Abstract
This study examined how repeated olanzapine (OLZ) or clozapine (CLZ) treatment in adolescence alters sensitivity to the same drug in adulthood in the phencyclidine (PCP) hyperlocomotion model. Male adolescent Sprague-Dawley rats (postnatal day (P) 44–48) were first treated with OLZ (1.0 or 2.0 mg/kg, subcutaneously (sc)) or CLZ (10.0 or 20.0 mg/kg, sc) and tested in the PCP (3.2 mg/kg, sc)-induced hyperlocomotion model for five consecutive days. Then a challenge test with OLZ (0.5 mg/kg) or CLZ (5.0 mg/kg) was administered either during adolescence (~P 51) or after the rats matured into adults (~P 76 and 91). During adolescence, repeated OLZ or CLZ treatment produced a persistent inhibition of PCP-induced hyperlocomotion across the five test days. In the challenge test during adolescence, rats previously treated with OLZ did not show a significantly stronger inhibition of PCP-induced hyperlocomotion than those previously treated with vehicle (VEH). In contrast, those previously treated with CLZ showed a weaker inhibition than the VEH controls. When assessed in adulthood, the enhanced sensitivity to OLZ and the decreased sensitivity to CLZ were detected on ~P 76, even on ~P 91 in the case of OLZ. These findings suggest that adolescent OLZ or CLZ exposure can induce long-term alterations in antipsychotic response that persist into adulthood.
Keywords: Olanzapine, clozapine, adolescent drug treatment, phencyclidine, prepulse inhibition, motor activity, sensitization, tolerance
Introduction
In recent years, there has been a significant increase in the number of children and adolescents who are being treated with antipsychotic drugs. One survey in the US showed a six-fold increase in the number of prescriptions of antipsychotics for patients ≤20 years from 1993–2002 (Olfson et al., 2006). The percentage of the user population accounted for by children and adolescents (i.e. by patients <18 years old) has doubled from 7% in 1996–1997 to 15% in 2004–2005 (Domino and Swartz, 2008). Also, the annualized rate of any antipsychotic use per 1000 children increased from 0.78 (1999–2001) to 1.59 (2007) (Olfson et al., 2010). Clinical studies demonstrate that atypical antipsychotics are effective for pediatric patients with early-onset schizophrenia and schizoaffective disorder, although they also cause more severe antipsychotic-related adverse side effects (e.g. extrapyramidal side effects, sedation, prolactin elevation, weight gain) in pediatric patients than in adult patients (Findling et al., 2010; Kumra et al., 2008; Sikich et al., 2008).
Current research on children and adolescent antipsychotic treatment has been mostly focusing on the efficacy, tolerability, and side effect profiles of individual drugs. There is a general lack of research on the long-term consequences of antipsychotic treatment on the brain and behavioral development of patients. Preclinical studies suggest that synaptic connections, receptor densities — especially those among the dopamine and serotonin systems — in the prefrontal cortex, striatum, and hippocampus undergo dramatic maturational changes during adolescence that may have implications for understanding the unique clinical response and side effects associated with adolescent antipsychotic treatment (Benes et al., 2000; Brenhouse and Andersen, 2011). Animal work also shows that antipsychotic exposure during adolescence alters various neuroreceptors, including dopamine D1, D2 and D4 receptors (Moran-Gates et al., 2006), serotonin 5-HT1A and 5-HT2A receptors (Choi et al., 2010), and ionotropic glutamate N-Methyl-D-aspartate (NDMA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (Choi et al., 2009) in unique ways not seen in adult animals. All these findings strongly suggest that antipsychotic exposure during adolescence could alter the trajectory of the brain and behavioral development of pediatric patients which in turn, may change their later response to drug treatment as adults. As pediatric treatment occurs during the period of rapid brain and behavioral development, there is a need to evaluate the possible short-term and long-term impacts of antipsychotic medications on psychological and brain functions in order to determine their risk/benefits.
The present study examined the extent to which early antipsychotic exposure during adolescence affects “exposure-dependent” alterations in responsiveness to its pharmacological actions later during adulthood. Such alterations can be manifested as either tolerance, which is characterized by progressive reductions in responsiveness to certain drug effects, or sensitization (also known as reverse-tolerance), which is characterized by progressive increases in responsiveness to certain drug effects (Carlezon et al., 2004). In recent years, we have demonstrated that, in adult rats, repeated treatment of olanzapine (OLZ) or clozapine (CLZ) (two atypical antipsychotic drugs commonly used in the treatment of pediatric schizophrenic patients) (Almandil et al., 2011; Sikich et al., 2008) induces a potentiated (sensitization) or a decreased (tolerance) inhibition of the phencyclidine (PCP)-induced hyperlocomotion, respectively (Feng et al., 2013; Sun et al., 2009; Zhang and Li, 2012; Zhao et al., 2012), a preclinical test for antipsychotic activity (Gleason and Shannon, 1997). The present study extended this line of research and investigated the persistence of OLZ sensitization and CLZ tolerance from adolescence to adulthood in this model. Our general approach was to induce OLZ sensitization or CLZ tolerance during the adolescent period (postnatal day (P) 44–48) through repeated drug administration (termed the induction phase), and then tested its expression either during adolescence (P 51) or during adulthood (P 76 and P 91) (termed the expression phase). The alterations of antipsychotic efficacy (sensitization or tolerance) were indexed by the change of the ability of OLZ or CLZ to suppress PCP-induced hyperlocomotion upon re-exposure in a challenge test. To assess how adolescent antipsychotic treatment and PCP treatment affect the brain and behavioral functions and developments of adolescent rats, we also examined the prepulse inhibition (PPI) of acoustic startle response (ASR) periodically throughout their developmental period. PPI refers to the phenomenon of a reduction in the startle magnitude when the startling stimulus is preceded by a low-intensity prepulse. It measures the sensorimotor gating ability which is found to be deficient in patients with schizophrenia and in animals treated with dopamine agonists and NMDA antagonists (Geyer and Braff, 1987; Li et al., 2011a, 2011b; Swerdlow et al., 2008).
Materials and methods
Animals
Male Sprague-Dawley adolescent rats weighing 101–125 g on the delivery date, (average age=P 35) (McCutcheon and Marinelli, 2009) from Charles River Inc. (Portage, Michigan, USA) were used. They were housed two per cage, in 48.3×26.7×20.3 cm transparent polycarbonate cages under 12 h light/dark conditions (light on between 06:30–18:30 hours). Room temperature was maintained at 22 ±1°C with a relative humidity of 45–60%. Food and water was available ad libitum. Animals were allowed at least five days of habituation to the animal facility before being used in experiments. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Nebraska-Lincoln.
Drugs and choice of dose
The injection solution of phencyclidine hydrochloride (PCP, gift from National Institute on Drug Abuse Chemical Synthesis and Drug Supply Program) was obtained by mixing the drug with 0.9% saline. The dose of PCP (3.2 mg/kg, sc) was chosen based on our previous work (Feng et al., 2013; Sun et al., 2009; Zhang and Li, 2012; Zhao et al., 2012). This dose of PCP is shown to induce a robust hyperlocomotion effect without causing severe stereotypy (Gleason and Shannon, 1997; Kalinichev et al., 2008). OLZ and CLZ (gifts from the National Institute of Mental Health drug supply program) were tested in this study because although they share some receptor-binding properties (e.g. dopamine D2, and serotonin 5-HT2A) they differ in several long-term behavioral effects (Lieberman et al., 2008). They were dissolved in distilled sterile water with 1.0–1.5% glacial acetic acid. Two doses of OLZ (1.0 and 2.0 mg/kg) and CLZ (10.0 and 20.0 mg/kg) were tested. These doses of OLZ and CLZ acutely inhibit PCP-induced hyperlocomotion (Feng et al., 2013; Li et al., 2010; Sun et al., 2009; Zhang and Li, 2012). Furthermore, both drugs at these doses give rise to a clinically comparable range (40%–80%) of striatal dopamine D2 occupancy in rats, which is comparable to values observed in schizophrenic patients (Kapur et al., 2003). All drugs were administrated subcutaneously (sc) at 1.0 ml/kg.
