Abstract
Objectives
To assess the preoperative serum levels of CA 125 with its diagnostic role and to evaluate the p53 expression in patients of primary ovarian neoplasms. We also wished to judge their relationship with other parameters like clinical staging and histopathologic tumor type.
Materials and Methods
The present study was conducted on 86 patients during the study period of 2.5 years. Preoperative CA 125 levels were evaluated by an automated immunoassay analyzer. p53 expression was judged immunohistochemically with pre-diluted monoclonal antibody. An objective scoring was done depending on distinct nuclear immunopositivity.
Results
Median value of preoperative CA 125 levels was 32 U/mL in benign surface epithelial-stromal tumors (BSEST), 53 U/mL in borderline surface epithelial-stromal tumors (BOT), 346 U/mL in malignant surface epithelial-stromal tumors (MSEST) and 560 U/mL in serous adenocarcinomas (SAC). Most of ovarian tumors were in the FIGO stage I (64 cases, 74.4%), but higher stages (II, III, IV) were observed mostly in MSESTs. SACs displayed the maximum p53 expression. Considering the cut-off value of more than 35 U/mL in CA 125 levels, the sensitivity to diagnose MSESTs was 94.7%. Preoperative CA 125 levels strongly and positively correlated with FIGO staging and p53 expression. Similarly p53 expression strongly and positively correlated with FIGO staging and histopathological categories.
Conclusion
Higher values of preoperative CA 125 levels and higher expression p53 are associated with MSESTs and BOTs especially of serous type. They strongly correlate with each other and with tumor stage. But there is no serum CA 125 concentration that can clearly differentiate benign and malignant ovarian masses.
Keywords: CA 125, p53, Ovary, Immunohistochemistry, Epithelial ovarian cancer
Introduction
Ovarian cancer (OC) is one of the leading causes of mortality from gynecologic cancers. There is a significant geographical variation in age-standardized incidence and mortality rates of OC. OC especially the epithelial OCs often remains asymptomatic and clinically undetected in early stages, and thus most of the patients have widespread disease at the time of diagnosis. The need for the development of reliable serum biomarkers for early detection and prognostication of OC, which are both sensitive and specific, remains a long-awaited priority [1]. CA 125, the first and most widely used serum tumor marker test for epithelial cancer of the ovary, was introduced by Bast et al. in 1983 and was recognized by murine monoclonal antibody (OC 125) [2]. It is a high molecular weight glycoprotein which is raised in approximately 90 % of patients with advanced epithelial OC with a reported sensitivity of only 40–60 % in stage I OC. Since its development, measurement of the serum level of the CA 125 antigen has become a standard component of routine management of women with advanced OC. CA 125 levels of less than 35 U/mL are now accepted as normal by most of the authors [1, 3]. Although serum CA 125 levels have also been shown to be elevated often in patients with benign adnexal masses, CA 125 levels have an established role in differential diagnosis of OC, the monitoring of disease status during treatment, and surveillance during follow-up [3, 4].
p53 suppressor gene, located on the short arm of the 17 chromosome, has an essential role in regulation of normal cell cycle, cell cycle arrest, apoptotic response, and initiating carcinogenesis [5]. Mutations of the p53 gene are the most common and most frequently studied molecular alterations in human cancer. Many studies have investigated their significance in diagnosis, prognosis, and treatment in tumors of various sites [6]. The wild-type p53 protein has a shorter half-life of 15–30 min and is rapidly removed from the nucleus, whereas the mutant forms have a prolonged half-life, which favors intranuclear accumulation, becoming detectable immunohistochemically [7]. Although the nucleotide sequencing is the most reliable technique to detect gene mutation, it is labor intensive, time consuming, and therefore, currently has limited application in clinical pathology practice. Immunohistochemical analysis of p53 expression is therefore commonly used as a surrogate for mutational analysis [6]. Mutations of the p53 gene, as determined by mutation analysis and/or positive immunohistochemical staining for p53, are common in OC and have been associated with poor clinical outcome [7].
The present study was conducted to assess the preoperative serum levels of CA 125 by immunoassay with its diagnostic role in malignant epithelial neoplasms and the p53 expression by immunohistochemistry (IHC) in patients diagnosed with primary ovarian neoplasms along with relevant clinicopathological parameters. We also wished to evaluate their relationship with other parameters like clinical staging and histopathologic tumor type.
