Abstract
Background
HIV-infected adults are at increased risk of severe malaria and death. Malaria prevention in people living with HIV (PLHIV) consists of several interventions, including cotrimoxazole (CTX) prophylaxis and insecticide-treated nets (ITNs). We conducted a systematic review of the available evidence.
Methods
MEDLINE, EmBase, Global Health, CINAHL, SOCA, and African Index Medicus were used to identify articles relevant to the CTX prophylaxis and ITNs interventions from 1995 to July 2014. For each individual study, we assessed the quality of evidence and the impact of the 2 interventions on the outcomes of mortality, morbidity, retention in care, quality of life, and/or prevention of ongoing HIV transmission. For each outcome, we summarized the quality of the overall body of evidence, the expected impact, and costing and cost-effectiveness (CE).
Findings
The overall quality of evidence regarding malaria-related morbidity was rated as “good” for CTX prophylaxis and “fair” for ITN use; the expected “impact” of these interventions on morbidity was rated “high” and “uncertain,” respectively. Three studies that addressed the costing and CE of ITN provision for malaria prevention in PLHIV consisted of 2 full “level 1” and 1 partial “level 2” economic evaluations.
Conclusions
CTX prophylaxis is effective in reducing malaria-related morbidity among PLHIV. Limited evidence is available with respect to the impact and the CE of ITN use and/or provision in this population.
Keywords: cotrimoxazole, insecticide-treated nets, malaria prevention, HIV, resource-limited settings
INTRODUCTION
There are important interactions between HIV and malaria at both the population and individual levels.1 In 2012, there were 2.3 million new HIV infections globally (1.9–2.7 million) and 1.6 million (1.4–1.9 million) AIDS-related deaths2; in the same year, an estimated 207 million cases of malaria (uncertainty interval: 135–287 million) caused approximately 627,000 deaths (uncertainty interval: 473,000–789,000).3 People living with HIV (PLHIV) are at increased risk for clinical malaria and may be at higher risk for treatment failure,4 and the adverse effects of malaria (anemia, low birth weight, preterm delivery) are amplified, particularly in pregnant women with HIV.5–8 Thus, identification of the best methods for reducing the incidence of malaria in adults with HIV infection could improve clinical and public health outcomes, especially in areas where both diseases are highly prevalent.
On World AIDS Day 2014, the World Health Organization (WHO) released a supplement to the 2013 “WHO consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection,”9 in which it provides an updated set of recommendations regarding cotrimoxazole (CTX) prophylaxis among PLHIV.10 The WHO states that “CTX prophylaxis is recommended for adults (including pregnant women) with severe or advanced HIV clinical disease (WHO stages 3 or 4) and/or with CD4 ≤350 cells per cubic millimeter. In settings where malaria and/or severe bacterial infections are highly prevalent, CTX prophylaxis should be initiated regardless of CD4 cell count or WHO stage.” The WHO also states that “CTX prophylaxis may be discontinued in adults (including pregnant women) with HIV infection, who are clinically stable on antiretroviral therapy (ART), with evidence of immune recovery and virologic suppression. In settings where malaria and/or severe bacterial infections are highly prevalent, CTX prophylaxis should be continued regardless of CD4 cell count or WHO clinical stage.”10
To reduce the risks associated with placental malaria, WHO recommends that HIV-infected pregnant women (as well as uninfected pregnant women and women whose HIV status is unknown) receive intermittent malaria preventive treatment during pregnancy (IPTp), although per WHO's 2006 guidance for CTX prophylaxis among PLHIV,11 and WHO's 2013 policy brief for the implementation of IPTp using sulfadoxine–pyrimethamine (IPTp-SP) to prevent malaria among pregnant women,8 IPTp is not required in pregnant women on CTX. This recommendation reflects the potential toxicity of combining CTX with other sulfa-based medications used for IPTp (eg, Sulfadoxine-pyrimethamine).8,11
This review aims at assessing the quality of evidence related to the efficacy of CTX prophylaxis for malaria prevention among HIV-infected adults, including pregnant women, and will address the question of whether CTX provided for general prophylaxis in women with HIV is adequate for the prevention of malaria-related morbidity.
Consistent and correct use of insecticide-treated nets (ITNs) is another key intervention for malaria prevention. ITNs, including long-lasting insecticidal nets (LLINs), reduce the incidence of malaria and associated morbidity, particularly among children and pregnant women (the most susceptible groups of populations to malaria and its consequences),12 and have successfully contributed to the reduction in malaria and all-cause mortality over the past decade.13,14
The WHO's “Recommendations for Achieving Universal Coverage with Long-Lasting Insecticidal Nets in Malaria Control” (September 2013) emphasize the need to ensure that all populations at risk of malaria have access to ITNs.14 The WHO also recommends that existing public health programs (eg, combined mass distribution and immunization campaigns) should be leveraged and used as platforms to maximize efforts to achieve “universal coverage” of ITNs.15
In line with the other papers in this JAIDS supplement, this review summarizes currently available information about CTX prophylaxis and ITNs as malaria prevention in PLHIV and their impact on key outcomes. Because the focus of this supplement is on adult patients with HIV, we include only literature pertinent to the adult population. We did not address indoor residual spraying and prompt and effective case management of malaria illness that targets adults, some of whom may be HIV infected, nor did we address malaria prevention in children.
METHODS
We conducted a search of 6 medical literature databases—MEDLINE, EmBase, Global Health, CINAHL, SOCA, and African Index Medicus—to identify articles relevant to the use of CTX prophylaxis and ITNs/LLINs for malaria prevention interventions from 1995 to July 2014. A more detailed description of the search terms applied and the geographic filters used can be found in “Evaluation of evidence for impact of HIV care and support interventions in resource-limited countries: Introduction” in this Supplement.16 Specific additional search terms were used (“MALARIA” or “MALARIAE” or “MALARIAL” or “MALARIALS” or “MALARIOUS” or “MALARI”).
Search outputs (titles and abstracts) were reviewed by the authors to identify potentially relevant studies. Papers that appeared to contain relevant data were read in their entirety; included articles (1) studied PLHIV, (2) were conducted in developing countries (low- or middle-income), (3) used a CTX prophylaxis intervention or insecticide-treated nets for prevention of malaria, and (4) reported on at least 1 of the 5 outcomes of interest (mortality, morbidity, retention in HIV care, quality of life, or prevention of HIV transmission) or on costing or cost-effectiveness (CE). Those that satisfied criteria for inclusion were abstracted and summarized on the basis of study design [eg, randomized control trial (RCT) and cohort study], comparison group(s), number of participants, and assessment of impact on the outcome(s) of interest [expressed as hazard ratios (HR), odds ratios (OR), incidence rate ratios (IRR), or relative risk (RR) and the respective 95% confidence intervals (CIs) if available]. An assessment of the quality of each study was made on the basis of study design, number of participants, and internal and external validity and rated as “strong,” “medium,” or “weak.” More details of the assessment of quality are described in “Evaluation of evidence for impact of HIV care and support interventions in resource-limited countries: Introduction.”16 Health economic studies were assessed based on an agreed-upon set of categories as follows: level 1 full economic evaluation (includes either cost-effectiveness analysis, cost–utility analysis, cost–benefit analysis), level 2 partial economic evaluations (ie, cost analysis, cost-description studies, cost–outcome descriptions), or level 3 randomized trials and studies reporting more limited information, such as estimates of resource use or costs associated with intervention(s) and comparator(s).
