Abstract
Background
Outbreaks of vector-borne diseases (VBDs) such as dengue and malaria can overwhelm health systems in resource-poor countries. Environmental management strategies that reduce or eliminate vector breeding sites combined with improved personal prevention strategies can help to significantly reduce transmission of these infections.
Objective
The aim of this study was to assess the knowledge, attitudes, and practices (KAPs) of residents in western Jamaica regarding control of mosquito vectors and protection from mosquito bites.
Methods
A cross-sectional study was conducted between May and August 2010 among patients or family members of patients waiting to be seen at hospitals in western Jamaica. Participants completed an interviewer-administered questionnaire on sociodemographic factors and KAPs regarding VBDs. KAP scores were calculated and categorized as high or low based on the number of correct or positive responses. Logistic regression analyses were conducted to identify predictors of KAP and linear regression analysis conducted to determine if knowledge and attitude scores predicted practice scores.
Findings
In all, 361 (85 men and 276 women) people participated in the study. Most participants (87%) scored low on knowledge and practice items (78%). Conversely, 78% scored high on attitude items. By multivariate logistic regression, housewives were 82% less likely than laborers to have high attitude scores; homeowners were 65% less likely than renters to have high attitude scores. Participants from households with 1 to 2 children were 3.4 times more likely to have high attitude scores compared with those from households with no children. Participants from households with at least 5 people were 65% less likely than those from households with fewer than 5 people to have high practice scores. By multivariable linear regression knowledge and attitude scores were significant predictors of practice score.
Conclusion
The study revealed poor knowledge of VBDs and poor prevention practices among participants. It identified specific groups that can be targeted with vector control and personal protection interventions to decrease transmission of the infections.
Key Words: dengue, Jamaica, KAP, malaria, vector-borne diseases
Introduction
Dengue fever is the most common human arboviral infection globally and is responsible for more illness and deaths than any other arboviral disease.1 It is an acute mosquito-transmitted viral disease characterized by fever, headache, muscle and joint pains, rash, nausea, and vomiting. An estimated 3900 million people in 128 countries are at risk for dengue infection.2 The incidence and geographical distribution of dengue have greatly increased in recent years. Currently, it is estimated that 390 million dengue infections occur each year, with about 100 million manifesting clinically with varying degrees of severity of the disease3; a small proportion progress to severe dengue. With the growing incidence of severe dengue epidemics since the 1970s, the World Health Organization (WHO) has reported cases across the Americas, South-East Asia, and the Western Pacific exceeding 1.2 million in 2008 and more than 3 million in 2013, based on official data submitted by member states.4 These numbers continue to increase: 2.35 million cases of dengue were reported in the Americas alone in 2013; of this number, 37,687 were cases of severe dengue.4 The dengue virus is carried and spread by species of mosquitoes in the genus Aedes. Of these species, the primary vector is Aedes aegypti. Other Aedes species that transmit dengue include Aedes albopictus, Aedes polynesiensis, and Aedes scutellaris.
Dengue is endemic in Jamaica, with epidemics reported as recently as in 2012. In that year, 93% of American missionaries returning from Jamaica showed serologic evidence of recent or past infection with the Dengue Virus.5 The Dengue Virus-1 serotype was first reported in the Americas region in 1977 after an outbreak that began in Jamaica and expanded to Cuba, Puerto Rico, and Venezuela and eventually to the rest of the Caribbean countries, Mexico, Central America, and the northern countries of South America.6 A study conducted in Jamaica in 2009 found the seroprevalence of dengue immunoglobulin (Ig)G and dengue IgM antibodies to be 100% and 3.6%, respectively.7 The high seroprevalence of dengue IgG antibodies suggests that the Jamaican population might be at increased risk for dengue hemorrhagic fever and dengue shock syndrome. It was recently reported that in a 2007–2008 epidemic of dengue in Jamaica, 3165 tested sera showed seropositivity for dengue, leptospirosis, and malaria at rates of 38.4%, 6%, and 6.5%, respectively.8 This highlights the presence of 3 concurrent epidemics with dengue seroprevalence being the highest in the country. Between January and December 2012, the Jamaican Ministry of Health recorded 5384 clinically suspected cases of dengue fever, with 732 laboratory-confirmed cases and 10 confirmed dengue-related deaths.9
The Jamaican Ministry of Health has been working tirelessly to prevent dengue outbreaks and fatalities through the implementation of control interventions.9, 10, 11 As with many community health problems, the knowledge, attitudes, and practices (KAPs) of the population play a major role in implementation of control measures. However, little is known of the KAPs of Jamaicans in the control and prevention of vector-borne diseases (VBDs). Although the WHO declared Jamaica free of malaria in 1965,12 between November 6, 2006 and February 3, 2007, the Jamaican Ministry of Health confirmed 280 cases of malaria due to Plasmodium falciparum on the island.13 This outbreak was brought under control in September 2007.14 In light of the recent outbreak of the Chikungunya virus leading to a declaration of a “national emergency” in Jamaica, it is very important now, more than ever, to assess and address matters that are imperative to preventing future VBD outbreaks and epidemics in the country.15 The goal of this study was to assess KAPs of selected communities in western Jamaica regarding control of VBDs such as dengue and malaria.
