscopy “malaria PCR-detected malaria”: “positive PCR” for malaria, irrespective of the microscopy result | HIV infection and pregnancy, aged 15 yrs or older, over 34 wk of gestation |
| Khoza et al14 | Not reported | NA | HIV-infected patients admitted at a major teaching hospital |
| Klement et al27 | FU duration: from 14 to 28 wk gestation until birth | Malaria: positive test, 1 symptom or more, including fever | HIV 1 infection, pregnancy, <28 wk of gestation, CD4 cell count ≥200 cells/μL, hemoglobin ≥7 g/dL |
| Kohler et al44 | FU duration: 1 yr | NA | ART ineligible patients before and after free CTX provision |
| Lim et al32 | FU duration: 0.8 yrs | “AIDS-defining illness” definition based on the “1993 Centers for Disease Control and Prevention (CDC)” | Patients included in the TREAT Asia HIV Observational Database (TAHOD) |
| Lowrance et al15 | FU duration: 0.5 yrs | NA | Participants aged 15 yrs or older, WHO stage 3 or 4 or a CD4 count cell ≤200 cells/μL |
| Madec et al16 | Median = 1.08 | Enrollees aged 13 yrs or older, WHO stage 4 or a CD4 count cell ,200 cells/μL | |
| Manyando et al40 | SR (see individual studies) | SR (see individual studies) | SR (see individual studies) |
| Maynart et al33 | Mean = 0.7 | Infections (including bacterial pneumonia, PCP, enteritis) | HIV 1 (HIV 1 and HIV 2), aged 15 yrs or older, with CD4 count below 400 copies/μL, no progressive infection |
| Mermin et al34 | FU duration: 1 yr | Malaria: fever and a positive blood smear; diarrhea: “3 or more loose or watery stools in a 24-hr period” | HIV-1 infected individuals and their HIV-negative household members |
| Msellati et al45 | CS: duration of recruitment: 0.3 yrs | NA | HIV-infected adult patients |
| Mwaungulu et al17 | FU duration: 1.5 yrs | NA | TB patients (PTB and EPTB; smear+ and smear−) |
| Newman et al28 | CSS (recruitment duration: 1 yr) | Positive placental blood smear defined as parasite density ≥1 parasite/μL | HIV-infected and uninfected pregnant women |
| Nunn et al18 | Range: 0-3.8 yrs of FU | NA | HIV-infected adults being treated for TB |
| Nunn et al19 | FU duration: 2.5 yrs (participants were followed up for a minimum of 1 yr) | NA | Women with HIV infection WHO stage 2 or 3 Recent delivery |
| Pitter et al48 | CE study | CE study | CE study |
| Polyak et al35 | FU duration: 1 yr | Malaria defined as rapid diagnostic test or smear positive with fever, pneumonia, and diarrhea (systematically ascertained) | HIV-infected adults who had been on ART for >18 mo and had CD4 count >350/μL |
| Suthar et al39 | SRM | NA | SRM |
| Van Oosterhout et al20 | FU duration: 0.5 yrs | NA | ART-naive individuals aged 18 yrs or older initiating ART |
| Walker et al36 | Median = 4.9 yrs | “New WHO stage 4,” “new or recurrent WHO stage 3 or 4,” and malaria (“clinical” or “microscopic” diagnosis) | From the “DART randomized trial of management strategies in HIV-infected symptomatic (WHO stage 2-4)” Aged 18 yrs or older, starting ART CD4 cell counts <200 cells/μL No history of ART uptake (except PMTCT) |
| Watera et al37 | Person-years of FU: 1463 (before and after CTX prophylaxis introduction) | “Primary outcomes”: fever and “other clinical signs, and positive laboratory tests” | HIV-seropositive adults (aged older than 15 yrs) |
| “Less well-defined morbid events” (secondary outcomes): clinical signs without laboratory tests results | |||
| Wiktor et al38 | Median = 0.87 | Morbidity assessed through “hospital admission rates” | HIV 1 infection and (HIV 1 and HIV 2) infected clients. “Sputum smear-positive PTB” |
| Yazdanpanah et al49 | CE study | CE study | CE study |
| Zachariah et al21 | FU duration: 1 yr | NA | HIV-infected TB patients |
CTX, cotrimoxazole; DALY, disability adjusted life-years; DART, Development of Anti-Retroviral Therapy; EPTB, extra-pulmonary tuberculosis; FU, follow-up; PTB, pulmonary tuberculosis; TB, tuberculosis; WHO, World Health Organization.