Materials and Methods
The present single-center, prospective, cross-sectional, analytical study was conducted on 86 patients of primary ovarian neoplasms in the Department of Pathology in association with the Department of Gynecology and Obstetrics after obtaining the proper approval from ethical committee of the institution and informed consent from the patients, during the study period of 2.5 years (01.07.2010–31.12.2012). All the clinically and radiologically suspected cases, who were postoperatively confirmed of having primary ovarian neoplasms on histopathologic examination during the study period, were included in the study.
Preoperative blood samples were collected between 1 and 7 days before surgery. The samples were allowed to clot at room temperature completely and were then centrifuged at 3,000 rpm for 5 min. Serum was separated, aliquoted, and stored at −20° C until analyzed. The sera were evaluated for CA 125 levels by an automated chemiluminescence immunoassay analyzer (ARCHITECT i2000, Abbott Diagnostics, Abbott Park, IL) using the principle of CMIA (chemiluminescent microparticle immunoassay) to measure analyte concentrations in the samples. In addition to relevant clinical features and staging according to FIGO criteria, detailed histopathologic examination was carried on Hematoxylin and Eosin(H and E)-stained sections of 3–5-µm thickness prepared from formalin-fixed, paraffin-embedded (FFPE) tissue received following cytoreductive surgery. Tumors were classified morphologically according to the current World Health Organization (WHO) system.
Immunohistochemical staining was done for p53 with pre-diluted ready-to-use monoclonal mouse IgG2b antibody (DO7, BioGenex, USA) on FFPE tissue sections mounted on poly-l-Lysine-coated slides. Following the process of baking, dewaxing, and rehydration, antigen retrieval was carried out by means of pressure cooking. Incubation with monoclonal primary antibody in humidifying chamber was done for about 2 h at room temperature after using peroxide block. Secondary antibody conjugated with horseradish peroxidase enzyme (Polymer-HRP) was applied for 30 min. Thereafter, di-amino benzidine (DAB) was added for 10 min as a substrate chromogen solution to produce a brown color reacting with the plenty of HRP molecules attached with the polymer. Finally, the slides were immersed in Harris Hematoxylin for counterstaining. Nuclear staining was considered a positive reaction. Scoring for p53 expression was based on the proportion of cells in a given tumor specimen exhibiting distinct nuclear immunopositivity. The p53 scoring results were transformed into a six-tiered scale—0 (negative or occasional positive cells), 1 + (<10 % cells positive), 2 + (10–25 % cells positive), 3 + (26–50 % cells positive), 4 + (51–75 % cells positive), or 5 + (>75 % cells positive).
Statistical Analysis
