Abstract
Neuromuscular ultrasound is an emerging technology for the evaluation of conditions affecting nerve and muscle, with the majority of research focusing on focal neuropathies. Despite this focus, researchers have also investigated the ultrasonographic changes that occur in the nerves and muscles of those with more diffuse polyneuropathies and motor neuron diseases, and this review will detail the findings in these conditions. Specific findings are discussed in this paper, but general themes will also be presented and include the following: hereditary polyneuropathies show diffuse nerve enlargement whereas immune-mediated polyneuropathies show more patchy involvement; nerve enlargement is more profound in demyelinating than axonal polyneuropathies; and muscle changes in motor neuron diseases include heterogeneous increases in echogenicity, atrophy, readily detectable fasciculations, and increased subcutaneous tissue thickness.
Keywords: Ultrasound, polyneuropathy, motor neuron disease, amyotrophic lateral sclerosis, Charcot-Marie-Tooth
Introduction
The first studies related to neuromuscular ultrasound were published in the early 1980s by Heckmatt and Dubowitz and focused on muscle ultrasound in boys with Duchenne's muscular dystrophy (Heckmatt et al., 1982). In the late 1980s, Fornage published the first paper on nerve ultrasound, and in 1992 Buchberger and colleagues described the ultrasonographic changes in focal nerve disease (Fornage, 1988; Buchberger et al., 1992). Subsequently, the field of neuromuscular ultrasound began to grow, but the first publications on diffuse nerve conditions such as polyneuropathy and ALS did not occur until 1999 (Heinemeyer and Reimers, 1999). This review will evaluate the relevant literature regarding the ultrasonographic evaluation of nerve and muscle in individuals with polyneuropathies and motor neuron diseases and how it can be used in diagnosis and as a biomarker of disease progression.
High-resolution ultrasonography of peripheral nerves encompasses identifying specific nerves, differentiating nerves from nearby structures, determining nerve size, assessing nerve echotexture, and measuring nerve vascularity. Nerves can be scanned quickly over long lengths, allowing for efficient and painless assessment of both distal and proximal aspects of nerves. Nerve enlargement, measured as cross-sectional area (CSA), is the most common parameter described in the neuromuscular ultrasound literature. CSA has good validity and intra-observer and inter-observer reliability (Tagliafico et al., 2012; Cartwright et al., 2013).
Hereditary Polyneuropathy (Charcot-Marie Tooth Disease)
Heinemeyer and Reimers published the first study imaging the radial, ulnar, median, and sciatic nerves in patients with confirmed Charcot-Marie Tooth (CMT) 1A (Heinemeyer and Reimers, 1999) in 1999. Interestingly, the authors did not find significant nerve size or structural differences between patients with hereditary motor and sensory neuropathy and healthy subjects. In this study, the median nerve distal to the forearm could be adequately visualized in only 20 of the 50 healthy individuals, which is likely explained by the use of a lower frequency 7.5 MHz transducer, and this lack of differentiating ultrasonographic features has not been demonstrated in subsequent studies.
Martinoli et al. in 2002 described the main features of the median nerve in CMT patients using high-resolution (12.5 MHz transducer) ultrasound (Martinoli et al., 2002). The mid-forearm median nerves of 24 patients with genetically-confirmed CMT 1A and 2 were imaged. CMT 1A patients showed larger nerve area and fascicular diameter compared to CMT 2 and X-linked CMT patients, and no correlation between CSA or fascicular diameter with height, body mass, or sex was observed. The peripheral nerves in CMT 1 were further described in 2009 by 2 groups. Zaidman et al. compared 11 patients with either genetically-confirmed CMT 1A or nerve conduction studies consistent with an inherited demyelinating polyneuropathy to 90 healthy children and adults (Zaidman et al., 2009) This study employed a nerve size index (NSI) that adjusted nerve measurements for height, derived from linear regression, to help describe expected nerve size. The height of pediatric and adult controls correlated with average nerve CSA along the length of the ulnar and median nerves, which was observed independent of BMI, age, or gender. Explicit CSA values were not published; however, in CMT 1A patients, the average NSI of the median and ulnar nerves were enlarged 3.5 times compared to controls. Additionally, nerve enlargement was diffuse and present in each CMT 1A patient.
In 2013, Pazzaglia et al. characterized the ultrasonographic features of the sural nerve in CMT 1A (Pazzaglia et al., 2013). A total of 20 CMT 1A patients underwent ultrasound evaluation of the sural nerve bilaterally and the right ulnar nerve. The sural nerve CSA was not increased in the majority of patients. Of the 40 total sural nerve CSA measurements, 34 were within normal range. Only 2 patients had enlarged sural nerve CSA on either side. Also observed was an increased ulnar nerve CSA present in CMT1A. The authors found inverse relations between CSA of the ulnar nerve and BMI (R = −0.8, P <0.0002) and between bilateral sural nerve CSA and age (R = −0.4, P = 0.00003).
The safety, noninvasiveness, and painlessness of high-resolution ultrasound are intuitively advantageous in pediatric patients, and ultrasound has been used to investigate nerve CSA in pediatric CMT 1A patients. Yiu et al. in 2015 published the largest pediatric-dedicated neuromuscular ultrasound study in CMT to date (Yiu et al., 2015). The CSA of the median, ulnar, tibial, and sural nerves in 29 children were compared to age- and sex-matched controls. Nerve CSA was significantly increased in children with CMT 1A compared to controls (1.9- to 3.5-fold increase, P < 0.001), which was observed even in the youngest patient (19 months old). The mean differences in nerve CSA ranged from 5.4 mm2 (median nerve at the wrist) to 11.3 mm2 (tibial nerve at the ankle). This remained after adjusting for differences in height between the 2 groups. The sural nerve at the ankle was noted to have a larger CSA in CMT 1A children (4.0 mm2 vs. 1.2 mm2, P < 0.001). This is in discrepancy with the aforementioned Cartwright and Pazzaglia studies, which showed the absence of sural nerve enlargement in the presence of upper extremity nerve enlargement in respective adult subjects. It is unclear why this discrepancy was observed, but Yiu et al. state this may reflect differences between early and late stages of disease, with more sural nerve axon loss as the disease progresses. This study also found a strong positive linear correlation of nerve CSA with age, height, and weight in both the CMT 1A and control groups. CMT 1A status disproportionately increased the regression coefficient of nerve CSA with age at all nerve sites excluding the sural nerve (1.9 to 5.9 times, P ≤ 0.001).