Because of the heterogeneity of study populations, study methods, settings, and outcomes, we did not perform quantitative synthesis of study results overall. However, we rated the overall quality of the body of evidence related to each outcome as “good,” “fair,” or “poor” based on criteria agreed on a priori and described in the introductory paper. Similarly, the expected impact on each outcome was rated as “high,” “medium,” “low,” or “uncertain.” These criteria are described in “Evaluation of evidence for impact of HIV care and support interventions in resource-limited countries: Introduction.”16
RESULTS
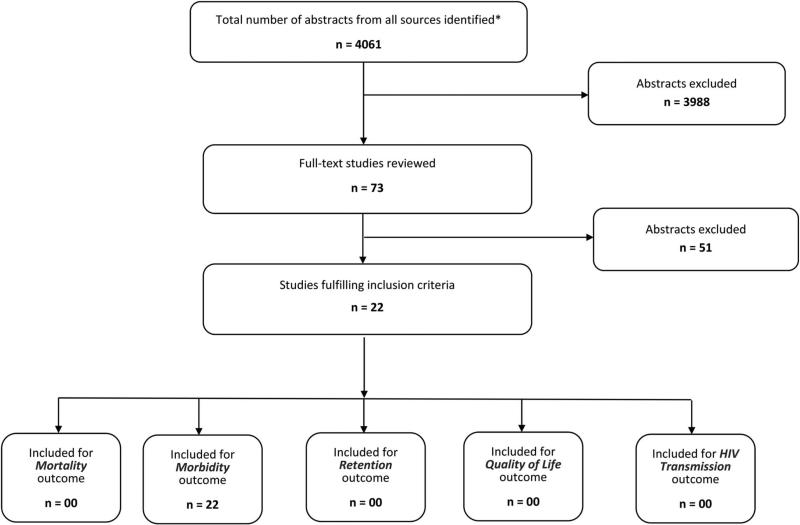
Searches identified 4061 unduplicated abstracts of which 73 appeared to contain pertinent information and were therefore read in their entirety to determine whether they satisfied criteria for inclusion (Fig. 1). Twenty-two articles met inclusion criteria. Morbidity (variably defined across reviewed articles) was the only outcome addressed in the included articles; none of the 22 addressed mortality, retention in care, quality of care, or HIV transmission. Table S1 (see Supplemental Digital Content, http://links.lww.com/QAI/A656) summarizes the study design, sample size, findings, and quality of evidence rating of the 22 articles meeting final inclusion criteria.
FIGURE 1.
Study flow diagram. *Duplicate citations removed.
The included studies had a wide variety of statistical designs and analyses, including 5 RCTs,17,19,20,24,28 13 observational studies (OSs),18,21–23,26,27,29–35 1 systematic review (SR),25 and 3 health economics studies (see Table S1, Supplemental Digital Content, http://links.lww.com/QAI/A656).36–38
The 22 studies varied with respect to the participants’ ART status. Of the 20 assessed individual studies (excluding the SR and 1 study that did not report on ART status),18,25 14 (including 4 RCTs,19,20,24,28 9 OS,21,23,27,29–34 and 1 CE study38) enrolled participants on ART; 6 studies included participants not on ART (1 RCT,17 3 OS,22,26,31,35 and 2 CE studies36). Of the 19 included studies that addressed malaria-related morbidity outcomes (excluding the 3 costing and CE studies), 17 studies addressed CTX prophylaxis17–33; 4 studies addressed provision and/or use of ITNs32–35 (including 2 studies that also addressed CTX prophylaxis32,33 and 2 that addressed ITN use alone).34,35 The 3 health economics studies also addressed ITNs.
CTX Prophylaxis for Malaria Prevention (Excluding Costing and CE Studies,36–38 the SR,25 and a Study That Did Not Report on the ART Status18) (n = 15)
Summary of Results of Individual Studies in Persons Who Did Not Receive ART (n = 4)
The study by Anglaret et al,17 an RCT in which malaria was addressed as a secondary outcome, reported an estimated malaria HR of 0·16 (0.04–0.73) among participants on CTX for morbidity events defined as “at least 1 severe malaria event.” Mermin et al26 (2004), in a longitudinal prospective study that aimed at assessing the association between CTX prophylaxis and morbidity (including malaria), mortality, CD4 cell count, and viral load in adult PLHIV, showed an estimated 72% reduction in malaria incidence in enrollees on CTX. Reduction in malaria-related morbidity was found in both studies, irrespective of CD4 cell count. The study by Hamel et al was a prospective, open-label, non-RCT, whose main purpose was to assess the potential impact of daily CTX on the emergence of “significant changes in antifolate and CTX resistance among common organisms,” including Plasmodium falciparum. HIV-infected subjects with CD4 cell count <350 per microliter (and receiving CTX) had an estimated 90% decrease in “incidence density” of “first or only infection” with P. falciparum compared with HIV-infected persons with CD4 cell count >350 per microliter (who were not receiving CTX) (adjusted rate ratio: 0.10, 95% CI: 0.07 to 0.15, P < 0.025). The “incidence density” for “all episodes of P. falciparum parasitemia” was found to be reduced as well. This protective effect against P. falciparum infection among enrollees who received CTX did not change after adjusting for factors that included “prior P. falciparum parasitemia, IPTp-SP, ITN use, and housing type, in Poisson regression models” (P < 0.001).22
The study by Watera et al,31 an OS involving a historical comparison, did not show an effect of CTX prophylaxis on frequencies of “febrile and other morbidity event rates.” It did however show a reduction in the incidence of malaria (IRR: 0.31, 95% CI: 0.13 to 0.72).31
Summary of Results of Individual Studies in Persons Who Received ART (n = 11)
Comparison “On CTX” vs. “Off CTX” (n = 4)
Walker et al30 estimated the effects of CTX prophylaxis on “clinical outcomes” (including malaria) in a retrospective study, which enrolled HIV-infected ART-naive adults who were started on ART, with CD4 cell counts <200 per microliter. This observational analysis used data from the “routine versus clinically driven laboratory monitoring of HIV antiretroviral therapy in Africa” (DART) study, conducted in 2 centers, in Uganda and in Zimbabwe. Because of low malaria endemicity in Zimbabwe, the analysis of the effect of CTX on malaria was confined to Uganda. The study showed a statistically significant reduction in the frequency of malaria with diagnosis based on either symptoms or positive microscopy, in persons on CTX (OR: 0.74, 95% CI: 0.63 to 0.88, P = 0.0005); the effect was sustained until 72 weeks of follow-up [between 48 and 72 weeks on ART, persons receiving CTX prophylaxis had an OR of developing malaria of 0.50 (95% CI: 0.33 to 0.74, P = 0.001)].30 Mermin et al (2006) was a 4-phase prospective longitudinal study whose primary purpose was to address the effect of ART on the frequency of “clinical malaria” in PLHIV and to determine the “additive effects” of CTX prophylaxis, ART, and ITNs. The study showed a statistically significant association between CTX prophylaxis and reduced malaria incidence, defined as a febrile episode combined with a positive microscopic examination (adjusted IRR: 0.24, 95% CI: 0.15 to 0.38).32
The study by Iliyasu et al33 was a cross-sectional study whose primary aim was to assess the “prevalence and predictors” (including CTX prophylaxis and ITNs use) of malaria among adult PLHIV. Most of the respondents in this study (86.0%) were receiving CTX prophylaxis and 53.5% reported use of ITNs. The study did not demonstrate a significant difference in prevalence of “clinical malaria” (symptomatic parasitemia) among those who reported use of CTX prophylaxis, compared with those not having been on CTX prophylaxis (adjusted odds ratios (aOR): 1.27, 95% CI: 0.98 to 1.64, P = 0.13).33 Statistically significant differences were, however, identified using a multivariate analysis aimed at addressing potential associations between reported “clinical malaria” with baseline CD4 cell count <350 per microliter vs. >600 per microliter and non-use of ITNs (aOR: 2.41, 95% CI: 1.23 to 3.74, P = 0.023; and 1.97, 95% CI: 1.17 to 2.85, P = 0.017, respectively).33
Saracino et al,29 in a prospective longitudinal study, enrolled consecutively hospitalized adults who received “HIV testing, malaria blood smear, and, in a subgroup of patients, a rapid malaria test (RDT).” The study aimed at assessing the association between a positive malaria blood smear and/or RDT and the prevalence of “clinical malaria” (symptomatic parasitemia) with concomitant HIV infection and, secondarily, with the use of CTX and/or ART. 66.7% of enrollees were HIV positive and 35.4% of HIV-positive patients reported having a CTX prophylaxis prescription at the time of admission. The study showed a statistically significant association between CTX prophylaxis and positive malaria testing (OR: 0.20, 95% CI: 0.14 to 0.61, P < 0.001).29
