Abstract
Background
Skin cancer is a growing public health problem in South Africa due to its high ambient ultraviolet radiation environment. The purpose of this study was to estimate the annual health system costs of cutaneous melanoma, squamous cell carcinoma (SCC) and basal cell carcinoma (BCC) in South Africa, incorporating both the public and private sectors.
Methods
A cost-of-illness study was used to measure the economic burden of skin cancer and a ‘bottom-up’ micro-costing approach. Clinicians provided data on the patterns of care and treatments while national costing reports and clinician fees provided cost estimates. The mean costs per melanoma and per SCC/BCC were extrapolated to estimate national costs using published incidence data and official population statistics. One-way and probabilistic sensitivity analyses were undertaken to address the uncertainty of the parameters used in the model.
Results
The estimated total annual cost of treating skin cancers in South Africa were ZAR 92.4 million (2015) (or US$15.7 million). Sensitivity analyses showed that the total costs could vary between ZAR 89.7 to 94.6 million (US$15.2 to $16.1 million) when melanoma-related variables were changed and between ZAR 78.4 to 113.5 million ($13.3 to $19.3 million) when non-melanoma-related variables were changed. The primary drivers of overall costs were the cost of excisions, follow-up care, radical lymph node dissection, cryotherapy and radiation therapy.
Conclusion
The cost of managing skin cancer in South Africa is sizable. Since skin cancer is largely preventable through improvements to sun-protection awareness and skin cancer prevention programs, this study highlights these healthcare resources could be used for other pressing public health problems in South Africa.
Keywords: Cost-of-illness, Melanoma, Squamous cell carcinoma, Basal cell carcinoma, Skin cancer
Background
In South Africa, among the white population, there is one of the highest incidences of malignant melanoma in the world and concern for skin cancer overall has grown in recent years. The estimated yearly incidence of malignant melanoma is 4.76 per 100,000 persons overall and 19.2 per 100,000 in whites [1]. In 2009, the Western Cape of South Africa’s incidence for whites was unofficially reported as high as 69 per 100,000 population [2]. South Africans are especially susceptible to skin cancer due to their exposure to year-round high ambient solar ultraviolet radiation (UV) and latitude (22–34°S) [3]. In a population of 54 million, the racial mix within South Africa shows a diverse population, consisting of black (80.2 %), white (8.4 %), coloured (8.8 %) and Asian/Indian (2.5 %) populations [4]. “Coloured” is a Statistics South Africa non-derogatory term referring to people of mixed race in South Africa. The skin pigmentation of South African populations varies widely and although whites are most susceptible to skin cancer, skin cancer occurs in all persons regardless of their skin pigment. Squamous cell carcinoma (SCC), basal cell carcinoma (BCC) and cutaneous melanoma (CM) have the highest incidence in white people, followed by coloureds and has considerably lower incidence in both blacks and Asian/Indians [1]. However, black South Africans often present to doctors late when their melanoma has already metastasized. Research has also found that there is a high risk of developing SCC in human immunodeficiency virus (HIV)-positive South Africans [5].
The South African healthcare system has made progress towards meeting the Millennium Development Goals, although in order to reach these goals and continue an upwards trajectory, significant improvements still remain necessary [6]. The increasing incidence of skin cancers will demand larger amounts of scarce healthcare resources and will compound the stress already placed on a strained public healthcare system. The expected rising incidence of skin cancer has already been seen for CM in the Cape [7] and possibly for all cancers, including CM, in the country as a whole [8]. Targeting diseases like skin cancer which are largely preventable through better awareness and promotion of healthy behaviours among its citizens is crucial to minimizing this healthcare resource burden.
The purpose of this study was to estimate the yearly health system costs of CM, SCC and BCC in South Africa, incorporating both the public and private sectors. In doing so, it will provide a better understanding of this disease burden, the health resources used in its current management, and the potential cost savings that might arise from prevention programs [9].
Methods
Overview
A cost-of-illness study was used to measure the economic burden of skin cancer including malignant CM, SCC and BCC. A ‘bottom-up’ micro-costing approach was taken in order to estimate the economic burden of skin cancer diagnosis and treatment [10, 11]. The bottom-up approach identifies the patterns of care for skin cancer, assigns unit costs to each specific intervention in the care pathway and aggregates the total costs of care incurred by patients [11]. This enables extrapolation of costs on a national level based on the numbers of patients receiving each type of treatment using incidence data. We abided by all ethics principles and since we used publicly available, population level data, with no individual details, ethical clearance was not needed.
Model
A model was constructed to describe the key pathways of care in skin cancer management in South Africa using the computer program TreeAge Pro Version 2015 (TreeAge Software Inc, Massachusetts,USA). This tool is useful because it can accommodate the continuum of care from diagnosis through to treatment and follow-up. The model combines probabilities of each care pathway and their costs. In the absence of South African published skin cancer clinical guidelines, the pathways were created by a team of currently practising and experienced South African dermatologists. Consequently, these pathways reflect the current ‘real life’ patterns for private and public skin cancer care. The patterns of care aligned well with several international clinical practice guidelines for management of malignant skin cancers including those from the United States (US) National Comprehensive Cancer Network, Cancer Care Ontario, European Society for Medical Oncology and the Australian Cancer Network [12]. Separate pathways were developed for CM versus SCC and BCC (Fig. 1). The time horizon was 12 months because for most cases, treatments were completed within this period.
