Abstract
The aim of this study was to explore the relationship between neutrophil-related factors, including neutrophil-lymphocyte ratio (NLR) and the responses of neutrophil to granulocyte colony-stimulating factors (RNG), and the prognosis of patients with locally advanced cervical squamous cell carcinoma (LACSCC) undergoing cisplatin-based concurrent chemoradiotherapy (CCCRT). A total of sixty LACSCC patients were enrolled in this study. We analyzed the association of NLR or RNG with clinicopathologic characteristics of these patients. The prognostic factors were evaluated by univariate and multivariate survival analysis. The optimal cut-off value of the NLR was determined to be 2.0 for the overall survival (OS). A higher level of the NLR was associated with younger age (P = 0.017) and higher baseline platelet count (P = 0.040). NLR was identified to be the only independent prognostic factor for OS by multivariate analysis (P = 0.037). The median RNG was 3.01, with a range of 1.19–16.84. RNG level was significantly associated with lymph node metastasis of these patients (P = 0.023). And higher RNG was identified as being a closely independent poor prognostic factor for OS (P = 0.055). This study showed that NLR and RNG may be used as potential biomarkers for survival prediction in patients with LACSCC receiving CCCRT.
1. Introduction
Cervical cancer is the second most common type of cancer and the leading cause of cancer death in female in developing countries [1]. In patients with advanced stage disease, the standard treatment is cisplatin-based concurrent chemoradiotherapy (CCCRT), followed by brachytherapy [2]. Tumor size, lymph node status, International Federation of Gynecology and Obstetrics (FIGO) stage, and pretreatment hemoglobin level were reported to be independent prognostic factors for locally advanced cervical cancer [3, 4]. However, to further improve the treatment outcome of these patients, more prognostic factors are still needed.
Recently, neutrophil-lymphocyte ratio (NLR) was evaluated as a prognostic indicator in many types of cancer including gastrointestinal tract malignancies [5], hepatocellular carcinoma [6], pancreatic cancer [7], and non-small-cell lung cancer [8]. Although the prognostic significance of NLR has also been investigated in cervical cancer [9–12], the value of NLR in survival prediction of patients with locally advanced cervical squamous cell carcinoma (LACSCC) who received CCCRT remains unknown.
Neutropenia is the most common therapy related toxicity of LACSCC patients who received CCCRT [13, 14]. The duration of neutropenia can be minimized with the use of granulocyte colony-stimulating factors (G-CSFs) [15]. However, the responses of neutrophil to G-CSFs (RNG) among patients are variable [16–18], which may impact the prognosis of LACSCC. To the best of our knowledge, the prognostic value of RNG in LACSCC has never been investigated.
In current study, we hypothesized that neutrophil-related factors, including NLR and RNG, were prognostic indicators of patients with LACSCC who underwent CCCRT. The prognostic values of NLR and RNG in LACSCC were evaluated.
2. Materials and Methods
2.1. Patient Population
The study included 60 consecutive patients with pathologically confirmed cervical cancer who underwent CCCRT from June 2009 to June 2010 at General Hospital of Ningxia Medical University. Clinicopathologic information of these patients, including age, pathologic diagnosis, histologic grade, tumor size, lymph node status, parametrial invasion, FIGO stage, baseline hemoglobin level, and platelet count, was obtained from medical records. Patients with hematologic, autoimmune, or infectious diseases were excluded. This study was approved by the ethics committee of our hospital.
2.2. Treatment and Follow-Up
The pretreatment evaluation included a review of the patient's history, physical examination, performance status, gynecologic examination, chest X-ray, complete blood count, blood chemistry, and abdominal-pelvic magnetic resonance imaging (MRI). Cystoscopy and sigmoidoscopy were performed when indicated. Radiotherapy included external beam radiotherapy up to 50 Gy and low-dose rate brachytherapy, six applications of 6 Gy. Chemotherapy consisted of weekly intravenous cisplatin administration (40 mg/m2) for 5 cycles concomitant with external pelvic radiation. Treatment response was clinically assessed according to RECIST version 1.1 [19]. Treatment toxicity was classified according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE; version 4.0) [20].
The patients were followed up every three months for the first two years, in six-month intervals for the next three years, and every year thereafter. During the routine follow-up, imaging studies including CT or MRI and chest X-ray were performed annually and when tumor recurrence was suspected based on clinical findings or imaging studies, biopsy of that lesion was performed on a case-by-case basis. Overall survival (OS) time was defined as the interval between date of the completion of treatment and death, or the last follow-up, and progression-free survival (PFS) time was defined as the period from date of the completion of treatment to the occurrence of local recurrence or distant metastasis or the last follow-up. Patient follow-up was maintained until death or the cut-off date of June 2015.
