Abstract
The first year of life is an important period for emergence of fear in humans. While animal models have revealed developmental changes in amygdala circuitry accompanying emerging fear, human neural systems involved in early fear development remain poorly understood. To increase understanding of the neural foundations of human fear, it is important to consider parallel cognitive development, which may modulate associations between typical development of early fear and subsequent risk for fear-related psychopathology. We, therefore, examined amygdala functional connectivity with rs-fcMRI in 48 neonates (M = 3.65 weeks, SD = 1.72), and measured fear and cognitive development at 6-months-of-age. Stronger, positive neonatal amygdala connectivity to several regions, including bilateral anterior insula and ventral striatum, was prospectively associated with higher fear at 6-months. Stronger amygdala connectivity to ventral anterior cingulate/anterior medial prefrontal cortex predicted a specific phenotype of higher fear combined with more advanced cognitive development. Overall, findings demonstrate unique profiles of neonatal amygdala functional connectivity related to emerging fear and cognitive development, which may have implications for normative and pathological fear in later years. Consideration of infant fear in the context of cognitive development will likely contribute to a more nuanced understanding of fear, its neural bases, and its implications for future mental health.
Keywords: Amygdala, Fear, Cognitive development, Infancy, Resting state fMRI
1. Introduction
1.1. Typical development of fear in infancy
Fear is a basic emotion involving perception of potential danger. The importance of fear for survival and adaptive functioning is highlighted by the conservation of fear-based learning and behavior, and the underlying neural circuitry across species (Milad and Quirk, 2012, Phelps and LeDoux, 2005). The developmental trajectory of fear in human infants is typically characterized by increasing fearfulness over the first year of life (Carranza Carnicero et al., 2000, Gartstein and Rothbart, 2003, Gartstein et al., 2010). In particular, a steep increase in fearfulness has been observed beginning at approximately 6-months-of-age (Braungart-Rieker et al., 2010) when a fear of strangers (Brooker et al., 2013, Waters et al., 1975), and increased allocation of attention to human facial expressions indicative of danger (Nelson and Dolgin, 1985, Peltola et al., 2009a, Peltola et al., 2009b) begin to emerge.
While a large part of the child and adult literature focus on pathologic aspects of fear, the increase in fear around 6 months of age in infants has been interpreted as supporting infants’ ability to successfully navigate the environment (as it coincides with increased capacity to independently navigate and explore surroundings, Leppanen and Nelson, 2012). At the same time, infant fear plays an important role in facilitating attachment with caregivers, as the presence of caregivers regulates fear and reduces distress (Ainsworth and Bell, 1970, Hofer, 1994, Landers and Sullivan, 2012). In fact, higher levels of fear during infancy have been associated with increased capacity to benefit from supportive caregiving (Belsky et al., 2007, Belsky and Pluess, 2013). Moreover, early fearfulness is also associated with subsequent development of prosocial emotions (Baker et al., 2012, Kochanska et al., 2002), and lower levels of aggression and disruptive behavior problems (Biederman et al., 2001, Rothbart and Bates, 2006).
1.2. Links between early fear and subsequent pathological fear
Although fear is adaptive, and typical developmental trajectories involve increasing fear during the first year of life, high levels of fearfulness during early development have also been associated with development of anxiety disorders. Higher levels of fearful temperament (and particularly a well characterized type of fearful temperament termed behavioral inhibition) during infancy and early childhood have been linked to the subsequent emergence of anxiety symptoms (Gartstein et al., 2010, Kagan and Snidman, 1999), and increased frequency of anxiety disorders in childhood and adolescence (Clauss and Blackford, 2012). Consistently high levels of fearfulness over time during toddlerhood and early childhood (Chronis-Tuscano et al., 2009, Hirshfeld et al., 1992), and extremes of fearful temperament during infancy and toddlerhood (Pérez-Edgar and Fox, 2005, Schwartz et al., 1999), are particularly strong predictors of emerging anxiety in adolescence. However, despite these findings, the association between early temperamental fear and subsequent anxiety is modest (Degnan and Fox, 2007, Nigg, 2006).
1.3. Disentangling maladaptive versus adaptive fear development by considering parallel cognitive development
In light of somewhat conflicting evidence regarding the implications of early fear development, investigators have sought to examine factors that interact with early fearfulness to determine healthy versus maladaptive developmental trajectories (Degnan et al., 2010). One fruitful approach that has emerged for disentangling maladaptive versus adaptive fear development is examination of fear in parallel with cognitive development. Patterns of negative emotional reactivity, including fearfulness, have generally been conceptualized as strong risk factors for psychopathology in the context of low cognitive capacity to regulate emotional and behavioral responses (Nigg, 2006, Rothbart and Bates, 2006). In line with this idea, children with high levels of fearful or inhibited temperament accompanied by higher cognitive skills, and particularly ability to shift attention, are less likely to develop anxiety symptoms compared to fearful children with lower cognitive skills (Degnan and Fox, 2007, Fox and Pine, 2012, White et al., 2011). This pattern extends to infancy. During the first year of life, higher levels of negative affect (including fear) predict subsequent internalizing symptoms only for infants with poor capacity to orient attention and regulate emotions (Gartstein et al., 2012). Thus development of cognitive skills may mitigate the chances of normative fear development leading to fear-related psychopathology.
Other research goes further and suggests that the combination of higher fear and early emerging cognitive skills during infancy may be associated with more optimal emotional and cognitive outcomes. For example, Crockenberg and Leerkes (2006) reported that in the context of good attentional control at 6 months, high distress to novelty at 6 months predicted lower levels of anxiety symptoms at 2.5 years (Crockenberg and Leerkes, 2006). A combination of both high negative reactivity and high frequency of regulatory behaviors (including attention orienting, seeking comfort, and avoidance) in response to a frightening stimulus at 15 months, has been found to predict the highest levels of executive functioning at 4-years compared to all other combinations of reactivity and regulation (Ursache et al., 2013). Taken together these results suggest that parallel examination of cognitive development is an important starting point for understanding the implications of fear during infancy for ongoing development and behavioral health.
1.4. Early brain connectivity as a predictor of fear development
In the current study we attempt to determine whether markers of functional brain organization in the neonatal period precede and relate to the emergence of fear at 6 months-of-age. Importantly, we do this in the context of variation in cognitive development to contribute to a more nuanced understanding of fear development. We focus on amygdala connectivity due to the animal literature indicating an important role for the amygdala in the early development of fear (Barr et al., 2009, Bauman et al., 2004, Bliss-Moreau et al., 2010, Moriceau and Sullivan, 2006, Moriceau et al., 2006, Raper et al., 2013). In humans, the amygdala functions in a coordinated manner with multiple subcortical and cortical brain regions including sensorimotor, emotion, memory and higher order attention centers (Das et al., 2005, Gabard-Durnam et al., 2014, Gee et al., 2013a, Gee et al., 2013b, Qin et al., 2014, Qin et al., 2012, Roy et al., 2009, Stein et al., 2007). Previous work in children and adults has highlighted coordinated functioning of the amygdala with several of these brain regions, including the anterior insula (aI) and medial prefrontal cortex (MPFC), as particularly important for understanding the neural basis of typical and pathological fear (Callaghan et al., 2014, Etkin et al., 2011, Etkin and Wager, 2007, Milad and Quirk, 2012, Qin et al., 2014, Rabinak et al., 2011, Sripada et al., 2012). However, the role of amygdala connectivity in the emergence of fear during infancy has not been investigated.
