Abstract
Toxin-producing blooms of dinoflagellates in the genus Alexandrium have plagued the inhabitants of the Salish Sea for centuries. Yet the environmental conditions that promote accelerated growth of this organism, a producer of paralytic shellfish toxins, is lacking. This study quantitatively determined the growth response of two Alexandrium isolates to a range of temperatures and salinities, factors that will strongly respond to future climate change scenarios. An empirical equation, derived from observed growth rates describing the temperature and salinity dependence of growth, was used to hindcast bloom risk. Hindcasting was achieved by comparing predicted growth rates, calculated from in situ temperature and salinity data from Quartermaster Harbor, with corresponding Alexandrium cell counts and shellfish toxin data. The greatest bloom risk, defined at μ>0.25 d−1, generally occurred from April through November annually; however, growth rates rarely fell below 0.10 d−1. Except for a few occasions, Alexandrium cells were only observed during the periods of highest bloom risk and paralytic shellfish toxins above the regulatory limit always fell within the periods of predicted bloom occurrence. While acknowledging that Alexandrium growth rates are affected by other abiotic and biotic factors, such as grazing pressure and nutrient availability, the use of this empirical growth function to predict higher risk time frames for blooms and toxic shellfish within the Salish Sea provides the groundwork for a more comprehensive biological model of Alexandrium bloom dynamics in the region and will enhance our ability to forecast blooms in the Salish Sea under future climate change scenarios.
Key index words: Alexandrium, harmful algae, growth rate, temperature, salinity, Salish Sea, Puget Sound, HAB, hindcast
INTRODUCTION
Dinoflagellates in the genus Alexandrium (Halim) produce a suite of potent neurotoxins, collectively called paralytic shellfish toxins (PSTs). Saxitoxin (STX) is the most potent PST and can accumulate in shellfish to levels that are unsafe for human consumption (>80 µg STX equivalents 100 · g−1 shellfish tissue). If contaminated shellfish are consumed, an illness called paralytic shellfish poisoning (PSP) can result. Gastrointestinal and neurological symptoms of PSP include vomiting and muscle paralysis, with death occurring in extreme cases (Quayle 1969, Kao 1993).
The Salish Sea is a coastal waterway that spans the U.S. State of Washington and British Columbia, Canada, and includes Puget Sound, the Strait of Juan de Fuca, and the Strait of Georgia. The earliest documented cases of PSP in the Salish Sea are from 1793 when four people were sickened (one fatally) after consuming mussels during the exploratory voyage of Captain George Vancouver (Vancouver 1798). Today, toxic blooms of Alexandrium occur regularly in the Salish Sea and elsewhere around the world (summarized in Anderson et al. 2012), resulting in numerous and widespread annual shellfish harvesting closures due to unsafe levels of PSTs accumulating in shellfish tissues. The shellfish industry in Puget Sound, WA is valued at over $50 million annually (based on 2008 and 2009 data compiled by the Pacific Coast Shellfish Growers Association). Even though shellfish are routinely monitored for biotoxins and commercial and recreational shellfish harvesting areas are closed when regulatory limits for human consumption are exceeded, illnesses have occurred, in particular when warnings were ignored or misinterpreted. For example, nine cases of PSP were reported in 2012 when people had harvested and consumed mussels from areas in the Salish Sea that were closed to recreational harvest (PSEMP 2013).
The species of Alexandrium responsible for toxic blooms in the Salish Sea historically has been identified as Alexandrium catenella (Whedon & Kofoid) Balech. This is synonymous with A. tamarense (Lebour) Balech Group I, a provisional species name proposed by Lilly et al. (2007). However, the name A. fundyense (Balech) has recently been proposed to replace all Group I strains of the A. tamarense species complex (John et al. 2014). In light of this recent work and recognizing alternative recommendations from other taxonomists (Wang et al. 2014), we will refer here only to the genus name, Alexandrium.
Much of our understanding of Alexandrium bloom ecology in the Salish Sea has been inferred from extensive shellfish toxicity records. The data from Washington State reveal an apparent increase in the frequency, duration, and geographic scope of Alexandrium blooms in Puget Sound since the 1950s (Rensel 1993, Trainer et al. 2003). The shellfish toxicity records also indicate that the typical “bloom season” for Alexandrium in Puget Sound is between July and November annually, but with high interannual variability (Moore et al. 2009). However, the relationship between shellfish toxicity levels and the abundance of vegetative cells of Alexandrium in Salish Sea waters is complex. Concurrent measurements during the 2006 bloom season found that an increase in shellfish toxicity was always preceded by an increase in cell numbers, but an increase in cell numbers did not always precede an increase in shellfish toxicity (Dyhrman et al. 2010). An improved understanding of the factors that govern the growth and toxicity of Alexandrium in the Salish Sea is required to anticipate changes in bloom abundance, frequency and spatial extent that will occur due to large scale climate pressures, thereby better understanding the risk to human health.
The two studies that have directly examined the effect of environmental factors on Alexandrium growth in the Salish Sea found that a temperature threshold of 13°C exists (Nishitani and Chew 1984), and optimal temperature and salinity ranges for growth were between 13–17°C temperature and 15–40 salinity (Norris and Chew 1975). These optimal ranges for growth were determined qualitatively by observing whether the cultures grew (defined simply by cell numbers at least doubling compared to the initial level), died, or exhibited no growth (Norris and Chew 1975). Quantitative growth responses to a wider range of temperature and salinity conditions that cells may experience are needed to better understand the present and future ecology and toxicity of Alexandrium in the Salish Sea.
Previous work has suggested that regional climate variability may contribute to the interannual differences in Alexandrium abundance and long-term increase in shellfish toxicity in the Salish Sea (Moore et al. 2010). Because sea surface temperature appeared to be a strong driver of historical toxic PST events (Moore et al. 2011), it is anticipated to be a key factor in regulating future bloom intensity. In an effort to build upon this knowledge and to assist with modeling the potential growth response of Alexandrium to future climate-driven changes in the Salish Sea, the present study provides experimentally-derived growth responses to temperature and salinity, two significant environmental drivers of phytoplankton responses. Other drivers of Alexandrium bloom dynamics are not considered here, and these may also be sensitive to climate change. These drivers are acknowledged in Moore et al. (2015) for Salish Sea Alexandrium blooms and reviewed by Wells et al. (2015) for harmful algal blooms (HABs) in general. Nevertheless, the occurrence of a temperature and salinity window in space and time that is favorable for Alexandrium growth is fundamental to mapping bloom risk, thereby mitigating the impacts of this organism in the Salish Sea and beyond.
