Abstract
Diabetic cardiomyopathy is a common complication in patients with diabetes and is associated with underlying chronic inflammation and cardiac cell death, subsequently leading to heart failure (HF). ELAV-like protein 1 (ELAVL1) plays a critical role in the progression of inflammation and HF. However the role of ELAVL-1 in inflammation induced cardiac cell death (pyroptosis) under hyper glycemic condition remains elusive. Our data demonstrates that ELAVL1 expression augmented with a concomitant increase in caspase-1 and IL-1 beta expression in human hearts and human ventricular cardiomyocytes under hyperglycemic condition. Furthermore, ELAVL1 knockdown abrogates TNF-α induced canonical pyroptosis via NLRP3, caspase-1 and IL-1beta suppression. Bioinformatics analysis and target validation assays showed that miR-9 directly targets ELAVL1. Interestingly, miRNA-9 expression significantly reduced in high glucose treated cardiomyocytes and in human diabetic hearts. Inhibition of miR-9 upregulates ELAVL1 expression and activates caspase-1. Alternatively, treatment with miR-9 mimics attenuates hyperglycemia-induced ELAVL1 and inhibits cardiomyocyte pyroptosis. Taken together our study highlights the potential therapeutic implications of targeting miR-9/ELAVL1 in preventing cardiomyocyte cell loss during HF in diabetics.
Keywords: microRNA-9, diabetic cardiomyopathy, ELAVL1, inflammation, pyroptosis
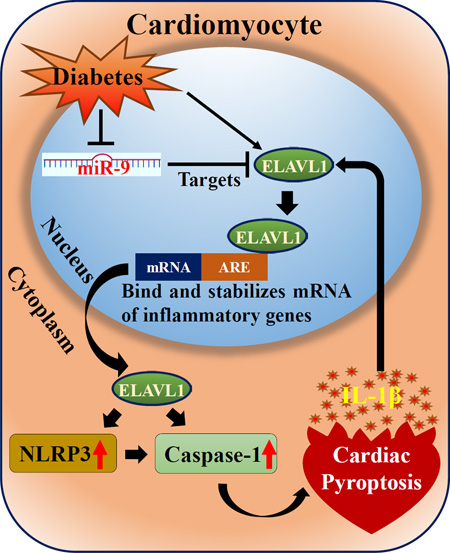
Graphical abstract
Schematic representation that illustrate high glucose induced cardiac pyroptosis and regulatory role of ELAVL1 and miR-9.
1. Introduction
Diabetic cardiomyopathy (DCM), characterized by diastolic dysfunction and increased ventricular mass, is the leading cause of mortality among patients with diabetes [1, 2]. High glucose (HG) induces the inflammatory cascade plays an important role in several diabetic complications [3]. Pyroptosis, a highly inflammatory form of programmed cell death (that shares the unique characteristics with both apoptosis and necrosis), is mediated through caspase-1 [4]. Caspase-1 activation results in processing of proforms of IL-1 beta and IL-18 into active forms. Studies have demonstrated pyroptosis-mediated cardiac cell death in the myocardium of diabetic mice and rats and that caspase-1-deficient mice were protected from ischemia/reperfusion-induced cardiac apoptosis [5, 6]. Hence strategies to limit the process are novel avenues in preventing DCM-related cardiac dysfunction and remodeling.
Numerous studies have shown that miRNAs (endogenous, small non-coding RNAs) are involved in a wide variety of biological processes, including cell proliferation, differentiation, metastasis, apoptosis and immune responses and also serve as prognostic markers in the development and progression of cardiovascular diseases [7–9]. Recently, we reported, that transplantation of miR-377 silenced hCD34 (+) cells promote neovascularization and improved left ventricular function in a mouse model of heart failure (HF) [10]. However, studies to show the participation of miRNAs in cardiac pyroptosis, specifically in DCM are limited.
Emerging studies has displayed the essential roles of microRNAs (miRNAs) and RNA binding proteins that interact with the cis-regulatory elements of transcripts to regulate gene expression [11]. Among the RNA binding proteins, ELAVL1 is ubiquitously expressed and binds to AU-rich element (ARE) - and U-rich element (URE)-containing and stabilizes the mRNAs [12]. Knockdown of ELAVL1 mimicked anti-inflammatory responses and attenuated post-MI inflammatory response and left ventricular dysfunction in IL-10-null mice [13]. The aim of this present study is to determine if diabetes promotes cardiomyocyte pyroptosis and elucidate the role of miRNA-9 in cardiomyocyte pyroptosis.
Here, we demonstrate that hyperglycemia augments ELAVL1 and NLRP3 expression and persuade caspase-1 mediated pyroptosis. Hyperglycemia down-regulates expression of miR-9 in failed human diabetic heart tissues. Also, inhibition of miR-9 up-regulates of ELAVL1, NLRP3 and caspase-1 expression. Taken together, in the present study we identified the crucial role of miR-9 and ELAVL1 in pathogenesis of hyperglycemia-induced cardiomyocyte pyroptosis in diabetic condition.
2. Materials and Methods
2.1. Cell culture
Immortalized human cardiomyocytes were purchased from Applied Biological Materials and cultured in Prigrow I medium with 10% FBS and 1% penicillin/streptomycin in a humidified atmosphere of 95% air and 5% CO2 at 37°C.
