Abstract
Background
Observational studies indicate that higher volumes of physical activity are associated with improved disease outcomes among colon cancer survivors. The aim of this report is to describe the purpose, study design, methods, and recruitment results of the COURAGE trial, a National Cancer Institute (NCI) sponsored, phase II, randomized, dose-response exercise trial among colon cancer survivors.
Methods/Results
The primary objective of the COURAGE trial is to quantify the feasibility, safety, and physiologic effects of low-dose (150 min·wk−1) and high-dose (300 min·wk−1) moderate-intensity aerobic exercise compared to usual-care control group over six months. The exercise groups are provided with in-home treadmills and heart rate monitors. Between January and July 2015, 1,433 letters were mailed using a population-based state cancer registry; 126 colon cancer survivors inquired about participation, and 39 were randomized onto the study protocol. Age was associated with inquiry about study participation (P<0.001) and randomization onto the study protocol (P<0.001). No other demographic, clinical, or geographic characteristics were associated with study inquiry or randomization. The final trial participant was randomized in August 2015. Six month endpoint data collection was completed in February 2016.
Discussion
The recruitment of colon cancer survivors into an exercise trial is feasible. The findings from this trial will inform key design aspects for future phase 2 and phase 3 randomized controlled trials to examine the efficacy of exercise to improve clinical outcomes among colon cancer survivors.
Keywords: physical activity, quality-of-life, survivorship, lifestyle, energy balance, obesity
1 INTRODUCTION
There are 103,000 people diagnosed annually with colon cancer in the United States [1]. Among those diagnosed, 39% will have localized colon cancer (confined to the primary site; stage I-II), 36% will have regional colon cancer (spread to regional lymph nodes; stage III), and 20% will have metastatic disease (spread to distant organs; stage IV) [1, 2]. Among those without metastatic disease, five year survival rates for localized and regional colon cancer are 90% and 70%, respectively [1]. Surgery is the primary treatment modality for localized and regional colon cancer, with curative resection occurring in 80-85% of patients [3, 4]. Those with regional disease may also receive adjuvant chemotherapy to reduce the risk of recurrent disease [5]. Despite the efficacy of surgical resection and adjuvant chemotherapy, 20-50% of patients with localized and regional colon cancer develop recurrent disease [6, 7]. Eighty percent of recurrences occur within the first three years after treatment, and 91% of patients who develop a recurrence by three years, die before five years [8]. Consequently, there exists a need to identify additional adjuvant therapies that can be prescribed at the conclusion of standard colon cancer therapy (e.g., surgery and chemotherapy) to minimize the risk of recurrence. Such adjuvant therapies may include the modification of lifestyle behaviors.
Physical activity or exercise is a modifiable lifestyle behavior that is associated with disease outcomes among colon cancer survivors. Among 832 stage III colon cancer survivors, participation in approximately 300 min·wk−1 (18 to 27 MET-hours) of physical activity after diagnosis was associated with a 45-49% improvement in disease-free survival (defined as cancer recurrence or death from any cause), and a 29-63% improvement in overall mortality [9]. This observation has been replicated in multiple cohorts of men [10, 11] and women [11-13], and is independent of known demographic, clinico-pathologic, and treatment-related prognostic factors [9-13]. A consistent finding in all of these cohort studies is that post-diagnosis physical activity is associated with disease outcomes in a dose-response fashion, such that larger doses of physical activity or exercise, up to approximately 300 minutes per week (min·wk−1), is associated with more favorable disease outcomes [9-13]. This dose-response pattern has been confirmed in several meta-analyses [14-16]. However, it is unknown if doses of exercise as large as 300 min·wk−1 are behaviorally feasible and have tolerable safety profiles for colon cancer survivors when compared to smaller doses of exercise, such as 150 min·wk−1 as is currently recommended by the American Cancer Society [17], American College of Sports Medicine [18], and the National Comprehensive Cancer Network [19]. Furthermore, the biological or biobehavioral pathways through which exercise may impact disease outcomes among colon cancer survivors are unknown. Evaluating potential biomarkers and/or mediators involved in the anti-cancer effects of exercise and the sensitivity of such biomarkers and/or mediators to respond to different doses of exercise will help to identify the optimal dose of exercise to improve outcomes and guide clinical decisions and recommendations.
2 AIMS OF THIS REPORT
The aims of this report are two-fold. First, we describe the purpose, study design, and methods of the COURAGE trial, a National Cancer Institute (NCI) sponsored, phase II, randomized, dose-response exercise trial of two distinct doses of moderate-intensity aerobic exercise compared to a usual-care control group among colon cancer survivors. Second, we present recruitment results to describe what demographic, clinical, or geographic characteristics are associated with inquiry about study participation and randomization onto the study protocol. Identifying the characteristics associated with inquiry about study participation and randomization onto the study protocol will provide empirical evidence to describe trial generalizability to the broader population of colon cancer survivors.
3 STUDY OBJECTIVES & OUTCOMES
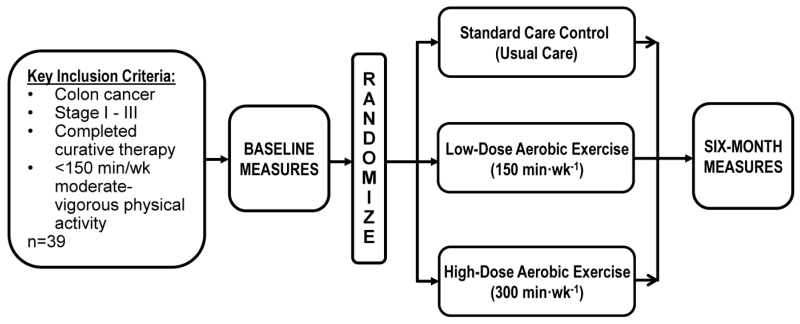
The primary objective of the COURAGE trial is to quantify the feasibility, safety, and physiologic effects of low-dose (150 min·wk−1) aerobic exercise, high-dose (300 min·wk−1) aerobic exercise, or usual-care control, among non-metastatic colon cancer survivors over six months (Figure 1). The primary outcomes include exercise adherence, adverse events, soluble intercellular adhesion molecule-1 (sICAM-1) and soluble vascular adhesion molecule-1 (sVCAM-1) prognostic biomarkers. Key secondary outcomes include visceral adipose tissue (VAT), and fasting insulin. Exploratory outcomes include the enumeration of circulating tumor cells (CTCs), functional status, and patient-reported outcomes and quality of life measures.
Figure 1.
Study Schema
4 METHODS
All study activities as described below were reviewed and approved by the University of Pennsylvania Human Subjects Protection Programs. The trial was registered with ClinicalTrials.gov as NCT02250053.
4.1 Eligibility Criteria
To balance the goals of recruiting a homogeneous study cohort with recruitment feasibility we a priori implemented a phased recruitment strategy which systematically broadened eligibility criteria in each successive phase of recruitment. In phase 1 (most strict) the inclusion criteria were as follows: histologically-confirmed stage II-III colon cancer; completed surgical resection and adjuvant chemotherapy ≤24 months before entering the study; ≤120 min·wk−1 of self-reported moderate or vigorous intensity physical activity using the Paffenbarger Physical Activity Questionnaire [20]; age ≥18 years; written physician approval; no additional surgery planned within the six month intervention (including colostomy reversal); and the ability to walk unaided for six minutes. In phase 2 (less restrictive) the inclusion criteria were broadened to increase the baseline self-reported physical activity level from ≤120 min·wk−1 to <150 min·wk−1, and expanded the time since completing surgical resection and adjuvant chemotherapy from ≤24 months to ≤36 months. In phase 3 (least restrictive) the inclusion criteria were broadened from histologically-confirmed stage II-III colon cancer to histologically-confirmed stage I-III colon cancer, and expanded the time since completing surgical resection and adjuvant chemotherapy to any period.
During all phases of recruitment, the exclusion criteria were as follows: history of another primary cancer (other than non-melanoma skin-cancer); evidence of metastatic colon cancer; planning to receive any additional adjuvant chemotherapy or surgery (i.e., ostomy reversal); pregnant or breast feeding; unable to provide baseline blood sample; cardiac conditions, including the following: myocardial infarction or coronary revascularization procedure within the past three months, uncontrolled hypertension, defined as a systolic blood pressure ≥180 mm Hg or diastolic blood pressure ≥100 mm Hg, high-risk or uncontrolled heart arrhythmias, clinically significant heart valve disease, decompensated heart failure, or known aortic aneurysm; and any other condition which, in the opinion of the investigator, may impede testing of the study hypothesis or make it unsafe to engage in the exercise program.