CTX and Continuation/Discontinuation Individual studies (n = 2)
The study by Campbell et al19 was an RCT in which HIV-infected persons stable on ART and with CD4 cell counts above 200 per microliter were assigned to either continuation or discontinuation of CTX prophylaxis. Participants were assessed primarily for malaria and diarrhea-related outcomes. Enrollees who discontinued CTX prophylaxis were at a significantly higher risk of developing clinical malaria compared with those who continued CTX prophylaxis (RR of malaria 32.5, 95% CI: 8.6 to 275.0, P < 0.001). This study was stopped in its fourth month, as recommended by the Data Safety and Monitoring Board (DSMB); consequently, it was impossible to exclude a “short-lived rebound effect” as an explanation of the increased incidence of malaria illness.19 “Rebound effects” are thought to be a result of an “impairment of the immunity protective effect,” as a consequence of the use of antimalarial therapies39–41 (it has been suggested that the role of “persisting asymptomatic and polyclonal P. falciparum infections,” which occur in the absence of malaria prophylaxis in settings of intense malaria transmission, could be a potential explanation of the observed “rebound effect” after the interruption of prophylaxis).41–43
The study by Polyak et al (an RCT) aimed to determine the impact of CTX prophylaxis discontinuation on morbidity among HIV-infected adults who had been on ART for more than 18 months and who had a CD4 cell count >350 per microliter (CD4 count at enrollment was estimated at 595 cells/μL, and median ART duration was 4.5 years).28 The primary end-point consisted of a “composite of morbidity (malaria, pneumonia, and diarrhea) and mortality.” Enrollees who discontinued CTX prophylaxis had a significantly higher risk of the predefined end-point compared with those who continued CTX prophylaxis (combined morbidity/mortality IRR: 2.27, 95% CI: 1.52 to 3.38, P < 0.001). Malaria, defined here as a febrile episode confirmed with either a positive RDT or smear examination, was deemed to be the “driver” of the higher risk finding (IRR estimated at 33.02, 95% CI: 4.52 to 241.02, P = 0.001).28
Comparison of CTX to Other Antimalarial Drugs Among Pregnant Women (n = 5)
Five individual studies, including 2 RCTs20,24 and 3 OS,21,23,27 addressed the effect of CTX on malaria end-points in HIV-infected pregnant women, compared with either IPTp-SP or mefloquine (MQ). Klement et al24 (an RCT) assessed the risk of developing malaria in HIV-infected pregnant women with a CD4 cell count >200 per microliter. The study compared the effectiveness of CTX prophylaxis with that of IPTp-SP in preventing malaria during pregnancy in a P. falciparum–endemic area in Togo. In the CTX arm, 75.4% of women did not develop malaria (defined as a thick blood smear or RDT-confirmed case of a symptomatic malaria episode) compared with 84.7% of women in the IPTp-SP arm. The difference was estimated at 9.3%, 95% CI: −0.53 to 19.1, not meeting the predefined “non-inferiority” criterion. The authors did not conclude that CTX was inferior to IPTp-SP regarding malaria-free survival (the study was designed to assess “non-inferiority”), but the difference in point estimates of malaria-free rates suggests that CTX could potentially be inferior to IPTp-SP. It is noteworthy that the author did not report on the proportions of enrollees who received 2 vs 3 doses of IPTp-SP; the third dose of IPTp-SP was given “when possible.”24 It is therefore possible that CTX prophylaxis would have been shown to be inferior had the subjects received the WHO-recommended 3 doses of IPTp-SP.8
In the RCT conducted by Denoeud-Ndam et al,20 CTX was found to be non-inferior when compared with CTX plus MQ in preventing microscopically diagnosed placental malaria in HIV-infected women with CD4 cell counts <350 cells per microliter. However, using polymerase chain reaction (PCR)–based diagnosis, placental malaria was significantly less likely to be diagnosed in women who received CTX + MQ compared with those who received CTX only (0/105 and 5/103, respectively, P = 0.03).20
The study by Dow et al21 was a retrospective study whose main purpose was to assess the effect of CTX prophylaxis on malaria during pregnancy, low birth weight, and preterm birth in women enrolled in the “Breastfeeding, Antiretroviral, and Nutrition” RCT, which was conducted in Malawi between 2004 and 2009. The implementation of CTX prophylaxis 2 years after the start of this RCT represented an opportunity to assess the effect of CTX prophylaxis. CTX prophylaxis was implemented in 2006. The analysis showed a statistically significant protective effect of CTX prophylaxis against malaria (defined as “the first episode after the second prenatal visit,” clinically diagnosed and laboratory-confirmed), as compared with IPTp (malaria HR: 0.35, 95% CI: 0.20 to 0.60). However, a “sensitivity analysis” of malaria rates in CTX-ineligible women (with CD4 counts >500/μL) in the 2 time-periods indicated that women were at lower risk of malaria after 2006; after adjustment for time-period, CTX prophylaxis was no longer found to be protective against malaria (aHR 0.66, 95% CI: 0.28 to 1.52).21
In Kapito et al, data from a cross-sectional study that was conducted to assess the effects of iron supplementation on “maternal morbidities” were used to assess the prevalence of malaria parasitemia and anemia in HIV-infected pregnant women who received CTX prophylaxis, with or without IPTp-SP, compared with women who received IPTp-SP only. Women enrolled in this study were aged 15 or older, less than 34 weeks pregnant, and were attending a routine antenatal care routine visit. Participants were required to respond to a questionnaire and submit to a physical examination, followed by malaria laboratory testing (smear microscopy and PCR).23 Enrollees had a median CD4 cell count of 423 cells per microliter (ranging from 11 to 1528 cells/μL), use of nets was reported by 59.6% of enrolled women, and 48.5% of the women reported ART. After adjusting for potential confounders, women taking CTX, with or without IPTp-SP, were less likely to have “microscopic” or “PCR-detected” malaria infection, compared with women who received IPTp-SP alone (infection OR: 0.09, 95% CI: 0.01 to 0.66 and 0.43, 95% CI: 0.19 to 0.97, respectively). Possible confounding factors, such as “ITN use, ART use,” and the change in the newly implemented national malaria prevention guidelines, may have prevented a more accurate analysis and interpretation of the study's findings, a challenge that could not be addressed, given the non-randomization nature of this study.23
Newman et al conducted a cross-sectional study that aimed at comparing the prevalence of placental malaria in HIV-infected pregnant women receiving CTX prophylaxis with that in HIV-uninfected pregnant women receiving IPTp-SP. After adjusting for factors including “gravidity, age, and season at the time of delivery,” no significant difference was found in terms of placental malaria prevalence at delivery, between HIV-infected women who received CTX prophylaxis as compared with HIV-uninfected women on IPTp-SP.27
Systematic review addressing CTX prophylaxis malaria-related morbidity
In the study by Manyando et al,25 which included some of the individual studies in this review,23,26,27,30,32 CTX was found to reduce malaria incidence by 46%–97%.