2.3. Definition of NLR and RNG
All baseline white cells and differential counts were obtained within one week before CCCRT. The NLR was defined as the absolute neutrophil count divided by the absolute lymphocyte count. During the CCCRT, some patients may develop neutropenia. The absolute neutrophil count of first neutropenia during treatment was defined as N 1. After the G-CSFs therapy, the absolute neutrophil count was defined as N 2. RNG was calculated as N 2/N 1.
2.4. Statistical Analysis
The associations between NLR or RNG and the clinicopathologic variables were analyzed by a chi-square test. The overall and progression-free survival curves were calculated according to the Kaplan-Meier method with the log-rank test. Prognostic factors with significance values of P less than 0.05 in a univariate analysis were entered into a multivariate analysis, which was conducted using the Cox proportional hazards model with the backward likelihood method. All reported P values were two-sided, and P less than 0.05 was considered statistically significant. SPSS 13.0 (SPSS Inc., Chicago, IL) was used for the statistical analysis.
3. Results
3.1. Patient Characteristics and Treatment Outcome
The enrolled 60 LACSCC patients had a median age of 53 years (range 36 to 80 years). Histologically, all primary tumors were squamous cell carcinoma. Staging was performed according to FIGO staging system classification. Clinicopathologic characteristics of these patients are listed in Table 1. All patients received the external beam radiotherapy and brachytherapy as indicated in the protocol. For myelosuppression and infectious complications, only 34 (56.67%) patients received 5 cycles of cisplatin-based concurrent chemotherapy. According to RECIST, the complete response rate of the whole cohort is 56.7%. The median follow-up time of the censored patients was 58 (range, 7–70) months. During the follow-up period, 23 patients were recurrent, and 19 patients were dead. The 5-year PFS and OS of the whole cohort were 56.60% and 61.30%, respectively.
Table 1.
Association between NLR and clinicopathologic characteristics of LACSCC patients.
| Clinicopathologic characteristics | NLR, n (%) | P value | |
|---|---|---|---|
| ≥2.0 | <2.0 | ||
| Age | |||
| ≤50 years | 21 (77.7) | 6 (22.3) | 0.017 |
| >50 years | 15 (45.4) | 18 (54.6) | |
| Histologic grade | |||
| Well and moderately differentiated | 18 (58.0) | 13 (42.0) | 0.797 |
| Poorly differentiated | 18 (62.0) | 11 (38.0) | |
| Tumor size | |||
| ≤4 cm | 9 (45.0) | 11 (55.0) | 0.105 |
| >4 cm | 27 (67.5) | 13 (32.5) | |
| Parametrial invasion | |||
| No | 16 (55.1) | 13 (44.9) | 0.595 |
| Unilateral | 14 (60.8) | 9 (39.2) | |
| Bilateral | 6 (75.0) | 2 (25.0) | |
| Clinical lymph node involvement | |||
| cN0 | 24 (54.5) | 20 (45.5) | 0.234 |
| cN1 | 12 (75.0) | 4 (25.0) | |
| FIGO stage | |||
| II | 20 (55.5) | 16 (44.5) | 0.432 |
| III | 16 (66.6) | 8 (33.4) | |
| Hemoglobin levels at diagnosis (g/dL) | |||
| ≤113 | 7 (53.8) | 6 (46.2) | 0.751 |
| >113 | 29 (61.7) | 18 (38.3) | |
| Platelets at diagnosis (g/dL) | |||
| ≤320 | 9 (90.0) | 1 (10.0) | 0.040 |
| >320 | 27 (54.0) | 23 (46.0) | |
| Response | |||
| CR | 20 (58.8) | 14 (41.2) | 1.000 |
| Non-CR | 16 (61.5) | 10 (38.5) | |
NLR, neutrophil-lymphocyte ratio; LACSCC, locally advanced cervical squamous cell carcinomas; FIGO, International Federation of Gynecology and Obstetrics; CR, complete response.
3.2. The Prognostic Value of NLR
The NLR value of the whole study population ranged from 0.99 to 6.91 with a median of 2.40. The optimal cut-off value of the NLR was determined to be 2.0 for the OS. We analyzed the association of the different NLR levels with clinicopathologic characteristics of patients. There were no significant differences in the clinicopathologic characteristics between the patients with high NLR level and those with low level, except age and baseline platelet count. It was found that a higher NLR level (≥2.0) was associated with younger age (P = 0.017) and higher baseline platelet count (P = 0.040). However, the NLR was not significantly associated with the response of cervical cancer to CCCRT (Table 1).
