Dear Editor,
As a mitochondrial deacetylase, SIRT3 deacetylates many enzymes involved in central metabolism and maintains mitochondrial proteostasis (Verdin et al., 2010; Papa and Germain, 2014). Substrates of SIRT3 include components of the respiratory complexes, proteins involved in fatty acid oxidation and TCA cycle (Yu et al., 2012). SIRT3 activates MnSOD to maintain reactive oxygen species (ROS) homeostasis and a loss of SIRT3 contributes to the age-associated diseases (McDonnell et al., 2015; Qiu et al., 2010). SIRT3 plays dual roles functioning as a tumor suppressor or a promoter in tumorigenesis and progression (Alhazzazi et al., 2011). On one hand, SIRT3 regulates the cellular ROS level and maintains genomic stability, and mediates metabolic reprogramming to prevent tumorigenesis (Finley and Haigis, 2012). As a result, the low expression of SIRT3 has been found in breast cancer, glioblastoma, colon cancer, osteosarcoma, prostate, and ovarian cancers (Kim et al., 2010; Finley and Haigis, 2012). On the other hand, SIRT3 is a prosurvival factor that modulates p53 activities and is upregulated in oral cancer, the node-positive breast cancer, and bladder cancer (Ashraf et al., 2006; Alhazzazi et al., 2011). These results suggest that SIRT3 possesses the tumor-type dependent function and its precise role needs to be elucidated in the context of a specific cancer. Clear cell renal cell carcinoma (ccRCC) is the most common histological subtype of renal cancer (Cohen and McGovern, 2005). The aims of the present study were to examine the expression of SIRT3 in ccRCC and to characterize effects of SIRT3 on tumorigenesis and progression using 293T human embryonic kidney cells as the model system that has cancer stem cell-like features (Debeb et al., 2010).
Equal amounts of proteins extracted from 18 paired ccRCC lesions and associated pericarcinous tissue samples were analyzed by Western blotting and the representative Western blot images of eight paired samples were shown in Fig. 1A, indicating that the expression levels of SIRT3 were lower in ccRCC than those in normal tissues. The gray scale analysis of the Western blot data for all eighteen paired samples showed that the SIRT3 expression was statistically down-regulated in ccRCC tissues (Fig. 1B), suggesting that the low expression of SIRT3 is important for ccRCC progression.
Figure 1.
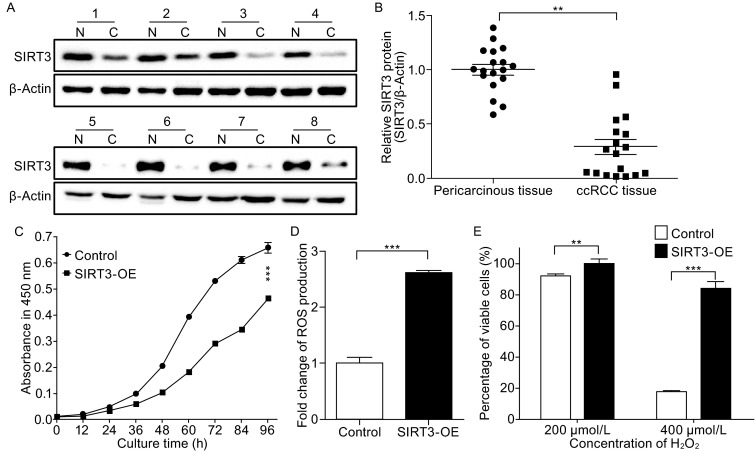
Downregulation of SIRT3 in ccRCC compared to associated pericarcinous tissues and characterization of SIRT3 overexpression cells. (A) Representative Western blot images of the expression levels of SIRT3 of eight paired samples, N (pericarcinous tissue), C (ccRCC tissue). (B) The gray scale analysis of SIRT3 presented in (A). (C) Growth curve of SIRT3-OE and the control cells. (D) Graphical representation of ROS levels of SIRT3-OE cells compared to the control cells. and (E) Survival rate of SIRT3-OE cells and the control cells treated with different concentration of H2O2 for 12 h. Data were analyzed using student’s t-test. *P < 0.05, **P < 0.01 and ***P < 0.001. *P < 0.05 is considered statistically significant. Error bars represent ±SEM
To understand the role of SIRT3 in tumorigenesis and progression of ccRCC, stable cells overexpressing SIRT3 were established in 293T cells. The overexpression of SIRT3 in 293T cells (SIRT3-OE) was examined by Western blotting (Fig. S1), confirming that the expression level of SIRT3 in SIRT3-OE cells was four fold higher than that in control cells. The SIRT3 overexpression in 293T led to a decrease in proliferation rates (Fig. 1C). The ROS level in SIRT3-OE cells is two and half fold higher than that in the control cells as detected using the CellROX® Deep Red kit (Fig. 1D). To determine the susceptibility of SIRT3-OE cells to oxidative stress, cells were treated with various concentrations of hydrogen peroxide for 12 h. The cell viability was measured using CCK-8 assay. The effects of hydrogen peroxide were represented as the percentage of viable cells after 12 h treatment (Fig. 1E). When cells were treated with 400 µmol/L H2O2 for 12 h, the percentages of viable cells were 20% and 90% for the control and SIRT3-OE cells, respectively (Fig. 1E). This declares that SIRT3-OE cells are more resistant to H2O2 treatment.