In 2015, Noto et al. examined the median, sural, and great auricular nerves and the 6th cervical nerve root in patients with CMT 1A, myelin protein zero (MPZ), neurofilament light polypeptide (NEFL), early growth response 2 (EGR2), and rho guanine nucleotide exchange factor 10 (ARHGEF10) associated forms of CMT (Noto et al., 2015). The study confirmed the uniform enlargement of nerves, including the nerve root, in CMT 1A. CMT 1A nerve enlargement was greater than the increased CSA in median nerves of individuals with MPZ, EGR2, and ARHGEF10 mutations. Measurements of the CMT2J patient were nearly the same as the mean control values except for the median nerve at the wrist, and NEFL mutations demonstrated CSAs comparable to controls. They concluded that nerve ultrasound may aid in in CMT subtype differentiation, when used in combination with nerve conduction studies.
In 2009, Cartwright et al. published the first study using ultrasound to measure nerve size in patients with CMT 1B. CMT 1B is caused by mutations in the myelin protein zero (MPZ) gene on chromosome 1q22, leading to demyelinating CMT (Cartwright et al., 2009). The investigators recruited 12 patients with CMT 1B, and 8 of the 12 CMT family members had cranial neuropathies manifesting as hemifacial spasms and trigeminal neuralgia. It was unknown if the observed cranial neuropathies were secondary to this family's particular MPZ mutation or mutation in a linked gene. Compared to healthy controls, all 12 CMT 1B patients had larger CSA of the median and vagus nerves. The median nerve at the mid-forearm measured 20.42 mm2 vs. 6.76 mm2 in controls (P < 0.0001). Interestingly, the mean CSA of the median nerve in this family was found to be smaller at the wrist than at the mid-forearm (20.42 mm2 to 13.67 mm2). The authors offered that the process resulting in nerve enlargement in demyelinating CMT may be hindered due to the anatomic restrictions within the carpal tunnel, and this should be considered when evaluating distal nerve entrapment in those with demyelinating CMT. The vagus nerve measured 8.50 mm2 vs. 5.88 mm2 (P = 0.018). The sural nerve of those with CMT 1B was slightly smaller than controls (5.00 mm2 vs. 6.33 mm2, P = 0.046). The authors speculated the sural nerve may have more length-dependent axon loss.
Ultrasound may be helpful in differentiating axonal and demyelinating subtypes of CMT. A study has shown a significantly increased CSA of the median nerve in the predominantly demyelinating CMT 1A, whereas in axonal CMT 2A nerve fascicle diameter is enlarged (Schreiber et al., 2013). It should be noted that although the mean median nerve CSA and fascicle diameter appeared larger than the CMT 2A group, this difference was not statistically significant. This study did perform nerve conduction studies on individuals with CMT 1A and CMT 2A. In CMT 1A, a significant inverse association between median nerve CSA and fascicle diameter with conduction velocity and action potential amplitude was observed. Spearman rank correlation coefficients were reported with the strongest correlations seen with median nerve fascicle diameter at the forearm compared to motor amplitude (K −0.93) and sensory amplitude (K −0.87). In CMT 2A, a significant inverse association between the motor and sensory amplitudes and the median nerve CSA was observed. The authors reported Spearman rank correlation coefficients for CSA at the wrist compared to motor amplitude (K −0.92) and CSA at the forearm vs. sensory amplitude (K −0.91). The study is limited by the size of the study (12 CMT 1A patients, 7 CMT 2A patients) but does suggest correlation between ultrasound ad electrodiagnosis.
Consistent with Schreiber et al., the previously mentioned Noto et al. study also identified a significant negative correlation between the CSAs of the median nerve and the motor conduction velocities in corresponding segments. The decreased motor conduction velocities in CMT 1A are a reflection of the progressive histopathological alteration of myelination which may parallel increased CSA (Noto et al., 2015).
Hereditary Neuropathies: Summary and Recommendations
Although each study of neuromuscular CMT was somewhat different, with a variety of genetic mutations, ultrasound devices, transducer frequencies, nerves studied, and techniques employed, some general conclusions can now be postulated. It appears that those with demyelinating forms of CMT have larger nerve CSA than those with axonal CMT, and those with axonal CMT have larger CSA than healthy controls. This finding is particularly notable in the CSA of the median and ulnar nerves in the forearm, whereas the sural nerve showed only minimal differences between CMT and healthy controls. Additionally, the nerve enlargement is diffuse in the nerves affected, rather than multifocal. No clear abnormalities in nerve echogenicity or vascularity have been reported. Many studies commented on enlarged fascicle size in those with CMT, but that has not been systematically quantified. Correlation between neuromuscular ultrasound and electrodiagnostic features shows an inverse association in both demyelinating and axonal CMT (Schreiber et al., 2013).
Immune-Mediated Polyneuropathies
Multifocal Motor Neuropathy (MMN)
The initial study of MMN was by Beekman et al., who evaluated 21 patients with MMN and a mean disease duration of 12.4 years (range 2 to 39 years) (Beekman et al., 2005). Ultrasound of the brachial plexus, median, ulnar, and radial nerves was performed bilaterally at multiple levels. 19 out of the 21 patients imaged had multiple nerve enlargements. The authors saw no correlation between the number of enlargements and disease duration, and the nerve enlargement was noted to be multifocal.
Padua et al. in 2012 demonstrated the multifocal, patchy nature of MMN in 2 patients (Padua et al., 2012). These patients had severe motor deficits due to long-standing disease. The patients exhibited inter- and intra-nerve variability in non-mutual nerves. In addition to presenting a possible distribution pattern of ultrasound changes in 12 MMN patients, Kerasnoudis et al confirmed significantly higher CSA values of the median, ulnar, and tibial nerves compared to controls (Kerasnoudis et al., 2014). Pathologic CSA changes were seen at distinct proximal and distal sites along the anatomic course of the imaged peripheral nerves, highlighting the multifocal nature of MMN. Intra- and inter-nerve variability existed, and consistent with the pathophysiology of MMN, no ultrasonographic changes were noted in the sural nerve. A significant correlation between ultrasonographic and electrophysiological findings between the compound muscle action potential (CMAP) amplitude and CSA of the median nerve was detected (r = 0.851, P < 0.001). Individuals with MMN had significantly lower values of motor conduction velocity and CMAPs, both of which were detectable in all 12 MMN patients. However, in those with very severe or chronic MMN, such that nerve conduction studies are unmeasurable or too low to detect block or slowing, ultrasound may be of use by detecting nerve enlargement.