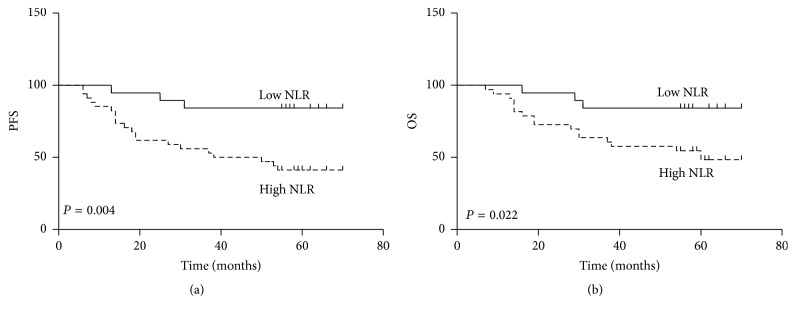
Compared with a lower NLR, a higher NLR was associated with significant worse PFS (P = 0.004) and OS (P = 0.022) (Figure 1). Other significant prognostic indicators identified by univariate analysis included lymph node metastasis (P = 0.029) and FIGO stage (P = 0.044) for OS. These variables were selected for multivariate analysis using a backward likelihood method, and only NLR (P = 0.037) was identified to be independent prognostic factor for OS. For PFS, only lymph node status (P = 0.032) was identified as an independent prognostic factor in univariate analysis. The detailed results were shown in Tables 2 and 3.
Figure 1.
Kaplan-Meier survival curves for progression-free survival (PFS) (a) and overall survival (OS) (b) of LACSCC patients with a high NLR and those with a low NLR.
Table 2.
Univariate survival analysis of PFS and OS in patients with LACSCC.
| Clinicopathologic characteristics | PFS | P value | OS | P value | ||
|---|---|---|---|---|---|---|
| Mean ± SD (months) | 95% CI | Mean ± SD (months) | 95% CI | |||
| Age | ||||||
| ≤50 years | 40.24 ± 4.51 | −1.775–19.821 | 0.100 | 45.36 ± 4.01 | −4.218–15.562 | 0.255 |
| >50 years | 49.26 ± 3.23 | 51.03 ± 3.04 | ||||
| Histologic grade | ||||||
| Well and moderately differentiated | 47.72 ± 3.27 | −16.953–5.247 | 0.295 | 49.71 ± 3.69 | −11.964–8.132 | 0.704 |
| Poorly differentiated | 41.87 ± 4.68 | 47.79 ± 3.31 | ||||
| Tumor size | ||||||
| ≤4 cm | 44.84 ± 3.60 | −13.095–9.726 | 0.769 | 48.79 ± 3.17 | −9.832–11.016 | 0.910 |
| >4 cm | 46.52 ± 4.02 | 48.20 ± 3.87 | ||||
| Parametrial invasion | ||||||
| No | 43.68 ± 3.01 | −22.467–7.181 | 0.877 | 46.18 ± 2.43 | −21.708–4.791 | 0.295 |
| Unilateral | 51.32 ± 4.13 | 54.63 ± 3.15 | ||||
| Bilateral | 40.25 ± 2.58 | 44.27 ± 4.21 | ||||
| Clinical lymph node involvement | ||||||
| cN0 | 48.80 ± 2.87 | 1.140–25.259 | 0.032 | 51.72 ± 2.55 | 1.283–22.960 | 0.029 |
| cN1 | 35.60 ± 5.96 | 39.60 ± 5.55 | ||||
| FIGO stage | ||||||
| II | 45.78 ± 3.51 | −10.341–12.072 | 0.878 | 52.57 ± 2.77 | 0.286–19.813 | 0.044 |
| III | 44.91 ± 4.32 | 42.52 ± 4.28 | ||||
| Hemoglobin levels at diagnosis (g/dL) | ||||||
| ≤113 | 44.14 ± 6.41 | −11.144–14.545 | 0.792 | 47.08 ± 6.16 | −9.923–13.816 | 0.743 |
| >113 | 45.84 ± 2.97 | 49.02 ± 2.64 | ||||
| Platelets at diagnosis (g/dL) | ||||||
| ≤320 | 36.50 ± 8.30 | −25.057–3.521 | 0.136 | 42.60 ± 7.69 | −20.208–5.738 | 0.269 |
| >320 | 47.27 ± 2.75 | 49.83 ± 2.49 | ||||
| Response | ||||||
| CR | 48.09 ± 3.47 | −4.490–17.499 | 0.241 | 52.11 ± 2.89 | −0.9463–18.740 | 0.075 |
| Non-CR | 41.58 ± 4.26 | 43.22 ± 4.15 | ||||
| NLR | ||||||
| ≥2.0 | 46.94 ± 3.49 | −9.711–14.331 | 0.701 | 43.06 ± 3.61 | −21.842–0.356 | 0.043 |
| <2.0 | 44.63 ± 4.96 | 54.16 ± 3.15 | ||||
PFS, progression-free survival; OS, overall survival; LACSCC, locally advanced cervical squamous cell carcinomas; FIGO, International Federation of Gynecology and Obstetrics; CR, complete response; NLR, neutrophil-lymphocyte ratio.
Table 3.