Methods
Study Setting
At the time of this study, the population of Jamaica was estimated at about 2.7 million.16 This study was conducted in the 4 parishes under the Western Region Health Authority (WRHA) namely, St. James, Westmoreland, Hanover, and Trelawny (Fig. 1 ), with a total population of 472,611 (17% of the entire country).17
Figure 1.
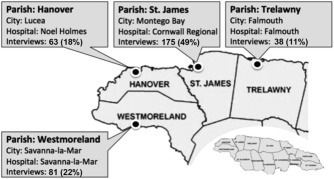
Number and percentage of participants sampled in the hospitals in the 4 parishes of Western Jamaica.
Study Design and Participants
A cross-sectional study was carried out between May and August 2010 among people visiting the Cornwall Regional Hospital in St. James, the Falmouth Hospital in Trelawny, the Noel Holmes Hospital in Hanover, and the Savanna-la-Mar Hospital in Westmoreland. Participants were either patients or family members of patients waiting to be seen by a health care official. Participants were recruited from the outpatient clinics, the pharmacy areas, and the emergency departments of the 4 hospitals. The aim of the study was explained to potential participants and they were asked to participate.
Upon approval, participants read the informed consent and were allowed to ask questions. After all questions were answered and clarifications were made, the participants were asked to sign the informed consent form indicating their understanding and agreement to participate. Participation was strictly voluntary and no incentives were provided. The Institutional Review Board of the University of Alabama at Birmingham, the Advisory Panel on Ethics and Medico-Legal Affairs in the Ministry of Health, Jamaica, and the WRHA of Jamaica approved the study protocol before its implementation.
Study Instrument and Data Collection
Data were collected from each participant using a pretested questionnaire administered by a study interviewer. The questionnaire addressed the following areas: sociodemographic characteristics (sex, age, marital status, education, employment status, occupation, income, and number of people in household), knowledge of and experience with VBDs, attitude toward VBDs, and practices of VBD control.
Data Analysis
Characteristics of the study population are presented as means, SDs, ranges, and frequency tabulations. Each participant was assigned a separate score for knowledge, attitude, and practice based on the number of correct or appropriate responses. Each appropriate answer was assigned 1 point (incorrect responses were assigned zero points). The knowledge scale ranged from 0 to 17 points, the attitude scale from 0 to 6 points, and the practice scale from 0 to 10 points. The scores were further dichotomized into low (poor) or high (good) as follows: knowledge (low = 0–7 points; high = 8–17 points); attitude (low = 0–3 points; high = 4–6 points); practice (low = 0–5 points, high = 6–10 points). Logistic regression analysis was used to calculate crude and adjusted odds ratios as well as corresponding 95% confidence intervals. Each variable was first entered separately into bivariate logistic regression models to evaluate the crude association with sociodemographic characteristics. All predictors were then entered in multivariable logistic regression to get adjusted odds ratios. Bivariate and multivariable linear regression analyses were conducted to identify predictors of practice score. Data were analyzed using the JMP Statistical software.
Results
Sociodemographic Characteristics of the Study Population
In all, 361 individuals participated in the study. Of these, 175 (48.5%) were from St. James, 81 (22.4%) were from Westmoreland, 63 (17.5%) were from Hanover, and 38 (10.5%) were from Trelawny (Fig. 1); 4 (1.1%) respondents did not disclose their parish. The mean age of study participants was 33 years (SD 13.1). The majority of respondents (76.5%) were women; almost two-thirds (64%) were single, widowed, or divorced; and almost 58% had completed secondary school (66% of men and 55% of women). Approximately 59% were unemployed (Table 1 ). Of the 104 participants who provided information about income, 78.7% reported weekly income above J$5,000 (∼US $50). The remaining 21% made less than J$5000 per week. More than 33% of participants reported at least 5 individuals living in their households, and 77% reported house ownership. Approximately 43% reported having no children in their households, whereas 47% had 1 to 2 children. Men and women did not differ according to vector and vector control KAP scores (Table 1).
Table 1.