1.5. The role of the caregiving environment
In examining neonatal amygdala connectivity as a precursor of emerging fear and cognitive development during infancy, it is imperative to consider the role of the caregiving environment. Evidence suggests that early developmental trajectories of fear are influenced by levels of positive and responsive caregiving (Braungart-Rieker et al., 2010, Gartstein et al., 2010). Work in animal models and with human children indicates that caregiving influences emerging fear via effects on developing amygdala circuitry (Gee et al., 2013a, Landers and Sullivan, 2012, Tottenham, 2014). An emerging body of work also suggests that certain early biological phenotypes (in the form of combinations of certain genes or aspects of nervous system functioning) confer sensitivity to the caregiving environment, such that negative environments will be associated with more detrimental outcomes, and positive environments will be associated with more gains (Belsky, 1997, Boyce and Ellis, 2005). Patterns of amygdala functioning (and associated physiological and behavioral measures) are posited to be a potentially important biological phenotype in determining sensitivity to the caregiving environment (Belsky and Pluess, 2013, Obradović and Boyce, 2009).
1.6. Present study
The present study therefore has the following aims. First, we aim to identify whether patterns of neonatal amygdala functional connectivity precede and relate to emerging fear at 6-months-of-age, an important time for typical development of fear behaviors. Second, we aim to establish whether patterns of amygdala connectivity at birth provide information relevant not only to development of fear, but to a more nuanced understanding of fear that incorporates cognitive development. Third, we aim to identify the extent to which functional brain connectivity at birth is relevant for emerging fear and cognition after accounting for the influence of the postnatal caregiving environment. As part of the third aim, we also investigate whether patterns of brain connectivity at birth confer sensitivity to the influence of postnatal caregiving on fear and cognitive development.
2. Methods and materials
2.1. Participants
Infants included in the study (N = 48) were part of an ongoing prospective longitudinal study for which mothers were recruited during the first trimester of pregnancy. Exclusionary criteria for infants were as follows: birth before 34 weeks gestation, and evidence of a congenital, genetic or neurologic disorder. Infants were approximately 1 month-of-age at the time of the fMRI scan, and 6.5-months-of-age at the time of the behavioral assessment. Detailed demographic information is presented in Table 1. All procedures were approved by the Institutional Review Board at University of California, Irvine.
Table 1.
Demographics.
| Mean (SD) | |
|---|---|
| Age in weeks | |
| Gestational age at birth | 39.3 (1.39) |
| Age at fMRI data collection | 3.65 (1.72) |
| Age at behavioral assessment | 28.0 (1.98) |
| Percentage | |
| Sex | |
| Male | 62.5 |
| Female | 37.5 |
| Race/ethnicity | |
| Caucasian non-Hispanic | 42.6 |
| African American non-Hispanic | 2.13 |
| Asian non-Hispanic | 10.6 |
| Multi-racial non-Hispanic | 10.6 |
| Caucasian Hispanic | 29.8 |
| Asian Hispanic | 2.13 |
| Multi-racial Hispanic | 2.13 |
| Highest level of maternal education | |
| High-school or test equivalent | 10.4 |
| Vocational school or some college | 50.0 |
| Associates degree | 4.20 |
| Bachelors or graduate level degree | 35.5 |
| Gross annual household income | |
| <$15,000 | 6.38 |
| $15,000–29,999 | 19.1 |
| $30,000–49,999 | 29.8 |
| $50,000–100,000 | 36.2 |
| >$100,000 | 8.51 |
2.2. MRI and fMRI data acquisition and processing
2.2.1. Data acquisition
Neuroimaging data was collected during natural sleep on a TIM Trio, Siemens Medical System 3.0T scanner. Neonates were swaddled and fitted with ear protection to reduce scanner noise. Waking and respiration were monitored throughout the scan to ensure the comfort and safety of the neonates. A high-resolution T2-weighted scan (TR = 3200 ms, echo time = 255 ms, resolution = 1 × 1 × 1 mm, 4.18 min) was collected as the anatomical reference image for the functional images. A high-resolution T1-weighted scan (MP-RAGE TR = 2400 ms, inversion time = 1200 ms, echo time = 3.16 ms, flip angle = 8°, resolution = 1 × 1 × 1 mm, 6.18 min) was also collected, and used in conjunction with the high-resolution T2-weighted scan for segmentation of the amygdala (described below). Functional images for rs-fcMRI were obtained using a gradient-echo, echoplanar imaging (EPI) sequence sensitive to blood oxygen level-dependent (BOLD) contrast (TR = 2000 ms; TE = 30 ms; FOV = 220 × 220 × 160 mm; flip angle = 77°). Full brain coverage was obtained with 32 ascending-interleaved 4 mm axial slices with a 1 mm skip. Steady-state magnetization was assumed after 4 frames (8 ∼ s). Functional data was obtained in a single scan consisting of 150 volumes for early participants (N = 8), and increased to 195 volumes (N = 40) in later stages of the study to increase the likelihood of acquiring a sufficient number of volumes for analysis. As discussed below, only functional scans with at least 4 min of data (after volume removal for motion) were included in the present study.
2.2.2. fMRI data preprocessing
Brain images were separated from the rest of the head tissue with the Brain Extraction Tool from the FMRIB Software Library (Beckmann et al., 2006, Smith et al., 2001, Smith, 2002), and additional refinement with an in-house technique to refine brain masks as necessary. Functional images were preprocessed to reduce artifacts (Miezin et al., 2000). These steps included:(i) removal of a central spike caused by MR signal offset, (ii) correction of odd versus even slice intensity differences attributable to interleaved acquisition without gaps, (iii) realignment, and (iv) intensity normalization to a whole brain mode value of 1000. Atlas transformation of the functional data was computed for each individual via the high-resolution T2 scan. The transformation involved calculation of a single matrix for each individual to facilitate registration both to a standard infant template (0- to 2-month age range; MRI Study of Normal Brain Development; Fonov et al., 2011, Fonov et al., 2009), and to the Talairach coordinate system (Talairach and Tournoux, 1988) (by aligning the infant template to a custom atlas-transformed, Lancaster et al., 1995 target template [711-2B] using a series of affine transforms (Michelon et al., 2003. Each run was then resampled in atlas space (Talairach and Tournoux, 1988), combining realignment and atlas transformation in one interpolation. All subsequent operations were performed on the atlas-transformed volumetric time series. Visual inspection of data resulted in the loss of two subjects for poor quality EPI scans, and one subject for a structural abnormality identified in the high resolution T2-weighted scan.
2.2.3. rs-fcMRI preprocessing
Additional preprocessing steps designed for rs-fcMRI were conducted to account for signal stemming from non-neuronal processes (Fair et al., 2012, Fox and Raichle, 2007). These steps followed established procedures described in previous work including, temporal band-pass filtering (0f < 0.1 Hz), regression of rigid body head motion parameters in 6 directions, regression of the whole brain signal, regression of ventricular signal averaged from a ventricular region mask, regression of white matter signal averaged from a white matter mask and regression of first order derivative terms for the whole brain, ventricular, and white matter signals (Fair et al., 2007, Fair et al., 2009, Fair et al., 2012). Due to research demonstrating that unfiltered nuisance regression on filtered data can introduce noise (Hallquist et al., 2013), we made sure that nuisance variables for regression were applied prior to bandpass filtering. Additional steps were taken to examine movement of a given frame relative to the previous frame, known as framewise displacement (FD; Fair et al., 2012, Power et al., 2012). We used a volume censoring approach, removing volumes associated with greater than 0.3 mm FD (and 1 preceding and 2 following volumes to account for temporal blurring; Power et al., 2012). We then removed scans with less than 4 min of data remaining, which resulted in removing an additional three infants from analyses. For the remaining infants (N = 48) scan length after volume removal was approximately 5 and a half minutes (M = 5.50, range = 4.17–6.30), and remaining FD was approximately 0.08 (M = 0.081, range = 0.047–0.134). Based on the recent recommendations, post-hoc analyses included adjustment for remaining FD to rule out effects of remaining motion on results (Fair et al., 2012, Power et al., 2015).
