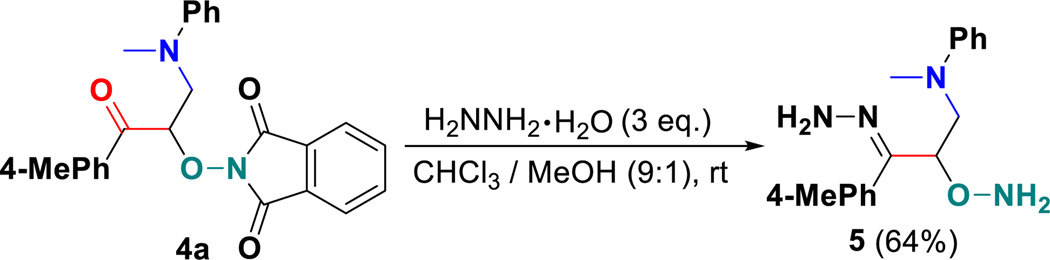Abstract
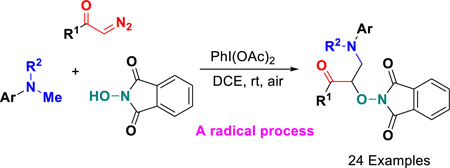
A novel three-component carbo-oxygenation of α-diazo carbonyls for flexible synthesis of unprecedented α-aminooxy-β-amino ketones has been established through metal-free C(sp3)–H functionalization from readily accessible N,N-dimethylanilines and N-hydroxyphthalimide. The reaction pathway involves in situ-generated phthalimide N-oxyl radical-triggered dediazotization/radical coupling sequence, leading to C-O and C-C bond formation.
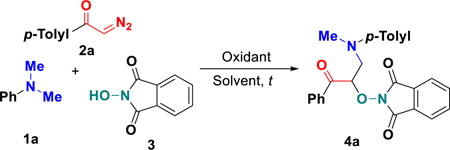
Graphical abstract
The search for high-efficient methodologies, particularly for those in atom-economic processes, has been actively pursued in organic community because of their significances in the construction of numerous targets.1 In the content, the direct and selective C(sp3)–H bond functionalization, due to its versatility and atom-economy potential, is a highly attractive. This strategy allows the direct conversion of C–H bonds to C-C and C–X bonds from simple precursors, thereby providing a practical technology for substantial challenging and intriguing syntheses.2 Arguably, transition-metal-catalyzed variants have occupied a dominant position,3 and a variety of transition metal species have been extensively utilized in unactivated C(sp3)–H bond functionalization such as gold,4 copper,5 palladium,6 iron7 and cobalt8 etc. But these procedures are generally considered not to be environmentally friendly.9 As a result, great efforts have been devoted to develop metal-free oxidative functionalization of C(sp3)-H bond,10 especially for ones adjacent to a nitrogen atom,11 through cross dehydrogenative coupling (CDC)12, which made it more powerful and applicable. Significant achievements have been made in C(sp3)–H bond activation adjacent to nitrogen atom by introducing diverse functional groups including indole,13 alkyne,14 cyano15 and pre-functionalized sp3 carbon.16 Despite these advances, to the best of our knowledge, there is no example on metal-free oxidative functionalization of sp3 C–H bond adjacent to nitrogen atom for carbo-oxygenation of α-diazo carbonyls via radical dediazotization with concomitant formation of C-C and C–O bonds.