Experiment 1: OLZ sensitization induced in adolescence and assessed in adolescence
In this experiment, we examined the potential increased inhibitory effect of repeated OLZ treatment on the PCP-induced hyperlocomotion in adolescent rats. The entire experiment was comprised of the following two phases: the induction phase and OLZ challenge test (expression) phase.
The induction phase
The apparatus was 16 motor activity monitoring boxes (48.3×26.7×20.3 cm transparent polycarbonate cages) equipped with a row of six photocell beams (7.8 cm between two adjacent photobeams) placed 3.2 cm above the floor of the cage. A computer with recording software (Aero Apparatus Sixbeam Locomotor System v1.4, Toronto, Canada) was used to detect the disruption of the photocell beams and recorded the number of beam breaks. Twenty-four adolescent rats (~P 42–43) were first randomly assigned to one of four treatment groups (n=6/group) and one of 16 test boxes: vehicle (VEH)+VEH (sterile water+saline), VEH+PCP; OLZ 1.0+PCP and OLZ 2.0+PCP. On the first two days (~P 42–43), rats were handled for 2 min and habituated to the locomotor activity apparatus for 30 min each day. On each of the next five consecutive days, adolescent rats (~P 44–48) were first injected with VEH (sterile water), OLZ 1.0 mg/kg, or OLZ 2.0 mg/kg, and then immediately placed in the boxes for 30 min. At the end of the 30-minute period, they were taken out and injected with PCP (3.2 mg/kg, sc) or saline and placed back in the boxes for another 60 min. Motor activity was measured in 5 min intervals throughout the entire 90-minute testing session.
The challenge test (expression phase) on ~P 51
Two days after the last OLZ test, all rats were returned to the locomotor activity boxes for one re-habituation session (30 min) (~P 50), followed by the OLZ sensitization test one day later (~P 51). On the challenge test day, all rats were first injected with a small dose of OLZ 0.5 mg/kg and then immediately placed in the motor activity boxes for 30 min. At the end of the 30-minute period, rats were taken out and injected with PCP (3.2 mg/kg) and placed back in the boxes for another 60 min. Motor activity was recorded for the entire 90-minute testing session.
Experiment 2: CLZ tolerance induced in adolescence and assessed in adolescence
In this experiment, we examined the effects of repeated CLZ treatment on PCP-induced hyperlocomotion during adolescence. The basic procedure was identical to that of Experiment 1 with the exceptions that twenty four adolescent rats were treated with two doses of CLZ (10.0 and 20.0 mg/kg, sc) during the induction phase and CLZ 5.0 mg/kg was used in the CLZ challenge test (~P 51). The four groups were: VEH+VEH (sterile water+saline), VEH+PCP (sterile water+PCP 3.2 mg/kg, sc), CLZ 10+PCP (CLZ 10.0 mg/kg+PCP 3.2 mg/kg), and CLZ 20+PCP (CLZ 20.0 mg/kg+PCP 3.2 mg/kg).
Experiment 3: OLZ sensitization induced in adolescence and assessed in adulthood
In this experiment, we examined the potential long-lasting OLZ sensitization from adolescence to adulthood. The entire experiment was comprised of the following three components: The induction phase in adolescence, OLZ challenge tests in adulthood, and PPI assessment. Table 1 details the timeline of events.
Table 1.
Timeline of events in experiments 3 and 4.
| Days of study | Approximate age | Manipulation |
|---|---|---|
| 1–2 | P 42–43 | Habituation to the motor activity boxes |
| 3–7 | P 44–48 | Five days of drug testing |
| 8 | P 49 | 1st PPI test |
| 9–26 | P 50–66 | Rest |
| 27 | P 67 | 2nd PPI test |
| 28–35 | P 68–74 | Rest |
| 36 | P 75 | Habituation to the motor activity boxes |
| 37 | P 76 | 1st drug challenge test: OLZ 0.5 mg/kg or CLZ 5.0 mg/kg followed by PCP 3.2 mg/kg (except the VEH+VEH group) |
| 38 | P 77 | 3rd PPI test |
| 39–50 | P 78–89 | Rest |
| 51 | P 90 | Habituation to the motor activity boxes |
| 52 | P 91 | 2nd drug challenge test: OLZ 0.5 mg/kg or CLZ 5.0 mg/kg followed by PCP 3.2 mg/kg |
CLZ: clozapine; OLZ: olanzapine; P: postnatal day; PCP: phencyclidine; PPI: prepulse inhibition; VEH: vehicle.
The induction phase in adolescence
Twenty-four adolescent rats (~P 42–43) were subjected to two days of apparatus habituation and five days of repeated OLZ drug testing (VEH, 1.0 mg/kg, or 2.0 mg/kg) under either PCP (3.2 mg/kg, sc) or saline.
OLZ challenge tests in adulthood on ~P 76 and 91
Twenty-nine days after the last PCP test, after the rats became adults (~P 75) (McCutcheon and Marinelli, 2009), they were returned to the locomotor activity boxes for one re-habituation session (30 min), followed by the first OLZ sensitization challenge test one day later (~P 76). The basic procedure was similar to that detailed in Experiment 1 with the exception that rats from the VEH+VEH group received a double injection of sterile water and saline. The second sensitization challenge test was conducted on ~P 91 in which all rats were first injected with OLZ 0.5 mg/kg, 30 min later, an injection of PCP 3.2 mg/kg.
PPI assessment
The PPI test apparatus and procedure have been described in detail previously (Chen et al., 2011; Li et al., 2011a, 2011b; Qiao et al., 2013). A total of three PPI tests were conducted throughout the developmental period. The first one was done during the late adolescent period (~P 49, one day after the five drug test days), the second one during the early adulthood period (~P 67). The third one (~P 77) was conducted one day after the 1st sensitization test. Each session lasted approximately 18 min and began with a 5-min period of 70 dB background noise (which continued throughout the duration of the session) followed by four different trial types: PULSE ALONE trials and three types of PREPULSE + PULSE trials, which consisted of a 20 ms 73, 76, or 82 dB prepulse (3, 6, and 12 dB above background) followed 100 ms later by a 120 dB pulse (40 ms). Each session was divided into four blocks. Blocks 1 and 4 were identical, each consisting of four PULSE ALONE trials. Blocks 2 and 3 were also identical and each consisted of eight PULSE ALONE trials and five of each PREPULSE + PULSE trial type. A total of 54 trials were presented during each test session. Trials within each block were presented in a pseudorandom order and were separated by a variable intertrial interval averaging 15 s (ranging from 9–21 s). Startle magnitude was defined as the maximum force (measured in Newtons) applied by the rat to the startle apparatus recorded over a period of 100 ms beginning at the onset of the pulse stimulus. Between each stimulus trial, 100 ms of activity was recorded when no stimulus was present: these trials were called NOSTIM trials and were not included in the calculation of intertrial intervals. Responses recorded during NOSTIM trials are considered a measure of gross motor activity within the PPI boxes. Startle responses from testing blocks 2 and 3 were used to calculate percentage prepulse inhibition (%PPI) for each acoustic prepulse trial type:
average startle response to
%PPI = 100 [( PREPULSE + PULSE trials) × 100 ]
average startle response to
PULSE ALONE trials
Experiment 4: CLZ tolerance induced in adolescence and assessed in adulthood
This experiment examined whether the tolerance effect induced by repeated CLZ treatment to adolescent rats in the PCP model persisted into adulthood. The basic procedure was identical to that of Experiment 3 with the exception that CLZ (10.0 and 20.0 mg/kg, sc) instead of OLZ, were administered during the adolescent period and one challenge dose of CLZ (5.0 mg/kg) was tested during the challenge tests (see Table 1). The four groups (n=6/group) were: VEH+VEH (sterile water+saline), VEH+PCP; CLZ 10.0+PCP and CLZ 20.0+PCP.
