Abstract
Background
Contemporary guidelines recommend angiotensin-converting-enzyme inhibitors (ACEi) or angiotensin-receptor blockers (ARB) for hypertensive patients with diabetes. However, there is limited data to evaluate the comparison between ACEi and ARB on end stage renal disease (ESRD) and major adverse cardiovascular events (MACE), in Asian diabetic patients.
Methods
We used the Taiwan Longitudinal Cohort of Diabetes Patients Database to perform a population-based dynamic cohort study. The comparison between ACEi and ARB on ESRD and MACE in diabetic patients was examined using the propensity score weighting method. We followed these patients until the occurrence of first study outcomes or end date of the study, whichever came first.
Results
There were 6898 and 12,758 patients in ACEi and ARB groups, respectively. The mean follow-up period was about 3.5 years in ESRD and 2.5 years in MACE. The incidence of ESRD was 0.44 % and 0.63 % per person-years in the ACEi and ARB group, respectively. The risk of ESRD was lower in the ACEi group than the ARB group [hazard ratio (HR) 0.69; 95 % confidence interval (CI) 0.54–0.88, P = 0.0025]. Among those without chronic kidney disease (CKD), the incidence of ESRD was 0.30 % and 0.37 % per person-years in the ACEi and ARB group, respectively. ACEi was similar to ARB in preventing ESRD for those without CKD (P = 0.11). Among those with CKD, the incidence of ESRD was 1.39 % and 2.34 % per person-years in the ACEi and ARB group, respectively. The ACEi group had a lower risk of ESRD than the ARB group (HR 0.61; 95 % CI 0.42–0.88, P = 0.008). The incidence of MACE was 9.33 % and 9.62 % per person-years in the ACEi and ARB group, respectively. There was no significant difference in the composite MACE outcome between the two groups (P = 0.42), but the ACEi group was associated with a higher risk of stroke than the ARB group (HR 1.12; 95 % CI 1.02–1.24, P = 0.02).
Conclusions
ACEi compared with ARB was associated with a lower incidence of ESRD, especially in those with CKD. Though ACEi and ARB had a similar risk of composite MACE outcome, ACEi had a slightly higher incidence of stroke than ARB, among the Asian diabetic patients.
Electronic supplementary material
The online version of this article (doi:10.1186/s12933-016-0365-x) contains supplementary material, which is available to authorized users.
Keywords: Angiotensin converting enzyme inhibitors, Angiotensin receptor blockers, Diabetes mellitus, Major adverse cardiovascular events, End-stage renal disease
Background
Diabetes mellitus (DM) is a global health problem and a major cause of cardiovascular disease and end-stage renal disease (ESRD) [1, 2]. According to current guidelines [1, 3], angiotensin-converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARB) are recommended for the treatment of hypertension, and especially for diabetic patients. Both drugs block angiotensin II, however ACEi are characterized by a decrease in the degradation of bradykinin leading to a release of nitric oxide and prostaglandins resulting in additional vasodilatation. The differences in the modes of action between ACEi and ARB may have clinical implications for diabetic patients [4, 5]. A recently published meta-analysis [6] showed that ACEi were associated with a significant reduction (14 %) in major cardiovascular events, but that ARB had no benefit on these outcomes. In these patients, management of cardiovascular risk factors and careful monitoring eGFR may represent opportunities to reduce the risk of major adverse cardiovascular events (MACE) [7]. In addition, these two agents have been reported to be beneficial in delaying the progression of kidney disease in patients with non-dialysis-dependent chronic kidney disease (CKD) [8–10]. However, studies comparing ACEi and ARB with regards to renoprotective effects are limited.
Cough occurs in 5–20 % of patients treated with ACEi. The mechanism may involve accumulation of prostaglandins, kinins, or substance P, as both bradykinin and substance P are degraded by ACE [11]. Furthermore, the incidence of discontinuing ACEi due to cough has been reported to be up to 30 % in Asian patients [12, 13]. Therefore, it is important to have a comparison of the efficacy of these two classes of drugs in Asian diabetic patients. Given the uncertainty surrounding the effect of ACEi/ARB, we performed a retrospective, population-based dynamic cohort study to compare the efficacy between ACEi and ARB in preventing ERSD and MACE in diabetic patients.
Research design and methods
Study population
The National Health Insurance (NHI) program, a compulsory universal health care system in Taiwan, was launched in 1995 and currently covers 23.72 million enrollees or about 99 % of the population. The National Health Research Institute (NHRI) is responsible for managing and maintaining all insurance claims data, and various datasets are released for research purposes after thorough review and after all personal information has been encrypted. Hence, informed consent was not required for this study. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approval by the Institutional Review Board of the Chang Gung Medical Foundation, Taiwan (103-7871C).