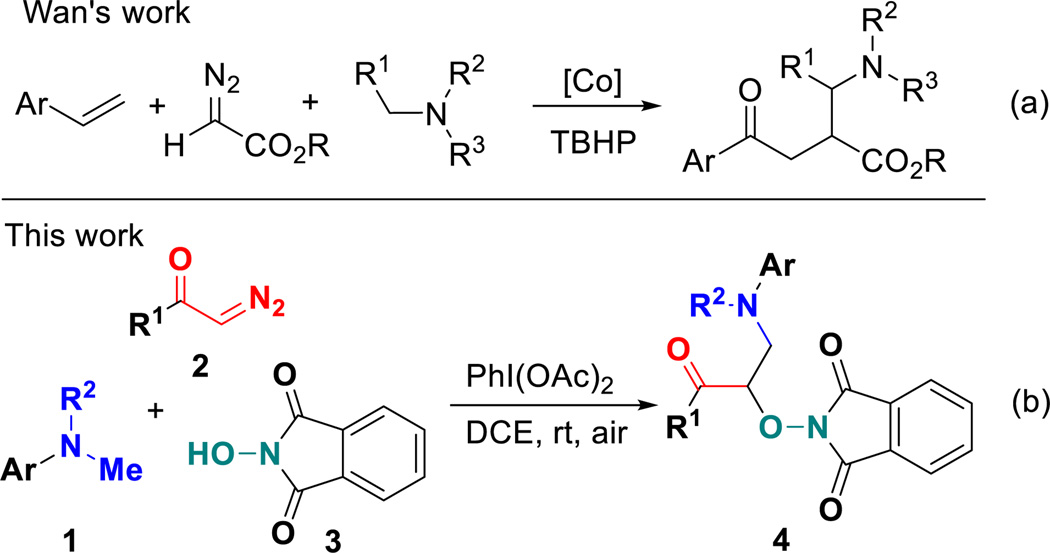
Diazo compounds are valuable synthetic intermediates as well as important structural units in many naturally occurring and bioactive compounds.17 In general, diazo compounds usually behaved as metal carbene precursors in organic synthesis and showed a high reactivity in transition-metal-catalyzed reactions.18 Recently, diazo compounds have been found to serve as good radical acceptors,19 enabling radical addition reactions for the C–C bond formation to construct important molecular frameworks. For instance, Wan and co-workers reported a cobalt-catalyzed oxidative multicomponent coupling of styrenes with diazo compounds and tertiary amines using t-butylhydroperoxide (TBHP) as oxidant, which involved the coupling between cobalt-based carbene radicals with α-aminoalkyl radicals to yield β-ester-γ-amino ketones (Scheme 1a).20 Enlightened by these interesting results and our ongoing interest in radical chemistry,21 we envisioned that under the right set of oxidative conditions, suitable diazo compounds could captured phthalimide N-oxyl (PINO) radicals, generated in situ from N-hydroxyphthalimide (NHPI),22 followed by radical dediazotization toward α-carbonylalkyl radicals, which are then intercepted by α-aminoalkyl radicals to access densely functionalized structures. Herein, we report the successful implementation of this analysis with a novel metal-free C(sp3)–H functionalization under one-pot oxidative conditions with use of simple starting materials such as substituted N,N-dimethylanilines 1, α-diazo carbonyls 2 and N-hydroxyphthalimide 3. It is particularly interesting and rare that three-component carbo-oxygenation of α-diazo carbonyls can be readily realized via metal-free PINO radical-triggered dediazotization in a function-group-compatible fashion (Scheme 1b). The present protocol allowed the simultaneous introduction of two different functional groups into one carbon atom via a radical process, affording a facile and reliable access to a series of new α-aminooxy-β-amino ketones 4 with synthetic potential.
Scheme 1.
Radical addition of α-diazo carbonyls
Our study commenced with the three-component reaction of N,N-dimethylaniline (1a) with 2-diazo-1-(p-tolyl)ethan-1-one (2a) and NHPI (3a) under air conditions using TBHP (70% in water) and Cu(OTf)2 (10 mol%). The reaction in 1,2-dichloroethane (DCE) at room temperature gave the desired product 4a in a 33% chemical yield (Table 1, entry 1). Encouraged by this result, we next optimized reaction conditions. Exchanging TBHP for phenyliodine bis(trifluoroacetate) (PIFA) completely compressed the reaction process (entry 2). In contrast, phenyliodine diacetate (PIDA) as an oxidant facilitated this dediazotized carbo-oxygenation and delivered a higher 42% yield (entry 3). The very lower conversion was observed as the usage of PIDA was increased (entry 4). Other metal salts including CuCl2 and FeCl2 were attempted for the system, and the results indicated that both metal catalysts behaved the higher catalytic activity than Cu(OTf)2 (entries 5–6). Delightedly, without metal catalysts, this transformation worked more efficiently, affording a 62% yield of 4a (entry 7). Screening followed by other aprotic solvents such as tetrahydrofuran (THF), dimethyl sulfoxide (DMSO) and acetonitrile (CH3CN) revealed that all these solvents met little success with respect to the reaction yields (entries 8–10). Elevating reaction temperatures deteriorated the reaction efficiency (entry 11). Similarly, use of Ar or O2 conditions is not beneficial for this transformation (entries 12–13). After careful optimizations, we found that fine-tuning stoichiometric ratio of three substrates in 2:2:1 (1a:2a:3) remarkably improved reaction efficiency and 83% yield of 4a was isolated (entry 15).