Sociodemographic Characteristics of Study Population∗
| Variables | Men n = 85 (24%) |
Women n = 276 (76%) |
Total N = 361 (100%) |
P |
|---|---|---|---|---|
| Age (y) | ||||
| <30 | 37 (43.5) | 132 (48.5) | 169 (47.3) | 0.286 |
| 30–49 | 32 (37.6) | 107 (39.3) | 139 (38.9) | |
| ≥50 | 16 (18.8) | 33 (12.1) | 49 (13.7) | |
| Marital status (%) | ||||
| Single/divorced | 52 (61.2%) | 176 (64.5%) | 228 (63.7%) | 0.581 |
| Married/living together | 33 (38.2%) | 97 (35.5%) | 130 (36.3%) | |
| Education (%) | ||||
| None | 1 (1.2%) | 15 (5.5%) | 16 (4.4%) | 0.048 |
| Primary | 13 (15.3%) | 38 (13.8%) | 51 (14.1%) | |
| Secondary | 56 (65.9%) | 151 (54.9%) | 207 (57.5%) | |
| University | 1 (1.2%) | 17 (6.2%) | 18 (5%) | |
| Technical/other | 14 (16.5%) | 54 (19.6%) | 68 (18.8%) | |
| Employment (%) | ||||
| Unemployed | 48 (56.5%) | 161 (59.2%) | 209 (58.5%) | 0.657 |
| Employed | 37 (43.5%) | 111 (40.8%) | 148 (41.5%) | |
| Weekly income (%) | ||||
| <J$2000 | 1 (1.2%) | 1 (0.4%) | 2 (0.5%) | |
| J$2000–J$5000 | 7 (8.2%) | 10 (3.6%) | 17 (4.7%) | |
| >J$5000 | 19 (22.4%) | 63 (22.8%) | 82 (22.7%) | |
| No response | 58 (68.2%) | 202 (73.2%) | 260 (72.1%) | |
| Total in household (%) | ||||
| 1–4 | 58 (68.2%) | 175 (64.1%) | 233 (65%) | 0.485 |
| ≥5 | 27 (31.8%) | 98 (78.40) | 125 (35%) | |
| House ownership (%) | ||||
| Tenant | 18 (21.2%) | 64 (23.9%) | 82 (23.2%) | |
| Owner | 67 (78.8%) | 204 (76.1%) | 271 (76.8%) | |
| Number of adults (%) | ||||
| 1–2 | 43 (50.6%) | 120 (43.5%) | 163 (45.5) | 0.432 |
| 3–4 | 29 (34.1%) | 96 (34.8%) | 125 (34.9) | |
| ≥5 | 13 (15.3%) | 57 (20.7%) | 70 (19.6) | |
| Number of children (%) | ||||
| None | 36 (42.4%) | 116 (42.5%) | 152 (42.5%) | 0.458 |
| 1–2 | 37 (43.5%) | 131 (48%) | 168 (47%) | |
| ≥3 | 12 (14.1%) | 26 (9.5%) | 38 (10.5%) | |
| Knowledge score (%) | ||||
| Low | 70 (85.4%) | 238 (87.5%) | 308 (87%) | 0.614 |
| High | 12 (14.6%) | 34 (12.5%) | 46 (13%) | |
| Attitude score (%) | ||||
| Low | 17 (20.2%) | 63 (23%) | 80 (22.3%) | 0.596 |
| High | 67 (79.8%) | 211 (77%) | 278 (77.7%) | |
| Practice score (%) | ||||
| Low | 68 (81%) | 212 (77.7%) | 280 (78.4%) | 0.521 |
| High | 16 (19%) | 61 (22.3%) | 77 (21.6%) | |
Numbers do not always add up to 361 because of missing responses.
Knowledge of Disease Transmission and Vector Control
Almost all of the study participants reported they had heard of dengue fever (97.5%) and malaria (95%; Table 2 ). However, only 11 (3%) reported a history of dengue fever, and 2 (0.6%) reported a history of malaria. Six percent reported having a family member or friend who had had dengue, and 3.3% reported having a family member or friend with a history of malaria. When asked about the mode of transmission of dengue fever, 45% of respondents correctly identified “mosquitoes” as the vectors of transmission. However, only 7.8% were able to identify the A aegypti mosquito as the vector for the disease. With regard to malaria, only 39% of study participants knew that the infection was transmitted by mosquitoes, and only 4% could correctly identify the Anopheles sp. mosquito as the main vector for malaria. With respect to vector biting times, only 2.5% of participants knew the biting time of A aegypti to be during the day. However, 86% of participants correctly identified the biting period of the malaria vector to be at night. Only 16% of respondents reported taking any measures to prevent dengue fever, and 12% reported taking measures to avoid malaria. Of the 11 participants who reported a history of dengue fever, 6 said they sought medical attention for the infection. Two participants with a history of malaria both reported seeking medical attention for the disease.
Table 2.
Knowledge of Vector-Borne Diseases
| Variable | Dengue |
Malaria |
||
|---|---|---|---|---|
| n | % | n | % | |
| Had dengue/malaria | ||||
| Yes | 11 | 3.1 | 2 | 0.6 |
| No | 350 | 96.9 | 359 | 99.4 |
| Had family or friend with dengue/malaria | ||||
| Yes | 22 | 6.1 | 12 | 3.3 |
| No | 339 | 93.9 | 349 | 96.7 |
| Mode of dengue/malaria transmission | ||||
| Mosquitoes | 164 | 45.4 | 141 | 39.1 |
| Not Mosquito | 197 | 54.6 | 220 | 60.9 |
| Identify mosquitos that transmit dengue or malaria | ||||
| Aedes/Anopheles | 28 | 7.8 | 14 | 3.9 |
| Others | 333 | 92.2 | 347 | 96.1 |
| Dengue/malaria mosquito biting periods | ||||
| Day time | 9 | 2.5 | 49 | 13.6 |
| Other times (night) | 352 | 97.5 | 312 | 86.4 |
| Took measures to prevent infection | ||||
| Yes | 59 | 16.3 | 43 | 12 |
| Not stated | 302 | 83.7 | 318 | 88 |
| Sought Medical Attention for Dengue | ||||
| Yes | 6 | 54.6 | 2 | 100 |
| No | 5 | 45.5 | 0 | 0 |
Attitudes Toward VBDs
Almost 70% of the participants agreed that malaria is a serious disease, whereas only 47% thought that dengue was a serious illness (Table 3 ). When the participants were asked what their course of action would be if they noticed they had symptoms of malaria or dengue fever, at least 94% said they would consult a physician. When asked if it were possible to get rid of mosquitoes in their communities, 69% said that it was. Although 55% of the participants responded that both the government and the community are responsible for mosquito management and keeping the environment safe, approximately 20% of the participants said that the government was solely responsible to take measures to prevent the disease and its transmission; 23% of participants believed it was their personal responsibility.