Data from the Longitudinal Cohort of Diabetes Patients (LHDB) released by the NHRI were used for this study. In LHDB, patients who were first diagnosed with DM from 1999 to 2010 were eligible for this study, if they: (1) had at least one hospitalization with DM as one of the discharge codes (A-code A181 before 2000 or ICD-9-CM code 250 after 2000) or used DM medications (Additional file 1); (2) had at least two outpatient visits due to DM in the same year; (3) had no hospitalizations or outpatient visits due to DM from 1996 to 1998; (4) were over 20 years of age. Due to the restriction set by the NHRI of releasing data no more than 10 % of the total population, an annual total of 120,000 eligible patients from 1999 to 2010 were randomly selected, and their original claims data including admissions, outpatient visits, and medications were recorded in the LHDB. The LHDB included data before a diagnosis of DM and treatment for patients with and without DM. The LHDB has been tested and confirmed by other studies to be representative of Taiwanese patients with DM [14–18]. In this study, ACEi and ARB were listed in the supplement. Patients in LHDB who had been prescribed ACEi or ARB before the first diagnosis of DM, or who had been prescribed neither ACEi nor ARB were excluded. ACEi users were those who took ACEi but not ARB. The ARB group were those who took ARB but not ACEi. The index date was defined as the calendar date of a first prescription of ACEi or ARB. Our selection further excluded if (1) the year of their first prescription was before 1996, or (2) they were younger than age 20 years of age on index date, or (3) they had ESRD before index date, or (4) they did not had hypertension before index date, or (5) cover rate less than 70 %. The follow-up period was from the index date until either the date of new onset of MACE or ESRD (when treated as an outcome variable), or 31 December 2011, whichever came first (Fig. 1). Note that the nature of LHDB and this study design is qualified as a dynamic cohort, in which eligible participants were recruited when newly diagnosed patients appeared during the study. The advantage of a dynamic cohort is that the number of participants does not decline over time, and that aging of the study participants over time does not weaken the study.
Fig. 1.
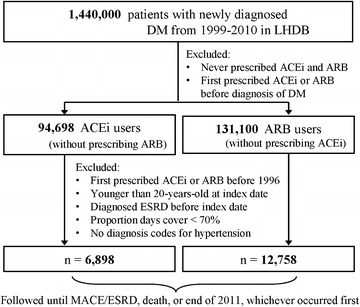
Flowchart of subject selection. LHDB longitudinal cohort of diabetes patients, DM diabetes mellitus, ACEi angiotensin converting enzyme inhibitors, ARB angiotensin receptor blockers, ESRD end stage renal disease, MACE major adverse cardiovascular events
A patient is considered to have reached the end points of the study, the occurrence of MACE, if he or she has bee diagnosed with one of the codes: International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes as follows: myocardial infarction (410), congestive heart failure (428), stroke (430–437), malignant dysrhythmia (426.0, 426.12–426.13, 426.51–426.52, 426.54, 427.1, 427.4, 427.41–427.42, 427.5), cardiogenic shock (785.51); or procedure codes of the Taiwan NHI for percutaneous coronary intervention (PCI) (33076A, 33076B, 33077A, 33077B, 33078A, 33078B), coronary artery bypass surgery (CABG) (68023A, 68023B, 68024A, 68024B, 68025A, 68025B), and thrombolysis therapy (B016526248, K000743248, K000744238) [19]. A previous MACE is defined as a hospitalization due to MACE before the index date. A new MACE is defined as a hospitalization with MACE as the primary diagnosis 14 days after the index date.
Patients with chronic kidney disease are defined as those who were diagnosed with ICD9-CM codes 580–589 at least twice at an outpatient clinic or a discharge. Patients with hyperlipidemia are defined as those who were diagnosed with ICD9-CM code 272 or A182 at least twice at an outpatient clinic or a discharge.
Patients with ESRD requiring chronic renal replacement therapy are eligible for a catastrophic illness certificate in Taiwan. Patients with a catastrophic illness certificate are entitled to a waiver for medical co-payment. Diagnostic information is sent to the insurance administration for a review by a panel of commissioned experts to review the diagnosis and approve the waiver. Consequently, ESRD is defined as patients with a catastrophic illness certificate for ESRD (ICD-9-CM code: 585).
Statistical analysis
We use the propensity score method to compare between ACEi and ARB on the ESRD and MACE in DM patients to eliminate the effects of unbalanced demographic and comorbid medical disorders at index date for observational data. The propensity score was the predicted probability of being in ACEi group derived from the fitted logistic regression, in which group status was regressed on covariates at index date (Table 1). Inverse probability of treatment weights (IPTWs) using the propensity scores was then applied to balance covariates across the two study groups. The standardized mean difference (SMD) rather than using statistical testing was made to examine the balance of covariates at index date between the two study groups, because balance is a property of the sample and not of an underlying population. The absolute value of SMD ≤ 0.1 indicates a negligible difference in covariates between the two study groups [20]. In time-to-event analyses, incident rate, crude hazard ratio (log-rank test) and adjusted hazard ratio (Cox’s proportional hazard model) were estimated, accounting for the weighted nature of two study groups with robust variance estimation [21]. Statistical significance was defined as a P value less than 0.05. All statistical analyses were performed using SAS 9.2 (SAS Institute Inc., Cary, NC, USA).