Statistical analysis
Motor activity data from the five consecutive drug test days were expressed as mean values ±standard error of the mean (SEM) and analyzed using a mixed-design analysis of variance (ANOVA) with the between-subjects factor being drug group and the within-subjects factor being test day, followed by Fisher’s least significant difference (LSD) tests to examine group difference. Data from the challenge tests were analyzed by one-way ANOVA followed by Fisher’s LSD (for more than three groups). %PPI data were analyzed using repeated measures ANOVAs with drug treatment group as a between-subjects factor and prepulse level as a within-subjects factor. All analyses were conducted using IBM SPSS Statistics 19, and p<0.05 was considered statistically significant.
Results
Experiment 1: OLZ sensitization induced in adolescence and assessed in adolescence
The induction phase
Figure 1(a) shows the mean motor activity of the four groups of rats during the 30-minute test period before PCP or vehicle injection throughout the five days of drug testing. Two-way repeated measures ANOVA revealed a main effect of group, F(3,20)=7.346, p=0.002 and test day, F(4,80)=22.952, p<0.001. Post-hoc Fisher’s LSD tests revealed that the two OLZ groups had significantly lower motor activity than the VEH+PCP group, all ps<0.03, confirming the inhibitory effect of OLZ treatment on spontaneous motor activity.
Figure 1.
Effect of repeated olanzapine (OLZ) (1.0 and 2.0 mg/kg) treatment on phencyclidine (PCP)-induced hyperlocomotion across the initial five test days (n=6/group) from ~postnatal day (P) 44–48 and on the re-habituation day (~P 50) and challenge test day (~P 51). (a) Locomotor activity during the 30-minute test period before vehicle (VEH) or PCP injection and (b) 60 min after PCP or VEH injection across the five test days is expressed as mean+standard error of the mean (SEM) for each group. (c) Locomotor activity measured for 30 min on the re-habituation day. On the OLZ challenge test day on ~P 51, locomotor activity was measured during the 30-minute test period before PCP injection (d) and during the 60-minute test period after PCP injection (e). * p<0.05 relative to the VEH+VEH group; # p<0.05 relative to the VEH+PCP group.
Figure 1(b) shows the mean motor activity during the 60-minute test period after PCP or VEH injection. Once again, two-way repeated measures ANOVA revealed a main effect of group, F(3,20)=40.817, p<0.001, and a significant group×day interaction, F(12,80)=2.910, p=0.002. The VEH+PCP group had significantly higher motor activity than the VEH+VEH group, p<0.001, indicating a strong psychomotor activation effect of this dose of PCP. The two OLZ groups had significantly lower motor activity than the VEH+PCP group, ps<0.001, confirming the inhibitory effect of OLZ treatment on PCP-induced hyperlocomotion. This inhibition was not complete though, as both OLZ groups still had significantly higher locomotion than the VEH+VEH group, ps<0.042.
Re-habituation session
On the re-habituation day (Figure 1(c)), rats previously treated with OLZ showed much higher motor activity than the other groups (a main effect of group, F(3,20)=11.755, p<0.001, and ps<0.005 vs the VEH groups), suggesting a possibly compensatory rebound effect against the motor inhibitory effect of OLZ during the drug withdrawal.
OLZ challenge test on ~P 51
One way ANOVA found no main effect of group in the 30-minute period before the PCP injection, F(3,20)=1.533, p=0.237 (Figure 1(d)). In the 60-minute test period after the PCP injection (Figure 1(e)), the main effect of group was also not significant, F(3,20)=1.879, p=0.166, although the OLZ 2.0+PCP group did show a relatively lower motor activity than the VEH+PCP group. These results suggest that repeated OLZ treatment caused a strong sensitized inhibition of PCP-induced hyperlocomotion during the drug test phase (i.e. the induction phase). However, the expression of such a sensitization effect three days later (still in adolescence) was relatively weak.
Experiment 2: CLZ tolerance induced in adolescence and assessed in adolescence
The induction phase
In the 30-minute test period throughout the five days of drug testing (Figure 2(a)), the effect of test day, F(4,80)=23.096, p<0.001, and the group×day interaction, F(12,80)=2.053, p=0.030 were significant, but the effect of group was not, F(3,20)=1.656, p=0.208. In the 60-minute period after PCP or VEH injection (Figure 2(b)), there was a main effect of group, F(3,20)=25.057, p<0.001, but no main effect of test day, F(4,80)=0.994, p=0.416, nor group×day interaction, F(12,80)=1.711, p=0.08. Post-hoc tests revealed that the VEH+PCP group had significantly higher motor activity than the VEH+VEH group, p<0.001. The CLZ 10+PCP group had significantly lower motor activity relative to the VEH+PCP group, p<0.001, but higher activity than the VEH+VEH group, p=0.038. In contrast, the CLZ 20+PCP group only showed significantly lower motor activity than the VEH+PCP group, p<0.001, but not than the VEH+VEH group, p=0.182. These results suggest that CLZ at 20.0 mg/kg, but not at 10.0 mg/kg completely abolished the motor activation effect of PCP and this inhibitory effect of CLZ maintained throughout the five days of testing.
Figure 2.
Effect of repeated clozapine (CLZ) (10.0 and 20.0 mg/kg) treatment on phencyclidine (PCP)-induced hyperlocomotion across the initial five test days (n=6/group) from ~postnatal day (P) 44–48 and on the re-habituation day (~P 50) and challenge test day (~P 51). (a) Locomotor activity in the 30 min before vehicle (VEH) or PCP injection and (b) 60 min after PCP or VEH injection across the five test days is expressed as mean+standard error of the mean (SEM) for each group. (c) Locomotor activity measured for 30 min on the re-habituation day. On the CLZ challenge test day on ~P 51, locomotor activity was measured during the 30-minute test period before PCP injection (d) and during the 60-minute test period after PCP injection (e). * p<0.05 relative to the VEH+VEH group; # p<0.05 relative to the VEH+PCP group.
Re-habituation session
Similar to the OLZ rats in Experiment 1, rats previously treated with CLZ (10.0 and 20.0 mg/kg) showed much higher motor activity than the other groups (Figure 2(c)). One-way ANOVA showed a significant effect of group, F(3,20)=5.025, p=0.009. Post-hoc test showed that the two CLZ groups had significantly higher motor activity than the VEH+PCP group, all ps<0.017, and the CLZ 20+PCP group also showed significantly higher motor activity relative to the VEH+VEH group, p=0.027, indicating a possibly compensatory rebound effect against the motor inhibitory effect of CLZ during the drug withdrawal.
CLZ challenge test on ~P 51
Figure 2(d) shows the mean motor activity during the 30-minute test period on the CLZ tolerance test on ~P 51. One-way ANOVA found a main effect of group, F(3,20)=3.386, p=0.038 due to the higher motor activity in the CLZ 20+PCP group, ps<0.015.
One-way ANOVA on the motor activity in the 60-minute test period after PCP injection found a main effect of group, F(3,20)=3.859, p=0.025. Again, the CLZ 20+PCP group had significantly higher motor activity than the CLZ naive groups (i.e. the VEH+VEH group, p=0.006 and the VEH+PCP group, p=0.013) (Figure 2(e)).
Overall, results from this experiment suggest that repeated CLZ treatment caused a persistent inhibition of PCP-induced hyperlocomotion during the induction phase in a dose-dependent fashion. Such a treatment caused a tolerance in the inhibition of PCP-induced hyperlocomotion later on.
