Abstract
Growth differentiation factor 11 (GDF11) and myostatin (MSTN or GDF8) are closely related members of the transforming growth factor β superfamily (TGFβ) and are often perceived to serve similar or overlapping roles. Yet, despite commonalities in protein sequence, receptor utilization and signaling, accumulating evidence suggests that these two ligands can have distinct functions in a number of situations. GDF11 is essential for mammalian development and has been suggested to regulate aging of multiple tissues, whereas MSTN is a well-described negative regulator of postnatal skeletal and cardiac muscle mass and modulates metabolic processes. In this review, we discuss the biochemical regulation of GDF11 and MSTN and their functions in the heart, skeletal muscle, and brain. We also highlight recent clinical findings with respect to a potential role for GDF11 and/or MSTN in humans with heart disease. Finally, we address key outstanding questions related to GDF11 and MSTN dynamics and signaling during development, growth, and aging.
Keywords: GDF11, GDF8, BMP11, MSTN, TGFβ, muscle
GDF11, also known as bone morphogenetic protein 11 (BMP11), and its homolog MSTN, (also known as GDF8) are closely related members of the TGFβ superfamily1, 2. MSTN plays an evolutionary conserved role in antagonizing postnatal muscle growth, limiting both the number and size of individual muscle fibers1. Hence, disruption of the myostatin gene or targeted inhibition of MSTN protein triggers hyper-muscular phenotypes in many mammals and fish3-5. MSTN function also has been implicated in postnatal glucose metabolism and adipogenesis6. GDF11, in contrast, plays a broad role during mammalian development, regulating anterior/posterior patterning, formation of the kidney, stomach, spleen and endocrine pancreas, and olfactory neurogenesis2, 7-11. GDF11's functions in postnatal tissues are less explored, partly due to the perinatal lethality of Gdf11-knockout mice2, 7, which exhibit homeotic skeletal transformations, cleft palate, and renal agenesis (Table 1). Recent work identified GDF11 as a candidate hormonal regulator of aging in a variety of different organs. Consistent with this function, boosting levels of GDF11 protein in aged mice improves age-related phenotypes in the heart12, brain13, and skeletal muscle14. In addition, two studies recently implicated GDF11 as a negative regulator of erythroid differentiation in mouse aging and thalassemia models15, 16.
Table 1.
Comparison of developmental expression patterns and phenotypes in GDF11- and Myostatin-deficient mice.
| Tissue/Phenotype | MSTN | GDF11 | |
|---|---|---|---|
| Predominant expression pattern | Developing and adult skeletal muscle1 | Primitive streak and tail bud Expressed in developing limb buds2, 7, 17, 164 |
|
| MSTN KO | GDF11 KO | MSTN/GDF11 DKO | |
| Premature lethality | No1, 7 | Yes – perinatal2, 7 | YES – born at expected ratio but none born alive2 |
| Bone | NR | Anterior homeotic transformation of the axial skeletal (transformation of posterior vertebrae to anterior identity) via altered HOX gene expression on A/P axis2; Increased frequency of cleft palate7 | More severe homeotic transformations than GDF11KO All have cleft palate; Additional skeletal defects, including limb defects (extra forelimbs, shortened limbs), Digit patterning defects (sixth digit)7 |
| Kidney | NR | Most have Renal agenesis165 | All have renal agenesis7 |
| Pancreas | NR | Reduced pancreas size due to exocrine hypoplasia; 2-4 fold increase in endocrine progenitor cells by E18166 Increased number of islet progenitors8 |
NR |
| Olfactory epithelium | NR | Increased number of olfactory neurons and neuronal progenitors10 | NR |
| Retina | NR | Increased number of retinal ganglion cells and reduced number of retinal amacrine cells and photoreceptors11 | NR |
| Skeletal muscle | Myofiber hyperplasia and hypertrophy1, 112, 167 | None reported (perinatal lethality) | NR (perinatal lethality) |
| Stomach | NR | Two-fold reduction in the thickness of gastric wall with reduced number of characteristic folds8 | NR |
| Fat | Increased BW with decreased lipid content, decreased serum lipid and triglyceride levels6, 102, 168 | None Reported– but analysis limited due to perinatal lethality | NR (perinatal lethality) |
| Heart | Increased HW and BW169 | NR (perinatal lethality) | NR (perinatal lethality) |
| Conditional MSTN KO in skeletal myofibers only (in MLC-cre X MSTN-flox) | Conditional GDF11 KO in skeletal myofibers only (in MLC-cre X GDF11-flox mice) | Conditional KO of GDF11 and MSTN in skeletal myofibers only | |
| Adult skeletal muscle | Two-fold increase in young adult muscle mass (due to hyperplasia and hypertrophy); not apparent at birth (emerges postnatally); more glycolytic fibers (IIB)7 | No increase in young adult muscle mass; no change in fiber type7 | Same as MSTN conditional KO; no increase in phenotypic severity7 |
| Conditional MSTN KO in cardiac myocytes only | Conditional GDF11 KO in cardiac myocytes only | Conditional KO of GDF11 and MSTN in cardiac myocytes only | |
| Cardiac myocytes | Did not prevent left ventricular decompensation after TAC97. Cardiac hypertrophy and hearth failure, but cardiac function is restored after several weeks88, 101 | N/A | N/A |
GDF11 and MSTN exhibit many similarities in their biosynthesis, regulation, receptor utilization, and intracellular signaling pathways. Yet, the consequences of loss of Gdf11 or MSTN expression in mice are phenotypically distinct. Comparative analysis suggests only partial functional redundancy. See text for details. NR - not reported
Further highlighting the differences in MSTN and GDF11, myostatin mRNA is predominantly detected in skeletal and cardiac muscle whereas GDF11 mRNA is detected broadly in numerous tissues17 and is most abundant in the kidney and spleen12. Both GDF11 and MSTN are found in the bloodstream, and while the functional implications of their circulation are still under investigation, their systemic presence implies that these proteins may act as hormonal signals. Given their high sequence similarity, it was expected that many of the features and functions of these two ligands should overlap. However, a growing number of studies have described disparities in their actions, sparking debate over their respective involvement in particular physiological processes. Here, we discuss the molecular properties of GDF11 and MSTN, their roles in regulating different organ systems, and the challenges encountered in studying these proteins, which have contributed to recent controversies regarding their biological roles.
BIOCHEMICAL REGULATION OF GDF11 AND MYOSTATIN
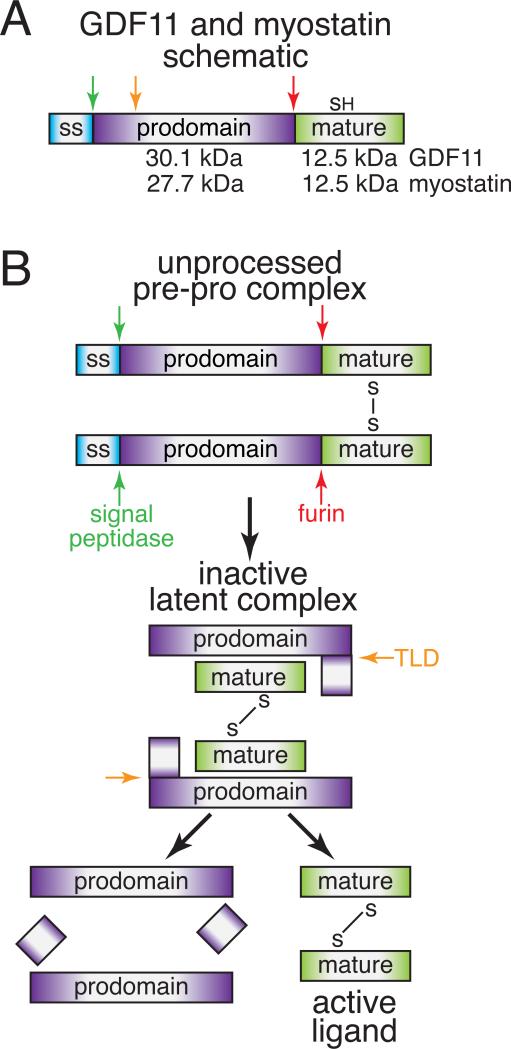
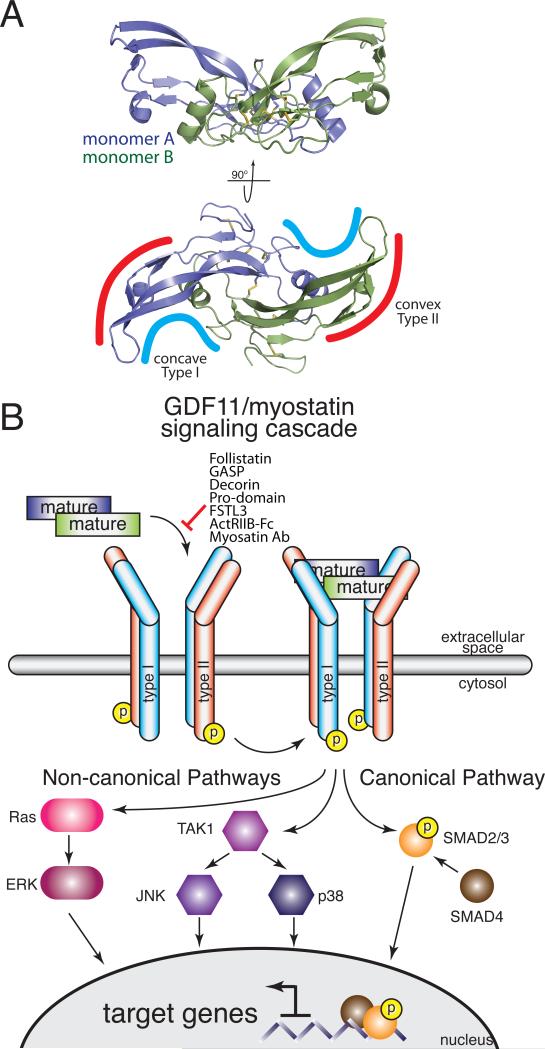
The TGFβ family comprises more than 30 structurally related, yet functionally distinct ligands. This family can be subdivided into three subclasses: the TGFβs, bone morphogenetic proteins (BMPs), and activin/MSTNs. GDF11 and MSTN belong to the activin/MSTN subclass and share 90% sequence identity within their mature, signaling domain. Similar to other TGFβ proteins, both GDF11 and MSTN are synthesized as precursor molecules where an N-terminal prodomain is cleaved from a C-terminal signaling or mature domain by a furin protease (Fig. 1A). The mature ligands are propeller-shaped, disulfide-linked dimers that initiate signal transduction by engaging two Type II receptors and two Type I receptors using convex and concave surfaces, respectively18 (Fig. 2).
Figure 1. Biosynthesis and proteolytic processing of GDF11 and MSTN.
A) Schematic of GDF11/MSTN monomer and relative position of proteolytic sites. B) Ordered proteolytic processing necessary to release an active dimer to elicit signaling.
Figure 2. Structure of MSTN and reported elements of GDF11/MSTN.
A) The symmetrical MSTN dimer forms two distinct interfaces, concave and convex, for receptor binding (PDB 3HH220). B) GDF11 and MSTN induced canonical and non-canonical signaling. Known extracellular regulators and pharmacological inhibitors of GDF11 and MSTN are listed.
The molecular structure of MSTN has been extensively investigated, including two X-ray crystal structures of MSTN in complex with two known antagonists19, 20. In contrast, GDF11 is less well characterized, and much of what is known for MSTN has been inferred for GDF11. However, the unbound X-ray crystal structure of GDF11 was recently determined revealing the classic propeller-shaped structure with subtle differences between myostatin and GDF11, particularly in receptor binding epitopes21. Therefore, while many structural and regulatory mechanisms are shared between these two ligands, growing evidence also points to unique features of GDF11 and MSTN biology.
Role of the Prodomain in Latency and Activation
While mature GDF11 and MSTN ligands share substantial sequence identity, their prodomains are only 52% identical (Fig. 3). Like other TGFβ members, the GDF11 and MSTN prodomains aid in folding of the mature dimeric ligand22, 23. However, unlike most TGFβ ligands, GDF11 and MSTN remain tightly bound to their prodomains after cleavage by furin-like proteases24-29, and are thereby held in a latent state, unable to bind receptors. Ligand activation requires additional cleavage of the prodomain by a Tolloid-like (TLD) metalloproteinase26, 27. Compared to other ligands, MSTN is inefficiently processed by furin, leaving a significant amount of unprocessed and presumably inactive protein24, 25. However, a SNP for the mutation K153R30 dramatically improves furin processing, but has no effects on TLD activation31 (Fig. 3B). Interestingly, this allelic variant was found at higher frequency in two centenarian cohorts, as compared to controls32, although the implications of this polymorphism in terms of longevity and maintenance of muscle mass and strength have yet to be definitively established32-41. While GDF11 has similar furin and TLD recognition sequences, it is not known if sequence variations, especially in the surrounding areas, which are more divergent, alter furin and/or TLD processing of GDF11 (Fig. 3B).