Table 3.
Attitudes of Study Population toward Vector-borne Diseases
| Variable | Dengue |
Malaria |
||
|---|---|---|---|---|
| n | % | n | % | |
| Is dengue or malaria serious? | 361 | 249 | ||
| Serious | 169 | 46.8 | 174 | 69.9 |
| Not serious | 192 | 53.2 | 75 | 30.1 |
| What to do in case of dengue fever or malaria symptoms? | ||||
| Consult a physician | 344 | 95.3 | 338 | 93.6 |
| Other actions | 17 | 4.7 | 23 | 6.4 |
| Is it possible to get rid of or control mosquitoes? (n = 361) | ||||
| Yes | 249 | 69 | ||
| No | 112 | 31 | ||
| Believe dengue is a problem in Jamaica (n = 361) | ||||
| Yes | 90 | 24.9 | ||
| No | 271 | 75.1 | ||
| Who is responsible for mosquito management (n = 358) | ||||
| Nobody | 1 | 0.3 | ||
| Themselves | 83 | 23.2 | ||
| Government | 73 | 20.4 | ||
| Both government and people | 196 | 54.7 | ||
| Don’t know | 5 | 1.4 | ||
Practices Regarding Vector Control
Table 4 shows practices regarding vector control in the study population. Sixty-five percent of respondents reported that they keep drinking water containers in and around their household, and 96% of those reported that they cover these containers. Approximately 57% of participants reported emptying their drinking containers regularly, whereas the other 43% reported rarely (not every week) or never emptying their drinking water storage containers. About 52% of participants reported having other water storage containers, and only 39% of those reported covering them. Also, 39% reported emptying other water containers at least every week. In addition to water containers, 12% of the respondents reported having flower pots or vases, and about 49% reported emptying the water in flower pots or vases at least every week. Approximately 20% of participants reported having containers containing or having the potential to hold water (eg, old car tires, stagnant or empty tins and cans, etc.) in and around the household. Although 79% reported that a garbage collection truck served their community, they expressed dissatisfaction with the schedule and consistency of garbage collection.
Table 4.
Practices to Reduce Vector-borne Disease Risk in Western Jamaica
| Total (N) | Yes (n) | Yes (%) | |
|---|---|---|---|
| Drinking water container in and around house | 357 | 232 | 65 |
| Cover drinking water container | 232 | 222 | 95.7 |
| Add anything to drinking water | 232 | 99 | 42.7 |
| Item added (bleach/other) | 361 | 90 | 24.9 |
| Empty drinking water containers | 361 | 205 | 56.8 |
| Other water container in and around house | 354 | 183 | 51.7 |
| Cover up water storage container? | 361 | 142 | 39.3 |
| Frequency of emptying water storage containers | 361 | 142 | 39.3 |
| Flower pots and vases in/around house | 354 | 43 | 12.1 |
| Empty flower pots and vases at least every week | 43 | 21 | 48.8 |
| Have water containers accumulating garbage | 361 | 71 | 19.7 |
| Empty other water containers | 183 | 56 | 30.6 |
| Served by garbage collection truck | 361 | 285 | 79 |
| Use of mosquito screen/mesh on windows | 361 | 71 | 19.7 |
| Have mosquito nets | 361 | 112 | 31 |
| Sleep under net during day | 112 | 46 | 41 |
| Sleep under net during night | 112 | 91 | 81.3 |
| Use of mosquitos coil during day | 361 | 57 | 15.8 |
| Use of mosquitos coil during night | 357 | 185 | 51.8 |
| Use of mosquitos repellant during day | 361 | 56 | 15.5 |
| Use of mosquito repellant during night | 361 | 138 | 38.2 |
| Empty other water containers | 183 | 56 | 30.6 |
A large proportion of the respondents (80%) reported not having mosquito screen/mesh on the windows of their household, and approximately 69% reported not having mosquito nets. When asked about mosquito net use, 41% of those with nets (13% of the total population) reported sleeping under mosquito nets during the day, and 81% of those with nets (25% of the total population) reported using mosquito nets at night. Regarding the use of mosquito coils and repellants by the respondents, only 16% reported using mosquito coils during the day, whereas 52% use coils during the night. A similar percentage (16%) reported using mosquito repellants during the day, but unlike the use of coils, only 38% used repellants at night.
Odds Ratios of Higher Knowledge Score with Sociodemographic Characteristics
In bivariate analysis, participants 30 to 49 years old were 2.34 times more likely to score higher on the knowledge scale than those younger than 30 years of age (Table 5 ). Moreover, single or divorced participants were 2.24 more likely than married people and couples living together to have higher knowledge score. Individuals with a prior history of dengue fever were 4 times more likely to have higher knowledge scores compared to those with no history of dengue. However, after appropriate adjustments were made, none of the sociodemographic variables were found to be statistically significant for knowledge score.
