Abstract
Itch, the unpleasant sensation that elicits a desire to scratch, is mediated by specific subtypes of cutaneous sensory neuron. Here, we identify a subpopulation of itch‐sensing neurons based on their expression of the receptor tyrosine kinase Ret. We apply flow cytometry to isolate Ret‐positive neurons from dorsal root ganglia and detected a distinct population marked by low levels of Ret and absence of isolectin B4 binding. We determine the transcriptional profile of these neurons and demonstrate that they express neuropeptides such as somatostatin (Sst), the NGF receptor TrkA, and multiple transcripts associated with itch. We validate the selective expression of Sst using an Sst‐Cre driver line and ablated these neurons by generating mice in which the diphtheria toxin receptor is conditionally expressed from the sensory neuron‐specific Avil locus. Sst‐Cre::AviliDTR mice display normal nociceptive responses to thermal and mechanical stimuli. However, scratching behavior evoked by interleukin‐31 (IL‐31) or agonist at the 5HT1F receptor is significantly reduced. Our data provide a molecular signature for a subpopulation of neurons activated by multiple pruritogens.
Keywords: DRG, sensory neurons, IL‐31, 5HT1F
Subject Categories: Neuroscience
Introduction
The perception of physical and chemical stimuli through the skin is initiated by peripheral sensory neurons that have their cell body in the DRG. The complexity of somatosensation is reflected by the fact that a myriad of sensations including touch, pain, itch, and temperature are recognized by the peripheral nervous system. It has long been debated whether this functional complexity arises from activation of specific subtypes of sensory neuron for each stimulus modality, or from encoding and summation of neuronal activity generated by neurons that can detect a broad range of stimuli 1. Recently, it has been proposed that the sensation of itch is a discrete sensory modality that utilizes a dedicated neuronal pathway tuned to perceive only this sensation 2, 3, 4.
Itch, the sensation that elicits a desire to scratch, serves a protective function against potentially harmful environmental irritants 5. Although unpleasant, itch is inherently different from pain, both in its sensory quality and its behavioral outcome (scratching compared to withdrawal). The neuronal pathways that mediate itch versus pain also appear to be distinct. In the spinal cord, ablation of neurons expressing receptors for the neuropeptides, gastrin‐releasing peptide (Grp) 4 or natriuretic polypeptide b (Nppb) 3, reduces itch responses to multiple pruritogens but does not affect nociceptive behavior. Similarly, subpopulations of C‐fiber primary afferents are activated by pruritogens 6, 7, 8 and ablation or selective activation of DRG neurons positive for the Mas‐related G protein‐coupled receptor A3 (MrgprA3) impacts upon scratching behavior but not pain 2.
Itch is further categorized by its dependence upon histaminergic or non‐histaminergic mechanisms. Histamine‐dependent itch is elicited through activation of the H1 receptor (HRH1) and signaling through phospholipase‐β3 (PLCβ3) and the ion channel TRPV1 9, 10. The existence of further histamine‐independent pathways is supported by observations that many chronic pruritic syndromes such as atopic dermatitis are resistant to antihistamine therapy 11. Mechanistically, histamine‐independent itch is likely to be mediated by activation of the ion channel TRPA1. For example, injection of the antimalarial agent chloroquine induces itch 12 via MrgprA3 receptors 13 functionally coupled to TRPA1 14, and TRPA1 is also required for itch produced by oxidative stress and leukocyte accumulation 15, 16. Other antihistamine‐resistant itch responses include those elicited by cytokines such as interleukin‐31 (IL‐31) released from T cells during allergic itch 17, 18. IL‐31 induces severe pruritus and may be a key mediator in atopic dermatitis 19.
Further information of the molecular profile of itch‐sensing neurons and identification of molecular markers for different subtypes of itch neuron would be valuable for understanding how different pruritogens activate itch pathways. Intriguingly, a recent study which took an unbiased approach to classify sensory neuron subtypes identified three populations of putative itch receptors 20. Each of these populations was enriched for transcripts associated with itch. However, from their gene expression profile, it was not clear whether they all signal only itch or can also contribute to other sensations such as pain.
To distinguish different populations of sensory neuron and ultimately define their function, we examined the expression pattern of the glial‐derived neurotrophic factor (GDNF) receptor Ret in mouse DRG. Almost every DRG neuron expresses at least one neurotrophic factor 21, and approximately 60 percent of cells are marked by Ret 22. The Ret tyrosine kinase is the signaling receptor for GDNF family ligands GDNF, neurturin, artemin, and persephin which bind via GPI‐anchored co‐receptors termed GFRα1–4 to initiate signaling through Ret 23. Two distinct waves of Ret expression arise during development with the first occurring prior to E11.5 and the second emerging subsequently 22, 24. Early Ret‐positive neurons develop into rapidly adapting mechanoreceptors and express the co‐receptor GFRα2 and high levels of Ret 25, 26. Late Ret‐positive neurons form a large heterogeneous group of non‐peptidergic nociceptors that can be distinguished by their binding of the plant lectin IB4 22, 24. A further population of Ret‐positive neuron co‐expresses the enzyme tyrosine hydroxylase (TH) 27, 28 and forms C‐fiber low threshold mechanoreceptors which have been implicated in the affective component of touch 29. Finally, a fourth population of Ret‐positive neurons that expresses the neurotrophin receptor TrkA in adult mice has also been described 22, 30. This population is rare, corresponding to around 10% of all Ret cells, and its role in vivo is unknown.
We sought to determine the function of this unique population of Ret‐positive sensory neuron. To this end, we took a genetic approach and generated mice where eGFP expression was driven from the Ret locus exclusively in peripheral sensory neurons 31, 32. We identified multiple subpopulations of Ret‐positive neurons in DRG which were quantified using flow cytometry. Microarray analysis of Ret‐expressing neurons that were negative for IB4 uncovered a sparse population of cells enriched in transcripts for TrkA, neuropeptides such as somatostatin (Sst), and pruritogen receptors. We validated the expression of Sst in this population using an Sst‐Cre driver line and generated a new mouse line to selectively ablate these neurons in vivo. Mice displayed normal nociceptive responses to thermal and mechanical stimuli. However, scratching behavior evoked by several classes of pruritogen was significantly reduced. Thus, Ret marks a population of itch receptors characterized by their co‐expression of Sst and multiple pruritogen receptors.
Results
Ret‐eGFP expression in primary sensory neurons
To examine Ret expression in the adult peripheral nervous system, ReteGFP/+ mice 31 were crossed with Avilcre/+ 32 mice to obtain heterozygote Avil‐Cre::ReteGFP/+ mice. This approach allowed us to target all DRG neurons and avoid extraneous GFP expression in surrounding tissues. Heterozygous mice were viable, exhibited no overt behavioral phenotype, and displayed robust eGFP fluorescence in peripheral sensory ganglia.
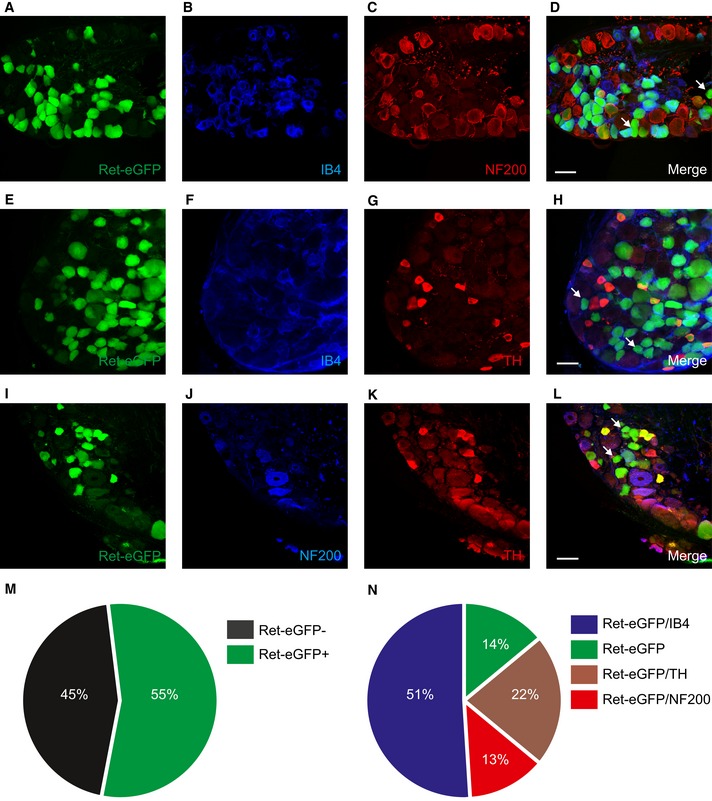
We investigated Ret‐eGFP distribution in DRG by co‐staining sections from Avil‐Cre::ReteGFP/+ mice with a selection of markers for different subtypes of sensory neuron. Ret‐eGFP was present in 55 percent of neurons (Fig 1M) and displayed a broad range of fluorescence intensities across different cells. We examined expression with IB4 and NF200, markers of non‐peptidergic nociceptors and myelinated neurons, respectively, and observed overlap with the majority of Ret‐eGFP‐positive neurons (Fig 1A–D), reflecting the early and late Ret neurons described previously 22, 24. We further investigated Ret‐eGFP co‐expression with TH, a marker of C‐fiber low threshold mechanoreceptors 27, 28. eGFP fluorescence was evident in many TH‐positive neurons and these cells were not co‐labeled with NF200 or IB4 (Fig 1E–L). Our analysis also indicated that a small proportion of Ret‐eGFP‐positive neurons were not marked by either IB4, NF200, or TH, suggesting the existence of a novel subtype of Ret‐expressing neuron. We quantified the overlap between Ret‐eGFP and each marker and determined that 14% of Ret‐eGFP‐positive neurons were negative for all markers (Fig 1N), supporting the idea that this population may reflect a functionally uncharacterized subset of primary afferent neuron.
Figure 1. Ret‐eGFP is expressed in multiple sensory neuron subsets.
-
A–LRet‐positive neurons largely overlap with markers for myelinated neurons (NF200), non‐peptidergic nociceptors (IB4), and C‐fiber low threshold mechanoreceptors (TH). However, some Ret‐positive neurons are negative for these markers (indicated by the arrows in D, H, and L) suggesting the existence of a further subset of Ret+ neurons. Scale bars, 50 μm.
-
MQuantification of the proportion of Ret‐eGFP‐positive neurons in DRG (n = 2,278 cells from three mice).
-
NQuantification of Ret‐eGFP co‐expression with other markers (n = 2,278 cells from three mice).