Continuous variables were presented with mean ± standard deviation (SD), median, minimum, and maximum values. Categorical variables were presented as frequencies and percentages. Diagnostic role was assessed by sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy. Association, correlation, and one-way analysis of variance (ANOVA) were judged by Chi-square test, Spearman’s and Kendall’s tau-b rank correlation, one-way ANOVA, and Kruskal–Wallis test, respectively. But the results of Chi-square test are not included as the assumption of Chi-square test, i.e., 80 % of the expected cell frequencies should exceed 5 and all expected cell frequencies should exceed 1, was not satisfied. P value of ≤0.05 was considered for statistical significance. All the analyses were done using IBM SPSS statistics software, version 20 and MedCalc software, version 12.3.0.0.
Results
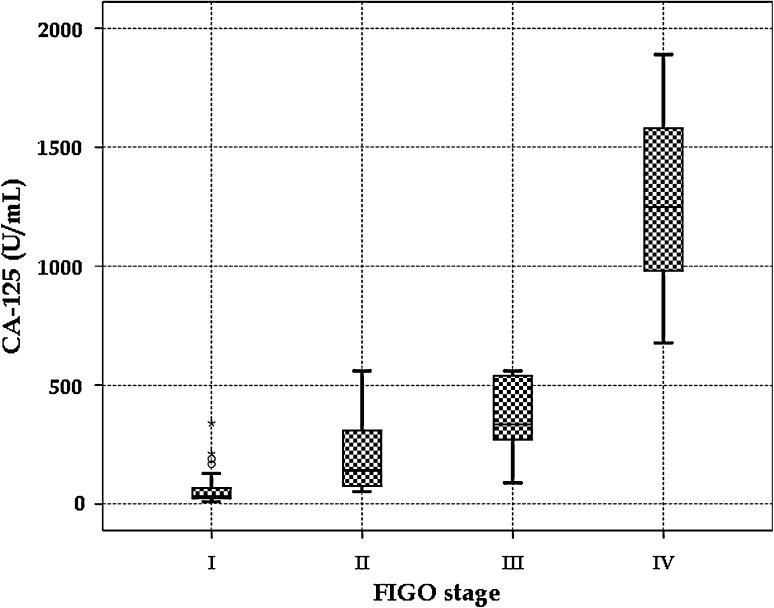
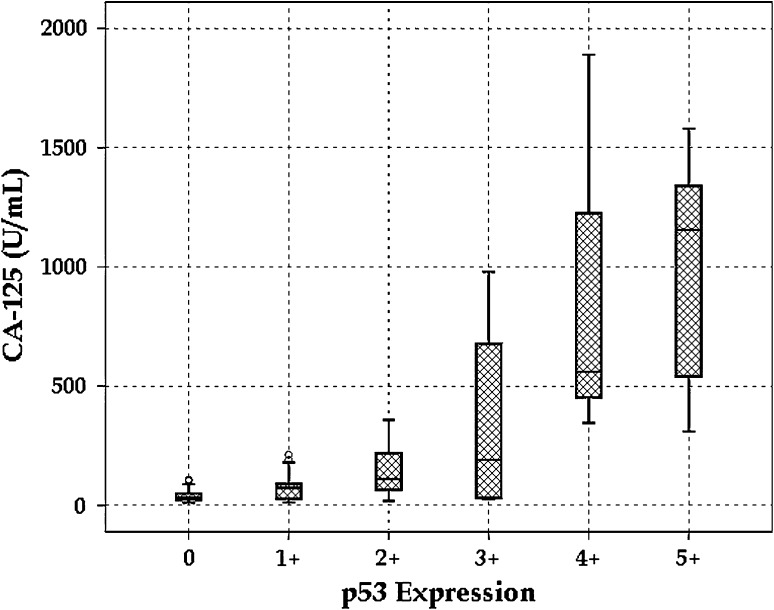
A total of 86 cases of primary ovarian neoplasms with a median age of 39 years were included in the study during the period of 2.5 years, of which 31 cases (36 %) were diagnosed as benign surface epithelial-stromal tumors (BSEST), five cases (5.8 %) as borderline surface epithelial-stromal tumors (BOT), 19 cases (22.1 %) as malignant surface epithelial-stromal tumors (MSEST), 27 cases (31.4 %) as germ cell tumors (GCT), and only four cases (4.7 %) were diagnosed as sex cord-stromal tumors (SCT). Although the median value of preoperative CA 125 levels was 56 U/mL in the total study group, it was 32 U/mL in BSESTs, 53 U/mL in BOT, 346 U/mL in MSESTs, and 560 U/mL in Serous adenocarcinomas (SAC). Most of ovarian tumors were in the FIGO stage I (64 cases, 74.4 %), but higher stages (II, III, IV) were observed in MSESTs, BOTs, and GCTs. Similarly, maximum number (42 cases, 48.8 %) of ovarian tumors immunohistochemically failed to display p53 expression. Higher levels (3 +, 4 + and 5 +) of p53 were expressed mostly in MSESTs, of which SAC displayed the maximum p53 expression (Table 1, Fig. 1 and 2). Considering the cut-off value of more than 35 U/mL in CA 125 levels, the sensitivity to diagnose MSESTs was 94.7 %, whereas the sensitivity to distinguish SACs from others was 100 %. It was 87.5 % and 93.8 %, respectively, if we include BOTs in the MSESTs, as the actual nature of the tumor was not established in BOTs. But the specificities as well as positive predictive values or precisions in all the cases were not more than 50 % reflecting the effect of high false positive values. Similarly, negative predictive values were more than 90 % as there was very small number of false negative cases. It was also noticed that the maximum diagnostic accuracy was 60.5 % (Table 2). On correlation analyses, preoperative CA 125 levels strongly and positively correlated with FIGO staging (Fig. 3) and p53 expression (Fig. 4). Similarly, p53 expression strongly and positively correlated with FIGO staging and histopathologic categories if it was arranged in the following order—SCTs, GCTs, BSESTs, BOTs, and MSESTs. But there was weak correlation between CA 125 levels and histopathologic categories. Analysis of variance judged parametrically by one-way ANOVA and non-parametrically by Kruskal–Wallis test revealed highly significant (P = 0.000) differences in CA 125 levels in different histopathologic categories, FIGO stages, and levels of p53 expression (Table 3).
Table 1.