Table 5.
Odds Ratios of Higher Knowledge Score with Sociodemographic Characteristics
| Variables | Odd Ratios |
|
|---|---|---|
| Crude (95% CI) | Adjusted (95% CI) | |
| Male vs Female | 1.2 (0.57–2.38) | 1.28 (0.39–3.79) |
| Age (y) | ||
| <30 | Referent | Referent |
| 30–49 | 2.34 (1.20–4.73) | 1.83 (0.65–5.24) |
| ≥50 | 1.16 (0.36–3.20) | 1.17 (0.21–5.10) |
| Marital status | ||
| Single and divorced vs Married and living together | 2.24 (1.11–4.92) | 2.04 (0.73–6.74) |
| Education | ||
| None | Referent | Referent |
| Primary | 0.48 (0.10–2.59) | 0.11 (0.00–3.91) |
| Secondary | 0.50 (0.15–2.32) | 0.31 (0.03–7.69) |
| University | 0.87 (0.14–5.40) | 0.44 (0.01–16.65) |
| Other | 1.04 (0.28–5.02) | 0.40 (0.03–10.46) |
| Employment | ||
| Unemployed vs employed | 1.51 (0.79–2.98) | 0.91 (0.32–2.70) |
| Occupation | ||
| Laborer | Referent | Referent |
| Student | 0.37 (0.02–4.16) | 0.32 (0.01–4.21) |
| Housewife | 1.79 (0.31–14.10) | 1.98 (0.26–19.30) |
| Other | 1.06 (0.27–7.11) | 1.06 (0.20–8.39) |
| Total in household | ||
| ≥5 vs 1–4 | 0.70 (0.34–1.35) | 0.83 (0.26–2.36) |
| Home ownership | ||
| Owner vs tenant | 1.27 (0.61–2.93) | 0.78 (0.25–2.69) |
| Number of adults | ||
| 1–2 | Referent | Referent |
| 3–4 | 0.84 (0.42–1.64) | 1.05 (0.34–3.09) |
| ≥5 | 0.48 (0.15–1.21) | 1.17 (0.31–4.03) |
| Number of children | ||
| None | Referent | Referent |
| 1–2 | 1.29 (0.67–2.55) | 1.32 (0.46–4.01) |
| ≥3 | 0.90 (0.25–2.62) | 1.05 (0.19–4.80) |
| Ever had dengue | ||
| Yes vs No | 4.04 (1.02–13.98) | – |
Adjusted for sociodemographic characteristics.
Odds Ratios of Higher Attitude Score with Sociodemographic Characteristics
In bivariate analysis, housewives were 75% less likely than laborers to have higher attitude score, and participants from households with 1 to 2 children were almost twice as likely to have higher attitude score than those from childless households. After adjustment, housewives were still less likely (82%) than laborers to have higher attitude scores, and participants from households with 1 to 2 children were 3.4 times more likely to have higher attitude scores than those from homes with no children. Home ownership became a significant variable after adjustment. Those who owned their homes were 65% less likely than renters to have higher attitude scores (Table 6 ).
Table 6.
Odds Ratios of Higher Attitude Score with Sociodemographic Characteristics
| Variables | Odd Ratios |
|
|---|---|---|
| Crude (95% CI) | Adjusted (95% CI) | |
| Male vs Female | 1.18 (0.66–2.20) | 0.99 (0.40–2.52) |
| Age groups | ||
| <30 | Referent | Referent |
| 30–49 | 1.59 (0.93–2.77) | 1.99 (0.85–4.94) |
| ≥50 | 1.64 (0.76–3.84) | 2.60 (0.76–11.07) |
| Marital status | ||
| Single and divorced vs Married and living together | 1.17 (0.70–1.96) | 1.32 (0.60–2.84) |
| Education | ||
| None | Referent | Referent |
| Primary | 0.78 (0.19–2.66) | 1.86 (0.07–23.10) |
| Secondary | 1.13 (0.30–3.41) | 2.46 (0.11–24.55) |
| University | 2.67 (0.44–21.63) | 3.46 (0.09–134.45) |
| Other | 1.70 (0.42–5.98) | 5.66 (0.23–69.81) |
| Employment | ||
| Unemployed vs employed | 0.90 (0.53–1.49) | 1.06 (0.44–2.53) |
| Occupation | ||
| Laborer | Referent | Referent |
| Student | 0.84 (0.15–4.33) | 0.67 (0.10–4.25) |
| Housewife | 0.25 (0.05–0.99) | 0.18 (0.03–0.98) |
| Other | 0.68 (0.15–2.21) | 0.59 (0.11–2.43) |
| Total in household | ||
| ≥5 vs 1–4 | 0.61 (0.37–1.02) | 0.69 (0.30–1.60) |
| Home ownership | ||
| Owner vs tenant | 1.09 (0.59–1.94) | 0.35 (0.12–0.93) |
| Number of adults | ||
| 1–2 | Referent | Referent |
| 3–4 | 1.13 (0.64–2.03) | 1.34 (0.55–3.38) |
| ≥5 | 0.72 (0.38–1.39) | 1.20 (0.45–3.38) |
| Number of children | ||
| None | Referent | Referent |
| 1–2 | 1.98 (1.16–3.39) | 3.41 (1.49–8.20) |
| ≥3 | 1.84 (0.79–4.82) | 2.15 (0.63–8.82) |
Adjusted for sociodemographic characteristics.