Table 1.
The demographic and comorbid medical disorders at index date, before and after propensity score weighting, among hypertensive and diabetic patients who were prescribed with either angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARB)
| Propensity score weighting | ||||||
|---|---|---|---|---|---|---|
| Before | After | |||||
| ACEi (n = 6898) | ARB (n = 12,758) | Standardized mean difference | ACEi (n = 6898) | ARB (n = 12,758) | Standardized mean difference | |
| Duration from DM to index datea (years) | 3.07 ± 3.15 | 3.69 ± 3.33 | −0.1931 | 3.17 ± 3.15 | 3.08 ± 2.26 | −0.0032 |
| Age (years) (mean ± SD) | 61.5 ± 13.0 | 62.4 ± 12.8 | −0.0711 | 61.5 ± 13.0 | 61.3 ± 9.7 | 0.0183 |
| Age group | 0.0944 | 0.0053 | ||||
| 20–49 (%) | 20.9 | 17.2 | 20.9 | 21.0 | ||
| 50–64 (%) | 39.4 | 40.3 | 39.4 | 39.5 | ||
| 65+ (%) | 39.7 | 42.4 | 39.7 | 39.5 | ||
| Male sex | 59.5 % | 53.1 % | 0.1271 | 59.5 % | 59.5 % | −0.0016 |
| Congestive heart failure | 4.51 % | 4.85 % | −0.0163 | 4.51 % | 4.51 % | −0.0000 |
| Stroke | 12.73 % | 14.70 % | −0.0572 | 12.73 % | 12.73 % | −0.0000 |
| Malignant dysrhythmia | 0.45 % | 0.49 % | −0.0054 | 0.45 % | 0.45 % | 0.0004 |
| Cardiogenic shock | 0.32 % | 0.23 % | 0.0176 | 0.32 % | 0.33 % | −0.0025 |
| MI/PCI | 5.00 % | 4.56 % | 0.0206 | 5.00 % | 4.98 % | 0.0010 |
| CABG | 0.46 % | 0.60 % | −0.0192 | 0.46 % | 0.46 % | 0.0012 |
| Thrombolysis therapy | 0.25 % | 0.24 % | 0.0023 | 0.25 % | 0.24 % | 0.0018 |
| Hyperlipidemia | 54.54 % | 59.81 % | −0.1066 | 54.54 % | 54.68 % | −0.0029 |
| Chronic kidney disease | 13.51 % | 16.73 % | −0.0901 | 13.51 % | 13.51 % | 0.0001 |
DM diabetes mellitus, ACEi angiotensin converting enzyme inhibitors, ARB angiotensin receptor blockers, MI myocardial infarction, PCI percutaneous coronary intevention, CABG coronary artery bypass graft surgery
aIndex date was the date first prescribed either ACEi or ARB
Results
The demographic characteristics and comorbid medical disorders at the index date are shown in Table 1. There were 6898 patients in ACEi and 12,758 patients in ARB groups, respectively. On average, patients were prescribed either ACEi or ARB 3 years after being diagnosed with DM. The mean age at the first prescription of either ACEi or ARB was 61.5 ± 13.0 and 62.4 ± 12.8 years, respectively. Approximately half (54.5 %) had hyperlipidemia, 13.5 % had CKD, 12.7 % had stroke and 5 % had myocardial infarction (MI) or PCI. A history of congestive heart failure, cardiogenic shock, CABG, and thrombolysis therapy were not common (from 0.25 to 4.51 %). After propensity score weighting, the age, gender and comorbid medical disorders were comparable with absolute values of standardized mean difference <0.1 between the two study groups.
During 26,809 person-years with a mean follow-up of 3.9 years in the ACEi group and 41,292 person-years with a mean follow-up of 3.2 years in the ARB group, the incidence (person-years) of ESRD was 0.44 % in the ACEi group and 0.63 % in the ARB group (Table 2). Figure 2 displays the Kaplan–Meier curves of ESRD-free rate among the ACEi and ARB group. The ACEi group had a lower incidence of ESRD than the ARB group (HR 0.69; 95 % CI 0.54–0.88, P = 0.0025).
Table 2.