Next, proteomic analysis was carried out on SIRT3-OE and control cells in biological replicates. Equal amounts of proteins from SIRT3-OE and the control cells were in-solution digested and labeled with TMT reagents. The generated tryptic peptides were fractionated using off-line HPLC and each fraction was further analyzed by nano-LC-MS/MS. Differentially expressed proteins were identified and quantified using the TMT-based quantification. We identified 7536 proteins in two biological replicates and the false-positive rate was estimated to be less than 1%. Based on the average reporter ion ratios (>1.5 or <0.67), 188 proteins were found to be differentially expressed between SIRT3-OE and control cells, in which 93 proteins were down-regulated and 95 were up-regulated (Tables S1 and S2). To understand the biological relevance of the differentially expressed proteins, the Gene Ontology (GO) was used to cluster differentially expressed proteins according to their associated biological processes. The annotations of gene lists are summarized via a pie plot based on the functional classification from Panther as shown in Fig. 2A. One hundred and eighty eight differentially expressed proteins participated in a variety of cellular processes including metabolic process, cellular process, and cellular component organization process. The primary metabolic process shows the dominant difference between SIRT3-OE and the control cells. About 25% of the down-regulated proteins are classified as mitochondrial proteins, indicating that SIRT3 overexpression has a great impact on the mitochondrial protein expressions. Five subunits of respiratory complex IV were down-regulated in SIRT3-OE cells including COX7C, COX6A1, COX7A2, COA7, and COA5 (Fig. 2B), suggesting that the SIRT3 overexpression disrupted the integrity of the respiratory complexes. We also identified that three proteins in the fatty acid β-oxidation pathway were downregulated in SIRT3-OE cells including enoyl-CoA hydratase, very long-chain specific acyl-CoA dehydrogenase and hydroxyacyl-coenzyme A dehydrogenase. More importantly, proteomic analysis showed that nine subunits of mitochondrial ribosomes were downregulated in SIRT3-OE cells (Fig. 2B). All these results indicated that SIRT3-overexpression disrupted mitochondrial proteostasis and contributed to mitochondrial dysfunction.
Figure 2.
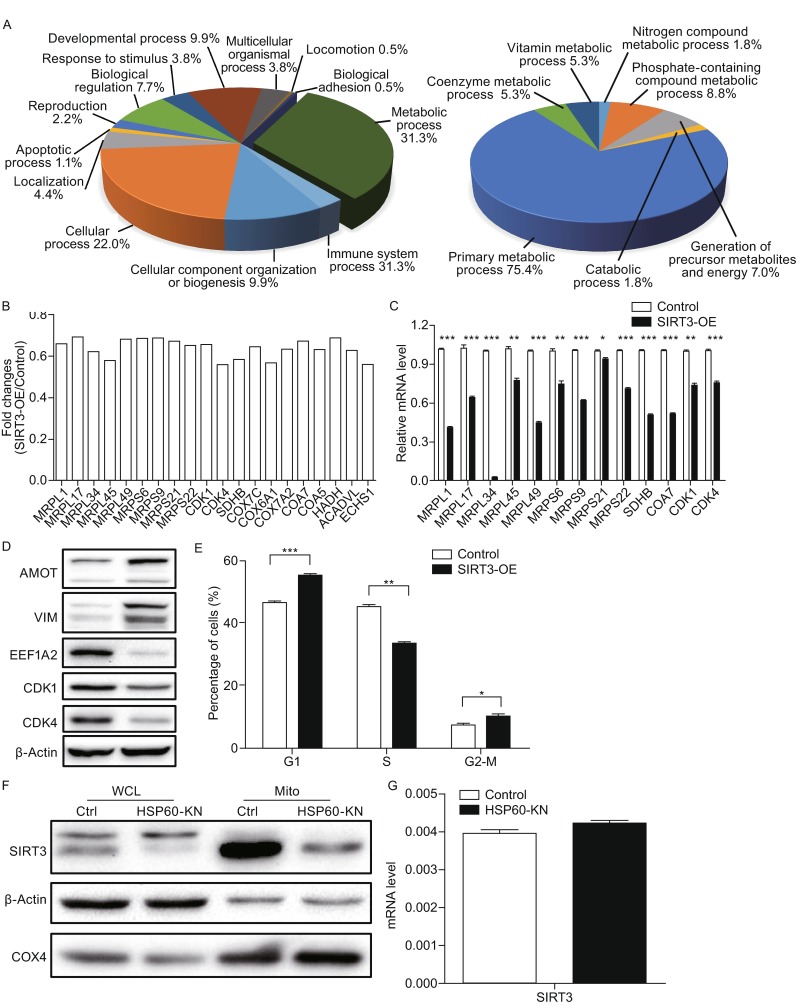
Proteomic, qPCR and Western blot analysis of differentially expressed proteins between SIRT3-OE and control cells. (A) GO analysis of the differentially expressed proteins in SIRT3-OE cells compared to the control cells with PANTHER (http://www.pantherdb.org). (B) Graphical representation of TMT ratios for proteins in SIRT3-OE cells compared to control cells. (C) qPCR analysis of mRNA expressions of selected mitochondrial ribosomal genes and other selected genes from SIRT3-OE cells and control cells. (D) Western blot analysis of selected proteins from SIRT3-OE and control cells. (E) Cell cycle analysis of SIRT3-OE cells and the control cells. (F) Western blotting images showing expression level of SIRT3 in HSP60-KN cells compared to the control cells, WCL (whole cell lysates), Mito (mitochondria); and (G) mRNA expression level of SIRT3 in HSP60-KN cells and control cells. Data were analyzed using student’s t-test. *P < 0.05, **P < 0.01 and ***P < 0.001. Error bars represent ±SEM