We utilized Avil‐Cre‐driven Ret‐eGFP expression to examine the peripheral and central projections of Ret‐positive sensory neurons. In the skin, Ret‐eGFP fluorescence was broadly distributed and present in free nerve endings terminating in the dermis and epidermis (Appendix Fig S1), and in lanceolate endings encircling hairs (Appendix Fig S1). Similarly in spinal cord sections, Ret‐eGFP was widely expressed across the dorsal horn. This was evident as a dense plexus of expression in lamina IIo corresponding to IB4‐positive non‐peptidergic nociceptors (Appendix Fig S2) and more diffusely through laminae III to V overlapping with NF200‐labeled mechanoreceptor inputs (Appendix Fig S2). Notably, we also detected Ret‐eGFP expression immediately ventral to IB4‐positive terminals that coincided with PKCγ, a marker for lamina IIi/III interneurons (Appendix Fig S2), and in lamina I co‐expressed with CGRP. Thus, RET‐eGFP expression distinguishes multiple populations of peripheral sensory neuron that are likely to be functionally distinct.
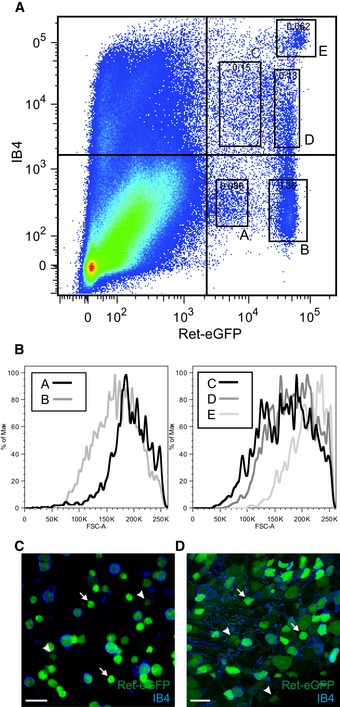
To obtain quantitative data on the distribution of Ret‐positive neuronal populations in DRG, we applied flow cytometric analysis to acutely dissociated neurons. We focused on levels of IB4 binding and native Ret‐eGFP fluorescence as this would allow for quantitative measurements in live cells. In line with histological data, we observed both IB4‐positive and IB4‐negative populations of Ret‐eGFP neuron (Figs 2A and EV1). Importantly, however, flow cytometric analysis revealed multiple well‐defined subpopulations delineated by their levels of eGFP fluorescence and IB4 binding (termed Ret‐eGFPLo:IB4Neg (A), Ret‐eGFPHi:IB4Neg (B), Ret‐eGFPLo:IB4Lo (C), Ret‐eGFPHi:IB4Lo (D), and Ret‐eGFPHi:IB4Hi (E)). There was a broad distribution in cell size across all populations as determined by forward scatter values, and no correlation between size and levels of IB4 binding or fluorescence (Fig 2B). We further validated flow cytometric analysis using fluorescent microscopy and observed that in both sensory neuron cultures (Fig 2C) and sections of DRG (Fig 2D) from Avil‐Cre::ReteGFP/+ mice, native eGFP fluorescence varied by an order of magnitude across IB4‐positive and IB4‐negative cells.
Figure 2. Multiple Ret‐positive subpopulations as determined by flow cytometric analysis of dissociated sensory neurons from Avil‐Cre::Ret+/eGFP mice.
-
AFlow cytometric analysis of dissociated sensory neurons plotted according to their level of endogenous Ret‐eGFP expression and IB4 binding (also indicated in Fig EV1). There are 5 well‐defined subsets, 2 of which do not bind to IB4 but display a differential level of eGFP intensity (termed Ret‐eGFPLo:IB4Neg, A, and Ret‐eGFPHi:IB4Neg, B, respectively). The other 3 subsets bind IB4 and display a range of eGFP intensities, termed Ret‐eGFPLo:IB4Lo, C, Ret‐eGFPHi:IB4Lo, D, and Ret‐eGFPHi:IB4Hi, E. Percent of total events are indicated in each box.
-
BNo correlation between the median cell size of different Ret+ subsets and eGFP intensity or IB4 binding. The graph shows the forward scatter values plotted against the normalized number of cells, displayed as the percent of Max.
-
C, DVariations in endogenous eGFP intensity are clearly visible with fluorescent microscopy. (C) Cultured neurons and (D) DRG section from Avil‐Cre::Ret+/eGFP mice displaying native eGFP fluorescence and stained with IB4. Different levels of eGFP intensity (high, indicated by arrows, and low, indicated by arrowheads) are detected across IB4+ and IB4− cells. Scale bar, 50 μm.
Figure EV1. Avil‐Cre::Ret+/ eGFP DRG cell sorting strategy.
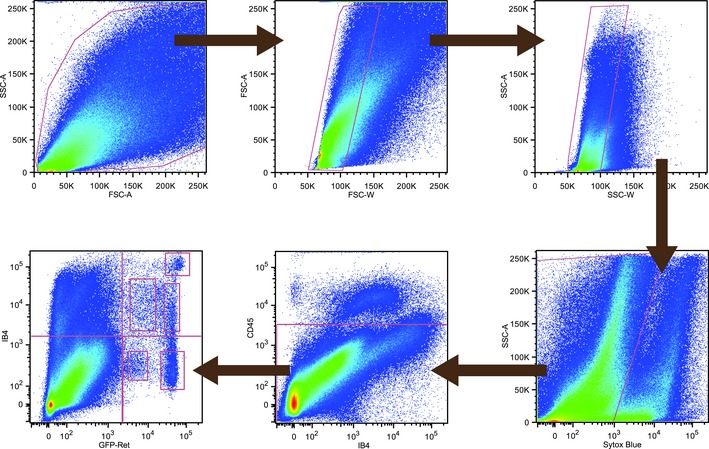
Hierarchical gating strategy for the isolation of live Ret+ sensory neurons. DRG cells were isolated from Avil‐Cre::Ret+/ eGFP mice, quantified, and selected according to their size and complexity. Dead cells and immune cells were excluded using the living dye Sytox Blue and an antibody against CD45. Live sensory neurons were defined by their levels of eGFP fluorescence and IB4 binding. The bottom left plot is identical to that in Fig 2A.
Transcription profiling of Ret‐eGFP‐ and IB4‐negative neurons
We reasoned that by defining the molecular composition of Ret‐eGFP populations, we may be able to gain clues as to their function. We focused on IB4‐negative neurons because flow cytometric data indicated that these cells formed two well‐defined and potentially homogeneous populations. Moreover, while one of these populations presumably corresponds to RA mechanoreceptors 25, 26, the other may reflect an as yet uncharacterized population of Ret‐positive neurons. We performed differential microarray screening on sorted Ret‐eGFPHi:IB4Neg and Ret‐eGFPLo:IB4Neg cells and determined that these two populations do indeed cluster into distinct, homogeneous subsets. We further identified several functional markers in each population that gave a first indication as to their identity. For example, Ret‐eGFPLo:IB4Neg cells were enriched in transcripts for TRPV1, CGRP (Calca), and the Ret co‐receptors GFRα1 and GFRα3 (Fig 3A), while Ret‐eGFPHi:IB4Neg neurons expressed significantly higher levels of Ret, GFRα2, and the mechanosensitive ion channel Piezo 2 (Fam38b) (Fig 3A). Intriguingly, neither population was enriched for TH. We thus speculate that Ret‐eGFPHi:IB4Neg neurons may correspond to RA mechanoreceptors, while Ret‐eGFPLo:IB4Neg cells represent a novel, functionally distinct subtype of nociceptor.
Figure 3. Transcriptional profiling of Ret‐eGFPHi:IB4Neg and Ret‐eGFPLo:IB4Neg sensory neurons.
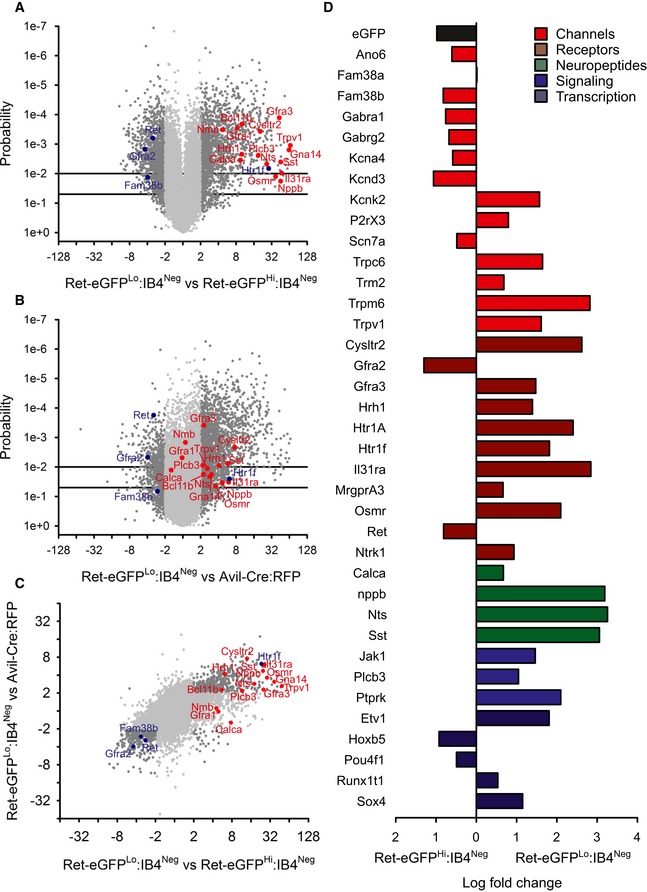
- Ret‐eGFPHi:IB4Neg and Ret‐eGFPLo:IB4Neg neurons display distinct expression profiles. A volcano plot of fold change expression in Ret‐eGFPLo:IB4Neg versus Ret‐eGFPHi:IB4Neg against probability. Ret has higher expression in the Ret‐eGFPHi:IB4Neg subset, which also shows an upregulation of the Ret co‐receptor Gfra2 and Fam38b, encoding for the mechanosensitive ion channel Piezo 2. The Ret‐eGFPLo:IB4Neg subset displays an array of molecules previously associated with itch (marked in red).
- Itch‐associated transcripts are enriched in the Ret‐eGFPLo:IB4Neg population compared to all DRG neurons. Volcano plot of fold change expression in Ret‐eGFPLo:IB4Neg neurons versus sorted neurons from Avil‐Cre::R26tdRFP mice against probability. Molecules linked to itch perception are significantly upregulated in the Ret‐eGFPLo:IB4Neg subset.
- Gene expression within the Ret‐eGFPLo:IB4Neg population is confirmed by triple comparison between Ret‐eGFPLo:IB4Neg, Ret‐eGFPHi:IB4Neg, and Avil‐Cre::R26tdRFP datasets.