Experiment 3: OLZ sensitization induced in adolescence and assessed in adulthood
The induction phase in adolescence
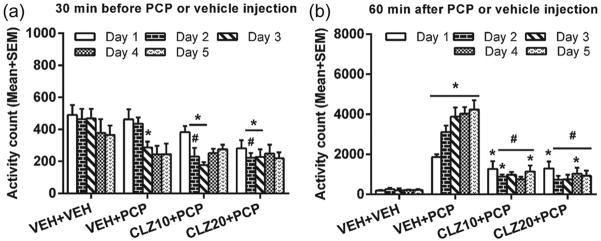
Figure 3(a) shows the mean motor activity of the four groups of rats during the 30-minute period before PCP or VEH injection throughout the five days of drug testing. Repeated measures ANOVA revealed a main effect of group, F(3,20)=6.174, p=0.004, and a main effect of test day, F(4,80)=18.767, p<0.001. Post-hoc analysis revealed that the two OLZ groups had significantly lower motor activity than the VEH+VEH group, ps<0.003. The OLZ (1.0 mg/kg) group also had significantly lower motor activity than the VEH+PCP group, p=0.029, confirming the strong inhibitory effect of OLZ on spontaneous motor activity.
Figure 3.
Effect of repeated olanzapine (OLZ) (1.0 and 2.0 mg/kg) treatment on phencyclidine (PCP)-induced hyperlocomotion across the five test days (n=6/group) during adolescence. (a) Locomotor activity in the 30 min before vehicle (VEH) or PCP injection and (b) 60 min after PCP or VEH injection across the five test days is expressed as mean+standard error of the mean (SEM) for each group. * p<0.05 relative to the VEH+VEH group; # p<0.05 relative to the VEH+PCP group; & p<0.05 relative to the OLZ 1.0+PCP group.
During the 60-minute period after PCP or VEH injection (Figure 3(b)), there was a significant main effect of group, F(3,0)=45.186, p<0.001, and a significant group×day interaction, F(12,80)=5.037, p<0.001. Post-hoc LSD tests revealed that the two OLZ (1.0 and 2.0 mg/kg) groups showed significantly lower motor activity compared to the VEH+PCP group, ps<0.001. The VEH+VEH group had significantly lower motor activity than the VEH+PCP group, p<0.001, the OLZ 1.0+PCP group, p<0.001, and marginally different from the OLZ 2.0+PCP group, p=0.05, indicating that the inhibition by OLZ was not completely lowered to the VEH level. The OLZ 2.0+PCP group also had significantly lower motor activity than the OLZ 1.0+PCP group, p<0.001.
Re-habituation session on ~P 75 and 90
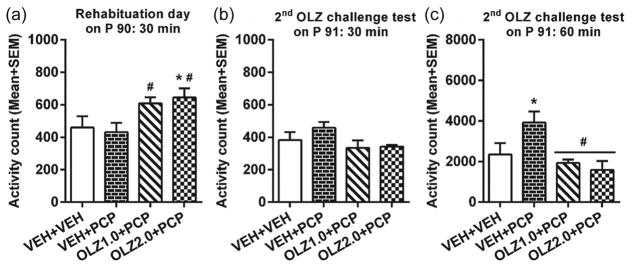
On the 1st re-habituation day (~P 75), rats previously treated with OLZ showed much higher motor activity than the other groups (Figure 4(a)). One-way ANOVA showed a significant effect of group, F(3,20)=4.308, p=0.017. Post-hoc tests showed that the OLZ 2.0+PCP group had significantly higher motor activity than the VEH+PCP and OLZ 1.0+PCP groups, all ps<0.008. Similarly on the 2nd re-habituation day (~P 90), the OLZ 2.0+PCP group still had significantly higher motor activity than the VEH+VEH group, p=0.033 and the VEH+PCP group, p=0.015. The OLZ 1.0+PCP group also had significantly higher motor activity than the VEH+PCP group, p=0.039 (Figure 5(a)). These results indicate a long-lasting compensatory rebound effect of adolescent OLZ treatment against the motor inhibitory effect of OLZ in adulthood.
Figure 4.
Locomotor activity on (a) the 1st re-habituation day (~postnatal day (P) 75) and (b) 1st olanzapine (OLZ) challenge day during the 30-minute test period before phencyclidine (PCP) or saline injection and (c) the 60-minute test period after PCP or vehicle (VEH) injection (~P 76). *p<0.05 relative to the VEH+VEH group; #p<0.05 relative to the VEH+PCP group.
Figure 5.
Locomotor activity on (a) the 2nd re-habituation day (~postnatal day (P) 90) and (b) 2nd olanzapine (OLZ) challenge day during the 30-minute test period before phencyclidine (PCP) injection and (c) the 60-minute test period after PCP injection (~P 91). * p<0.05 relative to the VEH+VEH group; # p<0.05 relative to the VEH+PCP group.
OLZ challenge tests in adulthood on ~P 76 and 91
Figure 4(b) shows the mean motor activity of the four groups of rats in the 1st 30-minute test period on the 1st OLZ challenge test. One way ANOVA revealed a main effect of group, F(3,20)=3.586, p=0.032. Post-hoc two group comparisons revealed that the OLZ 1.0+PCP group had significantly lower motor activity than the OLZ naive groups, all ps<0.022. One way ANOVA on the motor activity data in the 60-minute test period after PCP injection also revealed a main effect of group, F(3,20)=20.125, p<0.001. Again, the two OLZ-treated groups had significantly lower motor activity than the VEH+PCP, ps<0.002, indicating the OLZ sensitization effect (Figure 4(b) and 4(c)).
On the 2nd OLZ challenge test, one rat from the OLZ 2.0+PCP group jumped out the testing box, and its data were not used in the subsequent data analysis. The group effect in the first 30-minute test period was not significant (Figure 5(b)), F(3,19)=1.965, p=0.154, but the group effect in the second 60-minute test period was significant, F(3, 19)=5.110, p=0.009 (Figure 5(c)). Post-hoc two group comparisons revealed that both the OLZ 1.0+PCP and OLZ 2.0+PCP groups had significantly lower motor activity than the VEH+PCP group, p=0.005, and p=0.002, respectively, a finding similar to that observed on ~P 76. The VEH+PCP group still had significantly higher motor activity than the VEH+VEH group, p=0.023, indicating the persistence of PCP sensitization effect which was not abolished by acute OLZ treatment. Furthermore, the finding that both OLZ groups did not differ significantly from the VEH+VEH, ps>0.259, suggests that the OLZ sensitization effect reached a level high enough to eliminate the PCP sensitization effect.
Overall, results from this experiment suggest that repeated OLZ treatment during adolescence induced a strong sensitization effect during the adolescent treatment period and that this sensitization effect persisted into adulthood. This effect was long-lasting and dose-dependent. It manifested itself as an enhanced inhibition of PCP-induced hyperlocomotion (an index of antipsychotic activity) in the OLZ treated animals, and a rebound of spontaneous motor activity under the drug-free condition.
PPI assessment
PPI data from the three time points of testing (~P 49, 67 and 77) did not reveal any significant group difference, p=0.957, 0.708, and 0.286, respectively (data not shown). The group×prepulse level interactions were also not significant, p=0.447, 0.179, and 0.159, respectively. These findings suggest that repeated OLZ treatment and PCP treatment did not significantly impair the sensorimotor gating ability.