Age distribution, preoperative CA 125 levels, FIGO staging, and p53 expression in different primary ovarian neoplasms
| Histological diagnosis | No. of cases (Column %) | Age at diagnosis (year) | Preoperative CA 125 level (U/ml) | FIGO staging (Row %) | p53 Expression (Row %) |
|---|---|---|---|---|---|
| All ovarian tumors | 86 (100.0) | 40.1 ± 14.0 Median: 39 Min: 12 Max: 79 |
178.0 ± 340.2 Median: 56.0 Min: 11.3 Max: 1890.0 |
I: 64 (74.4) II: 10 (11.6) III: 6 (7.0) IV: 6 (7.0) |
0: 42 (48.8) 1 + : 14 (16.3) 2 + : 16 (18.6) 3 + : 5 (5.8) 4 + : 4 (4.7) 5 + : 5 (5.8) |
| I. Benign Surface epithelial-stromal tumors (BSEST) | 31 (36.0) | 38.1 ± 6.9 Median: 39 Min: 22 Max: 49 |
60.6 ± 54.1 Median: 32.0 Min: 12.6 Max: 212.0 |
I: 31 (100.0) II: 0 (0.0) III: 0 (0.0) IV: 0 (0.0) | 0: 16 (51.6) 1 + : 10 (32.3) 2 + : 5 (16.1) 3 + : 0 (0.0) 4 + : 0 (0.0) 5 + : 0 (0.0) |
| A. Serous cystadenoma | 19 (22.1) | 41.2 ± 4.8 Median: 41 Min: 32 Max: 49 |
72.6 ± 62.9 Median: 45.0 Min: 12.6 Max: 212.0 |
I: 19 (100.0) | 0:7 (36.8) 1 + : 7 (36.8) 2 + : 5 (26.3) |
| B. Mucinous cystadenoma | 11 (12.8) | 32.6 ± 6.9 Median: 34 Min: 22 Max: 42 |
43.7 ± 29.9 Median: 31.0 Min: 21.3 Max: 108.0 |
I: 11 (100.0) | 0: 8 (72.7) 1 + : 3 (27.3) |
| C. Brenner tumor* | 1 (1.2) | 39 | 18.2 | I: 1 (100.0) | 0: 1 (100.0) |
| II. Borderline Surface epithelial-stromal tumors (BOT) | 5 (5.8) | 48.6 ± 9.9 Median: 43 Min: 39 Max: 62 |
104.4 ± 143.1 Median: 53.0 Min: 22.3 Max: 359.5 |
I: 2 (40.0) II: 2 (40.0) III: 1 (20.0) IV: 0 (0.0) | 0: 0 (0.0) 1 + : 2 (40.0) 2 + : 2 (40.0) 3 + : 1 (20.0) 4 + : 0 (0.0) 5 + : 0 (0.0) |
| A. Borderline serous tumor* | 2 (2.3) | 56 & 62 | 31.0 & 359.0 | I: 1 (50.0) III: 1 (50.0) | 2 + : 1 (50.0) 3 + : 1 (50.0) |
| B. Borderline mucinous tumor | 3 (3.5) | 41.7 ± 2.3 Min: 39 Max: 43 |
44.0 ± 19.2 Min: 22.4 Max: 57.0 |
I: 1 (33.3) II: 2 (66.7) | 1 + : 2 (66.7) 2 + : 1(33.3) |
| III. Malignant Surface epithelial-stromal tumors (MSEST) | 19 (22.1) | 59.3 ± 8.7 Median: 59 Min: 46 Max: 79 |
586.3 ± 551.8 Median: 346.0 Min: 24.0 Max: 1890.0 |
I: 2 (10.5) II: 6 (31.6) III: 5 (26.3) IV: 6 (31.6) | 0: 1 (5.3) 1 + : 1 (5.3) 2 + : 4 (21.1) 3 + : 4 (21.1) 4 + : 4 (21.1) 5 + : 5 (26.3) |
| A. Serous adenocarcinoma | 14 (16.3) | 61.7 ± 8.3 Median: 60 Min: 48 Max: 79 |