- Validation of differential microarray screening by quantitative RT–PCR. Transcripts encoding ion channels, receptors, neuropeptides, signaling molecules, and transcription factors were selected for quantitative RT–PCR. Differential expression between Ret‐eGFPLo:IB4Neg and Ret‐eGFPHi:IB4Neg populations correlates with microarray analysis (n = 3).
We sought to define the function of the novel Ret‐eGFPLo:IB4Neg population and observed that this subset was highly enriched in molecules previously implicated in itch (Fig 3A, red dots). For example, transcripts for histamine‐dependent itch mediators HRH1, PLCβ3, and TRPV1 were all differentially expressed in Ret‐eGFPLo:IB4Neg neurons, as were itch‐associated neurotransmitters such as Nppb 3 and neuromedin B (Nmb) 33, and the co‐receptors for IL‐31, Il31ra, and Osmr. However, other molecules implicated in itch such as GRP, MrgprC11, and endothelin receptors were not over‐expressed in Ret‐eGFPLo:IB4Neg neurons, suggesting that these cells may contribute to a subtype of itch receptor.
To further investigate whether itch‐associated receptor transcripts were indeed specifically expressed in Ret‐eGFPLo:IB4Neg neuron, we performed a second microarray screen where we assessed differential expression in this population with respect to all DRG neurons. We sorted DRG neurons from Avil‐Cre::R26tdRFP mice in which the majority of peripheral sensory neurons are marked by RFP fluorescence (Appendix Fig S3) and subjected them to microarray analysis. Similar to differential screening between Ret‐eGFPLo:IB4Neg and Ret‐eGFPHi:IB4Neg populations, this dataset was also enriched in itch‐associated molecules (Fig 3B). Fold change levels were lower than for comparisons with Ret‐eGFPHi:IB4Neg neurons (Fig 3C), presumably reflecting the mixed molecular profile of all DRG neurons, and the fact that some transcripts such as Calca (CGRP) mark large populations of Ret‐negative neurons.
To validate the microarray analysis, we performed parallel quantitative RT–PCR analysis of 38 transcripts in Ret‐eGFPLo:IB4Neg and Ret‐eGFPHi:IB4Neg populations using a microfluidic platform (Fluidigm). We selected genes that represented not only itch‐associated transcripts but also ion channels, signaling molecules, and transcription factors with a demonstrated role in the peripheral nervous system. In agreement with microarray data, high differential expression between Ret‐eGFPLo:IB4Neg and Ret‐eGFPHi:IB4Neg neurons was evident for itch‐related genes such as the neuropeptide Nppb, the membrane receptors Il31ra, Osmr, Cysltr2, MrgprA3, and Hrh1, and the signaling molecule Plcb3. We also observed higher expression in this population for other markers such as Ntrk1 (TrkA), Calca (CGRP), and Trpv1 transcripts, marking it as a novel population of Ret‐positive neurons, as well as the ion channels Trpm6, Trpc6, Trpm2, and P2rx3 and the serotonin receptors Htr1f and Htr1a (Fig 3D and Appendix Fig S4). In the Ret‐eGFPHi:IB4Neg population, we detected almost 10‐fold higher expression of Ret and eGFP, validating the flow cytometry analysis. In addition, Gfra2 and the mechanosensitive ion channel Piezo 2 (Fam38b) (but not Piezo 1 (Fam38a)) were upregulated, supporting the assumption that these neurons function as RA mechanoreceptors. Intriguingly, we also observed that this population was enriched in transcripts involved in chloride transport including the GABA channel subunits α1 and γ2 (Gabra1, Gabrg2) and the putative chloride channel anoctamin 6 (Ano6) (Fig 3D and Appendix Fig S4).
SstCre as a surrogate marker for Ret‐eGFPLo:IB4Neg neurons
In order to investigate the function of Ret‐eGFPLo:IB4Neg neurons, we required a molecular tool with which to selectively manipulate this population. From the microarray and quantitative RT–PCR analysis, the neuropeptide somatostatin (Sst) was among the highest enriched transcripts in Ret‐eGFPLo:IB4Neg cells, displaying a 1,126‐fold higher expression compared to the Ret‐eGFPHi:IB4Neg population. Moreover, an SstCre driver line is available which has been well characterized in the CNS 34. We thus examined the expression pattern of SstCre‐mediated recombination in the PNS to determine whether it coincides with the Ret‐eGFPLo:IB4Neg population and could be used to genetically target these cells.
We first assessed the anatomical properties of SstCre‐positive neurons by crossing the SstCre driver line with ReteGFP mice to generate heterozygote Sst‐Cre::ReteGFP/+ mice. In DRG sections from these animals, we detected weak eGFP fluorescence in a sparse population of cells corresponding to 1.3% of all neurons (Fig 4I). We explored this expression pattern in more detail by co‐staining sections with IB4, NF200, and TH (Fig 4A–H). SstCre‐driven Ret‐eGFP expression was most evident in neurons not expressing any of these markers (85% of all Ret‐eGFP‐positive cells, Fig 4J), suggesting that SstCre may indeed mediate recombination in the Ret‐eGFPLo:IB4Neg population.
Figure 4. SstCre‐driven expression in DRG and skin.
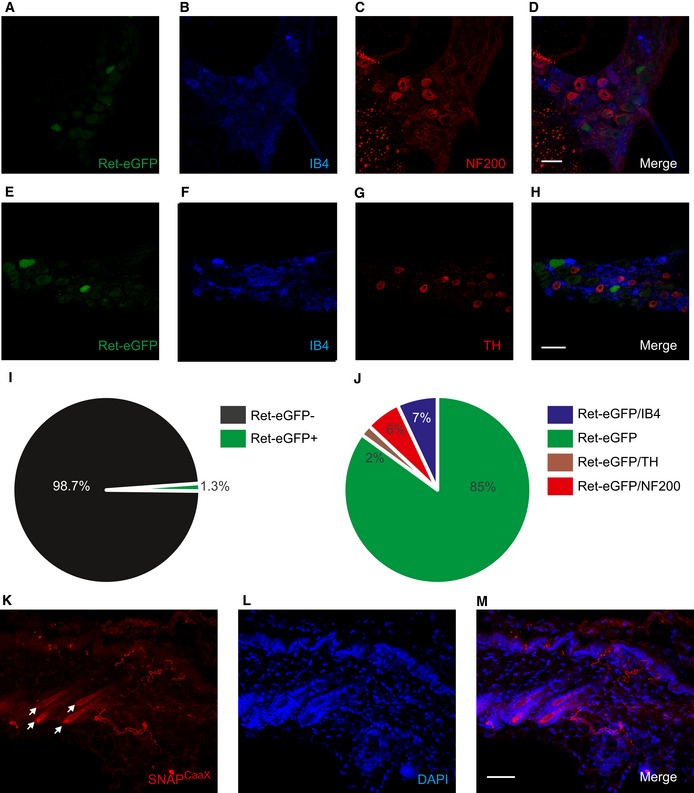
-
A–HSstCre‐mediated recombination of the Ret locus drives eGFP expression in sensory neurons that do not bind to IB4 or co‐express NF200 or TH. Triple immunostaining of DRG from Sst‐Cre::ReteGFP/+ mice with RetGFP (A), IB4 (B), NF200 (C), RetGFP (E), IB4 (F), and TH (G). Scale bars, 50 μm.
-
IQuantification of SstCre::Ret‐eGFP expression in DRG (n = 8,827 cells from three mice).
-
JQuantification of co‐expression of SstCre::Ret‐eGFP with neuronal markers (n = 8,827 cells from three mice).
-
K−MPeripheral projections of sensory neurons from Sst‐Cre::Rosa26SnapCaaX mice terminate in the hairy skin as free nerve endings. Non‐innervated hair follicles are indicated by arrows. Double labeling using TMR‐Star to label SNAP tag (K) and DAPI (L). Scale bars, 50 μm.
To investigate whether SstCre is selective for Ret‐eGFPLo:IB4Neg neurons, we crossed mice with a ubiquitous RFP reporter driven from the Rosa26 locus (Sst‐Cre::Rosa26RFP mice) and performed immunohistochemistry for neuronal markers on DRG sections. We again observed a low number of RFP‐positive cells (1.8% of total neurons, Fig EV2) that were mostly negative for IB4 and NF200 (87% of all Rosa26RFP‐positive cells, Fig EV2). These values were not significantly different from the number of Sst‐Cre::ReteGFP‐positive neurons (P = 0.36 for total cells and P = 0.7 for marker negative cells) implying that SstCre does not drive substantial recombination beyond the Ret‐eGFPLo:IB4Neg population.
Figure EV2. Sst‐Cre::Rosa26‐driven expression in sensory neurons.
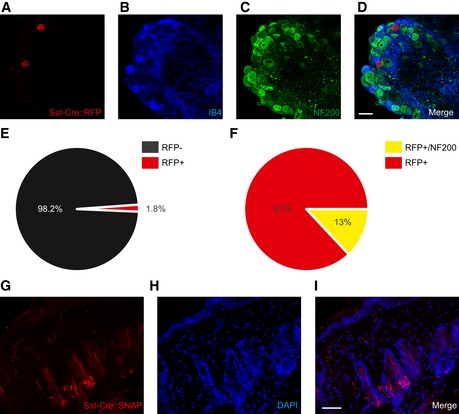
-
A–FSstCre‐mediated recombination of the Rosa26 locus drives RFP expression in a small population of sensory neurons. Triple immunostaining of DRG from Sst‐Cre::Rosa26RFP/+ mice with RFP (A), IB4 (B), and NF200 (C). (E) Quantification of SstCre:: Rosa26RFPexpression in DRG. (F) Quantification of co‐expression of SstCre::Rosa26RFP with neuronal markers (n = 2,592 cells from 3 mice). Scale bar, 50 μm.
-
G–ISNAP‐tag labeling of skin from Sst‐Cre::Rosa26SNAPCaaX/+ mice. Hair follicle innervation, often occurring in 3 hairs together, is occasionally observed in skin from Sst‐Cre::Rosa26SNAPCaaX mice. Scale bar, 50 μm.
We further examined the peripheral projections of SstCre‐positive sensory neurons using a Rosa26SNAPCaaX reporter mouse line that allows for highly sensitive detection of Cre‐positive cells 35. We labeled skin from Sst‐Cre::Rosa26SNAPCaax mice with fluorescent TMR‐Star SNAP substrate and observed prominent fluorescence mainly confined to neurons that formed free nerve endings and ran parallel to the dermal/epidermal border of the skin (Fig 4K–M). In occasional sections, we also detected a rare population of hair follicles that were innervated by SstCre‐positive neurons (1.9 ± 1% of hair follicles in 3 out of 13 sections, Fig EV2). Importantly, this staining pattern was absent from skin taken from control mice not expressing SstCre.