Data on the durability of the benefit of CTX are somewhat limited. Walker et al36 demonstrated a mortality benefit in ART patients receiving CTX up to 72 weeks after starting ART. However, the same study shows a reduction in the risk for new or recurrent WHO clinical stage 3 or 4 disease events and in the risk for malaria for up to 5 years. Consistent with these observations, the 2 studies addressing CTX discontinuation in persons on ART (both RCTs and both in malaria-endemic areas) demonstrated that discontinuation of CTX results in an increased risk for malaria.23,35
Limitations
This review provided useful information, but there were several limitations. Most studies were observational and therefore potentially confounded by other factors that may influence mortality and morbidity outcomes. Definitions of morbidity-related end-points varied across reviewed studies (Table 2). Some studies included both HIV-infected and HIV-uninfected persons, making the impact of CTX in HIV-infected persons difficult to ascertain. Several studies in HIV-infected pregnant women involved comparisons between CTX and other intermittent preventive treatment regimens to prevent malaria, making it difficult to assess the protective effect of CTX alone.
Research Gaps
Consistent with WHO recommendations, the benefit of CTX seems to persist in persons who are stable on ART in areas in which malaria and SBIs are common. However, more precise benchmarks for defining these thresholds, and therefore for determining when CTX might be discontinued, especially in malaria non-endemic areas, are unknown. The mechanism by which malaria occurs in persons discontinuing CTX merits more research; it is possible that waning immunity to malaria in those receiving CTX is time limited once the drug is discontinued. CTX seems to confer benefit against bacterial infections for which local antimicrobial resistance seems common; this is also true for protection against malaria in areas where resistance to SP is common. What is the mechanism of benefit, and is it possible that it will diminish with time? For HIV-infected pregnant women, CTX seems to offer comparable antimalarial benefit with IPTp, but uncertainties remain whether this protection is optimal and whether chemoprophylaxis against malaria should be supplemented in some way for HIV-infected pregnant women in malaria-endemic areas.
Programmatic Considerations
Nearly all countries have guidelines for the use of CTX, either stand-alone or incorporated into ARV treatment guidelines, and knowledge of the importance of CTX for HIV-infected persons has become essentially universal in recent years. However, implementation remains suboptimal, frequently because of drug stock-outs or diversion of existing stocks to treat bacterial infections in HIV-infected and non–HIV-infected patients. Therefore, in addition to continued guidance and training of healthcare providers concerning the importance of CTX, supply chains need to be strengthened to assure patient access to this drug. Considerations should be given at the health-facility level to maintain separate supplies of CTX for prophylaxis for HIV-infected persons. Monitoring and evaluation of implementation of CTX prophylaxis should be strengthened, so that data on the proportion of persons eligible for the drug who are in fact receiving it are available; quality improvement programs should be in place to address program deficiencies.
CONCLUSIONS
Our review emphasizes the existence of good quality consistent evidence of a protective effect of CTX on mortality and morbidity in HIV-infected adults in RLS, especially in sub-Saharan African settings. Evidence for mortality benefit is strongest in those with the lowest CD4 counts, but morbidity benefits are found in those with higher CD4 counts, especially in malaria-endemic areas. Questions remain on the durability of benefit, the appropriate time to discontinue prophylaxis in malaria–non-endemic areas, and whether CTX alone offers sufficient protection against malaria in HIV-infected pregnant women.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Gail Bang and Emily Weyant (Division of Epidemiology, Analysis and Library Services, Centers for Disease Control and Prevention, Atlanta, GA) for assisting with the literature searches. We would like also to thank Michel Tchuenche (Division of Global HIV/AIDS, Center for Global Health Centers for Disease Control and Prevention, Atlanta, GA) for his input in the cost-effectiveness studies review process.
Footnotes
Supported by the US President's Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
The findings and conclusions in this article are those of the authors and should not be construed to represent the positions of the US Department of State's Office of the US Global AIDS Coordinator and Health Diplomacy, the US Centers for Disease Control and Prevention, or the US Federal Government.
