Abstract
Aim
To investigate the genetic influence of circulating lactate level, a marker of oxidative capacity associated with diabetes.
Methods
We conducted a genome-wide association study of log-transformed plasma lactate levels in 6901 European-American participants in the Atherosclerosis Risk in Communities study. For regions that achieved genome-wide significance in European-American participants, we conducted candidate region analysis in African-American subjects and tested for interaction between metformin use and the index single nucleotide polymorphisms for plasma lactate in European-American subjects.
Results
The genome-wide association study in European-American subjects identified two genome-wide significant loci, GCKR (rs1260326, T allele β=0.08; P=1.8×10−47) and PPP1R3B/LOC157273 (rs9987289, A allele β=0.06; P=1.6×10−9). The index single nucleotide polymorphisms in these two loci explain 3.3% of the variance in log-transformed plasma lactate levels among the European-American subjects. In the African-American subjects, based on a region-significant threshold, the index single nucleotide polymorphism at GCKR was associated with plasma lactate but that at PPP1R3B/LOC157273 was not. Metformin use appeared to strengthen the association between the index single nucleotide polymorphism at PPP1R3B/LOC157273 and plasma lactate in European-American subjects (P for interaction=0.01).
Conclusions
We identified GCKR and PPP1R3B/LOC157273 as two genome-wide significant loci of plasma lactate. Both loci are associated with other diabetes-related phenotypes. These findings increase our understanding of the genetic control of lactate metabolism.
Introduction
Lactate is the end product of anaerobic glycolysis and is used clinically to indicate extreme states of decreased oxygen availability during vigorous exercise, hypoxia and ischaemia [1,2]. Blood lactate is associated with obesity, Type 2 diabetes and cardiovascular outcomes [3–7], suggesting that milder forms of decreased oxidative capacity occur in these conditions. Lactate is affected by other processes such as gluconeogenesis, for which lactate is a key substrate [2]. The discovery of genetic variants associated with plasma lactate may therefore inform our understanding of the sources of population variation in lactate and the biological mechanisms underlying its association with obesity and Type 2 diabetes.
Metformin is one of the most widely prescribed diabetes medications [8]. One of the rare adverse effects of metformin is severely elevated lactate levels [9]. Genetic association with plasma lactate as modified by metformin use may inform the biological mechanism underlying metformin's effect on plasma lactate levels.
We conducted a genome-wide association study of plasma lactate in European-American participants in the Atherosclerosis Risk in Communities (ARIC) study [10]. For the region with single nucleotide polymorphisms (SNPs) achieving genome-wide significance among European-American subjects, we conducted a candidate region analysis in African-American subjects, accounting for the potential difference in linkage disequilibrium between European-American subjects and African-American subjects. In addition, given that metformin is known to influence blood lactate levels [11], we tested for the interaction between metformin use and the index SNPs of the genome-wide association study (GWAS) loci for plasma lactate in European-American subjects.
Subjects and methods
Study population
The ARIC study is a prospective community-based cohort study [10]. Plasma lactate was measured at visit 4 of this study, 1996–1998. A total of 6901 European-American subjects and 1671 African-American subjects were included in the genetic association analysis. Subjects without measures of lactate, SNP data passing quality control, fasting blood sample or covariate data, those who took biguanides or thiazolidinediones, and those with lactate measures outside of three standard deviation units were excluded. Details are given in Table S1.
Plasma lactate measures and other variables
Plasma lactate was measured by quantifying the conversion of lactate to pyruvate using the Roche Hitachi 911 Auto-Analyzer (Roche Diagnostics, Indianapolis, IN, USA) [3]. Diabetes mellitus was defined as a fasting glucose level of at least 126 mg/dl, a non-fasting glucose level of at least 200 mg/dl, use of diabetes medication, or a self-reported physician diagnosis of diabetes. Diabetes medication use was based on the inspection of medication bottles. Measures of other variables (prevalent coronary heart disease, heart failure, HDL cholesterol, triglycerides, fasting glucose, smoking status and alcohol consumption) were also based on a standardized protocol and have been reported previously [5].