2.2.4. Amygdala regions of interest
Amygdala were segmented using a multi-modality, multi-template based automatic method combining T1 and T2 weighted high-resolution images (Wang et al., 2014), followed by manual correction in ITK-Snap (Yushkevich et al., 2006). Manual corrections of the automatic segmentations were performed with data re-aligned such that the anterior–posterior direction was positioned along hippocampal long axis. We employed a strict protocol of correcting the amygdala boundary definition using both the T1 and the T2 weighted image. Images were segmented in original and left-right mirrored presentation to account for asymmetric presentation biases (Maltbie et al., 2012) and averaged for the final segmentation. Scan/rescan stability tests for the automatic segmentation procedure have been conducted in a separate sample set. Reliability for manual correction was established for raters on this dataset via a standard reliability study, in which 5 datasets were triplicated and randomized. These 15 datasets were then segmented automatically, and manually corrected by two raters. For manual correction of these 15 datasets, agreement between the two raters (inter-rater correlation coefficient) and agreement within the same rater across the same dataset (intra-rater correlation coefficient) were both above 0.98.
For each infant, the individualized amygdala regions of interest (ROIs) were transformed to atlas space based on the atlas transform previously computed for the functional data and high-resolution T2 scan. These individualized amygdala ROIs were used as seed regions for rs-fcMRI analyses. Specifically, the time course of the BOLD signal was averaged across the voxels within each amygdala ROI, and then correlated with the time course for all other voxels throughout the brain. This resulted in whole-brain voxel-wise connectivity maps for both the left and right amygdala, which were used as predictors of the behavioral outcomes of interest in regression models employing Monte Carlo simulation to account for multiple comparisons (Forman et al., 1995; as described in more detail in Section 2.5).
2.3. Infant behavioral outcomes
2.3.1. Infant fear
Infant fear at 6 months was assessed via maternal report on the Infant Behavior Questionnaire-Revised (IBQ-R; Gartstein and Rothbart, 2003). Mothers rated infants’ engagement in specific fear-related behaviors from 1 (never) to 7 (always). This 16-item scale assesses “negative affect related to anticipated pain, distress and/or threat” (p. 198, Gartstein et al., 2012). This includes signs of distress and startle in response to rapid changes in sensory stimuli, and in response to novel physical and social stimuli. Items also measure slow or inhibited approach to novel stimuli. The scale score is calculated as the mean of all item ratings. Maternal report on this measure has been widely used to assess infant fear in the first year of life (Bornstein et al., 2015, Gartstein et al., 2010), and demonstrated good internal consistency (Gartstein and Rothbart, 2003, Parade and Leerkes, 2008), and convergent validity with laboratory observation (Braungart-Rieker et al., 2010, Gartstein et al., 2010, Parade and Leerkes, 2008).
2.3.2. Infant cognitive development
Infant cognitive development at 6-months was assessed with the Cognitive Scale composite score from Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III; Bayley, 2006). This is a widely used, standardized measure of infant development. The Cognitive Scale includes developmentally appropriate assessment of emerging sensorimotor, attention and memory skills, as well as interest in and understanding of the environment.
2.3.3. Differentiated fear phenotype
As a more nuanced/differentiated assessment of infant fear including cognitive development, an interaction term was created by multiplying infant fear and cognitive development scores. This continuous variable was used as an outcome in subsequent analyses. All analyses examining this outcome included the fear and cognitive development scores as covariates to adjust for their main effects.
2.4. Postnatal caregiving environment
2.4.1. Quality of the caregiving environment
The Home Observation for Measurement of the Environment (HOME) Inventory (Caldwell and Bradley, 2003) was used to index the quality of the caregiving environment when the infant was 6-month-of-age. Measurement is based on home observation and a semi-structured interview with mothers. Observers were trained, and achieved reliability with a certified administrator of this inventory (95% agreement on two consecutive videos). Analyses employed the total score, which is a summary measure that incorporates assessment of interactions between infants and caregivers (responsivity and acceptance), and the extent to which the physical environment is organized and supportive of infant development (learning materials and organization). In follow-up analyses, we additionally examined composites of subscales that are likely most relevant to the each behavioral outcome. For fear, we examined a composite of the responsivity and acceptance scales (mean), and for cognitive development, a composite of the learning materials and organization scales (mean).
2.4.2. Maternal depressive symptoms
The 20-item Center for Epidemiological Studies of Depression Scale (CESD; Radloff, 1977) was used to assess maternal depression concurrently with the IBQ-R due to evidence that maternal report of infant emotionality can be influenced by maternal mood (Parade and Leerkes, 2008). Mothers reported on symptoms of depression over the past week on a 4-point scale, ranging from 0 (rarely or none of the time) to 3 (most or all of the time). The mean score was used in analyses.
2.5. Analysis outline
Regression analyses were used to examine the associations between whole brain voxel-wise connectivity of the amygdala (left and right separately) during the neonatal period, and each behavioral outcome (fear, cognitive development and the differentiated fear phenotype) at 6 months age. Each regression model included whole brain voxel-wise connectivity of the left or right amygdala as the independent variable and one of the behavioral outcomes as the dependent variable. Infant gestational age at birth and age at scan were included as covariates in all analyses, and the fear and cognitive development scores (main effects) were included as covariates for the differentiated fear phenotype (interaction). For all of the primary results, post-hoc analyses were conducted including a covariate for remaining FD (micromovements remaining in the rs-fcMRI scan following removal of frames based on motion parameters) to assess for the potential confound of residual motion (Power et al., 2015). We examined associations between the outcomes of interest and additional potential confounds (maternal depression and infant sex) to determine whether to include them as covariates in post-hoc analyses. For the whole brain analyses, thresholding based on Monte Carlo simulation was implemented to account for multiple comparisons (Forman et al., 1995). Correction for p < 0.05 voxel clusters required a threshold of 53 contiguous voxels with a Z-value >2.25.
Post-hoc analyses examining the influence of the postnatal caregiving environment focused on functional connections identified in these whole brain regression analyses as associated with the behavioral outcomes. We focused specifically on functional connections, which have previously been shown to be particularly important for understanding the neural basis of typical and pathological fear in animal models, and human children and adults (Callaghan et al., 2014, Etkin et al., 2011, Etkin and Wager, 2007, Milad and Quirk, 2012, Qin et al., 2014, Rabinak et al., 2011, Sripada et al., 2012), such as amygdala-insula connectivity and amygdala-MPFC connectivity. This approach was taken to reduce multiple comparisons, and facilitate interpretability of results. Correlation coefficients (r) representing the strength of these connections were extracted using previously reported methods (Graham et al., 2015). In brief, a search algorithm from the 4dfp Suite of Image Processing Programs (ftp://ftp.imaging.wustl.edu/pub/raichlab/4dfp_tools/) was used to identify ROIs surrounding peak voxels within the whole brain thresholded results map. The time course of the signal was averaged across the voxels within each ROI and then correlated with the time course for the amygdala signal. Hierarchical linear regression models (one for each connection) were then used to examine: (1) whether the strength of these functional connections in neonates predicted 6-month behavioral outcomes above and beyond the influence of the postnatal caregiving environment, and (2) whether functional connection strength at birth interacted with the subsequent caregiving environment to predict 6-month behavioral outcomes (i.e. functional amygdala connectivity at birth was tested as a potential indicator of neural sensitivity to the environment). Previous covariates from the whole brain regressions were retained in analyses.
3. Results
3.1. Preliminary analyses
3.1.1. Infant fear and cognitive development measures
Internal consistency of the fear scale in this sample was high (α = 0.915). The distribution included a wide range of fearfulness (M = 2.84, SD = 1.11, range = 1.13–6.00). We created an age-residualized fear score to account for infant age at the time of IBQ assessment, which was used in all analyses. The distribution of cognitive development scores in the current sample was in line with expectations for typically developing infants (M = 103.4, SD = 8.70, range = 85–120).
3.1.2. Postnatal caregiving environment
The wide range of normally distributed scores on the HOME Inventory (M = 35.6, SD = 3.96, range = 25–44) indicated a high level of variability in the quality of the caregiving environment in this sample.