4.2 Participant Recruitment
Potentially-eligible study participants were recruited through the Pennsylvania Cancer Registry (PCR) [21]. The PCR is a member of the North American Association of Central Cancer Registries (NAACCR) that is responsible for collecting information on all new cases of cancer diagnosed and/or treated in the state of Pennsylvania (i.e., the PCR is population-based). The PCR has received Gold Certification from the NAACCR for excellence in areas of completeness, quality, and timeliness of cancer incidence reporting. Potentially-eligible study participants were listed in the PCR with international classification of diseases for oncology third edition (ICD-O-3) codes for colon cancer (C18.0, C18.2-C18.9). Cancer of the rectum (C20.9) was not eligible for participation. To minimize anticipated concerns regarding travel burden into the city of Philadelphia from surrounding suburbs, potentially-eligible participants were recruited from Philadelphia County and four surrounding counties (Bucks, Montgomery, Chester, and Delaware). Using an envelope with the University of Pennsylvania School of Medicine logo, potentially-eligible participants were sent one letter via postal mail that included an invitation to participate signed by the principal investigator, a one page flyer describing the study, the name and contact information (email, telephone) of the study coordinator, and a brochure describing the PCR. The one page flyer that described the study included statements that mentioned the provision of an in-home treadmill and an individualized exercise program. Mailings were completed in four successive waves as new incident data became available from the PCR (January, March, May, and July). This recruitment approach has been used by our research group in prior studies among breast cancer survivors [22].
4.3 Participant Screening
Screening was conducted via a telephone interview. The telephone interview included a brief description of the study, systematically queried callers about each of the above-described inclusion and exclusion criteria, and administered the physical activity readiness questionnaire (PAR-Q) [23]. The PAR-Q is a seven item questionnaire that identifies signs of cardiovascular disease, orthopedic conditions, and medications that could affect physiologic responses to an increase in physical activity. The PAR-Q has excellent sensitivity (close to 100%) and specificity (80%) for detecting medical contraindications to exercise [23]. After screening, eligible participants were invited to the University of Pennsylvania to meet with the study coordinator for 30-45 minutes to discuss the goals, objectives, risks, and benefits of the study in detail. At that time, written informed consent was obtained from eligible participants wishing to enroll in the study.
4.4 Study Measures
After obtaining written informed consent, the study coordinator sent a physician approval form to each of the participant’s physicians, which often included the primary care physician, surgical and/or medical oncologist, and other appropriate internal medicine specialists (e.g., cardiologist for participants with pre-existing cardiac conditions). The physician approval form included a brief description of the study, the results of the PAR-Q screening questionnaire, and a request that the physician review this information and provide approval that they believe their patient is medically fit to participate in a moderate-intensity aerobic exercise study. After obtaining written physician approval, the study participant completed a clinic visit at the University of Pennsylvania Center for Clinical and Translational Research Center. The specific measures of the clinic visit are described in detail below. All measures described below are completed at baseline and at six months (except clinical characteristics 4.4.1). All measures are conducted by staff blinded to study group.
4.4.1 Clinical Characteristics
Tumor-related characteristics including date of diagnosis and treatment completion, stage (American Joint Committee on Cancer, 7th edition [24]), primary tumor location within the colon, depth of tumor invasion (T-stage), number of positive resected lymph nodes (N-stage), grade of histologic differentiation, presence of lymphovascular invasion, and adjuvant chemotherapy are abstracted from the PCR database, medical record review, and from treatment summary reports obtained from the providing physician. Comorbidities and current medication and supplement use are obtained using the Chronic Disease Scale [25]. The above-described variables that were extracted from the PCR database were utilized to examine how demographic, clinical, or geographic characteristics were associated with inquiry about study participation and randomization onto the study protocol.
4.4.2 Blood Sample
A total of 50mL of blood is collected by a licensed phlebotomist. Blood is collected after a minimum of six hours of fasting, which is verified prior to venipuncture. Blood samples are processed using standardized laboratory procedures and aliquots of serum and EDTA-preserved plasma are stored in −80°C freezers. In addition to the biomarker assays described below, additional aliquot samples are stored for future exploratory analyses.
sICAM-1 is analyzed using an R&D systems enzyme linked immunoabsorbent assay [26, 27]. The inter-assay coefficient of variation and sensitivity are 5.4%, and 0.049-0.254 ng/mL, respectively. sVCAM-1 is analyzed using an R&D systems enzyme linked immunoabsorbent assay [26, 27]. The inter-assay coefficient of variation and sensitivity are 7.7%, and 0.17-1.26ng/mL, respectively. Fasting insulin is analyzed with a radioimmunoassay (Millipore). The inter-assay coefficient of variation is 6%, and sensitivity ranges from 2.0-200.0 μU/mL. Fasting glucose is analyzed with immuno-nephelometry assay. The inter-assay coefficient of variation is 2%, and sensitivity ranges from 0.6-45.0mmol/L. The measurement of glucose serves as a quality control to enable the attribution of elevated fasting levels to insulin resistance, rather than latent type 2 diabetes or blood samples that were collected in a non-fasting manner. The homeostatic model (HOMA) is calculated to quantify insulin resistance [28]. Other associated metabolic biomarkers including glycated hemoglobin (HbA1c), insulin-like growth factor-1 (IGF-1), insulin-like growth factor binding protein 3 (IGFBP3), and C-peptide are also analyzed.
CTCs from venous blood are isolated using geometrically enhanced differential immunocapture (GEDI) [29]. GEDI is a microfluidic platform that utilizes antibody coated obstacles to capture rare cells within anticoagulated whole blood. To capture CTCs, obstacles are coated with an antibody specific to epithelial cell-adhesion molecule (EpCAM), an epithelial cell-specific marker. After washes, captured cells are stained with the nuclear marker DAPI and fluorescently labeled antibodies to the leukocyte marker CD45 and the epithelial cell marker Pdx-1. Using fluorescence microscopy, Pdx1+/DAPI+/CD45- EpCAM captured cells with intact cellular morphology are counted as CTC’s by a blinded technician.
4.4.3 Anthropometric Measures
For all anthropometric measures participants wear study-provided medical scrubs (top and trousers). Height is measured using a wall-mounted digital stadiometer without shoes to the nearest 0.1 centimeter. Weight is measured using a calibrated digital scale to the nearest 0.1 kilogram. Height and weight are used to calculate body mass index (kg/m2). Waist and hip circumference are measured using a Gulick spring-loaded tape measure to the nearest 0.1 centimeter. Sagittal abdominal diameter is measured using an abdominal caliper (Holtain-Kahn) to the nearest 0.1 centimeter [30]. Body composition is measured using whole-body dual-energy x-ray absorptiometry (DXA; Hologic Discovery; APEX v 13.4 software). DEXA is used to quantify VAT (cm2), subcutaneous adipose tissue (cm2), total fat mass (kg), and lean mass (kg). DXA-derived VAT correlates with computed tomography derived VAT (r=0.93; P<0.001) [31], and is reliable across a large weight spectrum [32].
4.4.4 Cardiopulmonary & Functional Status
A resting electrocardiogram is completed to identify any clinically-significant cardiac conduction abnormalities [i.e., 3rd degree heart block, uncontrolled arrhythmia, or ST-segment depressions (>3 mm)] that may preclude participation in exercise. Functional status is measured using the six minute walk test (6MWT), following the guidelines of American Thoracic Society [33]. The outcome of the 6MWT is the distance walked in six minutes, recorded to the nearest meter. The 6MWT is reliable, and correlated with physiologic impairments of strength and power of the lower extremities [34]. The 6MWT is also used to identify persons who may develop signs and symptoms of cardiac distress, such as chest pain or severe shortness of breath during exertion, which may preclude participation in exercise.
4.4.5 Physical Activity
Physical activity is measured using both self-reported and objective measures. The Paffenbarger Physical Activity Questionnaire queries activities including flights of stairs climbed, blocks walked, and other leisure-time physical activities performed on a typical day or week [20]. The outcome of the Paffenbarger Physical Activity Questionnaire is the total energy expenditure associated with leisure-time physical activity, quantified in multiples of resting energy expenditures (METs) using the compendium of physical activities [35]. The ActiGraph model GT3X+ accelerometer is worn for seven consecutive days. Participants are provided with a diary to document accelerometer wear time. The ActiGraph is a valid and reliable objective measure of ambulatory activity [36]. The main outcomes obtained from the ActiGraph accelerometer will be the number of minutes of moderate-to-vigorous physical activity (≥3 METs) and activity-related energy expenditure (kcals), calculated using validated cut-points appropriate for adults [37].
4.4.6 Dietary Intake
Dietary intake is measured using three day dietary records collected during two weekdays and one weekend day [38]. Food records are entered into the Nutrition Data System for Research software (2009 version) by registered dietitians. The resulting data includes 165 micro- and macronutrient variables. Daily caloric intake is the primary covariate of interest to determine if any observed physiologic changes are due to exercise or the result of concurrent dietary alterations.