Provision and Use of Insecticide-Treated Nets (Excluding Health Economics studies)
Of the 19 studies that addressed malaria-related morbidity outcomes (excluding the 3 health economics studies), 4 studies addressed the use of ITNs.32–35
The study by Mermin et al32 (2006) was a 4-phase study in which 3 interventions (CTX prophylaxis, ART, and nets) were sequentially offered to participants who were enrolled at different points throughout the study's duration. CTX prophylaxis was found to have a protective effect against “clinical malaria” (“reported fever in the previous 2 days,” confirmed through both a thick and a thin smear exam). Malaria adjusted-IRR (aIRR) was estimated at 0.05, 95% CI: 0.03 to 0.08.32
A descriptive cross-sectional study reported by Olowookere et al34 enrolled a “convenience sample” of newly recruited PLHIV who were submitted to malaria screening, treated if diagnosed with malaria, educated about malaria infection, provided with an ITN, and finally evaluated for malaria infection on a monthly basis during a 3-month follow-up period. A decrease in prevalence of positive P. falciparum parasitemia from 59.7% at baseline to 5.1% at the end of the 3-month period was observed. The authors did not report on a precise value of the effect estimate.34
The study by Walson et al was a prospective cohort study that assessed the potential effect of “LLINs and simple point-of-use water filters” on the slowing of HIV-1 disease progression. The study's 2 primary end-points consisted of “time to CD4 cell count <350 cells per microliter” and “a composite end-point of time to CD4 cell count <350 cells per microliter and non-traumatic death.” Enrollment criteria included “HIV-1 infection, age >18, absence of a history of ART, a CD4 cell count >350 per microliter, and WHO stage I or II disease.”35 The study addressed malaria morbidity as a secondary end-point by measuring the incidence of both “self-reported” (symptomatic) and “laboratory-confirmed malaria.” Enrollees were followed for up to 24 months. Participants in the intervention cohort (receiving LLINs and water filters) were less likely to self-report malaria within the previous 3 months compared with participants in the control cohort (RR: 0.75, 95% CI: 0.60 to 0.93). “Clinically diagnosed” malaria was also less likely to be identified by a health care provider within the previous 3 months among subjects who received LLINs and water filters, compared with those who did not (RR: 0.66, 95% CI: 0.49 to 0.88). There was no difference in the use of CTX prophylaxis between the cohorts (99.7% and 99.8% in the intervention and in the control group of patients, respectively, P = 0.8).35 Finally, participants who received and used LLINs and point-of-use water filters were found to have a significantly lower risk of having their CD4 cell counts decline to below 350 cells per microliter (HR: 0.73, 95% CI: 0.57 to 0.95).35
The study by Iliyasu et al33 was a cross-sectional study that primarily aimed at estimating the “prevalence and predictors of malaria infection among HIV-positive patients attending a referral center.” The study showed that “clinical malaria” (symptoms and parasitemia) was significantly associated with the non-use of ITN (aOR: 1.97, 95% CI: 1.17 to 2.85, P = 0.017).33
Costing and Cost-effectiveness
CTX for Malaria Prevention
No relevant studies meeting the selection criteria for this review were found.
Provision of Insecticide-Treated Nets
Kern et al,38 in a study rated “level 1,” reported the “effectiveness, cost, and cost-effectiveness” of providing “LLINs and point-of-use water filters” to ART-naive HIV-infected adults and their family members. Data for this study were abstracted from an OS that enrolled 589 HIV-infected adults, conducted in Kenya, and which assessed the association between “LLITN use and water filters” and the progression of HIV-1 disease, as measured by “CD4 cell count decline” and/or “time to death.” Total costs averted were estimated at $34,975 [ART-related costs (because of delay in need for ART), costs averted by LLINs (malaria) and “costs averted” by water filters (diarrhea) were estimated at $24,395, $747, and $9,834, respectively]. A net cost savings of about US $26,000 was found for the intervention over 1.7 years. The authors concluded that the combined intervention cost of US $3100 per death averted or US $99 per disability-adjusted life year (DALY) averted, and that the estimated cost-effectiveness ratios were in the range of cost-effectiveness ratios for other prevention interventions for the 3 addressed diseases (HIV/AIDS, malaria, and diarrhea). It is noteworthy that the combined prevention interventions including LLINs averted substantial costs in terms of new ART initiations during the intervention period. For instance, the costs averted from malaria per se are small but the value of CTX and LLINs in terms of delaying ART costs is potentially important.38
Kahn et al36 (2011), in a study rated “level 2,” provided cost information for bednet provision as part of an “Integrated Prevention Campaign,” which consisted of the provision of nets, filters, HIV testing, and condoms. The study helped determine the necessary resources needed for implementation of the campaign. Findings showed a “projected unit cost” per person served, by disease, estimated at $6.27 for malaria (nets and training), $15.80 for diarrhea (filters and training), and $9.91 for HIV (test kits, counseling, condoms, and CD4 testing at each site).36
Kahn et al37 (2012), a level 1–rated study, addressed the CE of the aforementioned “Integrated Prevention Campaign” using a “spreadsheet-based model.” Existing data regarding the impact of the each of the 3 assessed interventions (VCT, LLINs, and water filters) on morbidity and mortality, along with the available costing information from the same campaign (in the study of Kahn et al. 2011), were used to determine estimates of averted deaths, DALYs, and cost savings. The model-based analysis included added costs associated with potential earlier ART initiation as well. Per 1000 campaign beneficiaries, the provision of LLINs allowed for the aversion of 4.31 deaths, 1304 malaria episodes, 125 DALYS, and $10,420 of costs. Findings were robust to the sensitivity analyses performed. Kahn et al37 concluded a more “favorable outcome” in their study, compared with the CE of existing published findings regarding malaria prevention–related interventions (a cost ranging from $2 to 15 per DALY averted44).
Quality of the Evidence and the Expected Impact on Mortality, Morbidity, and Retention in Care
CTX Prophylaxis
Of the 17 studies that addressed morbidity (including the 2 studies that addressed both CTX prophylaxis and ITNs use32,33), the quality of the evidence was rated as “strong” for 6 studies,17,19,20,24,25,28 “medium” for 10 studies,21–23,26,27,29,30,32–34 and “weak” for 1 study (Table 1; see Table S1, Supplemental Digital Content, http://links.lww.com/QAI/A656).18 The overall quality of the evidence in these studies was rated as “good.” The expected impact of CTX prophylaxis on malaria-related morbidity reduction was rated as “high” (Table 1).
TABLE 1.