3.1.3. Maternal depressive symptoms
Internal reliability of the CESD was high (α = 0.921), and the distribution was in line with expectations for a non-clinical sample of mothers (M = 10.3, SD = 9.86, range = 0–41).
3.1.4. Associations among main outcome variables and covariates
The main outcome variables (infant fear and cognitive development) were not significantly correlated (p > 0.1; Table 2). Furthermore, gestational age at birth, postnatal age at scan and maternal depressive symptoms were not associated with these main outcomes (Table 2). There were also no significant differences in the behavioral outcomes of interest depending on infant sex (p > 0.1). Due to the lack of significant associations between maternal depressive symptoms, infant sex and any of the main outcome variables, maternal depressive symptoms and infant sex were not adjusted for in any analyses. As expected the summary measure of the caregiving environment (HOME total) was strongly positively correlated with the two subscales (acceptance–responsivity and learning-organization). The two subscales were moderately correlated with one another. The learning-organization subscale demonstrated a negative association with maternal depressive symptoms, and the acceptance–responsivity subscale evidenced a positive association with infant cognitive development.
Table 2.
Correlations among study variables and covariates.
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| 1. Gestational age at birth | – | |||||
| 2. HOME total | 0.020 | – | ||||
| 3. HOME acceptance–responsivity | 0.093 | 0.788** | – | |||
| 4. HOME learning-organization | −0.014 | 0.675** | 0.348* | – | ||
| 5. Maternal CESD score | 0.002 | −0.209 | −0.09 | −0.403* | – | |
| 6. Infant fear | −0.197 | −0.012 | 0.091 | −0.039 | 0.183 | – |
| 7. Infant cognitive development | 0.104 | 0.238 | 0.366* | −0.017 | 0.084 | 0.183 |
p < 0.05.
p < 0.01.
3.2. Primary analyses
In this section we begin by examining the association between neonatal amygdala connectivity and 6-month fear, cognitive development, and the interaction between these two constructs (the differentiated fear phenotype). We follow this set of analyses by determining the role of the caregiving environment in the initial findings.
3.2.1. Fear
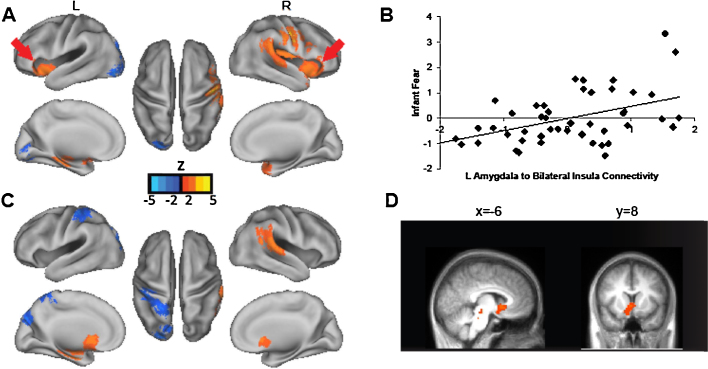
For the left amygdala, stronger positive connectivity to the bilateral aI (extending into the inferior frontal gyrus on the right), left parahippocampal gyrus (PHG), right putamen, right precentral gyrus (premotor cortex), right temporoparietal junction (TPJ) and right superior temporal gyrus (STG), predicted higher levels of infant fear at 6-months. Greater negative connectivity between the left amygdala and left occipital cortex (cuneus and lingual gyrus) also predicted higher fear (see Fig. 1 and Table S1).
Fig. 1.
Neonatal amygdala connectivity predicts infant fear at 6-months. Note. Panel (A) shows results for left amygdala. Greater positive connectivity to several regions including bilateral anterior insula (aI; red arrows) predicts higher fear at 6 months. Panel (B) illustrates the association between infant fear and connectivity strength from the left amygdala to the aI regions identified in the whole brain analyses (mean of the correlation coefficient for left amygdala-bilateral aI connectivity, standardized and adjusted for infant gestational age and age at scan). Panels (C and D) show results for the right amygdala indicating that greater positive connectivity to ventral striatum (VS), among other regions, predicts higher fear at 6 months. The scatter plot for right amygdala to VS connectivity and infant fear is similar to that for left amygdala and aI. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Findings for the right amygdala were distinct from the left amygdala. Greater positive connectivity to ventral striatum (VS), left parahippocampal gyrus, and right STG extending into TPJ predicted higher infant fear at 6-months. Greater negative connectivity between the right amygdala and left occipital cortex (cuneus) and postcentral gyrus also predicted higher fear (see Fig. 1 and Table S1). Post-hoc analyses indicated that the strength of these connections (both left and right amygdala) still significantly predicted higher fear after adjusting for remaining FD.
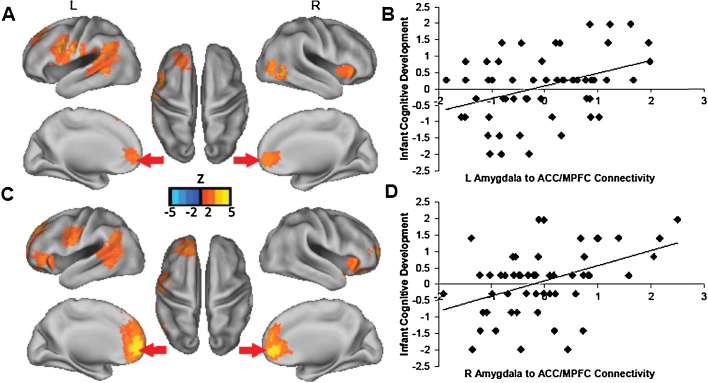
3.2.2. Cognitive development
The associations between neonatal amygdala connectivity and 6-month cognitive development were largely distinct from the fear findings, although an interesting overlap was also observed in the aI. For the left amygdala, stronger positive connectivity to the anterior cingulate cortex/MPFC (ACC/MPFC), left anterior, dorsal PFC (superior frontal gyrus [SFG] and medial frontal gyrus [MFG]), bilateral aI, left temporal cortex (STG and medial temporal gyrus [MTG]), and left pre- and postcentral gyrus predicted higher levels of cognitive development.
The pattern of findings for the right amygdala was similar to the findings for the left amygdala. For the right amygdala, stronger positive connectivity to the ACC/MPFC, dorsal MPFC, left anterior, dorsal PFC (SFG and MFG), left inferior frontal gyrus, bilateral aI, left temporal cortex (STG and MTG) and premotor cortex predicted higher cognitive development scores (see Fig. 2 and Table S2). Again, findings were consistent after adjusting for remaining FD.
Fig. 2.