MATERIALS AND METHODS
Isolates and culturing
Two strains of Alexandrium were isolated from the Salish Sea during bloom conditions in 2010. Alexandrium isolate NWFSC 439 was isolated from Guemes Channel (48° 31.218’N, 122° 39.677’W) in the central Salish Sea in July 2010 and isolate NWFSC 445 was isolated from Quartermaster Harbor (47° 22.361’N, 122° 27.325’W) in the southern Salish Sea in October 2010 (Fig. 1). An individual chain of cells was isolated and picked via a flame-drawn capillary tube and aseptically transferred to growth media. Cultures were initially grown in nutrient enriched, filter sterilized (0.2 µm, PES, Nalgene) Salish Sea seawater following Berges et al. (2001) and subsequent Corrigendum (2004), with the following modifications. Metals Stock I: FeCl3•6H2O, 1.772 g · L−1, Na2-EDTA, 2.442 g · L−1; Metals Stock II: Na2-EDTA, 3.086 g · L−1, MnSO4•H2O, 0.409 g · L−1. Selenium (Na2SeO3, 0.002 g · L−1) was prepared separately from the other metal stocks and added to achieve the final concentration of 5.8 × 10−9 M. Copper (CuSO4•5H2O, 0.010 g · L−1) was prepared separately from the other metal stocks, and added to achieve the final concentration of 4 × 10−9 M. Vitamin enrichments were unchanged, except that vitamin B12 was doubled in strength to 0.002 g · L−1 stock solution. Silicic acid was not added. Nitrate (NaNO3) and phosphate (Na2HPO4) were added to achieve final concentrations of 300 and 30 µM, respectively. Cultures were maintained in an environmental incubator at 12°C on a 14:10 h light:dark cycle and illuminated by soft-white fluorescent bulbs at an approximate photosynthetic photon flux density (PPFD) of 80–100 µmol photons · m−2 · s−1 until the experiments were initiated in December 2011. PPFD was measured using a 4π collector (Biospherical Instruments QSL-100 quantum scalar irradiance meter).
Fig. 1.
Map of the central and southern Salish Sea indicating the locations where isolates NWFSC 439 (Guemes Channel) and NWFSC 445 (Quartermaster Harbor) were collected.
Temperature gradient bar
Experiments were conducted using a temperature gradient bar (TGB) enclosed within a controlled light box modified from Watras et al. (1982). The TGB maintains a stable and uniformly distributed temperature gradient in an insulated aluminum bar (48" L × 9" W × 2" D). The warm end of the bar is heated by a 400W cartridge heater set to 30°C and the cool end is chilled by a refrigerated circulating bath containing water and antifreeze set to 0°C. Nineteen columns of 1" diameter holes were drilled along the length of the bar, each holding a 50 mL borosilicate glass culture tube (Kimble-Chase, Rochester, NY). Each column held six culture tubes at each of the experimental temperatures ranging from 4.5–27.8°C at ~1°C increments. A bank of six soft-white fluorescent bulbs (Plusrite, Ontario, CA), each lined up underneath the six rows of holes containing culture tubes to minimize variations, provided light from below on a 14:10 h light:dark cycle. The fluorescent bulbs were housed in a separate box compartment with a 1" thick Plexiglas top and cooled from one end with a 6" box fan. The PPFD measured in the culture tubes filled with growth medium during the light cycle ranged from 173–328 µmol photons · m−2 · s−1. These light intensities fall well within the range of intensities found within the photic zone in the Salish Sea (Fig. S1 in the Supporting Information).
Both Alexandrium isolates were tested in duplicate at each experimental temperature and salinity (10, 15, 20, 25, 30, and 35) in order to determine the growth response. The two isolates were acclimated from the maintenance conditions to approximate experimental conditions for 2 to 3 weeks before the experiments were conducted. Acclimating cells to each experimental temperature (19 total) was not practical so we instead acclimated 50 mL aliquots of each isolate to three temperatures along the gradient (7.4, 11.9, and 19.9°C) to minimize temperature shock. Cell acclimation at 10 salinity was also not feasible as cells did not accumulate enough biomass at that salinity, therefore cells acclimated at 15 salinity were used for the treatments at 10 salinity. Cell counts were performed after 2 to 3 weeks of growth at these acclimation temperatures and salinities. The acclimated cultures were distributed into fresh nutrient enriched seawater media in 50 mL glass culture tubes at initial concentrations of ~100 cells · mL−1. The nutrient enriched seawater used for the treatments was the same as that used to maintain the cultures except that nitrate and phosphate were reduced to initial concentrations of 200 and 20 µM, respectively. The total volume of culture and media in each tube was 30 mL.
Growth rate calculations
Growth was monitored daily using a model 10-AU fluorometer (Turner Designs, Sunnyvale, CA) by recording the in vivo fluorescence as relative fluorescence units (RFU) for each culture tube, and tubes were rotated daily within their temperature treatment column. Growth rates were determined during the exponential phase following the method of Brand et al. (1981). Maximum specific growth rates determined using this method compare well to growth rates determined using cell counts (Brand et al. 1981). For example, we determined that the maximum specific growth rates for a Salish Sea strain of Alexandrium calculated using the two methods differed by only 0.01 d−1 (culture conditions 14°C, 30 salinity, 14:10 h light:dark cycle, ~100 µmol photons · m−2 · s−1; results not shown). Water temperature was monitored every other day to ensure that the temperature gradient remained stable for the duration of all four experimental runs. Tubes were monitored for cell growth until two to three days into stationary growth. At that time, the contents of the tubes were harvested and replaced with 30 mL of water and returned to the TGB to maintain the constant temperature gradient for the remaining tubes along the bar. Specific growth rates (μ) were calculated from least-squares linear regression analyses of the exponential growth phase, determined from semi-log plots of RFU values (Guillard 1973).