Genetic association analysis methods
Genotyping of SNPs was performed using the Affymetrix 6.0 microarray. Details of genotype quality control and the generation of genetic principal components have been reported previously [12]. Over 38 million SNPs were imputed based on 1000 Genomes Phase I Intergrated Release Version 3 reference panels. Genotype imputation was performed using impute2 [13] after using ShapeIt (v1.r532) [14] for haplotype phasing. The criteria for SNPs included in imputation are: Hardy–Weinberg equilibrium P value >1e−5; SNP missingness <5%; and minor allele frequency >0.5% for European-American subjects and >1% for African-American subjects. This resulted in 711,589 SNPs used for imputation in European-American subjects and 806,416 in African-American subjects.
The association between natural log-transformed lactate and imputed SNP dosage was evaluated using linear regression adjusting for age, sex, study centre, BMI, waist circumference, and statistically significant principal components (principal components 1, 2, 3, and 5 in European-American subjects, and none was significant in African-American subjects). The genetic effect of each SNP was assumed to be additive. The genome-wide significance threshold was set at 5×10−8. For the genome-wide significant loci in European-American subjects, we performed analyses controlling for the index SNP to detect evidence for additional independent associations. In addition, for the index SNP at the genome-wide significant loci in European-American subjects, we performed inverse variance weighted fixed effect meta-analysis using Metal to combine the results from European-American and African-American subjects [15].
As allelic heterogeneity and differences in linkage disequilibrium pattern between European-American and African-American populations may give rise to different tag SNPs in an associated region, we evaluated the association of each genome-wide significant locus identified in European-American participants in the ARIC study in African-American participants using a candidate region approach based on a set of criteria similar to those developed by Liu et al. [16]. The significance threshold for candidate region association was set as 0.05 divided by the number of independent SNPs in the region. A region was defined as 500 kb or between the recombination hotspot on each side of the index SNP in the GWAS of European-American subjects, whichever was closer. The number of independent SNPs was estimated using plink [17] based on a variance inflation factor <2 with a window size of 50 SNPs and shift by five SNPs. We also conducted a post hoc power analysis for this candidate region approach based on the effect size of the index SNP in the GWAS of European-American subjects.
Plasma lactate is associated with prevalent and incident diabetes [3,4,18]. To gain insight into the relationship between lactate and diabetes-related loci, we interrogated the GWAS results of plasma lactate for index SNPs associated with diabetes-related traits (2-h glucose, fasting glucose and insulin) in the Catalog of Published Genome-wide Association Studies [19].
Interaction between metformin and index SNPs at GCKR and PPP1R3B/LOC157273
Given that both GCKR and PPP1R3B have important roles in hepatic glucose metabolism, which is also metformin's site of action [11,20–22], the index SNPs at GCKR andPPP1R3B and metformin may have synergistic effects on lactate levels; therefore, we tested for interaction between metformin use and the index SNPs at GCKR and PPPIR3B/LOC157273 for natural log-transformed plasma lactate levels in European-American subjects. Model 1 adjusted for age, sex and study centre. Model 2 further adjusted for other variables that are associated with plasma lactate or may be potential confounders: BMI, waist circumference, smoking status, alcohol consumption, prevalent coronary heart disease, prevalent heart failure, estimated GFR, natural log-transformed fasting glucose, insulin, triglycerides, HDL cholesterol and other diabetes medications (insulin, sulphonylureas, thiazolidinediones) [5,7,23]. Subjects who were excluded from the GWAS analysis because of use of biguanides or thiazolidinediones, or plasma lactate outside of three standard deviations were included in this analysis. The sample for this analysis was 6726 European-American subjects, with data for plasma lactate and all covariates. The GWAS of lactate in European-American subjects was performed using snptest (v2) [24]. All other analyses were conducted using R software.
Results
Study population characteristics
The median plasma lactate level in the European-American subjects included in the genome-wide association analysis was 6.4 mg/dl, their mean age was 63 years and 11% of them had diabetes. For the African-American subjects, the median lactate level was 7.3 mg/dl, the mean age was 62 years, and 20% had diabetes (Table S2).