765.1 ± 538.2 Median: 560.0 Min: 190.0 Max: 1890.0 |
II: 4 (28.6) III: 4 (28.6) IV: 6 (42.9) | 2 + : 2 (14.3) 3 + : 3 (21.4) 4 + : 4 (28.6) 5 + : 5 (35.7) |
| B. Mucinous adenocarcinoma* | 2 (2.3) | 46 & 49 | 24.1 & 58.0 | I: 2 (100.0) | 0: 1 (50.0) 3 + : 1 (50.0) |
| C. Endometrioid adenocarcinoma* | 2 (2.3) | 49 & 59 | 89.0 & 180.0 | II: 1 (50.0) III: 1 (50.0) | 1 + : 1 (50.0) 2 + : 1 (50.0) |
| D. Clear cell adenocarcinoma* | 1 (1.2) | 58 | 76.6 | II: 1 (100.0) | 2 + : 1 (100.0) |
| IV. Germ cell tumors (GCT) | 27 (31.4) | 27.0 ± 6.4 Median: 27 Min: 12 Max: 39 |
60.5 ± 64.5 Median: 43.0 Min: 11.4 Max: 340.6 |
I: 25 (92.6) II: 2 (7.4) III: 0 (0.0) IV: 0 (0.0) | 0: 21 (77.8) 1 + : 1 (3.7) 2 + : 5 (18.5) 3 + : 0 (0.0) 4 + : 0 (0.0) 5 + : 0 (0.0) |
| A. Mature cystic teratoma | 17 (19.8) | 28.6 ± 6.8 Median: 31 Min: 12 Max: 39 |
40.5 ± 25.3 Median: 32.0 Min: 11.4 Max: 102.0 |
I: 17 (100.0) | 0: 17 (100.0) |
| B. Immature teratoma* | 1 (1.2) | 16 | 56.0 | I: 1 (100.0) | 2 + : 1 (100.0) |
| C. Dysgerminoma | 3 (3.5) | 24.7 ± 3.8 Min: 22 Max: 29 |
72.0 ± 42.0 Min: 24.0 Max: 102.0 |
I: 2 (66.7) II: 1 (33.3) | 0: 1 (33.3) 1 + : 1 (33.3) 2 + : 1 (33.3) |
| D. Yolk sac tumor | 3 (3.5) | 25.3 ± 2.9 Min: 22 Max: 27 |
34.3 ± 18.7 Min: 23.2 Max: 56.0 |
I: 3 (100.0) | 0: 3 (100.0) |
| E. Embryonal carcinoma* | 1 (1.2) | 19 | 340.6 | I: 1 (100.0) | 2 + : 1 (100.0) |
| F. Mixed germ cell tumor* | 2 (2.3) | 26 & 31 | 102.0 &128.0 | I: 1 (50.0) II: 1 (50.0) | 2 + : 2 (100.0) |
| V. Sex cord-stromal tumors (SCT) | 4 (4.7) | 43.0 ± 8.5; Min: 31 Max: 49 |
33.5 ± 13.1 Min: 16.8 Max: 45.5 |
I: 4 (100.0) II: 0 (0.0) III: 0 (0.0) IV: 0 (0.0) | 0: 4 (100.0) 1 + : 0 (0.0) 2 + : 0 (0.0) 3 + : 0 (0.0) 4 + : 0 (0.0) 5 + : 0 (0.0) |
| A. Adult granulosa cell tumor* | 2 (2.3) | 48 & 49 | 31.3 & 45.0 | I: 2 (100.0) | 0: 2 (100.0) |
| B. Sertoli-Leydig cell tumor* | 1 (1.2) | 31 | 42.0 | I: 1 (100.0) | 0: 1 (100.0) |
| C. Fibroma* | 1 (1.2) | 43 | 16.3 | I: 1 (100.0) | 0: 1 (100.0) |
Median calculated for sample size ≥5; Figures in parentheses are column or row percentages
* Exact values are written in case of small number (<3) of observations
Fig. 1.
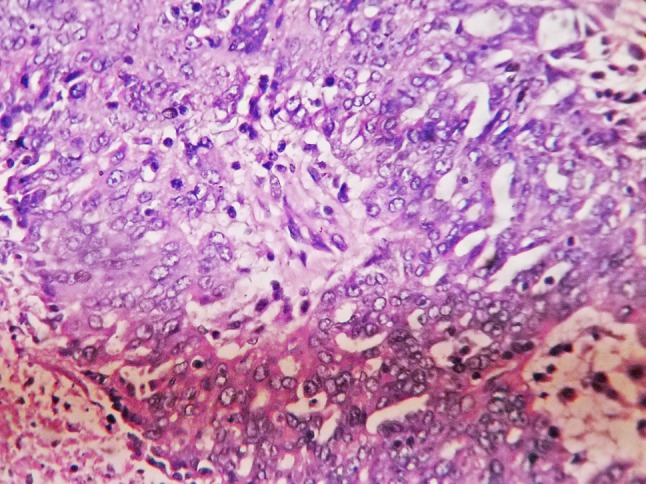
Photomicrograph of serous adenocarcinoma of ovary (H and E, ×400)
Fig. 2.