4.4.7 Patient-Reported Outcomes & Quality of Life Measures
In addition to the above-described physiologic measures, participants complete questionnaires relating to demographics, alcohol and smoking habits, and various self-report questionnaires that are known to influence the quality of life of colon cancer survivors [39, 40]. Overall quality of life is assessed using the Functional Assessment of Cancer Therapy-Colorectal (FACT-C) [41], and the SF-36 [42]. Pain is assessed using the Brief Pain Index [43]. Sleep quality is assessed using the Pittsburgh Sleep Quality Index [44]. Bowel function is assessed using the Assessment of Bowel Function Questionnaire [45]. Cancer-related fatigue is assessed using the Brief Fatigue Inventory [46]. Concerns about cancer recurrence are measured with the Fear of Cancer Recurrence Inventory [47].
4.4.8 Safety & Adverse Events
Participants in all study groups are asked each week by the study coordinator to identify any incident health events. All reported health events are graded in accordance with the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 [48]. At the end of the study, all participants complete a healthcare utilization and musculoskeletal injury questionnaire asking about any healthcare encounters and injuries experienced while in the study that may have not been previously reported [49, 50]. The data safety monitoring plan for this study focuses on monitoring by the principal investigator, along with prompt reporting of excessive adverse events and any serious adverse events to the National Institutes of Health and the Institutional Review Board at the University of Pennsylvania.
4.5 Randomization
After completion of baseline measurements, participants were stratified by stage of colon cancer (I vs. II vs. III) and randomized in a 1:1:1 ratio to either the low-dose (150 min·wk−1) aerobic exercise group, the high-dose (300 min·wk−1) aerobic exercise group, or the usual-care control group.
4.6 Treatment Plan
Participants randomized into the low-dose and high-dose exercise groups increase their exercise to 150 min·wk−1 and 300 min·wk−1, respectively. Participants randomized into the low-dose and high-dose exercise groups are provided with an in-home treadmill (LifeSpan Fitness, TR1200i, Salt Lake City, UT). Treadmills are ordered for participants on the day of randomization. Participants who were unable to accommodate a treadmill in their home were provided with a one-year health club membership (or other membership of similar monetary value) to a facility of the participants choosing. After treadmill delivery and setup, participants meet with a certified clinical exercise physiologist at the University of Pennsylvania to introduce the exercise prescription, and familiarize the participant to use of the treadmill, the completion of exercise logs, use of a heart rate monitor (described below), appropriate warm-up and cool-down, stretches, and proper footwear for aerobic exercise. The exercise physiologist provides ongoing behavioral support and monitoring of exercise adherence to the study protocol throughout the duration of the study. Behavioral support is individualized to each participant to include the benefits of exercise for colon cancer survivors, strategies to integrate exercise into day-to-day activities, how to identify and overcome barriers to exercise, recruiting friends and family members to provide support in reaching their exercise goals, and how to set simple, measureable, attainable, realistic, and timely (SMART) goals [51] to promote exercise self-efficacy and compliance [52]. The modes through which behavioral support is delivered are tailored to participant preference to maximize effectiveness of communication and include in-person sessions (as described below), complemented with weekly telephone/text, or email contact. If participants have upcoming scheduled medical procedures that may result in missed exercise (i.e., the day of a surveillance colonoscopy) the participant works with the exercise physiologist to develop a plan for maintaining optimal exercise adherence. If injury or illness prevents exercise for ≥1 week, minutes are allowed to be made-up upon return to exercise.
Exercise compliance is monitored through the use of self-reported and objective measures. All participants randomized to the low-dose and high-dose exercise groups are provided with an exercise log to record the date, modality of aerobic exercise used (any form of aerobic exercise will be acceptable, though most participants elect to use the study provided in-home treadmill), treadmill speed and incline, average heart rate obtained from a study-provided heart rate monitor, duration of exercise, completion of appropriate warm up and cool down, and any new or worsening musculoskeletal symptoms. Participants are provided with a Polar Heart Rate Monitor (Polar Electro Inc., RS400, Lake Success, NY). The heart rate monitors record up to 99 sessions of exercise. Heart rate monitor data is downloaded to a computer to enable objective monitoring of exercise adherence. Participant exercise logs and objective heart rate data are discussed and reviewed by the exercise physiologist to provide feedback including encouragement, problem solving, and long-term planning to the study participant.
For both the low-dose and high-dose exercise groups, the initial exercise dose prescribed in week one of the study is 60 min. This relatively low volume of exercise allows participants to gain confidence in exercising, allows for time to determine how exercise will be integrated into their schedule, and allows the participant to work towards a practical initial exercise goal that is not overwhelming. Exercise is titrated by 30 min·wk−1 as the participant successfully responds to the exercise dose prescribed in the prior week. During the exercise titration phase, participants meet with the exercise physiologist at the university research center each week to review the exercise completed in the prior week, discuss any new musculoskeletal symptoms, side effects, or barriers to exercise. At that time exercise is then titrated for the following week. The planned exercise volume of the low-dose and high-dose exercise groups are 150 min·wk−1 and 300 min·wk−1, respectively. In the absence of dose-limiting toxicity, low-dose and high-dose study participants have their exercise volume fully titrated by week 4 and 9 of the study, respectively. Delivered over six months, the planned total dose of exercise delivered to the low-dose and high-dose exercise groups is 3,720 minutes and 6,740 minutes, respectively. The intensity of all exercise is prescribed between 50 and 70% of the age-predicted maximum heart rate, consistent with that of moderate-intensity aerobic exercise (3-6 METs) [35]. Participants are allowed to complete exercise sessions that are ≥10 minutes to ≤75 minutes in length toward the goal of increasing behavioral feasibility of completing the planned exercise dose.
Exercise adherence is quantified using objective heart rate monitoring. However, heart rate monitors may fail due to technical error or exceed the available memory capacity. In our prior dose-response exercise trial this situation occurred <5% for all exercise sessions [53, 54]. In this scenario, objective monitoring is used to validate at least three weeks of self-report logs. Upon validation, self-report logs are accepted as a valid substitute for objective heart rate data until heart rate monitor function has been restored. Exercise adherence outcomes include the number of minutes of exercise completed within the target heart rate range for each week of the study and over six months, and the percent of prescribed minutes completed in each week of the study and over six months.
Participants randomized into the usual-care control group are asked to maintain their pre-study levels of physical activity and/or follow the recommendations provided by their physician. After completing six month measures, control group participants are provided with an in-home treadmill and individualized exercise program, similar to that prescribed to the two exercise groups. Upon study completion, all participants are allowed to keep their study-provided treadmills.
4.7 Statistical Considerations
Thirty nine participants were randomized to the three study arms (control, 150 min·wk−1, 300 min·wk−1), toward the goal of having 30 participants (10 per arm) with study endpoint data (76% completion rate). This sample size provides adequate statistical power for the primary outcome biomarkers (sICAM-1 and sVICAM-1) with a hypothesis for a linear dose-response trend. Against the hypothesis of a dose-response relationship with decreases of 22 and 44 units for low-dose and high-dose respectively for sICAM-1 and 74 and 148 units for sVCAM-1, 30 participants provide 80% power for two tests (one for sICAM-1 and one for sVCAM-1), each tested with a type I error rate of 0.025, to maintain the experiment-wise overall error rate of 0.05. The estimated change in sICAM-1 and sVCAM-1 is clinically meaningful and consistent with prior exercise interventions in non-cancer populations [27, 55-58].
The target sample size provides sufficient statistical power to examine key secondary outcomes that include VAT and fasting insulin. Against the hypothesis of a dose-response relationship with an increase in VAT of +8.6% (+14 cm2) in the control group, +1.7% (+2.9 cm2) in the low-dose group, and −6.9% (−11.6 cm2) in the high-dose group and a pooled standard deviation of ±17%, 30 participants provide 80% power tested with a type I error rate of 0.05. These changes are estimated from a prior dose-response exercise study [59-61]. Against the hypothesis of a dose-response relationship with an increase in fasting insulin of +1.1 μU/mL in the control group, –0.5 μU/mL in the low-dose group, and –0.9 μU/mL in the high-dose group, and a pooled standard deviation of ±0.7 μU/mL, 30 participants provide 80% power tested with a type I error rate of 0.05. These changes are estimated from a prior dose-response exercise study [62]. The exploratory outcomes for this trial include enumeration of CTCs, functional status, and patient-reported outcomes and quality of life measures. There are limited data to determine what proportion of colon cancer survivors who have completed adjuvant therapy for colon cancer will have enumerable CTCs. Further, it is unknown how exercise may impact CTC volume. Consequently, this outcome is considered exploratory with no directional hypothesis. We hypothesize that functional status, and patient-reported outcomes and quality of life measures will improve in dose-response fashion.