To obtain a more quantitative assessment of the degree of intersection between SstCre‐positive neurons and the Ret‐eGFPLo:IB4Neg population, we applied flow cytometric analysis to acutely dissociated neurons from Sst‐Cre::Ret‐eGFP mice. We followed an identical preparation protocol to that performed previously for Avil‐Cre::ReteGFP mice and used the same gates for analysis. In line with histological data, we observed very few IB4‐positive cells among the Sst‐Cre::ReteGFP population, and furthermore, native GFP fluorescence was low (Fig 5A). Indeed, when comparing this data directly to flow cytometric analysis of Avil‐Cre::ReteGFP neurons (Fig 5B), it was apparent that four out of the five subpopulations defined in earlier experiments were absent in SstCre mice and only the Ret‐eGFPLo:IB4Neg was present.
Figure 5. Flow cytometric and transcriptome analysis of Sst‐Cre::ReteGFP versus Ret‐eGFPHi:IB4Neg sensory neurons.
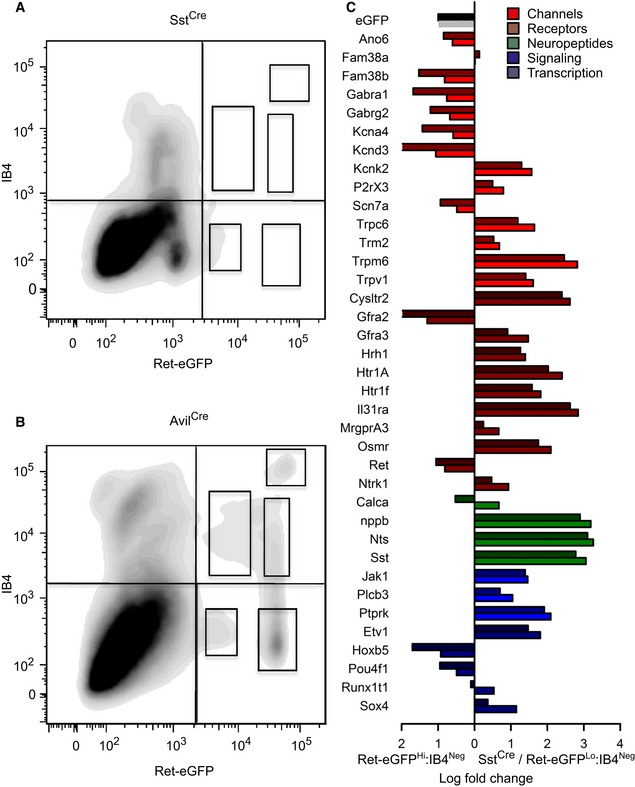
-
A, BContour diagram of dissociated sensory neurons from Sst‐Cre::ReteGFP/+ (A) and Avil‐Cre::ReteGFP/+neurons (B), plotted according to their native eGFP expression and IB4 binding. Only one subset of Ret‐eGFP cells can be defined within the Sst‐Cre::ReteGFP/+‐dissociated DRG neurons. This displays the same range of eGFP fluorescence as the Ret‐eGFPLo:IB4Neg population and does not bind to IB4.
-
CQuantitative RT–PCR analysis of selected transcripts in the Sst‐Cre::ReteGFP (darker colors) and Ret‐eGFPHi:IB4Neg (lighter colors) subsets displayed as differential expression compared to the Ret‐eGFPHi:IB4Neg subset. Almost all the genes that are upregulated in the Ret‐eGFPLo:IB4Neg compared to the Ret‐eGFPHi:IB4Neg subset are also enriched in the Sst‐Cre::ReteGFP population (n = 3).
Finally, we sought to validate the identity of SstCre‐positive sensory neurons by assessing their molecular composition compared to Ret‐eGFPLo:IB4Neg cells. We sorted Sst‐Cre::ReteGFP cells using the same gating paradigm as described previously and subjected cells to quantitative RT–PCR analysis. Strikingly, 36 out of 38 transcripts were regulated in the same direction as Ret‐eGFPLo:IB4Neg relative to Ret‐eGFPHi:IB4Neg cells (Fig 5C and Appendix Fig S5). Furthermore, levels of enrichment for both populations were remarkably similar, especially for the markers eGFP and Ret, and for itch‐associated transcripts such as Hrh1, Il31ra, Osmr, Cysltr2, Plcb3, and Nppb (Fig 5C and Appendix Fig S5). Collectively, these data imply that SstCre does indeed mark the Ret‐eGFPLo:IB4Neg population and that this population is largely homogenous.
Functional characterization of SstCre neurons
To examine the function of SstCre‐positive neurons, we reasoned that selective ablation of this population could uncover their role in driving distinct somatosensory behaviors. A well‐described and effective method for selectively ablating neurons in vivo involves Cre‐dependent expression of the diphtheria toxin receptor from the Rosa26 locus and subsequent treatment of animals with diphtheria toxin 36. However, because SstCre is expressed widely in the central nervous system 34, this approach would not be suitable for deleting only SstCre‐positive neurons in the peripheral nervous system. We thus generated a new mouse line where diphtheria toxin receptor is integrated into the sensory neuron‐specific Avil locus preceded by a loxP‐flanked stop cassette. Crossing these mice with a Cre driver line should therefore induce expression of the diphtheria toxin receptor only in peripheral sensory neurons, regardless of whether Cre is expressed in other tissues.
AviliDTR mice were generated using standard techniques for knock‐in to the Avil locus (Fig EV3). Mice were healthy, displayed normal fertility, and did not exhibit any obvious defects. To assess SstCre‐mediated recombination of the AviliDTR transgene, SstCre mice were crossed with AviliDTR animals to produce heterozygote Sst‐Cre::AviliDTR/+ mice, and expression of the diphtheria toxin receptor determined using immunohistochemistry. In control AviliDTR mice (without the Cre), we detected no diphtheria toxin receptor expression. However, in mice with the SstCre allele, a small number of diphtheria toxin receptor‐positive cells were evident in DRG that was also negative for IB4 (Fig 6A–C and G). We next investigated the efficiency of ablation of these cells by applying diphtheria toxin systemically in mice and evaluating the number of diphtheria toxin receptor cells using immunohistochemistry. We observed an almost complete loss of diphtheria toxin receptor immunoreactivity after toxin application (Fig 6D–F). To determine whether ablation also impacts upon endogenous Sst expression and does not affect other populations, we performed immunocytochemistry on dissociated DRG neurons plated on glass coverslips and labeled with Sst and NF200 antibodies, and IB4. Sst‐Cre::AviliDTR/+ mice treated with diphtheria toxin displayed a complete loss of Sst immunoreactivity with no change in the number of NF200‐ or IB4‐positive neurons (Fig EV4). Finally, we carried out quantitative RT–PCR on Sst and other transcripts associated with the Ret‐eGFPLo:IB4Neg population (Hrh1, MrgprA3, Il31ra, and Htr1f). All transcripts were strongly downregulated upon diphtheria toxin‐mediated ablation (Fig EV4), indicating that this strategy is an effective means of eliminating the SstCre population in vivo.
Figure EV3. Generation of Avili DTR mice.
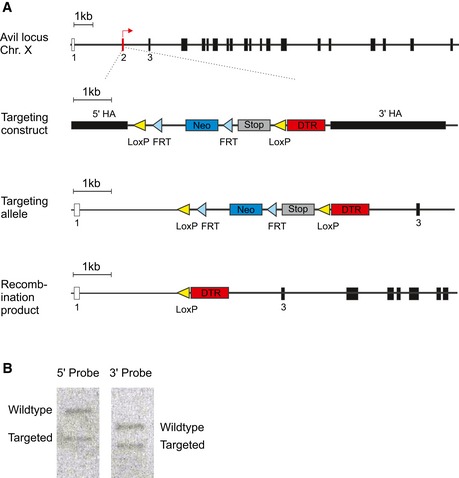
- Schematic diagram of the wild‐type Avil locus with the AviliDTR targeting construct, targeted allele, and recombination product.
- Southern blot of positive ES clone.
Figure 6. Diphtheria toxin‐mediated ablation of SstCre‐positive DRG neurons.
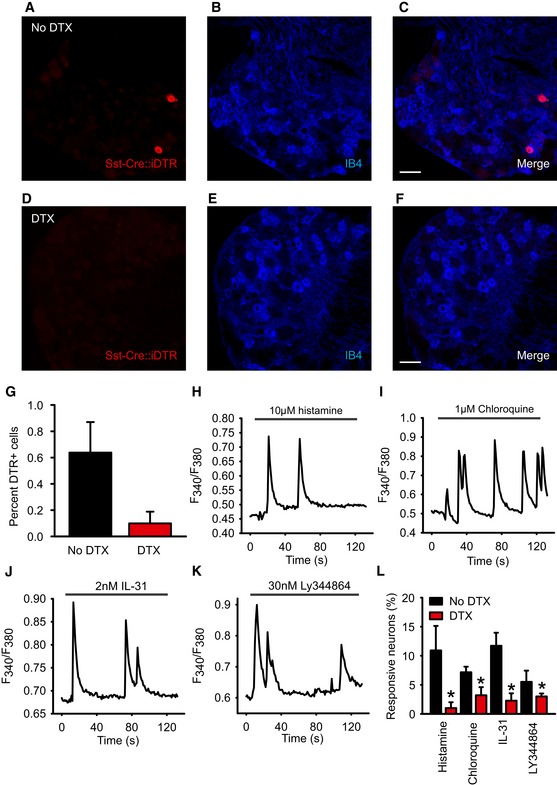
-
A–GAblation of Sst‐Cre::AviliDTR‐positive sensory neurons by injection of diphtheria toxin (DTX). Immunostaining of DRG from Sst‐Cre::AviliDTR mice with an antibody against the diphtheria toxin receptor and IB4 in the absence of diphtheria toxin (A–C) and after systemic injection of diphtheria toxin (D–F). (G) Quantification of diphtheria toxin receptor‐positive neurons before and after injection of diphtheria toxin.
-
H–KExamples of calcium flux in dissociated DRG upon application of histamine (H), chloroquine (I), IL‐31 (J), and 5HT1F agonist Ly344864 (K).
-
LQuantification of percent responding neurons to the indicated pruritogens before or after systemic injection of diphtheria toxin in Sst‐Cre::AviliDTR mice.
Figure EV4. Characterization of Avili DTR mice.