Fig. 1.
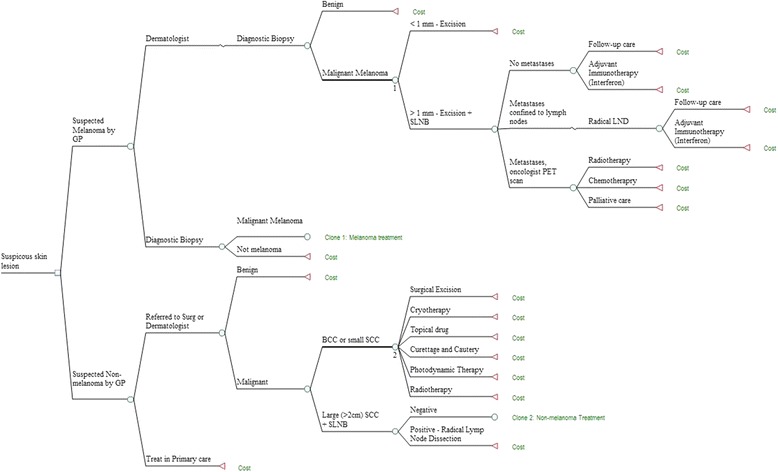
Structure of the model
Model inputs
Assumptions were necessary to complete the cost and probability values for the pathways and these are clearly stated underneath and in Tables 1 and 2.
Table 1.
Estimated probabilities, sensitivity values and sources
| Probabilities | Used in model | Sensitivity valuesa | Sources/assumptions |
|---|---|---|---|
| Melanoma | |||
| Proportion of cases of suspected melanoma are seen in public or private setting | 20 %/80 % | 15 %/85 %, 25 %/75 % | Expert opinion, persons with white skin are more susceptible to skin cancers and seen in private settings |
| Proportion of cases seen by GP who suspects a melanoma refers to a dermatologist or surgeon | 80 % | 70 %, 90 % | Assumption based on convenience and high likelihood in public hospital to refer to specialist |
| Proportion of suspected melanoma that were malignantb | 80 % | 75 %, 85 % | Expert opinionc, Fong 2014 [12] |
| Melanoma is surgically excised | 100 % | 87.9 % | Expert opinion (all melanomas including advanced) Vallejo-Torres 2014 [10] |
| Melanoma is greater than 1 mm thick | 30 % | 20 %, 40 % | Expert opinionc |
| Melanoma greater than 1 mm thick has metastasized | 20 % | 15 %, 25 % | Expert opinionc |
| Melanoma with no metastases is treated with interferon 2b alpha | 3 % | 2 %, 4 % | Expert opinionc, Fong 2014 [12] |
| Melanoma greater than 1 mm thick has metastases in lymph nodes | 30 % | 25 %, 35 % | Expert opinionc, published literature - ranges from 4 to 44 % |
| Melanoma with lymph node metastases is treated with radical LND | 100 % | – | Expert opinionc: All those with SLNB get RLND. |
| Melanoma is treated by radiotherapy | 5 % | 4 %, 6 % | Expert opinionc, most with metastases will only get palliative care, Fong 2014 [12] |
| Melanoma is treated with chemotherapy | 10 % | 5 %, 7 % | Expert opinionc, Fong 2014 [12] |
| Non-melanoma (NM)SCC or BCC | |||
| NM is treated by a GP in the public setting | 100 % | – | Expert opinionc, all seen first by a GP, (same for Aust. and England) |
| NM case is referred to a dermatologist | 60 % | 50 %, 70 % | Expert opinionc 60 % for dermatologist or surgeon |
| Suspected NM is confirmed to be malignantc | 85 % | 80, 90 % | Expert opinionc |
| SCC is >2 cm diameter | 10 % | 8 %, 12 % | Expert opinionc |
| Large SCC is positive and surgeon treats by radical LND | 20 % | 15 %, 20 % | Expert opinionc, Fong 2014 [12] |
| NM is treated by: | |||
| Surgical excision | 80 % | 86.0 % | Expert opinionc, Vallejo-Torres 2014 [10], Fong 2014 [12] |
| Cryotherapy | 10 % | 3.1 % | As above |
| Curette and diathermy/electrodesiccation | 5 % | 7.5 % | As above |
| Topical cream | 3 % | 0.5 % | As above |
| Photodynamic therapy | 1 % | 0.8 % | As above |
| Radiotherapy | 1 % | 1.7 % | As above |
LND lymph node dissection, GP general practitioner, SCC squamous cell carcinoma, BCC basal cell carcinoma
aThe sensitivity values are the high and low estimates used in the sensitivity analysis. These are based on sources in the literature or judged as plausible ranges around the best estimate used in the model base case. For probabilistic sensitivity analyses, beta distributions were assigned to probabilities to account for uncertainty
bExpert opinion is from practicing dermatologists and practicing doctors
cLesions suspected of being Malignant are often investigated and later diagnosed as benign
Table 2.