Grimm et al. reported significant differences in ultrasound between MMN and ALS (Grimm et al., 2015). They studied 17 patients with ALS and 8 patients with MMN and found more nerve enlargement in MMN (P < 0.001). However, single nerve enlargements were observed in ALS and controls as well. As expected, no differences in the sural nerve existed between ALS, MMN, and controls. ROC curve analysis revealed a reliable boundary value (sensitivity of 87.5%, specificity 94.1%) of nerve enlargement in at least 4 out of 10 landmarks to differentiate MMN from ALS.
Guillain-Barre Syndrome
A 2009 study by Zaidman et al. included 17 patients with GBS (Zaidman et al., 2009). The authors found the median and ulnar nerves of these patients were 1.4-times larger than controls. Ultrasound abnormalities were appreciated in 38% of these patients within 4 weeks of disease onset, and no correlation with nerve conduction study parameters was found (including motor latency, CMAP, or F-wave). Interestingly, 2 of these patients imaged within the first two weeks of disease had nerve enlargement detected by ultrasound before significant changes could be detected by electrodiagnosis.
Almeida et al. performed serial ultrasound of the upper and lower extremity nerves in an 8-year-old boy at months 2, 4, and 5 after GBS (Almeida et al., 2012). Single nerve fascicles were enlarged in some nerves with normal CSA. Nerves early in recovery did appear hypoechoic. Some of the nerve CSAs returned to normal, suggesting that nerve enlargement may reverse with improvement in symptoms.
Kerasnoudis et al. described the correlation of nerve ultrasound, electrodiagnostic testing, and clinical findings in 41 post-GBS patients (mean 3.4 years after onset) (Kerasnoudis et al., 2013). There were electrophysiologic signs of permanent axon loss in the majority of peripheral nerves. Ultrasound showed higher CSA of the ulnar, radial, tibial nerves, and brachial plexus compared to controls. Three statistically significant findings were observed with electrophysiology: the post-GBS group had longer distal motor latency in the fibular nerve and F-wave latency in the tibial nerve; lower motor conduction velocity and CMAPs in the median, ulnar, and tibial nerves; and lower values of the sensory conduction velocity and sensory nerve action potentials in the ulnar, radial, fibular, and tibial nerves. No correlation between sonographic and electrophysiological findings was found. Neither electrodiagnostic testing nor nerve ultrasound correlated closely with functional disability. The post-GBS group showed significantly higher intra- and inter-nerve CSA variability for the median, ulnar, fibular, tibial nerves and the brachial plexus.
Grimm et al. presented the first study with ultrasound findings in the acute phase of the disease (within 1 week of symptom onset) (Grimm et al., 2014). The vagal nerve and peripheral nerves, including the 6th cervical nerve root, were imaged in 18 patients 1-3 days after onset. They found significantly increased CSA in all peripheral nerves except the proximal ulnar nerve and sural nerves in patients with GBS. However, although the differences were significant, many of the enlarged sites were within published reference ranges. This may be due to the early evaluation of the disease. The vagus nerve was enlarged most dramatically in patients with autonomic dysfunction. Both Grimm et al. and the previously mentioned Kerasnoudis study found no correlation between nerve enlargement and severity of disease.
Chronic Inflammatory Demyelinating Polyneuropathy
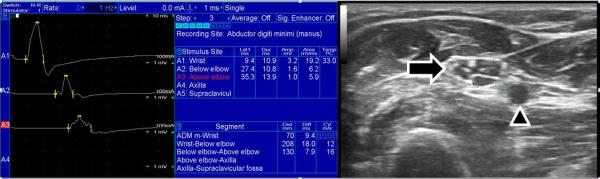
Ultrasonographic nerve enlargement was first reported in CIDP in 2004, and correlation between nerve enlargement and sites of motor conduction velocity slowing was also detected (Matsuoka et al., 2004) (see the Figure for an example of nerve enlargement at the site of conduction block). In 2014, Zaidman and Pestronk retrospectively compared serial clinical, electrodiagnostic, and nerve ultrasonography in 23 patients with CIDP (Zaidman and Pestronk, 2014). Thirteen of these patients achieved remission, defined as stable clinical improvement that persisted with reductions in medication or as continued improvement on stable, low-dose treatment. Patients with enlarged nerves at baseline who achieved remission had a greater reduction in nerve size compared to those not achieving remission (−41% vs. +7%, P = 0.04).
Figure 1.
The ulnar motor nerve conduction study and ulnar nerve ultrasound in an individual with CIDP are depicted. The nerve conduction studies show a drop in amplitude when stimulation at the wrist is compared to the forearm. Since no median-ulnar anastomosis was detected, and similar amplitude drops were detected in other nerves, this is consistent with a conduction block. At the site of ulnar conduction block in the forearm the ulnar nerve cross-sectional area was greatly increased (28 mm2, indicated by arrow) compared with our reference range (normal < 10 mm2). Additionally, large fascicles can be seen within the nerve. The arrowhead points at the ulnar artery.
Di Pasquale et al. described nerve ultrasound findings at noncompressible sites of the median, ulnar, and fibular nerves in 19 patients with CIDP (Di Pasquale et al., 2015). Abnormal nerve segments detected by ultrasound were associated more frequently with demyelinating electrophysiology. Additionally, the degree of CSA enlargement within a nerve segment correlated with longer disease duration, lower Medical Research Council score, and higher Inflammatory Neuropathy Cause and Treatment disability score.
A previous study by Jang et al. in 2014 did not find a correlation between nerve enlargement and clinical status (Jang el al., 2014). They did find nerve enlargement correlating with electrophysiologic dysfunction of nerves, as well as widely distributed nerve enlargement most notably in proximal regions and non-entrapment sites. Motor nerve conduction velocities of the median, ulnar, and tibial nerves negatively correlated with their respective CSA. Sural sensory nerve conduction velocity also showed a negative correlation with sural nerve CSA.
In addition to nerve enlargement in CIDP patients, a pilot study has demonstrated increased epineural blood flow (Goedee et al., 2014). The increased vascularization may represent proximal nerve inflammation and help in monitoring disease activity.