RESULTS
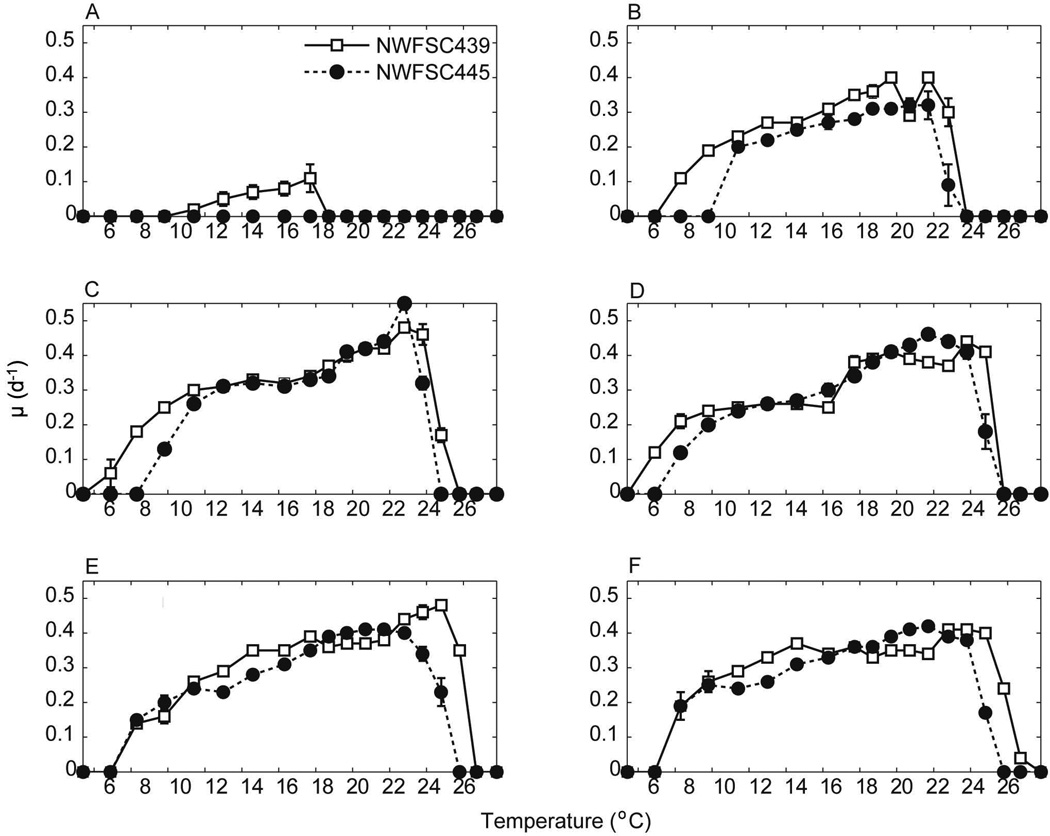
Both isolates of Alexandrium grew optimally (>0.25 d−1) over a wide range of experimental temperatures (~10–24°C) and salinities (15–35; Fig. 2). The maximum average specific growth rate was 0.52 d−1. Growth of both isolates was inhibited at temperatures below ~7.7°C and above ~24°C, and at 10 salinity, where isolate NWFSC 439 achieved a maximum growth rate of only ~0.10 d−1 and isolate NWFSC 445 exhibited no growth. At salinities ranging from 15–35, both isolates achieved maximum growth rates of >0.30 d−1. Above ~10°C, the growth response became less sensitive to temperature variations, exhibiting a broad range of temperatures (~10–24°C) supporting optimal growth (>0.25 d−1). Above ~24°C (depending on salinity and isolate) growth rates dropped abruptly to near zero.
Fig. 2.
Average Alexandrium specific growth rates (μ, d−1) for each isolate shown at a range of temperatures (°C) and salinities of (A) 10, (B) 15, (C) 20, (D) 25, (E) 30, and (F) 35. The mean value of replicate (n=2) growth tubes is shown with error bars indicating the range. Error bars that are not visible fall within the size of the symbol.
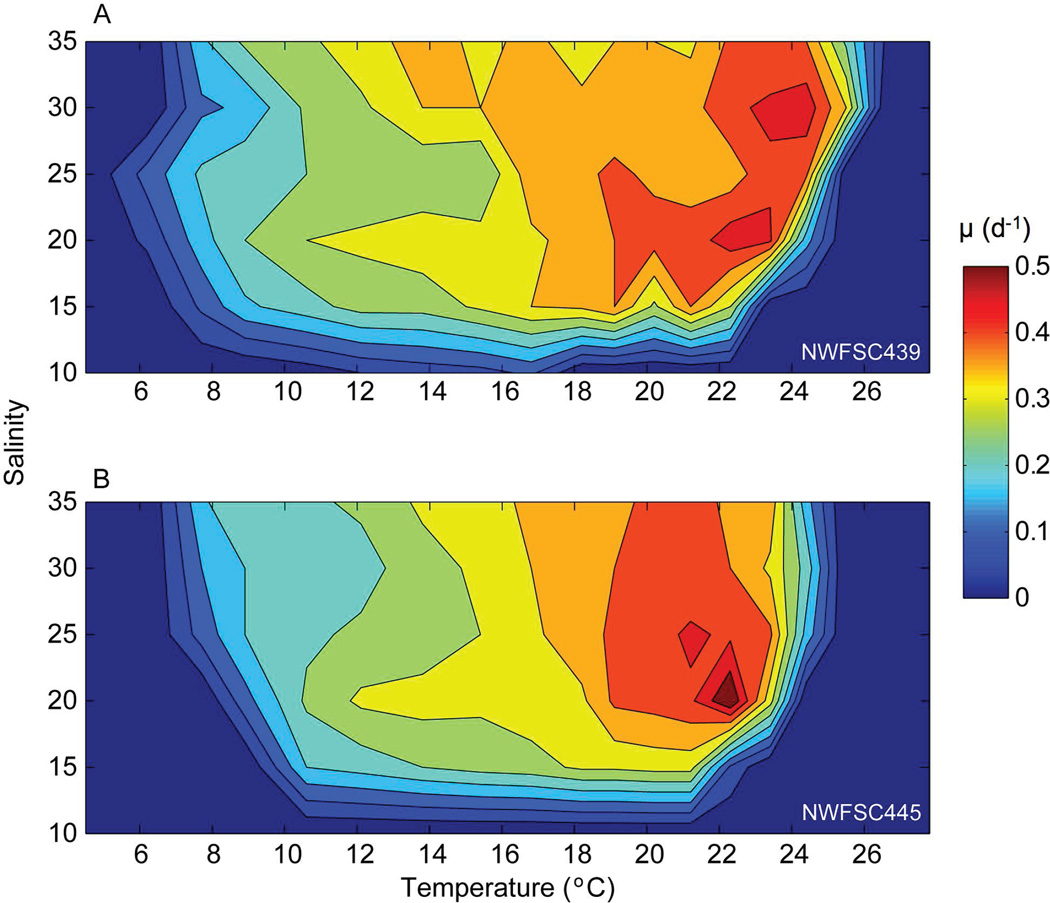
The growth rates observed for both isolates of Alexandrium, are shown as contour maps (Fig. 3), where darker colors indicate faster growth. The highest growth rates (>0.35 d−1) for NWFSC 439 fell within a range of salinity from 15–35 and temperature ~17–24°C, while the higher growth rates for NWFSC 445 fell within a range of salinity from 20–35 and temperature ~18–23°C. Overall, NWFSC 445 showed a slightly narrower range of temperature for optimal growth rates (11–23°C) than NWFSC 439 (9–25°C).