To identify characteristics associated with inquiry about study participation and randomization onto the study protocol we had 80% power to detect an odds ratio as small as 1.3 and 1.7, respectively. Both sets of analyses were tested with the type I error rate of 0.05.
4.8 Statistical Analysis
The feasibility of exercise is quantified by comparing the proportion of participants in each group who achieve ≥80% of their prescribed exercise dose. The safety of exercise is quantified by comparing the proportion of participants who experience adverse events in each of the three study groups. For each of the primary and secondary physiologic outcomes, a linear mixed-effects regression model is used to compare the change in biomarker levels for each group while adjusting for the baseline value of the dependent variable. All analyses follow an intention-to-treat approach. Models are fit to include dose as a linear term, and model fit is examined using standard methods. Alternatives to a linear dose trend include transformations of dose, such as average actual dose, log-transformed dose per week, or second-order polynomial (i.e., dose and dose2). In the absence of a dose-response relationship, each of the two intervention groups (low-dose and high-dose exercise) is compared to the control arm using a two-sided t-test.
Characteristics associated with inquiry about study participation and randomization onto the study protocol are identified using two sets of logistic regression analyses: one set to compare characteristics between those who inquired about study participation versus those who do not inquire about participation; and one set to compare characteristics between those who were randomized onto the study protocol versus those who were not randomized onto the study protocol. Characteristics with P<0.20 in univariable models are entered simultaneously in a multivariable model.
5 RESULTS
Between January and July 2015, 1,435 letters were delivered to potentially-eligible persons with an ICD-O-3 code for colon cancer (Table 1). Two recipients of the study letter expressed concern about disclosure of private health information (0.001%). These two recipients clarified their concerns with the principal investigator and were provided with the appropriate contact at the PCR. These two participants were excluded from the analyses presented herein reducing the analytic sample to 1,433. Colon cancer survivors who were invited to participate in this trial were representative of colon cancer survivors in the United States with respect to age, sex, race, and disease stage [1]. The median distance from the University of Pennsylvania to the zip-code centroid of survivors invited to participate was 20 kilometers [interquartile 25-75% range: 10-33] and ranged from 0.4 (~4 city blocks) to 71 kilometers.
Table 1.
Demographic, Clinical, and Recruitment Characteristics Associated with Study Inquiry and Randomization
| Invited to Participate (n=1,433) |
Inquired about Participation (n=126) |
Randomized (n=39) |
|||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Characteristic | n (%) or Median [IQR] |
n (%) or Median [IQR] |
OR (95% CI)a | P | n (%) or Median [IQR] |
OR (95% CI)b | P |
| Age,c y | 68 [58-77] | 59 [53-59] | 0.82 (0.76-0.88) | <0.001 | 55 [47-61] | 0.69 (0.60-0.78) | <0.001 |
| <70 y | 769 (54%) | 95 (75%) | 1.00 | 36 (92%) | 1.00 | ||
| ≥70 y | 664 (46%) | 31 (25%) | 0.35 (0.23-0.53) | <0.001 | 3 (8%) | 0.09 (0.03-0.30) | <0.001 |
| Sex, n (%) | |||||||
| Male | 710 (50%) | 55 (44%) | 1.00 | 15 (38%) | 1.00 | ||
| Female | 723 (50%) | 71 (56%) | 1.29 (0.90-1.87) | 0.167 | 24 (62%) | 1.59 (0.83-3.06) | 0.164 |
| Race, n (%) | |||||||
| White | 1,086 (76%) | 97 (77%) | 1.00 | 31 (79%) | 1.00 | ||
| Nonwhite | 320 (22%) | 29 (23%) | 1.02 (0.66-1.57) | 0.943 | 8 (21%) | 1.59 (0.83-3.06) | 0.164 |
| Missing or Unknown | 27 (2%) | 0 (0%) | — | — | 0 (0%) | — | — |
| Time Since Diagnosis,c mo | 16 [10-22] | 16 [11-21] | 15 [10-21] | ||||
| ≤12 mo. | 466 (32%) | 37 (29%) | 1.00 | 14 (36%) | 1.00 | ||
| 12-18 mo. | 384 (27%) | 36 (29%) | 1.20 (0.74-1.94) | 0.458 | 8 (20%) | 0.69 (0.28-1.65) | 0.403 |
| ≥18 mo. | 583 (41%) | 53 (42%) | 1.16 (0.75-1.80) | 0.509 | 17 (44%) | 0.97 (0.47-1.99) | 0.933 |
| Anatomical Location, n (%) | |||||||
| Left Colon | 534 (38%) | 57 (45%) | 1.00 | 18 (46%) | 1.00 | ||
| Right Colon | 866 (60%) | 67 (53%) | 0.70 (0.48-1.02) | 0.061 | 21 (54%) | 0.71 (0.38-1.35) | 0.298 |
| Unknown or Multiple | 33 (2%) | 2 (2%) | 0.54 (0.13-2.31) | 0.407 | 0 (0%) | — | — |
| AJCC Stage,d n (%) | |||||||
| I | 349 (24%) | 28 (22%) | 1.00 | 5 (13%) | 1.00 | ||
| II | 523 (37%) | 44 (35%) | 1.05 (0.64-1.73) | 0.838 | 14 (36%) | 1.89 (0.67-5.30) | 0.225 |
| III | 561 (39%) | 54 (43%) | 1.22 (0.76-1.97) | 0.412 | 20 (51%) | 2.54 (0.95-6.84) | 0.064 |
| Invasion Through Bowel Wall (T Stage), n (%) |
|||||||
| T1 | 247 (17%) | 23 (18%) | 1.00 | 4 (10%) | 1.00 | ||
| T2 | 174 (12%) | 10 (8%) | 0.59 (0.27-1.28) | 0.184 | 2 (5%) | 0.71 (0.13-3.90) | 0.690 |
| T3 | 773 (54%) | 74 (59%) | 1.03 (0.63-1.68) | 0.903 | 25 (64%) | 2.03 (0.70-5.89) | 0.193 |
| T4 | 239 (17%) | 19 (15%) | 0.84 (0.44-1.59) | 0.594 | 8 (21%) | 2.10 (0.62-7.08) | 0.230 |
| Lymph Node Involvement (N Stage), n (%) |
|||||||
| N0 (0) | 872 (61%) | 72 (57%) | 1.00 | 19 (49%) | 1.00 | ||
| N1 (1-3) | 381 (27%) | 34 (27%) | 1.09 (0.71-1.67) | 0.696 | 15 (38%) | 1.84 (0.92-3.66) | 0.082 |
| N2 (≥4) | 180 (12%) | 20 (16%) | 1.39 (0.82-2.34) | 0.219 | 5 (13%) | 1.28 (0.47-3.48) | 0.625 |
| Grade of Differentiation, n (%) | |||||||
| Well (low) | 123 (9%) | 6 (5%) | 1.00 | 2 (5%) | 1.00 | ||
| Moderate (intermediate) | 940 (66%) | 85 (67%) | 1.94 (0.83-4.54) | 0.127 | 29 (74%) | 1.92 (0.45-8.17) | 0.374 |
| Poor or Undifferentiated | 275 (19%) | 25 (20%) | 1.94 (0.78-4.88) | 0.154 | 5 (13%) | 1.12 (0.21-5.85) | 0.893 |
| (high) | |||||||
| Unknown | 95 (6%) | 10 (8%) | 2.29 (0.80-6.56) | 0.121 | 3 (8%) | 1.97 (0.32-12.05) | 0.462 |
| Lymphovascular Invasion, n (%) |
|||||||
| Absent | 876 (61%) | 73 (58%) | 1.00 | 23 (59%) | 1.00 | ||
| Present | 391 (27%) | 36 (29%) | 1.11 (0.73-1.69) | 0.609 | 12 (31%) | 1.17 (0.58-2.38) | 0.657 |
| Unknown | 166 (12%) | 17 (13%) | 1.25 (0.72-2.19) | 0.423 | 4 (10%) | 0.91 (0.31-2.68) | 0.872 |
| Chemotherapy, n (%) | 709 (50%) | 77 (61%) | 1.68 (1.15-2.44) | 0.007 | 28 (72%) | 2.66 (1.32-5.39) | 0.006 |
| Month Study Letter Mailed, n (%) |
|||||||
| January | 865 (60%) | 85 (67%) | 1.00 | 31 (79%) | 1.00 | ||
| March | 100 (7%) | 7 (6%) | 0.69 (0.31-1.54) | 0.365 | 1 (3%) | 0.27 (0.04-2.01) | 0.202 |
| May | 119 (8%) | 6 (5%) | 0.49 (0.21-1.14) | 0.098 | 2 (5%) | 0.46 (0.11-1.94) | 0.291 |
| July | 349 (24%) | 28 (22%) | 0.80 (0.51-1.25) | 0.328 | 5 (13%) | 0.39 (0.15-1.01) | 0.053 |
| Distance to Institution,c km | 20 [10-34] | 21 [9-26] | 1.03 (0.97-1.09) | 0.327 | 20 [9-40] | 1.07 (0.97-1.17) | 0.182 |
Odds Ratio (OR) and 95% Confidence Interval (CI) comparing those who inquired about participation to those who were invited to participate.