REFERENCES
- 1.World Health Organization [August 15, 2014];Guidelines on Co-Trimoxazole Prophylaxis for HIV-Related Infections Among Children, Adolescents and Adults. 2006 Available at: http://www.who.int/entity/hiv/pub/guidelines/ctxguidelines.pdf.
- 2.World Health Organization [December 12, 2014];Cotrimoxazole prophylaxis for malaria and bacterial infections in people with HIV. 2014 Available at: http://www.who.int/hiv/topics/arv/CTX_FS_FINAL-261114.pdf?ua=1.
- 3.World Health Organization/UNAIDS [August 15, 2014];Provisional WHO/UNAIDS secretariat recommendations on the use of co-trimoxazole prophylaxis in adults and children living with HIV/AIDS in Africa. 2000 Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2704444/pdf/AFHS0101-0030.pdf.
- 4.Date AA, Vitoria M, Granich R, et al. Implementation of co-trimoxazole prophylaxis and isoniazid preventive therapy for people living with HIV. Bull World Health Organ. 2010;88:253–259. doi: 10.2471/BLT.09.066522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boeree MJ, Harries AD, Zijlstra EE, et al. Co-trimoxazole in HIV-1 infection. Lancet. 1999;354:354. doi: 10.1016/S0140-6736(05)75236-3. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization [August 15, 2013];Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2013 Available at: http://www.who.int/hiv/pub/guidelines/arv2013/. [PubMed]
- 7.Kaplan JE, Hamm TE, Forhan S, et al. The impact of HIV care and support interventions on key outcomes in low and middle-Income countries: a literature review–introduction. J Acquir Immune Defic Syndr. 2015;68(suppl 3):S253–S256. doi: 10.1097/QAI.0000000000000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alemu AW, Sebastian MS. Determinants of survival in adult HIV patients on antiretroviral therapy in Oromiyaa, Ethiopia. Glob Health Action. 2010;3:5398. doi: 10.3402/gha.v3i0.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amuron B, Levin J, Birunghi J, et al. Mortality in an antiretroviral therapy programme in Jinja, south-east Uganda: a prospective cohort study. AIDS Res Ther. 2011;8:39. doi: 10.1186/1742-6405-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boeree MJ, Sauvageot D, Banda HT, et al. Efficacy and safety of two dosages of cotrimoxazole as preventive treatment for HIV-infected Malawian adults with new smear-positive tuberculosis. Trop Med Int Health. 2005;10:723–733. doi: 10.1111/j.1365-3156.2005.01433.x. [DOI] [PubMed] [Google Scholar]
- 11.Fairall LR, Bachmann MO, Louwagie GM, et al. Effectiveness of antiretroviral treatment in a South African program: a cohort study. Arch Intern Med. 2008;168:86–93. doi: 10.1001/archinternmed.2007.10. [DOI] [PubMed] [Google Scholar]
- 12.Grimwade K, Sturm AW, Nunn AJ, et al. Effectiveness of cotrimoxazole prophylaxis on mortality in adults with tuberculosis in rural South Africa. AIDS. 2005;19:163–168. doi: 10.1097/00002030-200501280-00008. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann CJ, Fielding KL, Charalambous S, et al. Reducing mortality with cotrimoxazole preventive therapy at initiation of antiretroviral therapy in South Africa. AIDS. 2010;24:1709–1716. doi: 10.1097/QAD.0b013e32833ac6bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khoza S, Mkudu V, Mthethwa J, et al. Use of cotrimoxazole prophylaxis in HIV infected in patients at a referral hospital. Cent Afr J Med. 2010;56:26–30. [PubMed] [Google Scholar]
- 15.Lowrance D, Makombe S, Harries A, et al. Lower early mortality rates among patients receiving antiretroviral treatment at clinics offering cotrimoxazole prophylaxis in Malawi. J Acquir Immune Defic Syndr. 2007;46:56–61. [PubMed] [Google Scholar]