Table 1.
Optimization of the reaction conditionsa
 | |||||
|---|---|---|---|---|---|
| Entry | Oxidant (eq.) | Cat. (mol%) | Solvent | t / °C | Yield b(%) |
| 1 | TBHP (2.0) | Cu(OTf)2 (10) | DCE | rt | 33 |
| 2 | PIFA (2.0) | Cu(OTf)2 (10) | DCE | rt | trace |
| 3 | PIDA (2.0) | Cu(OTf)2 (10) | DCE | rt | 42 |
| 4 | PIDA (4.0) | Cu(OTf)2 (10) | DCE | rt | 10 |
| 5 | PIDA (2.0) | CuCl2 (10) | DCE | rt | 49 |
| 6 | PIDA (2.0) | FeCl2 (10) | DCE | rt | 48 |
| 7 | PIDA (2.0) | - | DCE | rt | 62 |
| 8 | PIDA (2.0) | - | THF | rt | 35 |
| 9 | PIDA (2.0) | - | DMSO | rt | trace |
| 10 | PIDA (2.0) | - | CH3CN | rt | 30 |
| 11 | PIDA (2.0) | - | DCE | 50 | 53 |
| 12 | PIDA (2.0) | - | DCE | rt | 46c |
| 13 | PIDA (2.0) | - | DCE | rt | 60d |
| 14 | PIDA (2.0) | - | DCE | rt | 71e |
| 15 | PIDA (2.0) | - | DCE | rt | 83f |
Reaction conditions: N,N-dimethyl aniline (1a, 0.5 mmol), α-diazoketones (2a, 0.25 mmol), NHPI (3, 0.25 mmol), oxidant, solvent (2.0 mL), air.
Isolated yield based on NHPI.
Under Ar conditions.
Under O2 conditions.
The ratio of 1a:2a:3 in 2:1.5:1.
The ratio of 1a:2a:3 in 2:2:1.
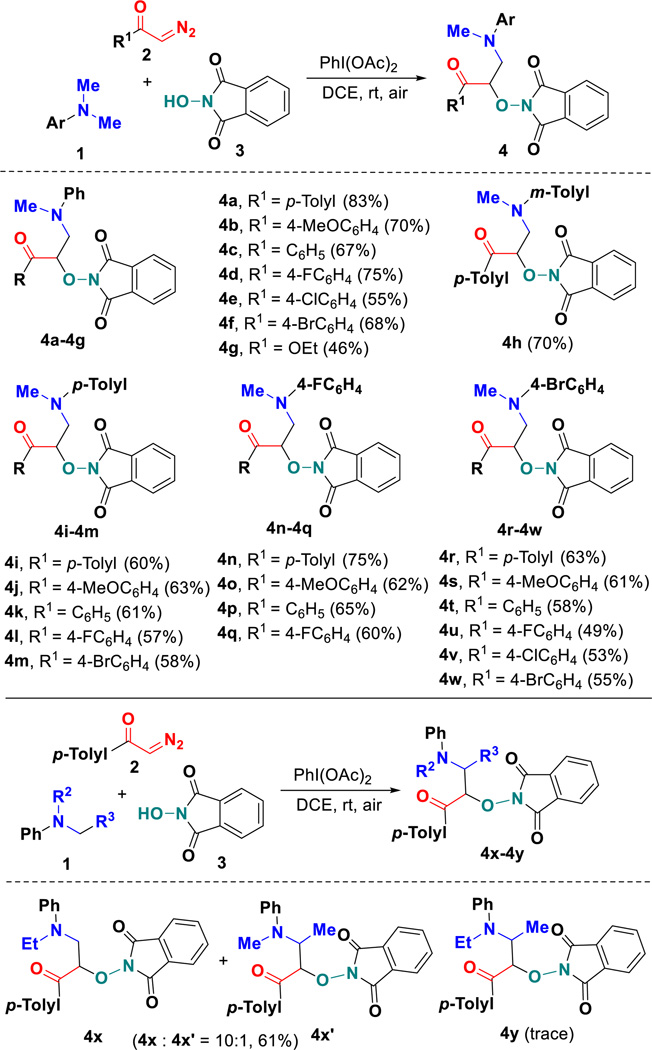
With the optimized reaction conditions in hand, we then set out to evaluate the generality of this metal-free radical coupling using a variety of N,N-dimethylanilines 1 and α-diazo carbonyls 2 (Scheme 2). Upon repeating the reaction with N,N-dimethylaniline 1a and NHPI, substrates 2 possessing either electronically neutral, poor, or rich substituents on the phenyl ring all work well, efficiently transforming into the corresponding poly-functionalized α-aminooxy-β-amino ketones 4a–4f in good to excellent yields. The variant of functional groups including methyl, methoxy, fluoro, chloro and bromo can tolerate the oxidative conditions well. Alternatively, ethyl diazoacetate (EDA) was an adaptable diazo component, which could be successful engaged in this transformation under the standard conditions to give access to α-aminooxy-β-amino esters 4g, albeit with 46% yield. Next, the scope with respect to the N,N-dimethylaniline coupling partner was systematically investigated. As we have expected, this metal-free radical strategy tolerates various N,N-dimethylanilines bearing both electron-rich and electron-deficient functional groups at different positions of aromatic ring, leading to the assemble of α-aminooxy-β-amino ketones 4h–4w with structural diversity (Scheme 2). Functional groups like methyl, fluoride and bromide located at the aniline ring were compatible in these radical reactions. The presence of electron-donating substituents (methyl and methoxy) at para-position of α-diazo