Estimated unit costs (2014/2015 South African Rand ZAR and USD) and sources
| Private sector | Public sector | Weighted costa(ZAR) | Weighted costa(USD) | Source | |
|---|---|---|---|---|---|
| Cost of a dermatologist or surgeon visit | 458 | 268 | 298 | 51 | UPFS tariffs #1012 + #1010 (facility fee level 2) |
| Cost of a GP visit | 335 | 158 | 186 | 32 | UPFS tariff #1011 + #1010 (facility fee level 2), private: review of invoices |
| Cost of a diagnostic biopsy (includes immunohistology) | 750 | 593 | 618 | 105 | UPFS tariff #1112 + #1110 (facility fee level 2), private: R750 dxbiopsy + excision |
| Cost of excision | 4643 | 593 | 1241b | 211 | UPFS tariff #1112 + #1110 (facility fee level 2), private: review of invoices |
| Cost of an excision with reconstructive surgery | 4433 | 2091 | 2466 | 418 | UPFS tariff #1612 cosmetic surgery cat A -specialist \practitioner + 75 facility feeb |
| Cost of cryotherapy | 1257 | 593 | 699 | 119 | UPFS tariff #1112 + #1110 (facility fee level 2),b |
| Cost of topical cream | 548 | 548 | 548 | 93 | Master procurement list - Imiquimod 5 % 12 sachets #180346230 |
| Cost of photodynamic therapy | 2514 | 1186 | 1399 | 237 | UPFS tariff #1112 + #1110 (facility fee level 2), need 2 sessionsb |
| Cost of curettage and cautery | 1257 | 593 | 699 | 119 | UPFS tariff #1112 + #1110 (facility fee level 2),b |
| Cost of radiotherapy - whole course | 62334 | 29403 | 34672 | 5884 | Annex N for Radiology - MR planning for radiotherapy course (oncol radiotherapy not available) Have used Aust. Price $7325 for whole coursec |
| Cost of chemotherapy - whole course | 31185 | 31185 | 31185 | 5292 | Dacarbazine was not on the procurement list therefore price is based on fotemustine, AU $7769 per coursec |
| Cost of treatment for SCC or BCC in primary care by GP | 1018 | 480 | 566 | 96 | UPFS tariff #1112 + #1010 (facility fee level 2),b |
| Cost of adjuvant immunotherapy with interferon - interferon alpha, 1 syringe injection | 367 | 367 | 367 | 62 | Master procurement list interferon alpha 2b syringe 9 MIU/0.5 mL #180348820 - daily injection for 10–24 weeks, then 3 ×/week for up to 6 mths |
| Cost of PET scan | 11195 | 4224 | 5339 | 906 | UPFS tariff #1952 + #1950 (facility fee level 2), private: review of invoices |
| Cost of a radical lymph node dissection | 6632 | 593 | 1559 | 265 | UPFS tariff #1112 + #1010 (facility fee level 2), |
| Cost of sentinel lymph node biopsy | 750 | 593 | 618 | 105 | UPFS tariff #1112 + #1010 (facility fee level 2),b |
| Cost of follow up | 1694 | 158 | 404 | 69 | UPFS tariff #1031 + #1010 (facility fee level 2) |
| Cost of hospitalisation - 1 night for melanoma with SLNB | 2105 | 993 | 1171 | 199 | UPFS tariff #0612 + #0610 (facility fee level 2) same as day patient #0663b |
UPFS Uniform Patient Fee Schedule, GP general practitioner, SLNB sentinel lymph node biopsy, PETpositron emission tomography, SCC squamous cell carcinoma, BCC basal cell carcinoma, MR magnetic radiation
aWeighting based on 16 % private and 84 % public [23]. Sensitivity analyses are based on the private and public sector values
bIn the absence of a private fee, it was assumed a 2.12 to 1.00 ratio of private to public based on the cost of a GP visit differential between private and public sectors
cConverted to ZAR by http://eppi.ioe.ac.uk/costconversion/default.aspx
Note: Sensitivity values were created with a 15 % change in the public sector probability when weighting the cost. (low = 84 %*0.85;high = 84 %*1.15). For probabilistic sensitivity analyses, gamma distributions were assigned to costs to account for uncertainty
Probabilities
A thorough literature search was performed to identify published studies in the medical literature and organisational websites that report on the patterns of skin cancer care in South Africa. There were no data on the probabilities of different treatment options typically used in South Africa, and we relied on two clinical experts for these estimates. The estimates were varied around plausible ranges and tested in sensitivity analyses. Table 1 provides the values used in the model, their ranges tested and sources. For simplicity, and due to data restrictions, a number of assumptions were necessary. These included: a person would only develop one skin cancer in the 12-month period; a person who received sentinel lymph node dissection would have an overnight stay in hospital prior to the procedure; all suspected melanomas had a diagnostic biopsy; treatments were all one-offs; benign lesions received no treatment; and no re-treatments occurred.