- 16.Madec Y, Laureillard D, Pinoges L, et al. Response to highly active antiretroviral therapy among severely immuno-compromised HIV-infected patients in Cambodia. AIDS. 2007;21:351–359. doi: 10.1097/QAD.0b013e328012c54f. [DOI] [PubMed] [Google Scholar]
- 17.Mwaungulu FB, Floyd S, Crampin AC, et al. Cotrimoxazole prophylaxis reduces mortality in human immunodeficiency virus-positive tuberculosis patients in Karonga District, Malawi. Bull World Health Organ. 2004;82:354–363. [PMC free article] [PubMed] [Google Scholar]
- 18.Nunn AJ, Mwaba P, Chintu C, et al. Role of co-trimoxazole prophylaxis in reducing mortality in HIV infected adults being treated for tuberculosis: randomised clinical trial. BMJ. 2008;337:a257. doi: 10.1136/bmj.a257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nunn AJ, Mwaba PB, Chintu C, et al. Randomised, placebo-controlled trial to evaluate co-trimoxazole to reduce mortality and morbidity in HIV-infected post-natal women in Zambia (TOPAZ). Trop Med Int Health. 2011;16:518–526. doi: 10.1111/j.1365-3156.2011.02731.x. [DOI] [PubMed] [Google Scholar]
- 20.Van Oosterhout JJ, Ndekha M, Moore E, et al. The benefit of supplementary feeding for wasted Malawian adults initiating ART. AIDS Care. 2010;22:737–742. doi: 10.1080/09540120903373581. [DOI] [PubMed] [Google Scholar]
- 21.Zachariah R, Spielmann MP, Chinji C, et al. Voluntary counselling, HIV testing and adjunctive cotrimoxazole reduces mortality in tuberculosis patients in Thyolo, Malawi. AIDS. 2003;17:1053–1061. doi: 10.1097/00002030-200305020-00015. [DOI] [PubMed] [Google Scholar]
- 22.Bulabula AM, Mayanga Mafwila, et al. Malaria prevalence in HIV patients under cotrimoxazole. Case of Kindu, Maniema, D.R. Congo. Preliminary results. Ital J Trop Med. 2009;14:1–2. [Google Scholar]
- 23.Campbell JD, Moore D, Degerman R, et al. HIV-infected ugandan adults taking antiretroviral therapy with CD4 counts >200 cells/muL who discontinue cotrimoxazole prophylaxis have increased risk of malaria and diarrhea. Clin Infect Dis. 2012;54:1204–1211. doi: 10.1093/cid/cis013. [DOI] [PubMed] [Google Scholar]
- 24.Denoeud-Ndam L, Zannou DM, Fourcade C, et al. Cotrimoxazole prophylaxis versus mefloquine intermittent preventive treatment to prevent malaria in HIV-infected pregnant women: two randomized controlled trials. J Acquir Immune Defic Syndr. 2014;65:198–206. doi: 10.1097/QAI.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 25.Dow A, Kayira D, Hudgens MG, et al. The effect of cotrimoxazole prophylactic treatment on malaria, birth outcomes, and postpartum CD4 count in HIV-infected women. Infect Dis Obstet Gynecol. 2013;2013:340702. doi: 10.1155/2013/340702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapito-Tembo A, Meshnick SR, Van Hensbroek MB, et al. Marked reduction in prevalence of malaria parasitemia and anemia in HIV-infected pregnant women taking cotrimoxazole with or without sulfadoxine-pyrimethamine intermittent preventive therapy during pregnancy in Malawi. J Infect Dis. 2011;203:464–472. doi: 10.1093/infdis/jiq072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klement E, Pitche P, Kendjo E, et al. Effectiveness of co-trimoxazole to prevent Plasmodium falciparum malaria in HIV-positive pregnant women in sub-Saharan Africa: an open-label, randomized controlled trial. Clin Infect Dis. 2014;58:651–659. doi: 10.1093/cid/cit806. [DOI] [PubMed] [Google Scholar]
- 28.Newman PM, Wanzira H, Tumwine G, et al. Placental malaria among HIV-infected and uninfected women receiving anti-folates in a high transmission area of Uganda. Malar J. 2009;8:254. doi: 10.1186/1475-2875-8-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anglaret X, Chene G, Attia A, et al. Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Cote d'Ivoire: a randomised trial. Cotrimo-CI Study Group. Lancet. 1999;353:1463–1468. doi: 10.1016/s0140-6736(98)07399-1. [DOI] [PubMed] [Google Scholar]