Neonatal amygdala connectivity predicts infant cognitive development at 6-months. Note. Panel (A) shows results for left amygdala. Greater positive connectivity to rostral anterior cingulate cortex/anterior medial prefontal cortex (ACC/MPFC; red arrows), and several other cortical regions, predicts higher cognitive development scores 6 months. Panel (B) illustrates the association between infant cognitive development and the correlation coefficient indicating connectivity strength from the left amygdala to the ACC/MPFC region (standardized and adjusted for infant gestational age and age at scan). Panels (C and D) show similar results for the right amygdala. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
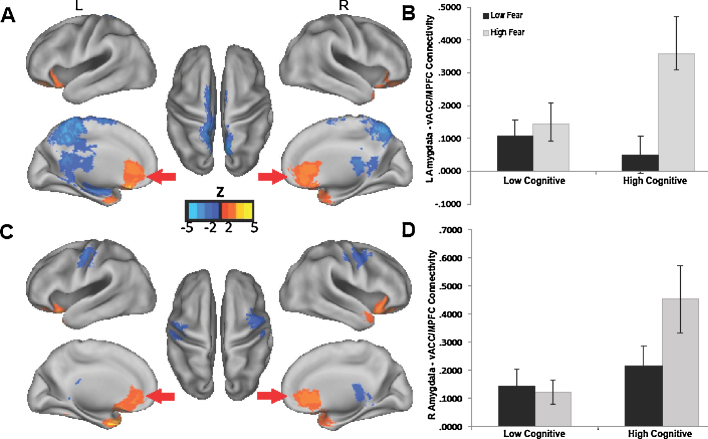
3.2.3. Differentiated fear phenotype (interaction between fear and cognitive development)
For the left amygdala, positive connectivity with the ACC/MPFC, medial orbital frontal cortex (OFC), left PHG, right temporal pole, bilateral IFG, and bilateral cerebellum, predicted the interaction between fear and cognitive development. Negative connectivity with parietal cortex (posterior cingulate cortex [PCC], precuneus and superior parietal lobule), cerebellum, and a subcortical cluster (extending into thalamus, caudate and hippocampus) also predicted the interaction between fear and cognition. For the right amygdala, positive connectivity with the ACC/MPFC, bilateral PHG, bilateral temporal pole, right IFG, and left cerebellum, predicted the interaction between fear and cognition. Negative connectivity with the left somatosensory and right premotor cortices also predicted the interaction term (Fig. 3 and Table S3). Interestingly, the ACC/MPFC region identified in these analyses (with a dorsal peak of x = 3, y = 31, z = 4 and ventral peak of x = −2, y = 20, z = −20) was ventral and posterior to that identified in the analyses focused on cognitive development (with a dorsal peak of x = 10, y = 43, z = 29 and ventral peak of x = 10, y = 43, z = −8; Fig. 2, Fig. 3; Tables S2 and S3). Therefore, we refer the MPFC region identified in the interaction analysis as the ventral ACC/MPFC (vACC/MPFC). Again, findings were consistent after adjusting for remaining FD.
Fig. 3.
Neonatal amygdala connectivity predicts specific combinations of infant fear and cognitive development at 6-months. Note. Panels (A and B) shows results for left amygdala. Greater positive connectivity to ventral anterior cingulate cortex/medial prefrontal cortex (vACC/MPFC), among other regions, predicts infant fear at 6 months differently depending on infants’ cognitive development. The bar graph in Panel (B) illustrates the interaction based on median splits of infant fear and cognitive development. Panels (C and D) show similar results for the right amygdala. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
We next ran a post-hoc analysis to probe the interaction, and determine how the association between amygdala-vACC/MPFC connectivity and fear varied depending on level of cognitive development. We focused specifically on amygdala-vACC/MPFC connectivity due to the extensive research documenting the importance of this connection for regulation of fear in animal models and human children and adults (Banks et al., 2007, Etkin et al., 2011, Fossati, 2012, Gee et al., 2013b, Milad and Quirk, 2012, Phelps et al., 2004). To statistically probe the interaction in a manner consistent with the continuous variables used in the analyses, we generated simple slopes (Aiken and West, 1991), which represented the association between amygdala-vACC/MPFC connectivity and infant fear depending on the level of infants’ cognitive development. Higher and lower cognition were defined as one standard deviation above and below the mean cognitive composite score (with mean remaining FD included as an additional covariate). For both the left and right amygdala, stronger connectivity to the vACC/MPFC was associated with higher fear only for infants with higher cognition (β = 0.425, p = 0.008 for left amygdala and β = 0.351, p = 0.028 for right amygdala). For infants with lower cognition, connectivity between the amygdala and vACC/MPFC was not significantly associated with fear (β = −0.402, p = 0.125 for left amygdala or β = −0.386, p = 0.144 for right amygdala). Repeating this analysis for all vACC/MPFC peaks revealed a consistent pattern of results. Thus, stronger positive connectivity between the amygdala and vACC/MPFC in neonates specifically predicted a phenotype of higher fear combined with higher cognitive development at 6-months. Although typically plotted with line graphs, the interaction is depicted with a bar graph in Fig. 3 because this provides the most intuitive visual representation of the results. This early pattern of connectivity that predicts the combination of higher fear and cognition may have implications for ongoing development of the capacity to use cognitive skills to adaptively regulate and make use of negative emotions.
3.3. Postnatal caregiving environment
Because of the impact of the caregiving environment on brain and behavioral development, we next conducted post-hoc analyses to determine whether the above findings were modulated by this environmental factor. All analyses included infant gestational age at birth and age at scan as covariates. After examining the total summary score of the home environment, follow-up analyses focused on caregiving subscales potentially more pertinent to the specific behavioral outcomes of interest (caregiver acceptance–responsivity for infant fear, and learning materials-organization for cognitive development).
3.3.1. Fear
As noted previously, to reduce multiple comparisons and increase interpretability of results, these analyses focused on functional connections with the strongest existing empirical and theoretical links to development of typical and pathological fear. For infant fear, this analysis therefore focused on left amygdala to bilateral aI connectivity. A regression model with left amgygdala to bilateral aI connectivity and the caregiving environment as predictors, explained a significant amount of the variance in infant fear at 6 months (R2 = 0.317; p = 0.004). Left amygdala to bilateral aI connectivity remained a significant predictor (β = 0.506, p = 0.001) of infant fear while the caregiving environment was not significantly associated with infant fear (β = −0.103, p = 0.466). Including the interaction between amygdala to aI connectivity and caregiving did not explain a significant amount of additional variance (R2 = 0.000, p = 0.921), and the interaction did not significantly predict infant fear (β = −0.015, p = 0.921). Follow-up analyses employed an identical model, but with the more specific measure of caregiver acceptance–responsivity in place of the summary measure. The findings replicated those reported above, and are therefore not reported in detail. These results suggest that neonatal amygdala connectivity to bilateral insula predicts infant fear at 6-months-of-age independent of the postnatal caregiving environment (as measured by the HOME Inventory).
3.3.2. Cognitive development
With regard to cognitive development we focused on left and right amygdala connectivity to ACC/MPFC—consistent with the original findings noted above. The regression model with left amgygdala to ACC/MPFC connectivity and the caregiving environment as predictors explained approximately 23% of the variance in infant cognitive development at 6 months (R2 = 0.230, p = 0.017). Left amygdala to ACC/MPFC connectivity remained a significant predictor (β = 0.459, p = 0.003), and caregiving was not significantly associated with cognitive development (β = 0.050, p = 0.728). Including the interaction between amygdala to ACC/MPFC and caregiving did not explain a significant amount of additional variance (R2 = 0.000, p = 0.973), and the interaction did not significantly predict infant cognitive development (β = 0.005, p = 0.973). For right amygdala to ACC/MPFC connectivity, the model explained approximately 20% of the variance in infant cognitive development (R2 = 0.208, p = 0.030). Right amygdala to ACC/MPFC connectivity remained a significant predictor (β = 0.406, p = 0.005), and the caregiving environment was not significantly associated with cognitive development (β = 0.116, p = 0.404). Including the interaction between amygdala to ACC/MPFC and caregiving did not explain a significant amount of additional variance (R2 = 0.030, p = 0.192), and the interaction did not significantly predict infant cognitive development (β = −0.193, p = 0.192). Follow-up analyses with the more specific measure of caregiver learning-organization replicated those for the summary measure, and are therefore not reported in detail. We additionally examined caregiver acceptance–responsivity due to its positive association with infant cognitive development (Table 2). The regression model with left amgygdala to ACC/MPFC connectivity and caregiver acceptance–responsivity as predictors explained approximately 29% of the variance in infant cognitive development at 6 months (R2 = 0.286, p = 0.004). Left amygdala to ACC/MPFC connectivity remained a significant predictor (β = 0.420, p = 0.003), and acceptance–responsivity was associated with cognitive development at the trend level (β = 0.251, p = 0.062). Including the interaction between amygdala to ACC/MPFC and acceptance–responsivity did not explain a significant amount of additional variance (R2 = 0.002, p = 0.749), and the interaction did not significantly predict infant cognitive development (β = 0.046, p = 0.973). For right amygdala to ACC/MPFC connectivity, the model including acceptance–responsivity explained approximately 27% of the variance in infant cognitive development (R2 = 0.270, p = 0.006). Right amygdala to ACC/MPFC connectivity remained a significant predictor (β = 0.388, p = 0.005), and acceptance–responsivity was also positively associated with cognitive development (β = 0.280, p = 0.038). Including the interaction between amygdala to ACC/MPFC and acceptance–responsivity did not explain a significant amount of additional variance (R2 = 0.031, p = 0.167), and the interaction did not significantly predict infant cognitive development (β = −0.183, p = 0.167). In line with the amygdala-insula connectivity and fear findings, the association between neonatal amygdala-ACC/MPFC connectivity and cognitive development at 6-months did not appear to be modulated by the postnatal caregiving environment, as assessed with the HOME Inventory.