Immune-mediated Plexitis
Neuralgic amyotrophy, or idiopathic brachial plexitis or Parsonage-Turner Syndrome, predominantly affects the motor nerves of the brachial plexus nerves and is characterized byunilateral episodes of severe neuropathic pain followed by paresis and sensory loss. No clear ultrasonographic pattern has been established in this disease in the literature. In our lab, we have not detected ultrasonographic abnormalities in the brachial plexus, but interestingly have noted in several cases that that anterior interosseous and/or posterior interosseous has been unilaterally enlarged on the affected side. Further ultrasonographic investigation is warranted in these individuals.
Immune-mediated Polyneuropathies: Summary and Recommendations
While research is somewhat limited regarding neuromuscular ultrasound in those with immune-mediated polyneuropathies, some general trends have been noted. As opposed to hereditary causes, those with immune-mediated polyneuropathies have patchy nerve enlargement. Interestingly, the site of focal enlargement does not always correlate with slowed conduction velocity, so electrodiagnosis and ultrasonography may truly be complementary diagnostic modalities in those with immune-mediated polyneuropathies. In addition to patchy nerve enlargement, focal areas of increased nerve vascularity have been described in immune-mediated polyneuropathies, but the meaning of this increased vascularity is unknown and deserves further investigation. The nature of the nerve enlargement in these conditions does not seem to be permanent, as improvement in strength and electrodiagnostic parameters correlates with decreased nerve CSA.
Diabetic Polyneuropathy
Surprisingly few studies have assessed the neuromuscular ultrasound findings in diabetic polyneuropathy. Watanabe et al. evaluated the median nerve at the carpal tunnel and tibial nerve at the ankle in 20 patients with diabetes using ultrasound and motor nerve conduction studies, performed only in the upper limbs (Watanabe et al., 2009). CSA in the carpal tunnel correlated with a reduced motor conduction velocity. The study found a slight increase in CSA with lower echogeneity in patients with diabetic polyneuropathy. In this study it is unclear if the changes seen in the diabetic polyneuropathy group were actually due to diabetic polyneuropathy or entrapment. The authors suggest asymptomatic median mononeuropathy may exist in diabetic patients, and the combination of nerve conduction studies and ultrasound may detect estimate median mononeuropathy at the wrist in these individuals.
Liu et al. in 2012 imaged the sural nerve in patients with type 2 diabetes mellitus (Liu et al., 2012). The authors reported significant CSA differences among controls, diabetic patients, and diabetic patients with electrodiagnostically confirmed polyneuropathy. The values published were 1.44 ± 0.34 mm2 in controls, 1.52 ± 0.35 mm2 in diabetic patients without neuropathy, and 1.88 ± 0.50 mm2 in diabetic patients with neuropathy, which were statistically significant but show considerable overlap and would be difficult to differentiate with ultrasound alone in a given individual.
Also in 2012, Riazi et al. examined the association between CSA of the posterior tibial nerve and presence of diabetic polyneuropathy in 98 patients (Riazi et al., 2012). The CSA of the posterior tibial nerve was measured at up to 5 cm proximal to the medial malleolus. The most significant values were reported at 3 cm above the malleolus with controls measuring 17.69 ± 5.05 mm2 and patients measuring 22.59 ± 7.00 mm2. Conversely, Hobson-Webb et al. found no significant differences in fibular (at the fibular head or popliteal fossa) or sural nerve CSA, diameter, or echogenicity between controls and 25 patients with diabetic polyneuropathy (Hobson-Webb et al., 2013). 17 patients had no response on sural sensory nerve action potential testing.
Even more recently, Wang et al. were able to measure foot muscle atrophy in diabetic patients with and without polyneuropathy using high-frequency ultrasound (Wang et al., 2014). They measured the diameter, thickness, and CSA of the extensor digitorum brevis (EDB) and thickness of the muscles of the first interstitium. The results showed these parameters were significantly smaller in those with diabetic polyneuropathy than those with no diabetic polyneuropathy and controls (P < 0.01).
Diabetic Polyneuropathy: Summary and Recommendations
Further research is certainly needed regarding the neuromuscular ultrasound findings in those with diabetic polyneuropathy, but the current body of literature shows that those with diabetic polyneuropathy do not have profound nerve enlargement, decreased echogenicity, or increased vascularity compared to controls. While subtle increases in nerve CSA can be demonstrated in those with diabetic polyneuropathy, these changes would be difficult to detect in a given individual as the absolute differences from controls are small. Further investigation of nerve fascicles, perhaps with very high-resolution ultrasound transducers, is worth pursuing. In addition to changes within the nerves, the decreases in intrinsic foot muscle thickness are of interest, and may eventually prove to be a responsive biomarker of disease progression.
Other Polyneuropathies
Vasculitic Polyneuropathy (Mononeuritis Multiplex)
Systemic vasculitides can affect the epineurial vessels to produce sensory and/or motor deficits and pain in different nerve regions. Thus, localization of lesions in these neuropathies may be difficult. Ito et al. in 2007 aimed to determine the ability of ultrasound to morphologically evaluate vasculitic neuropathy of the tibial nerve at the medial ankle (Ito et al., 2007). Compared to controls, the tibial nerve was hypoechoic and significantly larger in those with vasculitic neuropathies (13.5 ± 3.7 mm2 vs. 7.2 ± 1.5 mm2, P < 0.05). The authors hypothesized that the localized nerve enlargement is due to active vasculitic-granulomatous lesions in the epineurium and intrafascicular edema, which were found in biopsy specimens. However, it is notable that imaging in this study focused only on the tibial nerve at the ankle, rather than the site of suspected nerve involvement.
A study in 2014 by Grimm et al. measured the CSA of peripheral nerves in 14 patients with systemic vasculitic neuropathies (Grimm et al., 2014). Underlying diagnoses included Sjögren's syndrome, necrotizing lupus-vasculitis, Churg-Strauss, granulomatous disease with polyangiitis, and essential mixed cryoglobulinemia. The mean CSAs were significantly increased most prominently in the tibial and fibular nerve (P < 0.01). Nerve enlargement was found in 22 out of 31 clinically and electrophysiologically affected nerves (median, ulnar, fibular, tibial, sural). The authors concluded that focal CSA enlargement detected by peripheral nerve ultrasound could help facilitate earlier diagnosis and therapy in those with vasculitic neuropathies. Similarly, it has also been postulated that nerve ultrasound might be able to help guide sites for nerve biopsies and increased diagnostic yield of the biopsy in suspected vasculitic neuropathy (Chipman et al., 2009). Perhaps a nerve with unobtainable nerve conduction studies responses with enlargement seen on ultrasound may be a high-yield target for biopsy, when indicated.