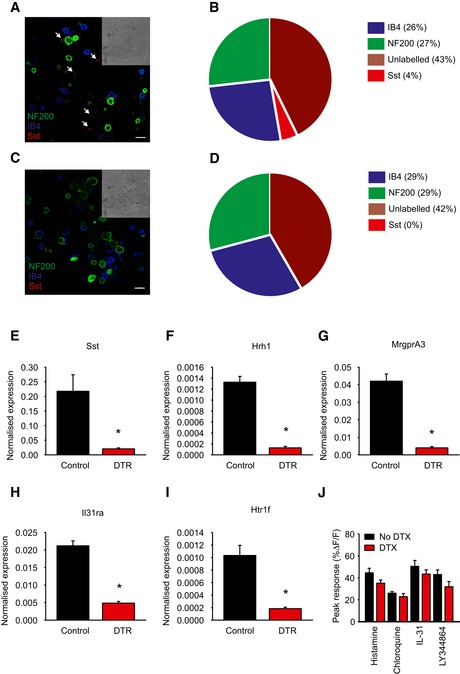
-
ADRG neurons from control AviliDTR/+ mice treated with diphtheria toxin and labeled with antibodies against NF200, Sst, and IB4, and arrows indicate Sst positive. Mice were treated with diphtheria toxin as described below, and DRG cells were isolated, dissociated, and plated on glass coverslips before staining. Scale bar, 30 μm.
-
BQuantification of the number of NF200, Sst, IB4, and unlabeled neurons in control mice.
-
CAcutely dissociated DRG neurons from Sst‐Cre::AviliDTR/+ mice treated with diphtheria toxin and labeled with antibodies against NF200 and Sst, and IB4. Scale bar, 30 μm.
-
DQuantification of the number of NF200, Sst, IB4, and unlabeled neurons in Sst‐Cre::AviliDTR/+ mice after ablation.
-
E–IQuantitative RT–PCR for the indicated transcripts (Sst, Hrh1, MrgprA3, Il31ra, Htr1f) in DRG from control AviliDTR and Sst‐Cre::AviliDTR mice treated with diphtheria toxin. Values are normalized to ubiquitin levels. Error bars indicate SEM, and asterisk indicates P < 0.05, n = 3.
-
JPeak calcium flux to the indicated pruritogen in Sst‐Cre::AviliDTR mice treated with vehicle or diphtheria toxin, n = 4–6 mice. Asterisk denotes P < 0.05, t‐test, and error bars indicate SEM.
To explore the function of SstCre‐positive cells, we initially performed calcium imaging on acutely dissociated DRG and investigated their activation by pruritogens. Based upon transcriptome analysis, we selected four receptor classes highly enriched in this population encompassing histamine‐dependent (histamine) and histamine‐independent signaling (chloroquine and IL‐31), and a class of receptor, 5HT1F not previously implicated in itch, but which couples to the inhibition of adenylate cyclase 37 and may therefore inhibit neuronal activity. Experiments were carried out on DRG from control AviliDTR mice and Sst‐Cre::AviliDTR mice, both treated systemically with diphtheria toxin. Application of histamine to cells evoked a characteristic phasic calcium response in approximately 10% of neurons from control mice, which upon ablation was reduced to less than 1% (Fig 6H and L). Chloroquine and IL‐31 activated a similar number of neurons in control mice with a corresponding decrease in the number of responsive cells in DRG from Sst‐Cre::AviliDTR mice (Fig 6I, J and L). Finally, we examined the function of the 5HT1F receptor by applying the 5HT1F agonist LY344864 to neurons. Unexpectedly, we found that application of low concentrations of LY344864 evoked calcium flux in a small population of neurons that was also reduced upon ablation of SstCre neurons (Fig 6K and L). In all cases, responses to pruritogens which remained after ablation were quantitatively similar to those from control cultures (Fig EV4).
The AviliDTR strategy opens up the possibility to determine how ablation of the SstCre population impacts upon mouse behavior, and thus translate in vitro observations in DRG to events occurring in sensory neuron endings in the skin. To explore this, we first examined nociceptive thresholds in control AviliDTR and Sst‐Cre::AviliDTR mice treated with diphtheria toxin. We observed no significant difference in thermal withdrawal thresholds in the hot plate test (P = 0.17, Fig 7A) or mechanical thresholds to calibrated von Frey filaments (P = 0.32, Fig 7B). We next considered responses to pruritogens by quantifying scratching behavior after intradermal injection of agonists tested in isolated DRG. We first assayed responses in control (AviliDTR) and Sst‐Cre::AviliDTR mice in the absence of diphtheria toxin. All compounds including the 5HT1F agonist LY344864 evoked robust scratching behavior compared to vehicle (Figs 7C–F and EV5). We then applied diphtheria toxin systemically to these mice and reexamined scratching responses. Strikingly, we observed a significant reduction in scratching behavior in Sst‐Cre::AviliDTR mice evoked by IL‐31 and LY344864, but not by histamine or chloroquine (Figs 7C–F and EV5). Responses in control AviliDTR mice were not influenced by diphtheria toxin application, indicating that SstCre neurons (and thus the Ret‐eGFPLo:IB4Neg population) mediate itch sensation in vivo.
Figure 7. Reduced scratching behavior in Sst‐Cre::AviliDTR mice after diphtheria toxin‐mediated ablation.
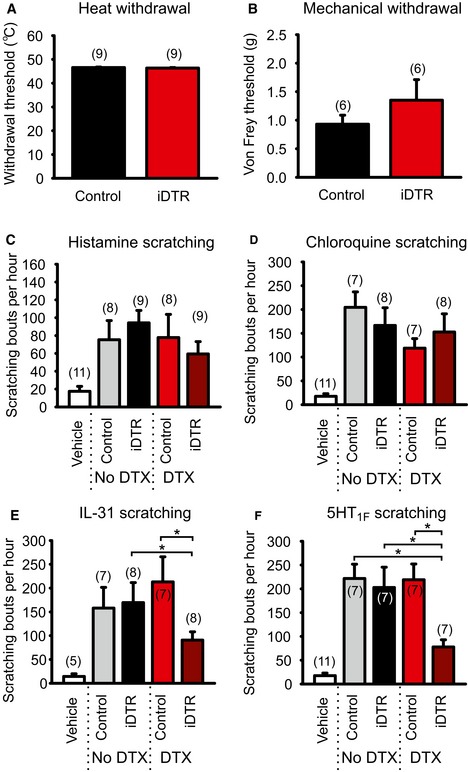
-
A, BNociceptive withdrawal thresholds in control (AviliDTR without the Cre) and Sst‐Cre::AviliDTR mice after systemic diphtheria toxin injection. No significant differences in thermal (A) or mechanical (B) withdrawal reflexes after ablation of SstCre‐positive neurons.
-
C–FScratching behavior after a single intradermal injection of the indicated pruritogen observed for 1 h. All compounds evoked a significant increase in the number of scratching bouts compared to vehicle alone in control (AviliDTR) or Sst‐Cre::AviliDTR mice. Diphtheria toxin injection in control mice (AviliDTR) had no effect on scratching behavior evoked by histamine (C), chloroquine (D), IL‐31 (E), or 5HT1F agonist Ly344864 (F) but significantly reduced responses to IL‐31 or Ly344864 in Sst‐Cre::AviliDTR mice.
Figure EV5. Scratching times in Sst‐Cre::Avili DTR mice after diphtheria toxin‐mediated ablation.
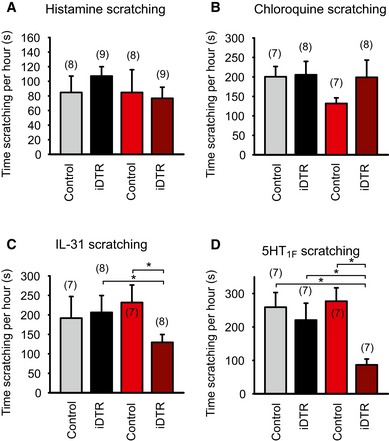
-
A–DTime spent scratching after a single intradermal injection of the indicated pruritogen observed for 1 h. All compounds evoked a significant increase in the time of scratching compared to vehicle alone in control (AviliDTR) or Sst‐Cre::AviliDTR mice. Diphtheria toxin injection in control mice (AviliDTR) had no effect on scratching time evoked by histamine (A), chloroquine (B), IL‐31 (C), or 5HT1F agonist Ly344864 (D) but significantly reduced responses to histamine, IL‐31, or Ly344864 in Sst‐Cre::AviliDTR mice. n numbers indicated above bars, and asterisk denotes P < 0.05, two‐way RM ANOVA, Holm–Sidak multiple comparison, and error bars indicate SEM.
Discussion
Here, we show that a previously uncharacterized population of sensory neurons that express the receptor tyrosine kinase Ret function as itch receptors. Using flow cytometry of dissociated DRG neurons, we isolated this population based upon its weak expression of Ret and absence of IB4 binding. We demonstrate that these neurons express markers such as TrkA and Sst, and importantly are highly enriched for transcripts associated with itch sensation. Anatomical and functional analysis of these cells indicates that they form a rare subtype of DRG neuron and function to detect itch inducing stimuli.
Three populations of Ret‐positive sensory neuron have been previously identified and characterized. Early Ret neurons develop into RA mechanoreceptors 25, 26, while late Ret neurons become C‐fiber low threshold mechanoreceptors and non‐peptidergic nociceptors 22, 28. Intriguingly, a fourth population of Ret‐positive neurons has also been described that expresses TrkA in adult mice but has unknown function 22, 30. This population is scarce, corresponding to around 10% of all Ret‐positive cells 22, 30, and presumably this rarity has made further analysis difficult. By taking advantage of the sensitivity and analytic power of flow cytometry, we have isolated these cells and performed gene expression profiling to determine their function.
We initially used histochemistry to identify populations of Ret‐eGFP‐positive neuron expressing markers of mechanoreceptors (NF200), non‐peptidergic nociceptors (IB4), and C‐fiber low threshold mechanoreceptors (TH). However, limited by the number of markers that can be applied simultaneously to the same section, we turned to flow cytometry to analyze Ret expression in more detail. We based our analysis on the observation that Ret expression levels differ considerably across the DRG and that low Ret levels correlate with GFRα1/3 expression 24, while high Ret‐expressing neurons express GFRα2 and form RA mechanoreceptors 25, 26. By co‐labeling cells with IB4, we were able to distinguish multiple subtypes of Ret‐positive neurons of which a Ret‐low, IB4‐negative population was clearly evident. That this population corresponds to the fourth, uncharacterized type of Ret neuron is supported by several lines of evidence. Firstly, it is IB4 negative, indicating that it is not part of the non‐peptidergic nociceptor population. Secondly, it expresses almost 10‐fold less Ret transcript compared to Ret‐eGFPHi:IB4Neg neurons. Thirdly, Gfra3 is upregulated 29‐fold in these cells, while Gfra2 has 21‐fold higher expression in the Ret‐eGFPHi:IB4Neg population. Finally, we detected many differentially expressed genes such as TrkA, CGRP, and TRPV1, in the Ret‐eGFPLo:IB4Neg population which are known to be absent from C‐LTMRs or RA mechanoreceptors 28. While we did not perform a similar analysis on IB4‐positive neurons, flow cytometry data indicate that further three populations can also be differentiated here which may also form distinct functional populations.