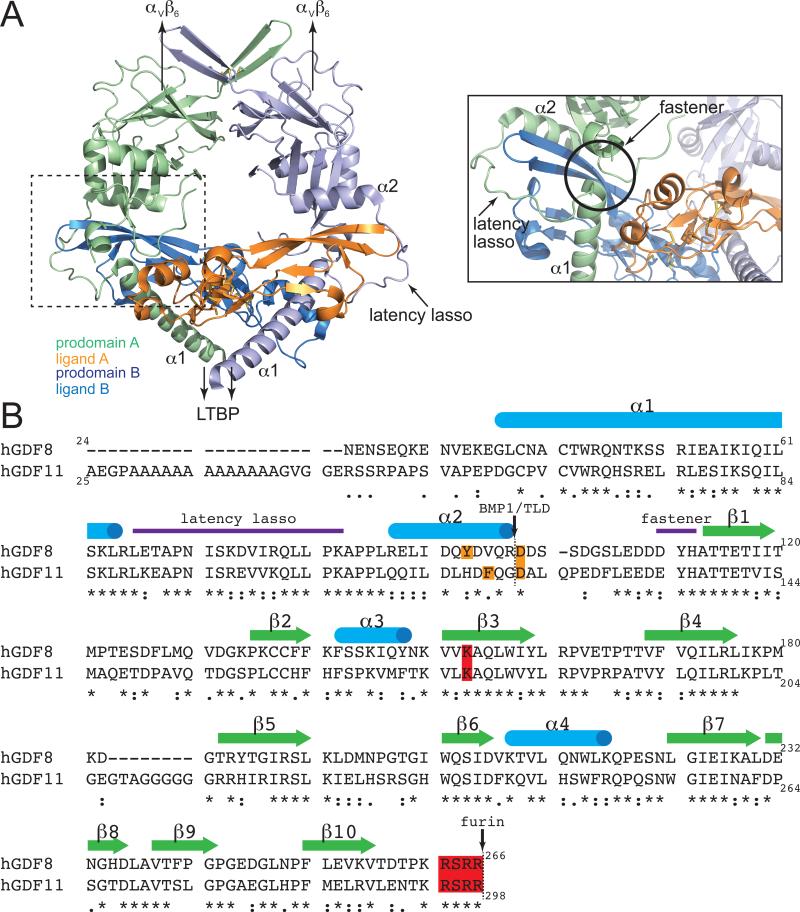
Figure 3. Structural organization of the TGFβ1 prodomain and comparison of GDF11 and MSTN prodomains.
A) Structure of the TGFβ1-prodomain latent complex (PDB 3RJR 42). Key regions identified in the TGFβ prodomain for conferring inhibition of the mature domain are highlighted (dotted box on the left is shown as the inset). B) Sequence alignment of human GDF11 and myostatin prodomains with topology based on the TGFβ structure. Known proteolytic sites and residues important for each proteolytic event are highlighted (furin: red; TLD: orange).
Structural details of the latent state have yet to be described for GDF11 and MSTN. Still, despite differences in mechanism of activation (discussed below), the structure of TGFβ1 in complex with its prodomain42 offers general insight into the molecular interactions driving latency. The latent structure of TGFβ1 has a ring-like appearance orchestrated by a centrally positioned mature dimer blanketed by the prodomains from each monomer42 (Fig. 3A). Ligand inhibition is mediated through interactions of the helical N-terminus (α1) with the Type I receptor site and a ‘latency lasso’ where the prodomain wraps around the ligand fingertips toward the Type II receptor site42. Consistent with this structure, MSTN prodomain residues 43-115 are necessary and sufficient to inhibit ligand activity43, 44. Interestingly, this region contains the TLD proteolytic site17,18 and is highly conserved with GDF11, suggesting that it serves a similar role in regulating GDF11 (Fig. 3B).
Nonetheless, the mechanism for TGFβ activation differs from that of GDF11 and MSTN45, 46. The TGFβ latent complex exists in the extracellular matrix (ECM) covalently bound to the latent transforming growth factor β protein 1 (LTBP1) via N-terminal disulfide linkages, a feature not known to occur for GDF11 and MSTN47-49. Additionally, the apex of the TGFβ latent complex interacts with αVβVI integrin45, 46. The combination of these two interactions tether the latent TGFβ complex at both ends, such that cellular contractile forces release the mature TGFβ ligand from the prodomain42, 45, 46. Although there is no evidence that MSTN or GDF11 latent complexes are covalently bound to a LTBP, these latent complexes do interact with the ECM components LTBP3 and perlecan25, 50. The purpose of these interactions remains unknown, but possibly relates to ligand activation, as binding to LTBP3 can prevent furin processing and overexpression of LTBP3 in skeletal muscle increase muscle mass25. Thus, interactions with ECM components may fine-tune the activity of MSTN and GDF11 and dissimilarities in GDF11 and MSTN prodomain sequences (Fig. 3) could allow for unique ECM interactions across tissues.
Receptor Utilization by GDF11 and Myostatin
Similar to the activin-type ligands, both GDF11 and MSTN predominantly utilize the Type II receptors activin receptor kinase II-A (ActRIIA) and ActRIIB and the Type I receptors activin receptor-like kinase 4 (ALK4) and ALK5 to elicit signal transduction via SMADs 2 and 328, 51, 52 (Fig. 2B). GDF11 also can signal through an additional Type I receptor, ALK7, although its biological role remains undetermined51.
Unlike the BMPs, the TGFβ and activin/MSTN sub-classes exhibit high affinity for the Type II receptor and low affinity for the Type I receptor18. The Type II receptors for the BMP and activin sub-classes bind on the concave surface of the fingers whereas TGFβs bind the Type II receptor more distally, towards the fingertips18. This positioning facilitates a cooperative binding interaction between the Type II and Type I receptor, as shown by the structure of the TGFβ3:TBRII:ALK5 receptor complex53. In contrast, the receptors bind BMPs on opposite sides of the fingers, and thus are unable to interact with one another, as described in the BMP2:ActRIIB:ALK3 complex54. The ternary receptor configuration for activin/MSTN has yet to be determined, as detailed binding and structural studies have been hampered by the low affinity for their Type I receptors18.
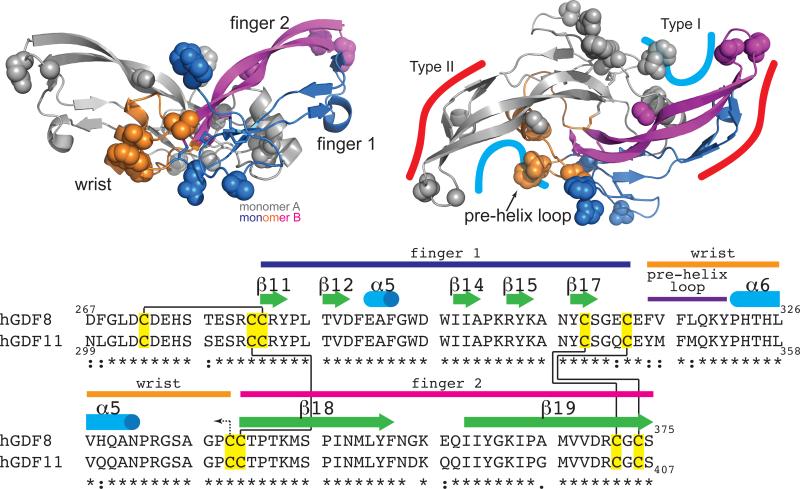
Using structural data as a guide to denote the approximate receptor interfaces53, 55-57, it is likely that GDF11 and MSTN bind Type II receptors similarly since residues in this location are identical (Fig. 4). However, residues in the Type I site, specifically the pre-helix loop and wrist helix (Fig. 4), are divergent between GDF11 and MSTN, suggesting that Type I receptor binding might differ, especially in the utilization of ALK7. Supporting this notion, introduction of the MSTN pre-helix loop into Activin A confers signaling through ALK520. Similar chimeric protein studies should help to reveal the biological consequences of sequence differences between GDF11 and MSTN at the receptor interface. Furthermore, with growing evidence indicating the importance of co-receptors in assembling TGFβ ligand complexes58-65, further studies are needed to define their roles in GDF11 and MSTN signaling.
Figure 4. Structural organization and comparison of GDF11 and MSTN mature domains.
MSTN dimer is shown where one monomer colored to show three sub-divisions of the ligand (finger 1: blue; wrist: orange; finger 2: magenta), which correspond to the colors in the sequence alignment below. Residues that differ between GDF11 and myostatin are shown as spheres. Differences are localized predominantly to the Type I site. Topology in the sequence alignment is an extension of the topology shown in Figure 3 and delineated by the structure of myostatin (PDB 3HH220). Cysteines (yellow) and corresponding intramolecular (solid black line) and intermolecular (dotted line) disulfide linkages are shown.
Regulation of GDF11 and Myostatin by Extracellular Binding Proteins
Signaling by GDF11 and MSTN is regulated by extracellular binding proteins that are typically thought to function as antagonists. These include follistatin (FS), follistatin-like 3 (FSTL3), decorin, and growth/differentiation factor associated serum proteins 1 and 2 (GASP1, GASP2)28, 66-70. Structural studies indicate that two FS or FSTL3 molecules symmetrically embrace the ligand to block both receptor epitopes19, 20. FSTL3 and FS similarly contain an N-terminal domain followed by tandem follistatin domains. The N-terminal domain binds in the concave Type I receptor slot where GDF11 and MSTN show the highest divergence19-21. However, mutagenesis studies and comparison to other FS-ligand structures indicate that the FS N-terminal domain is highly plastic and can accommodate diverse Type I interfaces19, 20, 71-73. Therefore, sequence differences likely have minimal impact on GDF11 and MSTN antagonism by FS. Sequence differences are also unlikely to impact the increased binding to cell surface-localized heparin/heparin sulfate and the subsequent acceleration of ligand degradation that occurs when FS is bound to MSTN20. This interaction is known to regulate MSTN signaling within skeletal muscle74, 75 and a similar mode of regulation may exist for GDF11. Interestingly, FSTL3 does not bind heparin and therefore readily escapes the cell surface to enter circulation76. Thus, the distinct localizations of FS-type antagonists provide an intriguing mechanism for differentially modulating the actions of circulating versus locally produced GDF11 and MSTN.
In contrast to broad TGFβ ligand antagonism by FS-type molecules, GASP1 and GASP2 selectively inhibit GDF11 and MSTN66, 77-80. GASP proteins contain six independent domains, a whey acidic protein domain, follistatin domain, Ig-like domain, 2 tandem kunitz domains, and a netrin-like domain66, 81. The follistatin domain is the primary driver of ligand antagonism80. Despite a similar domain layout, GASP1 binds MSTN in a unique 1:1 ratio, forming an asymmetric complex, whereas GASP2 binds symmetrically in a 2:1 ratio 68, similar to FS or FSTL319, 20, 77. Interestingly, C-terminal truncation of GASP1 induces 2:1 binding and weaker affinity for MSTN, similar to that of GASP277, 79, 80. GASP proteins antagonize signaling by preventing ligand binding to the Type II receptor79, 82, an intriguing mechanism given that GASP maintains unique specificity for GDF11 and MSTN despite conservation of the Type II receptor epitope among the activin/MSTN subclass. This observation suggests that steric forces and/or additional molecular contacts (e.g. in the Type I epitope) are likely important in defining this unique ligand-antagonist relationship.
In summary, GDF11 and MSTN are regulated by specific and non-specific interactions at nearly every step from biosynthesis to engagement with their cognate receptors. Given that sequence divergence exists between the GDF11 and MSTN prodomains, and to a lesser extent in the mature domains, it remains important to determine if the distinct biological functions of these proteins are driven in part by these molecular differences.
Signaling by GDF11 and Myostatin
Canonical TGFβ signaling is mediated through a series of SMAD proteins. Receptor binding by either GDF11 or MSTN induces phosphorylation and activation of the receptor-regulated SMAD (R-SMAD) proteins SMAD2 and SMAD3. Subsequently, the phosphorylated R-SMAD proteins assemble to form oligomeric complexes with the common SMAD (coSMAD) SMAD4, and this complex accumulates in the nucleus to regulate gene expression through direct and indirect DNA binding (Fig. 2B). Cellular responses to SMAD2/3 activation are highly context-dependent, and the presence or absence of particular transcriptional cofactors, DNA binding partners and chromatin modifiers can dramatically alter the ultimate output of ligand binding83, 84. GDF11 and MSTN may also signal through non-canonical (i.e., non-SMAD) pathways, including ERK, JNK, and p38 MAPK (Fig. 2B)85-88, adding further complexity.
The transcriptional targets of GDF11 and MSTN signaling remain incompletely defined. A recent study compared gene expression changes following stimulation of human primary muscle cells with GDF11 or MSTN87. Only a few differentially represented transcripts were identified, suggesting that GDF11 and MSTN gene regulation may be essentially identical87. However, this analysis was limited to a single cell type and neither ligand activated a robust gene-expression signature (the highest observed Fold Change values were only ~4-fold for either ligand). Thus, further experiments are needed to clarify potential differences in the transcriptional output of GDF11 and MSTN signaling in different cell types and physiological contexts.
GDF11 RELATED PATHWAYS IN THE HEART
The incidence of myocardial infarction and heart failure increases with age89, and aging increases mortality risk with any given infarction event90. TGFβ signaling regulates responses to myocardial ischemic injury91, 92, with recent studies suggesting possible roles for GDF11, MSTN and FSTL3 in the heart.
Myostatin in the heart
MSTN is best known for inhibiting skeletal muscle growth1, 3, but genetically engineered models have demonstrated an additional function in cardiac tissue. MSTN is expressed in fetal and adult hearts93 and its expression increases in patients with decompensated heart failure94 and congenital heart disease95. MSTN protein levels rapidly increase after ischemia96 and its circulating levels increase in mice after TAC-induced hypertrophy97. Germ-line inactivation of MSTN does not cause cardiac hypertrophy and does not attenuate cardiac fibrosis in dystrophin-deficient mice, indicating that MSTN does not function in cardiac muscle in a manner similar to skeletal muscle98. However, other experiments reveal that MSTN-null mice develop increased heart and body weights. MSTN−/− mice (28-30 month-old) were reported to have increased normalized heart mass at death compared to MSTN+/+ and MSTN+/− mice99. Aged mice with MSTN deletion have improved fractional shortening, smaller left ventricle diastolic diameters and less fibrosis compared to aged wild-type mice100. Heineke and colleagues compared deletion versus overexpression of MSTN in cardiomyocytes97. MSTN deletion in cardiomyocytes did not prevent left ventricular decompensation under pressure overload, whereas transgenic overexpression of MSTN in the heart inhibited cardiac growth97. Inducible genetic deletion of MSTN in adult mouse cardiomyocytes leads to dramatic deterioration of cardiac function and high mortality, as well as increased glycolysis and glycogen storage, revealing the importance of endogenous MSTN for adult cardiomyocyte metabolism101. Thus, MSTN likely participates in cardiac growth and metabolism, although the experimental findings have not been as consistent in the heart as in skeletal muscle.