H9C2 cells, a clonal line derived from embryonic rat heart, obtained from American Type Culture Collection (CRL-1446), were maintained in DMEM and supplemented with 10% (vol/vol) fetal bovine serum, 10 mM L-Glu, and 5 mg/mL penicillin/streptomycin in a humidified atmosphere of 95% air and 5% CO2 at 37°C.
2.2. Heart tissues samples from human patients
Heart tissue samples were obtained from failing human hearts at the time of transplantation at the Houston Methodist DeBakey Heart and Vascular Center, Houston Methodist Hospital, Houston, Texas, and immediately frozen in liquid nitrogen and stored at −80°C until use. Normal hearts were obtained from donor hearts not used for transplantation and were collected and stored in the same manner. All tissues were collected under an approved protocol by the Houston Methodist Research Institutional Review Board.
2.3. Dual-luciferase reporter assay to validate ELAVL1 as a target of mir-9
Culture human cardiomyocytes were co-transfected with control or miR-9 mimic or miR-9 inhibitor and a reporter plasmid containing the 3’ UTR of ELAVL1 (HmiT004694-a) inserted downstream of the luciferase reporter gene (pEZX-ELAVL1-UTR; GeneCopoeia). Briefly ELAVL1 UTR (200 ng) and 90 nm/l miR-9 mimic or miR-9 inhibitor the corresponding control empty luciferase reporter vector (CmiT000001-MT06) was mixed with Lipofectamine 2000 (Invitrogen) and added to 48-well plate containing human cardiomyocytes (1.3 × 104). After transfection (24 hrs), luciferase assay was performed on transfected cells using Dual-Luciferase reporter assay kit (Promega) as per manufacturer’s protocol.
2.4. In vitro transient transfection of cardiomyocytes with miRNA mimic and inhibitor
MiR-9 mimic, miR-9 inhibitor and respective negative control, (Ambion, Life Technologies), were used for this transient transfection. miRNA mimic and miRNA inhibitor negative control are random sequence miRNA mimic and miRNA inhibitor molecule that has been extensively tested and validated to produce no identifiable effects on known miRNA function. Cells were seeded in 6-well plates 24 hours prior to transfection. The miR-9 mimic and inhibitor was added at the final concentration of 90 nM after mixing with Lipofectamine Transfection Reagent (Invitrogen) according to the manufacturer’s instructions. After 48 hours, the cells from each group were harvested, for further analysis.
2.5. Quantitative RT-PCR and Western blot analysis
Expression of miR-9, ELAVL1 and NLRP3 were quantified at transcription level as described previously and expressed as fold change vs respective control. qRT-PCR was performed (Eppendorf thermocycler, Applied Biosystem) using a Taqman qPCR Kit Life Technologies Corporation Carlsbad, CA. Western blot analysis was carried out to measure the expression of protein at translational level. Cell lysates from various samples were analyzed as described previously. In short proteins resolved by electrophoresis and transferred in to a nitrocellulose filters. Then the blots were incubated with respective secondary antibodies. After incubation with the primary antibodies and horseradish peroxidase coupled secondary antibodies (Santa Cruz Biotechnology) the bands were detected by using chemiluminescence developing agents (SuperSignal West Pico, Thermo Scientific).
2.6. Immunofluorescence analysis
To observe pyroptosis, cultured human cardiomyocytes or tissue sections were fixed with 4% formaldehyde in PBS were permeabilized in 0.3% triton-100. Primary antibodies were be probed and stained with Alex Fluor secondary antibodies. Stained cells were then observed under Olympus Fluoview FV 1000 Laser Scan Confocal Microscope and the intensity of immunofluorescence were examined.
2.7. Histology and Immunohistochemistry
Serial sections from the Human heart tissues were stained with haematoxylin and eosin (H&E). For immunohistochemical analyses, endogenous peroxidase activity was quenched by incubating the slides in methanol and 0.6% H2O2 in methanol. In all cases, the primary antibody, was left overnight at 4°C. The slides were washed in TBS, and HRP-conjugated secondary antibody (Santa Cruz Biotechnology) was then added to incubate at room temperature for 45 min. The slides were washed in TBS and incubated with diaminobenzidine tetrahydrochloride as the substrate, and photographs were under light microscope.
2.8. Generation of stable ELAVL1-knockdown RAW 264.7 and stimulation
Mouse mononuclear/ macrophages Raw 264.7 cell line was maintained in RPMI (Clonitech, Palo Alto, CA, USA) with 10% FBS. Stable cell lines expressing either sh-Control or sh-ELAVL1 were obtained by lentiviral transduction as described earlier [13]. Recombinant TNF-α was obtained from PeproTech (Rocky Hill, NJ, USA) and LPS was obtained from Sigma-Aldrich (St. Louis, MO, USA). Control and ELAVL1-knockdown cells were stimulated with LPS (1µg/ml) or TNF-α (20 ng/ml) for 24 hrs.
2.9. Statistical analysis
All the values are expressed as means ± SEM. Statistical analysis of differences between the groups was performed by Student's “t”-test or one-way analysis of variance, followed by Tukey's multiple comparison test wherever necessary using GraphPad Prism 6 software. A value of P < 0.05 was considered statistically significant.