carbonyls 2 seemed to improve the efficiency of the reaction. Alternatively, N-ethyl-N-methylaniline was proven to be effective, but gave an inseparable mixture of regioisomers 4x and 4x’ with a total 61% yield in a 10:1 ratio by 1H NMR analysis (Scheme 2). However, N,N-diethylaniline did not work in this reaction, which may be caused by its steric hindrance. Note that this is the first reported procedure for the three-component synthesis of these new densely functionalized α-aminooxy-β-amino ketones through a metal-free PINO radical-triggered dediazotization.
Scheme 2.
Synthesis of α-aminooxy-β-amino ketones 4. aReaction conditions: All reactions were performed with 1 (1.0 mmol), 2 (1.0 mmol), 3 (0.5 mmol), PhI(OAc)2 (1.0 mmol), and dry DCE (2.0 mL) rt, under air conditions for 12 hours. bIsolated yield based on 3.
The structures of the resulting α-aminooxy-β-amino ketones 4 were determined by their NMR spectroscopy and HRMS. Furthermore, in the case of compound 4a, its structure was unambiguously confirmed by X-ray diffraction (see the Supporting Information)
As mentioned above, α-aminooxy-β-amino ketones are very useful synthetic intermediates and building blocks. Hence, we attempted to explore the feasibility of their further transformations. The preformed product 4a was subjected with hydrazine monohydrate (80% in water) in a mixed solvent of chloroform and methanol at room temperature. The reaction proceeded smoothly to afford the corresponding α-aminooxy-β-amino hydrazones 5 in a 64% yield (Scheme 3).
Scheme 3.
Further synthetic transformations
To gain a deeper mechanistic insight into this transformation, the following control experiments were conducted. When 2.0 equiv. of TEMPO (2,2,6,6-tetramethyl-1-piperidinyloxy), a well-known radical scavenger, was subjected to the reaction system under the standard conditions, a significantly decreased yield (30%) was obtained whereas a further increase of TEMPO (4.0 equiv) resulted in a higher yield (54%), indicated that TEMPO is beneficial to reaction process (Scheme S1a, see Supporting Information). A trace amount of the expected product 4a was observed with use of 2.0 equiv. of BHT (butylhydroxytoluene) (Scheme S1b). These experimental results supported the possibility of radical process. Subsequent investigation on an intermolecular deuterium-labelling competing reaction of 1a and [D6]-1a demonstrated a kinetic isotope effect of KH / KD = 2.13, which suggests that the C-H bond cleavage is involved in the rate-limiting step and N,N-dimethylanilines preferentially undergoes H-abstraction of C(sp3)-H bonds to form α-aminoalkyl radicals B (Scheme S1c).