Genome-wide significant loci in European-American subjects and candidate region association in African-American subjects
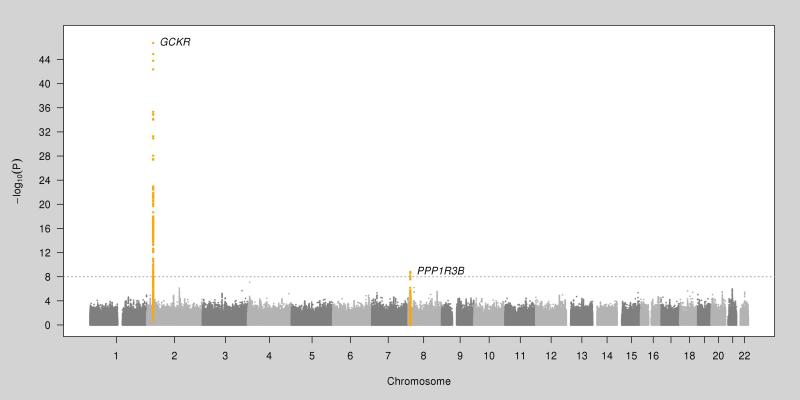
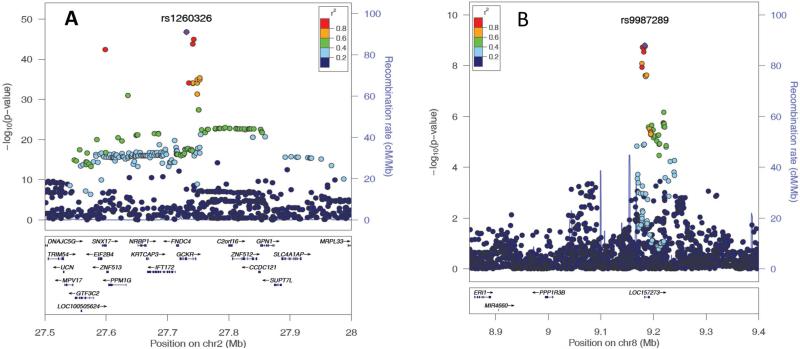
In the GWAS of the European-American subjects, SNPs in two loci attained genome-wide significance (rs1260326 at GCKR on chromosome 2, T allele β=0.08; P=1.8×10−47; rs9987289 at PPPR1B/LOC157273 on chromosome 8, A allele β=0.06; P=1.6×10−9; Table 1). Conditional association analysis of all SNPs at these two regions controlling for the respective index SNP did not identify additional genome-wide significant SNPs, suggesting the absence of multiple independent genetic associations. Figure 1 shows the plot of –log10(P value) by genomic position. Figures 2a and b show the regional association plots at GCKR and PPPR1B/LOC157273, respectively. In the meta-analysis of the index SNPs at GCKR and PPPR1B/LOC157273 combining the results from European-American and African-American subjects, the association of rs1260326 at GCKR was strengthened with no observed heterogeneity between the two populations (T allele β=0.08, P=4.3×10−52, I2=0), while the association of rs9987289 at PPPR1B/LOC157273 was weakened with substantial heterogeneity (A allele β=0.05; P=1.6×10−7, I2=89).
Table 1.
Genome-wide significant loci of plasma lactate identified in European-American subjects
| European-American subjects (n=6901) |
African-American subjects (n=1670) |
Meta-analysis | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP ID | Chr | Position (b37) |
Nearest gene |
SNP function |
Coded allele / Non- coded allele |
Coded allele freq. |
β | SE | P | Variance Explained |
Coded allele freq. |
β | SE | P | β | P | I2 |
| rs1260326 | 2 | 27730940 | GCKR | missense | T/C | 0.41 | 0.08 | 0.006 | 1.8E-47 | 2.8% | 0.15 | 0.08 | 0.02 | 2.3E-05 | 0.08 | 4.3E-52 | 0 |
| rs9987289 | 8 | 9183358 | PPP1R3B, LOC157273 | intron | A/G | 0.09 | 0.06 | 0.010 | 1.6E-09 | 0.5% | 0.18 | 0.006 | 0.02 | 7.5E-01 | 0.05 | 1.6E-07 | 89 |
Chr, chromosome; SNP, single nucleotide polymorphism.