Odds Ratio (OR) and 95% Confidence Interval (CI) comparing those who were randomized to those who were invited to participate.
Median and Interquartile [25-75%] range.
AJCC: American Joint Committee on Cancer (7th edition).
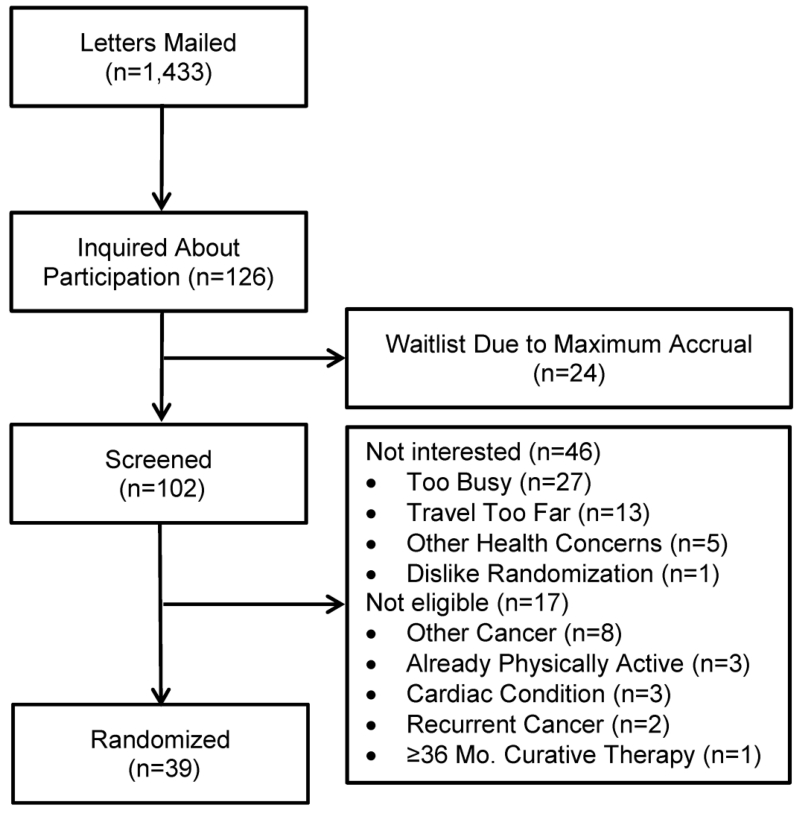
Among the 1,433 colon cancer survivors invited to participate, 126 (8.8%) inquired about participation (Figure 2). Eleven letters were mailed to screen one potentially-eligible participant. In univariate analysis, colon cancer survivors who inquired about participation were younger [Odds Ratio: 0.82 per 5-year increment (95% CI: 0.76-0.88); P<0.001; Table 2], and were more likely to be treated with chemotherapy [Odds Ratio: 1.68 (95% CI: 1.15-2.44); P=0.007], compared to those who did not inquire about participation. In a multivariable model, age was associated with inquiring about study participation [Odds Ratio: 0.83 per 5-year increment (95% CI: 0.77-0.89); P<0.001], but treatment with chemotherapy was no longer statistically significant [Odds Ratio: 1.43 (95% CI: 0.98-2.10); P=0.066].
Figure 2.
Consort Diagram
Among the 126 colon cancer survivors who inquired about participation, 102 were screened (81%). The remaining 24 inquiries were not screened due to the study reaching its accrual goal. Among the 102 colon cancer survivors screened, 46 (45%) were not interested in participating after learning more about the study. The most commonly cited reasons for not being interested in the study were: being too busy (n=27; 59%); traveling into the city of Philadelphia would be too burdensome (n=13; 28%); having other health concerns (n=5; 11%); and not wanting to be randomized to a group that may not include exercise (n=1; 2%). Among the 102 colon cancer survivors screened, 17 (17%) were not eligible for participation after completing the telephone interview. The most common reasons for ineligibility included: having a history of another cancer (n=8; 47%); currently exercising ≥150 min·wk−1 (n=3; 18%); having a cardiac condition (n=3; 18%); having recurrent or metastatic colon cancer (n=2; 12%); and being ≥36 months post cancer therapy (n=1; 5%).
Among the 102 colon cancer survivors who were screened, 39 (38%) were randomized onto the study protocol. Thirty seven letters were mailed to randomize one participant onto the study protocol. Thirty-two participants were recruited during phase 1 eligibility (82%; most strict, January and March letter mailings), two participants in phase 2 (5%; less strict, May letter mailing), and five participants in phase 3 (13%; least strict, July letter mailing). It is not clear why recruitment was most successful during the more restrictive phases of recruitment. This may be due in part to the month in which participants were recruited which may have influenced their willingness to participate in a trial of lifestyle modification. Compared with mailings in the month of January, mailings in the months of March, May, and July were associated with lower study inquiry and randomization rates, though these comparisons did not reach the threshold for statistical significance. No participants had their physician decline approval to participate in the study. In univariate analyses, colon cancer survivors who were randomized onto the study protocol were younger [Odds Ratio: 0.69 per 5-year increment (95% CI: 0.60-0.78); P<0.001], and were more likely to be treated with chemotherapy [Odds Ratio: 2.66 (95% CI: 1.32-5.39); P=0.006]. In a multivariable model, age was associated with randomization onto the study protocol [Odds Ratio: 0.70 per 5-year increment (95% CI: 0.61-0.79); P<0.001], and treatment with chemotherapy was attenuated to marginal statistical significance [Odds Ratio: 2.01 (95% CI: 0.98-4.13); P=0.056].
Among participants randomized to one of the two exercise groups, the median time from randomization to treadmill delivery and setup was 12 days [interquartile 25-75% range: 10-15]. The final trial participant was randomized in August 2015. Six month endpoint data collection was completed in February 2016.
6 DICUSSION
The COURAGE trial is an NCI sponsored, phase II, randomized, dose-response exercise trial that seeks to test the feasibility, safety, and physiologic effects of two distinct doses of moderate-intensity aerobic exercise compared to a usual-care control group among colon cancer survivors. The results of this trial will provide preliminary information regarding the appropriate dose of aerobic exercise to optimize biomarkers that may mediate the relationship between exercise and colon cancer recurrence and clarify important design aspects for future phase 2 and phase 3 randomized controlled trials.
The primary recruitment method for the COURAGE trial was postal mailings to potentially-eligible colon cancer survivors identified within the PCR. Historically the recruitment of survivors of colon cancer into lifestyle modification trials has been challenging [63-67]. The reasons for this have not been fully explained, but may be due in part to the fact that colon cancer survivors are often older, have other comorbid health conditions, or residual toxicity from treatment (i.e., neuropathy), each of which may influence willingness to participate in a program of lifestyle modification [68]. Several studies have described their experience in recruiting colon cancer survivors into trials of lifestyle modification. Pinto et al. recruited colon cancer survivors to a telephone-based physical activity intervention that aimed to increase participation in physical activity and improve self-reported quality-of-life outcomes [63]. Despite implementing various recruitment strategies (informational mailings, in-clinic recruitment, and community presentations), they were unable to recruit the required sample size of 134 participants; only randomizing 46 colon cancer survivors over 39 months (~1.5 participants per month). The Challenge trial is a randomized phase 3 trial that seeks to recruit high-risk stage II and stage III colon cancer survivors to a three year physical activity program that aims to improve disease-free survival [52]. When the Challenge trial was designed, recruitment of 962 colon cancer survivors was estimated to require 36 months (~27 participants per month). Despite being open at 20 centers in Canada and 22 centers in Australia, over 55-months, 250 colon cancer survivors have been randomized (~4.5 participants per month) [65]. At a single institution over seven months, the COURAGE trial randomized 39 colon cancer survivors (~5.5 participants per month). At the time the trial met its planned accrual goal and closed to enrollment, an additional 24 potentially-eligible colon cancer survivors expressed interest in participating and were waiting to be screened for trial enrollment.
Each of the above-described trials is unique in their eligibility criteria, intervention characteristics, and endpoints of interest, our recruitment experience indicates that utilizing population-based cancer registries to recruit colon cancer survivors into a lifestyle modification trial is feasible. The use of population-based cancer registries is disseminable to other cancer centers for accrual in multicenter trials, and may help to address an important barrier in the conduct of future lifestyle modification trials in this population. A potential limitation to the use of population-based cancer registries is the six to nine month delay that may occur from the time of diagnosis to entry into the registry. This limitation is of importance for trials that seek to modify behavior during or shortly after the completion of adjuvant therapy. To address this limitation, many cancer registries now offer rapid case ascertainment which accelerates the reporting process to within one month of diagnosis [69]. Another explanation to the high inquiry and screening rates in this study was the provision of an in-home treadmill. The mailed flyer did describe this novel feature of the study. As intended, this may have provided additional incentive to inquire about participation. Given the potential benefit of exercise to improve disease outcomes, developing methods to recruit colon cancer survivors to participate in lifestyle modification trials is of critically high importance.