Summary of the Body of the Evidence From All Studies by Outcome
| Overall Quality of Evidence |
Evidence From Economic Evaluation |
|||||
|---|---|---|---|---|---|---|
| Studies | Overall Quality of the Body of Evidence | Expected Impact of the Intervention* | Studies, Number of Studies With Costing/CE | Quality of Evidence From Economic Evaluation | Comments | |
| Morbidity | 22 (including 3 economic studies) | Good (CTX prophylaxis for malaria prevention) | High (CTX prophylaxis for malaria prevention) | No economic studies for CTX prophylaxis | CTX for malaria prevention: no relevant study was found | Of the 22 studies, 5 were RCTs,17,19,20,24,28 13 were OS,18,21–23,26–30,32–35 1 was a SR’ and 3 studies addressed exclusively costing and CE36–38 |
| 17 studies addressed the impact of CTX prophylaxis on the malaria-related morbidity outcome17–33 | Of the 5 RCTs, 4 enrolled participants on ART and 1 enrolled participants not on ART17 | |||||
| Of the 22 studies, only 2 assessed both CTX prophylaxis and the ITN intervention32,33 | Of the 13 OS, 8 enrolled ART patients21,23,26,29,30,32–34 and 4 enrolled patients not on ART.22,26,31,35 Information on ART status was unavailable for 1 OS18 | |||||
| Fair (ITNs/LLINs use/provision) | Uncertain (ITNs use) | 3 for ITNs provision/use | ITNs use intervention: Two LEVEL 1 studies reported: Robust findings in terms of effectiveness, costs and CE of providing LLINs and point-of-use water filters to ART-naive HIV-infected adults and their family members, in the context of a multi-site study in Kenya.37 | |||
| Very limited data on evidence that demonstrates the impact of ITNs on malaria morbidity reduction in the specific population of HIV-infected individuals32–35 | CE of LLINs part of an integrated prevention campaign including targeting malaria, HIV and diarrhea.38 | |||||
| One LEVEL 2 study provided a cost analysis information of bednet provision as part of a combination of three integrated interventions through a punctual campaign.36 | ||||||
Assessment of the expected impact of the intervention was based on published evidence. Additional considerations that would inform implementation decisions would have to take into account the CE information and country-specific contextual considerations.
The expected impact of the intervention was rated as high = intervention expected to have a high impact on the outcome; moderate = likely to have a moderate impact on the outcome; low = intervention expected to have a low impact on the outcome; and uncertain = available information is not adequate to assess estimated impact on the outcome.
ITNs/LLINs Provision and Use
Data were very limited regarding the impact of ITNs/LLINs provision and/or use on malaria morbidity reduction in HIV-infected persons. Four studies addressed morbidity32–35 (excluding the 3 health economics studies36–38 and including the 2 studies that addressed both ITNs use and CTX prophylaxis).32,33 The quality of each of the 4 studies was rated as “medium.” The overall quality of evidence was rated as “fair” and the impact of ITNs/LLINs was rated as “uncertain” (Table 1). The scarcity of the evidence did not allow for a more robust assessment of the potential impact of LLINs provision and/or use on malaria prevention.
DISCUSSION
There is clear evidence that rates of malaria (particularly P. falciparum malaria) infection and illness in people with HIV are reduced by CTX prophylaxis. The overall impact on morbidity and mortality in HIV-infected persons is more likely to drive use of CTX than the specific issue of malaria prevention—these considerations are summarized in the accompanying CTX review in this supplement (Assessment of the Impact of Cotrimoxazole Prophylaxis on Key outcomes Among HIV-Infected Adults in Low- and Middle-Income Countries: A Systematic Review).45 However, there are important malaria-specific questions related to CTX prophylaxis that should be considered. These include the question of whether additional or alternate interventions should be considered for pregnant women to prevent clinical and placental malaria and whether continuation of CTX prophylaxis remains a priority in malaria-endemic areas when it is no longer a priority for the purposes of general prophylaxis (eg, following immune reconstitution as a result of ART) or when universal access to and use of LLINs have been achieved.
Our review did not specifically focus on the issue of prevention of placental malaria. However, based on the currently available evidence, CTX prophylaxis is associated with reductions in placental malaria among pregnant women with HIV and does not seem to be inferior to other available interventions.
An important question for programs is whether the impact of CTX on malaria rates should influence decisions about discontinuation of CTX prophylaxis. Although the literature does not demonstrate the long-term impact of CTX on disease rates in persons on ART, continuation of CTX needs to be considered. In malaria-endemic areas, CTX discontinuation is associated with a marked increase in malaria rates.
Although very limited, existing evidence suggests that CTX prophylaxis does not result in a population-level increase in resistance to medications commonly used for malaria prevention or treatment, particularly IPTp-SP.46–49 Furthermore, concerns about the potential of widespread use of CTX prophylaxis implementation to increase the risk of antifolate resistant malaria may be less relevant because of the significant progress achieved in recent years regarding access to the artemisinin-based combination therapies, especially those that are antifolate-free.3 Also, IPTp-SP seems to remain effective in preventing the adverse consequences of malaria on maternal and fetal outcomes in P. falciparum–endemic areas.8
Limitations and Research Gaps
As described in the accompanying review of CTX prophylaxis (Assessment of the Impact of Cotrimoxazole Prophylaxis on Key Outcomes Among HIV-Infected Adults in Low- and Middle-Income Countries: A Systematic Review), available evaluations of malaria prevention in HIV are heterogeneous in terms of design and, particularly with respect to background rates of malaria, the definitions for end-points and durations of follow-up (Table 2). In the reviewed studies, malaria outcomes were defined variably (clinically, based on microscopy, rapid tests, and PCR), and assessment of the potential additional impact of LLINs to that of CTX prophylaxis was generally limited because of the non-availability of data related to quantified use of ITNs/LLINs. For instance, only 6 of the 17 individual studies that assessed CTX prophylaxis impact on malaria have reported data on precise use of LLINs.22–24,28,33,34 However, 3 of the 4 studies that addressed impact of LLINs on malaria morbidity quantified the use of nets (Table 2).33–35
TABLE 2.
F.U. Durations, Morbidity Definitions, Enrollment in Study Criteria, and ITNs Use
| F.U. Duration | Morbidity (Including Malaria) Definition When Applicable | Studies’ Enrollment Criteria | ITNs Use | |
|---|---|---|---|---|
| Anglaret et al17 | Mean = 0.9 yrs | Morbidity “potentially preventable by CTX,” including: infections with bacteria, toxoplasma, isospora, nocardia, Pneumocystis carinii, and malaria | Aged 18 yrs and older with HIV-1 or HIV-1 and HIV-2 dual seropositivity at stages 2 or 3 of the WHO staging system | Not reported |
| Bulabula et al18 | Cross-sectional study (duration of recruitment: 0.3 yrs) | Malaria prevalence (parasitemia): Positive smear for Plasmodia | HIV-infected and non-infected individuals | Not reported |
| Campbell et al19 | F.U. duration: 0.3 yrs | “Smear-positive episode of fever” and all cases were treated. Densities of >1250 parasites/μL (implying high likelihood that malaria was the cause of the “febrile illness,” “not incidental parasitemia” | Individuals aged 18 or older, with CD4 cell count below 250 cells/μL or WHO stage III or IV | Yes: ITNs provided free of charge to all participant women but no data on use |
| Denoeud-Ndam et al20 | F.U. duration: from 16- to 28-wk gestation until birth | Thick and thin blood smears stained with Giemsa and read by 2 independent microscopists, with conciliation of discrepancies by a third reader | HIV-infected pregnant women aged 18 or older and living permanently in the study area were enrolled between 16 and 28 wks of gestation | Yes: provided to all enrolled women but no data on use |
| Slide negative after 200 high-power fields were read | ||||