- 30.Badri M, Ehrlich R, Wood R, et al. Initiating co-trimoxazole prophylaxis in HIV-infected patients in Africa: an evaluation of the provisional WHO/UNAIDS recommendations. AIDS. 2001;15:1143–1148. doi: 10.1097/00002030-200106150-00009. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann CJ, Chaisson RE, Martinson NA. Cotrimoxazole prophylaxis and tuberculosis risk among people living with HIV. PLoS One. 2014;9:e83750. doi: 10.1371/journal.pone.0083750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim PL, Zhou J, Ditangco RA, et al. Failure to prescribe pneumocystis prophylaxis is associated with increased mortality, even in the cART era: results from the Treat Asia HIV observational database. J Int AIDS Soc. 2012;15:1. doi: 10.1186/1758-2652-15-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maynart M, Lievre L, Sow PS, et al. Primary prevention with cotrimoxazole for HIV-1-infected adults: results of the pilot study in Dakar, Senegal. J Acquir Immune Defic Syndr. 2001;26:130–136. doi: 10.1097/00042560-200102010-00004. [DOI] [PubMed] [Google Scholar]
- 34.Mermin J, Lule J, Ekwaru JP, et al. Effect of co-trimoxazole prophylaxis on morbidity, mortality, CD4-cell count, and viral load in HIV infection in rural Uganda. Lancet. 2004;364:1428–1434. doi: 10.1016/S0140-6736(04)17225-5. [DOI] [PubMed] [Google Scholar]
- 35.Polyak C, Yuhas K, Singa B, et al. CTX Prophylaxis discontinuation among ART-treated adults: a randomised non-Inferiority trial [Oral abstract 98].. Presented at: XXI Conference on Retroviruses and Opportunistic Infections; Boston, MA.. March 5, 2014. [Google Scholar]
- 36.Walker AS, Ford D, Gilks CF, et al. Daily co-trimoxazole prophylaxis in severely immunosuppressed HIV-infected adults in Africa started on combination antiretroviral therapy: an observational analysis of the DART cohort. Lancet. 2010;375:1278–1286. doi: 10.1016/S0140-6736(10)60057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watera C, Todd J, Muwonge R, et al. Feasibility and effectiveness of cotrimoxazole prophylaxis for HIV-1-infected adults attending an HIV/AIDS clinic in Uganda. J Acquir Immune Defic Syndr. 2006;42:373–378. doi: 10.1097/01.qai.0000221679.14445.1b. [DOI] [PubMed] [Google Scholar]
- 38.Wiktor SZ, Sassan-Morokro M, Grant AD, et al. Efficacy of trimethoprim-sulphamethoxazole prophylaxis to decrease morbidity and mortality in HIV-1-infected patients with tuberculosis in Abidjan, Cote d'Ivoire: a randomised controlled trial. Lancet. 1999;353:1469–1475. doi: 10.1016/s0140-6736(99)03465-0. [DOI] [PubMed] [Google Scholar]
- 39.Suthar AB, Granich R, Mermin J, et al. Effect of cotrimoxazole on mortality in HIV-infected adults on antiretroviral therapy: a systematic review and meta-analysis. Bull World Health Organ. 2012;90:128C–138C. doi: 10.2471/BLT.11.093260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manyando C, Njunju EM, D'Alessandro U, et al. Safety and efficacy of co-trimoxazole for treatment and prevention of Plasmodium falciparum malaria: a systematic review. PLoS One. 2013;8:e56916. doi: 10.1371/journal.pone.0056916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grimwade K, Swingler G. Cotrimoxazole prophylaxis for opportunistic infections in adults with HIV. Cochrane Database Syst Rev. 2003:CD003108. doi: 10.1002/14651858.CD003108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Auld AF, Mbofana F, Shiraishi RW, et al. Four-year treatment outcomes of adult patients enrolled in Mozambique's rapidly expanding antiretroviral therapy program. PLoS One. 2011;6:e18453. doi: 10.1371/journal.pone.0018453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clouse K, Shearer K, Bassett J, et al. Reduced loss to ART initiation among patients initiating cotrimoxazole prophylaxis therapy in Johannesburg, South Africa [TUPE737].. Presented at: XIX International AIDS Conference; Washington, DC.. 2012. [Google Scholar]