Transthyretin Mutations (Familial Amyloid Polyneuropathy)
The transthyretin (TTR) gene produces a homotetrameric plasma transport protein for thyroxine and the retinal-binding protein vitamin A complex. Mutations in this gene cause familial amyloid neuropathy, characterized by a small-diameter, length-dependent sensorimotor polyneuropathy with autonomic involvement. Granata et al. first utilized ultrasound for the evaluation of peripheral nerves in those with TTR mutations (Granata et al., 2014). The median, ulnar, fibular, tibial, and sural nerves of 7 patients with TTR-related neuropathy were imaged. Ultrasonographic abnormalities involving multiple nerves and sites were seen in 6 of the 7 patients. However, they did not find a consistent ultrasonographic pattern specific for TTR-related neuropathy; one patient had bilateral enlargement of ulnar nerve CSA at the elbow and another had unilateral ulnar nerve CSA enlargement. More studies are needed in this population to determine whether ultrasound can assist in diagnosis and monitoring of disease progression.
Acromegaly
Through unclear pathophysiology, acromegaly may affect peripheral nerves. In 2008, Tagliafico et al. examined the median and ulnar nerves of 34 nondiabetic, acromegalic patients with ultrasound and nerve conduction studies (Tagliafico et al., 2008). The median and ulnar nerve cross-sectional areas of these patients were significantly (P < 0.0001) larger at sites of the upper extremity compared to sex-, age-, and BMI-matched controls (median nerve at carpal tunnel 16.5 ± 4.4 mm2 vs. 7.4 ± 1.7 mm2; median nerve at mid-forearm 10.5 ± 2.4 mm2 vs. 5.5 ± 1.4 mm2; ulnar nerve at mid-forearm 9.5 ± 3.0 mm2 vs 5.3 ± 1.4 mm2; ulnar nerve at distal arm 13.1 ± 3.7 mm2 vs 6.6 ± 1.7 mm2). Nerve conduction studies were abnormal in 13 of 22 patients for which they were obtained. No statistical correlation was observed between nerve CSA and electrophysiological data. Insulin-like growth factor 1 (IGF1) levels correlated with cross-sectional area (r = 0.34) but not growth hormone (GH). A subsequent 2009 study by Resmini et al. examined these same patients 1 year after initial evaluation and demonstrated a reduction of cross-sectional areas of the mid-forearm median nerve and mid-forearm and distal ulnar nerve in line with the reduction of GH/IGF-I levels following treatment (Resmini et al., 2009).
Leprosy
Nerve tissue is involved in leprosy, a common cause of neuropathy world-wide. Temperature sensation is typically affected first before pain. Proprioception and vibratory sense are typically preserved. Ultrasound can be used to assess the peripheral nerves of individuals with leprosy.
In 2009, Elias et al. evaluated 21 patients with leprosy with ultrasound, which was the first report to correlate ultrasonographic findings with electrophysiologic findings in leprosy (Elias et al., 2009). The CSA of the ulnar nerve was examined in 3 regions. In addition to finding a significantly larger ulnar nerve CSA than in controls (P <0.01), the authors determined a maximum ulnar CSA cutoff value of 9.8 mm2 was the best discriminator between healthy controls and those with leprosy (sensitivity 0.91, specificity 0.90). Ultrasonographic abnormalities of these nerves include focal thickening, mixture of focal hyper and hypoechoic areas, and loss of fascicular pattern. Focal ulnar nerve thickening occurred in 90.5% of these patients with a tendency to be more severe above the medial epicondyle. Electrophysiologic abnormalities were found in 16 of the 19 ulnar nerves studied, and the 3 patients with normal electrophysiologic studies did have ultrasonographic abnormalities, which supports the use of ultrasound in these patients.
Visser et al. measured the epineurial thickness of the ulnar nerve above the elbow in 26 leprosy patients with high-resolution ultrasound (Visser et al., 2012). The mean epineurial thickness was 0.77 mm in symptomatic ulnar nerves, 0.58 mm in asymptomatic ulnar nerves, and 0.49 mm in healthy controls (P = 0.0001). A correlation existed between the CSA of the ulnar nerve and epineurial thickness (r = 0.64). No relation was observed between the presence of increased blood flow in the ulnar nerve and epineurial nerve thickening, though increased nerve vascularity was commonly detected in these patients.
Motor Neuron Diseases
Spinal Muscular Atrophy (SMA)
The spectrum of SMA phenotypes results from reductions of survival motor neuron protein resulting in motor neuron loss and progressive weakness. SMA is categorized based on age of disease onset: type I, the most common, with disease onset by age 6 months and death within 2 years; type II withonset of disease between ages 6 and 18 months; type III presents after 18 months of age; and type IV with adult-onset.
While there are no studies of nerve ultrasound in individuals with SMA, there are several investigations of muscle ultrasound. Heckmatt et al. first described changes seen with ultrasound imaging of children with neuromuscular diseases, particularly muscular dystrophy and SMA (Heckmatt et al., 1982). Muscular dystrophies were associated with homogeneously increased intensity of echo from muscle substance with loss of contrasting bone echo. Conversely, SMA showed heterogeneously increased muscle echogenicity, as well as increased depth of subcutaneous tissue. Wu et al. evaluated the biceps brachii, wrist extensors, quadriceps, and tibialis anterior of 25 patients with SMA types 2 and 3 to assess the value of quantitative ultrasound in these individuals (Wu et al., 2010). The investigators calculated a luminosity ratio for each muscle by comparing the echogenicity of the subcutaneous tissue to the echogenicity of the muscle. A higher ratio indicates increased muscle echo intensity and thus more involved muscle. In this study, quantitative ultrasound was able to discriminate between SMA types 2 and 3. SMA luminosity ratios were greater than controls and greatest in SMA type 2 (P < 0.001).
Amyotrophic Lateral Sclerosis
Early studies in ALS showed marked abnormalities with ultrasound, including reduced muscle thickness, increased muscle echo intensity, and fasciculations (Arts et al., 2008). Arts et al. in a longitudinal study assessed structural muscle changes in ALS over 6 months, and confirmed increased muscle echo intensity and decreased thickness at baseline in ALS patients. However, the pattern of muscle changes on ultrasound did not correlate with functional measures such as manual muscle testing and the revised ALS functional rating scale.