3. Results
3.1. Human diabetic heart shows up-regulation of inflammation and cardiac pyroptosis
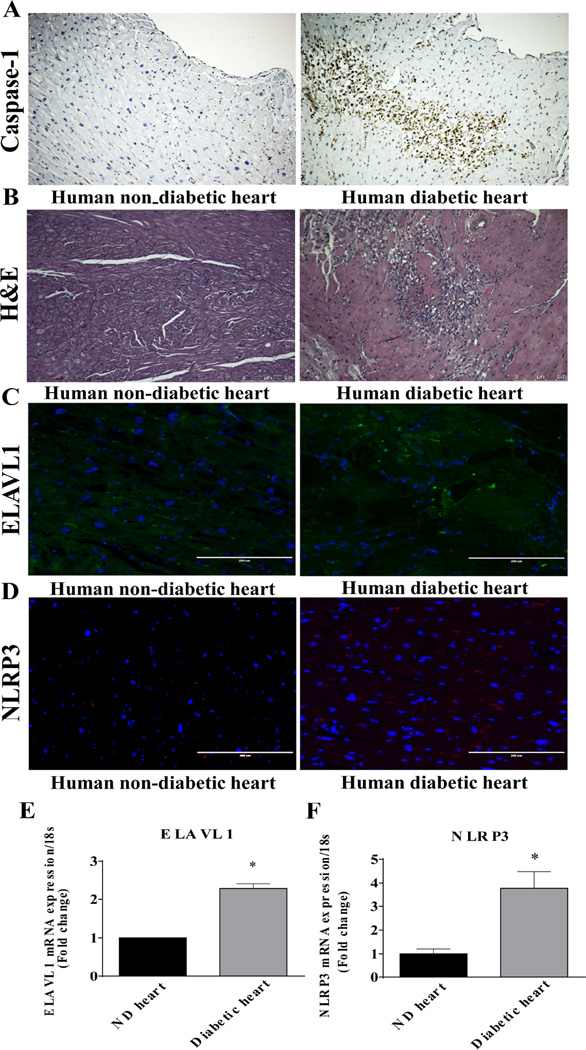
DCM is characterized by chronic inflammatory response, loss of cardiac cells leading to HF [14, 15]. Immunohistochemistry evaluation of heart shows increase in caspase-1 expression in human diabetic hearts as compared to non-diabetic heart tissue sections (Fig 2A). The increase in caspase-1 expression is associated with a higher infiltration of inflammatory cells into the myocardium of diabetic heart tissues (Fig 2B). Our previous reports have shown that an increase in inflammatory response in the myocardium after myocardial infarction corroborated with higher ELAVL1 expression levels [13]. In the present study, ELAVL1 expression is elevated in human diabetic heart both at protein (Fig 2C) and transcriptional level (Fig 2D, P<0.05). Interestingly, expression of NLRP3 also increased in diabetic failing hearts (Fig 2E and F, P<0.05).
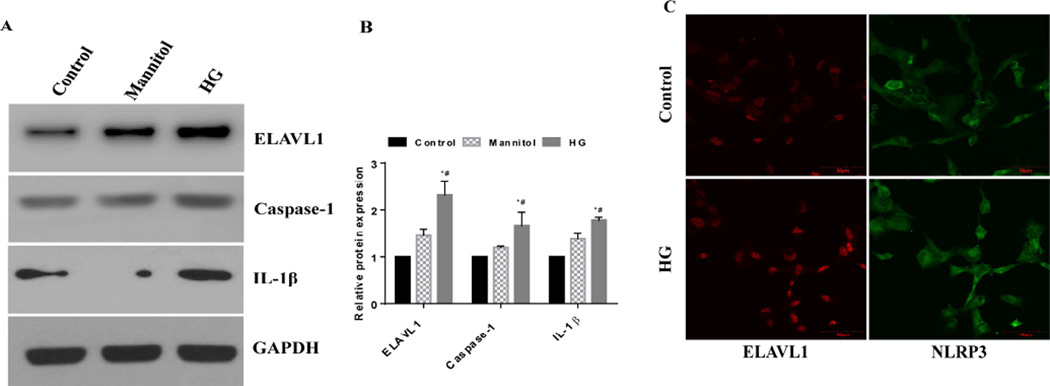
Figure 2. HG increases human cardiomyocyte pyroptosis.
(A) Western blot analysis of ELAVL1, caspase-1, IL-1β and GAPDH in human cardiomyocytes treated with none, mannitol and glucose. (B) Relative protein levels of ELAVL1, caspase-1 and IL-1β by densitometric analysis. (C) Immunofluorescence images showing the expression of ELAVL1 (green) and NLRP3 (red) in control and HG treated human cardio myocytes.
3.2 Hyperglycemia promotes pyroptosis in human ventricular cardiomyocyte
Hyperglycemia has been shown to activate inflammatory response and induce cell death. To determine the effect of hyperglycemia on pyroptosis and its correlation to inflammation, we exposed human cardiomyocytes to 35mM/L glucose (HG) to mimic hyperglycemia for 48 hrs. HG-treated cells show significant increase in ELAVL1, caspase-1 and IL-1β protein expression (Fig 1A, P<0.05). Furthermore, HG-treatment increases ELAVL 1 (Fig 1A, P<0.05) and inflammasome complex protein, NLRP3 expression (Fig 1B).
Figure 1. HG induced inflammatory signaling and cardiac pyroptosis in normal and failing human hearts.