Costs
Healthcare resource usage was identified through the treatment descriptions. The main components of costs in the model were: initial and follow-up consultations by general practitioners or specialists, pathology and treatment alternatives for example, surgical excisions, topical creams, photodynamic therapy etc. In the public health system, most patients will be seen by a general practitioner at the primary health care level and subsequently treated in a secondary level public hospital. Costs for public hospital services were derived from the National Public Hospital Tariff schedule [13]. Costs for medicines such as imiquimod 5 % cream and interferon 2b were sourced from the South African Master Procurement List [14]. Costs for patients in the private system were derived from fees charged by private practitioners with recent invoices reviewed as evidence of these. Although charges for services are not strictly economic costs, they do represent a monetary value of the present care provided to patients for skin cancer. Costs which were not in the above resources were converted into Rand from costs in the Australian healthcare system (Table 2). We combined the private and public costs into weighted averages for each item (Table 2) in recognition of the 84 % public and 16 % private system split in South Africa [8].
Analyses
All costs are presented in 2014/15 South African Rand (ZAR) and US dollars ($). The currency exchange rate was 1 ZAR = 0.1697 US using the Cochrane cost converter at http://eppi.ioe.ac.uk/costconversion/default.aspx which inflates and converts currencies simultaneously using purchasing power parities. The model aggregated all probabilities and costs to derive the mean cost per person for malignant CM or SCC/BCC. One-way sensitivity analysis was performed on all estimates to determine the reasonable variation in values where uncertainty and variation in clinical practice exists. Probability distributions were assigned to parameters with the highest amount of uncertainty and a probabilistic sensitivity analysis was performed to calculate the mean cost of CM and SCC/BCC skin cancer.
The mean costs per melanoma and per SCC/BCC were extrapolated to estimate national costs using published incidence data from 2000 to 2004 [1] and official population statistics [4]. Notifications of all histologically-confirmed cases of SCC, BCC and malignant CM are received by the South African National Cancer Registry (NCR). The NCR is the largest and most representative cancer registry in South Africa. Reporting processes are robust with quality-assurance measures in place [8]. However, between 2005 and 2011, this voluntary registry did not receive all private hospital registrations (up to 16 % of all patients). The impact of this was the underestimation of the true number of cases. A study by Singh et al. [8] has quantified this gap in case numbers for cancers overall (4 %) and our estimates were adjusted accordingly. The Singh [8] study did not include non-melanoma skin cancers, however it is assumed the reporting issue for these cancers would be equivalent. The ethnic group incidences sourced from Norval et al. [1] were not complete. The ethnic group of patients were unspecified in 93 % of cases. The study used a hot-deck imputation method to allocate surnames into ethnic groups, although a large proportion of surnames could not be allocated. A sub-group ‘unknown race’ was created to represent this population.
Benign skin lesions, suspected to be malignant, are often included in estimates of skin cancer cost [10, 15]. We also modelled the diagnostic or ‘screening’ costs of skin cancers where the medical consultation involved investigation of a suspicious skin lesion that was subsequently found to be benign and required no further treatment. The proportion of skin cancer investigations which were benign was sourced through the expert opinion of practising medical staff, although it is acknowledged the accuracy of the number of SCCs and BCCs treated by GPs is unknown.
Results
Our model predicted that the annual total cost of treating skin cancers in South Africa were ZAR 92.4 million ($15.7 million). This assumes all those diagnosed are treated as per local clinical practice however, despite some anecdotal evidence that loss to follow up may be high in the public sector in South Africa. The estimated costs were ZAR 81.6 million ($13.8 million) for non-melanoma skin cancer and ZAR 10.8 million ($1.8 million) for CM (Table 3). When lesions suspected to be skin cancers, but were diagnosed as benign, were considered, these additional costs were ZAR 45.1 million ($7.7 million). The total skin cancer costs per ethnic group were highest for whites (ZAR 30.5 million ($5.2 million) or 33.0 %), coloureds (ZAR 9.69 million ($1.6 million) or 10.5 %), black Africans (ZAR 7.84 million ($1.3 million) or 8.5 %) and lowest for Asians/Indians (ZAR 0.44 million ($0.1 million) or 0.5 %) (Table 3). The remaining ZAR 44.0 million ($7.5 million) (47.6 %) was attributed to persons of ‘unknown race’. The validity of the cost distribution across the ethnic subgroups is diminished due to the ‘unknown race’ subgroup representing almost half the total costs. Consequently, the estimates are grossly underestimated and are a limitation of the current data quality in South Africa.
Table 3.