Incidence (per 100 person-years) of outcomes among the ACEi and ARB users after propensity score weighting
| Incidence (95 % CI) | Hazard ratio (95 % CI) | |||
|---|---|---|---|---|
| ACEi (n = 6898) | ARB (n = 12,758) | ACEi vs. ARB | P value | |
| MACE | ||||
| Any MACEa | 9.33 (8.90−9.76) | 9.62 (9.16−10.08) | 1.03 (0.96−1.10) | 0.4244 |
| Cogestive heart failure | 1.48 (1.33−1.63) | 1.51 (1.35−1.67) | 1.06 (0.91−1.22) | 0.4538 |
| Stroke | 3.78 (3.53−4.03) | 3.56 (3.31−3.82) | 1.12 (1.02−1.24) | 0.0230 |
| Malignant dysrhythmia | 0.16 (0.12−0.21) | 0.15 (0.10−0.20) | 1.14 (0.73−1.77) | 0.5571 |
| Cardiogenic shock | 0.03 (0.01−0.06) | 0.04 (0.02−0.07) | 0.81 (0.31−2.07) | 0.6547 |
| MI or PCI | 1.77 (1.61−1.93) | 2.19 (2.00−2.39) | 0.89 (0.78−1.01) | 0.0646 |
| CABG | 0.18 (0.13−0.23) | 0.22 (0.16−0.28) | 0.89 (0.60−1.31) | 0.5438 |
| Thrombolysis therapy | 0.00 (0.00−0.02) | 0.02 (0.01−0.05) | 0.19 (0.02−1.66) | 0.1339 |
| ESRD | 0.44 (0.36−0.52) | 0.63 (0.53−0.73) | 0.69 (0.54−0.88) | 0.0025 |
ACEi angiotensin converting enzyme inhibitors, ARB angiotensin receptor blockers, CI confidence interval, MACE major adverse cardiovascular disease, MI myocardial infarction, CHF cogestive heart failure, PCI percutaneous coronary intevention, CABG coronary artery bypass graft surgery, ESRD end stage renal disease
aMACE = CHF or stroke or malignant dysrhythmia or cardiogenic shock or MI or PCI or CABG or thrombolysis therapy
Fig. 2.
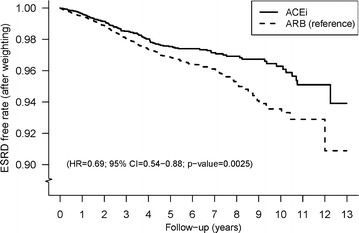
Overall end stage renal disease free rates for ACEi and ARB users after propensity score weighting. ACEi angiotensin converting enzyme inhibitors, ARB angiotensin receptor blockers, ESRD end stage renal disease
Furthermore, we evaluated the effect of CKD on the incidence of ESRD. There were 5966 and 10,623 patients without CKD in ACEi and ARB group, respectively. Among those without CKD, the mean age at the first prescription of either ACEi or ARB was 60.8 and 60.6 years, respectively. Approximately half (53.8 %) had hyperlipidemia, 12.2 % had stroke and 5 % had MI or PCI. A history of congestive heart failure, cardiogenic shock, CABG and thrombolysis therapy were not common (from 0.3 to 4.0 %). During 23,353 person-years with a mean follow-up of 3.9 years in ACEi group and 34,928 person-years with a mean follow-up of 3.3 years in ARB group, the incidence (person-years) of ESRD was 0.30 and 0.37 % in the ACEi and ARB group, respectively (Table 3). There were no significant differences in ESRD between the two groups (HR 0.76; 95 % CI 0.55–1.06, P = 0.11) (Fig. 3).
Table 3.
The demographic and comorbid medical disorders at index date after propensity score weighting among hypertensive and diabetic patients who were prescribed with either angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARB) stratified by chronic kidney disease or not
| Propensity score weighting | ||||||
|---|---|---|---|---|---|---|
| With CKD | Without CKD | |||||
| ACEi (n = 932) | ARB (n = 2135) | Standardized mean difference | ACEi (n = 5966) | ARB (n = 10,623) | Standardized mean difference | |
| Duration from DM to index date (years) | 3.50 ± 3.33 | 3.52 ± 2.09 | −0.0049 | 3.00 ± 3.11 | 3.01 ± 2.29 | −0.0035 |
| Age (years) (mean ± SD) | 65.5 ± 13.3 | 65.2 ± 8.8 | 0.0276 | 60.8 ± 12.9 | 60.6 ± 9.8 | 0.0175 |
| Age group (years) | 0.0123 | 0.0049 | ||||
| 20–49 (%) | 14.70 | 15.06 | 21.81 | 21.92 | ||
| 50–64 (%) | 32.08 | 32.25 | 40.56 | 40.69 | ||
| 65+ (%) | 53.22 | 52.69 | 37.63 | 37.39 | ||
| Male sex | 59.12 % | 59.21 % | −0.0019 | 59.52 % | 59.62 % | −0.0019 |
| Congestive heart failure | 8.05 % | 8.15 % | −0.0039 | 3.96 % | 3.94 % | 0.0006 |
| Stroke | 16.20 % | 16.15 % | 0.0014 | 12.19 % | 12.16 % | 0.0007 |
| Malignant dysrhythmia | 0.21 % | 0.21 % | 0.0014 | 0.49 % | 0.48 % | 0.0011 |
| Cardiogenic shock | 0.64 % | 0.73 % | −0.0102 | 0.27 % | 0.27 % | −0.0011 |
| MI/PCI | 5.15 % | 5.08 % | 0.0030 | 4.98 % | 4.96 % | 0.0008 |
| CABG | 0.11 % | 0.11 % | −0.0010 | 0.52 % | 0.51 % | 0.0008 |
| Thrombolysis therapy | 0.00 % | 0.00 % | −0.0002 | 0.28 % | 0.28 % | 0.0012 |
| Hyperlipidemia | 59.44 % | 59.86 % | −0.0085 | 53.77 % | 53.77 % | −0.0017 |
| Incidence of ESRDa | 1.39 | 2.34 | 0.30 | 0.37 | ||
| (95 % CI) | (1.00−1.78) | (1.80−2.89) | (0.23−0.37) | (0.29−0.46) | ||
DM diabetes mellitus, ACEi angiotensin converting enzyme inhibitors, ARB angiotensin receptor blockers, CKD chronic kidney diseases, MI myocardial infarction, PCI percutaneous coronary intevention, CABG coronary artery bypass graft surgery
aIncidence per 100 person-years
Fig. 3.