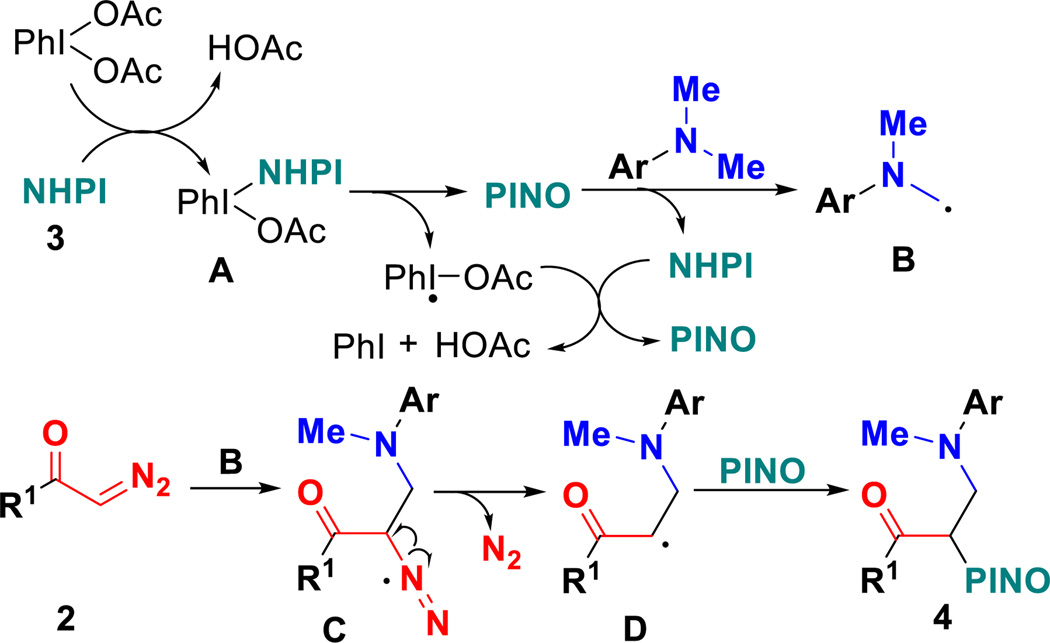
On the basis of the above experimental results and literature survey,20,22 we propose a plausible mechanism for forming products 4 as depicted in Scheme 4. A ligand exchange between PhI(OAc)2 and NHPI 3 would give intermediate A. Intermediate A is converted into a PINO radical by thermal homolytic cleavage due to the weak I-O bond. α-Aminoalkyl radicals B are formed through H-abstraction of C(sp3)-H bonds by PINO radical and regenerates NHPI, which undergoes second ligand exchange and thermal homolytic cleavage with iodobenzene acetate radicals to yield PINO. Then, the addition of α-aminoalkyl radicals B into α-diazo carbonyls 2 give radical C, followed by radical-triggered dediazotization to afford α-carbonylalkyl radicals D, which is trapped by PINO to convert into final products 4 by radical coupling.23
Scheme 4.
Proposed mechanism for forming products 4
In conclusion, we have established a novel metal-free three-component dehydrogenation coupling of N,N-dimethylanilines with α-diazo carbonyls and N-hydroxyphthalimide through direct dediazotized C(sp3)-H functionalization under mild oxidative condition. Considering the easy accessibility of starting materials and the benefits of the cheap hypervalent iodine reagents, this transformation enables the combination of three-component dehydrogenation coupling with radical dediazotization in a simple one-pot operation, providing an efficient and practical access to a wide range of unprecedented α–aminooxy-β-amino ketones with generally good yields. A detailed application of resulting β-amino ketones with chemical potential is currently underway in our laboratory.
Supplementary Material
Acknowledgments
We are grateful for financial support from the NSFC (Nos. 21232004, 21332005, and 21472071), PAPD of Jiangsu Higher Education Institutions, Robert A. Welch Foundation (D-1361, USA) and NIH (R33DA031860, USA), the Outstanding Youth Fund of JSNU (YQ2015003), NSF of Jiangsu Province (BK20151163), and the Open Foundation of Jiangsu Key Laboratory (K201505).