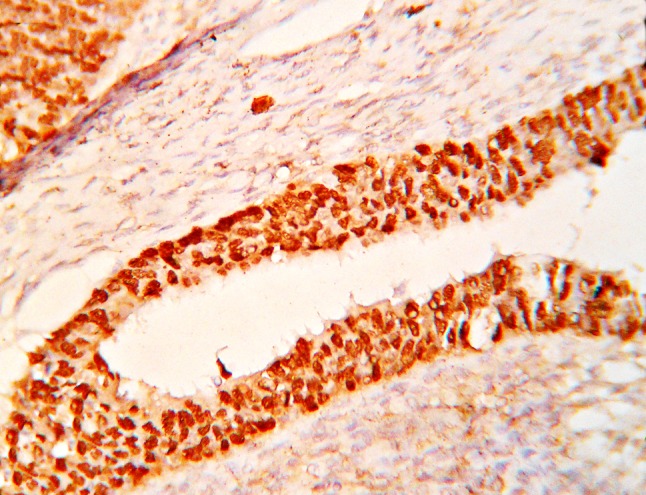
Photomicrograph showing p53 expression in serous adenocarcinoma of ovary (IHC, ×400)
Table 2.
Diagnostic statistics of CA 125 levels in different histopathologic diagnoses considering the differential value of >35 U/mL
| Histopathologic diagnostic categories | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | Diagnostic accuracy |
|---|---|---|---|---|---|
| Malignant Surface epithelial-stromal tumors (MSEST) | 94.7 | 49.3 | 34.6 | 97.1 | 59.3 |
| Serous adenocarcinoma | 100.0 | 47.2 | 26.9 | 100.0 | 55.8 |
| Mucinous adenocarcinoma* | 50.0 | 39.3 | 1.9 | 97.1 | 39.5 |
| Malignant surface epithelial-stromal tumors (MSEST) + Borderline surface epithelial-stromal tumors (BOT) | 87.5 | 50.0 | 40.4 | 91.2 | 60.5 |
| Serous adenocarcinoma + Borderline serous tumor | 93.8 | 47.1 | 28.9 | 97.1 | 55.8 |
| Mucinous adenocarcinoma + Borderline mucinous tumor* | 60.0 | 39.5 | 5.8 | 94.1 | 40.7 |
* Values may be unreliable due to the small number of observations
Fig. 3.
Box–Whisker plot showing distribution of CA 125 in relation to FIGO staging
Fig. 4.
Box–Whisker plot showing distribution of CA 125 in relation to p53 expression
Table 3.
Correlation analysis and one-way analysis of variance in different parameters of ovarian neoplasms
| Statistical approach | Statistical test | CA 125 versus HP categories | CA 125 versus FIGO staging | p53 versus CA 125 | p53 versus HP categories | p53 versus FIGO staging |
|---|---|---|---|---|---|---|
| Correlation analyses | Spearman’s rank correlation | ρ = 0.476 P = 0.000 |
ρ = 0.675 P = 0.000 |
ρ = 0.639 P = 0.000 |
ρ = 0.674 P = 0.000 |
ρ = 0.707 P = 0.000 |
| Kendall’s tau-b rank correlation | τ = 0.365 P = 0.000 |
τ = 0.568 P = 0.000 |
τ = 0.523 P = 0.000 |
τ = 0.602 P = 0.000 |
τ = 0.642 P = 0.000 |
|
| One-way analysis of variance | Parametric: One-way ANOVA | F = 14.335 P = 0.000 |
F = 148.688 P = 0.000 |
F = 26.852 P = 0.000 |
N/A* | N/A* |
| Non-parametric: Kruskal–Wallis test | χ2 = 30.396 P = 0.000 |
χ2 = 39.169 P = 0.000 |
χ2 = 37.532 P = 0.000 |
N/A* | N/A* |
* ANOVA is not applicable (N/A) in case of two categorical variables
Discussion
CA 125 antigen is a cell membrane glycoprotein expressed by various types of epithelial cells, and it is present in patients with a variety of cancers namely breast, endometrium, gastrointestinal tract, and lung in addition to the OC as well as in benign diseases of the uterus, liver, and gastrointestinal tract and benign tumors of the ovary and uterus [2]. Serum CA 125 level is a strong prognostic factor for overall survival and progression-free survival in OC. There is an inverse relationship between serum CA 125 levels and survival in OC. Serum levels of CA 125 are used to monitor responses to chemotherapy, relapse, and disease progression in OC patients. CA 125 can be elevated in the serum even before clinical development of primary and recurrent OC [1]. Mogensen et al. [8] studied on 184 female patients presenting with pelvic masses, of which ovarian tumors were diagnosed in 151 cases (91 carcinomas, 8 borderline, 52 benign). They concluded that increased preoperative CA 125 levels in patients with pelvic masses are highly suggestive of a malignant tumor and CA 125 should be an adjunct to the preoperative diagnostic armamentarium. The CA 125 measurements were also correlated to FIGO stage and histopathologic diagnosis in the 91 OCs. The study by Chen et al. [9] suggests that defining positive serum CA 125 levels as those greater than 35 U/mL is of limited clinical value because there is a high (39.9 %) false positive rate in patients with benign disease. The sensitivity, specificity, diagnostic accuracy, and positive predictive value of CA 125 to diagnose epithelial OC was 100, 60.1, 66.7, and 33.0 %, respectively. Similarly, the present study revealed high sensitivity and negative predictive value owing to few false negative cases and low specificity and positive predictive value due to high false positive cases.