Ultrasound also shows promise as a biomarker of disease progression. Lee et al. serially imaged muscles of 8 ALS patients and muscle thickness declined at a mean rate of −0.663 mm/month (P = 0.0014) (Lee et al., 2010). Cartwright found a thinner biceps/brachialis complex in the ALS patients than controls, and suggested muscle thickness as a biomarker (2.1 cm vs. 2.9 cm, P = 0.007) (Cartwright et al., 2011). This group also examined nerve CSA in 20 patients, and the median nerve in the mid-arm was smaller in the ALS group than in controls (10.5 mm2 vs. 12.7 mm2, P = 0.0023), though the difference was not profound and likely would not serve as an effective biomarker. Schreiber et al. did not find significant differences in median nerve CSA in their comparison of ALS patients and controls (Schreiber et al., 2015), though they measured the median nerve at more distal sites. Considerations in the relative proportion of motor and sensory fibers along the nerve's course may help explain this discrepancy. Schreiber et al. did find a significant distally-pronounced reduction of the ulnar nerve CSA, and suggested it as a potential marker for lower motor neuron involvement. Finally, diaphragm ultrasound is a newer field, and it too has promise as a biomarker in patients with ALS. Pinto et al. recently found a correlation between ultrasonographic thickness of the diaphragm and the number of functional motor units as assessed by phrenic nerve motor amplitude (Pinto et al., 2015).
Ultrasound has been found to be more sensitive for the detection of fasciculations than visual inspection or EMG. With ultrasound and EMG, Misawa et al. analyzed 474 muscles in 81 patients suspected of having ALS. EMG detected fasciculations in 71 out of 81 patients (88%), while ultrasound revealed fasciculations in 79 out of 81 patients (98%) (P = 0.02) (Misawa et al., 2011), and the combination of ultrasound and EMG substantially increased the diagnostic sensitivity of ALS when using the Awajji criteria. In another study, Arts et al. reported ultrasonography differentiating ALS from mimics with 96% sensitivity and 84% specificity (Arts et al., 2012). These mimics included hereditary spastic paresis, progressive supranuclear palsy, polyradiculopathy, myopathy, plexopathy, benign cramp fasciculation syndrome, metabolic- and toxic-induced fasciculations, and mononeuropathia multiplex. Additionally, ultrasound showed signs of lower motor neuron involvement in 15 of the 24 sites that were negative both clinically and on EMG. Utilizing ultrasound might aid in the search for lower motor neuron involvement in the diagnostic work up of ALS by reducing the number of muscles requiring needle examination.
Motor Neuron Diseases: Summary and Recommendations
In general, changes in nerve caliber and echogenicity are not marked in motor neuron disease, though small statistically significant decreases in nerve area have been reported. Much more prominent are the ultrasonographic changes in muscle in those with motor neuron disease, with the typical pattern consisting of heterogeneous increases in muscle echogenicity, muscle atrophy, and increased subcutaneous tissue thickness. In addition, ultrasound is the most sensitive test for detecting fasciculations. Ultrasonographic imaging of the diaphragm is a feasible and informative method for assessing motor neuron disease, and ALS in particular. Though further investigation is needed, ultrasound can demonstrate hemi-diaphragm atrophy, decreased ability of the diaphragm to thicken with inspiration, and decreased ability of the diaphragm to move in those with ALS. Diaphragm and muscle ultrasound have the potential to serve as responsive markers of disease progression in those with ALS.
Table 1.
Ultrasound Findings in Polyneuropathies and Motor Neuron Diseases
| Condition | Key Ultrasound Findings |
|---|---|
| Charcot-Marie Tooth Disease | Demyelinating forms have CSAs 2.0-4.0× that of controls in the median and ulnar nerves. Axonal forms have CSA 1.5-2.0× that of controls in the median and ulnar nerves. The sural nerve is only enlarged in children. |
| Multifocal Motor Neuropathy | Patchy increased CSA with inter- and intra-nerve variability; includes the brachial plexus Focal nerve enlargements can differentiate MMN from ALS Nerve enlargement does not correlate well with conduction block |
| Guillain-Barre Syndrome | Nerves may be enlarged before changes are detected by electrodiagnosis Nerve enlargement is reversible with recovery |
| Chronic Inflammatory Demyelinating Polyneuropathy | Patchy nerve enlargement The site of focal enlargement does not always correlate with slowed conduction velocity |
| Diabetic Polyneuropathy | Nerve changes are subtle and not particularly helpful for diagnosis Intrinsic foot muscle atrophy can be readily detected |
| Vasculitic Polyneuropathy | Minor increase in nerve CSA Can guide selection of nerve biopsy site |
| Amyloid Polyneuropathy | No standard abnormalities yet defined |
| Acromegaly | Diffuse enlargement of nerve CSA |
| Leprosy | Ulnar nerve CSA enlargement Increased epineurial thickness Increased vascularity |
| Spinal Muscular Atrophy | Patchy increase in muscle echotexture Increased subcutaneous tissue thickness |
| Amyotrophic Lateral Sclerosis | Normal to slightly decreased nerve CSA Muscle atrophy and increased echogenicity Ultrasound is more sensitive for the detection of fasciculations than visual inspection or EMG Diaphragm thinning and increased echogenicity May be a responsive biomarker of disease progression |