Colon cancer survivors who inquired about trial participation were younger than those who did not inquire about trial participation. Similarly, colon cancer survivors who were randomized onto the trial protocol were younger than those who were not randomized. This pattern is common to many trials, regardless of the intervention. In an analysis of 21 Southwest Oncology Group (SWOG) therapeutic trials that included 5,190 cancer patients with 21 types of cancer, trial enrollees were significantly younger than non-enrollees (P<0.001) [70]. These findings underscore the need for continued research to identify methods to communicate opportunities about clinical trial participation to older adults in an efficient and scalable manner. In our multivariable model, treatment with chemotherapy was of marginal statistical significance for both inquiry about study participation (P=0.066) and randomization onto the study protocol (P=0.056). This finding indicates the interest of patients with a higher probability of recurrence (i.e., high-risk stage II and stage III) to inquire and participate in a clinical trial of lifestyle modification. Collectively, these data indicate that with exception of age, COURAGE trial participants are similar to the broader community of colon cancer survivors on measured characteristics, such as sex, race, time since diagnosis, clinico-pathologic tumor features, and geographic proximity to the University of Pennsylvania.
In addition to obtaining important information about the feasibility and safety of the dose-response effects of exercise among colon cancer survivors, this trial will gather preliminary data regarding physiologic changes in biomarkers that are hypothesized to mediate the relationship between exercise and disease outcomes. sICAM-1 and sVCAM-1 are cell-adhesion molecules that promote the growth of existing micro-metastases, and promote CTC differentiation, contact inhibition, and apoptosis [71]. The down regulation of ICAM-1 attenuates the invasive potential of colon cancer cells and has been recommended as a therapeutic target [72]. VCAM-1 has also been recommended as a therapeutic target [73]. ICAM-1 and VCAM-1 have also been demonstrated to influence the metastatic potential of melanoma and gastric cancers [74, 75]. We hypothesize that exercise may inhibit both seeding of distant organs and the cultivation of the angiogenic milieu that is thought to be required for the growth of micro-metastases. We speculate that exercise may have direct effects on anti-cancer myokines, such as secreted protein acidic and rich in cysteine (SPARC) [76], and may also have indirect effects through pathways that include improved metabolic homeostasis (i.e., VAT and insulin). Several studies have demonstrated that abdominal adiposity, particularly VAT, is associated with poor colon cancer outcomes [77-82]. VAT associates with insulin among colon cancer survivors (r=0.519; P<0.001) [83], and is implicated in the recurrence of colon cancer [84, 85]. We hypothesize that exercise may reduce VAT and fasting insulin and potentially inhibit the growth of existing micro-metastases. As an exploratory aim, we will examine CTCs using the innovative GEDI platform [29]. We hypothesize that CTCs disseminate via the circulation during the earliest stages of recurrent metastatic growth, mirroring what occurs during the primary tumor setting. If this does indeed occur, we are uniquely situated to detect this phenomenon using GEDI. If exercise delays or inhibits metastases, this may be reflected in CTC concentration.
There are several strengths of this trial. The use of two intervention groups, each prescribed a distinct dose of exercise will allow us examine how feasibility, safety, and physiologic effects differ along the exercise dose curve. Given the eligibly criteria, these two different doses of exercise will require that some participants modestly increase their exercise and others to increase over 10-fold. The exercise intervention was designed to allow for scalability to larger phase 2 and ultimately a phase 3 clinical trial. The exercise program allows for flexibility, emphasizing a home-based program, blended with supervised training and ongoing behavioral and clinical support from an exercise physiologist. The provision of home-based treadmills serves a dual purpose of providing a reasonable incentive for participation, and promoting feasibility and favorable adherence to the exercise prescription over six months.
There exist several limitations to this trial. The primary limitation to this study is the small sample size which may limit interpretation of the study findings. However, this study was designed to gather important feasibility, safety, and preliminary physiologic data to refine important design aspects for future phase 2 and phase 3 trials. We acknowledge 300 min·wk−1 is a large weekly dose of aerobic exercise. We elected to compare 150 and 300 min·wk−1 of aerobic exercise to complement ongoing work which prescribes 225 min·wk−1 [52, 67] and to parallel the epidemiologic data that suggest a dose-response relationship with disease outcomes [9-16]. Our research team has been successful in promoting adherence to the prescription of 300 min·wk−1 of aerobic exercise in our prior studies [53, 54]. The high-dose exercise group received five additional in-person sessions with the exercise physiologist which may differentially impact adherence rates between the low-dose and high-dose exercise groups. Although VAT and fasting insulin are secondary outcomes of this trial, participants were not recruited on the basis of being overweight or obese at baseline and/or having high levels of fasting insulin. Consequently this may limit our ability to detect significant exercise-induced improvements in these outcomes. We considered the inclusion of survivors of rectal cancer, but restricted inclusion to colon cancer survivors, as there is limited evidence to support the benefit of exercise in improving rectal cancer outcomes [10].
7 CONCLUSION
In conclusion, the findings from this trial will be useful in understanding the feasibility, safety, and physiologic effects of two doses of aerobic exercise among colon cancer survivors. These findings contribute toward the goal of conducting a definitive trial to assess the effects of exercise on disease outcomes among colon cancer survivors.
Acknowledgements
This research was supported by R21-CA182767, F31-CA192560 and U54-CA155850 from the National Cancer Institute, P30-DK019525 from the National Institute of Diabetes and Digestive and Kidney Diseases, and UL1-TR000003 from the National Center for Research Resources and the National Center for Advancing Translation Science. This research was supported by discounts for treadmills from LifeSpan Fitness, LLC (Salt Lake City, UT). We gratefully thank the Pennsylvania Cancer Registry for their role in recruitment activities for this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
The authors declare no conflicts of interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- [1].Siegel R, DeSantis C, Jemal A. Colorectal cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64:104–17. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- [2].DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, et al. Cancer treatment and survivorship statistics, 2014. CA: A Cancer Journal for Clinicians. 2014 doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- [3].Clinical Outcomes of Surgical Therapy Study Group A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050–9. doi: 10.1056/NEJMoa032651. [DOI] [PubMed] [Google Scholar]
- [4].Lacy AM, Garcia-Valdecasas JC, Delgado S, Castells A, Taura P, Pique JM, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002;359:2224–9. doi: 10.1016/S0140-6736(02)09290-5. [DOI] [PubMed] [Google Scholar]
- [5].Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med. 2005;352:476–87. doi: 10.1056/NEJMra040958. [DOI] [PubMed] [Google Scholar]
- [6].André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–51. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- [7].Quasar Collaborative Group Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. The Lancet. 2007;370:2020–9. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]
- [8].Sargent DJ, Wieand HS, Haller DG, Gray R, Benedetti JK, Buyse M, et al. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2005;23:8664–70. doi: 10.1200/JCO.2005.01.6071. [DOI] [PubMed] [Google Scholar]