Fig. 3.
Contoured values of Alexandrium specific growth rate (μ, d−1) in response to temperature (°C) and salinity for (A) NWFSC 439 and (B) NWFSC 445.
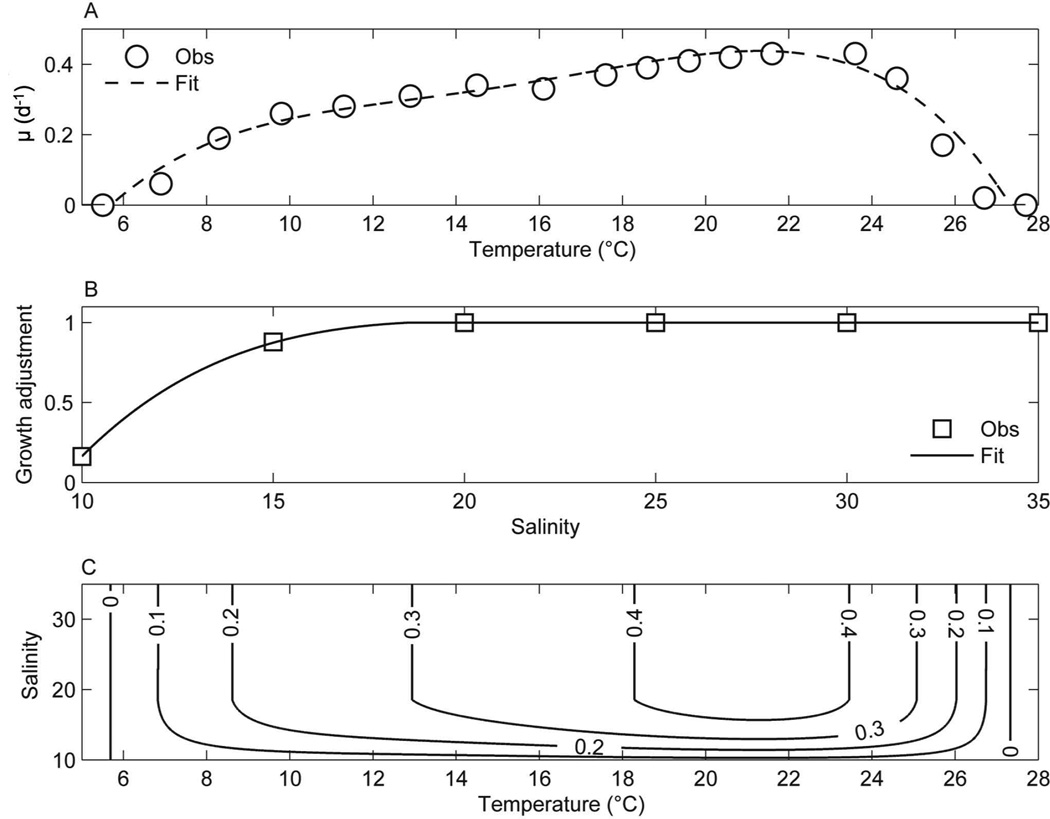
The growth rate data of both isolates of Alexandrium were combined and averaged for each temperature and salinity treatment. The temperature dependence of growth (μmax) was determined by fitting a polynomial function to the averaged Alexandrium growth data for a salinity (i.e., 20) that supported optimal growth (Equation 1; Fig. 4a). This modeled temperature response was then adjusted for the effect of salinity, with the salinity dependence determined in a similar way (μadj; Equation 2; Fig. 4b). The resulting growth function is given in Equation 3 and the modeled growth response is shown in Figure 4c.
| (1) |
| (2) |
| (3) |
Fig. 4.
Polynomial functions approximating the (A) temperature and (B) salinity dependence of Alexandrium growth. The curves are fitted (Fit) to the observed (Obs) growth rates using fourth order polynomial functions. The salinity dependence of growth is used to adjust the temperature response to approximate (C) Alexandrium growth rate (d−1; see Equations 1–3 in text).
The polynomial coefficient values for the equations describing the temperature and salinity dependence of growth (i.e., pT1, pT2, etc.) are given in Table 1. The resulting values for growth are sensitive to the number of significant figures of the coefficients for the polynomial functions.
Table 1.