Imputation quality: rs1260326, 0.98 in European-Americans, 0.95 in African-Americans; rs9987289, 0.99 in European-Americans, 0.71 in African-Americans.
FIGURE 1.
Plot of –log10(P-Value) by genomic position from the genome-wide association study of plasma lactate levels
FIGURE 2.
Regional association plots of the GCKR and PPP1R3B loci.
In African-American subjects, rs1260326 at GCKR was also associated with plasma lactate with an effect size similar to that seen among European-American subjects (T allele β=0.08, P=2.3×10−5, Table S3) based on the region-specific significance threshold (1.9×10−3). At the PPP1R3B/LOC157273 locus, none of the SNPs had a P value less than the region-specific threshold (6.3×10−4). Using the region-specific significance thresholds as the α levels, the post hoc power analysis showed that the sample size of African-American subjects in the ARIC study had >99% power to identify an association similar to rs1260326 at the GCKR locus, which explains 2.8% of phenotypic variance, and had only 29% power to identify an association similar to rs9987289 at the PPPR1B/LOC157273 locus, which explains only 0.5% of phenotypic variance.
From the Catalog of Published Genome-wide Association Studies [19], we retrieved 67 index SNPs with genome-wide significant association with diabetes-related traits. Besides the index SNPs at the GCKR and PPPR1B/LOC157273 loci, all other SNPs had P<1×10−6 in the GWAS of plasma lactate in European-American subjects (Table S4).
Interaction between metformin use and index SNPs at GCKR and PPP1R3B/LOC157273 in European-American subjects
At GCKR, the association between the T allele of rs1260326 and plasma lactate did not differ significantly by metformin use among those with diabetes (model 2, diabetes with metformin use: β= −0.01, P=0.90; diabetes without metformin use: β=0.09, P=1.1×10−7; P for interaction 0.32). At the PPP1R3B/ LOC157273 locus, the A allele of rs9987289 was associated with significantly higher lactate levels in those with diabetes taking metformin (β=0.25, P=0.01) compared with those with diabetes not taking metformin (β=0.06, P=0.02, P for interaction=0.02; Table 2).
Table 2.
Association of index single nucleotide polymorphisms at GCKR and PPP1R3B/LOC157273 with log(plasma lactate) in European-Americans, stratified by diabetes and metformin status
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| GCKR (rs1260326 T allele) | B (95% CI) | P | β (95% CI) | P |
| Participants with diabetes | ||||
| With metformin use | 0.07 (−0.049, 0.189) | 2.5E-01 | −0.01 (−0.12, 0.11) | 9.0E-01 |
| Without metformin use | 0.12 (0.084, 0.156) | 1.4E-10 | 0.09 (0.06, 0.12) | 1.1E-07 |
| Participants without diabetes | 0.09 (0.075, 0.099) | 1.2E-44 | 0.07 (0.06, 0.08) | 6.9E-36 |
| PPP1R3B/LOC157273 (rs9987289 A allele) | ||||
| Participants with diabetes | ||||
| With metformin use | 0.30 (0.09, 0.51) | 5.0E-03 | 0.25 (0.06, 0.43) | 1.0E-02 |
| Without metformin use | 0.08 (0.02, 0.14) | 9.4E-03 | 0.06 (0.01, 0.11) | 2.2E-02 |
| Participants without diabetes | 0.06 (0.04, 0.08) | 1.2E-08 | 0.05 (0.03, 0.07) | 5.0E-07 |
Sample size: diabetes with metformin use (n = 87), diabetes without metformin use (n = 715), no diabetes (n = 5924).
Model 1: adjusted for age, sex, centre.
Model 2: Model 1 + BMI, waist circumference, smoking, alcohol consumption, prevalent coronary heart disease, prevalent heart failure, estimated GFR, log(fasting glucose), log(insulin), log(triglycerides), HDL cholesterol, and other diabetes medication (thiazolidinedione, insulin and sulphonylureas).
P for interaction between diabetes with/without metformin use from Model 2: 0.32 for GCKR and 0.01 for PPP1R3B/LOC157273.