| Positive slides: parasite density was determined per 500 leukocytes (assumption of an average leukocyte count of 8000 cells/μL) | ||||
| Dow et al21 | F.U. duration: 0.54 yrs since study second antenatal visit, to assess probability of malaria-free survival | “First episode after the second prenatal visit” based on a positive blood smear with malaria symptoms | HIV-infected pregnant women, ART-naive, at least 14 yrs of age and less than 30 wk of gestation eligible for enrollment if hemoglobin levels >7 g/dL, CD4 cell count >250 cells/μL(>200 cells/μL before July 24, 2006) | Yes: no data on number provided and used |
| Hamel et al22 | Median = 0.46 yrs; mean = 0.38 yrs | “Clinical malaria”: Plasmodium falciparum infection with measured or reported fever or P. falciparum infection with parasite density ≥400 parasites/μL regardless of fever | Subjects aged 15 or older, who agreed to HIV testing, not severely ill nor in the first trimester of pregnancy, and who were not taking daily antibiotics for the treatment of a chronic illness (excluding tuberculosis) | Yes: data on use available |
| Kapito-Tembo et al23 | Cross-sectional | Presence of malaria parasites on microscopy | HIV-infected pregnant women aged 15 or older and with gestation over 34 wk attending the hospital's ANC clinic. Women with immediate life-threatening medical and obstetric conditions excluded | Yes: data on use available |
| “PCR-detected malaria infection” was defined as positive result of PCR for malaria regardless of microscopy results | ||||
| Klement et al24 | F.U. duration: From 14- to 28-wk gestation until birth | “Positive biological test,” a body temperature ≥37.5°C, and at “least 1 clinical sign”: asthenia, headache, myalgia, or abdominal pain | HIV type 1–infected pregnant women (≤28 wk of gestation, CD4 count >200 cells/μL, hemoglobin level 7 g/dL or greater) | Yes: all enrolled women received ITNs and data on use available |
| Manyando et al25 | SR | SR (see individual studies elsewhere in this table) | SR (see individual studies elsewhere in this table) | SR (see individual studies elsewhere in this table) |
| Mermin et al26 (2004) | Median FU time for individuals with HIV infection, before CTX: 0.42 yrs (IQR 0.4–0.44), and during CTX: 1.46 yrs (1.29–1.47) | Fever and a thick smear consistent with the presence of plasmodia | HIV-1–infected individuals and their HIV-negative household members | Not reported |
| Newman et al27 | Cross-sectional study (recruitment period: February 2008 and February 2009) | Positive placental blood smear: parasite density ≥1 parasite/μL | HIV-infected and infected pregnant women | Not reported |
| Polyak et al28 | F.U. duration: 1 yrs | RDT or smear positive with fever | HIV-infected adults who had been on ART for >18 mo and had CD4 count >350/μL | Yes: data on use available |
| Saracino et al29 | Cross-sectional study (from November to December 2010) | MBS or RDTs | All adult patients (>15 yrs, according to the hospital policy) consecutively hospitalized | Not reported |
| MBS: parasitemia was assessed using a semi-quantitative method | ||||
| Walker et al30 | Median: 4.9 yrs | New WHO stage 4 events, new or recurrent WHO stage 3 or 4 events, and malaria (“clinical” or “microscopic” diagnosis) | From the “DART RCT of management strategies” in HIV-infected symptomatic (WHO stage 2–4) adults aged 18 or more, starting ART with CD4 counts <200 cells per microliter, who reported no previous ART apart from to prevent mother-to-child transmission | Not reported |
| Watera et al31 | Person-yrs of F.U.: 1463 (before and after CTX prophylaxis introduction) | Participants who visited the clinic with a fever (temperature, “greater or equal to 37.5°C”) were examined by a physician who made a diagnosis (primary outcomes) based on “reported symptoms, observed signs, and the results of laboratory tests” | HIV-seropositive adults (aged older than 15 yrs) | Not reported |
| Mermin et al32 (2006) | Median F.U. before CTX was 0.42 yrs | Reported fever and “parasites seen on thick smear” | HIV-infected individuals aged 18 yrs or older | Yes: data on provision available. Not reported on use |
| During CTX 1.45 yrs, during CTX and ART: 0.34 yr | “Parasite density” calculated with WBC count of 8000/μL | |||
| During CTX, ART, and nets: 1.53 yrs | ||||
| Iliyasu et al33 | Cross-sectional study design (1-mo recruitment period) | Episode of fever confirmed as malaria when on blood film examination | HIV/AIDS patients attending the HIV clinic over a 1-mo period (June 1–30, 2012). All adults (≥18 yrs) with HIV infection with at least 1 clinic visit were included in the study (treatment-experienced or naive) | Yes: half of all respondents (53.5%) admitted to using ITN |
| Olowookere et al34 | Cross-sectional study (recruitment period from June to December 2010) | Positive blood film | New PLWHIV (irrespective of age) | Yes: data on use available |
| Walson et al35 | Mean F.U. time: control cohort: 1.85 yrs; intervention cohort: 1.67 yrs | Malaria: fever (>38.8°C) and positive RDT and/or “malaria thin and thick smear” | HIV-1–infected adults ineligible for ART initiation | Data on provision and use available |
| Diarrhea: “3 or more episodes of watery stools in a day” | Proportions of those who both have a net and sleep under it: “intervention group”: 97.3% | |||
| “Control group”: 82.4% (<0.001) | ||||
| Kahn et al36 (2011) Costing | Costing study | “Projected unit cost” per person served, by disease, estimated at $6.27 for malaria (nets and training) | CE study | Yes: data available for provision only. Not on use |
| Kahn et al37 (2012) CE | CE study | Per 1000 campaign beneficiaries, provision of LLINs led to the aversion of 4.31 deaths, 1304 malaria episodes, 125 DALYS, and $10.420 of costs | CE study | Yes: data available for provision only. Not on use |
| Kern et al38 CE | Costing study | “Net cost savings” of about US$ 26,000 for the intervention, over 1.7 yrs CE study | Costing study | Yes: data available from Walson et al 2013 |
DART, development of antiretroviral therapy; F.U., follow-up; MBS, malaria blood smear; RDT, rapid diagnostic test.
Moreover, it is difficult to evaluate the effectiveness of specific interventions that are provided in combination with other interventions (eg, vector control interventions including the use of ITNs and LLINs in combination with CTX prophylaxis and water filters). Evaluation of interventions that are provided in combination sometimes necessitates the use of creative study designs, such as the evaluation of sequential introduction of program elements.
The existing evidence showed non-inferiority of CTX prophylaxis compared with IPTp-SP (very likely 2 doses) for malaria prevention in HIV-infected pregnant women, especially in those with higher CD4 cell counts (≥350 cells/μL). However, more research is needed to better inform malaria prophylaxis policies for HIV-infected pregnant women by addressing the comparison of CTX prophylaxis to the effective provision of the “3-or-more-doses of IPTp-SP,” as recommended by the WHO for HIV-negative pregnant women in “moderate- and high-malaria transmission areas.”8 Studies should ascertain uptake of the “3-or-more-doses of IPTp-SP” and allow for longer follow-up durations and more focus on malaria incidence and birth outcomes as measured by the primary end-point. Finally, more operational research and program evaluation quality data are needed to guide the implementation of both CTX prophylaxis and IPTp-SP in a well-coordinated manner.
Programmatic Considerations
In settings where HIV and malaria diseases burden is high, HIV and malaria control programs (including major donors and stakeholders) have the potential and the opportunity to greatly benefit from further leveraging their own resources and maximizing the impact of their investments. Effective partnerships and close collaboration between the 2 programs and with other programs, including maternal and child health programs, should be encouraged. Distribution of free LLINs using prenatal clinics as a platform has, for instance, the potential for better coverage in the most vulnerable populations, namely pregnant women and children, irrespective of their HIV status. Such an approach has the potential of benefiting both the general and the HIV-infected populations, mitigating the negative impact of recurrent administrative and programmatic duplications, allowing for a better coordinated and harmonized design and implementation of malaria prevention policies, and contributing to more efficient commodities supply mechanisms (eg, procurement of LLINs) and use of human resources.