Polynomial coefficients for the equations estimating the Alexandrium growth response to temperature and salinity (Equations 1 and 2).
| Coefficients of the Temperature Polynomial | Coefficients of the Salinity Polynomial |
|---|---|
| pT1 = −3.102539342286946 × 10−5 | pS1 = −1.580671580671645 × 10−5 |
| pT2 = 1.874778556588596 × 10−3 | pS2 = 1.670064003397399 × 10−3 |
| pT3 = −4.159219720816749 × 10−2 | pS3 = −6.494062244062465 × 10−2 |
| pT4 = 4.192058763875539 × 10−1 | pS4 = 1.100886167552868 |
| pT5 = −1.352433098030715 | pS5 = −5.863714363714551 |
DISCUSSION
Salish Sea Alexandrium are euryhaline and grow over a wide range of temperatures. The lower salinity tolerance was 15 and the temperature tolerance range was ~7–25°C. Optimal growth occurred between ~10–24°C. Higher salinity dependent growth rates have been observed for A. tamarense strains at 20–25 salinity (Watras et al. 1982, Fauchot et al. 2005), but this pattern did not hold for Salish Sea Alexandrium which showed insensitivity to salinity changes over 20 (Fig. 4b). Prior work showed the tolerance ranges of Salish Sea Alexandrium to be from 15 to at least 40 salinity and 8–23°C temperature, with optimal growth occurring between 15–35 salinity and 13–20°C (Norris and Chew 1975). The optimal range of salinities identified by Norris and Chew (1975) is in agreement with our study; however, we identified a broader range of optimal temperatures for growth. Norris and Chew (1975) did not specify whether the Alexandrium cells were acclimated to the experimental conditions. If no acclimation took place, cells may have experienced shock when suddenly exposed to a different temperature, potentially resulting in a narrower range of temperature conditions identified as optimal. However, the difference in optimal temperature ranges reported in Norris and Chew (1975) and the present study may also be a consequence of selection as a result of culturing (Lakeman et al. 2009).
To our knowledge, this is the first time that specific growth rates for Salish Sea Alexandrium have been described for an extensive range of environmentally relevant temperatures and salinities. Norris and Chew (1975) used an index of growth to determine the tolerance and optimal ranges of temperature and salinity; the three measures of this index were a doubling of cell numbers from the initial levels, little or no growth (e.g., cells did not double from initial levels), or cell death. While this index was informative for broadly identifying temperature and salinity windows that support growth, it is less informative for modeling efforts. The high resolution and quantitative information describing Alexandrium growth rates generated by the present study will advance ongoing efforts to develop a more comprehensive biological model of Alexandrium bloom dynamics in the region that includes information on nutrients, light and mortality (Stock et al. 2005), and will help to evaluate future climate pathways and their effects on changes in the timing, duration and extent of PSP-causing blooms (Moore et al. 2015).
The effects of temperature and salinity on growth has been determined for a range of Alexandrium species isolated from various locations in the Mediterranean, Asia, Europe, South America and the Northwest Atlantic Ocean (Watras et al. 1982, Anderson et al. 1984, Parkhill and Cembella 1999, Etheridge and Roesler 2005, Laabir et al. 2011). While the temperature and/or salinity ranges used in each of the studies are ecologically relevant in the areas from which the strains were collected, and are therefore different, a general comparison of growth rates is useful to better understand similarities and differences among species and geographic regions. Studies using strains of Alexandrium tamarense from the Northwest Atlantic Ocean include Watras et al. (1982) who reported growth rates above 0.35 d−1 across a temperature range of 13–22.5°C at a salinity of 25.5. Anderson et al. (1984) reported no growth of A. tamarense below 7 or above 26°C with an optimum range between 11 and 22°C. Parkhill and Cembella (1999) reported that maximum growth rates of A. tamarense were achieved at 15°C and 25 salinity (0.50 divisions d−1) and that growth was inhibited at 10 salinity but did not differ significantly over the salinity range of 20–30. Etheridge and Roesler (2005) investigated A. fundyense strains and found that the highest growth rates were achieved at 15°C and no significant difference in growth was observed between the salinities of 15–35. Finally, Laabir et al. (2011) studied a strain of A. catenella from Thau Lagoon in the Mediterranean and found that the highest growth rates were observed between 35–40 salinity and between 15–27°C temperature, but that positive growth was observed at all salinities (10–40) and between the temperature range of 15–30°C. These results are broadly similar to the results found in our study, in particular the euryhaline nature of Alexandrium growth with no significant difference in growth observed at salinities between 15–35. However, the highest growth rates (>0.35 d−1) observed in our study occurred at a higher temperature range (~17–24°C) compared to the studies described above (with one exception being the warmer water Mediterranean study). One important conclusion illustrated by all of these studies is that temperature plays a larger role in regulating Alexandrium growth compared to salinity within the range of ecologically relevant conditions.