3.3.3. Differentiated fear phenotype (interaction between fear and cognitive development)
For the differentiated fear phenotype, analysis focused on left and right amygdala connectivity to vACC/MPFC. These analyses did not include a test for the moderating effects of the caregiving environment on the association between connectivity and the differentiated fear phenotype, because this would essentially involve testing a four-way interaction (between connectivity, caregiving, fear and cognitive development), which would not be appropriate given the current sample size. The regression model with left amygdala to vACC/MPFC connectivity and the caregiving environment as predictors, explained a significant amount of variance in the fear by cognition interaction (R2 = 0.211, p = 0.011). Left amygdala to vACC/MPFC connectivity remained a significant predictor (β = 0.449, p = 0.004), and the caregiving environment was not significantly associated with this interaction (β = −0.182, p = 0.215). For right amygdala to vACC/MPFC connectivity, the model also explained a significant amount of variance in the interaction between infant fear and cognitive development (R2 = 0.205, p = 0.013). Right amygdala to vACC/MPFC connectivity remained a significant predictor (β = 0.438, p = 0.004), and caregiving was not significantly associated with the interaction (β = −0.166, p = 0.257).
Overall, neonatal amygdala connectivity predicted infant fear, cognitive development and the differentiated fear phenotype at 6 months above and beyond effects of the caregiving environment. The caregiving environment also did not moderate the association between neonatal amygdala connectivity and fear or cognitive development, indicating that the neonatal connectivity patterns were generally consistent predictors of these outcomes across different caregiving environments.
4. Discussion
4.1. Summary of findings
In the current report we show that neonatal amygdala connectivity predicted both fearfulness and cognitive development at 6-months-of-age. Greater neonatal amygdala connectivity to several cortical and subcortical regions, including regions frequently implicated in fear in children and adults, such as the aI, were associated with emerging fear during the first year of life. The pattern of neonatal amygdala connectivity associated with more advanced cognitive development (e.g. a higher score on the cognitive measure relative to other infants in this typically developing sample) at 6-months included some intriguing overlap with the fear findings, but was also distinct in including anterior, dorsal and medial PFC regions. Yet another distinct pattern of neonatal amygdala connectivity predicted the differentiated fear phenotype, which takes into account cognitive development. Specifically, greater neonatal amygdala connectivity to vACC/MPFC predicted a phenotype characterized by higher fear in combination with more advanced cognitive development. The associations between neonatal amygdala connectivity and these outcomes did not appear to be modulated by variation in the caregiving environment.
4.2. Developmental framework for interpretation of findings
In discussing these findings, it is important to maintain a developmental framework. The first year of life, and particularly the beginning of the second-half of the first year, is a unique period for the development of fear. It is characterized by a developmentally typical increase in fear, as infants learn to effectively navigate their environments (Leppanen and Nelson, 2012). However, even in the context of typically developing fear, higher levels of fear have been associated with later emerging pathology (Gartstein et al., 2010). A better understanding of the earliest neural foundations of fear in the first year of life therefore has implications both for typical development of this important emotion, and for risk of fear-related psychopathology.
There are multiple factors that appear to influence whether high fear during infancy represents part of a typical developmental trajectory, or a risk factor for psychopathology. As reviewed previously, cognitive skills have emerged as a particularly important factor for modulating risk of psychopathology for individuals who display high fear or negative emotionality during infancy (Degnan and Fox, 2007, Nigg, 2006, Rothbart and Bates, 2006). We present evidence suggestive not only of patterns of connectivity that precede and predict emerging fear, but also of an early pattern of connectivity associated with a specific phenotype of higher fear combined with more advanced cognitive development. Based on previous work examining the interplay between fear and cognitive skills (Crockenberg and Leerkes, 2006, Gartstein et al., 2012), we propose that this phenotype may be indicative of a healthy developmental trajectory in which robust development of fear is balanced by parallel cognitive development. In contrast, high fear that is not paralleled by more rapid development of cognitive skills may be indicative of risk for fear-related psychopathology. Ongoing work will allow for testing this proposal, and for determining whether connectivity markers in the neonatal period can predict these outcomes. Nonetheless, this proposed model serves as a framework for interpreting the results of the current study.
4.3. Neonatal amygdala connectivity and infant fear at 6-months
Amygdala connections implicated in normative and pathological fear across the life span appear to have roots in a functional neural architecture evident by the time of birth. We expect that these functional connections will change substantially over the course of development (Gabard-Durnam et al., 2014, Gee et al., 2013b); however, the coordinated functioning of these regions in the neonatal period is consistent with evidence from animal models demonstrating that amygdala circuitry underlying fear behaviors is in place (and can be activated under conditions of duress) well before fear behaviors typically emerge during development (Landers and Sullivan, 2012, Moriceau et al., 2010, Moriceau et al., 2006).
Interestingly, the pattern of neonatal amygdala connectivity associated with subsequent fear at 6-months-of-age was consistent with recent work highlighting long range amygdala functional connections to cortical and subcortical brain regions in 6-month-old infants (Qiu et al., 2015). Specifically, Qiu and colleagues observed significant, positive amygdala connectivity with bilateral insula, basal ganglia, precentral gyrus and temporal cortex regions, among others, in 6-month-old infants. The results of the present study not only suggest that the integrity of these particular connections are important for fear at 6-months-of-age, but that coordinated functioning of the amygdala with these same regions is evident at birth.
In addition, there are remarkable similarities in the specific functional connections observed to predict fear at 6-months-of-age, and those associated with normative and pathological fear in older children and adults. In particular, the link between the neonatal amygdala and bilateral aI emerged as a significant predictor of infant fear at 6-months. Research with adults documents the role of amygdala to aI connectivity in identifying salient, or personally relevant, stimuli and interacting with other brain systems to facilitate flexible responding (as part of the “salience network”; Menon and Uddin, 2010, Menon, 2015, Seeley et al., 2007). It is not known whether this connection serves a similar purpose during infancy, but identification of stimuli as salient, or relevant for safety, is a logical precursor to emerging fear behaviors.
In line with this role in salience detection, amygdala to aI connectivity has been proposed to be important for integrating fear processing across multiple cortical and subcortical brain systems involved in different aspects of fear learning and behavior in adults (Baur et al., 2013, Phelps et al., 2001). Greater coordinated functioning between the amygdala and aI is observed following exposure to violence (van Marle et al., 2010) and perceived threat (van Wingen et al., 2011), and is more generally associated with higher levels of non-pathological fear (Baur et al., 2013). Interestingly, previous work is also in line with the lateralized findings in the present study, indicating a specific role for left amygdala to insula connectivity in fear in children and adults (Baur et al., 2013, Qin et al., 2014). In fact, resting state functional connectivity between the left amygdala and aI was found to explain 40% of the variance in state anxiety among a group of healthy adults (Baur et al., 2013). The results here suggest that these particular relationships are building as early as the neonatal period.
Higher neonatal amygdala to VS connectivity also predicted higher levels of fear at 6 months-of-age. This finding was not anticipated, but is interesting in the context of research with adults indicating a role for the VS in fear learning and prediction (Delgado et al., 2008, Jensen et al., 2003, Pohlack et al., 2012), ongoing flexible evaluation of fear-relevant stimuli (Schiller and Delgado, 2010), and generalization of fear based on features of an aversive stimulus in adults (Dunsmoor et al., 2011). Adolescents characterized as behaviorally inhibited during infancy and early childhood also show differences in striatal functioning during reward processing (Bar-Haim et al., 2009, Guyer et al., 2006, Helfinstein et al., 2011, Helfinstein et al., 2012). The current results raise questions about the role of early amygdala-VS connectivity in emerging fear.