(A) Caspase-1 expression in tissue sections of non-diabetic and diabetic human hearts assessed by immunohistochemical staining. Scale bar: 250µm. n=3. miR-9 expression is down-regulated in diabetic failing hearts. (B) HE staining of cross sectional tissue slices of control and diabetic failing human heart tissues (10X). Scale bar: 250µm. n=3. (C&D) Immunofluorescence images showing the expression of ELAVL1 (green) and NLRP3 (red) in non-diabetic and diabetic heart human heart tissue sections (n=3). Nuclei were stained with DAPI (blue). Scale bars (200µm). (E&F) qRT-PCR analysis of ELAVL1 and NLRP3 at mRNA levels in non-diabetic and diabetic human hearts. *p < 0.05; ± S.E.M.
3.3. ELAVL1 mediates TNF-α induced pyroptosis signaling
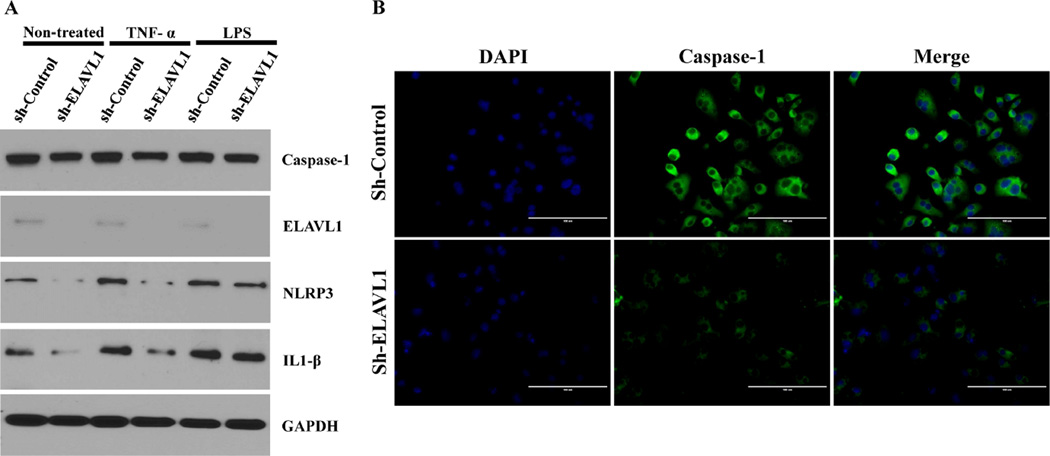
To elucidate the potential mechanistic role of ELAVL1 in pyroptosis, lentivirus based shRNA against ELAVL1 (sh-ELAVL1) or control shRNA (scrambled construct) -transduced RAW 264.7 (murine monocyte/macrophage) cells were treated with either LPS (mimics the non-canonical bacterial inflammasome pathway) or TNF-α (to mimics canonical inflammatory pathway without bacterial infection). Our findings show that caspase-1 expression at baseline is lower in ELAVL1 knockdown cells as compared to control-shRNA cells. Furthermore, LPS or TNF-α treatment stimulates caspase-1 expression as compared to untreated control-shRNA cells (Fig 3A and B). Interestingly, unlike in LPS-treated cells, ELAVL1 knockdown (shRNA-ELAVL1) cells show reduced caspase-1 expression in TNF-α stimulated cells. We found comparable caspase-1 protein expression in both shRNA-Control and shRNA-ELAVL1 cells when treated with LPS (Fig 3A and B).
Figure 3. ELAVL1 knockdown impairs canonical inflammasome pathway and failed to induce pyroptosis in mouse macropage RAW 264.7 cells.
(A) Knockdown of ELAVL1 displays diminished expression of caspase-1 in RAW 264.7 cells. Induction with inflammatory agent TNF-α did not show any further induction in KD cells, whereas induction was at significant level in non-canonical inflammasome pathway with LPS treatment. Western blot analysis shows the knockdown efficiency of ELAVL1 and it further describes TNF-α and LPS induced expression of NLRP3, IL-1β and GAPDH in control and ELAVL1 KD RAW 264.7 cells. (B) Immunofluorescence images showing expression of caspase-1 in control and ELAVL1 KD RAW 264.7 cells (40×). Scale bar: 100µm. n=3.
Next, we sought to identify the downstream signaling mechanisms through which ELAVL1 mediates pyroptosis. At baseline, Nod-like receptor pyrin domain containing 3 (NLRP3) and IL-1 beta expression was lower in ELAVL1 knockdown cells as compared to control-shRNA cells (Fig 3A). TNF-α and LPS treatment stimulates NLRP3 and IL-1beta expression as compared to untreated cells. Interestingly, TNF-α failed to induce NLRP3 and IL-1β expression in shRNA-ELAVL1 cells as compared to control-shRNA cells (Fig 3A). Furthermore, ELAVL1 knockdown did not affect NLRP3 and IL-1 beta expression in LPS-treated cells. Taken together, these data suggest that LPS (noncanonical) -induced pyroptosis is ELAVL1 independent and TNF-α (canonical) -induced pyroptosis is ELAVL1 dependent.