Footnotes
Electronic Supplementary Information (ESI) available. CCDC 1449860 (4a): [details of any supplementary information available should be included here]. See DOI: 10.1039/x0xx00000x
Notes and references
- 1.For selected reviews, see: Trost BM. Acc. Chem. Res. 2002;35:695. doi: 10.1021/ar010068z. Trost BM, Frederiksen MU, Rudd MT. Angew. Chem., Int. Ed. 2005;44:6630. doi: 10.1002/anie.200500136. Jiang B, Rajale T, Wever W, Tu S-J, Li G. Chem.-Asian J. 2010;5:2318. doi: 10.1002/asia.201000310. Peng B, Maulide N. Chem.-Eur. J. 2013;19:13274. doi: 10.1002/chem.201301522. Ohno H. Asian J. Org. Chem. 2013;2:18.
- 2.(a) Rouquet G, Chatani N. Angew. Chem., Int. Ed. 2013;52:11726. doi: 10.1002/anie.201301451. [DOI] [PubMed] [Google Scholar]; (b) Zhang S-Y, Zhang F-M, Tu Y-Q. Chem. Soc. Rev. 2011;40:1937. [Google Scholar]; (c) Hashiguchi BG, Bischof SM, Konnick MM, Periana RA. Acc. Chem. Res. 2012;45:885. doi: 10.1021/ar200250r. [DOI] [PubMed] [Google Scholar]
- 3.(a) Jazzar R, Hitce J, Renaudat A, Sofack-Kreutzer J, Baudoin O. Chem. Eur. J. 2010;16:2654. doi: 10.1002/chem.200902374. [DOI] [PubMed] [Google Scholar]; (b) Li B-J, Shi Z-J. Chem. Soc. Rev. 2012;41:5588. doi: 10.1039/c2cs35096c. [DOI] [PubMed] [Google Scholar]; (c) Che C-M, Lo VK-Y, Zhou C-Y, Huang J-S. Chem. Soc. Rev. 2011;40:1950. doi: 10.1039/c0cs00142b. [DOI] [PubMed] [Google Scholar]; (d) White MT, Grubbs RH. Acc. Chem. Res. 2009;42:1607. doi: 10.1021/ar900103e. [DOI] [PubMed] [Google Scholar]
- 4.Xie J, Pan C, Abdukader A, Zhu C. Chem. Soc. Rev. 2014;43:5245. doi: 10.1039/c4cs00004h. [DOI] [PubMed] [Google Scholar]
- 5.(a) Gephart RT, Warren TH. Organometallics. 2012;31:7728. [Google Scholar]; (b) Guo X-X, Gu D-W, Wu Z, Zhang W. Chem. Rev. 2015;115:1622. doi: 10.1021/cr500410y. [DOI] [PubMed] [Google Scholar]; (c) Miao J, Ge H. Eur. J. Org. Chem. 2015;2015:7859. [Google Scholar]
- 6.Liu G, Wu Y. Top. Curr. Chem. 2010;292:195. doi: 10.1007/128_2009_16. [DOI] [PubMed] [Google Scholar]
- 7.(a) Sun X, Li J, Huang X, Sun C. Curr. Inorg. Chem. 2012;2:64. [Google Scholar]; (b) Sun C-L, Li B-J, Shi Z-J. Chem. Rev. 2011;111:1293. doi: 10.1021/cr100198w. [DOI] [PubMed] [Google Scholar]
- 8.Su B, Cao Z-C, Shi Z-J. Acc. Chem. Res. 2015;48:886. doi: 10.1021/ar500345f. [DOI] [PubMed] [Google Scholar]
- 9.(a) Mousseau JJ, Charette AB. Acc. Chem. Res. 2013;46:412. doi: 10.1021/ar300185z. [DOI] [PubMed] [Google Scholar]; (b) Narayan R, Matcha K, Antonchick AP. Chem. Eur. J. 2015;21:14678. doi: 10.1002/chem.201502005. [DOI] [PubMed] [Google Scholar]; (c) Rohlmann R, Garcia Mancheno O. Synlett. 2013;24:6. [Google Scholar]
- 10.(a) Tobisu M, Chatani N. Angew. Chem., Int. Ed. 2006;45:1683. doi: 10.1002/anie.200503866. [DOI] [PubMed] [Google Scholar]; (b) Wang T, Jiao N. Acc. Chem. Res. 2014;47:1137. doi: 10.1021/ar400259e. [DOI] [PubMed] [Google Scholar]; (c) Chan TL, Wu Y, Choy PY, Kwong FY. Chem.-Eur. J. 2013;19:15802. doi: 10.1002/chem.201301583. [DOI] [PubMed] [Google Scholar]; (d) Schreiner PR, Fokin AA. Chem. Rec. 2004;3:247. doi: 10.1002/tcr.10070. [DOI] [PubMed] [Google Scholar]