The role of amygdala-aI and amygdala-VS functional connectivity in emerging fear in infancy is also of interest due to associations between these connections and pathological anxiety in adolescents and adults (Greenberg et al., 2010, Milad and Rauch, 2012, Rabinak et al., 2011, Roy et al., 2013, Simon et al., 2010, Sripada et al., 2012). In children 7 to 9 years of age, greater positive amygdala-aI and amygdala-VS connectivity was associated with higher levels of anxiety symptoms within a range including typical and potentially pathological anxiety (Qin et al., 2014). Ongoing longitudinal work will be needed to examine the relevance of these connections during the neonatal period as risk factors for pathological anxiety. However, we interpret the present results in the context of the typical developmental trajectory of emerging fear over the first year of life, and posit that connections specifically linked to the interaction between fear and cognitive development may be most relevant for understanding risk for pathological fear.
4.4. Neonatal amygdala connectivity and infant cognitive development at 6-months
The strong associations between neonatal amygdala connectivity and cognitive development at 6-months-of-age highlight the importance of examining the amygdala beyond the domain in which it is most frequently examined (emotional development). Indeed, the utility of distinguishing between brain regions based solely on their perceived role in emotional versus cognitive processes has increasingly been questioned (Pessoa, 2008, Salzman and Fusi, 2011). The pattern of neonatal connectivity associated with cognitive development in the current study was distinguished from the fear findings due to the inclusion of anterior, medial and dorsal PFC regions. In adults and children, these regions form important components of brain systems that support high level cognitive processes, such as purposeful control of attention (e.g. cingulo-opercular system; Dosenbach et al., 2008, Fair et al., 2007, Power et al., 2011). The medial PFC regions are part of the default mode network, which appears to be involved in self-generated, or spontaneous cognition (Andrews-Hanna et al., 2014, Andrews-Hanna, 2012). The present findings suggest that coordinated functioning between subcortical regions frequently studied in the context of emotion processing, and cortical regions more classically associated with cognition, begins at the earliest stages of development. These early connections may lay a foundation for how the amygdala interacts with multiple aspects of cognitive processing.
Interestingly, there was also overlap between the findings for fear and cognitive development. Specifically, greater amygdala-aI connectivity was associated with both higher levels of fear and more advanced cognitive development. As noted previously, this connection is part of the salience network, which is involved in detecting personally relevant stimuli (as noted previously; Menon and Uddin, 2010, Menon, 2015, Seeley et al., 2007), but it is also consistently implicated in the cingulo-opercular network (Power et al., 2011), which is important for task control (Dosenbach et al., 2008, Fair et al., 2007). Just as these processes (and particularly detection of salient stimuli) are likely important for emerging fear behaviors, they are also necessary prerequisites for development of many cognitive skills (Supekar and Menon, 2012). In addition, previous work in infants suggests that the insula is one of the most highly interconnected brain regions beginning in the neonatal period (Gao et al., 2011). The current results are consistent with an understanding of the aI as a highly interconnected brain region involved in multiple behavioral domains beginning in the earliest stages of development.
4.5. Neonatal amygdala connectivity as a predictor of the emerging interaction between fear and cognitive development
Coordinated functioning of the neonatal amygdala also appears to be relevant for a more nuanced understanding of emerging fear, which incorporates cognitive development. Interestingly, greater neonatal amygdala connectivity with vACC/MPFC was specifically associated with the phenotype of high fear combined with more advanced cognitive development. Research in adults has built on an extensive animal literature and demonstrated the importance of the MPFC (including the specific MPFC region identified here) for fear learning, appraisal, and regulation in the context of fear conditioning paradigms (Etkin et al., 2011, Linnman et al., 2011, Milad and Quirk, 2012, Milad et al., 2007, Phelps et al., 2004). Beyond fear conditioning paradigms, coordinated functioning of the MPFC and amygdala plays an important role in regulating emotions in a variety of different contexts (Banks et al., 2007, Etkin et al., 2011, Fossati, 2012), regardless of the specific strategy employed for emotion regulation (Hariri et al., 2003, Hariri et al., 2000, Ochsner and Gross, 2005). In line with this well established role in regulation of fear, amygdala-MPFC connectivity has been highlighted as playing a key role in pathological anxiety (Casey and Lee, 2015, Kim et al., 2011, Roy et al., 2013).
The pattern of greater neonatal amygdala-vACC/MPFC connectivity associated with high fear and more advanced cognitive skills may represent a foundation for the later emerging capacity to regulate fear via interactions between the amygdala and MPFC. Research in older children supports this idea to some extent, as developmental change in the coordinated functioning of the amygdala with a very similar ACC/MPFC region has been linked to the typical decline in separation anxiety from early childhood to adolescence (Gee et al., 2013b). The importance of coordinated amygdala-MPFC functioning for development of risk for fear-related psychopathology is highlighted by a growing body of work documenting effects of early life stress on subsequent emergence of anxiety symptoms mediated by changes in the development of amygdala-MPFC functional connectivity (Burghy et al., 2012, Callaghan et al., 2014, Gee et al., 2013a, Herringa et al., 2013).
It is important to note that stronger amygdala connectivity to MPFC was also found to predict higher cognitive development without consideration of fear. However, for cognition alone, the ACC/MPFC region identified was more anterior and dorsal compared to the region associated with the high fear and cognitive development phenotype. Thus neonatal amygdala to ACC/MPFC connectivity appears to be relevant for cognitive development in general, and more specifically related to cognitive development in the context of fear within a more ventral region. This finding is interesting in light of research in adults documenting differences in the role of ventral versus dorsal ACC/MPFC in processing and regulating negative emotions, and particularly fear (Etkin et al., 2011). Previous work in children suggests that younger children, and children with more fearful temperaments engage more ventral versus dorsal ACC during emotion processing and regulation (Perlman and Pelphrey, 2011). It will be important to build on the present findings by examining whether the pattern observed here in neonates is a precursor to the subsequent differentiated roles of dorsal and ventral ACC/MPFC regions in emotion processing and regulation.
4.6. Caregiving environment
The association between amygdala connectivity and infant fear, cognitive development, and the differentiated fear phenotype remained significant when considering the quality of the postnatal environment, suggesting an independent effect of neonatal amygdala connectivity strength on these outcomes. Furthermore, at least in the domains of fear and cognitive development, the strength of these particular amygdala connections did not determine inter-individual differences in propensity of responding to the postnatal caregiving environment. It is important to note that for amygdala connectivity linked to the differentiated fear phenotype we did not examine interaction with the caregiving environment due to the limitations of the sample size (although we did examine the independent effect of this connection when adjusting for variation in caregiving). Nonetheless, given previous evidence documenting effects of caregiving on the development of fear and cognition in infancy (Braungart-Rieker et al., 2010, Gartstein et al., 2010, Lugo-gil and Tamis-lemonda, 2008), and the fairly comprehensive assessment of the caregiving environment in the present study, these results provide support for neonatal amygdala connectivity as a robust predictor of development in these areas, and raise questions regarding the intrauterine environmental and genetic determinants of neonatal amygdala connectivity.