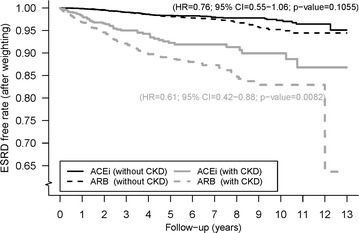
End stage renal disease free rates stratified by chronic kidney disease or not for ACEi and ARB users after propensity score weighting. ACEi angiotensin converting enzyme inhibitors, ARB angiotensin receptor blockers, ESRD end stage renal disease, CKD chronic kidney disease
There were 932 and 2135 patients with CKD in the ACEi and ARB group, respectively. The mean age was 65.5 years in the ACEi group and 65.2 years in the ARB group. Approximately half (59 %) had hyperlipidemia, 16.2 % had stroke, 8.1 % had CHF and 5.2 % had MI or PCI. A history of cardiogenic shock, CABG and thrombolysis therapy were not common (from 0 to 0.7 %). Among those with CKD, during 3457 person-years with a mean follow-up of 3.7 years in the ACEi group and 6364 person-years with a mean follow-up of 3.0 years in the ARB group, the incidence rate (person-years) of ESRD was 1.39 and 2.34 % in the ACEi and ARB group, respectively (Table 3). The ACEi group had a lower rate of the ESRD than with ARB group (HR 0.61; 95 % CI 0.42–0.88, P = 0.008) (Fig. 3).
During 19,203 person-years with a mean follow-up of 2.8 years in the ACEi group and 30,389 person-years with a mean follow-up of 2.4 years in the ARB group, the incidence rate (person-years) of any MACE was 9.33 and 9.62 % in the ACEi and ARB groups, respectively (Table 2). Figure 4 displays the Kaplan–Meier curves of MACE free rate for the two study groups. There were no significant differences in MACE between the two groups (HR 1.03; 95 % CI 0.96–1.10, P = 0.42). Stroke, MI or PCI and congestive heart failure were the most common MACE in both the ACEi and ARB groups, with incidence (person-years) of 3.78/3.56, 1.77/2.19, and 1.48/1.51 %, respectively. Note that the ACEi group had a higher incidence of stroke than the ARB group (HR 1.12; 95 % CI 1.02–1.24, P = 0.02) (Table 2). Figure 5 displays the Kaplan–Meier curves of stroke- free rate among the ACEi and ARB group.
Fig. 4.
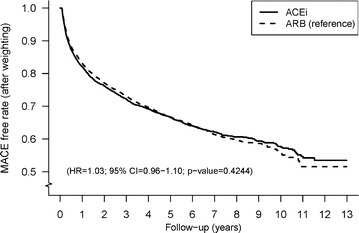
Overall major adverse-cardiovascular-events free rates for ACEi and ARB users after propensity score weighting. ACEi angiotensin converting enzyme inhibitors, ARB angiotensin receptor blockers, MACE major adverse cardiovascular events
Fig. 5.
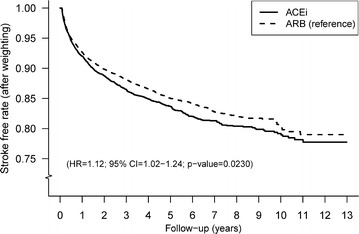
Overall stroke free rates for ACEi and ARB users after propensity score weighting. ACEi angiotensin converting enzyme inhibitors, ARB angiotensin receptor blockers
Discussion
In this study, we compared the long-term impact of ACEi and ARB in Asian diabetic patients, using a population-based dynamic cohort study design and propensity score weighting method. Our results demonstrated that ACEi compared with ARB was associated with a lower incidence of the progression to ESRD in diabetic patients, especially in the patients with CKD. In addition, though ACEi and ARB had a similar incidence of the composite MACE outcome, ARB had a lower incidence of the stroke than ACEi.