Multivariate survival analysis of OS in patients with LACSCC.
| Clinicopathologic characteristics | B | SE | Wald | P value | HR | 95% CI |
|---|---|---|---|---|---|---|
| Lymph node metastasis | 0.626 | 0.516 | 1.475 | 0.225 | 1.870 | 0.681–5.139 |
| FIGO stage | 0.188 | 0.495 | 0.144 | 0.704 | 1.207 | 0.457–3.185 |
| NLR | −1.316 | 0.631 | 4.350 | 0.037 | 0.268 | 0.078–0.924 |
OS, overall survival; LACSCC, locally advanced cervical squamous cell carcinomas; FIGO, International Federation of Gynecology and Obstetrics; NLR, neutrophil-lymphocyte ratio.
3.3. The Prognostic Value of RNG
In the whole cohort, 35 (58.33%) patients experienced neutropenia, including 19 Grade 1, 14 Grade 2, and 2 Grade 3 cases. RNG value of this subgroup patients ranged from 1.19 to 16.84 (median, 3.01). The median value 3.01 was selected as the cut-off of high and low RNG levels. The complete rates of 5 cycles concurrent chemotherapy in high and low RNG group were 55.56% and 58.82% (P = 0.832), respectively. The mean amounts of G-CSFs administration in high and low RNG group were 583.34 ± 69.66 μg and 566.71 ± 85.58 μg (P = 0.960), respectively. Table 4 shows the relationship of different levels of RNG and the clinicopathologic characteristics of patients. The RNG level was significantly associated with lymph node status of these patients (P = 0.023).
Table 4.
Association between RNG and clinicopathologic characteristics of LACSCC patients with neutropenia.
| Clinicopathologic characteristics | RNG, n (%) | P value | |
|---|---|---|---|
| ≥3.01 | <3.01 | ||
| Age | |||
| ≤50 years | 11 (52.4) | 10 (47.6) | 0.890 |
| >50 years | 7 (50.0) | 7 (50.0) | |
| Histologic grade | |||
| Well and moderately differentiated | 7 (50.0) | 7 (50.0) | 0.890 |
| Poorly differentiated | 11 (52.4) | 10 (47.6) | |
| Tumor size | |||
| ≤4 cm | 5 (55.6) | 4 (44.4) | 0.921 |
| >4 cm | 13 (50.0) | 13 (50.0) | |
| Parametrial invasion | |||
| No | 6 (42.9) | 8 (57.1) | 0.462 |
| Unilateral | 9 (64.3) | 5 (35.7) | |
| Bilateral | 3 (42.9) | 4 (57.1) | |
| Clinical lymph node involvement | |||
| cN0 | 7 (33.3) | 14 (66.7) | 0.023 |
| cN1 | 11 (78.6) | 3 (21.4) | |
| FIGO stage | |||
| II | 9 (52.9) | 8 (47.1) | 0.862 |
| III | 9 (50.0) | 9 (50.0) | |
| Hemoglobin levels at diagnosis (g/dL) | |||
| ≤113 | 4 (44.4) | 5 (55.6) | 0.921 |
| >113 | 14 (53.8) | 12 (46.2) | |
| Platelets at diagnosis (g/dL) | |||
| ≤320 | 3 (75.0) | 1 (25.0) | 0.638 |
| >320 | 15 (48.3) | 16 (51.7) | |
| Response | |||
| CR | 7 (36.8) | 12 (63.2) | 0.060 |
| Non-CR | 11 (68.8) | 5 (31.2) | |
RNG, responses of neutrophil to granulocyte colony-stimulating factors; LACSCC, locally advanced cervical squamous cell carcinomas; FIGO, International Federation of Gynecology and Obstetrics; CR, complete response.
According to the Kaplan-Meier analysis, the OS was significantly shorter for the patients in the high RNG group than for their low counterparts (52.94% versus 83.33%, P = 0.011) (Figure 2). However, the difference of PFS between these two groups did not meet statistical significance. We performed univariate analyses to determine the prognostic factors for OS of these patients. Lymph node status (P = 0.025) and RNG level (P = 0.002) were prognostic predictors for poor OS. The multivariate analysis confirmed that RNG level was a closely independent prognostic predictor for OS (P = 0.055). The detailed results were shown in Tables 5 and 6.
Figure 2.