- 44.Kohler PK, Chung MH, McGrath CJ, et al. Implementation of free cotrimoxazole prophylaxis improves clinic retention among antiretroviral therapy-ineligible clients in Kenya. AIDS. 2011;25:1657–1661. doi: 10.1097/QAD.0b013e32834957fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Msellati P, Juillet-Amari A, Prudhomme J, et al. Socio-economic and health characteristics of HIV-infected patients seeking care in relation to access to the Drug Access Initiative and to antiretroviral treatment in Cote d'Ivoire. AIDS. 2003;17(suppl 3):S63–S68. doi: 10.1097/00002030-200317003-00009. [DOI] [PubMed] [Google Scholar]
- 46.Abimbola TO, Marston BJ. The cost-effectiveness of cotrimoxazole in people with advanced HIV infection initiating antiretroviral therapy in sub-Saharan Africa. J Acquir Immune Defic Syndr. 2012;60:e8–e14. doi: 10.1097/QAI.0b013e3182478dc0. [DOI] [PubMed] [Google Scholar]
- 47.Goldie SJ, Yazdanpanah Y, Losina E, et al. Cost-effectiveness of HIV treatment in resource-poor settings- the case of Cote d'Ivoire. N Engl J Med. 2006;355:1141–1153. doi: 10.1056/NEJMsa060247. [DOI] [PubMed] [Google Scholar]
- 48.Pitter C, Kahn JG, Marseille E, et al. Cost-effectiveness of cotrimoxazole prophylaxis among persons with HIV in Uganda. J Acquir Immune Defic Syndr. 2007;44:336–343. doi: 10.1097/QAI.0b013e31802f12b5. [DOI] [PubMed] [Google Scholar]
- 49.Yazdanpanah Y, Losina E, Anglaret X, et al. Clinical impact and cost-effectiveness of co-trimoxazole prophylaxis in patients with HIV/AIDS in Cote d'Ivoire: a trial-based analysis. AIDS. 2005;19:1299–1308. doi: 10.1097/01.aids.0000180101.80888.c6. [DOI] [PubMed] [Google Scholar]
- 50.World Health Organization [August 15, 2014];A Strategic Framework for Malaria Prevention and Control during Pregnancy in the African Region. 2004 Available at: http://whqlibdoc.who.int/afro/2004/AFR_MAL_04.01.pdf.
- 51.Roll Back Malaria. [March 24, 2011];Global Strategic Plan 2005-2010. 2005 Available at: http://www.rollbackmalaria.org/forumV/docs/gsp_en.pdf.
- 52.Greenwood BM, David PH, Otoo-Forbes LN, et al. Mortality and morbidity from malaria after stopping malaria chemoprophylaxis. Trans R Soc Trop Med Hyg. 1995;89:629–633. doi: 10.1016/0035-9203(95)90419-0. [DOI] [PubMed] [Google Scholar]
- 53.Menendez C, Kahigwa E, Hirt R, et al. Randomised placebo-controlled trial of iron supplementation and malaria chemoprophylaxis for prevention of severe anaemia and malaria in Tanzanian infants. Lancet. 1997;350:844–850. doi: 10.1016/S0140-6736(97)04229-3. [DOI] [PubMed] [Google Scholar]
- 54.Mockenhaupt FP, Reither K, Zanger P, et al. Intermittent preventive treatment in infants as a means of malaria control: a randomized, double-blind, placebo-controlled trial in northern Ghana. Antimicrob Agents Chemother. 2007;51:3273–3281. doi: 10.1128/AAC.00513-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beck HP, Felger I, Vounatsou P, et al. Effect of iron supplementation and malaria prophylaxis in infants on Plasmodium falciparum genotypes and multiplicity of infection. Trans R Soc Trop Med Hyg. 1999;93(suppl 1):41–45. doi: 10.1016/s0035-9203(99)90326-7. [DOI] [PubMed] [Google Scholar]
- 56.Bereczky S, Liljander A, Rooth I, et al. Multiclonal asymptomatic Plasmodium falciparum infections predict a reduced risk of malaria disease in a Tanzanian population. Microbes Infect. 2007;9:103–110. doi: 10.1016/j.micinf.2006.10.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.