3.4. miR-9 regulates ELAVL1 expression in human ventricular cardiomyocytes
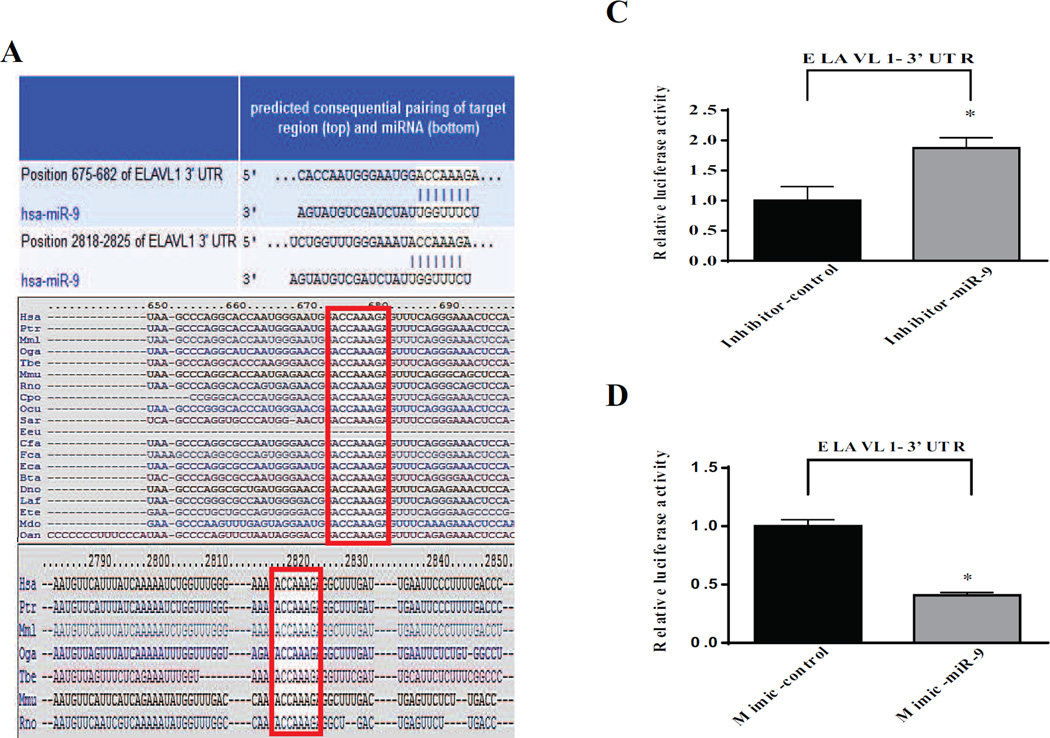
MicroRNAs (miRNAs) play key regulatory role in diverse biological process during development, physiology and disease, including HF [16]. miRNA target prediction analysis identified that ELAVL1 harbors complementary binding sites for several miRNAs including miRNA-9 (Position 675–682 and 2818–2825). Furthermore, computational analysis predicted that miR-9 has two conserved binding sites in the 3’-UTR of ELAVL1 (Fig 4A). Also, MiR-9 has been implicated in inflammation, fibrosis, insulin secretion and neuronal cell death suggesting its potential role in pathogenesis of cardiac disease process under metabolic stress [17–21].
Figure 4. Validation of ELAVL1 as a direct target of miR-9.
(A) Computational miR target prediction analysis shows that ELAVL1’s 3’UTR has complementary sequences for miR-9 at two different positions and highly conserved (white fonts) in most mammals. (B &C) Quantitative analysis dual-luciferase reporter assay. *p < 0.05, inhibitor-miR-9 versus inhibitor-control *p < 0.05; mimic-miR-9 versus mimic-control, n=3. (D) Expression of miR-9 downregulated in diabetic failing human heart. (E) Expression of ELAVL1, caspase-1 and IL-1β in human cardiomyocytes transfected with inhibitor-miR-9 or inhibitor-control. Expression of ELAVL1, caspase-1 and IL-1β in human cardiomyocytes transfected with mimic-miR-9 or respective negative control.
To further validate that ELAVL1 3’-UTR is a direct target of miR-9, human cardiomyocytes was co-transfected with dual luciferase reporter vector with ELAVL1 3-UTR along with transfection either with miR-9 inhibitor or miR-9 mimic or their respective negative controls. Inhibition of miR-9 significantly increased luciferase activity when compared to human cardiomyocytes transfected with miRNA inhibitor negative control transfected cells (Fig 4B). In contrast, miR-9 mimic transfected cells showed decrease in luciferase activity compared with miR mimic negative control transfected human cardiomyocytes (Fig 4C). These data evidently supports that miR-9 directly regulates ELAVL1.
3.5. Down-regulation of miR-9 in human diabetic failing heart
We further evaluated the effect of diabetes on miRNA-9 expression in human heart tissues. Diabetic heart tissues with increased caspase-1 expression (Fig 1A) was associated with significant down regulation of miR-9 expression as compared to non-diabetic healthy heart tissues (Fig 4D).
3.6. MiR-9 regulates pyroptosis signaling mechanism in cardiomyocytes
Next, we evaluated the effect of miRNA-9 loss- and gain- of function on ELAVL1 expression. Human ventricular cardiomyocytes transfected with miR-9 inhibitor significantly increases ELAVL1 expression when compared to cell transfected with appropriate mimic control (Fig 4E). On the contrary, miR-9 mimic shows a significant decrease in ELAVL1 expression (Fig 4E).