Cruickshank and co-workers [10] observed a significant correlation of CA 125 level with FIGO stage at presentation. Elevated CA 125 levels were found in patients with all types of epithelial tumors, SCTs, and even in Krukenberg tumors. We too observed strong positive (>0.5) correlations of CA 125 levels with FIGO staging and p53 expressions.
Information regarding the association of preoperative tumor marker findings and BOT is very limited. To date, the diagnosis of a BOT cannot be made solely with CA 125 measurements because of the considerable variability and overlap between patients with OC and healthy individuals. The high rate of false positive results make interpretation of CA 125, alone, difficult in gynecological patients. Therefore, combination with sonography and clinical exam remains essential for diagnosis and decision making [11]. Altaras et al. [12] experienced that the CA 125 is an invaluable indicator of the clinical status of the OC patients and opined that it could be a new tumor marker in patients with malignant GCTs. Our study also supports that elevated serum levels could be observed in cases of BSESTs, BOTs, GCTs, and SCTs in addition to MSESTs.
Detection of p53 in tumors by immunohistochemistry has been considered to be an indicator of p53 mutations because missense p53 mutations may stabilize p53. However, caution should be used in equating positive p53 immunohistochemistry in tumors with p53 mutation because accumulation of p53 in tumors has been observed without evidence of p53 mutation [13]. The study by Henriksen et al. [14] revealed that p53 gene may be involved in tumorigenesis, as its expression was detected in both borderline and malignant tumors, while normal ovaries and benign ovarian tumors were unstained with the p53 antibody. The presence of p53 was also related to dissemination of disease, residual tumor bulk, and poor differentiation as well as the presence of the proliferation variable like Ki-67. Høgdall et al. [7] observed a significant increase in the frequency of p53 tissue expression in OC with increasing FIGO stage (P < 0.00001). Multivariate Cox regression analysis found that less than 20 % tissue expression of p53 was associated with longer OC disease-specific survival. O’Neill et al. [15] noticed a statistically significant higher expression of p53 in high-grade compared with low-grade serous OCs. Giurgea et al. [5] found almost similar frequency of immunostaining in borderline tumors and low-grade invasive serous carcinomas in contrast to the significantly higher frequency of p53 mutations in high-grade serous carcinomas.
The present study clearly depicts that higher expression of p53 was mostly associated with serous type of MSESTs and BOTs. And the overexpression was strongly correlated with the stage of the disease.
Conclusion
Higher values of preoperative CA 125 levels are associated with MSESTs and BOTs especially of serous type. CA 125 levels also correlate strongly with tumor stage at initial diagnosis and with p53 expression. Similarly, higher expression p53 strongly correlates with the tumor stage and histopathologic diagnostic categories i.e., overexpression of p53 observed more in MSESTs than BSESTs or BOTs. Noticeably, serous adenocarcinomas displayed higher p53 expression than others.
But there is no serum CA 125 concentration that can clearly differentiate benign and malignant ovarian masses due to a significant number of false positive cases leading to low specificity and low positive predictive value, and therefore caution is needed in the interpretation of the results and it should be considered as an adjunct to other investigations.
Compliance with ethical requirements and Conflict of interest
The present study was conducted on patients of primary ovarian neoplasms in the Department of Pathology in association with the Department of Gynecology and Obstetrics after obtaining the proper approval from ethical committee of the institution and informed consent from the patients, during the study period of 2.5 years (01.07.2010–31.12.2012). The authors declare that they have no conflict of interest.
Dr. Ranjan Kumar Tiwari
worked as a post graduate trainee in the Department of Pathology in Institute of Post Graduate Medical Education and Research, Kolkata, India during 2010–2013. He is currently working as a fellow in the Department of Pediatric Oncology at Tata Medical Center, Kolkata.