Given the powerful regulatory actions of neurotrophic factors in the peripheral nervous system, a major emphasis of previous research has been to investigate how deletion of these factors impacts upon the survival, differentiation, and maintenance of distinct populations. Here, we have not considered the effects of Ret ablation on Ret‐eGFPLo:IB4Neg‐negative neurons, concentrating instead upon the initial functional characterization of this population. Of note, the number of Ret‐positive/TrkA‐positive neurons is not reduced in Ret nociceptor‐specific conditional knockout mice 30, suggesting that this population does not require Ret for its survival. It is, however, possible that the termination pattern of these neurons in the skin and spinal cord, and the expression of ion channels and signaling molecules are regulated by Ret signaling as has been shown for other populations 22, 24, 25, 26, 27, 28, 30, 38. The identification of markers and molecular tools with which to target Ret‐eGFPLo:IB4Neg‐negative cells described here would now allow for further investigation of these phenotypes.
Among the differentially expressed genes present in Ret‐eGFPLo:IB4Neg cells, we observed a strong enrichment of the neuropeptides CGRP, Nppb, neurotensin (Nts), and somatostatin (Sst). Of these, we focused our attention on Sst because of the availability of an Sst‐Cre driver line 34 which we reasoned could be used to target the Ret‐eGFPLo:IB4Neg population. Indeed, recombination of the Ret or Rosa26 locus by Sst‐Cre occurred in very few neurons of the DRG, resulting in fluorescence in mainly IB4‐ and NF200‐negative cells, analogous to the Ret‐eGFPLo:IB4Neg population. Moreover, flow cytometry analysis and transcriptional profiling revealed that these neurons overlap. Of note, Sst‐Cre‐mediated recombination of eGFP and RFP reporters resulted in a lower number of positive cells when compared to the Ret‐eGFPLo:IB4Neg population, or to antibody staining of Sst. We suggest that this may be due to the lower sensitivity of reporter fluorophores 35. Indeed, in ablation experiments which require minimal expression of the diphtheria toxin receptor to induce cell death, we observed around 10% reduction in the number of cells responding to pruritogens such as histamine.
To define the function of the Sst‐Cre/Ret‐eGFPLo:IB4Neg population, we generated a new mouse line which allows for selective ablation of Cre‐positive sensory neurons regardless of whether Cre recombinase is expressed in other tissues beyond the DRG. Using this mouse, we were able to selectively ablate Sst‐positive neurons and found that both cellular and behavioral responses to pruritogens but not nociceptive behavior were reduced upon ablation. Intriguingly, we also observed several differences between in vitro studies on isolated DRG and in vivo studies examining scratching behavior. For example, histamine‐ and chloroquine‐evoked responses were sensitive to diphtheria toxin ablation in isolated DRG, but were not affected at the whole animal level. One possible explanation for this discrepancy is that ablation of Sst‐Cre‐positive neurons does indeed eliminate those neurons which respond directly to the tested pruritogens. However, in vivo, alternative pathways involving other cell types may be evoked (especially by histamine and chloroquine) which in turn activate a broader spectrum of neurons to trigger scratching. It was also unexpected that scratching behavior was only partially reduced in vivo. Indeed, knockout mice for Nbbp show an almost complete loss of itch‐evoked behavior 3, and Nbbp was highly upregulated in Ret‐eGFPLo:IB4Neg neurons and is co‐expressed with Sst 29, 30. Since our data indicate that Sst neurons are fully ablated upon treatment with diphtheria toxin (Fig EV4), this would suggest that Nbbp is also expressed outside of the Sst population. Thus, the AviliDTR mouse line may be a useful tool for establishing functional overlap between different populations of sensory neuron.
We were intrigued by the high expression levels of the serotonin receptors Htr1f and Htr1a in Ret‐eGFPLo:IB4Neg neurons. Serotonin has a well‐established pruritogenic action when applied subcutaneously and this effect is mediated by 5‐HT2 receptors 40, 41. Transcripts for 5‐HT2 receptors were not upregulated in Ret‐eGFPLo:IB4Neg cells and we did not investigate further whether serotonin directly activates these cells. Instead, we focused on the 5HT1F receptor because it is coupled to Gi signaling and should therefore inhibit neuronal activation. Unexpectedly, we observed an apparent activation of Ret‐eGFP/IB4‐negative sensory neurons by the 5HT1F agonist LY344864 and robust scratching behavior when LY344864 was injected intradermally in control animals. A potential basis for this effect may come from the observation that many Gi‐coupled receptors are able to interact with G14 subunits promoting the stimulation of phospholipase C 42. Indeed, the transcript for G14 (Gna14) was among the most differentially expressed genes in Ret‐eGFPLo:IB4Neg neurons (Fig 5A–C), and thus, its presence could expand the signaling repertoire of these cells allowing for Gi‐coupled receptors such as 5HT1F to generate neuronal activity. Of note, 5HT1F receptor agonists have also been proposed as clinical targets for the treatment of migraine 43. Our data indicate that these compounds could have unwanted side effects via activation of Sst‐Cre/Ret‐eGFPLo:IB4Neg neurons and generation of itch.
In addition to the four pruritogen receptors examined here, many molecules previously implicated in itch sensation such as Cysltr2, Plcb3, and Nppb were enriched in the Ret‐eGFPLo:IB4Neg population. However, other itch‐associated transcripts were absent from the transcriptome data including GRP, MrgprC11, endothelin receptors, and TSLP receptor transcripts (Il7r and Crlf2) which play a critical role in the development of atopic dermatitis 44. These data support the recent finding that several populations of putative itch receptors exist in the mouse which may be tuned to selectively detect the multitude of irritants which can evoke scratching behavior 20. Our data on the expression profile of Ret‐eGFPLo:IB4Neg neurons provide a molecular inventory for one of these populations, and as such many more molecules with an as yet unexplored role in itch may be contained within this list. Moreover, this complexity suggests that therapeutic strategies for treating itch should target common signaling molecules or disrupt neuronal function, rather than inhibit specific receptors. The transcriptional profiling approach described here is a first step toward identifying these molecules.
Materials and Methods
Transgenic mouse lines
To study Ret expression in adult primary sensory neurons, we crossed RETeGFP/+ 31 mice with the DRG neuron‐specific Cre line Avilcre/+ 32 to obtain Avil‐Cre::RETeGFP/+ mice. We also used the Avilcre/+ line to mark sensory neurons within the DRG by crossing it with a Rosa26tdRFP strain 45 to generate Avil‐Cre::R26tdRFP/+ mice. To specifically mark Ret‐eGFPLo:IB4Neg neurons, we bred Sstcre/+ 34 and RETeGFP/+ mice, thus obtaining Sst‐Cre::ReteGFP/+ animals. To mark all Sst‐positive neurons, we crossed the SstCre line with the above‐mentioned Rosa26tdRFP strain, generating Sst‐Cre::R26tdRFP/+ mice. To visualize Sst‐positive nerve endings, the SstCre/+ mice were bred with Rosa26SnapCaaX/+ animals 35 to obtain Sst‐Cre::Rosa26SnapCaaX/+ offspring.
To ablate Sst‐positive sensory neurons, we crossed the SstCre line with an AviliDTR line, thus obtaining Sst‐Cre::AviliDTR/+ mice that express the diphtheria toxin receptor specifically in Sst‐positive DRG neurons.
The AviliDTR line was obtained by classical knock‐in of the iDTR cassette Lox‐STOP‐Lox‐DTR (kindly provided by the Waisman group in Mainz) into the Avil locus, to replace exon 2 and 66 bp upstream thereof (Fig EV3). The targeting construct was transfected into A9 ES cells. Individual ES cell clones underwent Southern blot screening to identify homologous recombinants. DNA was digested with PstI and HindIII and hybridized with 5′ or 3′ probe, respectively, obtaining 9,600 (wild‐type)‐ and 6,300 (targeted)‐bp DNA fragments by using the 5′ probe and 7,100 (wild‐type)‐ and 5,600 (targeted)‐bp DNA fragments by using the 3′ probe. Positive clones were injected into 8‐cell stage embryos to generate mice heterozygous for the targeted allele. To assess mouse genotype, PCR was performed with the following primers: aggagcgagggctcagttgggctgt (forward) and acaccaggttagcctttaagcctgc (reverse) for the wild‐type and ctgccacccaggttaccatggagagagg (forward) and cattctagttgtggtttgtccaaactca (reverse) for the mutant allele, giving PCR products of 313 and 287 bp, respectively. Heterozygous mice were all viable and exhibited no overt behavioral phenotype.
All mice were bred and maintained at the EMBL Mouse Biology Unit, Monterotondo, in accordance with Italian legislation (Art. 9, 27. Jan 1992, no 116) under license from the Italian Ministry of Health, and in compliance with the ARRIVE guidelines. The AviliDTR conditional allele has been deposited in the European Mutant Mouse Archive (www.emmanet.org).
Immunofluorescence
DRG and spinal cord from adult mice (6 weeks or older) were postfixed in 4% PFA for 30 min (DRG) or 2 h (SC), embedded in 12% bovine gelatin, and sectioned using a vibratome at 50–100 μm. Sections were incubated for 30 min in 50% ethanol and then overnight in 0.3% Triton X‐100, and 5% goat or donkey serum in PBS at 4°C containing one or more antibodies diluted as shown below. Secondary antibodies and streptavidin‐647 were diluted (1:1,000 and 1:600, respectively) in 0.3% Triton X‐100, 5% goat or donkey serum in PBS and left for 1–2 h at 4°C. Slides were mounted with Prolong gold antifade (Invitrogen, P36930).
For immunofluorescence analysis of the Avil‐Cre::RETeGFP/+ mouse skin, the hair was removed and skin collected and postfixed in 4% PFA for 8–12 h, incubated in 30% sucrose overnight, and frozen in OCT. 35‐μm sections were cut with a cryostat. Antibody staining was performed as described above.
For SNAP‐tag analysis of the Sst‐Cre::Rosa26SnapCaaX/+ mouse peripheral nerve endings, the back skin of the animals was injected intradermally with 10 μM of Snap‐Cell TMR‐Star (New England Biolabs, S9105S). After 6 h, the skin was collected and treated as described above. Once sectioned, the skin was additionally stained with DAPI (Invitrogen, D1306).