Cardiac effects of GDF11
GDF11 is expressed in cardiac tissue102 but at lower levels compared to spleen, kidney and skeletal muscle in mice12. We reported an anti-hypertrophic effect of GDF11 in aging mice12. Using heterochronic parabiosis, we reported reduced cardiac hypertrophy in aging mice that shared a common circulation with young mice. We further devised a sham parabiosis procedure wherein mice were joined but did not share a chimeric circulation. With heterochronic sham parabiosis, hypertrophy in old mice was not reduced, implying the presence of a circulating factor that regulated cardiac size. Proteomic studies identified GDF11 as a candidate for this anti-hypertrophic effect, and supplementing blood levels in old mice by daily intraperitoneal injection of recombinant GDF11 (rGDF11) protein reduced cardiomyocyte size and heart mass over 4 weeks12.
In contrast, Smith and colleagues reported recently that injections of the same quantity of rGDF11 employed by us in old mice (0.1 mg/kg, injected daily) did not alter cardiac structure or function103. These apparently contradictory results may be explained in part by the different sources of rGDF11 and by a potential dose-dependent effect of GDF11. Following the study by Smith and colleagues103, we performed a dose-response analysis, and demonstrated that for a given protein preparation, the reduction in cardiac mass by GDF11 is dose-dependent; for recent protein preparations with improved quality control of protein concentration, a dose of 0.5 mg/kg reduced cardiac mass in 9 days104. Injection of rGDF11 rapidly activates SMAD signaling in cardiac tissue of both young and old mice104, which together indicate that exogenous GDF11 can regulate cardiomyocyte size and hypertrophy and highlight that doses and protein preparations used could impact the results of in vivo studies105.
Given observations reported previously for genetic manipulation of MSTN expression88, 98, 99, it is likely that administration of recombinant MSTN could achieve a similar anti-hypertrophic effect, but this has yet to be tested. Furthermore, it remains to be determined if a similar effect can be achieved with other ligands that likewise activate the SMAD2/3 pathway, such as members of the TGFβs or activin sub-classes. Finally, while most studies to date have focused on SMAD activation as a readout of signaling activity, it will be interesting to determine if cross-talk with non-canonical pathways may achieve ligand-specific effects.
FSTL3 in cardiac tissue
FSTL3 is expressed in cardiac tissue106 and its expression increases in end-stage failing myocardium in humans107. Heart mass, left ventricular and systolic pressure, and systolic arterial pressure are increased in FSTL3-deficient mice compared to wild-type mice108. In addition, experimental cardiac injury induces myocardial expression of the prosurvival TGFβ ligand, activin A, and one of its antagonist regulators, FSTL3, where it is thought that the relative expression levels of these molecules dictate cell survival following insult. Interestingly, cardiomyocyte-specific deletion of FSTL3 reduces infarct size and apoptosis, suggesting a detrimental effect of endogenous FSTL3 on the heart, while overexpression of FSTL3 inhibits the prosurvival effect of activin A109. FSTL3 also regulates cardiac hypertrophy induced by pressure overload106. While no differences were seen between hearts of cardiac-specific FSTL3−/− and wild-type mice in standard physiological conditions, FSTL3−/− mice106 exhibited attenuated myocardial hypertrophy and reduced left ventricular dilatation, and systolic dysfunction and interstitial fibrosis were reduced106, 110 after transverse aortic constriction-induced pressure overload (TAC). These data suggest that endogenous FSTL3 regulates the heart in many circumstances and that induced expression of FSTL3 may have deleterious effects. It remains to be determined how cardiac insult and upregulation of FSTL3 may impact the heart over time with respect to GDF11 and MSTN levels, especially in the case for older populations where evidence suggests that GDF11 and/or MSTN levels decline with age104. Nevertheless, because FSTL3 inhibits multiple TGFβ family ligands111, cardiac effects of FSTL3 cannot be attributed to a particular ligand interaction at this time.
GDF11 RELATED PATHWAYS IN SKELETAL MUSCLE
Skeletal muscle is composed of multinucleated, non-dividing fibers. Repair of muscle fibers after severe damage invokes the regenerative activities of a unipotent population of muscle stem cells, known as satellite cells, which reside immediately adjacent to myofibers in adult muscle. Aging impairs both the homeostatic maintenance of muscle mass and muscle regenerative potential, and recent studies have focused on the potential role of GDF11-related signaling pathways in these age-associated changes.
Myostatin in muscle homeostasis and repair
Prevailing views on GDF11 function in skeletal muscle and satellite cells have been greatly influenced by analogy to MSTN due to the high homology of their mature, C-terminal ligand domains (Fig. 4). MSTN is expressed almost exclusively in mature and developing muscle and negatively regulates muscle mass. Targeted disruption of MSTN in mice produces a near doubling of adult muscle mass, with an increase in both the size and number of muscle fibers1 and a shift toward more glycolytic fiber types1, 112. Whether the hypertrophic and hyperplastic phenotype of MSTN-null muscle reflects in part a release of MSTN-mediated inhibition on muscle satellite cells remains unclear. While some studies suggest that MSTN inhibits satellite cell proliferation113, 114, others have reported that it has no impact115, 116. Likewise, some groups have found increased numbers of satellite cells in MSTN-null muscle117, while others report slightly lower numbers115. MSTN overexpression and supplementation studies suggest that high levels of MSTN can drive rapid muscle atrophy118, 119, although more moderate increases in MSTN do not detectably alter muscle mass120. Conversely, treatment of mice with the inhibitory MSTN propeptide induces muscle hypertrophy121.
GDF11 effects in muscle
In contrast to the profound effects of MSTN deficiency, muscle phenotypes are not prominent in GDF11-null mice, which exhibit defects in a variety of mesodermal, endodermal and ectodermal lineages and die within 24 hours after birth2. Nonetheless, double mutants lacking both MSTN and GDF11 exhibit increased penetrance of renal, palatal and skeletal abnormalities7, suggesting that MSTN can compensate to some degree for loss of GDF11 in GDF11-null mice. Studies to similarly assess possible redundancy of GDF11 and MSTN function in regulating muscle mass have been challenging, due to the fact that GDF11-null mice die prior to the age at which MSTN-null mice begin to exhibit muscle phenotypes. However, analysis of mice with muscle fiber specific deletion of GDF11 showed no changes in muscle mass or fiber type, and muscle-specific GDF11 deletion did not exacerbate the phenotype of MSTN-null animals7. These results suggest that GDF11 and MSTN may have distinct functions in skeletal muscle, or that GDF11's effects on muscle are mediated by GDF11 protein that is produced by non-muscle tissues.
GDF11 has been implicated as a negative regulator of muscle development by studies in chick embryos and in cultures of differentiating C2C12 cells and primary human and mouse muscle myoblasts. In these systems, GDF11, like MSTN, can block myogenic differentiation87, 122-124. Yet, other data suggest that GDF11's role in myogenesis may be more complex. Gdf11 mRNA levels in mouse skeletal muscle appear to peak during the most rapid phase of postnatal muscle growth and are higher in males than in females, despite males having greater muscle mass124. Gdf11 expression is also increased in the muscles of MSTN-null mice, which nonetheless exhibit accelerated muscle growth and increased muscle mass124. These data raise the possibility that GDF11's actions in muscle may not fully overlap with those of MSTN such that GDF11 cannot compensate fully for the loss of MSTN in muscle. Whether this lack of functional redundancy reflects differences in the biochemical properties and signaling activities of GDF11 and MSTN, or differences in their absolute levels in muscle, remains to be determined. Interestingly, ectopic GDF11 can induce expression of FS122, which as discussed above inhibits both GDF11 and MSTN signaling. Thus, GDF11 can initiate a negative feedback loop that may antagonize its own activity, as well as that of other activin/MSTN ligands. The existence of such a feedback mechanism suggests the likely importance of maintaining signaling by these ligands within a tight physiological range.
Experiments employing heterochronic parabiosis recently implicated GDF11 as a candidate regulator of aging phenotypes in the heart, muscle and brain12-14. Consistent with this notion, satellite cells isolated from the uninjured muscle of aged mice that received daily intraperitoneal injection of rGDF11 for four weeks showed improved myogenic activity in ex vivo clonal assays and a reduced burden of DNA damage. In addition, aged animals supplemented with rGDF11 showed accelerated recovery from muscle injury in a cryoinjury model14. rGDF11 treatment in aged mice also improved neuromuscular junctions and myofibrillar and mitochondrial morphology, and increased average exercise endurance and grip strength without any detectable changes in muscle mass or fiber caliber14. Notably, the dosage of rGDF11 in these studies was similar to that reported previously to lack effects on muscle size in an in vivo rMSTN dosing study120. Interestingly, young mice in this study showed no differences in regenerative capacity following treatment with the same amount of rGDF11, suggesting that GDF11's beneficial effects on muscle were age-dependent.
In contrast to the studies summarized above, a subsequent paper questioned the beneficial effects of rGDF11 on muscle aging, reporting instead that supplementation of rGDF11 in aged mice has no effect and in young mice impairs muscle repair. Yet, it is important to note that the study design employed by this group87 differed significantly from that in the earlier studies14. From a reagent standpoint, the authors used rGDF11 from a different protein source and employed a different dosing regimen. Further, they assessed muscle regeneration in a more severe, cardiotoxin-mediated damage model, which ablates the majority of resident satellite cells125, 126 and shows distinct temporal and spatial features of regeneration in comparison to cryoinjury127, 128. Thus, the different outcomes obtained in the two studies may reflect differences in experimental design, and comparison of the methods employed could provide an avenue for discovering key mechanisms that determine GDF11's in vivo effects. For instance, the inability of rGDF11 to accelerate repair in the satellite-cell depleting cardiotoxin injury model87 suggests that sustaining a sufficient pool of regenerative satellite cells may be essential for a beneficial effect on regeneration in response to rGDF11. Likewise, the possible negative impact of higher doses of rGDF11 in young mice87 could indicate that rGDF11 exhibits antagonistic pleiotropy in young versus old animals, or that the precise timing and dosage of rGDF11 administration is critical in determining its in vivo effects.
It is also important to remember that rGDF11's impact on muscle repair in vivo likely represents the integrated effects of this growth factor on many different target cells. GDF11 signaling has been implicated in a number of biological processes, including vasculogenesis13, a process clearly documented to influence the efficiency of muscle repair129. Indeed, if the primary actions of GDF11 in vivo are on vasculature, this may explain the coordinated responses to systemic administration of this protein seen in skeletal muscle, heart and brain, as each of these organs shows critical dependence on proper vascularization, a process which declines with increasing age130.
The potential effects of changing levels of MSTN on aging muscle have also been examined. As mentioned above, human genomics studies suggest an association of the K153R polymorphism, thought to increase MSTN activity by enhancing furin processing31, with longer lifespan32. Interestingly, homozygosity for the K153R allele is extremely rare, and most R allele-carrying centenarians are heterozygous for this variant, a genotype that does not appear to alter muscle phenotypes in aged individuals38, 39, 41. Studies in mice also suggest that alterations of MSTN signaling may impact lifespan. A recent study of male MSTN+/+, MSTN+/− and MSTN−/− mice (n=38-42 mice per group), revealed an increase in both median and maximum lifespan of heterozygous animals, which showed a 30% decrease in circulating MSTN levels, as compared to wild-type controls99. In contrast, the median and maximal lifespan of MSTN−/− mice did not differ from wild-type. Replication of this study, with inclusion of female animals, should be very illuminating, particularly as data from human subjects suggests sex-specific differences in the regulation of circulating MSTN levels with age131.
Analogous studies of lifespan in GDF11 mutant mice are complicated by the developmental phenotypes exhibited by both GDF11−/− and GDF11+/− mice. However, Pazdro and colleagues recently reported that levels of circulating GDF11 are heritable in genetically diverse inbred mouse strains and can be used to predict median lifespan, with higher GDF11 levels at middle age associated with longer lifespan132. In addition, an overall positive impact of higher GDF11 and/or MSTN levels on lifespan, as well as muscle function, has been corroborated in invertebrate models. For example, overexpression of myoglianin, the fly ortholog of both GDF11 and MSTN, delayed the onset of age-related neuromuscular dysfunction and extended lifespan, while its inhibition hastened neuromuscular decline and caused premature death133. Studies in shrimp likewise implicate the ancestral form of GDF11/MSTN in supporting muscle growth and survival134.
In summary, although more research is clearly needed, evidence across species implicates GDF11 as a candidate regulator of muscle homeostatic and regenerative function during aging, and suggests that raising levels of circulating GDF11 might be helpful for some age-related muscle pathologies. Emerging evidence also implicates both GDF11 and MSTN in regulating longevity and suggests that even subtle variations in ligand activity can alter phenotypic outcomes. While the mechanisms underlying these effects remain unclear at present, these data raise the possibility that systemic GDF11 could provide a useful biometric for mammalian aging.
GDF11 IN THE BRAIN
TGFβ family ligands have diverse and pleiotropic roles in the development and maintenance of the nervous system, and their effects can vary between ligands, target cell types, and an animal's developmental stage or age. Neurological effects of GDF11 have been best characterized in the developing olfactory epithelium, spinal cord and retina where GDF11 influences the timing and progression of neurogenesis as well as the ratios of different neural subtypes10, 11, 135. In the developing olfactory epithelium, GDF11 plays a critical role in an autoregulatory loop, wherein newly born olfactory receptor neurons (ORNs) and their immediate neuronal precursor cells (INPs) signal to neighboring cells to limit the production of more ORNs10. ORNs and INPs secrete GDF11, which drives cell cycle exit by inducing upregulation of the cyclin dependent kinase inhibitor p27Kip1. Loss of GDF11 augments proliferation of INPs and increases the number of differentiated neurons. Conversely, enhanced activation of GDF11 by genetic deletion of FS leads to sharp decreases in the numbers of both INPs and ORNs. In the spinal cords of mice lacking GDF11, neural progenitor cells fail to exit the cell cycle, the rate of neurogenesis is slowed, the ratios of neural subtypes are altered, and the positional identities of motor neurons are disrupted135-137. Interestingly, in the retina, GDF11 also controls the timing of neurogenesis, but through a mechanism that does not involve proliferation11. Instead, GDF11 controls the length of time that progenitor cells are competent to produce retinal ganglion cells, and thereby regulates the ratio of RGCs to photoreceptors and amacrine cells.