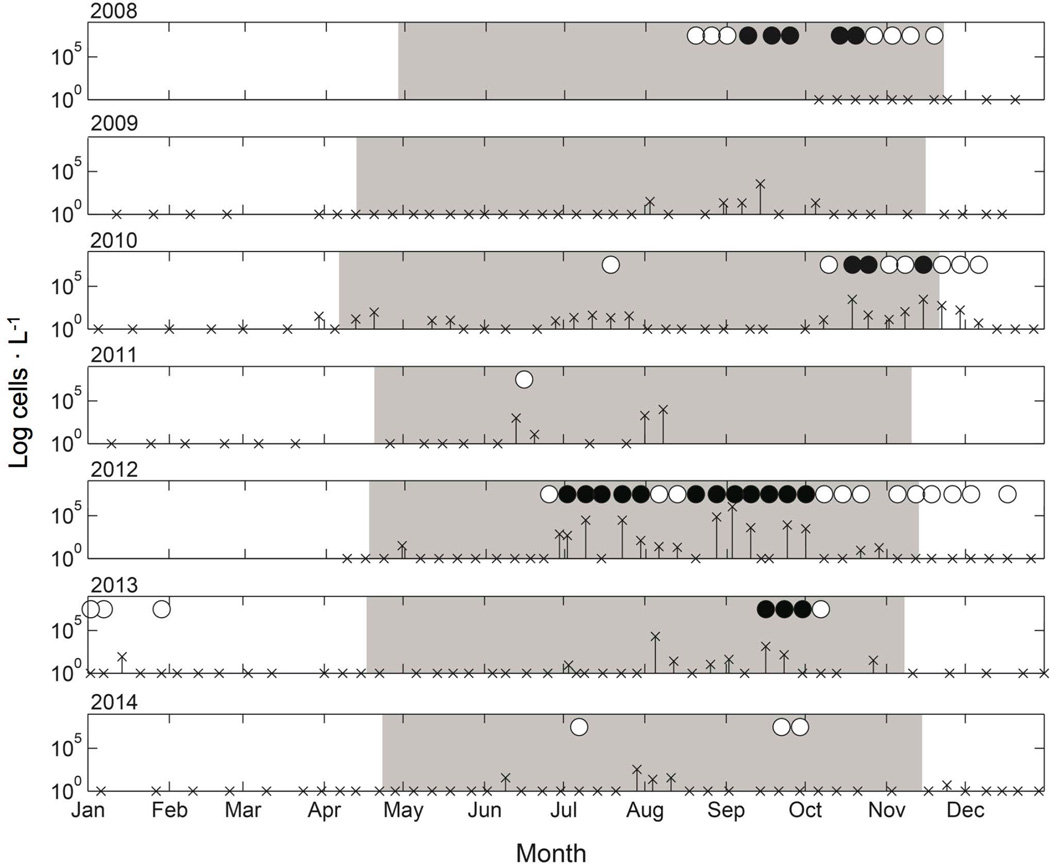
An empirical equation describing Salish Sea Alexandrium growth as a function of temperature and salinity has potential for identifying periods of bloom risk. Other factors, such as nutrient availability, light intensity, grazing and pathogen activity, will undoubtedly influence Alexandrium growth rates. Here, our use of temperature and salinity alone provides a conservative estimate of the risk of Alexandrium bloom events in Quartermaster Harbor. It is understood that the occurrence of a temperature and salinity window for Alexandrium growth does not necessarily mean that blooms will occur; rather, that the potential exists for blooms to develop if other factors are also favorable for growth. A similar approach of using derived equations to predict Alexandrium presence was used in New England salt ponds where it was concluded that temperature and salinity were the principal determinants of Alexandrium growth (Watras et al. 1982). To evaluate the utility of our approach, we compared calculated growth rates using in situ temperature and salinity measurements from Quartermaster Harbor with in situ Alexandrium cell count data and shellfish toxicity data from 2008–2014 (Fig. 5). Time periods with growth rates exceeding 0.25 d−1 are indicated by the shaded areas, and periods when shellfish saxitoxin concentrations exceeded the regulatory limit (black circles) or were detected but not above the regulatory limit (white circles) are shown (Fig. 5). Periods of bloom risk were strongly seasonal and primarily driven by variations in temperature. The greatest bloom risk (and risk of shellfish toxicity) generally occurred from April to November annually. Except for a few occasions, both Alexandrium cells and shellfish saxitoxin levels above the regulatory limit were observed only during the periods of bloom risk.
Fig. 5.
In situ Quartermaster Harbor Alexandrium concentrations (log cells · L−1) from 2008–2014. Shaded areas indicate periods when growth rates were predicted to be >0.25 d−1. Growth rates were calculated using our empirical equation (Equation 3) and in situ temperature and salinity data. Blue mussel toxin concentrations above the regulatory limit (black circle; >80 µg STX equivalents · 100 g−1 shellfish tissue) and below the regulatory limit (white circle) are shown.
Our calculated growth rates using in situ temperature and salinity measurements in Quartermaster Harbor rarely fall below 0.10 d−1, which is possibly due to our lack of adjustment for grazing and/or pathogen reduction. Additionally, cells are not always observed during the window of bloom risk, undoubtedly due to factors other than temperature and salinity that are known to affect growth rates, such as nutrient availability and light intensity. Finally, physical factors such as sampling frequency, tidal cycle, thermocline/pycnocline depth and surface mixing will have an impact on the observed cell concentrations used in this estimate of bloom risk. Despite the omission of these other factors, our analysis demonstrates that periods of potential PSP risk can be approximated using temperature and salinity alone.
This application of the empirical equation describing Salish Sea Alexandrium growth is a conservative approach for evaluating bloom risk, in that growth rates likely are overestimated. This is because growth rates were determined in the laboratory using monospecific cultures with ample nutrients, no mixing, and no grazing. Optimal temperature windows for growth established under these conditions are often wider than those observed in natural systems (Karentz and Smayda 1984, 1998). However, even using this conservative approach, detectable levels of toxin in shellfish occurred outside of the calculated period of bloom risk in 2010, 2012, and 2013 (Fig. 5). One possible explanation for this could be the increased cellular toxicity of Alexandrium when temperatures fall below the range for optimal growth. For example, preliminary toxicity data from this study indicate that growth conditions that promote slower growth rates (i.e. lower temperature) lead to higher cellular toxin concentrations in those treatments (data not shown). Higher cellular toxicity for slower growing cells has been observed in other studies (Anderson et al. 1990, Cembella 1998, Parkhill and Cembella 1999, Etheridge and Roesler 2005). Other possible explanations for toxins appearing in shellfish outside of the calculated period of bloom risk include ingestion of toxic cysts of Alexandrium that can be resuspended into the water column (Dale et al. 1978) and the retention of toxin by blue mussel for several weeks after cell concentrations abate (Bricelj and Shumway 1998).
In summary, we show that Salish Sea Alexandrium exhibit optimal growth over a wider range of temperatures (10–24°C) than previously identified. Salish Sea Alexandrium are euryhaline and show no significant difference in growth over a wide range of optimal salinities (15–35), demonstrating that temperature plays a larger role in regulating Alexandrium growth rates in the Salish Sea compared to salinity. A polynomial fit applied to the observed growth rates accurately captured the temperature and salinity dependence of Alexandrium growth, which when applied to in situ temperature and salinity data was able to predict higher risk time frames for blooms and toxic shellfish within the Salish Sea. Shellfish managers and HAB researchers can use this information to intensify biotoxin monitoring schedules during high risk periods when HABs may be impacting shellfish safety. These results will ultimately enhance our ability to forecast PSP-causing blooms in the Salish Sea under future climate change scenarios by providing the groundwork for a more comprehensive model that includes other key drivers of Alexandrium bloom dynamics. Ultimately, this approach can help scientists identify habitats that are high risk for blooms and can be used as a guide in other forecasting efforts for Alexandrium, an organism with global impacts.