Estimates of national costs of skin cancer (2014/15 ZAR, $US)
| Melanoma | BCC and SCC | Total | |
|---|---|---|---|
| Mean costs per suspected case | ZAR 3566 $605 | ZAR 2154 $366 | - |
| Mean costs per diagnosed case | ZAR 4197 $712 | ZAR 2767 $470 | - |
| <1 mm ZAR 2931 $497 | |||
| >1 mm ZAR 7149 $1213 | |||
| Incidence per 100,000a | 4.77 | 54.6 | 59.4 |
| million | million | million | |
| Total costs | ZAR 10.80 $1.8 | ZAR 81.60 $13.8 | ZAR 92.40 $15.7 |
| By racial group | |||
| Blacks | ZAR 2.09 $0.4 | ZAR 5.76 $1.0 | ZAR 7.84 $1.3 |
| Asians/indians | ZAR 0.05 $0.0 | ZAR 0.38 $0.1 | ZAR 0.44 $0.1 |
| Coloureds | ZAR 1.04 $0.2 | ZAR 8.66 $1.5 | ZAR 9.69 $1.6 |
| Whites | ZAR 3.67 $0.6 | ZAR 26.82 $4.6 | ZAR 30.49 $5.2 |
| Unknown race | ZAR 3.96 $0.7 | ZAR 40.06 $6.8 | ZAR 44.02 $7.5 |
| By Gender | |||
| Men | ZAR 6.10 $1.0 | ZAR 54.71 $9.3 | ZAR 60.81 $10.3 |
| Women | ZAR 4.70 $0.8 | ZAR 26.96 $4.6 | ZAR 31.67 $5.4 |
Sensitivity analyses showed that the annual cost of skin cancers could vary between ZAR 89.7 to ZAR 94.6 million ($15.2 to $16.1 million) when melanoma-related variables were changed and between ZAR 78.4 to ZAR 113.5 million ($13.3 to $19.3 million) when non-melanoma-related variables were changed. The primary driver in costs were the costs of follow-up and investigations, cost of excision and histopathology and the proportion of public patients, melanoma >1 mm and non-melanoma referrals.
Discussion
This is the first cost-of-illness study performed on skin cancer in South Africa. The total cost of treating skin cancer was estimated to be in the vicinity of ZAR 92.4 million ($15.7 million) a year in South Africa. This is likely to be underestimated due to not all BCC being monitored throughout South Africa and also excludes the costs associated with potential life lost and lost productivity, as included in other studies [16]. However, putting this finding into perspective, if skin cancer was prevented and the funds currently spent on diagnosis and treatment were redeployed, ZAR 92.4 million ($15.7 million) could provide two doses of human papilloma virus (HPV) vaccine to 305,000 girls in public schools (this translates to 67 % coverage of Grade 4 girls aged 9 and older, based on 2014 data)1. Cost-of-illness studies are limited for health policy decisions because they do not provide information on cost-effectiveness and therefore, cannot guide decisions about the wisest choice of interventions to be provided. However, skin cancer is preventable if sun exposure is not excessive and therefore cost studies are important and useful for raising awareness for preventable diseases, and they illustrate the costs that may be averted through prevention programs. Many studies have previously shown that prevention initiatives such as sunscreen promotion, educational programs, and multifaceted programs are cost-effective [17–22]. These programs have been effective in the context of organisational settings such as educational locations as well as workplaces and sporting bodies and at systemic levels through mass media campaigns.
Skin cancer is known to be caused by solar ultraviolet radiation and sun protection strategies are well-established in primary prevention. Preventing skin cancer through clothing, hats, sunscreen and seeking shade when outdoors, are known to reduce skin cancer development. In a strained healthcare system, understanding the impact of averting skin cancers emphasizes the economic importance of skin cancer prevention as a way of not only promoting health but freeing health resources for other conditions. Due to the lack of recent cancer statistics and monitoring systems, the study highlights the need for efficient surveillance and data capturing, increased research, improved awareness and informed prevention of skin cancer.
South Africa has a pluralistic health system, where the public and private sectors have radically different resources . Approximately 80 % of the population is served by an under-resourced and severely strained public health system where treatment is provided free of charge [23]. The remaining 20 % of the population (mostly those who are formally employed) receives world-class health care from private healthcare providers, through private medical insurance. Health resources are heavily skewed towards the private sector, which serves the minority of the country’s population. There are racial differences in health care utilisation: 60 % of white and coloured adults visit a health professional in a year, of which 81 % were to a private facility, compared to 44 % of black African adults, of which 34 % were to private facilities [24]. The lower rates of black South Africans seeking medical care are explained, in part, by high travel costs to attend health care, out-of-pocket cost burden, long queues, perceived disrespectful treatment by facility staff, medicine stock-outs, perceived ineffective care and a preference to see traditional healers [25]. This behaviour has affected the presentation of melanoma in black South Africans with nodal disease occurring in more than one third of patients at their initial visit and 15 % already having disseminated metastatic disease [26, 27]. The lack of concern for skin problems relative to other serious health worries for people with HIV/AIDS may also be an additional reason for late presentation. Because black South Africans are not immune to skin cancers and because of high HIV prevalence, sun protection messages remain important [28].