- [9].Meyerhardt JA, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24:3535–41. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- [10].Meyerhardt JA, Giovannucci EL, Ogino S, Kirkner GJ, Chan AT, Willett W, et al. Physical activity and male colorectal cancer survival. Arch Intern Med. 2009;169:2102–8. doi: 10.1001/archinternmed.2009.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Campbell PT, Patel AV, Newton CC, Jacobs EJ, Gapstur SM. Associations of recreational physical activity and leisure time spent sitting with colorectal cancer survival. J Clin Oncol. 2013;31:876–85. doi: 10.1200/JCO.2012.45.9735. [DOI] [PubMed] [Google Scholar]
- [12].Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, Colditz GA, et al. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24:3527–34. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- [13].Kuiper JG, Phipps AI, Neuhouser ML, Chlebowski RT, Thomson CA, Irwin ML, et al. Recreational physical activity, body mass index, and survival in women with colorectal cancer. Cancer Causes Control. 2012 doi: 10.1007/s10552-012-0071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Je Y, Jeon JY, Giovannucci EL, Meyerhardt JA. Association between physical activity and mortality in colorectal cancer: a meta-analysis of prospective cohort studies. International Journal of Cancer. 2013;133:1905–13. doi: 10.1002/ijc.28208. [DOI] [PubMed] [Google Scholar]
- [15].Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Ann Oncol. 2014;25:1293–311. doi: 10.1093/annonc/mdu012. [DOI] [PubMed] [Google Scholar]
- [16].Des Guetz G, Uzzan B, Bouillet T, Nicolas P, Chouahnia K, Zelek L, et al. Impact of physical activity on cancer-specific and overall survival of patients with colorectal cancer. Gastroenterology research and practice. 2013:2013. doi: 10.1155/2013/340851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012 doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- [18].Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, et al. American college of sports medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409–26. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- [19].National Comprehensive Cancer network NCCN Clinical Practice Guidelines in Oncology: Survivorship. 2013:2013. [Google Scholar]
- [20].Paffenbarger RS, Jr, Blair SN, Lee IM, Hyde RT. Measurement of physical activity to assess health effects in free-living populations. Med Sci Sports Exerc. 1993;25:60–70. doi: 10.1249/00005768-199301000-00010. [DOI] [PubMed] [Google Scholar]
- [21].Pennsylvania Department of Health . Pennsylvania Cancer Registry (PCR) [Google Scholar]
- [22].Rogerino A, Grant LL, Wilcox H, 3rd, Schmitz KH. Geographic recruitment of breast cancer survivors into community-based exercise interventions. Med Sci Sports Exerc. 2009;41:1413–20. doi: 10.1249/MSS.0b013e31819af871. [DOI] [PubMed] [Google Scholar]
- [23].Shephard RJ. Readiness for physical activity: President’s Council on Physical Fitness and Sports. 1994. [Google Scholar]
- [24].Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. Springer; New York: 2010. [Google Scholar]
- [25].Von Korff M, Wagner EH, Saunders K. A chronic disease score from automated pharmacy data. J Clin Epidemiol. 1992;45:197–203. doi: 10.1016/0895-4356(92)90016-g. [DOI] [PubMed] [Google Scholar]
- [26].Craft LL, Guralnik JM, Ferrucci L, Liu K, Tian L, Criqui MH, et al. Physical activity during daily life and circulating biomarker levels in patients with peripheral arterial disease. Am J Cardiol. 2008;102:1263–8. doi: 10.1016/j.amjcard.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Adamopoulos S, Parissis J, Kroupis C, Georgiadis M, Karatzas D, Karavolias G, et al. Physical training reduces peripheral markers of inflammation in patients with chronic heart failure. Eur Heart J. 2001;22:791–7. doi: 10.1053/euhj.2000.2285. [DOI] [PubMed] [Google Scholar]
- [28].Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–95. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- [29].Gleghorn JP, Pratt ED, Denning D, Liu H, Bander NH, Tagawa ST, et al. Capture of circulating tumor cells from whole blood of prostate cancer patients using geometrically enhanced differential immunocapture (GEDI) and a prostate-specific antibody. Lab Chip. 2010;10:27–9. doi: 10.1039/b917959c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zamboni M, Turcato E, Armellini F, Kahn H, Zivelonghi A, Santana H, et al. Sagittal abdominal diameter as a practical predictor of visceral fat. Int J Obes. 1998;22:655–60. doi: 10.1038/sj.ijo.0800643. [DOI] [PubMed] [Google Scholar]
- [31].Micklesfield LK, Goedecke JH, Punyanitya M, Wilson KE, Kelly TL. Dual-Energy X-Ray Performs as Well as Clinical Computed Tomography for the Measurement of Visceral Fat. Obesity. 2012;20:1109–14. doi: 10.1038/oby.2011.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bredella MA, Gill CM, Keating LK, Torriani M, Anderson EJ, Punyanitya M, et al. Assessment of abdominal fat compartments using DXA in premenopausal women from anorexia nervosa to morbid obesity. Obesity. 2013;21:2458–64. doi: 10.1002/oby.20424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- [34].Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, Taylor DW, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132:919–23. [PMC free article] [PubMed] [Google Scholar]
- [35].Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- [36].Bassett DR, Jr, Ainsworth BE, Swartz AM, Strath SJ, O’Brien WL, King GA. Validity of four motion sensors in measuring moderate intensity physical activity. Med Sci Sports Exerc. 2000;32:S471–80. doi: 10.1097/00005768-200009001-00006. [DOI] [PubMed] [Google Scholar]
- [37].Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–8. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- [38].Luhrmann PM, Herbert BM, Gaster C, Neuhauser-Berthold M. Validation of a self-administered 3-day estimated dietary record for use in the elderly. Eur J Nutr. 1999;38:235–40. doi: 10.1007/s003940050066. [DOI] [PubMed] [Google Scholar]
- [39].Harrington CB, Hansen JA, Moskowitz M, Todd BL, Feuerstein M. It’s not over when it’s over: long-term symptoms in cancer survivors--a systematic review. Int J Psychiatry Med. 2010;40:163–81. doi: 10.2190/PM.40.2.c. [DOI] [PubMed] [Google Scholar]
- [40].Committee on Cancer Survivorship: Institute of Medicine and national Research Board, editor. From Cancer Patient to Cancer Survivor: Lost in Transition. The National Academic Press; Washington, DC: 2006. [Google Scholar]
- [41].Ward WL, Hahn EA, Mo F, Hernandez L, Tulsky DS, Cella D. Reliability and validity of the Functional Assessment of Cancer Therapy-Colorectal (FACT-C) quality of life instrument. Qual Life Res. 1999;8:181–95. doi: 10.1023/a:1008821826499. [DOI] [PubMed] [Google Scholar]
- [42].Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- [43].Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–38. [PubMed] [Google Scholar]
- [44].Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- [45].Haddock MG, Sloan JA, Bollinger JW, Soori G, Steen PD, Martenson JA, et al. Patient assessment of bowel function during and after pelvic radiotherapy: results of a prospective phase III North Central Cancer Treatment Group clinical trial. J Clin Oncol. 2007;25:1255–9. doi: 10.1200/JCO.2006.09.0001. [DOI] [PubMed] [Google Scholar]
- [46].Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, et al. The rapid assessment of fatigue severity in cancer patients: Use of the brief fatigue inventory. Cancer. 1999;85:1186–96. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- [47].Simard S, Savard J. Fear of Cancer Recurrence Inventory: development and initial validation of a multidimensional measure of fear of cancer recurrence. Supportive care in cancer. 2009;17:241–51. doi: 10.1007/s00520-008-0444-y. [DOI] [PubMed] [Google Scholar]
- [48].US Department of Health and Human Services . Common terminology criteria for adverse events (CTCAE) version 4.0. National Institutes of Health, National Cancer Institute; 2009. p. 4. [Google Scholar]
- [49].Brown JC, Troxel AB, Schmitz KH. Safety of weightlifting among women with or at risk for breast cancer-related lymphedema: musculoskeletal injuries and health care use in a weightlifting rehabilitation trial. Oncologist. 2012;17:1120–8. doi: 10.1634/theoncologist.2012-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Warren M, Schmitz KH. Safety of strength training in premenopausal women: musculoskeletal injuries from a two-year randomized trial. Am J Health Promot. 2009;23:309–14. doi: 10.4278/ajhp.07081584. [DOI] [PubMed] [Google Scholar]
- [51].Weinberg RS. Goal setting and performance in sport and exercise settings: a synthesis and critique. Med Sci Sports Exerc. 1994;26:469–77. [PubMed] [Google Scholar]