Furthermore, to determine whether transient inhibition or overexpression of miR-9 regulates pyroptosis, we blocked miR-9 expression and determined its effect on caspase-1 and IL-1β expression. Human cardiomyocytes transfected with miR9 inhibitor showed an increase in the expression of caspase-1 and IL-1β as compared to inhibitor control transfected cells (Fig 4E). Conversely, expression levels of these proteins were down regulated in cardiomyocytes transfected with miR-9 mimics (Fig 4E).
4. Discussion
DCM is characterized by cardiomyocyte cell death leading to left ventricular dysfunction and HF. Pyroptosis is caspase-1 dependent programmed cell death that features plasma membrane rupture and release of proinflammatory intracellular contents (especially IL-1beta) and leads to cell death. Given the importance of pyroptosis in various pathophysiological conditions, it is essential to understand its molecular mechanisms. Nevertheless, the mechanism of pyroptosis in cardiomyocyte and more specifically under diabetic conditions remains elusive. Important novel findings in the present study includes: 1) human diabetic heart shows increased NLRP3 and caspase-1 expression and decrease in miR-9 and a corresponding increase in ELAVL1 expression; 2) in vitro, human ventricular cardiomyocytes exposed to HG show similar changes observed in human diabetic heart tissue; 3) canonical inflammasome activation induces caspase-1 and IL-1beta expression, which is ELAVL1-dependent, whereas noncanonical inflammasome activation and pyroptosis is independent of ELAVL1 and 4) for the first time we demonstrate that miR-9 mimic transfection or ELAVL1 knockdown inhibits pyroptosis. Our findings unravel new signaling mechanisms involved in hyperglycemia-induced cardiomyocyte cell death that potentially contributes to the pathophysiological of DCM.
HG-induced cardiomyocyte cell death causes loss of myocardial function leading to HF. Caspase-1 knockout mice show a lesser degree of cell death following myocardial infarction suggesting its role in cardiomyocyte death and therefore cardiovascular diseases [6]. In agreement with the above study, immunohistochemical analysis of human diabetic heart reveals an increase in caspase-1 expression. Furthermore, there was a corresponding increase in ELAVL1 and NLRP3 mRNA expression. Thus, it is conceivable that increased levels of caspase-1 and NLRP3 displayed the pivotal characteristics of cardiac pyroptosis in human diabetic heart. Intriguingly, human cardiomyocytes exposed to hyperglycemic conditions in vitro show similar results. IL-1β is a vital substrate of caspase-1 and both the molecules acts synergistically to orchestrate inflammation induced pyroptosis [22]. Together, our data suggests that diabetes induces caspase-1 dependent pyroptosis, which might potentially modulate the pathogenesis of DCM.
Next, we sought to elucidate the role of ELAVL1 in HG-induced cardiomyocyte pyroptosis. Previous studies from our lab has shown that ELAVL1, an mRNA-stabilizing protein, binds to AU-rich elements (ARE) in the 3’-UTR of various inflammatory cytokines including TNF-α and plays a key role in the progression of post-MI inflammatory response [13, 23, 24]. Previous reports have suggested two distinct pathways in inflammation-induced pyroptosis: (1) non-canonical inflammatory pathway triggered by bacterial LPS and (2) canonical pathway initiated by inflammatory agents like TNF-α [25]. ELAVL1 knockdown in mouse macrophages inhibits TNF-α induced caspase-1, NLRP3 and IL1-β expression, whereas LPS-induced expression of these proteins is not affected by ELAVL1 knockdown. These data suggest that TNF-α induced pyroptosis is ELAVL1-dependent but LPS-induced pyroptosis is ELAVL1-independent. NLRP3, a key member of inflammasome is critical for the activation of caspase-1 and secretion of IL-1β in response to inflammatory stimulants [26]. HG treated cardiomyocytes showed increase in ELAVL1 expression with a corresponding increase in NLRP3 expression. Furthermore, ELAVL1 knockdown significantly reduces NLRP3 expression. These findings suggest the possible involvement of NLRP3 in ELAVL1 regulated caspase-1 and IL-1beta expression and therefore pyroptosis.
Emerging studies have demonstrated that miRNAs are critical mediators of gene regulation and influences a wide variety of biological functions [27]. Aberrant expression of miRNAs plays crucial role in the pathogenesis of various diseases, including DCM [28]. MiR-9 is a highly conserved miRNA across species and is encoded by three distinct genomic loci miR-9-1, 9-2 and 9-3 that give rise to mature miR-9 species with identical sequences. Previous studies in inflammation revealed the importance of miR-9 in LPS mediated release of proinflammatory cytokines, and its progressive role in cancer metastasis [29–31]. Recent studies have implicated MiR-9 in cardiac hypertrophy [32, 33]. However, the role of miR-9 in DCM or HG induced inflammation and cardiac pyroptosis and its effect on cardiovascular diseases is very limited. The present study shows that miR-9 is down-regulated in human diabetic heart with a corresponding increase in ELAVL1 expression. Bioinformatics analysis reveals that ELAVL1 is a potential target for miR-9, which binds putatively at two different sites (position 675–682 and position 2818–2825). Dual luciferase reporter assay validates that ELAVL1 is a direct target of miR-9 in cardiomyocytes. Furthermore, our studies demonstrate that inhibition of miR-9 results in an elevation of ELAVL1 protein expression along with caspase-1 and IL-1β expression, while miR-9 mimic transfection reduces ELAVL1, caspase-1 and IL-1β expression. Taken together, these findings identify ELAVL1 as a direct target of miR-9 and suggest that modulating miR-9 expression serves as a novel therapeutic option for regulating ELAVL1-mediated disease processes, including diabetes-induced cardiomyocyte cell death and inflammation and cancer.