A recent systematic review of studies reporting national costs of skin cancers and cost-effectiveness studies of prevention programs indicates the significant economic burden of skin cancer around the world [29]. The review found that in 16 studies, as a ratio to the population size of the country, the highest annual direct health system costs, is felt in Australia, New Zealand, Sweden and Denmark. If the results of the present analysis were available at the time of the review, it would have showed that South Africa was lowest for melanoma burden but higher than Brazil for non-melanoma cost burden (as a ratio of 2013 euros to population size) [29].
This analysis is limited because it relies on simplifying assumptions and on limited expert opinion for the treatment probabilities in the model rather than on large observational studies. Although not ideal, such data is not available in South Africa. Nevertheless, our estimates are ‘real life’, contemporary and the types and frequencies of treatments are comparable to those of the general international literature and guidelines for skin cancer [12]. For example, excision is clearly the preferred and dominant approach for non-melanoma skin cancer and melanoma. In these types of modelling studies, gaps commonly occur and reliance on expert opinion for these estimates is often necessary [30]. Treatments of skin cancers are also changing. New targeted therapies for advanced melanoma (e.g., dabrafenib, ipilimumab) are now available in other countries and are very expensive. If these are accepted for use in South Africa, the estimated cost of ZAR 10.8 million (US$1.6 million) for treating CM will be even higher. Lastly, the incidence data used to extrapolate the total costs were calculated using data from 2000 to 2004, and may no longer be accurate, and under-reporting is strongly suspected for BCCs because health care services do not fully cover all areas of South Africa [1].
It is standard practice in health economics to acknowledge and transparently quantify the uncertainty present in modelling studies [30]. We undertook one-way sensitivity analyses to assess parameter uncertainty and the tornado figures (Figs. 2 and 3) outline the variables which impact the costing results the most. An additional use of the sensitivity analysis in this study is that it highlights which variables new research could concentrate on to obtain better estimates. Those with high uncertainty and significant influence on the results are displayed at the top of the tornado diagrams (Figs. 2 and 3). They include the costs of follow-up and investigations, cost of excision and histopathology, the proportion of public patients, melanoma size and non-melanoma referrals. Beyond skin cancer, dedicated investment for capturing medical surveillance data in order to create accurate costing models in the South African setting is necessary. This would assist decision-makers in allocating resources where the most public health gain and least costly choices are possible. An efficient health system is one where spending occurs wisely, wastage is eliminated and where the most South Africans can gain better health outcomes.
Fig. 2.
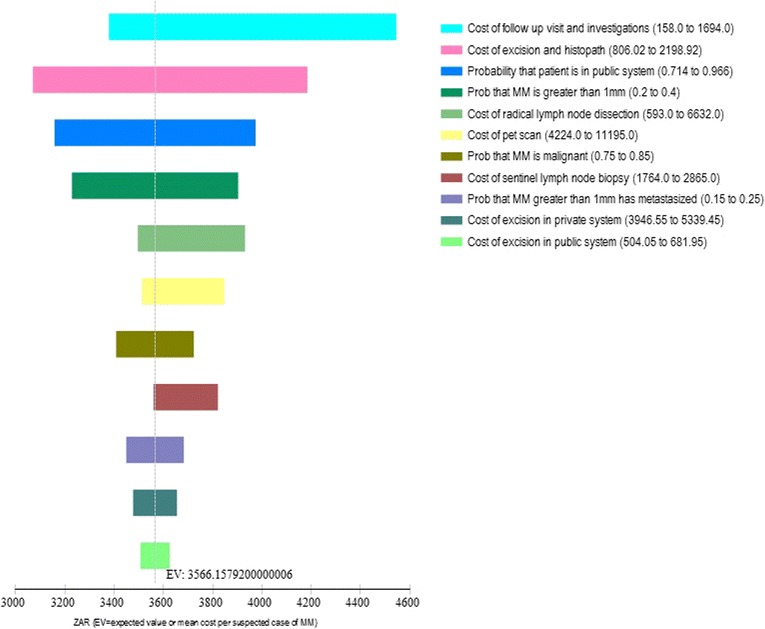
Results of sensitivity analysis for melanoma costs (ZAR)
Fig. 3.
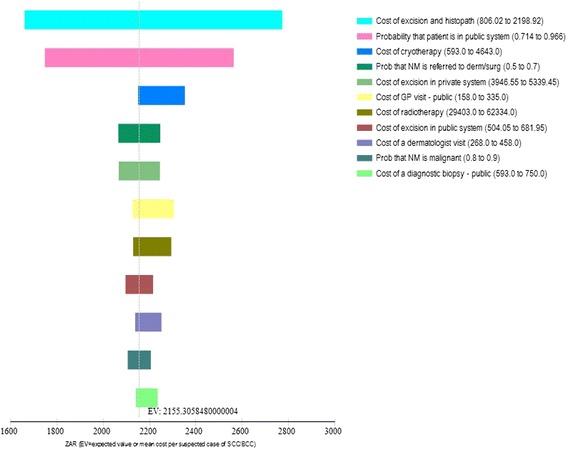
Results of sensitivity analysis for non-melanoma costs (ZAR)
Conclusion
In conclusion, this study is a first attempt to provide a snapshot on the financial burden of skin cancer in South Africa. Subject to the caveats herein, the cost of skin cancer in South Africa is substantial and improvements to sun-protection awareness and behaviours are likely to avoid skin cancer development. In doing so, the health of individuals will be improved while also releasing scarce healthcare resources for other pressing public health problems.