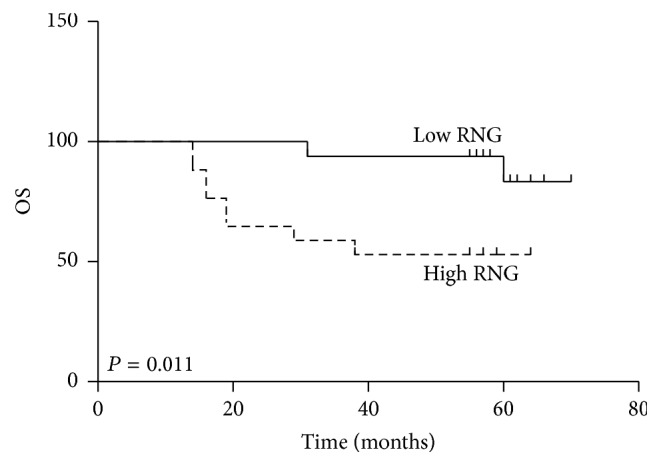
Kaplan-Meier survival curves for overall survival (OS) of LACSCC patients with a high RNG and those with a low RNG.
Table 5.
Univariate survival analysis of OS for LACSCC patients with neutropenia.
| Clinicopathologic characteristics | OS | P value | |
|---|---|---|---|
| Mean ± SD (months) | 95% CI | ||
| Age | |||
| ≤50 years | 45.21 ± 4.54 | −21.451–3.589 | 0.155 |
| >50 years | 54.14 ± 3.60 | ||
| Histologic grade | |||
| Well and moderately differentiated | 51.64 ± 4.19 | −8.242–17.422 | 0.471 |
| Poorly differentiated | 47.05 ± 4.42 | ||
| Tumor size | |||
| ≤4 cm | 47.33 ± 3.90 | −20.303–8.075 | 0.386 |
| >4 cm | 53.44 ± 4.40 | ||
| Parametrial invasion | |||
| No | 45.15 ± 3.19 | −27.924–13.195 | 0.420 |
| Unilateral | 54.07 ± 2.56 | ||
| Bilateral | 46.71 ± 4.37 | ||
| Clinical lymph node involvement | |||
| cN0 | 54.50 ± 2.96 | −26.016–1.912 | 0.025 |
| cN1 | 40.54 ± 5.76 | ||
| FIGO stage | |||
| II | 50.71 ± 3.88 | −16.257–9.215 | 0.057 |
| III | 47.19 ± 4.93 | ||
| Hemoglobin levels at diagnosis (g/dL) | |||
| ≤113 | 47.70 ± 3.95 | −9.524–18.138 | 0.530 |
| >113 | 52.00 ± 4.73 | ||
| Platelets at diagnosis (g/dL) | |||
| ≤320 | 49.24 ± 3.27 | −17.599–21.580 | 0.837 |
| >320 | 47.25 ± 10.49 | ||
| Response | |||
| CR | 44.80 ± 5.15 | −20.231–4.832 | 0.219 |
| Non-CR | 52.50 ± 3.60 | ||
| RNG | |||
| ≥3.01 | 40.06 ± 4.80 | 7.571–29.312 | 0.002 |
| <3.01 | 58.50 ± 2.00 | ||
OS, overall survival; LACSCC, locally advanced cervical squamous cell carcinomas; FIGO, International Federation of Gynecology and Obstetrics; CR, complete response; RNG, responses of neutrophil to granulocyte colony-stimulating factors.
Table 6.
Multivariate survival analysis of OS for LACSCC patients with neutropenia.
| Clinicopathologic characteristics | B | SE | Wald | P value | HR | 95% CI |
|---|---|---|---|---|---|---|
| Lymph node metastasis | 0.668 | 0.696 | 0.921 | 0.337 | 1.951 | 0.498–7.635 |
| FIGO stage | 0.326 | 0.753 | 0.188 | 0.665 | 1.385 | 0.317–6.056 |
| RNG | 1.833 | 0.956 | 3.678 | 0.055 | 6.252 | 0.961–40.687 |
OS, overall survival; LACSCC, locally advanced cervical squamous cell carcinomas; FIGO, International Federation of Gynecology and Obstetrics; RNG, responses of neutrophil to granulocyte colony-stimulating factors.
4. Discussion
It has been recognized that inflammation is an important regulator in the genesis, progression, and metastasis of malignant diseases [21]. Patient response to malignant tumors comprises not only changes in the tumor microenvironment, but also systemic inflammatory alternations [22]. More recently, NLR was defined as a potential marker to determine inflammation in various malignant diseases [5–8]. For cervical cancer, the association between NLR and clinicopathologic characteristics of patients, including age, tumor size, lymph node metastasis, FIGO stage, and depth of stromal infiltration, has been demonstrated. The correlation between the increase of NLR and prognosis of cervical cancer has also been found in these studies [9–12]. However, there was large heterogeneity in the disease stage and treatment modality of these studies; thus the prognostic value of NLR in cervical cancer, especially for LACSCC, remains controversial.