Among participants without diabetes and metformin use, the association of the index SNPs at GCKR and PPP1R3B/ LOC157273 with plasma lactate remained strong after adjusting for fasting glucose and insulin and other covariates in model 2 (rs1260326 T allele, β=0.07, P=6.9×10−36; rs9987289 A allele, β=0.05, P=5.0×10−7).
Discussion
Summary of findings
Among European-American subjects in the ARIC study, we identified two loci associated with plasma lactate levels, GCKR and PPP1R3B/LOC157273. The index SNP at GCKR was also associated with lactate among African-American subjects. In addition, among European-American subjects with diabetes, metformin use strengthened the association between the index SNP at PPP1R3B/LOC157273 and plasma lactate levels.
Results in the context of the literature
GCKR
At GCKR, the index SNP (rs1260326; C>T) encodes an amino acid change from proline to leucine (P446L) in the glucokinase regulator protein (GKRP). This variant is associated with lower fasting glucose and insulin levels [25,26] and lower risk for diabetes [27]. In the present study, the T allele was associated with higher lactate levels. GKRP binds to glucokinase (GCK) and inhibits its activities. GCK is a rate-limiting enzyme in hepatic glucose uptake and glycogen storage, and is a negative modifier of hepatic gluconeogenesis. GKRP with the T allele at rs1260326 shows reduced binding to GCK, thereby increasing GCK activity in the liver, promoting hepatic glucose uptake and lowering blood glucose [21,28]. These effects could also increase blood lactate. In carriers of the T allele, the rate of gluconeogenesis may be lower; therefore, lactate utilization will also be lower, as it is an important substrate for gluconeogenesis. Essentially, the GCKR polymorphism might influence Cori cycle kinetics, resulting in different glucose and lactate concentrations at steady state. As a result, the T allele at rs1260326 is associated with lower glucose and reduced risk of incident diabetes but higher lactate levels. Notably, higher blood lactate levels are associated with a higher risk of incident diabetes [4,18]; therefore, the positive association between lactate and incident diabetes probably reflects mechanisms independent of the GCKR pathway.
PPP1R3B/LOC157273
At the PPP1R3B/LOC157273 region, the minor allele (A) of rs4841132 is associated with higher fasting glucose and insulin levels in previous GWAS [29]. In the present study, the A allele at rs4841132 was associated with higher lactate levels (β=0.06, P=1.6×10−9) and was in high linkage disequilibrium with rs9987289, the index SNP at the PPP1R3B/LOC157273 (D’=1 and r2=1 based on 1000 Genomes Pilot 1 data) [30]. The minor allele at rs9987289 was associated with higher lactate, and this association was strengthened in individuals taking metformin. PPP1R3B encodes the regulatory subunit 3b of protein phosphatase 1 (PP1) and is highly expressed in both skeletal muscle and the liver [31]. rs9987289 is associated with expression levels of PPP1R3B in a large gene expression study of human liver tissue [32]. PPP1R3B suppresses deactivation of glycogen phosphorylase and enhances activation of glycogen synthase in the liver [33]. If the causal variant or variants underlying this association decrease glycogen production, then some portion of the glucose destined for glycogen may instead end as lactate via glycolysis.
Of the index SNPs in loci of diabetes-related traits in the Catalog of Published Genome-wide Association Studies, only the index SNPs at GCKR and PPP1R3B/LOC157273 reached genome-wide significance in the GWAS of plasma lactate in European-American subjects, and all other index SNPs had modest to negligible association with plasma lactate. We speculate that the strong association of the index variants at GCKR and PPP1R3B/LOC157273 with plasma lactate is a consequence of the involvement of the underlying causal variants in hepatic gluconeogenesis, which is a major determinant of plasma lactate levels, while the causal variants of the other diabetes-related loci are functional in other tissues or pathways and therefore had much weaker or little association with plasma lactate levels. For example, GCK is important in glucose metabolism in both β-cells and hepatocytes, and the index variant of GCK (rs4607517) in the GWASs of glycaemic traits is in high linkage disequilibrium with a variant in the β-cell-specific promoter of GCK and not variants in the hepatocyte-specific promoter [34]. This could explain why rs4607517 had little association with plasma lactate in our analysis (P=0.41). The strong associations of the index SNPs at GCKR and PPP1R3B/ LOC157273 with plasma lactate among European-American subjects without diabetes, after adjusting for fasting insulin, also supports the hypothesis that the genetic association of plasma lactate at these two loci are not mediated by insulin resistance or β-cell function.