Acknowledgements
Dr Marc Roscher, dermatologist; Dr Lester Davids, scientist at UCT; Dr Dagmar Whittaker, dermatologist; Patricia Kellett, National Cancer Registry and Dr Stephen Purcell and Dr Anil Bramdev, Lancet Laboratories. The CSIR Parliamentary Grant EECM022 funded this research during 2013–2014. Thomas Elliott is funded through a grant from the National Health and Medical Council of Australia through the Centre for Research Excellence in Sun and Health #1001456.
Abbreviations
- AIDS
Acquired Immunodeficiency Disease
- BCC
basal cell carcinoma
- CM
cutaneous melanoma
- HIV
human immunodeficiency virus
- HPV
Human papilloma virus
- NCR
National Cancer Registry
- SCC
Squamous cell carcinoma
- USD
United States Dollars
- ZAR
South African Rand
Footnotes
HPV vaccine was approved in the public sector in South Africa in 2014 (school-based program for girls in Grade 4 in government schools). Vaccines are to be provided to approximately 450,000 girls who will receive a 2 dose vaccine, at a cost of R157 per dose (GSK tender prices reported by MSF). In round 1 of vaccination reached 412,617 girls and round 2 reached 422,000 girls. Authors own calculations based on data included in: Richter, K (2014) Implementation of HPV vaccination in South Africa, https://www.phasa.org.za/implementation-hpv-vaccination-south-africa/ Sans Frontieres (2015). The Right Shot: Bringing Down Barriers to Affordable and Adapted Vaccines, 2nd Edition. January 2015. Downloaded from https://www.msf.org.za/msf-publications/new-msf-report-says-cost-sa-vaccines-too-high.
Competing interests
The author(s) declare that they have no competing interests.
Authors’ contributions
LG contributed to the design of the study, the model structure, participated in the analyses and drafted the manuscript. TE drafted the model, undertook the literature searches to populate the model, participated in the analyses and manuscript writing. CW conceived of the study, coordinated the clinical expertise required during the model development and contributed to manuscript writing. ND assisted with the costing sources for the model. WV participated in the model structure, model inputs and contributed clinical expertise to the topic. All authors read and approved the final manuscript.
References
- 1.Norval M, Kellett P, Wright CY. The incidence and body site of skin cancers in the population groups of South Africa. Photodermatol Photoimmunol Photomed. 2014;30(5):262–265. doi: 10.1111/phpp.12106. [DOI] [PubMed] [Google Scholar]
- 2.Melanoma Fact Sheet [http://www.melanoma.co.za/D_doccnr_MFS.asp]. Accessed 24 Feb 2015.
- 3.Wright CY, Norval M, Summers B, Davids LM, Coetzee G, Oriowo M. Solar ultraviolet radiation exposure and human health in South Africa: finding a balance. S Afr Med J. 2012;102(8):665–666. doi: 10.7196/samj.5921. [DOI] [PubMed] [Google Scholar]
- 4.Africa SS. Mid-year population estimates. In: Africa SS; 2014.
- 5.Stein L, Urban MI, O’Connell D, Yu XQ, Beral V, Newton R, Ruff P, Donde B, Hale M, Patel M, et al. The spectrum of human immunodeficiency virus-associated cancers in a South African black population: results from a case-control study, 1995–2004. Int J Cancer. 2008;122(10):2260–5. [DOI] [PubMed]
- 6.Pillay Y, Barron P. Progress towards the millennium development goals in SA. S Afr Med J. 2014;104(3 Suppl 1):223. doi: 10.7196/samj.7924. [DOI] [PubMed] [Google Scholar]
- 7.Jessop S, Stubbings H, Sayed R, Duncan-Smith J, Schneider JW, Jordaan HF. Regional clinical registry data show increased incidence of cutaneous melanoma in Cape Town. S Afr Med J. 2008;98(3):197–199. [PubMed] [Google Scholar]
- 8.Singh JMU E, Nattey C, Babb C, Sengayi M, Kellet P. South African National Cancer Registry: Effect of withheld data from private health systems on cancer incidence estimates. S Afr Med J. 2015;105(2):107–10. doi: 10.7196/samj.8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guy GP, Jr, Machlin SR, Ekwueme DU, Yabroff KR. Prevalence and costs of skin cancer treatment in the U.S., 2002–2006 and 2007-2011. Am J Prev Med. 2015;48(2):183–187. doi: 10.1016/j.amepre.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vallejo-Torres L, Morris S, Kinge JM, Poirier V, Verne J. Measuring current and future cost of skin cancer in England. J Public Health (Oxf) 2014;36(1):140–148. doi: 10.1093/pubmed/fdt032. [DOI] [PubMed] [Google Scholar]
- 11.Chapko MK, Liu CF, Perkins M, Li YF, Fortney JC, Maciejewski ML. Equivalence of two healthcare costing methods: bottom-up and top-down. Health Econ. 2009;18(10):1188–1201. doi: 10.1002/hec.1422. [DOI] [PubMed] [Google Scholar]
- 12.Fong ZV, Tanabe KK. Comparison of melanoma guidelines in the U.S.A., Canada, Europe, Australia and New Zealand: a critical appraisal and comprehensive review. Br J Dermatol. 2014;170(1):20–30. doi: 10.1111/bjd.12687. [DOI] [PubMed] [Google Scholar]
- 13.Team U. Uniform Patient Fee Schedule. In: Africa NDoHRoS. Pretoria; 2014.