Compared to its role in the developing nervous system, less is known about the role of endogenous GDF11 in the mature nervous system. Northern blot analysis of tissues from adult rats (3-4 weeks of age) showed high expression of Gdf11 in dental pulp and brain17. In the same study, RNA in situ hybridization identified specific areas of Gdf11 expression in the brain, including the dentate gyrus of the hippocampus, the hypothalamus and the Purkinje cell layer of the cerebellum17. It was recently demonstrated that systemic administration of rGDF11 in aged mice alters brain physiology13. Heterochronic parabiosis experiments showed that young systemic factors could reverse age-related neurogenic decline by enhancing neural stem cell production and thereby increase adult neurogenesis and olfactory discrimination capacities. Furthermore, youthful blood factors induced remodeling of the aged vasculature, restoring cerebrovascular blood flow to its youthful levels. Systemic injection of rGDF11 recapitulated several beneficial effects of heterochronic parabiosis, including vascular remodeling of the aged blood vessels in the subventricular zone and increased numbers of neural stem cells in this area. rGDF11 also induced the proliferation of brain capillary endothelial cells and activated the SMAD2/3 pathway in vitro. The in vivo effects of rGDF11 were not as prominent as those seen with heterochronic parabiosis, suggesting that additional factors may be present in the young circulation that exerts these CNS effects138. Future studies testing different concentrations of rGDF11104, different treatment lengths and co-injection of factors such as IGF-1 or EGF that affect neural stem cell proliferation and/or vascular behavior may clarify this issue.
The CNS effects of rGDF11 are due to exogenous/circulating protein and not to brain-derived GDF11, whose role in the adult/aged brain is not yet understood. It will be important to determine if systemically administered rGDF11 crosses the blood-brain-barrier and acts directly on neurons and neural stem cells, or if its effects on the CNS are a by-product of its cerebrovascular effects. It will likewise be exciting to determine if an auto-regulatory loop exists between circulating and endogenous GDF11 that could amplify its effects. Interestingly, the effects of systemic rGDF11 are consistent with a recent report that constitutive activation of ALK5, a type I receptor for GDF11 and multiple TGFβs, leads to increased adult neurogenesis and higher expression of c-Fos in newborn neurons in the adult hippocampus, more complex dendritic arborization, increased neural activity, and improved performance in memory tests139. In addition, TGFβ1 is neuroprotective in mouse models of Alzheimer's disease and excitotoxicity140, 141, and a recent study also reported that GDF10, which activates similar intracellular pathways as TGFβ1, β2, β3, and GDF11, improves brain vasculature, increases neurite outgrowth, and enhances performance in behavioral assays in a mouse model of stroke142.
CLINICAL IMPLICATIONS OF GDF11 and MSTN
Olson, Ganz and colleagues investigated whether GDF11 and MSTN might have similar cardioprotective properties in humans to those demonstrated in mice143. In 928 archived plasma samples in subjects with stable coronary heart disease (CHD) from the Heart and Soul prospective observational cohort, they measured circulating levels of ligand using modified aptamers as binding reagents143. This assay does not discriminate GDF11 from MSTN; thus the measured analyte is referred to here as GDF11+MSTN. The aims of the study were to characterize the association of plasma levels of GDF11+MSTN in humans with 1) age, 2) left ventricular hypertrophy and 3) cardiovascular events and all-cause mortality during a median 8.9 years of follow-up. The key findings were replicated in 971 subjects with stable CHD from the HUNT3 Norwegian cohort, with a median follow-up of 4.5 years.
GDF11, MSTN, and age
Analysis in patients revealed an inverse relationship of plasma GDF11+MSTN to age in the HUNT3 cohort (Fig. 5A), which was also observed in the Heart and Soul cohort143. Despite the narrower age range of these human subjects compared to the extremes of age reported in mice12, significantly lower levels of GDF11+MSTN were detected in older compared to younger individuals in both patient cohorts143. This cross-sectional analysis likely underestimates the decline in GDF11+MSTN with age, as GDF11+MSTN is inversely associated with all-cause mortality; thus, surviving older individuals enrolled in these cohorts likely had higher GDF11+MSTN concentrations than those older individuals who had died and whose potentially lower levels could not be captured. Although GDF11+MSTN levels were higher in males compared with females enrolled in this study, an age-related decline in GDF11+MSTN levels was detected in both sexes143. In support, a recent study used mass spectrometry to quantify circulating MSTN specifically in human subjects without chronic disease, and likewise reported higher MSTN concentrations in younger men compared to younger women, but found sex-specific differences in the effects of age on MSTN levels (higher MSTN concentrations in older versus younger women and lower MSTN concentrations in older versus younger men)131. Circulating levels of the antagonist FSTL3 increased with age in both sexes131. Thus, while this latter study131 analyzed a much smaller cohort (only 40 individuals of each age/sex), together these reports131, 143 highlight a potential influence of age, sex, and health status on GDF11+MSTN levels and are consistent with an age-related loss of function of these ligands in humans, either through diminished protein concentrations143 or increased inhibition of ligand activity131.
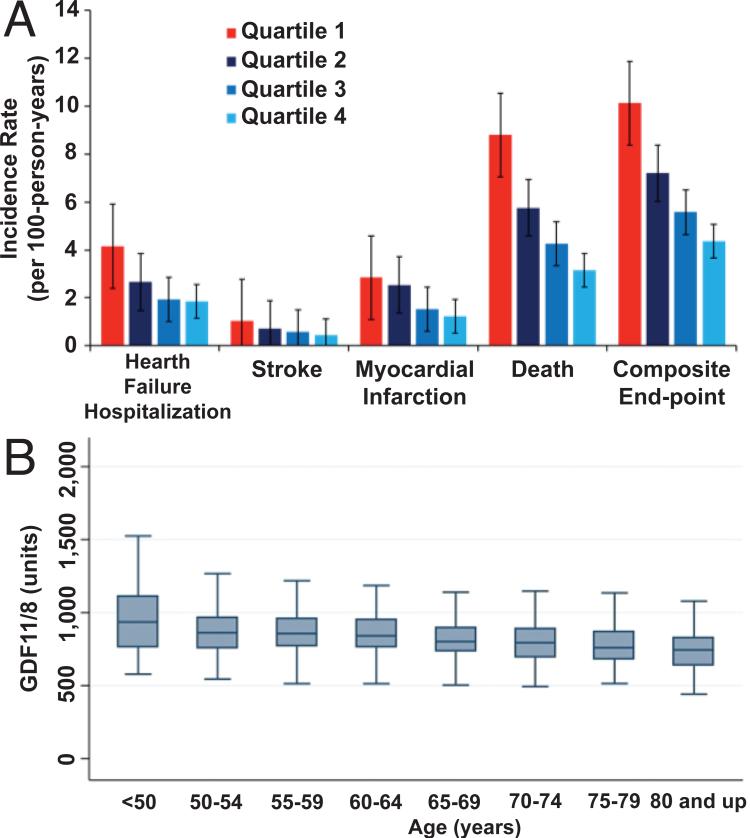
Figure 5. GDF11/MSTN in humans.
A) Incidence of heart failure hospitalization, stroke, myocardial infarction, all-cause death, and their composite end-point in the HUNT3 cohort, unadjusted, stratified by quartile of GDF11+MSTN. Quartile 1 is the referent quartile with the lowest GDF11+8 concentrations. P-values for trend are <0.001 for heart failure, death, and composite end-point, 0.004 for stroke, and 0.02 for myocardial infarction. B) GDF11+MSTN levels by age in HUNT3 cohort, unadjusted. Inner line = median, box 25th-75th percentile, outer whiskers denote adjacent value 1.5 times height of box. Units = relative fluorescence units. P-value is < 0.001. This figure is reproduced from Olson et al.143 with permission from the European Heart Journal.
GDF11, MSTN, and cardiovascular and all-cause mortality
A relationship of plasma GDF11+MSTN levels to cardiovascular events (heart failure hospitalization, stroke and myocardial infarction), all-cause deaths and their composite end-point were also demonstrated in 971 subjects in the HUNT3 cohort (Fig. 5B)143. In both the HUNT3 and Heart and Soul cohorts, there was a strong, graded relationship between plasma GDF11+MSTN and cardiovascular and mortality outcomes143. Participants in the highest quartile of GDF11+MSTN had markedly lower risk of individual types of events and their composite end-point than those in the lowest quartile, both unadjusted (Fig. 5B) and after multi-variable adjustment143.
GDF11, MSTN, and left ventricular hypertrophy
Of the 928 participants in the Heart and Soul cohort, 368 had left ventricular hypertrophy (LVH) by echocardiography143. There was a significant, inverse, graded relationship between GDF11/MSTN and LVH, with 31% of participants in the highest quartile of GDF11/MSTN having LVH compared with 46% in the lowest quartile143.
In sum, in two large independent cohorts of patients with stable CHD, lower levels of GDF11+MSTN were associated with notably higher rates of incident cardiovascular events and all-cause deaths as well as higher prevalence of LVH. Lastly, GDF11+MSTN levels are lower among older compared to younger individuals. Taken in the context of mechanistic studies in mice, these findings in humans support the hypothesis that GDF11 and/or MSTN protect against adverse cardiovascular events and all-cause deaths143. More broadly, these findings support the hypothesis that age-related decline in GDF11+MSTN contributes to cardiovascular aging and mortality in humans143 as it does in mice.
Future directions for clinical research
A significant interaction was present in the multi-racial Heart and Soul cohort between race and GDF11+MSTN for the composite cardiovascular and all-cause mortality outcome143. Whites compared to non-whites had both lower levels of GDF11+MSTN and a stronger link between plasma GDF11+MSTN and adverse outcomes. As noted previously, GDF11+MSTN levels also varied by sex143. Accordingly, future studies should investigate in greater detail any racial and sex differences in GDF11 and MSTN levels and their relationship to outcomes.
As discussed above, several endogenous inhibitors of GDF11 have been reported. These inhibitors may antagonize other ligands in the TGFβ family, including MSTN and activin67, 68, 79. Heidecker and Ganz investigated the association of GASP1, FSTL3 and FS with cardiovascular outcomes and all cause-mortality in the Heart and Soul and HUNT3 cohorts, using the same archived plasma samples as for the aforementioned GDF11+MSTN analyses144. High levels of FSTL3 were associated with increased risk of adverse cardiovascular events and all-cause mortality, and the effect of low levels of GDF11+MSTN and high levels of FSTL3 were additive. Subjects in the least favorable quartile of GDF11+MSTN (lowest) and its inhibitor FSTL3 (highest) had nearly 7-fold increased risk of cardiovascular events and all-cause mortality144. Thus, any future studies of human diseases will need to consider the levels of GDF11+MSTN, levels of their inhibitors and their interactions.
Any therapeutic targeting of GDF11 or MSTN in humans must consider its potential adverse effects. GDF11 has been reported to exacerbate anemia in the setting of ineffective erythropoiesis145. Blocking GDF11 alleviates anemia in a mouse model of beta-thalassemia and also in humans146. Analysis of observational cohort studies may provide much needed clarity whether levels of GDF11 and/or MSTN are associated with any additional adverse outcomes.
CURRENT CONTROVERSIES
The suggestion that GDF11 may serve as an evolutionarily conserved age-dependent hormone in multiple organ systems12-14, 133, raises possibilities for manipulating this signaling pathway to restore or preserve function to aging tissues. Yet, this provocative notion also presents a new and unexpected role for GDF11 within the TGFβ superfamily, and contrasts with studies of many other TGFβ family proteins in aging, which have frequently concluded that these molecules suppress healthy tissue function during aging, most notably by promoting tissue fibrosis and inflammation147-150. Thus, it is not surprising that there has been some skepticism and even controversy surrounding these results. It is likely that much of this controversy reflects the fact that GDF11 is a relatively under-studied member of the TGFβ superfamily, particularly compared to its close relative MSTN, and thus the tools for studying GDF11 biology are still evolving. In addition, given the substantial sequence conservation of GDF11 with MSTN, many have assumed that the functions of these proteins should be identical7, 87. Here, we discuss some of these controversies and suggest strategies to resolve the apparent discrepancies to advance our mechanistic understanding of GDF11 functions in organ physiology and aging.
Presence in circulation and direction of change with age of circulating GDF11 protein
In 2013, we reported a significant decline in systemic levels of GDF11 in aged, as compared to young mice12. This conclusion was based on an aptamer-driven analysis of serum from young (2 mo.) versus old (24 mo.) mice performed using Somalogics SomaMERs151, in combination with Western blotting using a monoclonal antibody from abcam, which, at the time, was reported to be specific for GDF11. Subsequent reports from our group104 and others87, 143, 152 revealed that the SomaMER and mAb used in these initial studies cross-react with MSTN, and so, while these studies are consistent with a reduction in the circulating pool of GDF11 and MSTN in aged animals, these data cannot discriminate the relative impact of aging on systemic levels of GDF11 specifically. A report from Egerman and colleagues argued that circulating levels of GDF11 might actually increase with age, based on the results of Western blot, RNA expression, and GDF11-specific immunoassay87. However, the Western results reported by this group were based on quantification of a ~25kDa immunoreactive band, the approximate size of the disulfide-linked mature ligand dimer, which was present in samples that had been reduced and denatured prior to Western blot analysis. Puzzled by this observation (since the blotting conditions should have reduced the dimeric ligand to monomer), we reproduced these experiments and confirmed that the ~25kDa band does increase with age. However, we further demonstrated that this band is comprised of serum immunoglobulin, known to increase with age153, and not GDF11 or MSTN104. As expected, under denaturing and reducing conditions, detection of GDF11 and MSTN in serum is limited to the ligand monomer of ~12.5kDa, which declines with age in the Egerman studies87, as in prior reports12, 14. In addition, the RNA expression analysis reported by Egerman et al. was limited to skeletal muscle, which expresses GDF11 at substantially lower levels (2-3 fold) than other tissues (e.g., the spleen and kidney12, which may represent the predominant sources of circulating GDF11 protein). Finally, their immunoassay results in rats and humans were extremely underpowered (e.g., only 9 individuals “>60 years old” were assessed in the human studies, with no evaluation of health status) and did not yield statistically significant differences for either species.