References
- 1.Gupta D, Lis CG. Role of CA125 in predicting ovarian cancer survival—a review of the epidemiological literature. J Ovarian Res. 2009;2:13. doi: 10.1186/1757-2215-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fritsche HA, Bast RC. CA 125 in ovarian cancer: advances and controversy. Clin Chem. 1998;44:1379–1380. [PubMed] [Google Scholar]
- 3.Kolwijck E, Thomas CM, Bulten J, et al. Preoperative CA-125 levels in 123 patients with borderline ovarian tumors: a retrospective analysis and review of the literature. Int J Gynecol Cancer. 2009;19:1335–1338. doi: 10.1111/IGC.0b013e3181a83e04. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs IJ, Skates S, Davies AP, et al. Risk of diagnosis of ovarian cancer after raised serum CA 125 concentration: a prospective cohort study. BMJ. 1996;313:1355–1358. doi: 10.1136/bmj.313.7069.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giurgea LN, Ungureanu C, Mihailovici MS. The immunohistochemical expression of p53 and Ki67 in ovarian epithelial borderline tumors. Correlation with clinicopathological factors. Rom J Morphol Embryol. 2012;53:967–973. [PubMed] [Google Scholar]
- 6.Yemelyanova A, Vang R, Kshirsagar M, et al. Immunohistochemical staining patterns of p53 can serve as a surrogate marker for TP53 mutations in ovarian carcinoma: an immunohistochemical and nucleotide sequencing analysis. Mod Pathol. 2011;24:1248–1253. doi: 10.1038/modpathol.2011.85. [DOI] [PubMed] [Google Scholar]
- 7.Høgdall EV, Christensen L, Høgdall CK, et al. Distribution of p53 expression in tissue from 774 Danish ovarian tumour patients and its prognostic significance in ovarian carcinomas. APMIS. 2008;116:400–409. doi: 10.1111/j.1600-0463.2008.00917.x. [DOI] [PubMed] [Google Scholar]
- 8.Mogensen O, Mogensen B, Jakobsen A. CA 125 in the diagnosis of pelvic masses. Eur J Cancer Clin Oncol. 1989;25:1187–1190. doi: 10.1016/0277-5379(89)90413-6. [DOI] [PubMed] [Google Scholar]
- 9.Chen DX, Schwartz PE, Li XG, et al. Evaluation of CA 125 levels in differentiating malignant from benign tumors in patients with pelvic masses. Obstet Gynecol. 1988;72:23–27. [PubMed] [Google Scholar]
- 10.Cruickshank DJ, Fullerton WT, Klopper A. The clinical significance of pre-operative serum CA 125 in ovarian cancer. Br J Obstet Gynaecol. 1987;94:692–695. doi: 10.1111/j.1471-0528.1987.tb03177.x. [DOI] [PubMed] [Google Scholar]
- 11.Lenhard MS, Nehring S, Nagel D, et al. Predictive value of CA 125 and CA 72-4 in ovarian borderline tumors. Clin Chem Lab Med. 2009;47:537–542. doi: 10.1515/CCLM.2009.134. [DOI] [PubMed] [Google Scholar]
- 12.Altaras MM, Goldberg GL, Levin W, et al. The value of cancer antigen-125 as a tumor marker in malignant germ cell tumors of the ovary. Gynecol Oncol. 1986;25:150–159. doi: 10.1016/0090-8258(86)90096-X. [DOI] [PubMed] [Google Scholar]
- 13.DiCioccio RA, Werness BA, Peng R, et al. Correlation of TP53 mutations and p53 expression in ovarian tumors. Cancer Genet Cytogenet. 1998;105:93–102. doi: 10.1016/S0165-4608(98)00011-9. [DOI] [PubMed] [Google Scholar]
- 14.Henriksen R, Strang P, Wilander E, et al. p53 expression in epithelial ovarian neoplasms: relationship to clinical and pathological parameters, Ki-67 expression and flow cytometry. Gynecol Oncol. 1994;53:301–306. doi: 10.1006/gyno.1994.1138. [DOI] [PubMed] [Google Scholar]
- 15.O’Neill CJ, Deavers MT, Malpica A, et al. An immunohistochemical comparison between low-grade and high-grade ovarian serous carcinomas: significantly higher expression of p53, MIB1, BCL2, HER-2/neu, and C-KIT in high-grade neoplasms. Am J Surg Pathol. 2005;29:1034–1041. [PubMed] [Google Scholar]