Odds Ratios of Higher Practice Score with Sociodemographic Characteristics
In bivariate analysis, participants with university education were 83% less likely than respondents with no education to have higher vector control practice scores (Table 7 ). However, this was not significant after adjustment. After adjustment, participants from households with at least 5 people were 65% less likely to have higher practice scores than those from smaller households.
Table 7.
Odds Ratios of Higher Practice Score with Sociodemographic Characteristics
| Variables | Odd Ratios |
|
|---|---|---|
| Crude (95% CI) | Adjusted (95% CI) | |
| Male vs. Female | 0.82 (0.43–1.48) | 0.55 (0.21–1.34) |
| Age groups | ||
| <30 | Referent | Referent |
| 30–49 | 0.87 (0.49–1.51) | 0.97 (0.44–2.11) |
| ≥50 | 1.05 (0.47–2.21) | 0.87 (0.24–2.75) |
| Marital status | ||
| Single and divorced vs Married and living together | 1.43 (0.40–1.19) | 1.54 (0.73–3.40) |
| Education | ||
| None | Referent | |
| Primary | 0.41 (0.12–1.35) | |
| Secondary | 0.34 (0.12–1.00) | |
| University | 0.17 (0.02–0.89) | |
| Other | 0.30 (0.09–0.99) | |
| Employment | ||
| Unemployed vs employed | 1.65 (0.98–2.84) | 1.28 (0.55–3.06) |
| Occupation | ||
| Laborer | Referent | Referent |
| Student | 0.99 (0.28–3.55) | 0.46 (0.10–2.10) |
| Housewife | 0.81 (0.22–2.95) | 0.43 (0.09–2.02) |
| Other | 0.48 (0.18–1.38) | 0.24 (0.07–0.83) |
| Total in household | ||
| ≥5 vs 1–4 | 0.58 (0.33–1.01) | 0.35 (0.13–0.83) |
| House ownership | ||
| Owner vs tenant | 0.95 (0.53–1.76) | 0.68 (0.28–1.67) |
| Number of adults | ||
| 1–2 | Referent | Referent |
| 3–4 | 1.51 (0.86–2.67) | 1.34 (0.59–3.05) |
| ≥5 | 1.27 (0.62–2.52) | 1.04 (0.39–2.65) |
| Number of children | ||
| None | Referent | Referent |
| 1–2 | 1.47 (0.85–2.57) | 2.17 (0.99–4.95) |
| ≥3 | 1.91 (0.82–4.27) | 1.27 (0.34–4.31) |
Adjusted for sociodemographic characteristics.
Linear Regression Analysis of Knowledge and Attitude Scores Against Practice Score
In bivariate linear regression analysis, knowledge score (P = 0.0335) and attitude score (P = 0.0453) were significant predictors of practice score. However, knowledge score was not a significant predictor of attitude score. In multivariate analysis with knowledge and attitude scores in the model, after controlling for attitude score, we found that practice score increased by 0.06 for every unit increase in knowledge score (Table 8 ). After controlling for knowledge score, practice score increased by 0.17 for every unit increase in attitude score (Table 8).
Table 8.
Linear Regression of Knowledge and Attitude Scores Against Practice Score
| Parameter Estimates | ||||
|---|---|---|---|---|
| Term | Estimate | Standard Error | t Ratio | Prob > |t| |
| Intercept | 2.799 | 0.435 | 6.43 | <0.0001∗ |
| Knowledge Score | 0.064 | 0.032 | 2.01 | 0.0447∗ |
| Attitude Score | 0.167 | 0.091 | 1.83 | 0.0681 |
Significant at P < .05.
Discussion
This study was conducted to assess the KAPs regarding malaria and dengue and their prevention in western Jamaica. We found that the study participants had poor knowledge of vectors and disease transmission; only 13% received high knowledge scores. This may be partly because Jamaica is not a country in which malaria is endemic, and only a small portion of individuals knew someone who had been diagnosed with dengue or malaria. Although the global burden of dengue is more prominent in Asia, the virus has been well documented in the Caribbean. In Jamaica, there have been several significant outbreaks of dengue fever, during which times the Ministry of Health has officially informed the Jamaican public of impending danger and put measures in place to control the spread of the diseases.9, 10, 11 However, these public awareness programs and control measures appear to be implemented only during outbreaks.
At the time of this study, there were no outbreaks of dengue, and this might explain the low level of knowledge of vector control. Furthermore, the respondents could not correctly identify the vectors that transmit these infections (Aedes sp. or Anopheles sp.) nor the times when they are vulnerable to infection by these vectors.