- 11.(a) Li C-J. Acc. Chem. Res. 2009;42:335. doi: 10.1021/ar800164n. [DOI] [PubMed] [Google Scholar]; (b) Campos KR. Chem. Soc. Rev. 2007;36:1069. doi: 10.1039/b607547a. [DOI] [PubMed] [Google Scholar]; (c) Doye S. Angew. Chem., Int. Ed. 2001;40:3351. doi: 10.1002/1521-3773(20010917)40:18<3351::aid-anie3351>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 12.(a) Girard SA, Knauber T, Li C-J. Angew. Chem., Int. Ed. 2014;53:74. doi: 10.1002/anie.201304268. [DOI] [PubMed] [Google Scholar]; (b) Scheuermann CJ. Chem.-Asian J. 2010;5:436. doi: 10.1002/asia.200900487. [DOI] [PubMed] [Google Scholar]; (c) Louillat M-L, Patureau FW. Chem. Soc. Rev. 2014;43:901. doi: 10.1039/c3cs60318k. [DOI] [PubMed] [Google Scholar]; (d) Li C-J, Yoo W-J. Top. Curr. Chem. 2010;292:281. doi: 10.1007/128_2009_17. [DOI] [PubMed] [Google Scholar]; (e) Zhang M, Zhang A. J. Heterocyclic Chem. 2012;49:721. [Google Scholar]
- 13.For selected examples, see: Zhang G, Ma Y, Cheng G, Liu D, Wang R. Org. Lett. 2014;16:656. doi: 10.1021/ol500045p. Dhineshkumar J, Lamani M, Alagiri K, Prabhu KR. Org. Lett. 2013;15:1092. doi: 10.1021/ol4001153. Ueda H, Yoshida K, Tokuyama H. Org. Lett. 2014;16:4194. doi: 10.1021/ol5018883. Tanoue A, Yoo W-J, Kobayashi S. Org. Lett. 2014;16:2346. doi: 10.1021/ol500661t.
- 14.For selected examples, see: Zhong J-J, Meng Q-Y, Liu B, Li X-B, Gao X-W, Lei T, Wu C-J, Li Z-J, Tung C-H, Wu L-Z. Org. Lett. 2014;16:1988. doi: 10.1021/ol500534w. Jin L, Feng J, Lu G, Cai C. Adv. Synth. Catal. 2015;357:2105.
- 15.(a) Sugiishi T, Nakamura H. J. Am. Chem. Soc. 2012;134:2504. doi: 10.1021/ja211092q. [DOI] [PubMed] [Google Scholar]; (b) Li Z, Li C-J. J. Am. Chem. Soc. 2004;126:11810. doi: 10.1021/ja0460763. [DOI] [PubMed] [Google Scholar]; (c) Li Z, Li C-J. Org. Lett. 2004;6:4997. doi: 10.1021/ol047814v. [DOI] [PubMed] [Google Scholar]; (c) Franz JF, Kraus WB, Zeitler K. Chem. Commun. 2015;51:8280. doi: 10.1039/c4cc10270c. [DOI] [PubMed] [Google Scholar]; (d) Rueping M, Koenigs RM, Poscharny K, Fabry DC, Leonori D, Vila C. Chem.-Eur. J. 2012;18:5170. doi: 10.1002/chem.201200050. [DOI] [PubMed] [Google Scholar]
- 16.(a) Liu Q, Li Y-N, Zhang H-H, Chen B, Tung C-H, Wu L-Z. Chem.-Eur. J. 2012;18:620. doi: 10.1002/chem.201102299. [DOI] [PubMed] [Google Scholar]; (b) Rueping M, Vila C, Bootwicha T. ACS Catal. 2013;3:1676. [Google Scholar]; (c) Nobuta T, Tada N, Fujiya A, Kariya A, Miura T, Itoh A. Org. Lett. 2013;15:574. doi: 10.1021/ol303389t. [DOI] [PubMed] [Google Scholar]
- 17.(a) Woo CM, Gholap SL, Lu L, Kaneko M, Li Z, Ravikumar PC, Herzon SB. J. Am. Chem. Soc. 2012;134:17262. doi: 10.1021/ja307497h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Nicolaou KC, Li H, Nold AL, Pappo D, Lenzen A. J. Am. Chem. Soc. 2007;129:10356. doi: 10.1021/ja074297d. [DOI] [PubMed] [Google Scholar]; (c) Marco-Contelles J, Molina MT. Curr. Org. Chem. 2003;7:1433. [Google Scholar]
- 18.For selected reviews, see: Hodgson DM, Pierard FYTM, Stupple PA. Chem. Soc. Rev. 2001;30:50. Shao Z, Zhang H. Chem. Soc. Rev. 2012;41:560. doi: 10.1039/c1cs15127d. Hu F, Xia Y, Ma C, Zhang Y, Wang J. Chem. Commun. 2015;51:7986. doi: 10.1039/c5cc00497g.