Supplementary Material
Average photosynthetic photon flux density (PPFD; µmol photons · m−2 · s−1) with depth in Quartermaster Harbor during spring, summer, and autumn months from 2007–2008. The shaded area represents the range of light values used in this study to determine the temperature and salinity dependence of Alexandrium growth. Previous work has found that, during daylight hours, most (>80%) Alexandrium cells were found between 0–5 m depth (Nishitani and Chew 1984) indicating that our experimental PPFD levels were ecologically-relevant to light intensities that are encountered by Salish Sea Alexandrium. Unpublished data were provided by Cheryl L. Greengrove, from Quartermaster Harbor Nitrogen Management Study.
Acknowledgments
The authors thank K. Bright, (American Gold Seafoods) and K. Rickerson (SoundToxins program) for collecting the bloom water used for Alexandrium isolation; J. A. Johnstone (Joint Institute for the Study of the Atmosphere and Ocean) for modeling the growth responses; S. Brugger for culture maintenance; and the Northwest Fisheries Science Center’s Pasco Research Station for building the temperature-gradient bar. We thank C. Greengrove and J. Masura (University of Washington Tacoma) for providing in situ Quartermaster Harbor light data, K. Rickerson and the SoundToxins partnership for Alexandrium cell counts, the King County Department of Natural Resources and Parks for temperature and salinity data and the Washington State Department of Health for shellfish toxicity data. This research was supported in part by a grant from the NOAA Ecology and Oceanography of Harmful Algal Bloom (ECOHAB) Program to the NOAA Northwest Fisheries Science Center. Support for D. M. Anderson was also provided through the Woods Hole Center for Oceans and Human Health, National Science Foundation Grant OCE-1314642 and National Institute of Environmental Health Sciences Grant 1-P01-ES021923-01. This is ECOHAB publication number 808.
Abbreviations
- PST
paralytic shellfish toxin
- STX
saxitoxin
- PSP
paralytic shellfish poisoning
- TGB
temperature gradient bar
- PPFD
photosynthetic photon flux density
- RFU
relative fluorescence units
Contributor Information
Brian D. Bill, Marine Biotoxins Program, Environmental and Fisheries Science Division, Northwest Fisheries Science Center, Seattle, Washington, USA.
Stephanie K. Moore, University Corporation for Atmospheric Research, Joint Office for Science Support. Visiting Scientist at Northwest Fisheries Science Center, Seattle, Washington, USA
Levi R. Hay, University of Washington. Contract to Northwest Fisheries Science Center, Seattle, Washington, USA
Donald M. Anderson, Woods Hole Oceanographic Institution, Woods Hole, Massachusetts, USA
Vera L. Trainer, Marine Biotoxins Program, Environmental and Fisheries Science Division, Northwest Fisheries Science Center, Seattle, Washington, USA
REFERENCES
- Anderson DM, Kulis DM, Binder BJ. Sexuality and cyst formation in the dinoflagellate Gonyaulax tamarensis: Cyst yield in batch cultures. J. Phycol. 1984;20:418–425. [Google Scholar]
- Anderson DM, Kulis DM, Sullivan JJ, Hall S, Lee C. Dynamics and physiology of saxitoxin production by the dinoflagellates Alexandrium spp. Mar. Biol. 1990;104:511–524. [Google Scholar]
- Anderson DM, Alpermann TJ, Cembella AD, Collos Y, Masseret E, Montersor M. The globally distributed genus Alexandrium: Multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae. 2012;14:10–35. doi: 10.1016/j.hal.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berges JA, Franklin DJ, Harrison PJ. Evolution of an artificial seawater medium: improvements in enriched seawater, artificial water over the last two decades. J. Phycol. 2001;37:1138–1145. [Google Scholar]
- Corrigendum. J. Phycol. 2004;40:619. [Google Scholar]
- Brand LE, Guillard RR, Murphy LS. A method for the rapid and precise determination of acclimated phytoplankton reproduction rates. J. Plankton Res. 1981;3:193–201. [Google Scholar]
- Bricelj VM, Shumway SE. Paralytic shellfish toxins in bivalve molluscs: Occurrence, transfer kinetics, and biotransformation. Rev. Fish. Sci. 1998;6:315–383. [Google Scholar]
- Cembella AD. Ecophysiology and metabolism of paralytic shellfish toxins in marine microalgae. In: Anderson DM, Cembella AD, Hallegraeff GM, editors. Physiological Ecology of Harmful Algal Blooms. Berlin: Springer-Verlag; 1998. pp. 381–426. [Google Scholar]
- Dale B, Yentsch CM, Hurst JW. Toxicity in resting cysts of the red-tide dinoflagellate Gonyaulux excavata from deeper water coastal sediments. Science. 1978;201:1223–1225. doi: 10.1126/science.201.4362.1223. [DOI] [PubMed] [Google Scholar]
- Dyhrman ST, Haley ST, Borchert JA, Lona B, Kollars N, Erdner DL. Parallel analyses of Alexandrium catenella cell concentrations and shellfish toxicity in the Puget Sound. Appl. Environ. Microbiol. 2010;76:4647–4654. doi: 10.1128/AEM.03095-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etheridge SM, Roesler CS. Effects of temperature, irradiance, and salinity on photosynthesis, growth rates, total toxicity, and toxin composition for Alexandrium fundyense isolates from the Gulf of Maine and Bay of Fundy. Deep-Sea Res. Part II. 2005;52:2491–2500. [Google Scholar]
- Fauchot J, Levasseur M, Roy S, Gagmon R, Weise AM. Environmental factors controlling Alexandrium tamarense (Dinophyceae) growth rate during a red tide event in the St. Lawrence Estuary (Canada) J. Phycol. 2005;41:263–272. [Google Scholar]
- Guillard RL. Division rates. In: Stein JR, editor. Handbook of Phycological Methods. Cambridge: Cambridge University Press; 1973. pp. 290–311. [Google Scholar]