Clinical studies of ACEi and ARB have shown that they reduce albuminuria and the loss of glomerular filtration rate and the need for dialysis in those with advanced renal disease [22]. Data from the ONTARGET trial [23], which focused on diabetic patients showed that the effect of telmisartan on dialysis was similar to ramipril with rates of hemodialysis of 0.6 and 0.56 %, respectively (P = 0.747). A retrospective cohort study, using Veterans Affairs databases showed that ACEIs are associated with lower ESRD development in diabetic patients compared with ARB (Odds ratio 0.10; 95 % CI 0.04–0.21, P = 0.02) [24]. In this study, focused on diabetic patients with hypertension, we demonstrated that ACEi was superior to ARB in preventing ESRD development (HR 0.69; 95 % CI 0.54–0.88, P = 0.0025). Lacourciere et al. demonstrated that among diabetic patients with normal glomerular filtration rate (GFR), the rate of decline in GFR with 1 year follow up is similar between patients taking either ACEi or ARB [25]. Our study with longer follow-up duration (mean 3.5 years) also showed the ACEi/ARB have similar effect in progression to ESRD among the diabetic patients without CKD. In addition, among the patients with CKD, our study demonstrated that ACEi was associated with a lower risk of ESRD than ARB (HR 0.61, 95 % CI 0.42–0.88, P = 0.008). We saw that ACEi with a better renoprotective effect than ARB, primary from these with CKD. There are two important differences between ACEi and ARB (angiotensin II type I antagonists). The first is the blockade of bradykinin degradation by ACEi and the second is the supraphysiologic activation of the Angiotensin II type 2 receptor (AT2) when ARB (AT1 antagonists) are administered [26]. AT2 receptor plays a counterregulatory protective role mediated via bradykinin and nitric oxide against the antinatriuretic and pressor actions of angiotensin II [27]. These mechanisms may explain why ACEi has better renoprotection than ARB. Some studies have suggested that dual blockage of renin–angiotensin system may provide additive benefit in diabetic nephropathy, but further studies are needed to validate such therapy, which should be used with caution [28].
In our study, the incident rates of cardiovascular comorbidities including myocardial infarction, congestive heart failure, PCI and stroke were relatively lower, as compared with the HOPE [29], TRANSCEND [30], or ONTARGET [31] studies. The ONTARGET study (Asian population 13.8 %, 1200 patients) compared the ACEi (ramipril) and the ARB (telmisartan) in patients with vascular disease or high-risk diabetes (37 %), and found that telmisartan was equivalent to ramipril in reducing mortality and morbidity from cardiovascular causes. We also found that treatment with ACEi and ARB had a similar incidence of the composite MACE outcome in Asian diabetic patients. On the other hand, we found that among diabetic patients, ACEi has a slightly higher risk of stroke than ARB (HR 1.12; 95 % CI 1.02–1.24, P = 0.023). In other words, our study demonstrated that ARB has a slightly better protective effect on stroke than ACEi among the diabetic patients. A meta-analysis [32], which did not focus on the diabetic patients, also showed that ARB was more protective than ACEi on the risk of stroke (odds ratio 0.92; 95 % CI:0.85–0.99, P = 0.037).
There are several limitations to this study. First, nearly all hospitals/clinics are linked to the National Health Insurance (NHI) program, which covered over 99 % of the population covered by the program during the study period. However, it is possible that we were not able to assess some cases of MACE. Second, several important clinical parameters are not available in the NHI claims database, including clinical/imaging information, severity of MACE, smoking history, albuminuria, LDL-cholesterol, and creatinine level. Third, the benefit of antiplatelet agents in primary prevention for cardiovascular events is still under debate, so we do not examine the effect of antiplatelet agents in MACE and ESRD. [33].
In conclusion, among Asian diabetic patients, ACEi appeared to have a lower risk of ESRD development than ARB, especially in those with chronic kidney disease. Despite the similar incidence of the composite MACE outcome, ARB was slightly more protective than ACEi on the risk of stroke.
Authors’ contributions
Conceived and designed the experiments: LSW, SHC, YHY, LCS. Performed the experiments: JRL, SHC, CTK. Analyzed the data: JRL, LCS. Contributed reagents/materials/analysis tools: GJC, WJC, CTK, MSW. Wrote the manuscript: LSW, LCS. Overall responsibility: YSY and LCS. All authors read and approved the final manuscript.