In this study, we assessed the clinicopathologic relevance and prognostic value of NLR in LACSCC patients who underwent CCCRT. We found that NLR was associated with some important clinicopathologic characteristics of LACSCC, including patient age and baseline platelet count. In addition, Kaplan-Meier analysis confirmed that patients with high NLR had a significantly poorer PFS and OS compared with patients with low NLR. Cox regression analysis revealed that NLR was an independent prognostic indicator for OS of patients with LACSCC, but not for PFS. These findings were also confirmed in other studies (Table 7). Lee et al. [9] reported that patients with cervical cancer of the higher NLR group were younger in age and had more advanced staged disease when compared with those of the lower NLR group. In multivariable analysis, higher pretreatment NLR was identified as being an independent poor prognostic factor for survival. Zhang et al. [11] evaluate the clinicopathologic and prognostic values of NLR in patients with cervical cancer undergoing primary radical hysterectomy with pelvic lymphadenectomy. They found NLR was highly associated with depth of stromal infiltration and lymph node metastasis. Multivariable analysis showed that the NLR was an independent prognostic marker for PFS, but not for OS. They concluded that the preoperative NLR may be used as a potential and easy biomarker for survival prognosis in patients with cervical cancer receiving initial radical hysterectomy with pelvic lymphadenectomy. Mizunuma et al. [12] retrospectively analyzed 56 patients with squamous cell carcinoma of the uterine cervix who underwent RT or concurrent chemotherapy and RT. They demonstrated that NLR was a significant prognostic factor for PFS and OS. Patients with a high NLR had significantly shorter PFS and OS than those with a low NLR. Furthermore, in comparison to a high NLR, a low NLR was significantly associated with a complete response. However, we observed no significant correlations between the NLR and response rate of CCCRT in this study. The possible reason for the differences may be attributed to the various stage of the enrolled patients and the different treatment regimens between these two studies.
Table 7.
The summary of NLR studies in cervical cancer.
| Patient number |
Median age (range, years) |
FIGO stage | Histology | Treatment | Median NLR/cut-off value of NLR |
Association of NLR with clinicopathologic characteristics |
Prognostic factor determined in multivariable analysis |
Reference |
|---|---|---|---|---|---|---|---|---|
| 1061 | 50 (21–85) | IB1-IVA | SCC, AC, ASC | Surgery + RT or CRT | 1.9/1.9 | Age, stage, treatment modality | NLR | [9] |
|
| ||||||||
| 111 | 42 (21–68) | IB2-IIB | SCC, non-SCC | NCT + surgery | 2.4/2.5 | FIGO stage | LN metastasis, lymphovascular space involvement | [10] |
|
| ||||||||
| 460 | 44 (24–78) | I-II | SCC, AC | Surgery + RT | 2.213/2.213 | Depth of stromal infiltration, LN metastasis | NLR, FIGO stage and LN metastasis | [11] |
|
| ||||||||
| 56 | 65.1 (35–89) | IB1-IV | SCC | RT or CRT | 2.4/2.5 | FIGO stage, mean SCC value, tumor size, LN metastasis, CR | NLR | [12] |
|
| ||||||||
| 60 | 53 (36–80) | II-III | SCC | CRT | 2.4/2.0 | Age, baseline platelet count | NLR | Current study |
NLR, neutrophil-lymphocyte ratio; FIGO, International Federation of Gynecology and Obstetrics; SCC, squamous cell carcinoma; AC, adenocarcinoma; ASC, adenosquamous cell carcinoma; RT, radiotherapy; CRT, chemoradiotherapy; NCT, neoadjuvant chemotherapy; LN, lymph node; CR, complete response.
The specific mechanisms as to the relationship between high NLR and poor survival of cancer have yet to be identified. There are some points that can be used for interpreting these results. Firstly, pretreatment neutrophil and lymphocyte numbers indicate the level of systemic inflammation. Inflammation is known to change the microenvironment of a tumor and promote angiogenesis and metastasis [21, 23]. Secondly, high-density circulating neutrophils may adversely affect the tumor-bearing host, resulting in a negative association between neutrophil density and patient survival [11]. Circulating lymphocyte has been shown to secrete cytokines, which prevent proliferation and metastasis of tumor cells and have an important function in cytotoxicity [24]. NLR can reflect the balance between host inflammatory response and immune response. The imbalance of NLR could lead to a negative association with oncologic outcome. Thirdly, neutrophils have been proved to contain and secrete vascular endothelial growth factor, IL-18, and matrix metalloproteinases, which directly contribute to tumor-related angiogenesis, tumor growth, and metastasis [25, 26].