Experiment 4: CLZ tolerance induced in adolescence and assessed in adulthood
The induction phase in adolescence
Figure 6(a) shows the mean locomotor activity of the four groups of rats during the 30-minute period before PCP or VEH injection throughout the five days of drug testing. Two-way repeated measures ANOVA revealed a main effect of group, F(3, 20)=5.321, p=0.007, test day, F(4,80)=7.781, p<0.001, and a significant group×day interaction, F(12,80)=2.033, p=0.032. Post-hoc LSD tests revealed that the VEH+VEH group had significantly higher motor activity than the CLZ 10+PCP group, p=0.005, and the CLZ 20+PCP group, p=0.002, indicating a strong motor inhibition by CLZ.
Figure 6.
Effect of repeated clozapine (CLZ) (10.0 and 20.0 mg/kg) treatment on phencyclidine (PCP)-induced hyperlocomotion across the five test days (n=6/group) during adolescence. (a) Locomotor activity in the 30 min before vehicle (VEH) or PCP injection and (b) 60 min after PCP or VEH injection across the five test days is expressed as mean+standard error of the mean (SEM) for each group. * p<0.05 relative to the VEH+VEH group; # p<0.05 relative to the VEH+PCP group.
During the 60-minute period after PCP or VEH injection (Figure 6(b)), two-way repeated measures ANOVA revealed a significant main effect of group, F(3,20)=49.740, p<0.001, a significant main effect of test day, F(4,80)=4.604, p=0.002, and a significant group×day interaction, F(12,80)=8.875, p<0.001. The two CLZ groups had significantly lower motor activity than the VEH+PCP group, all ps<0.001, confirming the strong inhibitory effect of CLZ treatment on PCP-induced hyperlocomotion. The VEH+VEH group showed significantly lower motor activity than the VEH+PCP group, p<0.001, the CLZ 10+PCP group, p<0.001, and the CLZ 20+PCP group, p=0.017, indicating the inhibition by CLZ was not completely back to the VEH level.
Re-habituation session on ~P 75 and 90
On the 1st re-habituation day, rats previously treated with CLZ showed much higher motor activity than the other groups (Figure 7(a)). One-way ANOVA confirmed this observation, F(3,20)=3.204, p=0.045. Post-hoc tests showed that the CLZ 20+PCP group had significantly higher motor activity than the VEH+VEH and VEH+PCP groups, ps<0.033. On the 2nd re-habituation day (~P 90), one day before the second CLZ tolerance test, this compensatory rebound effect disappeared, as there was no main effect of group, F(3,20)=0.532, p=0.665 (Figure 8(a)).
Figure 7.
Locomotor activity on (a) the 1st re-habituation day (~postnatal day (P) 75) and (b) 1st clozapine (CLZ) challenge day during the 30-minute test period before phencyclidine (PCP) injection and (c) the 60-minute test period after PCP or vehicle (VEH) injection (~P 76). * p<0.05 relative to the VEH+VEH group; # p<0.05 relative to the VEH+PCP group.
Figure 8.
Locomotor activity (a) on the 2nd re-habituation day (~postnatal day (P) 90) and (b) 2nd clozapine (CLZ) challenge day during the 30-minute test period before phencyclidine (PCP) injection and (c) the 60-minute test period after PCP injection (~P 91).
CLZ challenge tests in adulthood on ~P 76 and 91
Figure 7(b) shows the mean motor activity of the four groups of rats in the first 30-minute test period on the 1st CLZ challenge test. No significant group effect was found, F(3,20)=1.083, p=0.379. However, the significant group effect was found in the 60-minute test period after PCP injection (Figure 7(c)), F(3,20)=9.785, p<0.001. The CLZ 10+PCP group (but not the CLZ 20+PCP group) had significantly higher motor activity than the VEH+PCP group, p=0.019, indicating the CLZ tolerance effect. The CLZ groups also had significantly higher motor activity than the VEH+VEH group, all ps<0.018. On the 2nd challenge test (Figure 8(b) and 8(c)), the group effect was not significant, all ps>0.623.
Overall, results from this experiment suggest that, like OLZ, repeated CLZ treatment induced a sensitized inhibition of PCP-induced hyperlocomotion during the adolescent drug treatment period. When assessed in adulthood, a tolerance effect (i.e. decreased inhibition of the PCP-induced hyperlocomotion) was still detected.
PPI assessment
PPI data from the three time points of testing (~P 49, 67, and 77) did not reveal any significant group difference, p=0.286, 0.239, and 0.789, respectively (data not shown). The group×prepulse level interactions were also not significant, p=0.644, 0.088, and 0.232, respectively. These findings suggest that repeated CLZ treatment and PCP treatment did not significantly impair the sensorimotor gating ability.
Discussion
The present study uncovered an important phenomenon, that is, adolescent antipsychotic exposure can induce long-lasting alterations in the behavioral development of animals which, in turn, cause an increase or decrease of their later antipsychotic response as adults. Specifically, we showed that adolescent OLZ treatment induced a sensitization effect that persisted throughout the development into adulthood. This effect was long-lasting, detectable even more than 40 days since the last drug treatment. It was dose-dependent and manifested as an enhanced inhibition of PCP-induced hyperlocomotion (a validated measure of antipsychotic activity) and a rebound of spontaneous motor activity. In contrast, although adolescent CLZ treatment, like OLZ, induced a persistent inhibition of PCP-induced hyperlocomotion during the drug treatment period and caused an increase in spontaneous motor activity, when assessed for its short-term (i.e. three days) and long-term (i.e. >30 days) consequences, a decreased inhibition of PCP-induced hyperlocomotion (i.e. tolerance) was noticed in CLZ-pretreated rats, and this effect persisted into early adulthood but disappeared with the passage of time. In this regard, CLZ shares a similar inhibitory effect with OLZ during drug treatment (i.e. the induction phase) and drug withdrawal periods. However, CLZ differed from OLZ in the drug challenge test (i.e. the expression phase) in which a tolerance, rather than a sensitization effect was found.
The PCP-induced hyperlocomotion model is commonly used as a screening tool for the detection of antipsychotic activity. When given acutely, many antipsychotics inhibit the hyperlocomotor activity induced by acute administrations of PCP (Gleason and Shannon, 1997; Millan et al., 1999; Sun et al., 2010). In recent years, we have used the repeated PCP hyperlocomotion model to investigate the long-term effects of repeated antipsychotic treatment (Feng et al., 2013; Sun et al., 2009, Zhang and Li, 2012, Zhao et al., 2012). We and others show that in adult rats, repeated haloperidol, OLZ or CLZ treatment, but not anxiolytic (e.g. chlordiazepoxide) or antidepressant treatment (e.g. fluoxetine and citalopram), progressively potentiates the inhibition of PCP-induced hyperlocomotion across sessions and prolongs this action within sessions (Redmond et al., 1999; Sun et al., 2009; Zhao et al., 2012). When the long-term effects were assessed in a challenge test later, adult rats previously treated with OLZ showed an enhanced response to OLZ (i.e. sensitization), while those previously treated with CLZ showed a decreased response (i.e. tolerance) (Feng et al., 2013). The present study extended our adult work to adolescent animals by showing that even adolescent rats exhibited drug-induced alterations in antipsychotic response and these alterations were long-lasting. These significant findings are consistent with our recent study showing that OLZ sensitization and CLZ tolerance could be induced in adolescent rats in the conditioned avoidance response model (Qiao et al., 2013), another animal test of antipsychotic activity, suggesting the generality of these long-term antipsychotic effects independent of any particular behavioral model used to assess these effects.