Supplementary Material
Highlights.
Human diabetic heart shows increased ELAVL1, pyroptosis and decreased miR-9.
Human cardiomyocytes exposed to HG show similar changes.
KD of ELAVL1 impairs canonical inflammatory pathway associated pyroptosis.
miR-9 directly targets ELAVL1.
miR-9 mimic transfection or ELAVL1 knockdown inhibits pyroptosis.
Acknowledgments
This work was supported, in part, by the National Institutes of Health (NIH) grants 1R01HL116729 (to P.K.); HL091983, HL053354, HL108795 and HL108806 (to R.K.), American Heart Association Grant-in-aid GRNT 25860041 (to P.K.), American Heart Association Postdoctoral Fellowship 15POST25710392 (to R.A.T). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interests
The authors declare that they have no conflict of interests.
References
- 1.Voulgari C, Papadogiannis D, Tentolouris N. Diabetic cardiomyopathy: from the pathophysiology of the cardiac myocytes to current diagnosis and management strategies. Vascular health and risk management. 2010;6:883–903. doi: 10.2147/VHRM.S11681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Litwin SE. Diabetes and the heart: is there objective evidence of a human diabetic cardiomyopathy? Diabetes. 2013;62:3329–3330. doi: 10.2337/db13-0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaul K, Hodgkinson A, Tarr JM, Kohner EM, Chibber R. Is inflammation a common retinal-renal-nerve pathogenic link in diabetes? Current diabetes reviews. 2010;6:294–303. doi: 10.2174/157339910793360851. [DOI] [PubMed] [Google Scholar]
- 4.Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infection and immunity. 2005;73:1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrne BG, Dubuisson JF, Joshi AD, Persson JJ, Swanson MS. Inflammasome components coordinate autophagy and pyroptosis as macrophage responses to infection. mBio. 2013;4:e00620–e00612. doi: 10.1128/mBio.00620-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merkle S, Frantz S, Schon MP, Bauersachs J, Buitrago M, Frost RJ, Schmitteckert EM, Lohse MJ, Engelhardt S. A role for caspase-1 in heart failure. Circulation research. 2007;100:645–653. doi: 10.1161/01.RES.0000260203.55077.61. [DOI] [PubMed] [Google Scholar]
- 7.Yang B, Lin H, Xiao J, Lu Y, Luo X, Li B, Zhang Y, Xu C, Bai Y, Wang H, Chen G, Wang Z. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nature medicine. 2007;13:486–491. doi: 10.1038/nm1569. [DOI] [PubMed] [Google Scholar]
- 8.Chen Q, Xu J, Li L, Li H, Mao S, Zhang F, Zen K, Zhang CY, Zhang Q. MicroRNA-23a/b and microRNA-27a/b suppress Apaf-1 protein and alleviate hypoxia-induced neuronal apoptosis. Cell death & disease. 2014;5:e1132. doi: 10.1038/cddis.2014.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan Z, Sun X, Shan H, Wang N, Wang J, Ren J, Feng S, Xie L, Lu C, Yuan Y, Zhang Y, Wang Y, Lu Y, Yang B. MicroRNA-101 inhibited postinfarct cardiac fibrosis and improved left ventricular compliance via the FBJ osteosarcoma oncogene/transforming growth factor-beta1 pathway. Circulation. 2012;126:840–850. doi: 10.1161/CIRCULATIONAHA.112.094524. [DOI] [PubMed] [Google Scholar]
- 10.Joladarashi D, Srikanth Garikipati VN, Thandavarayan RA, Verma SK, Mackie AR, Khan M, Gumpert AM, Bhimaraj A, Youker KA, Uribe C, Suresh Babu S, Jeyabal P, Kishore R, Krishnamurthy P. Enhanced Cardiac Regenerative Ability of Stem Cells After Ischemia-Reperfusion Injury: Role of Human CD34(+) Cells Deficient in MicroRNA-377. Journal of the American College of Cardiology. 2015;66:2214–2226. doi: 10.1016/j.jacc.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciafre SA, Galardi S. microRNAs and RNA-binding proteins: a complex network of interactions and reciprocal regulations in cancer. RNA Biol. 2013;10:935–942. doi: 10.4161/rna.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdelmohsen K, Lal A, Kim HH, Gorospe M. Posttranscriptional orchestration of an anti-apoptotic program by HuR. Cell cycle. 2007;6:1288–1292. doi: 10.4161/cc.6.11.4299. [DOI] [PubMed] [Google Scholar]
- 13.Krishnamurthy P, Lambers E, Verma S, Thorne T, Qin G, Losordo DW, Kishore R. Myocardial knockdown of mRNA-stabilizing protein HuR attenuates post-MI inflammatory response and left ventricular dysfunction in IL-10-null mice. FASEB J. 2010;24:2484–2494. doi: 10.1096/fj.09-149815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burchfield JS, Xie M, Hill JA. Pathological ventricular remodeling: mechanisms: part 1 of 2. Circulation. 2013;128:388–400. doi: 10.1161/CIRCULATIONAHA.113.001878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poornima IG, Parikh P, Shannon RP. Diabetic cardiomyopathy: the search for a unifying hypothesis. Circulation research. 2006;98:596–605. doi: 10.1161/01.RES.0000207406.94146.c2. [DOI] [PubMed] [Google Scholar]