In the second part of our study, we examined the RNG levels in LACSCC and its correlation with patient prognosis. We found that high RNG level was significantly correlated with shorter OS and RNG level was a closely independent prognostic factor for OS of patients with LACSCC. RNG level was significantly associated with lymph node metastasis of LACSCC which has also been demonstrated. However, the mechanism underlying these results has not been elucidated yet. A possible explanation is that G-CSFs induced the production of myeloid derived suppressor cells (MDSCs), especially in the sensitive patients [27]. These MDSCs could cause rapid progression of cervical cancer, leukocytosis, and treatment resistance. Mabuchi et al. depleted MDSCs, which succeeded in inhibiting the progression of cervical cancer, leukocytosis and enhancing radiosensitivity [28]. Additionally, as the production of G-CSFs, neutrophil may also contribute to the progression of cervical cancer. An increased neutrophil count in the peripheral blood has been suggested to be a significant prognostic factor in patients with a variety of cancers, including metastatic melanoma, renal cell carcinoma, non-small-cell lung cancer, breast cancer, head and neck carcinoma, and sarcoma [29]. Vascular endothelial growth factor, interleukin-18, and matrix metalloproteinases, secreted by circulating neutrophils, contribute to the tumor-related angiogenesis, tumor growth, and metastasis [30–32].
Some limitations of this study should be noticed. Firstly, this study is limited by its retrospective design and single-institution experience. Secondly, the lack of analysis on the correlation of these findings in the bloodstream with the intratumoral infiltrate is a major limitation of this study. Finally, the cut-off value for NLR was 2.0, which was selected based on its prognostic value in our data set. It needs to be verified in a validation cohort.
In conclusion, this study highlights the potential of NLR and RNG as additional prognostic indicators in patients with LASCC who had been treated with CCCRT. However, the results of current study need to be validated in larger prospective studies in near future.
Acknowledgments
This work was supported by the National Nature Science Foundation of China (Grant no. 81460459).
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Torre L. A., Bray F., Siegel R. L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer Journal for Clinicians. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Haie-Meder C., Morice P., Castiglione M. Cervical cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Annals of Oncology. 2009;20(supplement 4):iv27–iv28. doi: 10.1093/annonc/mdp119. [DOI] [PubMed] [Google Scholar]
- 3.Pfaendler K. S., Tewari K. S. Changing paradigms in the systemic treatment of advanced cervical cancer. American Journal of Obstetrics and Gynecology. 2016;214(1):22–30. doi: 10.1016/j.ajog.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tangjitgamol S., Katanyoo K., Laopaiboon M., Lumbiganon P., Manusirivithaya S., Supawattanabodee B. Adjuvant chemotherapy after concurrent chemoradiation for locally advanced cervical cancer. Cochrane Database of Systematic Reviews. 2014;(12) doi: 10.1002/14651858.CD010401.pub2.CD010401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walsh S. R., Cook E. J., Goulder F., Justin T. A., Keeling N. J. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. Journal of Surgical Oncology. 2005;91(3):181–184. doi: 10.1002/jso.20329. [DOI] [PubMed] [Google Scholar]
- 6.Halazun K. J., Hardy M. A., Rana A. A., et al. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Annals of Surgery. 2009;250(1):141–151. doi: 10.1097/SLA.0b013e3181a77e59. [DOI] [PubMed] [Google Scholar]
- 7.Bhatti I., Peacock O., Lloyd G., Larvin M., Hall R. I. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratio. The American Journal of Surgery. 2010;200(2):197–203. doi: 10.1016/j.amjsurg.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 8.Sarraf K. M., Belcher E., Raevsky E., Nicholson A. G., Goldstraw P., Lim E. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. Journal of Thoracic and Cardiovascular Surgery. 2009;137(2):425–428. doi: 10.1016/j.jtcvs.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 9.Lee Y.-Y., Choi C. H., Kim H.-J., et al. Pretreatment neutrophil:lymphocyte ratio as a prognostic factor in cervical carcinoma. Anticancer Research. 2012;32(4):1555–1561. [PubMed] [Google Scholar]
- 10.Wang D., Wu M., Feng F.-Z., et al. Pretreatment neutrophil-to-lymphocyte and platelet-tolymphocyte ratios do not predict survival in patients with cervical cancer treated with neoadjuvant chemotherapy and radical hysterectomy. Chinese Medical Journal. 2013;126(8):1464–1468. doi: 10.3760/cma.j.issn.0366-6999.20122672. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y., Wang L., Liu Y., et al. Preoperative neutrophil-lymphocyte ratio before platelet-lymphocyte ratio predicts clinical outcome in patients with cervical cancer treated with initial radical surgery. International Journal of Gynecological Cancer. 2014;24(7):1319–1325. doi: 10.1097/IGC.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 12.Mizunuma M., Yokoyama Y., Futagami M., Aoki M., Takai Y., Mizunuma H. The pretreatment neutrophil-to-lymphocyte ratio predicts therapeutic response to radiation therapy and concurrent chemoradiation therapy in uterine cervical cancer. International Journal of Clinical Oncology. 2015;20(5):989–996. doi: 10.1007/s10147-015-0807-6. [DOI] [PubMed] [Google Scholar]