Another noticeable manifestation of long-term consequences of adolescent OLZ or CLZ treatment was the rebound of spontaneous motor activity on the re-habituation days when all rats were tested drug-free and presumably in the drug withdrawal state. Rats treated with OLZ or CLZ were more active than those treated with VEH or even PCP. This effect was also dose-dependent and persisted from adolescence into adulthood. Interestingly, although repeated OLZ and CLZ gave rise to opposite effects (sensitization vs tolerance) on the inhibition of PCP-induced hyperlocomotion on the challenge tests, they all caused the same rebound effect on spontaneous motor activity. We are not aware of any previous report on this phenomenon. It resembles to some extent the antipsychotic withdrawal-induced behavioral hypersensitivity; a state of dopamine supersensitivity that is characterized by increased behavioral responses to the psychomotor-stimulating effects of dopamine agonists such as amphetamine or apomorphine thought to result from hypersensitivity of dopamine D2 receptors (especially in a state of high-affinity, D2 high) (Carvey et al., 1990; Kinon et al., 1984; Samaha et al., 2007; Sayers et al., 1975; Seeman, 2006). This issue needs to be further investigated to determine its clinical relevance and related mechanisms.
One seemingly strange finding was that the expression of OLZ sensitization appeared to be weaker in adolescence than in adulthood. This effect was almost non-detectable just three days after the last OLZ treatment, while it was robustly expressed at a time point more than 40 days since the last OLZ treatment. With this perspective, the strength of OLZ sensitization actually increased over time. This observation fits well with the description of “time-dependent sensitization,” referring to the observation that a brief exposure to an antipsychotic drug induces a clinical effect that grows with the passage of time (Antelman et al., 2000). In Antelman’s earlier work, he found that a single exposure to a clinically low dose of antipsychotic produced changes that were shown up to eight weeks later (Antelman, 1986). The strengthening of OLZ sensitization effect over time has also been reported in adult rats in the conditioned avoidance response model (Swalve and Li, 2012). On the other hand, CLZ tolerance appeared to be weakened with the passage of time, suggesting that the drug memory mechanisms involved in CLZ tolerance may degrade over time (i.e. forgetting). The significance of this temporal difference between OLZ and CLZ effects may warrant further research.
Besides, in the PCP-induced hyperlocomotion test, OLZ sensitization has also been reported in other tests, such as the conditioned avoidance response test (Swalve and Li, 2012; Zhang and Li, 2012), the prepulse inhibition of acoustic startle procedure (Li et al., 2011) and the operant responding procedure (Varvel et al., 2002), although the term “OLZ sensitization” may not have been used. Similarly, CLZ-induced tolerance has also been observed in other tasks, including the drug discrimination task (Goudie et al., 2007a, 2007b) and conditioned avoidance response test (Li et al., 2010, 2012; Sanger, 1985). Among many antipsychotic drugs, CLZ appears to be the only one that causes a tolerance, rather than a sensitization effect. Another difference between OLZ and CLZ was that CLZ tolerance seems to be weaker than OLZ sensitization in terms of its persistence. While OLZ sensitization was detected at both adulthood test time points (~P 76 and ~P 91), CLZ tolerance was only detectable on ~P 76. One caveat to this conclusion is that the 1st CLZ challenge on ~P 76 might have affected the 2nd CLZ challenge on ~P 91 differently from that of the 1st OLZ challenge on the 2nd OLZ challenge, leading to the persistence of OLZ sensitization and the disappearance of CLZ tolerance on ~P 91. Because we did not test these effects in animals not previously challenged on ~P 76, we could not determine the exact causes of this time course OLZ–CLZ difference. If this difference reflects the superior therapeutic effect and favorable side effects of CLZ, understanding the underlying mechanisms may help us develop and identify CLZ-like drugs, as CLZ appears to be more effective than OLZ and haloperidol in the treatment of childhood onset schizophrenia (Kumra et al., 2008).
In this study, we also assessed how adolescent antipsychotic and PCP treatments affected the PPI performance, a measure of the sensorimotor gating ability which is found to be deficient in patients with schizophrenia and in animals treated with dopamine agonists and NMDA antagonists (Geyer and Braff, 1987; Li et al., 2011a, 2011b; Swerdlow et al., 2008). We did not find any long-lasting detrimental effect by repeated OLZ or CLZ treatment. This result is consistent with our recent adolescence antipsychotic study using another animal model of antipsychotic activity (Qiao et al., 2013) and others (Llorente-Berzal et al., 2012; Meyer et al., 2010). Other PCP studies show that PPI in rats is disrupted during, but not after, chronic PCP-treatment (Martinez et al., 1999; Schwabe et al., 2005), suggesting that there is a dissociation between the brain neurochemical effects of chronic PCP treatment and its behavioral effects (Jentsch et al., 1998). It is also possible that in order to reveal any long-lasting effect of adolescent antipsychotic treatment and PCP effects in adulthood, we need to challenge the animals with either a dopamine agonist or an NMDA antagonist. This work is certainly warranted.
What are the neurobiological mechanisms that account for OLZ sensitization and CLZ tolerance as observed in this study? Because various neuroreceptors targeted by antipsychotic drugs, such as dopaminergic, adrenergic, serotonergic receptors, undergo dramatic changes from the adolescent period to adulthood (e.g. an inverted U-shape curve of development) (Andersen et al., 2000; Lidow et al., 1991; Tarazi et al., 1998; Teicher et al., 1995), it is possible that antipsychotic exposure in adolescence may alter the expression and function of these neuroreceptors (Choi et al., 2009, 2010; Moran-Gates et al., 2006) which “imprint” an antipsychotic drug memory trace (Li et al., 2007). The finding that OLZ sensitization and CLZ tolerance had survived the adolescent brain re-organization period (Brenhouse and Andersen, 2011) indicates that certain drug-induced brain changes may be relatively permanent. We recently investigated the neurochemical basis of OLZ sensitization and CLZ tolerance in adult rats. Our results suggest that dopamine D2/3 receptors and serotonin 5-HT2A/2C receptors may play a role in these effects (Li et al., 2010). Using a conditioned avoidance response model, another animal model of antipsychotic activity (Wadenberg and Hicks, 1999), we found that pretreatment of quinpirole, a selective D2/D3 dopaminergic receptor agonist, attenuated OLZ sensitization but enhanced CLZ tolerance. The likely brain sites include various limbic structures, such as the nucleus accumbens (NAc) shell, ventral tegmental area (VTA), ventral part of lateral septal nucleus (LSv), central amygdaloid nucleus (CeA), and medial prefrontal cortex (mPFC) (Zhao et al., 2012). In this regard, the induction and maintenance of adolescent OLZ sensitization and CLZ tolerance may involve the neuroplasticity initiated by D2/3 blockade in the above mentioned sites. This idea is consistent with the findings showing that repeated administration of OLZ (5.0 mg/kg, once daily), but not CLZ (20.0 mg/kg, twice daily), from P 22–P 42 caused an increase of D2 receptor levels in the hippocampus and D4 levels in the NAc and caudate-putamen (CPu) in juvenile brains (Moran-Gates et al., 2006). Apparently, actions on other neurotransmitter systems (e.g. serotonergic, adrenergic, glutamatergic) in other brain areas during adolescence may also contribute to the long-lasting sensitization and tolerance effects. Future work should systematically investigate the role of these receptors in the mediation of OLZ and CLZ tolerance from adolescence to adulthood. Furthermore, the contribution of pharmacokinetic differences between OLZ and CLZ (half-life: 2.5 h vs 1.5 h, respectively) (Kapur et al., 2003) to their different behavioral patterns may also need to be explored.
Antipsychotic drugs are not only used to treat schizophrenia or schizoaffective disorders in children and adolescents, but also autism, attention deficit and hyperactivity disorder (ADHD), Tourette’s disorder, disruptive behaviors, bipolar disorder, and mood disorders (Correll et al., 2011). Because individuals with these disorders are likely to receive antipsychotic treatment upon becoming adults, the present work thus has implications for clinical practice involving adolescent antipsychotic treatments, provided that the same sensitization or tolerance effect in healthy animals could be identified in human patients with schizophrenia who may already have some neuropathology at an early age, thus may respond to treatment differently from normal individuals. One lesson that can be learned from this preclinical study is that there is a possibility of long-term impacts of early antipsychotic exposure on the brain and behavioral development and functions. Therefore, the choice of specific drug, drug dose and treatment schedule for individual patients needs to take their past treatment histories into consideration.