Acknowledgements
This study is based in part on data from the National Health Insurance.Research Database provided by the National Health Insurance Administration, Ministry of Health and Welfare and managed by National Health Research Institutes, Taiwan. The interpretation and conclusions contained herein do not represent those of the National Health Insurance Administration, Ministry of Health and Welfare or National Health Research Institutes.
This work was supported by grants from Taiwan Ministry of Science and Technology (102-2628-B-182-011-MY3).
Competing interests
The authors declare that they have no competing interests.
Additional file
10.1186/s12933-016-0365-x The drugs analyzed are listed as following.
Contributor Information
Lung-Sheng Wu, Email: r5278@adm.cgmh.org.tw.
Shang-Hung Chang, Email: afen.chang@gmail.com.
Gwo-Jyh Chang, Email: gjchang@mail.cgu.edu.tw.
Jia-Rou Liu, Email: jiarou@mail.cgu.edu.tw.
Yi-Hsin Chan, Email: s851047@cgmh.org.tw.
Hsin-Fu Lee, Email: 8805033@cgmh.org.tw.
Ming-Shien Wen, Email: wenms123@adm.cgmh.org.tw.
Wei-Jan Chen, Email: wjchen@adm.cgmh.org.tw.
Yung-Hsin Yeh, Email: yeongshinn@adm.cgmh.org.tw.
Chi-Tai Kuo, Email: chitai@adm.cgmh.org.tw.
Lai-Chu See, Phone: 886-3-2218800-5119, Email: lichu@mail.cgu.edu.tw.
References
- 1.American Diabetes Association Standards of Medical Care in Diabetes-2014. Diabetes Care. 2014;37(Suppl 1):S14–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 2.KDOQI KDOQI Clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49(2 Suppl 2):S12–154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 3.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to Eight Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 4.Palmer SC, Mavridis D, Navarese E, Craig JC, Tonelli M, Salanti G, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet. 2015;385(9982):2047–2056. doi: 10.1016/S0140-6736(14)62459-4. [DOI] [PubMed] [Google Scholar]
- 5.Padwal R, Lin M, Eurich DT. The comparative effectiveness of angiotensin-converting enzyme inhibitors and angiotensin ii receptor blockers in patients with diabetes. J Clin Hypertens. 2015 doi: 10.1111/jch.12647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng J, Zhang W, Zhang X, Han F, Li X, He X, et al. Effect of angiotensin-converting enzyme inhibitors and angiotensin II receptor blocker on all-cause mortality, cardiovascular deaths, and cardiovascular events in patients with diabetes mellitus: a meta-analysis. JAMA Intern Med. 2014;174(5):773–785. doi: 10.1001/jamainternmed.2014.348. [DOI] [PubMed] [Google Scholar]
- 7.Soriano CL, Johansson B, Rodriguez LA. Cardiovascular events and all-cause mortality in a cohort of 57,946 patients with type 2 diabetes: associations with renal function and cardiovascular risk factors. Cardiovasc Diabetol. 2015;14:38. doi: 10.1186/s12933-015-0204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 9.Giatras I, Lau J, Levey AS. Effect of angiotensin-converting enzyme inhibitors on the progression of non diabetic renal disease: a meta-analysis of randomized trials: angiotensin-converting enzyme inhibition and Progressive Renal Disease Study Group. Ann Intern Med. 1997;127:337–345. doi: 10.7326/0003-4819-127-5-199709010-00001. [DOI] [PubMed] [Google Scholar]
- 10.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Pariving HH, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 11.Israili ZH, Hall WD. Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy. A review of the literature and the pathophysiology. Ann Intern Med. 1992;117:234–242. doi: 10.7326/0003-4819-117-3-234. [DOI] [PubMed] [Google Scholar]
- 12.Chan WK, Chan TY, Luk WK, Leung VK, Li TH, Critchley JA, et al. A high incidence of cough in Chinese subjects treated with angiotensin converting enzyme inhibitors. Eur J Clin Pharmacol. 1993;44:299–300. doi: 10.1007/BF00271377. [DOI] [PubMed] [Google Scholar]
- 13.Ng LP, Goh PS. Incidence of discontinuation of angiotensin-converting enzyme inhibitors due to cough, in a primary healthcare centre in Singapore. Singapore Med J. 2014;55:146–149. doi: 10.11622/smedj.2014034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen SW, Tsan YT, Chen JD, Hsieh HI, Lee CH, Lin HH, et al. Use of thiazolidinediones and the risk of colorectal cancer in patients with diabetes: a nationwide, population-based, case–control study. Diabetes Care. 2013;36(2):369–375. doi: 10.2337/dc11-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang SH, Wu LS, Lee CH, Kou CT, Liu JR, Wen MS, et al. Simvastatin–ezetimibe combination therapy is associated with a lower rate of major adverse cardiovascular events in type II diabetics than high potency statins alone: a population-based dynamic cohort study. Int J Cardiol. 2015;190:20–25. doi: 10.1016/j.ijcard.2015.04.121. [DOI] [PubMed] [Google Scholar]