Studies from this group and one other103, also suggested that circulating levels of GDF11 may be very low compared to MSTN, leading a third group154 to argue that changes in circulating GDF11 levels are “mostly irrelevant”. However, published mass spectrometry data clearly demonstrate the presence of GDF11 protein in both human and mouse sera155, and, many critically important bioactive hormones are present at very low levels (i.e. pg/ml) in circulation, including glucagon, IL6, and TNF-α156. Thus, it is difficult to infer biological relevance from protein concentration alone. Future studies to develop highly specific reagents for measuring systemic GDF11 levels using either quantitative mass spectrometry or antibody-based immunoassays that can reliably distinguish GDF11 from MSTN in the blood of both humans and mice, something that has not yet been accomplished87, 103, will be essential to clarify the overall abundance and age-related changes of circulating GDF11 and MSTN proteins. Adding to the complexity, current methods131, 154, 157, 158 only measure the ‘total’ amount of protein and cannot account for the status of the ligand in the serum (e.g. free, latent or in complex with an extracellular antagonist). Finally, it is still unclear if GDF11 and MSTN act predominantly as systemic hormones or if they may exert their most potent effects locally through autocrine/paracrine mechanisms, which complicates interpretation of the impact on peripheral tissues of changing levels of these proteins in the blood. Thus, it will be important to clarify which cell types produce GDF11 and what pathophysiological stimuli may alter GDF11 and/or MSTN expression.
Effect of GDF11 supplementation on skeletal muscle regeneration in old and young mice
Studies investigating the effects of exogenous rGDF11 on muscle regeneration have reported apparently discrepant conclusions. While we reported14 that rGDF11 reverses age-specific muscle phenotypes and improves muscle strength, endurance and regenerative potential, with no discernable effects in young mice, Egerman et al. argued instead that rGDF11 supplementation has no effect in aged mice and may slow regeneration in young mice87. As discussed above, these different conclusions likely arise from differences in protein source and experimental design. Of particular importance is the use by Egerman et al. of a more severe cardiotoxin injury model125, 128, as compared to the milder cryoinjury model applied in our studies14 and those that originally demonstrated rejuvenation of muscle repair by heterochronic parabiosis159. Furthermore, in their studies of young mice, Egerman et al. used a markedly different dosing schedule, shortening the pre-injury treatment window from 28 to 3 days and lengthening post-injury treatment from 7 to 14 days. They also employed an unconventional analysis strategy that selectively focused on tiny “fibers” that lacked apparent nuclei. The reported in vitro studies, also, were quite different in design, with one group analyzing cells purified from uninjured muscle14, and the other cells purified from cardiotoxin-damaged muscle87, with distinct culture conditions and time points of analysis. Thus, discrepancies in the reported effects of rGDF11 on muscle repair and muscle satellite cells may reflect differences in the recombinant protein itself, in protein dosage and treatment schedule, in the severity or method of muscle damage and the consequent size of the residual muscle stem cell pool, or in the particular culture conditions and analysis strategies employed. Future studies to compare these experimental conditions side-by-side are necessary to identify the key experimental variables that underlie the different outcomes. In addition, the development and use of genetic gain- and loss-of-function models, as well as careful dose-titration assays in mice of different ages and different muscle injury paradigms will be helpful in establishing phenotypic thresholds for the possible context-specific effects of rGDF11 in skeletal muscle.
Effect of GDF11 supplementation on cardiac hypertrophy
Our studies published in 201312 and 2015104 reported an anti-hypertrophic effect of rGDF11 administration by comparing heart weight-tibia length (HW/TL) ratio in treated and control aging mice, while the study by Smith and colleagues reported no effects on the heart103. As discussed above, this disagreement may relate to dose-dependent effects of rGDF11 on cardiac mass, but it is also important to emphasize that Smith and colleagues did not observe changes in body weight in rGDF11 injected mice103, while we reported a significant decrease in body weight in aging mice with exogenous rGDF11104. Observations from our laboratories indicate that exogenous rGDF11 decreases body weight in old mice in a dose dependent manner (unpublished observation and12, 104). While rGDF11 directly activates cardiomyocyte SMAD signaling in cardiomyocytes104, it is also possible that the reduction in cardiac size with exogenous rGDF11 in vivo is due to an indirect effect of rGDF11. A systemic signal, possibly initiated by a reduction in body size or a change in a specific tissue, is quite plausible. For example, there is clear evidence for cross talk between adipose tissue and cardiac160, 161 and skeletal133, 162, 163 tissues. Thus, any interpretation of a direct effect of rGDF11 on the myocardium must be considered in the context of its systemic effects.
In conclusion, GDF11 has emerged as an intriguing candidate in the regulation of vertebrate aging and considerable progress has been made in the analysis of its role in the progression of age-associated disease. Future investigation of GDF11 and MSTN biology and biochemistry will clarify the similarities and differences in the functions of these proteins and advance our understanding of organismal aging and disease.
Supplementary Material
Acknowledgements
Thank you to M. Painter for helpful discussions.
Sources of Funding
This study was supported in part by the National Institutes of Health, the Graduate Dean Fellowship and the Glenn Foundation for Medical Research (R01AG032977, R01AG040019, and R03AG049657 to RTL; R56 AG048917 and R01 AG033053 to AJW; RO1GM114640 to TBT; GD Fellowship to RGW).
Non-standard abbreviations and non-standard acronyms
- ActR
Activin Receptor Kinase
- ALK
Activin Receptor-like Kinase
- BMP11
Bone Morphogenetic Protein 11
- CNS
Central Nervous System
- EGF
Epidermal Growth Factor
- BW
Body Weight
- CHD
Coronary Heart Disease
- FS
Follistatin
- FSTL3
Follistatin-like 3
- GASP
Growth and Differentiation Factor-Associated Serum Protein
- GDF11
Growth Differentiation Factor 11
- GDF8
Growth Differentiation Factor 8
- HW/TL
Heart Weight/Tibia Length
- IGF1
Insulin-like Growth Factor 1
- IL6
Interleukin 6
- INPs
Immediate Neuronal Precursors
- LTBP
Latent Transforming Growth Factor β Protein
- LVH
Left Ventricular Hypertrophy
- MSTN
Myostatin
- ORNs
Olfactory Receptor Neurons
- rGDF11
Recombinant GDF11
- SMAD
Mothers Against Decapentaplegic
- SNP
Single Nucleotide Polymorphism
- TAC
Transverse Aortic Constriction
- TGFβ
Transforming Growth Factor β
- TLD
Tolloid-like
- TNFα
Tumor Necrosis Factor α
Footnotes
DISCLOSURES
Harvard University and Brigham and Women's Hospital have filed for intellectual property on GDF11, listing Dr. Wagers, Rubin and Dr. Lee as inventors.
REFERENCES
- 1.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new tgf-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 2.McPherron AC, Lawler AM, Lee SJ. Regulation of anterior/posterior patterning of the axial skeleton by growth/differentiation factor 11. Nat Genet. 1999;22:260–264. doi: 10.1038/10320. [DOI] [PubMed] [Google Scholar]
- 3.McPherron AC, Lee SJ. Double muscling in cattle due to mutations in the myostatin gene. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:12457–12461. doi: 10.1073/pnas.94.23.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mosher DS, Quignon P, Bustamante CD, Sutter NB, Mellersh CS, Parker HG, Ostrander EA. A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs. PLoS genetics. 2007;3:e79. doi: 10.1371/journal.pgen.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medeiros EF, Phelps MP, Fuentes FD, Bradley TM. Overexpression of follistatin in trout stimulates increased muscling. Am J Physiol Regul Integr Comp Physiol. 2009;297:R235–242. doi: 10.1152/ajpregu.91020.2008. [DOI] [PubMed] [Google Scholar]
- 6.McPherron AC, Lee SJ. Suppression of body fat accumulation in myostatin-deficient mice. The Journal of clinical investigation. 2002;109:595–601. doi: 10.1172/JCI13562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McPherron AC, Huynh TV, Lee SJ. Redundancy of myostatin and growth/differentiation factor 11 function. BMC developmental biology. 2009;9:24. doi: 10.1186/1471-213X-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harmon EB AA, Smart NG, Gu X, Osborne DH, Kim SK. Gdf11 modulates ngn3+ islet progenitor cell number and promotes beta-cell differentiation in pancreas development. Development. 2004;131:6163–6174. doi: 10.1242/dev.01535. [DOI] [PubMed] [Google Scholar]
- 9.JP L. The function of growth/differentiation factor 11 (gdf11) in rostrocaudal patterning of the developing spinal cord. Development. 2006;133:2865074. doi: 10.1242/dev.02478. [DOI] [PubMed] [Google Scholar]
- 10.Wu HH, Ivkovic S, Murray RC, Jaramillo S, Lyons KM, Johnson JE, Calof AL. Autoregulation of neurogenesis by gdf11. Neuron. 2003;37:197–207. doi: 10.1016/s0896-6273(02)01172-8. [DOI] [PubMed] [Google Scholar]
- 11.Kim J, Wu HH, Lander AD, Lyons KM, Matzuk MM, Calof AL. Gdf11 controls the timing of progenitor cell competence in developing retina. Science. 2005;308:1927–1930. doi: 10.1126/science.1110175. [DOI] [PubMed] [Google Scholar]
- 12.Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, Sinha M, Dall'Osso C, Khong D, Shadrach JL, Miller CM, Singer BS, Stewart A, Psychogios N, Gerszten RE, Hartigan AJ, Kim MJ, Serwold T, Wagers AJ, Lee RT. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–839. doi: 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344:630–634. doi: 10.1126/science.1251141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sinha M, Jang YC, Oh J, Khong D, Wu EY, Manohar R, Miller C, Regalado SG, Loffredo FS, Pancoast JR, Hirshman MF, Lebowitz J, Shadrach JL, Cerletti M, Kim MJ, Serwold T, Goodyear LJ, Rosner B, Lee RT, Wagers AJ. Restoring systemic gdf11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014;344:649–652. doi: 10.1126/science.1251152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suragani RN, colleagues Transforming growth factor-beta superfamily ligand trap ace-536 corrects anemia by promoting late-stage erythropoiesis. Nature medicine. 2014;20:408–414. doi: 10.1038/nm.3512. [DOI] [PubMed] [Google Scholar]
- 16.Dussiot M, colleagues An activin receptor iia ligand trap corrects ineffective erythropoiesis in beta-thalassemia. Nature medicine. 2014;20:398–407. doi: 10.1038/nm.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakashima M, Toyono T, Akamine A, Joyner A. Expression of growth/differentiation factor 11, a new member of the bmp/tgfbeta superfamily during mouse embryogenesis. Mech Dev. 1999;80:185–189. doi: 10.1016/s0925-4773(98)00205-6. [DOI] [PubMed] [Google Scholar]
- 18.Yadin D, Knaus P, Mueller TD. Structural insights into bmp receptors: Specificity, activation and inhibition. Cytokine Growth Factor Rev. 2015 doi: 10.1016/j.cytogfr.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Cash JN, Angerman EB, Kattamuri C, Nolan K, Zhao H, Sidis Y, Keutmann HT, Thompson TB. Structure of myostatin.Follistatin-like 3: N-terminal domains of follistatin-type molecules exhibit alternate modes of binding. The Journal of biological chemistry. 2012;287:1043–1053. doi: 10.1074/jbc.M111.270801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cash JN, Rejon CA, McPherron AC, Bernard DJ, Thompson TB. The structure of myostatin:Follistatin 288: Insights into receptor utilization and heparin binding. The EMBO journal. 2009;28:2662–2676. doi: 10.1038/emboj.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Padyana AK, Vaidialingam B, Hayes DB, Gupta P, Franti M, Farrow NA. Crystal structure of human gdf11. Acta crystallographica. Section F, Structural biology communications. 2016;72:160–164. doi: 10.1107/S2053230X16001588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrison CA, Al-Musawi SL, Walton KL. Prodomains regulate the synthesis, extracellular localisation and activity of tgf-beta superfamily ligands. Growth Factors. 2011;29:174–186. doi: 10.3109/08977194.2011.608666. [DOI] [PubMed] [Google Scholar]
- 23.Gray AM, Mason AJ. Requirement for activin a and transforming growth factor--beta 1 pro-regions in homodimer assembly. Science. 1990;247:1328–1330. doi: 10.1126/science.2315700. [DOI] [PubMed] [Google Scholar]
- 24.McFarlane C, Langley B, Thomas M, Hennebry A, Plummer E, Nicholas G, McMahon C, Sharma M, Kambadur R. Proteolytic processing of myostatin is auto-regulated during myogenesis. Dev Biol. 2005;283:58–69. doi: 10.1016/j.ydbio.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 25.Anderson SB, Goldberg AL, Whitman M. Identification of a novel pool of extracellular pro-myostatin in skeletal muscle. The Journal of biological chemistry. 2008;283:7027–7035. doi: 10.1074/jbc.M706678200. [DOI] [PubMed] [Google Scholar]