- John U, Litaker W, Montresor M, Murray S, Brosnahan ML, Anderson DM. Proposal to reject the name Gonyaulux catenella (Alexandrium catenella) (Dinophyceae) Taxon. 2014;63:932–933. doi: 10.12705/634.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao CY. Paralytic shellfish poisoning. In: Falconer IR, editor. Algal Toxins in Seafood and Drinking Water. London: Academic Press; 1993. pp. 75–86. [Google Scholar]
- Karentz D, Smayda TJ. Temperature and seasonal occurrence patterns of 30 dominant phytoplankton species in Narragansett Bay over a 22-year period (1959–1980) Mar. Ecol. Prog. Ser. 1984;18:277–293. [Google Scholar]
- Karentz D, Smayda TJ. Temporal patterns and variations in phytoplankton community organization and abundance in Narragansett Bay during 1959–1980. J. Plankton Res. 1998;20:145–168. [Google Scholar]
- Laabir M, Jauzein C, Genovesi B, Masseret E, Grzebyk D, Cecchi P, Vaquer A, Perrin Y, Collos Y. Influence of temperature, salinity and irradiance on the growth and cell yield of the harmful red tide dinoflagellate Alexandrium catenella colonizing Mediterranean waters. J. Plankton Res. 2011;33:1550–1563. [Google Scholar]
- Lakeman MB, von Dassow P, Cattolico RA. The strain concept in phytoplankton ecology. Harmful Algae. 2009;8:746–758. [Google Scholar]
- Lilly EL, Halanych KM, Anderson DM. Species boundaries and global biogeography of the Alexandrium tamarense complex (Dinophyceae) J. Phycol. 2007;43:1329–1338. [Google Scholar]
- Moore SK, Johnstone JA, Banas NS, Salathé EP., Jr Present-day and future climate pathways affecting Alexandrium blooms in Puget Sound, WA, USA. Harmful Algae. 2015;48:1–11. doi: 10.1016/j.hal.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Moore SK, Mantua NJ, Salathé EP., Jr Past trends and future scenarios for environmental conditions favoring the accumulation of paralytic shellfish toxins in Puget Sound shellfish. Harmful Algae. 2011;10:521–529. [Google Scholar]
- Moore SK, Mantua NJ, Hickey BM, Trainer VL. The relative influences of El Niño Southern Oscillation and Pacific Decadal Oscillation on paralytic shellfish toxin accumulation in Pacific Northwest shellfish. Limnol. Oceanogr. 2010;6:2262–2274. [Google Scholar]
- Moore SK, Mantua NJ, Trainer VL, Hickey BM. Recent trends in paralytic shellfish toxins in Puget Sound, relationships to climate, and capacity for prediction of toxic events. Harmful Algae. 2009;8:463–477. [Google Scholar]
- Nishitani L, Chew KK. Recent developments in paralytic shellfish poisoning research. Aquaculture. 1984;39:317–329. [Google Scholar]
- Norris L, Chew KK. Effect of environmental factors on growth of Gonyaulax catenella. In: LoCicero VR, editor. Proceedings of the First International Conference on Toxic Dinoflagellate Blooms. Boston: Massachusetts Science and Technology Foundation; 1975. pp. 143–152. [Google Scholar]
- Parkhill J-P, Cembella AD. Effects of salinity, light and inorganic nitrogen on growth and toxigenicity of the marine dinoflagellate Alexandrium tamarense from northeastern Canada. J. Plankton Res. 1999;21:939–955. [Google Scholar]
- PSEMP Marine Waters Workgroup. Moore SK, Stark K, Bos J, Williams P, Newton J, Dzinbal K, editors. Puget Sound marine waters: 2012 overview. 2013 URL: http://www.psp.wa.gov/downloads/psemp/PSmarinewaters_2012_overview.pdf.
- Quayle DB. Paralytic shellfish poisoning in British Columbia. Bull. Fish. Res. Board Can. 1969;169:1–68. [Google Scholar]
- Rensel J. Factors controlling Paralytic Shellfish Poisoning (PSP) in Puget Sound, Washington. J. Shellfish Res. 1993;12:371–376. [Google Scholar]
- Stock CA, McGillicuddy DJ, Jr, Solow AR, Anderson DM. Evaluating hypotheses for the initiation and development of Alexandrium fundyense blooms in the western Gulf of Maine using a coupled physical-biological model. Deep-Sea Res. Part II. 2005;52:2715–2744. [Google Scholar]
- Trainer VL, Eberhart B-TL, Wekell JC, Adams NG, Hanson L, Cox F, Dowell J. Paralytic shellfish toxins in Puget Sound, Washington State. J. Shellfish Res. 2003;22:213–223. [Google Scholar]
- Vancouver G. A voyage of discovery to the North Pacific Ocean and round the world, 1791–1795. In: Robinson GG, Robinson J, editors. Vol. 2, Fourth book, Chapter 2. London: Paternoster-Row and J. Edwards; 1798. pp. 260–287. [Google Scholar]
- Wang L, Zhuang Y, Zhang H, Lin X, Lin S. DNA barcoding species in Alexandrium tamarense complex using ITS and proposing designation of five species. Harmful Algae. 2014;31:100–113. doi: 10.1016/j.hal.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Watras CJ, Chisholm SW, Anderson DM. Regulation of growth in an estuarine clone of Gonyaulax tamarensis Lebour: Salinity-dependent temperature responses. J. Exp. Mar. Biol. Ecol. 1982;62:25–37. [Google Scholar]
- Wells ML, Trainer VL, Smayda TJ, Karlson BSO, Trick CW, Kudela RM, Ishikawa A, Bernard S, Wulff A, Anderson DM, Cochlan WP. Harmful algal blooms and climate change: Learning from the past and present to forecast the future. Harmful Algae. 2015;49:68–93. doi: 10.1016/j.hal.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Average photosynthetic photon flux density (PPFD; µmol photons · m−2 · s−1) with depth in Quartermaster Harbor during spring, summer, and autumn months from 2007–2008. The shaded area represents the range of light values used in this study to determine the temperature and salinity dependence of Alexandrium growth. Previous work has found that, during daylight hours, most (>80%) Alexandrium cells were found between 0–5 m depth (Nishitani and Chew 1984) indicating that our experimental PPFD levels were ecologically-relevant to light intensities that are encountered by Salish Sea Alexandrium. Unpublished data were provided by Cheryl L. Greengrove, from Quartermaster Harbor Nitrogen Management Study.