CONCLUSIONS
Our review suggests a potentially high impact of CTX prophylaxis for malaria prevention in adult PLHIV, including pregnant women. The evidence similarly shows a potential higher risk of malaria when CTX is discontinued in areas with high malaria prevalence. Because of very limited evidence, the expected impact of ITNs distribution and use on malaria prevention in PLHIV seems uncertain.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Gail Bang and Emily Weyant (Division of Epidemiology, Analysis and Library Services, Centers for Disease Control and Prevention, Atlanta, GA) for assisting with the literature searches. The authors also thank Michel Tchuenche (Division of Global HIV/AIDS, Centers for Disease Control and Prevention, Atlanta, GA) for his input in the review process of cost-effectiveness studies, and Peter McElroy (Malaria Branch, Division of Parasitic Diseases and Malaria, Centers for Disease Control and Prevention, Atlanta, GA) for his valuable comments from the malaria technical area's perspective.
Supported by the US President's Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention.
Footnotes
The authors have no conflicts of interest to disclose.
The findings and conclusions in this article are those of the authors and should not be construed to represent the positions of the US Department of State's Office of the US Global AIDS Coordinator and Health Diplomacy, the US Centers for Disease Control and Prevention, or the US Federal Government.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
REFERENCES
- 1.Van Geertruyden JP. Interactions between malaria and human immunodeficiency virus anno 2014. Clin Microbiol Infect. 2014;20:278–285. doi: 10.1111/1469-0691.12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UNAIDS The Gap Report. [October 6, 2014];Joint United Nations Programme on HIV/AIDS (UNAIDS) The Gap Report 2014. 2014 Available at: http://www.unaids.org/sites/default/files/en/media/unaids/contentassets/documents/unaidspublication/2014/UNAIDS_Gap_report_en.pdf.
- 3.World Health Organization [October 6, 2014];World Malaria Report 2013. 2013. Available at: http://www.whoint/malaria/publications/world_malaria_report_ 2013/report/en/.
- 4.World Health Organization [October 15, 2014];Guidelines for the Treatment of Malaria Second Edition March 2010. Available at: http://whqlibdoc.who.intx/publications/2010/9789241547925_eng.pdf?ua=1.
- 5.Aoussi EF, Tanon KA, Ehui E, et al. Paludisme et infection à VIH en Afrique subsaharienne: encore un couple maudit? [in French]. Sante. 2011;21:174–177. doi: 10.1684/san.2011.0255. [DOI] [PubMed] [Google Scholar]
- 6.Slutsker L, Marston BJ. HIV and malaria: interactions and implications. Curr Opin Infect Dis. 2007;20:3–10. doi: 10.1097/QCO.0b013e328012c5cd. [DOI] [PubMed] [Google Scholar]
- 7.Steketee RW, Wirima JJ, Bloland PB, et al. Impairment of a pregnant woman's acquired ability to limit Plasmodium falciparum by infection with human immunodeficiency virus type-1. Am J Trop Med Hyg. 1996;55:42–49. doi: 10.4269/ajtmh.1996.55.42. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization [October 25, 2013];WHO Policy Brief for the Implementation of Intermittent Preventive Treatment of Malaria in Pregnancy Using Sulfadoxine-pyrimethamine. 2013 Available at: http://wwwwhoint/entity/malaria/publications/atoz/Policy_brief_(IPTp-SP)_implementation_11april2013.pdf.pdf.
- 9.World Health Organization [August 14, 2013];Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. 2013 Available at: http://www.who.int/hiv/pub/guidelines/arv2013/. [PubMed]
- 10.World Health Organization [December 12, 2014];Cotrimoxazole Prophylaxis for Malaria and Bacterial Infections in People with HIV. 2014 Available at: http://www.who.int/hiv/topics/arv/CTX_FS_FINAL-261114.pdf?ua=1.
- 11.World Health Organization [August 15, 2014];Guidelines on Co-trimoxazole Prophylaxis for HIV-related Infections Among Children, Adolescents and Adults. 2006 Available at: http://www.who.int/entity/hiv/pub/guidelines/ctxguidelines.pdf.
- 12.Centers for Disease Control and Prevention [September 4, 2014];Insecticide-Treated Bed Nets. 2014 Available at: http://www.cdc.gov/malaria/malaria_worldwide/reduction/itn.html.
- 13.Centers for Disease Control and Prevention [September 4, 2014];CDC Global Health Strategy 2012-2015. 2012 Available at: http://www.cdc.gov/globalhealth/strategy/pdf/CDC-GlobalHealthStrategy.pdf.
- 14.President Malaria Initiative [October 10, 2014];President's Malaria Initiative Technical Guidance. 2014 Available at: http://www.pmi.gov/docs/default-source/default-document-library/tools-curricula/fy2015_pmi_technical_guidance_april2014.pdf?sfvrsn=4.
- 15.World Health Organization [October 10, 2014];WHO Recommendations for Achieving Universal Coverage with Long-lasting Insecticidal Nets in Malaria Control. 2013 Available at: http://www.who.int/malaria/publications/atoz/who_recommendations_universal_coverage_llins.pdf.
- 16.Kaplan JE, Hamm TE, Forhan S, et al. The impact of HIV care and support interventions on key outcomes in low and middle-income countries: a literature review–Introduction. J Acquir Immune Defic Syndr. 2015;68(suppl 3):S253–S256. doi: 10.1097/QAI.0000000000000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anglaret X, Chene G, Attia A, et al. Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Cote d'Ivoire: a randomised trial. Cotrimo-CI Study Group. Lancet. 1999;353:1463–1468. doi: 10.1016/s0140-6736(98)07399-1. [DOI] [PubMed] [Google Scholar]
- 18.Bulabula AM, Mayanga Mafwila, et al. Malaria prevalence in HIV patients under cotrimoxazole. Case of Kindu, Maniema, D.R. Congo. Preliminary Results. Ital J Trop Med. 2009;14:1–2. [Google Scholar]
- 19.Campbell JD, Moore D, Degerman R, et al. HIV-infected ugandan adults taking antiretroviral therapy with CD4 counts .200 cells/muL who discontinue cotrimoxazole prophylaxis have increased risk of malaria and diarrhea. Clin Infect Dis. 2012;54:1204–1211. doi: 10.1093/cid/cis013. [DOI] [PubMed] [Google Scholar]
- 20.Denoeud-Ndam L, Zannou DM, Fourcade C, et al. Cotrimoxazole prophylaxis versus mefloquine intermittent preventive treatment to prevent malaria in HIV-infected pregnant women: two randomized controlled trials. J Acquir Immune Defic Syndr. 2014;65:198–206. doi: 10.1097/QAI.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 21.Dow A, Kayira D, Hudgens MG, et al. The effect of cotrimoxazole prophylactic treatment on malaria, birth outcomes, and postpartum CD4 count in HIV-infected women. Infect Dis Obstet Gynecol. 2013;2013:340702. doi: 10.1155/2013/340702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamel MJ, Greene C, Chiller T, et al. Does cotrimoxazole prophylaxis for the prevention of HIV-associated opportunistic infections select for resistant pathogens in Kenyan adults? Am J Trop Med Hyg. 2008;79:320–330. [PubMed] [Google Scholar]