- 26.Wolfman NM, McPherron AC, Pappano WN, Davies MV, Song K, Tomkinson KN, Wright JF, Zhao L, Sebald SM, Greenspan DS, Lee SJ. Activation of latent myostatin by the bmp-1/tolloid family of metalloproteinases. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:15842–15846. doi: 10.1073/pnas.2534946100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ge G, Hopkins DR, Ho WB, Greenspan DS. Gdf11 forms a bone morphogenetic protein 1-activated latent complex that can modulate nerve growth factor-induced differentiation of pc12 cells. Mol Cell Biol. 2005;25:5846–5858. doi: 10.1128/MCB.25.14.5846-5858.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:9306–9311. doi: 10.1073/pnas.151270098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thies RS, Chen T, Davies MV, Tomkinson KN, Pearson AA, Shakey QA, Wolfman NM. Gdf-8 propeptide binds to gdf-8 and antagonizes biological activity by inhibiting gdf- 8 receptor binding. Growth Factors. 2001;18:251–259. doi: 10.3109/08977190109029114. [DOI] [PubMed] [Google Scholar]
- 30.Ferrell RE, Conte V, Lawrence EC, Roth SM, Hagberg JM, Hurley BF. Frequent sequence variation in the human myostatin (gdf8) gene as a marker for analysis of muscle-related phenotypes. Genomics. 1999;62:203–207. doi: 10.1006/geno.1999.5984. [DOI] [PubMed] [Google Scholar]
- 31.Szlama G, Trexler M, Buday L, Patthy L. K153r polymorphism in myostatin gene increases the rate of promyostatin activation by furin. FEBS letters. 2015;589:295–301. doi: 10.1016/j.febslet.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 32.Garatachea N, Pinos T, Camara Y, Rodriguez-Romo G, Emanuele E, Ricevuti G, Venturini L, Santos-Lozano A, Santiago-Dorrego C, Fiuza-Luces C, Yvert T, Andreu AL, Lucia A. Association of the k153r polymorphism in the myostatin gene and extreme longevity. Age. 2013;35:2445–2454. doi: 10.1007/s11357-013-9513-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhatt SP, Nigam P, Misra A, Guleria R, Luthra K, Jain SK, Qadar Pasha MA. Association of the myostatin gene with obesity, abdominal obesity and low lean body mass and in non-diabetic asian indians in north india. PloS one. 2012;7:e40977. doi: 10.1371/journal.pone.0040977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santiago C, Ruiz JR, Rodriguez-Romo G, Fiuza-Luces C, Yvert T, Gonzalez-Freire M, Gomez-Gallego F, Moran M, Lucia A. The k153r polymorphism in the myostatin gene and muscle power phenotypes in young, non-athletic men. PloS one. 2011;6:e16323. doi: 10.1371/journal.pone.0016323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seibert MJ, Xue QL, Fried LP, Walston JD. Polymorphic variation in the human myostatin (gdf-8) gene and association with strength measures in the women's health and aging study ii cohort. Journal of the American Geriatrics Society. 2001;49:1093–1096. doi: 10.1046/j.1532-5415.2001.49214.x. [DOI] [PubMed] [Google Scholar]
- 36.Ben-Zaken S, Meckel Y, Nemet D, Rabinovich M, Kassem E, Eliakim A. Frequency of the mstn lys(k)-153arg(r) polymorphism among track & field athletes and swimmers. Growth hormone & IGF research : official journal of the Growth Hormone Research Society and the International IGF Research Society. 2015;25:196–200. doi: 10.1016/j.ghir.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Corsi AM, Ferrucci L, Gozzini A, Tanini A, Brandi ML. Myostatin polymorphisms and age-related sarcopenia in the italian population. Journal of the American Geriatrics Society. 2002;50:1463. doi: 10.1046/j.1532-5415.2002.50376.x. [DOI] [PubMed] [Google Scholar]
- 38.Garatachea N, Lucia A. Genes, physical fitness and ageing. Ageing research reviews. 2013;12:90–102. doi: 10.1016/j.arr.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez-Freire M, Rodriguez-Romo G, Santiago C, Bustamante-Ara N, Yvert T, Gomez-Gallego F, Serra Rexach JA, Ruiz JR, Lucia A. The k153r variant in the myostatin gene and sarcopenia at the end of the human lifespan. Age. 2010;32:405–409. doi: 10.1007/s11357-010-9139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X, Wang SJ, Tan SC, Chew PL, Liu L, Wang L, Wen L, Ma L. The a55t and k153r polymorphisms of mstn gene are associated with the strength training-induced muscle hypertrophy among han chinese men. Journal of sports sciences. 2014;32:883–891. doi: 10.1080/02640414.2013.865252. [DOI] [PubMed] [Google Scholar]
- 41.Tosun Tasar P, Sahin S, Karaman E, Oz A, Ulusoy MG, Duman S, Berdeli A, Akcicek F. Myostatin gene polymorphism in an elderly sarcopenic turkish population. Genetic testing and molecular biomarkers. 2015;19:457–460. doi: 10.1089/gtmb.2015.0033. [DOI] [PubMed] [Google Scholar]
- 42.Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T, Springer TA. Latent tgf-beta structure and activation. Nature. 2011;474:343–349. doi: 10.1038/nature10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang MS, Liang LF, Wang S, Ratovitski T, Holmstrom J, Barker C, Stotish R. Characterization and identification of the inhibitory domain of gdf-8 propeptide. Biochemical and biophysical research communications. 2004;315:525–531. doi: 10.1016/j.bbrc.2004.01.085. [DOI] [PubMed] [Google Scholar]
- 44.Takayama K, Noguchi Y, Aoki S, Takayama S, Yoshida M, Asari T, Yakushiji F, Nishimatsu S, Ohsawa Y, Itoh F, Negishi Y, Sunada Y, Hayashi Y. Identification of the minimum peptide from mouse myostatin prodomain for human myostatin inhibition. J Med Chem. 2015;58:1544–1549. doi: 10.1021/jm501170d. [DOI] [PubMed] [Google Scholar]
- 45.Annes JP, Chen Y, Munger JS, Rifkin DB. Integrin alphavbeta6-mediated activation of latent tgf-beta requires the latent tgf-beta binding protein-1. J Cell Biol. 2004;165:723–734. doi: 10.1083/jcb.200312172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang W, Zhang Y, Li Y, Wu Z, Zhu D. Myostatin induces cyclin d1 degradation to cause cell cycle arrest through a phosphatidylinositol 3-kinase/akt/gsk-3 beta pathway and is antagonized by insulin-like growth factor 1. The Journal of biological chemistry. 2007;282:3799–3808. doi: 10.1074/jbc.M610185200. [DOI] [PubMed] [Google Scholar]
- 47.Gleizes PE, Beavis RC, Mazzieri R, Shen B, Rifkin DB. Identification and characterization of an eight-cysteine repeat of the latent transforming growth factor-beta binding protein-1 that mediates bonding to the latent transforming growth factor-beta1. The Journal of biological chemistry. 1996;271:29891–29896. doi: 10.1074/jbc.271.47.29891. [DOI] [PubMed] [Google Scholar]
- 48.Saharinen J, Taipale J, Keski-Oja J. Association of the small latent transforming growth factor-beta with an eight cysteine repeat of its binding protein ltbp-1. The EMBO journal. 1996;15:245–253. [PMC free article] [PubMed] [Google Scholar]
- 49.Miyazono K, Olofsson A, Colosetti P, Heldin CH. A role of the latent tgf-beta 1-binding protein in the assembly and secretion of tgf-beta 1. The EMBO journal. 1991;10:1091–1101. doi: 10.1002/j.1460-2075.1991.tb08049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sengle G, Ono RN, Sasaki T, Sakai LY. Prodomains of transforming growth factor beta (tgfbeta) superfamily members specify different functions: Extracellular matrix interactions and growth factor bioavailability. The Journal of biological chemistry. 2011;286:5087–5099. doi: 10.1074/jbc.M110.188615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andersson O, Reissmann E, Jornvall H, Ibanez CF. Synergistic interaction between gdf1 and nodal during anterior axis development. Dev Biol. 2006;293:370–381. doi: 10.1016/j.ydbio.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 52.Rebbapragada A, Benchabane H, Wrana JL, Celeste AJ, Attisano L. Myostatin signals through a transforming growth factor beta-like signaling pathway to block adipogenesis. Mol Cell Biol. 2003;23:7230–7242. doi: 10.1128/MCB.23.20.7230-7242.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Groppe J, Hinck CS, Samavarchi-Tehrani P, Zubieta C, Schuermann JP, Taylor AB, Schwarz PM, Wrana JL, Hinck AP. Cooperative assembly of tgf-beta superfamily signaling complexes is mediated by two disparate mechanisms and distinct modes of receptor binding. Mol Cell. 2008;29:157–168. doi: 10.1016/j.molcel.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 54.Allendorph GP, Vale WW, Choe S. Structure of the ternary signaling complex of a tgfbeta superfamily member. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:7643–7648. doi: 10.1073/pnas.0602558103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Radaev S, Zou Z, Huang T, Lafer EM, Hinck AP, Sun PD. Ternary complex of transforming growth factor-beta1 reveals isoform-specific ligand recognition and receptor recruitment in the superfamily. The Journal of biological chemistry. 2010;285:14806–14814. doi: 10.1074/jbc.M109.079921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thompson TB, Woodruff TK, Jardetzky TS. Structures of an actriib:Activin a complex reveal a novel binding mode for tgf-beta ligand:Receptor interactions. The EMBO journal. 2003;22:1555–1566. doi: 10.1093/emboj/cdg156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keller S, Nickel J, Zhang JL, Sebald W, Mueller TD. Molecular recognition of bmp-2 and bmp receptor ia. Nature structural & molecular biology. 2004;11:481–488. doi: 10.1038/nsmb756. [DOI] [PubMed] [Google Scholar]
- 58.Babitt JL, Huang FW, Wrighting DM, Xia Y, Sidis Y, Samad TA, Campagna JA, Chung RT, Schneyer AL, Woolf CJ, Andrews NC, Lin HY. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nature genetics. 2006;38:531–539. doi: 10.1038/ng1777. [DOI] [PubMed] [Google Scholar]
- 59.Babitt JL, Zhang Y, Samad TA, Xia Y, Tang J, Campagna JA, Schneyer AL, Woolf CJ, Lin HY. Repulsive guidance molecule (rgma), a dragon homologue, is a bone morphogenetic protein co-receptor. The Journal of biological chemistry. 2005;280:29820–29827. doi: 10.1074/jbc.M503511200. [DOI] [PubMed] [Google Scholar]
- 60.Bianco C, Adkins HB, Wechselberger C, Seno M, Normanno N, De Luca A, Sun Y, Khan N, Kenney N, Ebert A, Williams KP, Sanicola M, Salomon DS. Cripto-1 activates nodaland alk4-dependent and -independent signaling pathways in mammary epithelial cells. Mol Cell Biol. 2002;22:2586–2597. doi: 10.1128/MCB.22.8.2586-2597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheifetz S, Bellon T, Cales C, Vera S, Bernabeu C, Massague J, Letarte M. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. The Journal of biological chemistry. 1992;267:19027–19030. [PubMed] [Google Scholar]
- 62.Finnson KW, Tam BY, Liu K, Marcoux A, Lepage P, Roy S, Bizet AA, Philip A. Identification of cd109 as part of the tgf-beta receptor system in human keratinocytes. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20:1525–1527. doi: 10.1096/fj.05-5229fje. [DOI] [PubMed] [Google Scholar]
- 63.Guardiola O, Lafuste P, Brunelli S, Iaconis S, Touvier T, Mourikis P, De Bock K, Lonardo E, Andolfi G, Bouche A, Liguori GL, Shen MM, Tajbakhsh S, Cossu G, Carmeliet P, Minchiotti G. Cripto regulates skeletal muscle regeneration and modulates satellite cell determination by antagonizing myostatin. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E3231–3240. doi: 10.1073/pnas.1204017109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kemaladewi DU, de Gorter DJ, Aartsma-Rus A, van Ommen GJ, ten Dijke P, t Hoen PA, Hoogaars WM. Cell-type specific regulation of myostatin signaling. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26:1462–1472. doi: 10.1096/fj.11-191189. [DOI] [PubMed] [Google Scholar]
- 65.Samad TA, Rebbapragada A, Bell E, Zhang Y, Sidis Y, Jeong SJ, Campagna JA, Perusini S, Fabrizio DA, Schneyer AL, Lin HY, Brivanlou AH, Attisano L, Woolf CJ. Dragon, a bone morphogenetic protein co-receptor. The Journal of biological chemistry. 2005;280:14122–14129. doi: 10.1074/jbc.M410034200. [DOI] [PubMed] [Google Scholar]
- 66.Hill JJ, Qiu Y, Hewick RM, Wolfman NM. Regulation of myostatin in vivo by growth and differentiation factor-associated serum protein-1: A novel protein with protease inhibitor and follistatin domains. Molecular endocrinology. 2003;17:1144–1154. doi: 10.1210/me.2002-0366. [DOI] [PubMed] [Google Scholar]
- 67.Sidis Y, Mukherjee A, Keutmann H, Delbaere A, Sadatsuki M, Schneyer A. Biological activity of follistatin isoforms and follistatin-like-3 is dependent on differential cell surface binding and specificity for activin, myostatin, and bone morphogenetic proteins. Endocrinology. 2006;147:3586–3597. doi: 10.1210/en.2006-0089. [DOI] [PubMed] [Google Scholar]
- 68.Amthor H, Nicholas G, McKinnell I, Kemp CF, Sharma M, Kambadur R, Patel K. Follistatin complexes myostatin and antagonises myostatin-mediated inhibition of myogenesis. Dev Biol. 2004;270:19–30. doi: 10.1016/j.ydbio.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 69.Miura T, colleagues Decorin binds myostatin and modulates its activity to muscle cells. Biochemical and biophysical research communications. 2006;340:675–680. doi: 10.1016/j.bbrc.2005.12.060. [DOI] [PubMed] [Google Scholar]
- 70.Hill JJ, Davies MV, Pearson AA, Wang JH, Hewick RM, Wolfman NM, Qiu Y. The myostatin propeptide and the follistatin-related gene are inhibitory binding proteins of myostatin in normal serum. The Journal of biological chemistry. 2002;277:40735–40741. doi: 10.1074/jbc.M206379200. [DOI] [PubMed] [Google Scholar]