- [52].Courneya KS, Booth CM, Gill S, O’Brien P, Vardy J, Friedenreich CM, et al. The Colon Health and Life-Long Exercise Change trial: a randomized trial of the National Cancer Institute of Canada Clinical Trials Group. Curr Oncol. 2008;15:279–85. doi: 10.3747/co.v15i6.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Schmitz KH, Williams NI, Kontos D, Domchek S, Morales KH, Hwang W, et al. Dose Response Effects of Aerobic Exercise on Estrogen Among Women at High Risk for Breast Cancer: A Randomized Controlled Trial. Breast Cancer Res Treat. 2015 doi: 10.1007/s10549-015-3604-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Schmitz KH, Williams NI, Kontos D, Kurzer M, Schnall M, Domchek S, et al. Women In Steady Exercise Research (WISER) Sister: Study Design and Methods. Contemporary clinical trials. 2015 doi: 10.1016/j.cct.2014.12.016. [DOI] [PubMed] [Google Scholar]
- [55].Hamdy O, Ledbury S, Mullooly C, Jarema C, Porter S, Ovalle K, et al. Lifestyle modification improves endothelial function in obese subjects with the insulin resistance syndrome. Diabetes Care. 2003;26:2119–25. doi: 10.2337/diacare.26.7.2119. [DOI] [PubMed] [Google Scholar]
- [56].Leinonen E, Hurt-Camejo E, Wiklund O, Hulten LM, Hiukka A, Taskinen MR. Insulin resistance and adiposity correlate with acute-phase reaction and soluble cell adhesion molecules in type 2 diabetes. Atherosclerosis. 2003;166:387–94. doi: 10.1016/s0021-9150(02)00371-4. [DOI] [PubMed] [Google Scholar]
- [57].Tonjes A, Scholz M, Fasshauer M, Kratzsch J, Rassoul F, Stumvoll M, et al. Beneficial effects of a 4-week exercise program on plasma concentrations of adhesion molecules. Diabetes Care. 2007;30:e1. doi: 10.2337/dc06-1760. [DOI] [PubMed] [Google Scholar]
- [58].Saetre T, Enoksen E, Lyberg T, Stranden E, Jorgensen JJ, Sundhagen JO, et al. Supervised exercise training reduces plasma levels of the endothelial inflammatory markers E-selectin and ICAM-I in patients with peripheral arterial disease. Angiology. 2011;62:301–5. doi: 10.1177/0003319710385338. [DOI] [PubMed] [Google Scholar]
- [59].Slentz CA, Aiken LB, Houmard JA, Bales CW, Johnson JL, Tanner CJ, et al. Inactivity, exercise, and visceral fat. STRRIDE: a randomized, controlled study of exercise intensity and amount. J Appl Physiol. 2005;99:1613–8. doi: 10.1152/japplphysiol.00124.2005. [DOI] [PubMed] [Google Scholar]
- [60].Slentz CA, Duscha BD, Johnson JL, Ketchum K, Aiken LB, Samsa GP, et al. Effects of the amount of exercise on body weight, body composition, and measures of central obesity: STRRIDE--a randomized controlled study. Arch Intern Med. 2004;164:31–9. doi: 10.1001/archinte.164.1.31. [DOI] [PubMed] [Google Scholar]
- [61].Slentz CA, Houmard JA, Kraus WE. Exercise, abdominal obesity, skeletal muscle, and metabolic risk: evidence for a dose response. Obesity. 2009;17:S27–33. doi: 10.1038/oby.2009.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Houmard JA, Tanner CJ, Slentz CA, Duscha BD, McCartney JS, Kraus WE. Effect of the volume and intensity of exercise training on insulin sensitivity. J Appl Physiol (1985) 2004;96:101–6. doi: 10.1152/japplphysiol.00707.2003. [DOI] [PubMed] [Google Scholar]
- [63].Pinto BM, Papandonatos GD, Goldstein MG, Marcus BH, Farrell N. Home-based physical activity intervention for colorectal cancer survivors. Psychooncology. 2013;22:54–64. doi: 10.1002/pon.2047. [DOI] [PubMed] [Google Scholar]
- [64].Ligibel JA, Meyerhardt J, Pierce JP, Najita J, Shockro L, Campbell N, et al. Impact of a telephone-based physical activity intervention upon exercise behaviors and fitness in cancer survivors enrolled in a cooperative group setting. Breast Cancer Res Treat. 2012;132:205–13. doi: 10.1007/s10549-011-1882-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Courneya KS, Vardy J, Gill S, Jonker D, O’Brien P, Friedenreich CM, et al. Update on the Colon Health and Life-Long Exercise Change trial: A phase III study of the impact of an exercise program on disease-free survival in colon cancer survivors. Current Colorectal Cancer Reports. 2014;10:321–8. [Google Scholar]
- [66].van Waart H, Stuiver MM, van Harten WH, Geleijn E, Kieffer JM, Buffart LM, et al. Effect of Low-Intensity Physical Activity and Moderate- to High-Intensity Physical Exercise During Adjuvant Chemotherapy on Physical Fitness, Fatigue, and Chemotherapy Completion Rates: Results of the PACES Randomized Clinical Trial. J Clin Oncol. 2015;33:1918–27. doi: 10.1200/JCO.2014.59.1081. [DOI] [PubMed] [Google Scholar]
- [67].Meyerhardt J. Exercise and Metformin in Colorectal and Breast Cancer Survivors. 2013. p. 2013. [Google Scholar]
- [68].Brown JC, Schmitz KH. The prescription or proscription of exercise in colorectal cancer care. Med Sci Sports Exerc. 2014;46:2202–9. doi: 10.1249/MSS.0000000000000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Beskow LM, Sandler RS, Weinberger M. Research recruitment through US central cancer registries: balancing privacy and scientific issues. Am J Public Health. 2006;96:1920. doi: 10.2105/AJPH.2004.061556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Unger JM, Barlow WE, Martin DP, Ramsey SD, Leblanc M, Etzioni R, et al. Comparison of survival outcomes among cancer patients treated in and out of clinical trials. J Natl Cancer Inst. 2014;106:dju002. doi: 10.1093/jnci/dju002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Paschos KA, Canovas D, Bird NC. The role of cell adhesion molecules in the progression of colorectal cancer and the development of liver metastasis. Cell Signal. 2009;21:665–74. doi: 10.1016/j.cellsig.2009.01.006. [DOI] [PubMed] [Google Scholar]
- [72].Howard K, Lo KK, Ao L, Gamboni F, Edil BH, Schulick R, et al. Intercellular adhesion molecule-1 mediates murine colon adenocarcinoma invasion. J Surg Res. 2014;187:19–23. doi: 10.1016/j.jss.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Schlesinger M, Bendas G. Vascular cell adhesion molecule-1 (VCAM-1)—An increasing insight into its role in tumorigenicity and metastasis. International Journal of Cancer. 2015;136:2504–14. doi: 10.1002/ijc.28927. [DOI] [PubMed] [Google Scholar]
- [74].Giavazzi R, Chirivi RG, Garofalo A, Rambaldi A, Hemingway I, Pigott R, et al. Soluble intercellular adhesion molecule 1 is released by human melanoma cells and is associated with tumor growth in nude mice. Cancer Res. 1992;52:2628–30. [PubMed] [Google Scholar]
- [75].Nakashio T, Narita T, Sato M, Akiyama S, Kasai Y, Fujiwara M, et al. The association of metastasis with the expression of adhesion molecules in cell lines derived from human gastric cancer. Anticancer Res. 1997;17:293–9. [PubMed] [Google Scholar]
- [76].Aoi W, Naito Y, Takagi T, Tanimura Y, Takanami Y, Kawai Y, et al. A novel myokine, secreted protein acidic and rich in cysteine (SPARC), suppresses colon tumorigenesis via regular exercise. Gut. 2013;62:882–9. doi: 10.1136/gutjnl-2011-300776. [DOI] [PubMed] [Google Scholar]
- [77].Rickles AS, Iannuzzi JC, Mironov O, Deeb A, Sharma A, Fleming FJ, et al. Visceral obesity and colorectal cancer: are we missing the boat with BMI? Journal of Gastrointestinal Surgery. 2013;17:133–43. doi: 10.1007/s11605-012-2045-9. [DOI] [PubMed] [Google Scholar]
- [78].Moon HG, Ju YT, Jeong CY, Jung EJ, Lee YJ, Hong SC, et al. Visceral obesity may affect oncologic outcome in patients with colorectal cancer. Ann Surg Oncol. 2008;15:1918–22. doi: 10.1245/s10434-008-9891-4. [DOI] [PubMed] [Google Scholar]
- [79].Guiu B, Petit JM, Bonnetain F, Ladoire S, Guiu S, Cercueil J, et al. Visceral fat area is an independent predictive biomarker of outcome after first-line bevacizumab-based treatment in metastatic colorectal cancer. Gut. 2010;59:341–7. doi: 10.1136/gut.2009.188946. [DOI] [PubMed] [Google Scholar]
- [80].Ballian N, Lubner MG, Munoz A, Harms BA, Heise CP, Foley EF, et al. Visceral obesity is associated with outcomes of total mesorectal excision for rectal adenocarcinoma. J Surg Oncol. 2012;105:365–70. doi: 10.1002/jso.22031. [DOI] [PubMed] [Google Scholar]
- [81].Haydon AM, Macinnis RJ, English DR, Giles GG. Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut. 2006;55:62–7. doi: 10.1136/gut.2005.068189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Prizment AE, Flood A, Anderson KE, Folsom AR. Survival of women with colon cancer in relation to precancer anthropometric characteristics: the Iowa Women’s Health Study. Cancer Epidemiology Biomarkers & Prevention. 2010;19:2229–37. doi: 10.1158/1055-9965.EPI-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Jiang B, Zhang X, Du L, Wang Y, Liu D, Han C, et al. Possible roles of insulin, IGF-1 and IGFBPs in initiation and progression of colorectal cancer. World journal of gastroenterology: WJG. 2014;20:1608. doi: 10.3748/wjg.v20.i6.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Sandhu MS, Dunger DB, Giovannucci EL. Insulin, insulin-like growth factor-I (IGF-I), IGF binding proteins, their biologic interactions, and colorectal cancer. J Natl Cancer Inst. 2002;94:972–80. doi: 10.1093/jnci/94.13.972. [DOI] [PubMed] [Google Scholar]
- [85].Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001;131:3109S–20S. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]