- 13.Jakubowicz J., Blecharz P., Skotnicki P., Reinfuss M., Walasek T., Luczynska E. Toxicity of concurrent chemoradiotherapy for locally advanced cervical cancer. European Journal of Gynaecological Oncology. 2014;35(4):393–399. doi: 10.12892/ejgo24342014. [DOI] [PubMed] [Google Scholar]
- 14.Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. Journal of Clinical Oncology. 2008;26(35):5802–5812. doi: 10.1200/JCO.2008.16.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aapro M. S., Bohlius J., Cameron D. A., et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. European Journal of Cancer. 2011;47(1):8–32. doi: 10.1016/j.ejca.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Foley C., Mackey M. C. Mathematical model for G-CSF administration after chemotherapy. Journal of Theoretical Biology. 2009;257(1):27–44. doi: 10.1016/j.jtbi.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 17.Brooks G., Provencher G., Lei J., Mackey M. C. Neutrophil dynamics after chemotherapy and G-CSF: the role of pharmacokinetics in shaping the response. Journal of Theoretical Biology. 2012;315:97–109. doi: 10.1016/j.jtbi.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 18.Craig M., Humphries A. R., Nekka F., Bélair J., Li J., Mackey M. C. Neutrophil dynamics during concurrent chemotherapy and G-CSF administration: mathematical modelling guides dose optimisation to minimise neutropenia. Journal of Theoretical Biology. 2015;385:77–89. doi: 10.1016/j.jtbi.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Eisenhauer E. A., Therasse P., Bogaerts J., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) European Journal of Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Soria J.-C., Wu Y.-L., Nakagawa K., et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomised trial. The Lancet Oncology. 2015;16(8):990–998. doi: 10.1016/s1470-2045(15)00121-7. [DOI] [PubMed] [Google Scholar]
- 21.Mantovani A., Allavena P., Sica A., Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 22.Diakos C. I., Charles K. A., McMillan D. C., Clarke S. J. Cancer-related inflammation and treatment effectiveness. The Lancet Oncology. 2014;15(11):e493–e503. doi: 10.1016/S1470-2045(14)70263-3. [DOI] [PubMed] [Google Scholar]
- 23.Balkwill F., Mantovani A. Inflammation and cancer: back to Virchow? The Lancet. 2001;357(9255):539–545. doi: 10.1016/s0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 24.Ding P.-R., An X., Zhang R.-X., et al. Elevated preoperative neutrophil to lymphocyte ratio predicts risk of recurrence following curative resection for stage IIA colon cancer. International Journal of Colorectal Disease. 2010;25(12):1427–1433. doi: 10.1007/s00384-010-1052-0. [DOI] [PubMed] [Google Scholar]
- 25.Ardi V. C., Kupriyanova T. A., Deryugina E. I., Quigley J. P. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(51):20262–20267. doi: 10.1073/pnas.0706438104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jablonska E., Puzewska W., Grabowska Z., Jablonski J., Talarek L. VEGF, IL-18 and NO production by neutrophils and their serum levels in patients with oral cavity cancer. Cytokine. 2005;30(3):93–99. doi: 10.1016/j.cyto.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Wesolowski R., Markowitz J., Carson W. E., III Myeloid derived suppressor cells—a new therapeutic target in the treatment of cancer. Journal for Immunotherapy of Cancer. 2013;1, article 10 doi: 10.1186/2051-1426-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mabuchi S., Matsumoto Y., Kawano M., et al. Uterine cervical cancer displaying tumor-related leukocytosis: a distinct clinical entity with radioresistant feature. Journal of the National Cancer Institute. 2014;106(7) doi: 10.1093/jnci/dju147.dju147 [DOI] [PubMed] [Google Scholar]
- 29.Kosumi K., Baba Y., Ishimoto T., et al. Neutrophil/lymphocyte ratio predicts the prognosis in esophageal squamous cell carcinoma patients. Surgery Today. 2016;46(4):405–413. doi: 10.1007/s00595-015-1197-0. [DOI] [PubMed] [Google Scholar]
- 30.Di Carlo E., Forni G., Musiani P. Neutrophils in the antitumoral immune response. Chemical Immunology and Allergy. 2003;83:182–203. doi: 10.1159/000071561. [DOI] [PubMed] [Google Scholar]
- 31.Schaider H., Oka M., Bogenrieder T., et al. Differential response of primary and metastatic melanomas to neutrophils attracted by IL-8. International Journal of Cancer. 2003;103(3):335–343. doi: 10.1002/ijc.10775. [DOI] [PubMed] [Google Scholar]
- 32.Shamamian P., Schwartz J. D., Pocock B. J. Z., et al. Activation of progelatinase A (MMP-2) by neutrophil elastase, cathepsin G, and proteinase-3: a role for inflammatory cells in tumor invasion and angiogenesis. Journal of Cellular Physiology. 2001;189(2):197–206. doi: 10.1002/jcp.10014. [DOI] [PubMed] [Google Scholar]