- 16.Chang SH, Wu LS, Chiou MJ, Liu JR, Yu KH, Kou CF, et al. Association of metformin with lower atrial fibrillation risk among patients with type 2 diabetes mellitus: a population-based dynamic cohort and in vitro studies. Cardiovasc Diabetol. 2014;13:123. doi: 10.1186/s12933-014-0123-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shih CJ, Chen HT, Chao PW, Kou SC, Li SY, Yang CY, et al. Angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers and the risk of major adverse cardiac events in patients with diabetes and prior stroke. J Hypertens. 2016;34(3):567–575. doi: 10.1097/HJH.0000000000000804. [DOI] [PubMed] [Google Scholar]
- 18.Fung CS, Wan EY, Wong CK, Jiao F, Chan AK. Effect of metformin monotherapy on cardiovascular diseases and mortality: a retrospective cohort study on Chinese type 2 diabetes mellitus patients. Cardiovasc Diabetol. 2015;9(14):137. doi: 10.1186/s12933-015-0304-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang SH, Wu LS, Chiou MJ, Liu JR, Yu KH, Kou CF, et al. Association of metformin with lower atrial fibrillation risk among patients with type 2 diabetes mellitus: a population-based dynamic cohort and in vitro studies. Cardiovasc Diabetol. 2014;13:123. doi: 10.1186/s12933-014-0123-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33:1242–1258. doi: 10.1002/sim.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higers KF, Mann JF. ACE inhibitors versus AT(1) receptor antagonists in patients with chronic renal disease. J Am Soc Nephrol. 2002;13:1100–1108. doi: 10.1681/ASN.V1341100. [DOI] [PubMed] [Google Scholar]
- 23.Mann JF, Anderson C, Gao P, Gerstein HC, Boehm M, Ryden L, et al. Dual inhibition of the renin-angiotensin system in high-risk diabetes and risk for stroke and other outcomes: results of the ONTARGET trial. J Hypertens. 2013;31:414–421. doi: 10.1097/HJH.0b013e32835bf7b0. [DOI] [PubMed] [Google Scholar]
- 24.Campbell HM, Khan N, Raisch DW, Borrego ME, Sather MR, Murata GH. Angiotensin-converting enzyme inhibitors versus angiotensin receptor blockers for end stage renal disease/mortality in type 2 diabetes. Diabetes Res Clin Pract. 2013;102(3):233–241. doi: 10.1016/j.diabres.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Lacourciere Y, Belanger A, Godin C, Halle JP, Ross S, Wright N, et al. Long-term comparison of losartan and enalapril on kidney function in hypertensive type 2 diabetics with early nephropathy. Kidney Int. 2000;58(2):762–769. doi: 10.1046/j.1523-1755.2000.00224.x. [DOI] [PubMed] [Google Scholar]
- 26.Burnier M. Angiotensin II type I receptor blockers. Circulation. 2001;103(6):904–912. doi: 10.1161/01.CIR.103.6.904. [DOI] [PubMed] [Google Scholar]
- 27.Siragy HM, Inagami T, Carey RM. Sustained hypersensitivity to angiotensin II and its mechanism in mice lacking the subtype-2(AT2) angiotensin receptor. Proc Natl Acad Sci USA. 1999;96(11):6506–6510. doi: 10.1073/pnas.96.11.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren F, Tang L, Cai Y, Yuan X, Huang W, Luo L, Zhou J, Zheng Y. Meta-analysis: the efficacy and safety of combined treatment with ARB and ACEi on diabetic nephropathy. Ren Fail. 2015;37(4):548–561. doi: 10.3109/0886022X.2015.1012995. [DOI] [PubMed] [Google Scholar]
- 29.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcome Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 30.Yusuf S, Teo KK, Andrerson C, Pogue J, Dyal L, Copland I, et al. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomized controlled trial. Lancet. 2008;372:1174–1183. doi: 10.1016/S0140-6736(08)61193-9. [DOI] [PubMed] [Google Scholar]
- 31.Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 32.Reboldi G, Angeli F, Cavallini C, Gentile G, Mancia G, Verdecchia P, et al. Comparison between angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on the risk of myocardial infarction, stroke and death: a meta-analysis. J Hypertens. 2008;26(7):1282–1289. doi: 10.1097/HJH.0b013e328306ebe2. [DOI] [PubMed] [Google Scholar]
- 33.Halvorsen S, Andreotti F, ten Berg JM, Cattaneo M, Coccheri S, Marchioli R, et al. Aspirin therapy in primary cardiovascular disease prevention: a position paper of the European Society of Cardiology working group on thrombosis. J Am Coll Cardiol. 2014;64:319–327. doi: 10.1016/j.jacc.2014.03.049. [DOI] [PubMed] [Google Scholar]