qPCR analysis was conducted and showed that the mRNA expression levels of these nine mitochondrial ribosomal genes were lower in SIRT3-OE cells than those in the control cells (Fig. 2C). qPCR analysis also confirmed the downregulation of other genes including SDHB, COA7, CDK1, and CDK4 (Fig. 2C). Additionally, Western blotting was employed to examine expressions of the selected proteins and showed that the expression levels of EEF1A2, CDK1, and CDK4 were down-regulated whereas those of vimentin and angiomotin were upregulated in SIRT3-OE cells (Fig. 2D), consistent with the proteomic results displayed in Tables S1 and S2. The above results also showed that SIRT3-OE cells grew slower than the control cells (Fig. 1C), indicating that cell cycle progression varies between those two cells. Indeed, the cell cycle analysis of SIRT3-OE and control cells showed that SIRT3-OE cells had the higher G1-phase accumulation (Fig. 2E), in consistent with the down-regulation of CDK1 and CDK4 in SIRT3-OE cells.
SIRT3 is known to play a crucial role in maintenance of mitochondrial proteostasis. Proteomic analysis showed that multiple subunits of respiratory complex IV were down-regulated in SIRT3-OE cells, which compromised the integrity and assembly of respiratory complexes, leading to over-production of ROS (Fig. 1D). Excessive ROS can negatively regulate cell growth and may activate the Nrf2/Keap1 pathway that protects SIRT3-OE cells from oxidative stress (Fig. 1E). An alternative explanation for the high resistance to oxidative stress exhibited in SIRT3-OE cells is that deacetylation of antioxidant proteins by SIRT3 enhances their enzymatic activities, which needs to be confirmed in the future study. Three key proteins in fatty acid β-oxidation pathway were also down-regulated in SIRT3-OE cells (Table S2). Growth and proliferation of tumor cells require fatty acids for synthesis of membranes and signaling molecules. Disruption of fatty acid oxidation pathway causes a decrease in both acetyl-CoA production that is essential for the de novo lipid synthesis and NADH and FADH2 generation that are important for ATP and citrate production (Carracedo et al., 2013). Moreover, SIRT3-meidated disruption of fatty acid β-oxidation can lead to the accumulation of fatty acids to induce lipotoxicity. Cyclin-dependent kinase 1 (CDK1) and cyclin-dependent kinase 4 (CDK4) were found to be downregulated in SIRT3-OE cells as confirmed by qPCR and Western blotting. CDK4 is associated with D-type cyclins to promote cell-cycle entry and progression through G1 by inactivating the retinoblastoma protein Rb (Sherr and Roberts, 1999). SIRT3-induced CDK4 downregulation causes the prolonged G1 cell cycle arrest that contributes to the slower growth of SIRT3-OE cells as compared to the control cells.