- 23.Kapito-Tembo A, Meshnick SR, van Hensbroek MB, et al. Marked reduction in prevalence of malaria parasitemia and anemia in HIV-infected pregnant women taking cotrimoxazole with or without sulfadoxine-pyrimethamine intermittent preventive therapy during pregnancy in Malawi. J Infect Dis. 2011;203:464–472. doi: 10.1093/infdis/jiq072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klement E, Pitche P, Kendjo E, et al. Effectiveness of co-trimoxazole to prevent Plasmodium falciparum malaria in HIV-positive pregnant women in sub-Saharan Africa: an open-label, randomized controlled trial. Clin Infect Dis. 2014;58:651–659. doi: 10.1093/cid/cit806. [DOI] [PubMed] [Google Scholar]
- 25.Manyando C, Njunju EM, D'Alessandro U, et al. Safety and efficacy of co-trimoxazole for treatment and prevention of Plasmodium falciparum malaria: a systematic review. PLoS One. 2013;8:e56916. doi: 10.1371/journal.pone.0056916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mermin J, Lule J, Ekwaru JP, et al. Effect of co-trimoxazole prophylaxis on morbidity, mortality, CD4-cell count, and viral load in HIV infection in rural Uganda. Lancet. 2004;364:1428–1434. doi: 10.1016/S0140-6736(04)17225-5. [DOI] [PubMed] [Google Scholar]
- 27.Newman PM, Wanzira H, Tumwine G, et al. Placental malaria among HIV-infected and uninfected women receiving anti-folates in a high transmission area of Uganda. Malar J. 2009;8:254. doi: 10.1186/1475-2875-8-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polyak C, Yuhas K, Singa B, et al. CTX Prophylaxis discontinuation among ART-treated adults: a randomised non-Inferiority trial [Oral abstract 98].. Paper presented at: XXI Conference on Retroviruses and Opportunistic Infections; Boston. March 5, 2014. [Google Scholar]
- 29.Saracino A, Nacarapa EA, Da Costa Massinga EA, et al. Prevalence and clinical features of HIV and malaria co-infection in hospitalized adults in Beira, Mozambique. Malar J. 2012;11:241. doi: 10.1186/1475-2875-11-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker AS, Ford D, Gilks CF, et al. Daily co-trimoxazole prophylaxis in severely immunosuppressed HIV-infected adults in Africa started on combination antiretroviral therapy: an observational analysis of the DART cohort. Lancet. 2010;375:1278–1286. doi: 10.1016/S0140-6736(10)60057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watera C, Todd J, Muwonge R, et al. Feasibility and effectiveness of cotrimoxazole prophylaxis for HIV-1-infected adults attending an HIV/AIDS clinic in Uganda. J Acquir Immune Defic Syndr. 2006;42:373–378. doi: 10.1097/01.qai.0000221679.14445.1b. [DOI] [PubMed] [Google Scholar]
- 32.Mermin J, Ekwaru JP, Liechty CA, et al. Effect of co-trimoxazole prophylaxis, antiretroviral therapy, and insecticide-treated bednets on the frequency of malaria in HIV-1-infected adults in Uganda: a prospective cohort study. Lancet. 2006;367:1256–1261. doi: 10.1016/S0140-6736(06)68541-3. [DOI] [PubMed] [Google Scholar]
- 33.Iliyasu Z, Babashani M, Abubakar IS, et al. Clinical burden and correlates of HIV and malaria co-infection, in northwest Nigeria. Acta Trop. 2013;128:630–635. doi: 10.1016/j.actatropica.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 34.Olowookere SA, Adeleke NA, Abioye-Kuteyi EA, et al. Use of insecticide treated net and malaria preventive education: effect on malaria parasitemia among people living with AIDS in Nigeria, a cross-sectional study. Asia Pac Fam Med. 2013;12:2. doi: 10.1186/1447-056X-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walson JL, Sangare LR, Singa BO, et al. Evaluation of impact of long-lasting insecticide-treated bed nets and point-of-use water filters on HIV-1 disease progression in Kenya. AIDS. 2013;27:1493–1501. doi: 10.1097/QAD.0b013e32835ecba9. [DOI] [PubMed] [Google Scholar]
- 36.Kahn JG, Harris B, Mermin JH, et al. Cost of community integrated prevention campaign for malaria, HIV, and diarrhea in rural Kenya. BMC Health Serv Res. 2011;11:346. doi: 10.1186/1472-6963-11-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kahn JG, Muraguri N, Harris B, et al. Integrated HIV testing, malaria, and diarrhea prevention campaign in Kenya: modeled health impact and cost-effectiveness. PLoS One. 2012;7:e31316. doi: 10.1371/journal.pone.0031316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kern E, Verguet S, Yuhas K, et al. Provision of bednets and water filters to delay HIV-1 progression: cost-effectiveness analysis of a Kenyan multisite study. Trop Med Int Health. 2013;18:916–924. doi: 10.1111/tmi.12127. [DOI] [PubMed] [Google Scholar]
- 39.Greenwood BM, David PH, Otoo-Forbes LN, et al. Mortality and morbidity from malaria after stopping malaria chemoprophylaxis. Trans R Soc Trop Med Hyg. 1995;89:629–633. doi: 10.1016/0035-9203(95)90419-0. [DOI] [PubMed] [Google Scholar]
- 40.Menendez C, D'Alessandro U, Ter Kuile FO. Reducing the burden of malaria in pregnancy by preventive strategies. Lancet Infect Dis. 2007;7:126–135. doi: 10.1016/S1473-3099(07)70024-5. [DOI] [PubMed] [Google Scholar]
- 41.Mockenhaupt FP, Reither K, Zanger P, et al. Intermittent preventive treatment in infants as a means of malaria control: a randomized, double-blind, placebo-controlled trial in northern Ghana. Antimicrob Agents Chemother. 2007;51:3273–3281. doi: 10.1128/AAC.00513-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beck HP, Felger I, Vounatsou P, et al. Effect of iron supplementation and malaria prophylaxis in infants on Plasmodium falciparum genotypes and multiplicity of infection. Trans R Soc Trop Med Hyg. 1999;93(suppl 1):41–45. doi: 10.1016/s0035-9203(99)90326-7. [DOI] [PubMed] [Google Scholar]
- 43.Bereczky S, Liljander A, Rooth I, et al. Multiclonal asymptomatic Plasmodium falciparum infections predict a reduced risk of malaria disease in a Tanzanian population. Microbes Infect. 2007;9:103–110. doi: 10.1016/j.micinf.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 44.Laxminarayan R, Mills AJ, Breman JG, et al. Advancement of global health: key messages from the disease control priorities project. Lancet. 2006;367:1193–1208. doi: 10.1016/S0140-6736(06)68440-7. [DOI] [PubMed] [Google Scholar]
- 45.Saadani Hassani A, Marston BJ, Kaplan JE. Assessment of the impact of co-trimoxazole prophylaxis on key outcomes among HIV-infected adults in low- and middle-income countries: a systematic review. J Acquir Immune Defic Syndr. 2015;68(suppl 3):S257–S269. doi: 10.1097/QAI.0000000000000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ter Kuile FO, van Eijk AM, Filler SJ. Effect of sulfadoxinepyrimethamine resistance on the efficacy of intermittent preventive therapy for malaria control during pregnancy: a systematic review. JAMA. 2007;297:2603–2616. doi: 10.1001/jama.297.23.2603. [DOI] [PubMed] [Google Scholar]
- 47.Malamba SS, Mermin J, Reingold A, et al. Effect of cotrimoxazole prophylaxis taken by human immunodeficiency virus (HIV)-infected persons on the selection of sulfadoxine-pyrimethamine-resistant malaria parasites among HIV-uninfected household members. Am J Trop Med Hyg. 2006;75:375–380. [PubMed] [Google Scholar]
- 48.Thera MA, Sehdev PS, Coulibaly D, et al. Impact of trimethoprimsulfamethoxazole prophylaxis on falciparum malaria infection and disease. J Infect Dis. 2005;192:1823–1829. doi: 10.1086/498249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laufer MK, Plowe CV. Cotrimoxazole prophylaxis and malaria in Africa: have the important questions been answered? Am J Trop Med Hyg. 2006;75:373–374. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.