- 16.Joladarashi D, Thandavarayan RA, Babu SS, Krishnamurthy P. Small engine, big power: microRNAs as regulators of cardiac diseases and regeneration. International journal of molecular sciences. 2014;15:15891–15911. doi: 10.3390/ijms150915891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramachandran D, Roy U, Garg S, Ghosh S, Pathak S, Kolthur-Seetharam U. Sirt1 and mir-9 expression is regulated during glucose-stimulated insulin secretion in pancreatic beta-islets. The FEBS journal. 2011;278:1167–1174. doi: 10.1111/j.1742-4658.2011.08042.x. [DOI] [PubMed] [Google Scholar]
- 18.Sangiao-Alvarellos S, Pena-Bello L, Manfredi-Lozano M, Tena-Sempere M, Cordido F. Perturbation of hypothalamic microRNA expression patterns in male rats after metabolic distress: impact of obesity and conditions of negative energy balance. Endocrinology. 2014;155:1838–1850. doi: 10.1210/en.2013-1770. [DOI] [PubMed] [Google Scholar]
- 19.Fierro-Fernandez M, Busnadiego O, Sandoval P, Espinosa-Diez C, Blanco-Ruiz E, Rodriguez M, Pian H, Ramos R, Lopez-Cabrera M, Garcia-Bermejo ML, Lamas S. miR-9-5p suppresses pro-fibrogenic transformation of fibroblasts and prevents organ fibrosis by targeting NOX4 and TGFBR2. EMBO reports. 2015;16:1358–1377. doi: 10.15252/embr.201540750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chakraborty S, Zawieja DC, Davis MJ, Muthuchamy M. MicroRNA signature of inflamed lymphatic endothelium and role of miR-9 in lymphangiogenesis and inflammation. American journal of physiology. Cell physiology. 2015;309:C680–C692. doi: 10.1152/ajpcell.00122.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei N, Xiao L, Xue R, Zhang D, Zhou J, Ren H, Guo S, Xu J. MicroRNA-9 Mediates the Cell Apoptosis by Targeting Bcl2l11 in Ischemic Stroke. Molecular neurobiology. 2015 doi: 10.1007/s12035-015-9605-4. [DOI] [PubMed] [Google Scholar]
- 22.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nature reviews. Microbiology. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krishnamurthy P, Rajasingh J, Lambers E, Qin G, Losordo DW, Kishore R. IL-10 inhibits inflammation and attenuates left ventricular remodeling after myocardial infarction via activation of STAT3 and suppression of HuR. Circulation research. 2009;104:e9–e18. doi: 10.1161/CIRCRESAHA.108.188243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajasingh J, Bord E, Luedemann C, Asai J, Hamada H, Thorne T, Qin G, Goukassian D, Zhu Y, Losordo DW, Kishore R. IL-10-induced TNF-alpha mRNA destabilization is mediated via IL-10 suppression of p38 MAP kinase activation and inhibition of HuR expression. FASEB J. 2006;20:2112–2114. doi: 10.1096/fj.06-6084fje. [DOI] [PubMed] [Google Scholar]
- 25.Broz P. Immunology: Caspase target drives pyroptosis. Nature. 2015 doi: 10.1038/nature15632. [DOI] [PubMed] [Google Scholar]
- 26.Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nature immunology. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vidigal JA, Ventura A. The biological functions of miRNAs: lessons from in vivo studies. Trends in cell biology. 2015;25:137–147. doi: 10.1016/j.tcb.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tyagi AC, Sen U, Mishra PK. Synergy of microRNA and stem cell: a novel therapeutic approach for diabetes mellitus and cardiovascular diseases. Current diabetes reviews. 2011;7:367–376. doi: 10.2174/157339911797579179. [DOI] [PubMed] [Google Scholar]
- 29.Ma L, Young J, Prabhala H, Pan E, Mestdagh P, Muth D, Teruya-Feldstein J, Reinhardt F, Onder TT, Valastyan S, Westermann F, Speleman F, Vandesompele J, Weinberg RA. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nature cell biology. 2010;12:247–256. doi: 10.1038/ncb2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang L, Chao J, Kook YH, Gao Y, Yao H, Buch SJ. Involvement of miR-9/MCPIP1 axis in PDGF-BB-mediated neurogenesis in neuronal progenitor cells. Cell death & disease. 2013;4:e960. doi: 10.1038/cddis.2013.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao H, Ma R, Yang L, Hu G, Chen X, Duan M, Kook Y, Niu F, Liao K, Fu M, Hu G, Kolattukudy P, Buch S. MiR-9 promotes microglial activation by targeting MCPIP1. Nature communications. 2014;5:4386. doi: 10.1038/ncomms5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J, Chintalgattu V, Shih T, Ai D, Xia Y, Khakoo AY. MicroRNA-9 is an activation-induced regulator of PDGFR-beta expression in cardiomyocytes. Journal of molecular and cellular cardiology. 2011;51:337–346. doi: 10.1016/j.yjmcc.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 33.Wang K, Long B, Zhou J, Li PF. miR-9 and NFATc3 regulate myocardin in cardiac hypertrophy. The Journal of biological chemistry. 2010;285:11903–11912. doi: 10.1074/jbc.M109.098004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.