- 71.Cash JN, Angerman EB, Keutmann HT, Thompson TB. Characterization of follistatintype domains and their contribution to myostatin and activin a antagonism. Molecular endocrinology. 2012;26:1167–1178. doi: 10.1210/me.2012-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stamler R, Keutmann HT, Sidis Y, Kattamuri C, Schneyer A, Thompson TB. The structure of fstl3.Activin a complex. Differential binding of n-terminal domains influences follistatin-type antagonist specificity. The Journal of biological chemistry. 2008;283:32831–32838. doi: 10.1074/jbc.M801266200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thompson TB, Lerch TF, Cook RW, Woodruff TK, Jardetzky TS. The structure of the follistatin:Activin complex reveals antagonism of both type i and type ii receptor binding. Dev Cell. 2005;9:535–543. doi: 10.1016/j.devcel.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 74.Datta-Mannan A, Yaden B, Krishnan V, Jones BE, Croy JE. An engineered human follistatin variant: Insights into the pharmacokinetic and pharmocodynamic relationships of a novel molecule with broad therapeutic potential. J Pharmacol Exp Ther. 2013;344:616–623. doi: 10.1124/jpet.112.201491. [DOI] [PubMed] [Google Scholar]
- 75.Winbanks CE, Weeks KL, Thomson RE, Sepulveda PV, Beyer C, Qian H, Chen JL, Allen JM, Lancaster GI, Febbraio MA, Harrison CA, McMullen JR, Chamberlain JS, Gregorevic P. Follistatin-mediated skeletal muscle hypertrophy is regulated by smad3 and mtor independently of myostatin. J Cell Biol. 2012;197:997–1008. doi: 10.1083/jcb.201109091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sidis Y, Schneyer AL, Keutmann HT. Heparin and activin-binding determinants in follistatin and fstl3. Endocrinology. 2005;146:130–136. doi: 10.1210/en.2004-1041. [DOI] [PubMed] [Google Scholar]
- 77.Walker RG, Angerman EB, Kattamuri C, Lee YS, Lee SJ, Thompson TB. Alternative binding modes identified for growth and differentiation factor-associated serum protein (gasp) family antagonism of myostatin. The Journal of biological chemistry. 2015;290:7506–7516. doi: 10.1074/jbc.M114.624130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Szlama G, Kondas K, Trexler M, Patthy L. Wfikkn1 and wfikkn2 bind growth factors tgfbeta1, bmp2 and bmp4 but do not inhibit their signalling activity. FEBS J. 2010;277:5040–5050. doi: 10.1111/j.1742-4658.2010.07909.x. [DOI] [PubMed] [Google Scholar]
- 79.Lee YS, Lee SJ. Regulation of gdf-11 and myostatin activity by gasp-1 and gasp-2. Proc Natl Acad Sci U S A. 2013;110:E3713–3722. doi: 10.1073/pnas.1309907110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kondas K, Szlama G, Trexler M, Patthy L. Both wfikkn1 and wfikkn2 have high affinity for growth and differentiation factors 8 and 11. The Journal of biological chemistry. 2008;283:23677–23684. doi: 10.1074/jbc.M803025200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Trexler M, Banyai L, Patthy L. A human protein containing multiple types of protease-inhibitory modules. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:3705–3709. doi: 10.1073/pnas.061028398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Szlama G, Trexler M, Patthy L. Latent myostatin has significant activity and this activity is controlled more efficiently by wfikkn1 than by wfikkn2. FEBS J. 2013;280:3822–3839. doi: 10.1111/febs.12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Massague J. Tgfbeta signalling in context. Nature reviews. Molecular cell biology. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Macias MJ, Martin-Malpartida P, Massague J. Structural determinants of smad function in tgf-beta signaling. Trends in biochemical sciences. 2015;40:296–308. doi: 10.1016/j.tibs.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Philip B, Lu Z, Gao Y. Regulation of gdf-8 signaling by the p38 mapk. Cellular signalling. 2005;17:365–375. doi: 10.1016/j.cellsig.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 86.Yang W, Chen Y, Zhang Y, Wang X, Yang N, Zhu D. Extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase pathway is involved in myostatinregulated differentiation repression. Cancer research. 2006;66:1320–1326. doi: 10.1158/0008-5472.CAN-05-3060. [DOI] [PubMed] [Google Scholar]
- 87.Egerman MA, Cadena SM, Gilbert JA, Meyer A, Nelson HN, Swalley SE, Mallozzi C, Jacobi C, Jennings LL, Clay I, Laurent G, Ma S, Brachat S, Lach-Trifilieff E, Shavlakadze T, Trendelenburg AU, Brack AS, Glass DJ. Gdf11 increases with age and inhibits skeletal muscle regeneration. Cell Metab. 2015;22:164–174. doi: 10.1016/j.cmet.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Biesemann N, Mendler L, Kostin S, Wietelmann A, Borchardt T, Braun T. Myostatin induces interstitial fibrosis in the heart via tak1 and p38. Cell Tissue Res. 2015;361:779–787. doi: 10.1007/s00441-015-2139-2. [DOI] [PubMed] [Google Scholar]
- 89.Shih H, Lee B, Lee RJ, Boyle AJ. The aging heart and post-infarction left ventricular remodeling. J Am Coll Cardiol. 2011;57:9–17. doi: 10.1016/j.jacc.2010.08.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maggioni AP, Maseri A, Fresco C, Franzosi MG, Mauri F, Santoro E, Tognoni G. Agerelated increase in mortality among patients with first myocardial infarctions treated with thrombolysis. The investigators of the gruppo italiano per lo studio della sopravvivenza nell'infarto miocardico (gissi-2). The New England journal of medicine. 1993;329:1442–1448. doi: 10.1056/NEJM199311113292002. [DOI] [PubMed] [Google Scholar]
- 91.Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (tgf)-beta signaling in cardiac remodeling. J Mol Cell Cardiol. 2011;51:600–606. doi: 10.1016/j.yjmcc.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Koitabashi N, Danner T, Zaiman AL, Pinto YM, Rowell J, Mankowski J, Zhang D, Nakamura T, Takimoto E, Kass DA. Pivotal role of cardiomyocyte tgf-beta signaling in the murine pathological response to sustained pressure overload. The Journal of clinical investigation. 2011;121:2301–2312. doi: 10.1172/JCI44824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sharma M, Kambadur R, Matthews KG, Somers WG, Devlin GP, Conaglen JV, Fowke PJ, Bass JJ. Myostatin, a transforming growth factor-beta superfamily member, is expressed in heart muscle and is upregulated in cardiomyocytes after infarct. Journal of cellular physiology. 1999;180:1–9. doi: 10.1002/(SICI)1097-4652(199907)180:1<1::AID-JCP1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 94.George I, Bish LT, Kamalakkannan G, Petrilli CM, Oz MC, Naka Y, Sweeney HL, Maybaum S. Myostatin activation in patients with advanced heart failure and after mechanical unloading. European journal of heart failure. 2010;12:444–453. doi: 10.1093/eurjhf/hfq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bish LT, George I, Maybaum S, Yang J, Chen JM, Sweeney HL. Myostatin is elevated in congenital heart disease and after mechanical unloading. PloS one. 2011;6:e23818. doi: 10.1371/journal.pone.0023818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Castillero E, Akashi H, Wang C, Najjar M, Ji R, Kennel PJ, Sweeney HL, Schulze PC, George I. Cardiac myostatin upregulation occurs immediately after myocardial ischemia and is involved in skeletal muscle activation of atrophy. Biochemical and biophysical research communications. 2015;457:106–111. doi: 10.1016/j.bbrc.2014.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Heineke J, Auger-Messier M, Xu J, Sargent M, York A, Welle S, Molkentin JD. Genetic deletion of myostatin from the heart prevents skeletal muscle atrophy in heart failure. Circulation. 2010;121:419–425. doi: 10.1161/CIRCULATIONAHA.109.882068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cohn RD, Liang HY, Shetty R, Abraham T, Wagner KR. Myostatin does not regulate cardiac hypertrophy or fibrosis. Neuromuscular disorders : NMD. 2007;17:290–296. doi: 10.1016/j.nmd.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mendias CL, Bakhurin KI, Gumucio JP, Shallal-Ayzin MV, Davis CS, Faulkner JA. Haploinsufficiency of myostatin protects against aging-related declines in muscle function and enhances the longevity of mice. Aging cell. 2015;14:704–706. doi: 10.1111/acel.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Morissette MR, Stricker JC, Rosenberg MA, Buranasombati C, Levitan EB, Mittleman MA, Rosenzweig A. Effects of myostatin deletion in aging mice. Aging cell. 2009;8:573–583. doi: 10.1111/j.1474-9726.2009.00508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Biesemann N, Mendler L, Wietelmann A, Hermann S, Schafers M, Kruger M, Boettger T, Borchardt T, Braun T. Myostatin regulates energy homeostasis in the heart and prevents heart failure. Circulation research. 2014;115:296–310. doi: 10.1161/CIRCRESAHA.115.304185. [DOI] [PubMed] [Google Scholar]
- 102.McPherron AC. Metabolic functions of myostatin and gdf11. Immunology, endocrine & metabolic agents in medicinal chemistry. 2010;10:217–231. doi: 10.2174/187152210793663810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Smith SC, Zhang X, Zhang X, Gross P, Starosta T, Mohsin S, Franti M, Gupta P, Hayes D, Myzithras M, Kahn J, Tanner J, Weldon SM, Khalil A, Guo X, Sabri A, Chen X, MacDonnell S, Houser SR. Gdf11 does not rescue aging-related pathological hypertrophy. Circulation research. 2015;117:926–932. doi: 10.1161/CIRCRESAHA.115.307527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Poggioli T, Vujic A, Yang P, Macias-Trevino C, Uygur AN, Loffredo FS, Pancoast JR, Cho M, Goldstein J, Tandias RM, Gonzalez E, Walker RG, Thompson TB, Wagers AJ, Fong YW, Lee RT. Circulating growth differentiation factor 11/8 levels decline with age. Circulation research. 2015 doi: 10.1161/CIRCRESAHA.115.307521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McNally EM. Questions and answers about myostatin, gdf11, and the aging heart. Circulation research. 2016:118. doi: 10.1161/CIRCRESAHA.115.307861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shimano M, Ouchi N, Nakamura K, Oshima Y, Higuchi A, Pimentel DR, Panse KD, Lara-Pezzi E, Lee SJ, Sam F, Walsh K. Cardiac myocyte-specific ablation of follistatinlike 3 attenuates stress-induced myocardial hypertrophy. The Journal of biological chemistry. 2011;286:9840–9848. doi: 10.1074/jbc.M110.197079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lara-Pezzi E, Felkin LE, Birks EJ, Sarathchandra P, Panse KD, George R, Hall JL, Yacoub MH, Rosenthal N, Barton PJ. Expression of follistatin-related genes is altered in heart failure. Endocrinology. 2008;149:5822–5827. doi: 10.1210/en.2008-0151. [DOI] [PubMed] [Google Scholar]
- 108.Mukherjee A, Sidis Y, Mahan A, Raher MJ, Xia Y, Rosen ED, Bloch KD, Thomas MK, Schneyer AL. Fstl3 deletion reveals roles for tgf-beta family ligands in glucose and fat homeostasis in adults. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1348–1353. doi: 10.1073/pnas.0607966104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Oshima Y, Ouchi N, Shimano M, Pimentel DR, Papanicolaou KN, Panse KD, Tsuchida K, Lara-Pezzi E, Lee SJ, Walsh K. Activin a and follistatin-like 3 determine the susceptibility of heart to ischemic injury. Circulation. 2009;120:1606–1615. doi: 10.1161/CIRCULATIONAHA.109.872200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Panse KD, Felkin LE, Lopez-Olaneta MM, Gomez-Salinero J, Villalba M, Munoz L, Nakamura K, Shimano M, Walsh K, Barton PJ, Rosenthal N, Lara-Pezzi E. Follistatinlike 3 mediates paracrine fibroblast activation by cardiomyocytes. Journal of cardiovascular translational research. 2012;5:814–826. doi: 10.1007/s12265-012-9400-9. [DOI] [PubMed] [Google Scholar]
- 111.Shi Y, Massague J. Mechanisms of tgf-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 112.Girgenrath S, Song K, Whittemore LA. Loss of myostatin expression alters fiber-type distribution and expression of myosin heavy chain isoforms in slow- and fast-type skeletal muscle. Muscle Nerve. 2005;31:34–40. doi: 10.1002/mus.20175. [DOI] [PubMed] [Google Scholar]
- 113.Garikipati DK, Rodgers BD. Myostatin inhibits myosatellite cell proliferation and consequently activates differentiation: Evidence for endocrine-regulated transcript processing. The Journal of endocrinology. 2012;215:177–187. doi: 10.1530/JOE-12-0260. [DOI] [PubMed] [Google Scholar]
- 114.George RM, Biressi S, Beres BJ, Rogers E, Mulia AK, Allen RE, Rawls A, Rando TA, Wilson-Rawls J. Numb-deficient satellite cells have regeneration and proliferation defects. Proc Natl Acad Sci U S A. 2013;110:18549–18554. doi: 10.1073/pnas.1311628110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Amthor H, Otto A, Vulin A, Rochat A, Dumonceaux J, Garcia L, Mouisel E, Hourde C, Macharia R, Friedrichs M, Relaix F, Zammit PS, Matsakas A, Patel K, Partridge T. Muscle hypertrophy driven by myostatin blockade does not require stem/precursor-cell activity. Proc Natl Acad Sci U S A. 2009;106:7479–7484. doi: 10.1073/pnas.0811129106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee SJ, Huynh TV, Lee YS, Sebald SM, Wilcox-Adelman SA, Iwamori N, Lepper C, Matzuk MM, Fan CM. Role of satellite cells versus myofibers in muscle hypertrophy induced by inhibition of the myostatin/activin signaling pathway. Proc Natl Acad Sci U S A. 2012;109:E2353–2360. doi: 10.1073/pnas.1206410109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R. Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol. 2003;162:1135–1147. doi: 10.1083/jcb.200207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Durieux AC, Amirouche A, Banzet S, Koulmann N, Bonnefoy R, Pasdeloup M, Mouret C, Bigard X, Peinnequin A, Freyssenet D. Ectopic expression of myostatin induces atrophy of adult skeletal muscle by decreasing muscle gene expression. Endocrinology. 2007;148:3140–3147. doi: 10.1210/en.2006-1500. [DOI] [PubMed] [Google Scholar]