- 14.Registry SAMP. Master Procurement Catalogue November 2014. In: HEALTH NDO. Pretoria; 2014.
- 15.Girgis A, Clarke P, Burton RC, Sanson-Fisher RW. Screening for melanoma by primary health care physicians: a cost-effectiveness analysis. J Med Screen. 1996;3(1):47–53. doi: 10.1177/096914139600300112. [DOI] [PubMed] [Google Scholar]
- 16.Guy GP, Ekwueme DU. Years of potential life lost and indirect costs of melanoma and non-melanoma skin cancer: a systematic review of the literature. Pharmacoeconomics. 2011;29(10):863–874. doi: 10.2165/11589300-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 17.Garattini L, Cainelli T, Tribbia G, Scopelliti D. Economic evaluation of an educational campaign for early diagnosis of cutaneous melanoma. Pharmacoeconomics. 1996;9(2):146–155. doi: 10.2165/00019053-199609020-00006. [DOI] [PubMed] [Google Scholar]
- 18.Gordon LG, Scuffham PA, van der Pols JC, McBride P, Williams GM, Green AC. Regular sunscreen use is a cost-effective approach to skin cancer prevention in subtropical settings. J Invest Dermatol. 2009;129(12):2766–2771. doi: 10.1038/jid.2009.141. [DOI] [PubMed] [Google Scholar]
- 19.Hirst N, Gordon L, Gies P, Green AC. Estimation of avoidable skin cancers and cost-savings to government associated with regulation of the solarium industry in Australia. Health Policy. 2009;89(3):303–311. doi: 10.1016/j.healthpol.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Hirst NG, Gordon LG, Scuffham PA, Green AC. Lifetime cost-effectiveness of skin cancer prevention through promotion of daily sunscreen use. Value Health. 2012;15(2):261–268. doi: 10.1016/j.jval.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Kyle JW, Hammitt JK, Lim HW, Geller AC, Hall-Jordan LH, Maibach EW, De Fabo EC, Wagner MC. Economic evaluation of the US Environmental Protection Agency’s SunWise program: sun protection education for young children. Pediatrics. 2008;121(5):e1074–1084. [DOI] [PubMed]
- 22.Shih ST, Carter R, Sinclair C, Mihalopoulos C, Vos T. Economic evaluation of skin cancer prevention in Australia. Prev Med. 2009;49(5):449–453. doi: 10.1016/j.ypmed.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Health NDo. National Health Insurance Green Paper. In: Health NDo. Pretoria; 2011.
- 24.McLaren ZM, Ardington C, Leibbrandt M. Distance decay and persistent health care disparities in South Africa. BMC Health Serv Res. 2014;14:541. doi: 10.1186/s12913-014-0541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris B, Goudge J, Ataguba JE, McIntyre D, Nxumalo N, Jikwana S, Chersich M. Inequities in access to health care in South Africa. J Public Health Policy. 2011;32 Suppl 1:S102–123. [DOI] [PubMed]
- 26.Hudson DA, Krige JE. Melanoma in black South Africans. J Am Coll Surg. 1995;180(1):65–71. [PubMed] [Google Scholar]
- 27.Lodder JV, Simson W, Becker PJ. Malignant melanoma of the skin in black South Africans: a 15-year experience. S Afr J Surg. 2010;48(3):76–79. [PubMed] [Google Scholar]
- 28.Krige JE, Isaacs S, Hudson DA, King HS, Strover RM, Johnson CA. Delay in the diagnosis of cutaneous malignant melanoma. A prospective study in 250 patients. Cancer. 1991;68(9):2064–2068. doi: 10.1002/1097-0142(19911101)68:9<2064::AID-CNCR2820680937>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 29.Gordon LG, Rowell D. Health system costs of skin cancer and cost-effectiveness of skin cancer prevention and screening: a systematic review. Eur J Cancer Prev. 2015;24(2):141–149. doi: 10.1097/CEJ.0000000000000056. [DOI] [PubMed] [Google Scholar]
- 30.Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD. Model parameter estimation and uncertainty: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force--6. Value Health. 2012;15(6):835–842. doi: 10.1016/j.jval.2012.04.014. [DOI] [PubMed] [Google Scholar]