References
- Almeida V, Mariotti P, Veltri S, Erra C, Padua L. Nerve ultrasound follow-up in a child with Guillain-Barré syndrome. Muscle Nerve. 2012;46:270–275. doi: 10.1002/mus.23325. [DOI] [PubMed] [Google Scholar]
- Arts IMP, Overeem S, Pillen S, et al. Muscle ultrasonography: a diagnostic tool for amyotrophic lateral sclerosis. Clin Neurophysiol. 2012;123:1662–1667. doi: 10.1016/j.clinph.2011.11.262. [DOI] [PubMed] [Google Scholar]
- Arts IMP, van Rooij FG, Overeem S, et al. Quantitative muscle ultrasonography in amyotrophic lateral sclerosis. Ultrasound Med Biol. 2008;34:354–361. doi: 10.1016/j.ultrasmedbio.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Beekman R, van den Berg LH, Franssen H, et al. Ultrasonography shows extensive nerve enlargements in multifocal motor neuropathy. Neurology. 2005;65:305–307. doi: 10.1212/01.wnl.0000169179.67764.30. [DOI] [PubMed] [Google Scholar]
- Buchberger W, Judmaier W, Birbamer G, Lener M, Schmidauer C. Carpal tunnel syndrome: diagnosis with high-resolution sonography. AJR Am J Roentgenol. 1992;159:793–798. doi: 10.2214/ajr.159.4.1529845. [DOI] [PubMed] [Google Scholar]
- Cartwright MS, Brown ME, Eulitt P, Walker FO, Lawson VH, Caress JB. Diagnostic nerve ultrasound in Charcot-Marie-Tooth disease type 1B. Muscle Nerve. 2009;40:98–102. doi: 10.1002/mus.21292. [DOI] [PubMed] [Google Scholar]
- Cartwright MS, Demar S, Griffin LP, Balakrishnan N, Harris JM, Walker FO. Validity and reliability of nerve and muscle ultrasound. Muscle Nerve. 2013;47:515–521. doi: 10.1002/mus.23621. [DOI] [PubMed] [Google Scholar]
- Cartwright MS, Walker FO, Griffin LP, Caress JB. Peripheral nerve and muscle ultrasound in amyotrophic lateral sclerosis. Muscle Nerve. 2011;44:346–351. doi: 10.1002/mus.22035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipman JN, Mott RT, Stanton CA, Cartwright MS. Ultrasonographic Tinel sign. Muscle Nerve. 2009;40:1033–1035. doi: 10.1002/mus.21461. [DOI] [PubMed] [Google Scholar]
- Di Pasquale A, Morino S, Loreti S, Bucci E, Vanacore N, Antonini G. Peripheral nerve ultrasound changes in CIDP and correlations with nerve conduction velocity. Neurology. 2015;84:803–809. doi: 10.1212/WNL.0000000000001291. [DOI] [PubMed] [Google Scholar]
- Elias J, Nogueira-Barbosa MH, Feltrin LT, et al. Role of Ulnar Nerve Sonography in Leprosy Neuropathy With Electrophysiologic Correlation. J Ultrasound Med. 2009;28:1201–1209. doi: 10.7863/jum.2009.28.9.1201. [DOI] [PubMed] [Google Scholar]
- Fornage BD. Peripheral nerves of the extremities: imaging with US. Radiology. 1988;167:179–182. doi: 10.1148/radiology.167.1.3279453. [DOI] [PubMed] [Google Scholar]
- Goedee HS, Brekelmans GJF, Visser LH. Multifocal enlargement and increased vascularization of peripheral nerves detected by sonography in CIDP: a pilot study. Clin Neurophysiol. 2014;125:154–159. doi: 10.1016/j.clinph.2013.05.025. [DOI] [PubMed] [Google Scholar]
- Granata G, Luigetti M, Coraci D, et al. Ultrasound evaluation in transthyretin-related amyloid neuropathy. Muscle Nerve. 2014;50:372–376. doi: 10.1002/mus.24168. [DOI] [PubMed] [Google Scholar]
- Grimm A, Décard BF, Axer H. Ultrasonography of the peripheral nervous system in the early stage of Guillain-Barré syndrome. J. Peripher. Nerv. Syst. 2014;19:234–241. doi: 10.1111/jns.12091. [DOI] [PubMed] [Google Scholar]
- Grimm A, Décard BF, Athanasopoulou I, Schweikert K, Sinnreich M, Axer H. Nerve ultrasound for differentiation between amyotrophic lateral sclerosis and multifocal motor neuropathy. J. Neurol. 2015;262:870–880. doi: 10.1007/s00415-015-7648-0. [DOI] [PubMed] [Google Scholar]
- Grimm A, Décard BF, Bischof A, Axer H. Ultrasound of the peripheral nerves in systemic vasculitic neuropathies. J. Neurol. Sci. 2014;347:44–49. doi: 10.1016/j.jns.2014.09.017. [DOI] [PubMed] [Google Scholar]
- Heckmatt JZ, Leeman S, Dubowitz V. Ultrasound imaging in the diagnosis of muscle disease. J. Pediatr. 1982;101:656–660. doi: 10.1016/s0022-3476(82)80286-2. [DOI] [PubMed] [Google Scholar]
- Heinemeyer O, Reimers CD. Ultrasound of radial, ulnar, median, and sciatic nerves in healthy subjects and patients with hereditary motor and sensory neuropathies. Ultrasound Med Biol. 1999;25:481–485. doi: 10.1016/s0301-5629(98)00187-2. [DOI] [PubMed] [Google Scholar]
- Hobson-Webb LD, Massey JM, Juel VC. Nerve ultrasound in diabetic polyneuropathy: correlation with clinical characteristics and electrodiagnostic testing. Muscle Nerve. 2013;47:379–384. doi: 10.1002/mus.23625. [DOI] [PubMed] [Google Scholar]
- Ito T, Kijima M, Watanabe T, Sakuta M, Nishiyama K. Ultrasonography of the tibial nerve in vasculitic neuropathy. Muscle Nerve. 2007;35:379–382. doi: 10.1002/mus.20673. [DOI] [PubMed] [Google Scholar]
- Jang JH, Cho CS, Yang K-S, Seok HY, Kim B-J. Pattern analysis of nerve enlargement using ultrasonography in chronic inflammatory demyelinating polyneuropathy. Clin Neurophysiol. 2014;125:1893–1899. doi: 10.1016/j.clinph.2013.12.115. [DOI] [PubMed] [Google Scholar]
- Kerasnoudis A, Pitarokoili K, Behrendt V, Gold R, Yoon M-S. Correlation of nerve ultrasound, electrophysiological, and clinical findings in post Guillain-Barré syndrome. J. Peripher. Nerv. Syst. 2013;18:232–240. doi: 10.1111/jns5.12037. [DOI] [PubMed] [Google Scholar]