Acknowledgments
Research reported in this paper was supported by the National Institute of Mental Health of the National Institutes of Health under award number R01MH085635 to Ming Li. The authors would like to thank Jun Gao, Shinn-Yi Chou, and Natashia Swalve for their thoughtful comments on an earlier version of this manuscript.
Footnotes
The authors declare that there are no conflicts of interest.
References
- Almandil NB, Wong IC. Review on the current use of antipsychotic drugs in children and adolescents. Arch Dis Child Educ Pract Ed. 2011;96:192–196. doi: 10.1136/archdischild-2011-300054. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AT, Rutstein M, et al. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37:167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Antelman SM, Kocan D, Edwards DJ, et al. Behavioral effects of a single neuroleptic treatment grow with the passage of time. Brain Res. 1986;385:58–67. doi: 10.1016/0006-8993(86)91547-7. [DOI] [PubMed] [Google Scholar]
- Antelman SM, Levine J, Gershon S. Time-dependent sensitization: The odyssey of a scientific heresy from the laboratory to the door of the clinic. Mol Psychiatry. 2000;5:350–356. doi: 10.1038/sj.mp.4000721. [DOI] [PubMed] [Google Scholar]
- Benes FM, Taylor JB, Cunningham MC. Convergence and plasticity of monoaminergic systems in the medial prefrontal cortex during the postnatal period: Implications for the development of psychopathology. Cereb Cortex. 2000;10:1014–1027. doi: 10.1093/cercor/10.10.1014. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Developmental trajectories during adolescence in males and females: A cross-species understanding of underlying brain changes. Neurosci Biobehav Rev. 2011;35:1687–1703. doi: 10.1016/j.neubiorev.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Konradi C. Understanding the neurobiological consequences of early exposure to psychotropic drugs: Linking behavior with molecules. Neuropharmacology. 2004;47(Suppl 1):47–60. doi: 10.1016/j.neuropharm.2004.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvey PM, Kao LC, Zhang TJ, et al. Dopaminergic alterations in cotreatments attenuating haloperidol-induced hypersensitivity. Pharmacol Biochem Behav. 1990;35:291–300. doi: 10.1016/0091-3057(90)90158-e. [DOI] [PubMed] [Google Scholar]
- Chen J, Wang Z, Li M. Multiple ‘hits’ during postnatal and early adulthood periods disrupt the normal development of sensorimotor gating ability in rats. J Psychopharmacol. 2011;25:379–392. doi: 10.1177/0269881109354929. [DOI] [PubMed] [Google Scholar]
- Choi YK, Gardner MP, Tarazi FI. Effects of risperidone on glutamate receptor subtypes in developing rat brain. Eur Neuropsychopharmacol. 2009;19:77–84. doi: 10.1016/j.euroneuro.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YK, Moran-Gates T, Gardner MP, et al. Effects of repeated risperidone exposure on serotonin receptor subtypes in developing rats. Eur Neuropsychopharmacol. 2010;20:187–194. doi: 10.1016/j.euroneuro.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll CU, Kratochvil CJ, March JS. Developments in pediatric psychopharmacology: Focus on stimulants, antidepressants, and antipsychotics. J Clin Psychiatry. 2011;72:655–670. doi: 10.4088/JCP.11r07064. [DOI] [PubMed] [Google Scholar]
- Domino ME, Swartz MS. Who are the new users of antipsychotic medications? Psychiatr Serv. 2008;59:507–514. doi: 10.1176/appi.ps.59.5.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng M, Sui N, Li M. Environmental and behavioral controls of the expression of clozapine tolerance: Evidence from a novel across-model transfer paradigm. Behav Brain Res. 2013;238:178–187. doi: 10.1016/j.bbr.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findling RL, Johnson JL, McClellan J, et al. Double-blind maintenance safety and effectiveness findings from the Treatment of Early-Onset Schizophrenia Spectrum (TEOSS) study. J Am Acad Child Adolesc Psychiatry. 2010;49:583–594. doi: 10.1016/j.jaac.2010.03.013. quiz 632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Braff DL. Startle habituation and sensorimotor gating in schizophrenia and related animal models. Schizophr Bull. 1987;13:643–668. doi: 10.1093/schbul/13.4.643. [DOI] [PubMed] [Google Scholar]
- Gleason SD, Shannon HE. Blockade of phencyclidine-induced hyperlocomotion by olanzapine, clozapine and serotonin receptor subtype selective antagonists in mice. Psychopharmacology (Berl) 1997;129:79–84. doi: 10.1007/s002130050165. [DOI] [PubMed] [Google Scholar]
- Goudie AJ, Cole JC, Sumnall HR. Olanzapine and JL13 induce cross-tolerance to the clozapine discriminative stimulus in rats. Behav Pharmacol. 2007a;18:9–17. doi: 10.1097/FBP.0b013e328014138d. [DOI] [PubMed] [Google Scholar]
- Goudie AJ, Cooper GD, Cole JC, et al. Cyproheptadine resembles clozapine in vivo following both acute and chronic administration in rats. J Psychopharmacol. 2007b;21:179–190. doi: 10.1177/0269881107067076. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR, Roth RH. Subchronic phencyclidine administration increases mesolimbic dopaminergic system responsivity and augments stress- and psychostimulant-induced hyperlocomotion. Neuropsychopharmacology. 1998;19:105–113. doi: 10.1016/S0893-133X(98)00004-9. [DOI] [PubMed] [Google Scholar]
- Kalinichev M, Robbins MJ, Hartfield EM, et al. Comparison between intraperitoneal and subcutaneous phencyclidine administration in Sprague-Dawley rats: A locomotor activity and gene induction study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:414–422. doi: 10.1016/j.pnpbp.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Kapur S, Vanderspek SC, Brownlee BA, et al. Antipsychotic dosing in preclinical models is often unrepresentative of the clinical condition: A suggested solution based on in vivo occupancy. J Pharmacol Exp Ther. 2003;305:625–631. doi: 10.1124/jpet.102.046987. [DOI] [PubMed] [Google Scholar]
- Kinon BJ, Merson D, Kane JM. Effect of daily dose of chronic haloperidol and chronic apomorphine on behavioral hypersensitivity in the rat. Psychopharmacology (Berl) 1984;84:347–351. doi: 10.1007/BF00555211. [DOI] [PubMed] [Google Scholar]
- Kumra S, Oberstar JV, Sikich L, et al. Efficacy and tolerability of second-generation antipsychotics in children and adolescents with schizophrenia. Schizophr Bull. 2008;34:60–71. doi: 10.1093/schbul/sbm109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Fletcher PJ, Kapur S. Time course of the antipsychotic effect and the underlying behavioral mechanisms. Neuropsychopharmacology. 2007;32:263–272. doi: 10.1038/sj.npp.1301110. [DOI] [PubMed] [Google Scholar]