We used the following primary antibody dilutions: mouse anti‐NF200 (Sigma Aldrich, N0142) 1:500, goat anti‐CGRP (Santa Cruz, SC8856) 1:500, rabbit anti‐PKCγ (Santa Cruz, SC‐211) 1:100, isolectin GS‐B4‐biotin XX conjugate (Invitrogen I21414) 1:100, rabbit anti‐RFP (Rockland, 600‐401‐379) 1:200, rabbit anti‐TH (Millipore, AB152) 1:1,000, rabbit anti‐PGP9.5 antibody (Dako, Z5116) 1:200.
To visualize the cells expressing the diphtheria toxin receptor, we used goat anti‐human HB‐EGF antibody (R&D Systems, AF‐259‐NA).
Sst, NF200, and IB4 staining was performed on acutely dissociated neurons from Sst‐Cre::AviliDTR/+ and control AviliDTR/+ (without the Cre), after treatment with diphtheria toxin. Cells were dissociated with 1 mg/ml collagenase IV and 0.05% trypsin (Gibco, 25300‐054) for 25 min each at 37°C and subsequently plated on glass coverslips treated with poly‐L‐lysine. Cells were fixed with 4% PFA for 5 min and permeabilized 10 min with 0.1% Triton X‐100/PBS. Blocking with 3% normal goat serum in 0.01% Tween‐20/PBS was performed for 20 min and cells were then stained overnight at 4°C with rat anti‐Sst (1:50 concentration, MAB354, Millipore), mouse anti‐NF200 (1:1,000), and IB4 (1:100). After washing with PBS, secondary antibody staining was performed for 1.5 h. Glass coverslips were then mounted with Prolong gold antifade.
All images were visualized with a Leica SP5 confocal microscope and analyzed with ImageJ.
Flow cytometry and microarray analysis
To quantitatively analyze the distribution of Ret+ sensory neurons in DRG, we used flow cytometry on Avil‐Cre::RETeGFP/+ mice: DRG were collected from 10 adult Avil‐Cre::RETeGFP/+ mice (6 weeks or older) and pooled. Cells were dissociated with 1 mg/ml collagenase IV and 0.05% trypsin (Gibco, 25300‐054) for 25 min each at 37°C and subsequently kept on ice in 1% FBS in PBS. Cells were labeled in suspension with 1:50 anti‐mouse CD16/32 (eBioscience 14‐0161‐85) for 5 min and isolectin GS‐B4‐biotin XX conjugate (1:100) for 10 min. After washing, cells were incubated immediately with a cocktail containing streptavidin‐Alexa 647 and anti‐CD45‐PeCy7 for 5 min, washed, and resuspended in 1% FBS in PBS.
To isolate the whole sensory neuron population, we applied flow cytometric analysis and subsequent sorting on Avil‐Cre::R26tdRFP/+ mice: DRG were collected from three adult Avil‐Cre::R26tdRFP/+ mice (6 weeks or older) and pooled. Cell dissociation was performed as described above. Cells were then labeled in suspension with 1:50 anti‐mouse CD16/32 (eBioscience 14‐0161‐85) for 5 min, and after washing, cells were incubated in anti‐CD45‐PeCy7 (1:200) for 5 min, washed, and resuspended in 1% FBS in PBS.
To analyze the distribution of Ret‐ and Sst‐positive sensory neurons, we used flow cytometry on Sst‐Cre::ReteGFP/+ animals. Sensory neurons from 10 mice were collected, isolated, and stained following the same protocol used for the analysis of Avil‐Cre::RETeGFP/+ mice.
Flow cytometric online and offline analyses were performed in a FACS Aria III SORP (BD Bioscience) using FACS Diva software (BD Bioscience) and FlowJo (Tree Star, Inc.), respectively. For gene expression analysis, DRG cells were sorted (see Fig EV1) using an 85‐μm nozzle (40 PSI) directly into RLT buffer (RNeasy kit, Qiagen) and RNA was purified according to manufacturer instructions. Two rounds of RNA amplification, labeling, and hybridization to Affymetrix® MOE430 2.0 GeneChip® expression arrays were performed by the EMBL Gene Core Facility. Microarray data were analyzed as detailed in the Appendix. All data have been submitted to ArrayExpress with the accession code E‐MTAB‐1836.
Fluidigm
To validate the microarray data, we used the microfluidic platform Fluidigm to perform quantitative RT–PCR. DRG from Avil‐Cre::RETeGFP/+ mice were collected and processed as described above. Cells were sorted directly into 5 μl of CellsDirect (Invitrogen, PN 11753‐100 and 11753‐500) 2× Buffer, and combined with 0.2 μl of RT/Taq mix, 1 μl of assay pool (formed by 38 individual assay pairs that were previously pooled and diluted to a final concentration of 500 nM), and 2.8 μl of water. The samples went through target‐specific RT and pre‐amplification (16 cycles) following manufacturer instructions. Pre‐amplified cDNA was then diluted 1:10 and combined together with 2.5 μl 2× SsoFast Evagreen Supermix (Bio‐Rad, PN 172‐5211) and 0.25 μl 20× Sample Loading Reagent (Fluidigm, PN 100‐0388) and loaded onto the sample inlets of a 48.48 Fluidigm Dynamic Array. All 38 individual assays pairs (100 μM) were diluted by combining 0.25 μl of each assay with 2.25 μl of TE Buffer (TEKnova, PN T0224) and 2.5 μl of Assay Loading Reagent (Fluidigm, PN 85000736). The 48.48 chip was then loaded on the IFC Controller MX and 30 cycles of qPCR were performed on the Fluidigm BioMark HD system. Data were analyzed using Fluidigm Real‐Time Analysis Software.
To compare the level of expression of the 38 genes between Ret‐eGFPLo:IB4Neg‐ and Sst‐Cre::ReteGFP‐positive sensory neurons, we used Fluidigm platform to perform qRT–PCR on DRG neurons sorted from Sst‐Cre::ReteGFP/+ animals. We followed the same procedure described above.
qRT–PCR
DRG were dissected from adult Sst‐Cre::AviliDTR/+ and control AviliDTR/+ (without the Cre) mice treated with 40 μg/kg of diphtheria toxin (2 injections, the second injection occurring 72 h after the first one). Mice were sacrificed 8 days after the second DTX injection. RNA was isolated using Trizol and reverse‐transcribed using Superscript II (Invitrogen). Quantitative RT–PCR was then performed with LightCycler 480 SYBR Green I Master kit (Roche, 04707516001) following manufacturer instructions and using Roche LightCycler 480 instrument. The expression of each gene was calculated relatively to ubiquitin using the 2−ΔΔCt method.
Calcium imaging
DRG from adult Sst‐Cre::AviliDTR/+ and control AviliDTR/+ (without the Cre) mice were collected and incubated in 1 mg/ml collagenase IV (Sigma Aldrich, C5138) and 0.05% trypsin (Gibco, 25300‐054) for 25 min each at 37°C. They were then suspended in DRG medium (DMEM (Gibco, 41966‐029) with the addition of 10% heat inactivated FBS (PAA, A15101), 0,8% glucose and 100 U of penicillin/streptomycin (Gibco, 15140‐122)). Cells were plated in a droplet of medium on glass coverslips treated with poly‐L‐lysine and left to attach for 3 h at 37°C.
Cells were then incubated in 3 μM Fura2‐AM (Invitrogen, F1221) in CIB (140 mM NaCl, 4 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 4.55 mM NaOH, 5 mM glucose, 10 mM HEPES, pH 7.4) and calcium imaging was performed as described 46. Concentrations of compounds used were as follows: histamine, 1 μM (Sigma, H7250); LY344864, 30 nM (Abcam, ab120592); IL‐31, 2 nM (Peprotech, 210‐31); chloroquine, 500 μM (Sigma, C 6628).
Behavior
To evaluate the scratching response of animals prior and after ablation of the Sst‐positive sensory neurons, we removed the hair of the neck of 6‐ to 8‐week‐old Sst‐Cre::AviliDTR/+ and control AviliDTR/+ mice with an electric shaver. The animals were acclimatized for 2–3 days and the following day they were injected intradermally with 50 μl solution in the nape of the neck with one of the following reagents: 10 mM histamine (Sigma, H7250) in saline, 1 mM LY344864 (Abcam, ab120592) in saline, 5.6 μM IL‐31 (Peprotech, 210‐31) in 0.1% BSA/PBS, 12.5 mM chloroquine (Sigma, C 6628) in saline. Control injections were performed with appropriate vehicle. Animals were observed for 60 min and bouts of scratching counted. One bout was defined as an event of scratching lasting from when the animal lifted the hind paw to scratch until it returned it to the floor or started licking it.
The actual time of scratching was also assessed, considered as the time from when the animal started scratching until it put its hind paw down.
The same animals were then injected i.p. with 40 μg/kg of DTX (Sigma D0564). After 72 h, a second injection of DTX was performed. After 5 days, mice were again acclimatized for 2–3 days, and at the 8th day after the second DTX injection, scratching behavior was assessed again, using the same reagent of the first test, at the same concentration.
For the hot plate and the von Frey tests, Sst‐Cre::AviliDTR/+ and AviliDTR/+ (without the Cre) mice were injected twice with 40 μg/kg of DTX, with the second injection occurring 72 h after the first one. The tests were performed 8 days after the second DTX injection, after 2–3 days of acclimatization.
For the hot plate test, each animal was placed on the hot plate (Ugo Basile) and left to acclimatize for 30 min. A gradient from 42 to 49°C was applied and stopped as soon as a paw withdrawal or licking was observed. This was repeated for 2–3 days. On the day of the actual test, animals were placed again on the hot plate and the gradient was applied: The temperature at which a paw withdrawal or licking was observed was registered as individual threshold for thermal nociception.
For the von Frey test, animals were acclimatized by placing them in cages on a mesh platform and by applying von Frey filaments (North Coast Medical) on the plantar surface of the hind paw. The actual test was performed following the simplified up–down method proposed by Bonin et al 47.
Sample sizes were determined from the power of the statistical test performed. No animals were excluded and all experiments were performed blinded with order of testing randomized.
Author contributions
The study was designed by PAH and KKS. KKS, LI, RD, CM, LC, LN, EP, CP, MP, KSS, FCR, and DB performed experiments and TP performed bioinformatics analysis. PAH wrote the paper with contributions from other authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Expanded View Figures PDF
Review Process File
Acknowledgements
We thank Dr. Sanjay Jain for providing us with the RETeGFP/+ mice, Dr. Ari Waisman for supplying the iDTR cassette for the Adv‐iDTR mouse, Paul Collier and the EMBL Genomics Core Facility for Fluidigm and Microarray procedures, Maria Kamber and the EMBL Monterotondo Mouse Phenotyping, Giulia Bolasco and the Microscopy facility for technical support of our work, and Violetta Paribeni and Stefano Tatti for mouse husbandry.