- 119.Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, McPherron AC, Wolfman NM, Lee SJ. Induction of cachexia in mice by systemically administered myostatin. Science. 2002;296:1486–1488. doi: 10.1126/science.1069525. [DOI] [PubMed] [Google Scholar]
- 120.Stolz LE, Li D, Qadri A, Jalenak M, Klaman LD, Tobin JF. Administration of myostatin does not alter fat mass in adult mice. Diabetes, obesity & metabolism. 2008;10:135–142. doi: 10.1111/j.1463-1326.2006.00672.x. [DOI] [PubMed] [Google Scholar]
- 121.Lach-Trifilieff E, Minetti GC, Sheppard K, Ibebunjo C, Feige JN, Hartmann S, Brachat S, Rivet H, Koelbing C, Morvan F, Hatakeyama S, Glass DJ. An antibody blocking activin type ii receptors induces strong skeletal muscle hypertrophy and protects from atrophy. Mol Cell Biol. 2014;34:606–618. doi: 10.1128/MCB.01307-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gamer LW, Cox KA, Small C, Rosen V. Gdf11 is a negative regulator of chondrogenesis and myogenesis in the developing chick limb. Dev Biol. 2001;229:407–420. doi: 10.1006/dbio.2000.9981. [DOI] [PubMed] [Google Scholar]
- 123.Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ. Myostatin reduces akt/torc1/p70s6k signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol. 2009;296:C1258–1270. doi: 10.1152/ajpcell.00105.2009. [DOI] [PubMed] [Google Scholar]
- 124.Jeanplong F, Falconer SJ, Thomas M, Matthews KG, Oldham JM, Watson T, McMahon CD. Growth and differentiation factor-11 is developmentally regulated in skeletal muscle and inhibits myoblast differentiation. Open J. of Molec. and Integrative Physiol. 2012;2:127–138. [Google Scholar]
- 125.Polesskaya A, Seale P, Rudnicki MA. Wnt signaling induces the myogenic specification of resident cd45+ adult stem cells during muscle regeneration. Cell. 2003;113:841–852. doi: 10.1016/s0092-8674(03)00437-9. [DOI] [PubMed] [Google Scholar]
- 126.Boldrin L, Neal A, Zammit PS, Muntoni F, Morgan JE. Donor satellite cell engraftment is significantly augmented when the host niche is preserved and endogenous satellite cells are incapacitated. Stem cells. 2012;30:1971–1984. doi: 10.1002/stem.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gayraud-Morel B, Chretien F, Flamant P, Gomes D, Zammit PS, Tajbakhsh S. A role for the myogenic determination gene myf5 in adult regenerative myogenesis. Dev Biol. 2007;312:13–28. doi: 10.1016/j.ydbio.2007.08.059. [DOI] [PubMed] [Google Scholar]
- 128.Gayraud-Morel B, Chretien F, Tajbakhsh S. Skeletal muscle as a paradigm for regenerative biology and medicine. Regenerative medicine. 2009;4:293–319. doi: 10.2217/17460751.4.2.293. [DOI] [PubMed] [Google Scholar]
- 129.Liadaki K, Casar JC, Wessen M, Luth ES, Jun S, Gussoni E, Kunkel LM. Beta4 integrin marks interstitial myogenic progenitor cells in adult murine skeletal muscle. J Histochem Cytochem. 2012;60:31–44. doi: 10.1369/0022155411428991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kovacic JC, Moreno P, Nabel EG, Hachinski V, Fuster V. Cellular senescence, vascular disease, and aging: Part 2 of a 2-part review: Clinical vascular disease in the elderly. Circulation. 2011;123:1900–1910. doi: 10.1161/CIRCULATIONAHA.110.009118. [DOI] [PubMed] [Google Scholar]
- 131.Bergen HR, 3rd, Farr JN, Vanderboom PM, Atkinson EJ, White TA, Singh RJ, Khosla S, LeBrasseur NK. Myostatin as a mediator of sarcopenia versus homeostatic regulator of muscle mass: Insights using a new mass spectrometry-based assay. Skelet Muscle. 2015;5:21. doi: 10.1186/s13395-015-0047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhou Y, Jiang Z, Harris EC, Reeves J, Chen X, Pazdro R. Circulating concentrations of growth differentiation factor 11 are heritable and correlate with life span. J Gerontol A Biol Sci Med Sci. 2016 doi: 10.1093/gerona/glv308. [DOI] [PubMed] [Google Scholar]
- 133.Demontis F, Patel VK, Swindell WR, Perrimon N. Intertissue control of the nucleolus via a myokine-dependent longevity pathway. Cell Rep. 2014;7:1481–1494. doi: 10.1016/j.celrep.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lee JH, Momani J, Kim YM, Kang CK, Choi JH, Baek HJ, Kim HW. Effective rnasilencing strategy of lv-mstn/gdf11 gene and its effects on the growth in shrimp, litopenaeus vannamei. Comp Biochem Physiol B Biochem Mol Biol. 2015;179:9–16. doi: 10.1016/j.cbpb.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 135.Shi Y, Liu JP. Gdf11 facilitates temporal progression of neurogenesis in the developing spinal cord. J Neurosci. 2011;31:883–893. doi: 10.1523/JNEUROSCI.2394-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liu JP, Laufer E, Jessell TM. Assigning the positional identity of spinal motor neurons: Rostrocaudal patterning of hox-c expression by fgfs, gdf11, and retinoids. Neuron. 2001;32:997–1012. doi: 10.1016/s0896-6273(01)00544-x. [DOI] [PubMed] [Google Scholar]
- 137.Liu JP. The function of growth/differentiation factor 11 (gdf11) in rostrocaudal patterning of the developing spinal cord. Development. 2006;133:2865–2874. doi: 10.1242/dev.02478. [DOI] [PubMed] [Google Scholar]
- 138.Villeda SA, Plambeck KE, Middeldorp J, Castellano JM, Mosher KI, Luo J, Smith LK, Bieri G, Lin K, Berdnik D, Wabl R, Udeochu J, Wheatley EG, Zou B, Simmons DA, Xie XS, Longo FM, Wyss-Coray T. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nature medicine. 2014;20:659–663. doi: 10.1038/nm.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.He Y, Zhang H, Yung A, Villeda SA, Jaeger PA, Olayiwola O, Fainberg N, Wyss-Coray T. Alk5-dependent tgf-beta signaling is a major determinant of late-stage adult neurogenesis. Nat Neurosci. 2014;17:943–952. doi: 10.1038/nn.3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Brionne TC, Tesseur I, Masliah E, Wyss-Coray T. Loss of tgf-beta 1 leads to increased neuronal cell death and microgliosis in mouse brain. Neuron. 2003;40:1133–1145. doi: 10.1016/s0896-6273(03)00766-9. [DOI] [PubMed] [Google Scholar]
- 141.Tesseur I, Zou K, Esposito L, Bard F, Berber E, Can JV, Lin AH, Crews L, Tremblay P, Mathews P, Mucke L, Masliah E, Wyss-Coray T. Deficiency in neuronal tgf-beta signaling promotes neurodegeneration and alzheimer's pathology. J Clin Invest. 2006;116:3060–3069. doi: 10.1172/JCI27341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li S, Nie EH, Yin Y, Benowitz LI, Tung S, Vinters HV, Bahjat FR, Stenzel-Poore MP, Kawaguchi R, Coppola G, Carmichael ST. Gdf10 is a signal for axonal sprouting and functional recovery after stroke. Nat Neurosci. 2015;18:1737–1745. doi: 10.1038/nn.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Olson KA, Beatty AL, Heidecker B, Regan MC, Brody EN, Foreman T, Kato S, Mehler RE, Singer BS, Hveem K, Dalen H, Sterling DG, Lawn RM, Schiller NB, Williams SA, Whooley MA, Ganz P. Association of growth differentiation factor 11/8, putative anti-ageing factor, with cardiovascular outcomes and overall mortality in humans: Analysis of the heart and soul and hunt3 cohorts. European heart journal. 2015;36:3426–3434. doi: 10.1093/eurheartj/ehv385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Heidecker B, Olson K, Beatty A, Dubin R, Kato S, Lawn R, Murthy A, Regan M, Sterling D, Whooley M, Ganz P. Low levels of growth differentiation factor 11 and high levels of its inhibitor follitatin-like 3 are associated with adverse cardiovascular outcomes in humans. Journal of the American College of Cardiology. 2015;65:10_S. [Google Scholar]
- 145.Paulson RF. Targeting a new regulator of erythropoiesis to alleviate anemia. Nature medicine. 2014;20:334–335. doi: 10.1038/nm.3524. [DOI] [PubMed] [Google Scholar]
- 146.Arlet JB, Dussiot M, Moura IC, Hermine O, Courtois G. Novel players in betathalassemia dyserythropoiesis and new therapeutic strategies. Current opinion in hematology. 2016 doi: 10.1097/MOH.0000000000000231. [DOI] [PubMed] [Google Scholar]
- 147.Burks TN, Andres-Mateos E, Marx R, Mejias R, Van Erp C, Simmers JL, Walston JD, Ward CW, Cohn RD. Losartan restores skeletal muscle remodeling and protects against disuse atrophy in sarcopenia. Sci Transl Med. 2011;3:82ra37. doi: 10.1126/scitranslmed.3002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 149.Xu M, Palmer AK, Ding H, Weivoda MM, Pirtskhalava T, White TA, Sepe A, Johnson KO, Stout MB, Giorgadze N, Jensen MD, LeBrasseur NK, Tchkonia T, Kirkland JL. Targeting senescent cells enhances adipogenesis and metabolic function in old age. eLife. 2015:4. doi: 10.7554/eLife.12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Naito AT, colleagues Complement c1q activates canonical wnt signaling and promotes aging-related phenotypes. Cell. 2012;149:1298–1313. doi: 10.1016/j.cell.2012.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gold L, Ayers D, colleagues Aptamer-based multiplexed proteomic technology for biomarker discovery. PloS one. 2010;5:e15004. doi: 10.1371/journal.pone.0015004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hathout Y, colleagues Large-scale serum protein biomarker discovery in duchenne muscular dystrophy. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:7153–7158. doi: 10.1073/pnas.1507719112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Natsuume-Sakai S, Motonishi K, Migita S. Quantitative estimations of five classes of immunoglobulin in inbred mouse strains. Immunology. 1977;32:861–866. [PMC free article] [PubMed] [Google Scholar]
- 154.Rodgers BD, Eldridge JA. Reduced circulating gdf11 is unlikely responsible for agedependent changes in mouse heart, muscle, and brain. Endocrinology. 2015;156:3885–3888. doi: 10.1210/en.2015-1628. [DOI] [PubMed] [Google Scholar]
- 155.Souza TA, Chen X, Guo Y, Sava P, Zhang J, Hill JJ, Yaworsky PJ, Qiu Y. Proteomic identification and functional validation of activins and bone morphogenetic protein 11 as candidate novel muscle mass regulators. Molecular endocrinology. 2008;22:2689–2702. doi: 10.1210/me.2008-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jedrychowski MP, Wrann CD, Paulo JA, Gerber KK, Szpyt J, Robinson MM, Nair KS, Gygi SP, Spiegelman BM. Detection and quantitation of circulating human irisin by tandem mass spectrometry. Cell metabolism. 2015;22:734–740. doi: 10.1016/j.cmet.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Breitbart A, colleagues Highly specific detection of myostatin prodomain by an immunoradiometric sandwich assay in serum of healthy individuals and patients. PloS one. 2013;8:e80454. doi: 10.1371/journal.pone.0080454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.White TA, LeBrasseur NK. Myostatin and sarcopenia: Opportunities and challenges - a mini-review. Gerontology. 2014;60:289–293. doi: 10.1159/000356740. [DOI] [PubMed] [Google Scholar]
- 159.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 160.Lopez-Jaramillo P. The role of adiponectin in cardiometabolic diseases: Effects of nutritional interventions. The Journal of nutrition. 2016 doi: 10.3945/jn.114.202432. [DOI] [PubMed] [Google Scholar]
- 161.Parker-Duffen JL, Walsh K. Cardiometabolic effects of adiponectin. Best practice & research. Clinical endocrinology & metabolism. 2014;28:81–91. doi: 10.1016/j.beem.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dong J, Dong Y, Dong Y, Chen F, Mitch WE, Zhang L. Inhibition of myostatin in mice improves insulin sensitivity via irisin-mediated cross talk between muscle and adipose tissues. International journal of obesity. 2015 doi: 10.1038/ijo.2015.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shan T, Liang X, Bi P, Kuang S. Myostatin knockout drives browning of white adipose tissue through activating the ampk-pgc1alpha-fndc5 pathway in muscle. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27:1981–1989. doi: 10.1096/fj.12-225755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gamer LW, Wolfman NM, Celeste AJ, Hattersley G, Hewick R, Rosen V. A novel bmp expressed in developing mouse limb, spinal cord, and tail bud is a potent mesoderm inducer in xenopus embryos. Dev Biol. 1999;208:222–232. doi: 10.1006/dbio.1998.9191. [DOI] [PubMed] [Google Scholar]
- 165.Esquela AF, Lee SJ. Regulation of metanephric kidney development by growth/differentiation factor 11. Dev Biol. 2003;257:356–370. doi: 10.1016/s0012-1606(03)00100-3. [DOI] [PubMed] [Google Scholar]
- 166.Dichmann DS, Yassin H, Serup P. Analysis of pancreatic endocrine development in gdf11-deficient mice. Dev Dyn. 2006;235:3016–3025. doi: 10.1002/dvdy.20953. [DOI] [PubMed] [Google Scholar]
- 167.Amthor H, Macharia R, Navarrete R, Schuelke M, Brown SC, Otto A, Voit T, Muntoni F, Vrbova G, Partridge T, Zammit P, Bunger L, Patel K. Lack of myostatin results in excessive muscle growth but impaired force generation. Proc Natl Acad Sci U S A. 2007;104:1835–1840. doi: 10.1073/pnas.0604893104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lin J, Arnold HB, Della-Fera MA, Azain MJ, Hartzell DL, Baile CA. Myostatin knockout in mice increases myogenesis and decreases adipogenesis. Biochemical and biophysical research communications. 2002;291:701–706. doi: 10.1006/bbrc.2002.6500. [DOI] [PubMed] [Google Scholar]
- 169.Rodgers BD, Interlichia JP, Garikipati DK, Mamidi R, Chandra M, Nelson OL, Murry CE, Santana LF. Myostatin represses physiological hypertrophy of the heart and excitation-contraction coupling. The Journal of physiology. 2009;587:4873–4886. doi: 10.1113/jphysiol.2009.172544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.