To further identify factors that regulate SIRT3 stability, we isolated the SIRT3 complexes from SIRT3-OE cells. Protein components of the SIRT3 complexes were separated on a 1D SDS-PAGE gel and changes in band intensities were identified between SIRT3-OE and the control cells (Fig. S2). These bands were excised, digested by trypsin and analyzed by LC-MS/MS, resulting in the identification of HSP60 as the major binding partner of SIRT3. Similarly, immunoprecipitation of HSP60 from 293T cells was carried out and showed that SIRT3 bound to HSP60, suggesting that SIRT3 directly interacts with HSP60. In order to confirm that HSP60 regulates SIRT3 stability, we established the HSP60 knockdown cells. Western blotting showed that SIRT3 was down regulated in HSP60 knockdown cells as compared to the control cells (Fig. 2F). On the other hand, qPCR analysis revealed that the SIRT3 mRNA level was unchanged between these two cells, suggesting that HSP60 regulated SIRT3 stability (Fig. 2G). This is consistent with an early study showing that two murine SIRT3 isoforms interacted with HSP60 (Yang et al., 2011). HSP60 is the major mitochondrial chaperone and is essential in maintenance of mitochondrial proteostasis. More work is needed to examine the molecular mechanisms of SIRT3 degradation in HSP60-knockdown cells. Nevertheless, our results propose that HSP60 is important in the maintenance of SIRT3 protein stability.
Taken together, we demonstrate that SIRT3 overexpression disrupts mitochondrial proteostasis that causes overproduction of ROS and the cell cycle arrest to suppress cell proliferation, proposing that the low level expression of SIRT3 is important for tumorigenesis and progression in ccRCC. Our data also show that HSP60 mediates the stability of SIRT3 and proteomics is a powerful approach to decipher the complex cellular processes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
FOOTNOTES
We thank the Protein Chemistry Facility at the Center for Biomedical Analysis of Tsinghua University for sample analysis. This work was supported in part by the National Natural Science Foundation of China (Grant No. 31270871 to H.T.D) and MOEC 2012Z02293 (H.T.D), the National Basic Research Program (973 Program) (No. 2014CBA02005 to H.T.D) and the Global Science Alliance Program of Thermo-Fisher Scientific.
Xiaofei Wang, Haiping Tang, Yuling Chen, Binghuan Chi, Shiyu Wang, Yang Lv, Di Wu, Renshan Ge, and Haiteng Deng declare that they have no conflict of interest. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
References
- Alhazzazi TY, Kamarajan P, Verdin E, Kapila YL. SIRT3 and cancer: tumor promoter or suppressor? Biochim Biophys Acta. 2011;1816:80–88. doi: 10.1016/j.bbcan.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf N, Zino S, Macintyre A, Kingsmore D, Payne AP, George WD, Shiels PG. Altered sirtuin expression is associated with node-positive breast cancer. Br J Cancer. 2006;95:1056–1061. doi: 10.1038/sj.bjc.6603384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carracedo A, Cantley LC, Pandolfi PP. Cancer metabolism: fatty acid oxidation in the limelight. Nat Rev Cancer. 2013;13:227–232. doi: 10.1038/nrc3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005;353:2477–2490. doi: 10.1056/NEJMra043172. [DOI] [PubMed] [Google Scholar]
- Debeb BG, Zhang X, Krishnamurthy S, Gao H, Cohen E, Li L, Rodriguez AA, Landis MD, Lucci A, Ueno NT, et al. Characterizing cancer cells with cancer stem cell-like features in 293T human embryonic kidney cells. Mol Cancer. 2010;9:180. doi: 10.1186/1476-4598-9-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley LW, Haigis MC. Metabolic regulation by SIRT3: implications for tumorigenesis. Trends Mol Med. 2012;18:516–523. doi: 10.1016/j.molmed.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Patel K, Muldoon-Jacobs K, Bisht KS, Aykin-Burns N, Pennington JD, van der Meer R, Nguyen P, Savage J, Owens KM, et al. SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell. 2010;17:41–52. doi: 10.1016/j.ccr.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell E, Peterson BS, Bomze HM, Hirschey MD. SIRT3 regulates progression and development of diseases of aging. Trends Endocrinol Metab. 2015;26:486–492. doi: 10.1016/j.tem.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa L, Germain D. SirT3 regulates the mitochondrial unfolded protein response. Mol Cell Biol. 2014;34:699–710. doi: 10.1128/MCB.01337-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12:662–667. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- Verdin E, Hirschey MD, Finley LW, Haigis MC. Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling. Trends Biochem Sci. 2010;35:669–675. doi: 10.1016/j.tibs.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Chen KY, Tong Q. Murine Sirt3 protein isoforms have variable half-lives. Gene. 2011;488:46–51. doi: 10.1016/j.gene.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Dittenhafer-Reed KE, Denu JM. SIRT3 protein deacetylates isocitrate dehydrogenase 2 (IDH2) and regulates mitochondrial redox status. J Biol Chem. 2012;287:14078–14086. doi: 10.1074/jbc.M112.355206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