EMBO Reports (2016) 17: 585–600
References
- 1. Ma Q (2010) Labeled lines meet and talk: population coding of somatic sensations. J Clin Invest 120: 3773–3778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN et al (2013) A subpopulation of nociceptors specifically linked to itch. Nat Neurosci 16: 174–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mishra SK, Hoon MA (2013) The cells and circuitry for itch responses in mice. Science 340: 968–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF (2009) Cellular basis of itch sensation. Science 325: 1531–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ikoma A, Steinhoff M, Stander S, Yosipovitch G, Schmelz M (2006) The neurobiology of itch. Nat Rev Neurosci 7: 535–547 [DOI] [PubMed] [Google Scholar]
- 6. Schmelz M, Schmidt R, Weidner C, Hilliges M, Torebjork HE, Handwerker HO (2003) Chemical response pattern of different classes of C‐nociceptors to pruritogens and algogens. J Neurophysiol 89: 2441–2448 [DOI] [PubMed] [Google Scholar]
- 7. Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjork HE (1997) Specific C‐receptors for itch in human skin. J Neurosci 17: 8003–8008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davidson S, Zhang X, Yoon CH, Khasabov SG, Simone DA, Giesler GJ Jr (2007) The itch‐producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci 27: 10007–10014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, Han SK (2009) TRPV1‐expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci USA 106: 11330–11335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Han SK, Mancino V, Simon MI (2006) Phospholipase Cbeta 3 mediates the scratching response activated by the histamine H1 receptor on C‐fiber nociceptive neurons. Neuron 52: 691–703 [DOI] [PubMed] [Google Scholar]
- 11. Yosipovitch G, Papoiu AD (2008) What causes itch in atopic dermatitis? Curr Allergy Asthma Rep 8: 306–311 [DOI] [PubMed] [Google Scholar]
- 12. Ajayi AA, Oluokun A, Sofowora O, Akinleye A, Ajayi AT (1989) Epidemiology of antimalarial‐induced pruritus in Africans. Eur J Clin Pharmacol 37: 539–540 [DOI] [PubMed] [Google Scholar]
- 13. Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y et al (2009) Sensory neuron‐specific GPCR Mrgprs are itch receptors mediating chloroquine‐induced pruritus. Cell 139: 1353–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wilson SR, Gerhold KA, Bifolck‐Fisher A, Liu Q, Patel KN, Dong X, Bautista DM (2011) TRPA1 is required for histamine‐independent, Mas‐related G protein‐coupled receptor‐mediated itch. Nat Neurosci 14: 595–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu T, Ji RR (2012) Oxidative stress induces itch via activation of transient receptor potential subtype ankyrin 1 in mice. Neurosci Bull 28: 145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fernandes ES, Vong CT, Quek S, Cheong J, Awal S, Gentry C, Aubdool AA, Liang L, Bodkin JV, Bevan S et al (2013) Superoxide generation and leukocyte accumulation: key elements in the mediation of leukotriene B(4)‐induced itch by transient receptor potential ankyrin 1 and transient receptor potential vanilloid 1. FASEB J 27: 1664–1673 [DOI] [PubMed] [Google Scholar]
- 17. Akiyama T, Carstens E (2013) Neural processing of itch. Neuroscience 250: 697–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cevikbas F, Wang X, Akiyama T, Kempkes C, Savinko T, Antal A, Kukova G, Buhl T, Ikoma A, Buddenkotte J et al (2013) A sensory neuron‐expressed IL‐31 receptor mediates T helper cell‐dependent itch: Involvement of TRPV1 and TRPA1. J Allergy Clin Immunol 133: 448–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dillon SR, Sprecher C, Hammond A, Bilsborough J, Rosenfeld‐Franklin M, Presnell SR, Haugen HS, Maurer M, Harder B, Johnston J et al (2004) Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol 5: 752–760 [DOI] [PubMed] [Google Scholar]
- 20. Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, Hjerling‐Leffler J, Haeggstrom J, Kharchenko O, Kharchenko PV et al (2015) Unbiased classification of sensory neuron types by large‐scale single‐cell RNA sequencing. Nat Neurosci 18: 145–153 [DOI] [PubMed] [Google Scholar]
- 21. Marmigere F, Ernfors P (2007) Specification and connectivity of neuronal subtypes in the sensory lineage. Nat Rev Neurosci 8: 114–127 [DOI] [PubMed] [Google Scholar]
- 22. Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD (1997) IB4‐binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron 19: 849–861 [DOI] [PubMed] [Google Scholar]
- 23. Airaksinen MS, Saarma M (2002) The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci 3: 383–394 [DOI] [PubMed] [Google Scholar]
- 24. Luo W, Wickramasinghe SR, Savitt JM, Griffin JW, Dawson TM, Ginty DD (2007) A hierarchical NGF signaling cascade controls Ret‐dependent and Ret‐independent events during development of nonpeptidergic DRG neurons. Neuron 54: 739–754 [DOI] [PubMed] [Google Scholar]
- 25. Luo W, Enomoto H, Rice FL, Milbrandt J, Ginty DD (2009) Molecular identification of rapidly adapting mechanoreceptors and their developmental dependence on ret signaling. Neuron 64: 841–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bourane S, Garces A, Venteo S, Pattyn A, Hubert T, Fichard A, Puech S, Boukhaddaoui H, Baudet C, Takahashi S et al (2009) Low‐threshold mechanoreceptor subtypes selectively express MafA and are specified by Ret signaling. Neuron 64: 857–870 [DOI] [PubMed] [Google Scholar]
- 27. Franck MC, Stenqvist A, Li L, Hao J, Usoskin D, Xu X, Wiesenfeld‐Hallin Z, Ernfors P (2011) Essential role of Ret for defining non‐peptidergic nociceptor phenotypes and functions in the adult mouse. Eur J Neurosci 33: 1385–1400 [DOI] [PubMed] [Google Scholar]
- 28. Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, Gong S, Jankowski MP, Luo W, Heintz N, Koerber HR et al (2011) The functional organization of cutaneous low‐threshold mechanosensory neurons. Cell 147: 1615–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Olausson H, Wessberg J, Morrison I, McGlone F, Vallbo A (2010) The neurophysiology of unmyelinated tactile afferents. Neurosci Biobehav Rev 34: 185–191 [DOI] [PubMed] [Google Scholar]
- 30. Golden JP, Hoshi M, Nassar MA, Enomoto H, Wood JN, Milbrandt J, Gereau RWt, Johnson EM Jr, Jain S (2010) RET signaling is required for survival and normal function of nonpeptidergic nociceptors. J Neurosci 30: 3983–3994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jain S, Golden JP, Wozniak D, Pehek E, Johnson EM Jr, Milbrandt J (2006) RET is dispensable for maintenance of midbrain dopaminergic neurons in adult mice. J Neurosci 26: 11230–11238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zurborg S, Piszczek A, Martinez C, Hublitz P, Al Banchaabouchi M, Moreira P, Perlas E, Heppenstall PA (2011) Generation and characterization of an Advillin‐Cre driver mouse line. Mol Pain 7: 66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jensen RT, Battey JF, Spindel ER, Benya RV (2008) International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol Rev 60: 1–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Fu Y, Lu J, Lin Y et al (2011) A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 71: 995–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang G, de Castro Reis F, Sundukova M, Pimpinella S, Asaro A, Castaldi L, Batti L, Bilbao D, Reymond L, Johnsson K et al (2015) Genetic targeting of chemical indicators in vivo . Nat Methods 12: 137–139 [DOI] [PubMed] [Google Scholar]
- 36. Buch T, Heppner FL, Tertilt C, Heinen TJ, Kremer M, Wunderlich FT, Jung S, Waisman A (2005) A Cre‐inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat Methods 2: 419–426 [DOI] [PubMed] [Google Scholar]
- 37. Adham N, Kao HT, Schecter LE, Bard J, Olsen M, Urquhart D, Durkin M, Hartig PR, Weinshank RL, Branchek TA (1993) Cloning of another human serotonin receptor (5‐HT1F): a fifth 5‐HT1 receptor subtype coupled to the inhibition of adenylate cyclase. Proc Natl Acad Sci USA 90: 408–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Honma Y, Kawano M, Kohsaka S, Ogawa M (2010) Axonal projections of mechanoreceptive dorsal root ganglion neurons depend on Ret. Development 137: 2319–2328 [DOI] [PubMed] [Google Scholar]
- 39. Li CL, Li KC, Wu D, Chen Y, Luo H, Zhao JR, Wang SS, Sun MM, Lu YJ, Zhong YQ et al (2016) Somatosensory neuron types identified by high‐coverage single‐cell RNA‐sequencing and functional heterogeneity. Cell Res 26: 83–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yamaguchi T, Nagasawa T, Satoh M, Kuraishi Y (1999) Itch‐associated response induced by intradermal serotonin through 5‐HT2 receptors in mice. Neurosci Res 35: 77–83 [DOI] [PubMed] [Google Scholar]
- 41. Nojima H, Carstens E (2003) 5‐Hydroxytryptamine (5‐HT)2 receptor involvement in acute 5‐HT‐evoked scratching but not in allergic pruritus induced by dinitrofluorobenzene in rats. J Pharmacol Exp Ther 306: 245–252 [DOI] [PubMed] [Google Scholar]
- 42. Ho MK, Yung LY, Chan JS, Chan JH, Wong CS, Wong YH (2001) Galpha(14) links a variety of G(i)‐ and G(s)‐coupled receptors to the stimulation of phospholipase C. Br J Pharmacol 132: 1431–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goadsby PJ (2005) New targets in the acute treatment of headache. Curr Opin Neurol 18: 283–288 [DOI] [PubMed] [Google Scholar]
- 44. Wilson SR, The L, Batia LM, Beattie K, Katibah GE, McClain SP, Pellegrino M, Estandian DM, Bautista DM (2013) The epithelial cell‐derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 155: 285–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Luche H, Weber O, Nageswara Rao T, Blum C, Fehling HJ (2007) Faithful activation of an extra‐bright red fluorescent protein in “knock‐in” Cre‐reporter mice ideally suited for lineage tracing studies. Eur J Immunol 37: 43–53 [DOI] [PubMed] [Google Scholar]
- 46. Caspani O, Zurborg S, Labuz D, Heppenstall PA (2009) The contribution of TRPM8 and TRPA1 channels to cold allodynia and neuropathic pain. PLoS One 4: e7383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bonin RP, Bories C, De Koninck Y (2014) A simplified up‐down method (SUDO) for measuring mechanical nociception in rodents using von Frey filaments. Mol Pain 10: 26 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Review Process File