Most participants regarded dengue and malaria seriously enough to say that they would consult a physician if they experienced symptoms of the infections. The majority also believed that it was possible to control mosquitoes. However, only 47% believed that dengue was a serious illness, and 75% did not believe that dengue was a problem in Jamaica. It is interesting that more than 50% of respondents believe that both the government and the communities are responsible for mosquito control, whereas 20% believe that it is the sole responsibility of the government. Seventy-nine percent expressed dissatisfaction with the schedule and consistency of garbage collection, demonstrating the need for the government to reassess the efficiency of or revamp these services. The findings from this study demonstrate that poor knowledge and poor practices regarding vector control may be due to literacy level, as the majority of study participants had only secondary education or less. However, the study did not find significant results between education level and knowledge scores or education level and practice scores.
With regard to attitude scores, we found that homeowners were 65% less likely than renters to obtain high attitude scores. It is possible that renters feel an obligation to keep their homes and immediate surroundings clean to prevent problems with landlords and other tenants. This tendency to comply with rental or leasing agreements translates into the tenant assuming responsibility for cleanliness around the home, as well as the health and contractual risks associated with not doing so. Homeowners, on the other hand, are more likely to have higher income and live in better, cleaner, and more secluded neighborhoods, and so are less likely to be concerned about vector control. They may be more likely to pay for cleaning services and have more reliable garbage collection services, thus maintaining good sanitation in their environment.
Laborers were found to be 82% more likely than housewives to have high attitude scores. This may be explained by the fact that laborers work outside of their homes and have a higher likelihood of interacting with other people in their communities or workplace, some of whom may have experienced the effects of VBDs. Therefore, laborers may be more likely to engage with others in discussions on health, cleanliness, and other infection prevention matters that improve their understanding of VBDs and the need to maintain a positive attitude toward the prevention of these diseases.
Participants from households with 1 to 2 children were 3.4 times more likely than those from childless households to have high attitude scores. Parental beliefs have been found to be strongly associated with receipt of preventive health care by their children.18, 19 We posit that the presence of children in the household places responsibility on the parents or guardians to maintain a clean and safe environment to prevent their children (and themselves) from becoming ill. This personal awareness of health risks, therefore, translates into higher attitude scores in households with children than in households without children.
With regard to practice scores, participants from households with fewer than 5 occupants had a 65% greater likelihood of having higher practice scores than those from households with 5 or more occupants. This suggests that people in smaller households have a relatively greater likelihood of keeping their homes and surroundings clean and of taking preventive and protective measures to control vectors and vector bites compared with larger households. Furthermore, occupants of larger households were more likely to be living under more stringent social and economic conditions (such as overcrowding, fewer resources, and lower income). Therefore, they may be less likely to have the resources to keep their surroundings clean and to buy protective gear such as mosquito nets, repellants, and mesh screens.
Knowledge and attitude scores were significant predictors of practice score. This indicates that those with better knowledge and attitude were more likely to take precautionary measures to prevent infection by the vectors. In the literature, knowledge and attitude scores have not always been found to be predictive of practice scores. Knowledge and attitude scores did not predict practice score on KAP toward medicines among school teachers in Nepal,20 or regarding condom use at last intercourse among Filipino-American adolescents.21 Also, knowledge score weakly predicted attitude score but not practice score in a survey on osteoporosis among a sample of Malaysian university students.22 However, higher knowledge score was found to be a strong predictor of higher attitude and practice scores among military servicemen in Singapore shortly after the peak of an influenza epidemic caused by a novel strain (H1N1) of influenza A23 and of better adoption of precautionary practices during a severe acute respiratory syndrome (SARS) epidemic in Hong Kong.24 It is possible that higher perceived threat of deadly contagious infections such as influenza and SARS led to higher rates of precautionary practices and that better knowledge increased the practice of preventive measures.
In the present study, knowledge of the dangers of dengue infection seemed to have influenced practice. This emphasizes the need for the government and the Ministry of Health officials to find ways of providing and improving access to educational materials and programs across the country to provide citizens with the opportunity to understand the risks for VBDs and prevention practices that can be undertaken to decrease or prevent transmission of such diseases in the country.
This study has certain limitations that must be taken into consideration when interpreting the results. First, the assessments of attitudes and practices toward VBDs and vector control have relied on self-reported data collected through interviews and could potentially be affected by social desirability bias. However, the low practice scores obtained by the majority of the participants indicate that this may not be so. Second, although participants were recruited from all of the government-operated hospitals under the WRHA (which serves a wide population area), it should be noted that these facilities might be underutilized by individuals of upper-middle to high socioeconomic status.
Conclusion
Despite the limitations of the study, the findings contribute to our understanding of KAP regarding VBDs in western Jamaica and can be used to develop interventions designed to improve vector control and reduce transmission of these diseases in the region and possibly the country.
Acknowledgments
The authors acknowledge the participants and the staff of the Epidemiology Research Unit of the WRHA for their support and for making the study possible.
Footnotes
This research was supported by the Minority Health International Research Training (MHIRT) grant #T37 MD001448 from the National Institute on Minority Health and Health Disparities, National Institutes of Health, and the Ministry of Health, Jamaica. All authors have access to the data and participated in writing the manuscript.