- Li M, He E, Volf N. Time course of the attenuation effect of repeated antipsychotic treatment on prepulse inhibition disruption induced by repeated phencyclidine treatment. Pharmacol Biochem Behav. 2011a;98:559–569. doi: 10.1016/j.pbb.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, He W, Chen J. Time course of prepulse inhibition disruption induced by dopamine agonists and NMDA antagonists: Effects of drug administration regimen. Pharmacol Biochem Behav. 2011b;99:509–518. doi: 10.1016/j.pbb.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Li M, Sun T, Mead A. Clozapine, but not olanzapine, disrupts conditioned avoidance response in rats by antagonizing 5-HT(2A/2C) receptors. J Neural Transm. 2012;119:497–505. doi: 10.1007/s00702-011-0722-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Sun T, Zhang C, et al. Distinct neural mechanisms underlying acute and repeated administration of antipsychotic drugs in rat avoidance conditioning. Psychopharmacology (Berl) 2010;212:45–57. doi: 10.1007/s00213-010-1925-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS, Rakic P. Synchronized overproduction of neurotransmitter receptors in diverse regions of the primate cerebral cortex. Proc Natl Acad Sci U S A. 1991;88:10218–10221. doi: 10.1073/pnas.88.22.10218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman JA, Bymaster FP, Meltzer HY, et al. Antipsychotic drugs: Comparison in animal models of efficacy, neurotransmitter regulation, and neuroprotection. Pharmacol Rev. 2008;60:358–403. doi: 10.1124/pr.107.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente-Berzal A, Mela V, Borcel E, et al. Neurobehavioral and metabolic long-term consequences of neonatal maternal deprivation stress and adolescent olanzapine treatment in male and female rats. Neuropharmacology. 2012;62:1332–1341. doi: 10.1016/j.neuropharm.2011.07.031. [DOI] [PubMed] [Google Scholar]
- McCutcheon JE, Marinelli M. Age matters. Eur J Neurosci. 2009;29:997–1014. doi: 10.1111/j.1460-9568.2009.06648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez ZA, Ellison GD, Geyer MA, et al. Effects of sustained phencyclidine exposure on sensorimotor gating of startle in rats. Neuropsychopharmacology. 1999;21:28–39. doi: 10.1016/S0893-133X(98)00137-7. [DOI] [PubMed] [Google Scholar]
- Meyer U, Spoerri E, Yee BK, et al. Evaluating early preventive antipsychotic and antidepressant drug treatment in an infection-based neurodevelopmental mouse model of schizophrenia. Schizophr Bull. 2010;36:607–623. doi: 10.1093/schbul/sbn131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ, Brocco M, Gobert A, et al. Contrasting mechanisms of action and sensitivity to antipsychotics of phencyclidine versus amphetamine: Importance of nucleus accumbens 5-HT2A sites for PCP-induced locomotion in the rat. Eur J Neurosci. 1999;11:4419–4432. doi: 10.1046/j.1460-9568.1999.00858.x. [DOI] [PubMed] [Google Scholar]
- Moran-Gates T, Gan L, Park YS, et al. Repeated antipsychotic drug exposure in developing rats: Dopamine receptor effects. Synapse. 2006;59:92–100. doi: 10.1002/syn.20220. [DOI] [PubMed] [Google Scholar]
- Olfson M, Blanco C, Liu L, et al. National trends in the outpatient treatment of children and adolescents with antipsychotic drugs. Arch Gen Psychiatry. 2006;63:679–685. doi: 10.1001/archpsyc.63.6.679. [DOI] [PubMed] [Google Scholar]
- Olfson M, Crystal S, Huang C, et al. Trends in antipsychotic drug use by very young, privately insured children. J Am Acad Child Adolesc Psychiatry. 2010;49:13–23. doi: 10.1097/00004583-201001000-00005. [DOI] [PubMed] [Google Scholar]
- Qiao J, Li H, Li M. Olanzapine sensitization and clozapine tolerance: From adolescence to adulthood in the conditioned avoidance response model. Neuropsychopharmacology. 2013;38:513–524. doi: 10.1038/npp.2012.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond AM, Harkin A, Kelly JP, et al. Effects of acute and chronic antidepressant administration on phencyclidine (PCP) induced locomotor hyperactivity. Eur Neuropsychopharmacol. 1999;9:165–170. doi: 10.1016/s0924-977x(98)00023-6. [DOI] [PubMed] [Google Scholar]
- Samaha AN, Seeman P, Stewart J, et al. “Breakthrough” dopamine supersensitivity during ongoing antipsychotic treatment leads to treatment failure over time. J Neurosci. 2007;27:2979–2986. doi: 10.1523/JNEUROSCI.5416-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger DJ. The effects of clozapine on shuttle-box avoidance responding in rats: Comparisons with haloperidol and chlordiazepoxide. Pharmacol Biochem Behav. 1985;23:231–236. doi: 10.1016/0091-3057(85)90562-3. [DOI] [PubMed] [Google Scholar]
- Sayers AC, Burki HR, Ruch W, et al. Neuroleptic-induced hypersensitivity of striatal dopamine receptors in the rat as a model of tardive dyskinesias. Effects of clozapine, haloperidol, loxapine and chlorpromazine. Psychopharmacologia. 1975;41:97–104. doi: 10.1007/BF00421063. [DOI] [PubMed] [Google Scholar]
- Schwabe K, Brosda J, Wegener N, et al. Clozapine enhances disruption of prepulse inhibition after sub-chronic dizocilpine- or phencyclidine-treatment in Wistar rats. Pharmacol Biochem Behav. 2005;80:213–219. doi: 10.1016/j.pbb.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Seeman P. Targeting the dopamine D2 receptor in schizophrenia. Expert Opin Ther Targets. 2006;10:515–531. doi: 10.1517/14728222.10.4.515. [DOI] [PubMed] [Google Scholar]
- Sikich L, Frazier JA, McClellan J, et al. Double-blind comparison of first- and second-generation antipsychotics in early-onset schizophrenia and schizo-affective disorder: Findings from the treatment of early-onset schizophrenia spectrum disorders (TEOSS) study. Am J Psychiatry. 2008;165:1420–1431. doi: 10.1176/appi.ajp.2008.08050756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Hu G, Li M. Repeated antipsychotic treatment progressively potentiates inhibition on phencyclidine-induced hyperlocomotion, but attenuates inhibition on amphetamine-induced hyperlocomotion: Relevance to animal models of antipsychotic drugs. Eur J Pharmacol. 2009;602:334–342. doi: 10.1016/j.ejphar.2008.11.036. [DOI] [PubMed] [Google Scholar]
- Sun T, Zhao C, Hu G, et al. Iptakalim: A potential antipsychotic drug with novel mechanisms? Eur J Pharmacol. 2010;634:68–76. doi: 10.1016/j.ejphar.2010.02.024. [DOI] [PubMed] [Google Scholar]
- Swalve N, Li M. Parametric studies of antipsychotic-induced sensitization in the conditioned avoidance response model: Roles of number of drug exposure, drug dose, and test-retest interval. Behav Pharmacol. 2012;23:380–391. doi: 10.1097/FBP.0b013e32835651ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Weber M, Qu Y, et al. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology (Berl) 2008;199:331–388. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine and serotonin transporters in rat caudate-putamen and nucleus accumbens septi. Neurosci Lett. 1998;254:21–24. doi: 10.1016/s0304-3940(98)00644-2. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Hostetter JC., Jr Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Brain Res Dev Brain Res. 1995;89:167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Vann RE, Wise LE, et al. Effects of antipsychotic drugs on operant responding after acute and repeated administration. Psychopharmacology (Berl) 2002;160:182–191. doi: 10.1007/s00213-001-0969-y. [DOI] [PubMed] [Google Scholar]
- Wadenberg ML, Hicks PB. The conditioned avoidance response test re-evaluated: Is it a sensitive test for the detection of potentially atypical antipsychotics? Neurosci Biobehav Rev. 1999;23:851–862. doi: 10.1016/s0149-7634(99)00037-8. [DOI] [PubMed] [Google Scholar]
- Zhang C, Li M. Contextual and behavioral control of antipsychotic sensitization induced by haloperidol and olanzapine. Behav Pharmacol. 2012;23:66–79. doi: 10.1097/FBP.0b013e32834ecac4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Sun T, Li M. Neural basis of the potentiated inhibition of repeated haloperidol and clozapine treatment on the phencyclidine-induced hyperlocomotion. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38:175–182. doi: 10.1016/j.pnpbp.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]