Interaction between metformin and lactate-associated variants
Metformin decreases the uptake of lactate as a substrate of hepatic gluconeogenesis and thus increases lactate levels [35]. There was no interaction between the index SNP at GCKR and metformin with regard to lactate levels. As the index SNP at GCKR and metformin are both associated with higher lactate through decreased gluconeogenesis, at least in part, this lack of interaction suggests that the effect of these factors is not multiplicative. In contrast, PPP1R3B/LOC157273 was associated with higher lactate among subjects with diabetes taking metformin than among those not taking metformin. The causal variant(s) at PPP1R3B/LOC157273 may be associated with increased conversion of glucose to lactate because the conversion of glucose to glycogen is decreased; therefore, among those taking metformin, excess lactate produced cannot be converted back to glucose via gluconeogenesis because metformin decreases the rate of gluconeogenesis. In addition to inhibiting gluconeogenesis, metformin also lowers glucose levels by suppressing glucagon signalling, which mediates the conversion of glycogen to glucose [35], while PPP1R3B is involved in the pathway of glycogen synthesis from glucose [31]. If decreased glycogenolysis attributable to metformin leads to a response that reduces glycogen synthesis, then the synergistic effect between metformin and the lactate-increasing variant near PPP1R3B is biologically plausible. This is the first genome-wide association study of plasma lactate in a population-based cohort that includes subjects of both European and African origin. The sample size of African-American subjects in the ARIC study had enough power to identify genetic association of moderate effect sizes, such as the association at GCKR, and had low power to identify genetic association of small effect size, such as the association at PPP1R3B/LOC157273, which also had substantial allelic heterogeneity between European-American and African-American subjects. The present findings have not been replicated in other European-American populations; however, our findings have high biological plausibility, because the genes are associated with glucose homeostasis and share common pathways with lactate metabolism.
In conclusion, GCKR and PPP1R3B/LOC157273 are two genome-wide significant loci with variants influencing plasma lactate among European-American subjects. The index SNP at GCKR was also associated with lactate among African-American subjects. SNPs in both regions are associated with other quantitative phenotypes related to diabetes. In addition, metformin modified the effect of the index variant at PPP1R3B/LOC157273 on plasma lactate levels. These findings further our understanding of lactate metabolism and mechanisms through which metformin increases lactate.
Supplementary Material
What's new?
We report the first genome-wide association study of plasma lactate levels in individuals with European ancestry and the identification of GCKR and PPP1R3B as genome-wide significant loci of lactate levels. The association at GCKR was also significant in individuals of African ancestry at the gene level.
We also identified a significant interaction between metformin and the index single nucleotide polymorphism at PPP1R3B on lactate levels.
Our findings add to our knowledge on the mechanism of metformin and lactate metabolism.
Acknowledgements
The authors thank the staff and participants of the ARIC study for their important contributions.
Funding sources
The ARIC Study was carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C), R01HL087641, R01HL59367 and R01HL086694; National Human Genome Research Institute contract U01HG004402; and National Institutes of Health (NIH) contract HHSN268200625226C. Infrastructure was partly supported by Grant Number UL1RR025005, a component of the NIH and NIH Roadmap for Medical Research. A.T. is supported by the NIH/NIDDK T32 DK007732 Renal Disease Epidemiology Training Program.
Footnotes
Competing interests
None declared.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Table S1. Participants included in genetic association analysis.
Table S2. Study population characteristics.
Table S3. Association between the GCKR and PPPIR3B/LOC157273 loci and plasma lactate in African-American subjects in the Atherosclerosis Risk in Communities study (N=1670).
Table S4. Association between log(plasma lactate) and index single nucleotide polymorphisms in European-American subjects included in the Atherosclerosis Risk in Communities study at known loci of diabetes-related traits.