- 19.(a) Lu H, Dzik WI, Xu X, Wojtas L, de Bruin B, Zhang XP. J. Am. Chem. Soc. 2011;133:8518. doi: 10.1021/ja203434c. [DOI] [PubMed] [Google Scholar]; (b) Dzik WI, Xu X, Zhang XP, Reek JNH, de Bruin B. J. Am. Chem. Soc. 2010;132:10891. doi: 10.1021/ja103768r. [DOI] [PubMed] [Google Scholar]; (c) Zheng J, Qi J, Cui S. J. Org. Chem. 2015;80:9224. doi: 10.1021/acs.joc.5b01684. [DOI] [PubMed] [Google Scholar]; (d) Kim S, Cho JR. Bull. Korean Soc. 1993;14:664. [Google Scholar]; (e) Dang H-S, Roberts BP. J. Chem. Soc. Perkin Trans. 1. 1996:769. [Google Scholar]
- 20.(a) Zhang J, Jiang J, Xu D, Luo Q, Wang H, Chen J, Li H, Wang Y, Wan X. Angew. Chem., Int. Ed. 2015;54:1231. doi: 10.1002/anie.201408874. [DOI] [PubMed] [Google Scholar]; (b) Jiang J, Liu J, Yang L, Shao Y, Cheng J, Bao X, Wan X. Chem. Commun. 2015;51:14728. doi: 10.1039/c5cc05183e. [DOI] [PubMed] [Google Scholar]
- 21.(a) Chen Z-Z, Liu S, Hao W-J, Xu G, Wu S, Miao J-N, Jiang B, Wang S-L, Tu S-J, Li G. Chem. Sci. 2015;6:6654. doi: 10.1039/c5sc02343b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Qiu J-K, Jiang B, Zhu Y-L, Hao W-J, Wang D-C, Sun J, Wei P, Tu S-J, Li G. J. Am. Chem. Soc. 2015;137:8928. doi: 10.1021/jacs.5b05735. [DOI] [PubMed] [Google Scholar]; (c) Zhu Y-L, Jiang B, Hao W-J, Wang A-F, Qiu J-K, Wei P, Wang D, Li G, Tu S-J. Chem. Commun. 2016;52:1907. doi: 10.1039/c5cc08895j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.For selected examples, see: Lee JM, Park EJ, Cho SH, Chang S. J. Am. Chem. Soc. 2008;130:7824. doi: 10.1021/ja8031218. Yao H, Yamamoto K. Chem.-Asian J. 2012;7:1542. doi: 10.1002/asia.201200017. Ghosh R, Olofsson B. Org. Lett. 2014;16:1830. doi: 10.1021/ol500478t. Xia X-F, Gu Z, Liu W, Wang H, Xia Y, Gao H, Liu X, Liang Y-M. J. Org. Chem. 2015;80:290. doi: 10.1021/jo502327r. Melone L, Punta C. Beilstein J. Org. Chem. 2013;9:1296. doi: 10.3762/bjoc.9.146.
- 23.(a) Fischer H. Chem. Rev. 2001;101:3581. doi: 10.1021/cr990124y. [DOI] [PubMed] [Google Scholar]; (b) Studer A. Chem. Eur. J. 2001;7:1159. doi: 10.1002/1521-3765(20010316)7:6<1159::aid-chem1159>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]; (c) Studer A. Chem. Soc. Rev. 2004;33:267. doi: 10.1039/b307652k. [DOI] [PubMed] [Google Scholar]; (d) Studer A, Schulte T. Chem. Rec. 2005;5:27. doi: 10.1002/tcr.20033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.