References
- 1.World Health Organization (WHO) WHO. Dengue haemorrhagic fever: Diagnosis, treatment, prevention, and control. 2nd ed. WHO; Geneva, Switzerland: 1997. World Health Report. Communicable disease surveillance and response. [Google Scholar]
- 2.Brady O.J., Gething P.W., Bhatt S. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012;6:e1760. doi: 10.1371/journal.pntd.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatt S., Gething P.W., Brady O.J. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO media center fact sheets. Available at: http://www.who.int/mediacentre/factsheets/fs117/en/. Accessed September 20, 2015.
- 5.Moncayo A.C., Baumblatt J., Thomas D. Dengue among American missionaries returning from Jamaica, 2012. Am J Trop Med Hyg. 2015;92:69–71. doi: 10.4269/ajtmh.14-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan American Health Organization Dengue in the Caribbean, 1977. Sci Publ Pan Am Health Organ. 1979;375:1–182. [Google Scholar]
- 7.Brown M.G., Vickers I.E., Salas R.A., Smikle M.F. Seroprevalence of dengue virus antibodies in healthy Jamaicans. Hum Antibodies. 2009;18:123–126. doi: 10.3233/HAB-2009-0207. [DOI] [PubMed] [Google Scholar]
- 8.Lindo J., Brown P.D., Vickers I., Brown M., Jackson S.T., Fuller E.L. Leptospirosis and malaria as causes of febrile illness during a dengue epidemic in Jamaica. Pathog Glob Health. 2013;107:329–334. doi: 10.1179/2047773213Y.0000000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Government of Jamaica; Jamaica Information Service. 28 fogging machines purchased as part of the Dengue Outbreak Control Programme. Available at: http://jis.gov.jm/28-fogging-machines-purchased-as-part-of-the-dengue-outbreak-control-programme/. Accessed September 20, 2015.
- 10.The Government of Jamaica; Jamaica Information Service. Govt. commits additional $14 million to tackle Dengue. Available at: http://jis.gov.jm/govt-commits-additional-14-million-to-tackle-dengue/. Accessed September 20, 2015.
- 11.The Government of Jamaica; Jamaica Information Service. Cabinet approves additional $111M to continue Dengue eradication. Available at: http://jis.gov.jm/cabinet-approves-additional-111m-to-continue-dengue-eradication/. Accessed September 20, 2015.
- 12.Jones M. A “textbook pattern”? Malaria control and eradication in Jamaica, 1910–1965. Med History. 2013;57:397–419. doi: 10.1017/mdh.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Government of Jamaica; Jamaica Information Service. Jamaica regains malaria free status. Available at: http://jis.gov.jm/jamaica-regains-malaria-free-status/. Accessed September 20, 2015.
- 14.World Health Organization. Global alert and response disease outbreak news. Available at: http://www.who.int/csr/don/2007_02_09/en/. Accessed September 20, 2015.
- 15.Public Radio International. Health and medicine. Available at: http://www.pri.org/stories/2014-10-21/jamaica-declares-state-emergency-try-stop-spread-painful-chikungunya-virus. Accessed September 20, 2015.
- 16.Statistical Institute of Jamaica. Population by sex: 2012-2013. Available at http://statinja.gov.jm/Demo_SocialStats/population.aspx. Accessed September 20, 2015.
- 17.Statistical Institute of Jamaica. 2011 census of population and housing. Available at: http://statinja.gov.jm/Census/Census2011/Census%202011%20data%20from%20website.pdf. Accessed September 20, 2015.
- 18.Nathanson C.A., Drachman R.H., Kirscht J.P. Mothers’ health beliefs and children’s clinic visits: a prospective study. J Community Health. 1977;3:125–135. doi: 10.1007/BF01674234. [DOI] [PubMed] [Google Scholar]
- 19.Hughes S.C., Wingard D.L. Parental beliefs and children’s receipt of preventive care: Another piece of the puzzle? Health Serv Res. 2008;43(1 Pt 1):287–299. doi: 10.1111/j.1475-6773.2007.00759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jha N., Bajracharya O., Shankar P.R. Knowledge, attitude and practice towards medicines among school teachers in Lalitpur district, Nepal before and after an educational intervention. BMC Public Health. 2013;13:652. doi: 10.1186/1471-2458-13-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maxwell A.E., Bastani R., Warda U.S. Knowledge and attitudes toward condom use–do they predict behavior among Filipino Americans? Ethn Dis. 2000;10:113–124. [PubMed] [Google Scholar]
- 22.Khan Y.H., Sarriff A., Khan A.H., Mallhi T.H. Knowledge, attitude and practice (KAP) survey of osteoporosis among students of a tertiary institution in Malaysia. Trop J Pharm Res. 2014;13:155–162. [Google Scholar]
- 23.Yap J., Lee V.J., Yau T.Y., Ng T.P., Tor P. Knowledge, attitudes and practices towards pandemic influenza among cases, close contacts, and healthcare workers in tropical Singapore: a cross-sectional survey. BMC Public Health. 2010;10:442. doi: 10.1186/1471-2458-10-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leung G.M., Quah S., Ho L.M. A tale of two cities: Community psycho behavioral surveillance in Hong Kong and Singapore during the severe acute respiratory syndrome epidemic. Infect Control Hosp Epidemiol. 2004;25:1033–1041. doi: 10.1086/502340. [DOI] [PubMed] [Google Scholar]