References
- 1.Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin Proc. 2013;88:1127–1140. doi: 10.1016/j.mayocp.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Hall G. Lactate kinetics in human tissues at rest and during exercise. Acta Physiol. 2010;199:499–508. doi: 10.1111/j.1748-1716.2010.02122.x. [DOI] [PubMed] [Google Scholar]
- 3.Crawford SO, Hoogeveen RC, Brancati FL, Astor BC, Ballantyne CM, Schmidt MI, et al. Association of blood lactate with type 2 diabetes: the Atherosclerosis Risk in Communities Carotid MRI Study. Int J Epidemiol. 2010;39:1647–1655. doi: 10.1093/ije/dyq126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Juraschek SP, Selvin E, Miller ER, Brancati FL, Young JH. Plasma lactate and diabetes risk in 8045 participants of the atherosclerosis risk in communities study. Ann of Epidemiol. 2013;23:791–796. e794. doi: 10.1016/j.annepidem.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsushita K, Williams EK, Mongraw-Chaffin ML, Coresh J, Schmidt MI, Brancati FL, et al. The association of plasma lactate with incident cardiovascular outcomes: the ARIC Study. Am J Epidemiol. 2013;178:401–409. doi: 10.1093/aje/kwt002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shantha GP, Wasserman B, Astor BC, Coresh J, Brancati F, Sharrett AR, et al. Association of blood lactate with carotid atherosclerosis: the Atherosclerosis Risk in Communities (ARIC) Carotid MRI Study. Atherosclerosis. 2013;228:249–255. doi: 10.1016/j.atherosclerosis.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crawford SO, Ambrose MS, Hoogeveen RC, Brancati FL, Ballantyne CM, Young JH. Association of lactate with blood pressure before and after rapid weight loss. Am J Hypertens. 2008;21:1337–1342. doi: 10.1038/ajh.2008.282. [DOI] [PubMed] [Google Scholar]
- 8.Sargen MR, Hoffstad OJ, Wiebe DJ, Margolis DJ. Geographic variation in pharmacotherapy decisions for U.S. Medicare enrollees with diabetes. J Diabetes Complications. 2012;26:301–307. doi: 10.1016/j.jdiacomp.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Berlo-van de Laar IR, Vermeij CG, Doorenbos CJ. Metformin associated lactic acidosis: incidence and clinical correlation with metformin serum concentration measurements. J Clin Pharm Ther. 2011;36:376–382. doi: 10.1111/j.1365-2710.2010.01192.x. [DOI] [PubMed] [Google Scholar]
- 10.ARIC. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 11.Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333:550–554. doi: 10.1056/NEJM199508313330903. [DOI] [PubMed] [Google Scholar]
- 12.Tin A, Astor BC, Boerwinkle E, Hoogeveen RC, Coresh J, Kao WH. Genome-wide significant locus of beta-trace protein, a novel kidney function biomarker, identified in European and African Americans. Nephrol Dial Transplant. 2013;28:1497–1504. doi: 10.1093/ndt/gfs591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howie B, Fuchsberger C, Stephens M, Marchini J, Abecasis GR. Fast and accurate genotype imputation in genome-wide association studies through pre-phasing. Nat Genet. 2012;44:955–959. doi: 10.1038/ng.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delaneau O, Zagury JF, Marchini J. Improved whole-chromosome phasing for disease and population genetic studies. Nat Methods. 2013;10:5–6. doi: 10.1038/nmeth.2307. [DOI] [PubMed] [Google Scholar]
- 15.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–2191. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu CT, Garnaas MK, Tin A, Kottgen A, Franceschini N, Peralta CA, et al. Genetic association for renal traits among participants of African ancestry reveals new loci for renal function. PLoS Genet. 2011;7:e1002264. doi: 10.1371/journal.pgen.1002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juraschek SP, Shantha GP, Chu AY, Miller ER, 3rd, Guallar E, Hoogeveen RC, et al. Lactate and risk of incident diabetes in a case-cohort of the atherosclerosis risk in communities (ARIC) study. PloS One. 2013;8:e55113. doi: 10.1371/journal.pone.0055113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.A Catalog of Published Genome-Wide Association Studies. National Human Genome Research Institute; 2014. [Google Scholar]