- Kerasnoudis A, Pitarokoili K, Behrendt V, Gold R, Yoon M-S. Multifocal motor neuropathy: correlation of nerve ultrasound, electrophysiological, and clinical findings. J. Peripher. Nerv. Syst. 2014;19:165–174. doi: 10.1111/jns5.12067. [DOI] [PubMed] [Google Scholar]
- Lee CD, Song Y, Peltier AC, Jarquin-Valdivia AA, Donofrio PD. Muscle ultrasound quantifies the rate of reduction of muscle thickness in amyotrophic lateral sclerosis. Muscle Nerve. 2010;42:814–819. doi: 10.1002/mus.21779. [DOI] [PubMed] [Google Scholar]
- Liu F, Zhu J, Wei M, Bao Y, Hu B. Preliminary evaluation of the sural nerve using 22-MHz ultrasound: a new approach for evaluation of diabetic cutaneous neuropathy. PLoS ONE. 2012;7:e32730. doi: 10.1371/journal.pone.0032730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinoli C, Schenone A, Bianchi S, et al. Sonography of the median nerve in Charcot-Marie-Tooth disease. AJR Am J Roentgenol. 2002;178:1553–1556. doi: 10.2214/ajr.178.6.1781553. [DOI] [PubMed] [Google Scholar]
- Matsuoka N, Kohriyama T, Ochi K, et al. Detection of cervical nerve root hypertrophy by ultrasonography in chronic inflammatory demyelinating polyradiculoneuropathy. J. Neurol. Sci. 2004;219:15–21. doi: 10.1016/j.jns.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Misawa S, Noto Y, Shibuya K, et al. Ultrasonographic detection of fasciculations markedly increases diagnostic sensitivity of ALS. Neurology. 2011;77:1532–1537. doi: 10.1212/WNL.0b013e318233b36a. [DOI] [PubMed] [Google Scholar]
- Noto Y, Shiga K, Tsuji Y, et al. Nerve ultrasound depicts peripheral nerve enlargement in patients with genetically distinct Charcot-Marie-Tooth disease. J. Neurol. Neurosurg. Psychiatr. 2015;86:378–384. doi: 10.1136/jnnp-2014-308211. [DOI] [PubMed] [Google Scholar]
- Padua L, Martinoli C, Pazzaglia C, et al. Intra- and internerve cross-sectional area variability: new ultrasound measures. Muscle Nerve. 2012;45:730–733. doi: 10.1002/mus.23252. [DOI] [PubMed] [Google Scholar]
- Pazzaglia C, Minciotti I, Coraci D, Briani C, Padua L. Ultrasound assessment of sural nerve in Charcot-Marie-Tooth 1A neuropathy. Clin Neurophysiol. 2013;124:1695–1699. doi: 10.1016/j.clinph.2013.02.020. [DOI] [PubMed] [Google Scholar]
- Pinto S, Alves P, Pimentel B, Swash M, de Carvalho M. Ultrasound for assessment of diaphragm in ALS. Clinical Neurophysiology. 2015 doi: 10.1016/j.clinph.2015.03.024. in press. [DOI] [PubMed] [Google Scholar]
- Resmini E, Tagliafico A, Nizzo R, et al. Ultrasound of peripheral nerves in acromegaly: changes at 1-year follow-up. Clin. Endocrinol. 2009;71:220–225. doi: 10.1111/j.1365-2265.2008.03468.x. [DOI] [PubMed] [Google Scholar]
- Riazi S, Bril V, Perkins BA, et al. Can ultrasound of the tibial nerve detect diabetic peripheral neuropathy? A cross-sectional study. Diabetes Care. 2012;35:2575–2579. doi: 10.2337/dc12-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S, Abdulla S, Debska-Vielhaber G, et al. Peripheral nerve ultrasound in amyotrophic lateral sclerosis phenotypes. Muscle Nerve. 2015;51:669–675. doi: 10.1002/mus.24431. [DOI] [PubMed] [Google Scholar]
- Schreiber S, Oldag A, Kornblum C, et al. Sonography of the median nerve in CMT1A, CMT2A, CMTX, and HNPP. Muscle Nerve. 2013;47:385–395. doi: 10.1002/mus.23681. [DOI] [PubMed] [Google Scholar]
- Tagliafico A, Cadoni A, Fisci E, Bignotti B, Padua L, Martinoli C. Reliability of side-to-side ultrasound cross-sectional area measurements of lower extremity nerves in healthy subjects. Muscle Nerve. 2012;46:717–722. doi: 10.1002/mus.23417. [DOI] [PubMed] [Google Scholar]
- Tagliafico A, Resmini E, Nizzo R, et al. Ultrasound measurement of median and ulnar nerve cross-sectional area in acromegaly. J. Clin. Endocrinol. Metab. 2008;93:905–909. doi: 10.1210/jc.2007-1719. [DOI] [PubMed] [Google Scholar]
- Visser LH, Jain S, Lokesh B, Suneetha S, Subbanna J. Morphological changes of the epineurium in leprosy: a new finding detected by high-resolution sonography. Muscle Nerve. 2012;46:38–41. doi: 10.1002/mus.23269. [DOI] [PubMed] [Google Scholar]
- Wang X, Chen L, Liu W, Su B, Zhang Y. Early detection of atrophy of foot muscles in Chinese patients of type 2 diabetes mellitus by high-frequency ultrasonography. J Diabetes Res. 2014:927069. doi: 10.1155/2014/927069. doi:10.1155/2014/927069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Ito H, Morita A. Sonographic evaluation of the median nerve in diabetic patients: comparison with nerve conduction studies. J Ultrasound Med. 2009;28:727–734. doi: 10.7863/jum.2009.28.6.727. [DOI] [PubMed] [Google Scholar]
- Wu JS, Darras BT, Rutkove SB. Assessing spinal muscular atrophy with quantitative ultrasound. Neurology. 2010;75:526–531. doi: 10.1212/WNL.0b013e3181eccf8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu EM, Brockley CR, Lee KJ, et al. Peripheral nerve ultrasound in pediatric Charcot-Marie-Tooth disease type 1A. Neurology. 2015;84:569–574. doi: 10.1212/WNL.0000000000001236. [DOI] [PubMed] [Google Scholar]
- Zaidman CM, Lozi MA, Pestronk A. Peripheral nerve size in normals and patients with polyneuropathy: an ultrasound study. Muscle Nerve. 2009;40:960–966. doi: 10.1002/mus.21431. [DOI] [PubMed] [Google Scholar]
- Zaidman CM, Pestronk A. Nerve size in chronic inflammatory demyelinating neuropathy varies with disease activity and therapy response over time: a retrospective ultrasound study. Muscle Nerve. 2014;50:733–738. doi: 10.1002/mus.24227. [DOI] [PMC free article] [PubMed] [Google Scholar]