- 20.Pernicova I, Korbonits M. Metformin-–mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol. 2014;10:143–156. doi: 10.1038/nrendo.2013.256. [DOI] [PubMed] [Google Scholar]
- 21.Beer NL, Tribble ND, McCulloch LJ, Roos C, Johnson PR, Orho-Melander M, et al. The P446L variant in GCKR associated with fasting plasma glucose and triglyceride levels exerts its effect through increased glucokinase activity in liver. Hum Mol Genet. 2009;18:4081–4088. doi: 10.1093/hmg/ddp357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo X, Zhang Y, Ruan X, Jiang X, Zhu L, Wang X, et al. Fasting-induced protein phosphatase 1 regulatory subunit contributes to postprandial blood glucose homeostasis via regulation of hepatic glycogenesis. Diabetes. 2011;60:1435–1445. doi: 10.2337/db10-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mongraw-Chaffin ML, Matsushita K, Brancati FL, Astor BC, Coresh J, Crawford SO, et al. Diabetes medication use and blood lactate level among participants with type 2 diabetes: the atherosclerosis risk in communities carotid MRI study. PloS One. 2012;7:e51237. doi: 10.1371/journal.pone.0051237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marchini J, Howie B, Myers S, McVean G, Donnelly P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat Genet. 2007;39:906–913. doi: 10.1038/ng2088. [DOI] [PubMed] [Google Scholar]
- 25.Ramos E, Chen G, Shriner D, Doumatey A, Gerry NP, Herbert A, et al. Replication of genome-wide association studies (GWAS) loci for fasting plasma glucose in African-Americans. Diabetologia. 2011;54:783–788. doi: 10.1007/s00125-010-2002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42:105–116. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaxillaire M, Cavalcanti-Proenca C, Dechaume A, Tichet J, Marre M, Balkau B, et al. The common P446L polymorphism in GCKR inversely modulates fasting glucose and triglyceride levels and reduces type 2 diabetes risk in the DESIR prospective general French population. Diabetes. 2008;57:2253–2257. doi: 10.2337/db07-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rees MG, Wincovitch S, Schultz J, Waterstradt R, Beer NL, Baltrusch S, et al. Cellular characterisation of the GCKR P446L variant associated with type 2 diabetes risk. Diabetologia. 2012;55:114–122. doi: 10.1007/s00125-011-2348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manning AK, Hivert MF, Scott RA, Grimsby JL, Bouatia-Naji N, Chen H, et al. A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat Genet. 2012;44:659–669. doi: 10.1038/ng.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Broad Institute SNP Annotation and Proxy Search (SNAP) http://www.broadinstitute.org/mpg/snap/ldsearchpw.php.
- 31.Newgard CB, Brady MJ, O'Doherty RM, Saltiel AR. Organizing glucose disposal: emerging roles of the glycogen targeting subunits of protein phosphatase-1. Diabetes. 2000;49:1967–1977. doi: 10.2337/diabetes.49.12.1967. [DOI] [PubMed] [Google Scholar]
- 32.Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doherty MJ, Moorhead G, Morrice N, Cohen P, Cohen PT. Amino acid sequence and expression of the hepatic glycogen-binding (GL)-subunit of protein phosphatase-1. FEBS Lett. 1995;375:294–298. doi: 10.1016/0014-5793(95)01184-g. [DOI] [PubMed] [Google Scholar]
- 34.Prokopenko I, Langenberg C, Florez JC, Saxena R, Soranzo N, Thorleifsson G, et al. Variants in MTNR1B influence fasting glucose levels. Nat Genet. 2009;41:77–81. doi: 10.1038/ng.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fulgencio JP, Kohl C, Girard J, Pegorier JP. Effect of metformin on fatty acid and glucose metabolism in freshly isolated hepatocytes and on specific gene expression in cultured hepatocytes. Biochem Pharmacol. 2001;62:439–446. doi: 10.1016/s0006-2952(01)00679-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.