In interpreting the findings regarding the caregiving environment, it is also important to note that we only examined connections found to predict infant fear and cognitive development. Among those connections, we focused on those previously found to be particularly important for understanding the neural basis of typical and pathological fear in children and adults. Such an approach was deemed necessary to retain the focus on development of fear and the differentiated fear phenotype, prevent excessive multiple comparisons and facilitate interpretability of results. However, it also increases the chance of missing aspects of neonatal amygdala connectivity that confer sensitivity to the caregiving environment. In addition, we only examined one assessment of the caregiving environment, and did not examine extreme variation in caregiving (i.e. abuse or neglect). Thus, our findings are not necessarily at odds with extensive research showing associations between the caregiving environment and developing connectivity between the amygdala and MPFC (Burghy et al., 2012, Callaghan et al., 2014, Gee et al., 2013a, Herringa et al., 2013). A more thorough examination of this phenomenon, including a larger sample size, and consideration of outcomes beyond the first 6-months of life, will be an important topic for future research.
4.7. Limitations and additional considerations
There are several important considerations for interpreting and building on the results reported here. First, this paper focuses on fear, and a broad measure of early cognitive development. However, the brain regions and connections highlighted in these findings likely play important roles in the development of a wide range of emotions (including positive emotions and other negatively valenced emotions), and the interplay between these emotions and various aspects of cognitive development. We take a step towards a multi-dimensional perspective on brain and behavioral development by including fear and cognitive development, but a more comprehensive approach, including multi-method assessment of a range of emotions and different cognitive skills will be an important next step. For example, future work should include specific assessment of attentional control, which has been highlighted as a cognitive skill that is particularly important for emotion regulation during infancy (Crockenberg and Leerkes, 2006), and closely associated with later development of fear-related psychopathology (Fox and Pine, 2012).
Establishing rs-fcMRI as a predictor of behavioral outcomes will necessitate the use of more advanced statistical modelling techniques, as well as inclusion of baseline measures of physiology and behavior to examine whether patterns of functional connectivity predict outcomes above and beyond these other measures. Building on the results of this study will also involve taking a systems level approach to characterizing functional brain organization. We used individualized amygdala regions of interest, and seed based analyses, as a first step in understanding neural foundations of fear in early infancy. However, research in children and adults has demonstrated that examining brain organization at the level of networks, and whole brain topology, allows for a more parsimonious and thorough characterization of functional brain organization relevant for emotional and cognitive development and mental health (Menon, 2011, Power et al., 2010, Vértes and Bullmore, 2015). Such an approach will likely be helpful in continuing to advance understanding of the links between early brain functioning and typical and atypical development of fear. Finally, we acknowledge that the amygdala is composed of multiple sub-nuclei, which may be differentially involved in development of fear (Qin et al., 2014). Future research will be needed to test appropriate methods for identifying and segmenting these sub-nuclei in neonates in order to understand their potentially distinct roles in early emotional and cognitive development.
4.8. Conclusions
The capacity to successfully navigate the world depends on fear, as well as other negative emotions, and particularly on the ability to use these emotions to respond adaptively to a variety of stimuli. The early patterns of amygdala connectivity linked to parallel high fear and cognitive development may facilitate both identification of, and flexible responding to meaningful stimuli in the environment. Ongoing longitudinal research will be needed to test the idea that this neurobehavioral phenotype lays a foundation for healthy, versus pathological development of fear over time. The current results highlight the potential utility of rs-fcMRI in this future work, and in other research seeking to gain an early window into complex developmental processes prior to their emergence at a behavioral level.
The results also raise questions about the potential contribution of the prenatal environment to the patterns of brain connectivity observed here in neonates. Previous work suggests that prenatal conditions, such as maternal stress and elevated maternal cortisol concentrations during pregnancy, as well as preconceptional conditions (e.g. maternal obesity), are associated with amygdala structure (Buss et al., 2012a) and function in offspring (Qiu et al., 2015), and with as development of fear (Davis et al., 2007) and cognitive skills (Buss et al., 2011, Buss et al., 2012b). Research examining the extent to which these early patterns of amygdala connectivity are associated with specific intrauterine conditions will be important for identifying modifiable conditions to inform prevention and intervention efforts.
Acknowledgments
Support for this work was provided by NIMH R01 MH091351 (Buss; Fetal Programming of the Newborn and Infant Human Brain), Supplement to R01 MH091351 (Buss & Fair; Fetal Programming of Brain Functional Connectivity in Neonates and Infants), NIMH F32 MH105283 (Graham; Early Neurobiological Predictors of Executive Functioning in Toddlers), R00 MH091238 (Fair), R01 MH096773 (Fair), and NICHD R01 HD060628 (Wadhwa; EMA Assessment of Biobehavioral Processes in Human Pregnancy). We are grateful to the families for participating in the study, to Eric Earl (Fair Neuroimaging Lab) for his hard work on the rs-fcMRI processing pipelines, and to Kimberly Lawerence, Everlyne Gomez and Virginia Allhusen (UCI) for their work in ensuring high quality behavioral data.
Contributor Information
Claudia Buss, Email: claudia.buss@charite.de.
Damien A. Fair, Email: faird@ohsu.edu.
References
- Aiken L.S., West S.G. Sage; Thousand Oaks, CA: 1991. Multiple Regression: Testing and Interpreting Interactions. [Google Scholar]
- Ainsworth M.D.S., Bell S.M. Attachment, exploration, and separation: illustrated by the behavior of one-year-olds in a strange situation. Child Dev. 1970;41(1):49–67. [PubMed] [Google Scholar]
- Andrews-Hanna J.R. The brain's default network and its adaptive role in internal mentation. The Neurosci. 2012;18(3):251–270. doi: 10.1177/1073858411403316. http://doi.org/10.1177/1073858411403316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J.R., Smallwood J., Spreng R.N. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann. N.Y. Acad. Sci. 2014;1316:29–52. doi: 10.1111/nyas.12360. http://doi.org/10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker E., Baibazarova E., Ktistaki G., Shelton K.H., van Goozen S.H.M. Development of fear and guilt in young children: stability over time and relations with psychopathology. Dev. Psychopathol. 2012;24(03):833–845. doi: 10.1017/S0954579412000399. http://doi.org/10.1017/S0954579412000399. [DOI] [PubMed] [Google Scholar]
- Banks S.J., Eddy K.T., Angstadt M., Nathan P.J., Phan K.L. Amygdala–frontal connectivity during emotion regulation. Soc. Cogn. Affect. Neurosci. 2007;2(4):303–312. doi: 10.1093/scan/nsm029. http://doi.org/10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y., Fox N.A., Benson B., Guyer A.E., Williams A., Nelson E.E. Neural correlates of reward processing in adolescents with a history of inhibited temperament. Psychol. Sci. 2009;20(8):1009–1018. doi: 10.1111/j.1467-9280.2009.02401.x. http://doi.org/10.1111/j.1467-9280.2009.02401.x.Neural. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr G.a., Moriceau S., Shionoya K., Muzny K., Gao P., Wang S., Sullivan R.M. Transitions in infant learning are modulated by dopamine in the amygdala. Nat. Neurosci. 2009;12(11):1367–1369. doi: 10.1038/nn.2403. http://doi.org/10.1038/nn.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman M.D., Lavenex P., Mason W.a., Capitanio J.P., Amaral D.G. The development of social behavior following neonatal amygdala lesions in rhesus monkeys. J. Cogn. Neurosci. 2004;16(8):1388–1411. doi: 10.1162/0898929042304741. http://doi.org/10.1162/0898929042304741. [DOI] [PubMed] [Google Scholar]
- Baur V., Hänggi J., Langer N., Jäncke L. Resting-state functional and structural connectivity within an insula-amygdala route specifically index state and trait anxiety. Biol. Psychiatry. 2013;73(1):85–92. doi: 10.1016/j.biopsych.2012.06.003. http://doi.org/10.1016/j.biopsych.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Bayley N. third ed. Harcourt Assessment; San Antonio, TX: 2006. Bayley Scales of Infant and Toddler Development. [Google Scholar]
- Beckmann C.F., Jenkinson M., Woolrich M.W., Behrens T.E.J., David E., Devlin J.T., Smith S.M. Applying FSL to the FIAC data: model-based and model-free analysis of voice and sentence repetition priming. Hum. Brain Mapp. 2006;27(5):380–391. doi: 10.1002/hbm.20246. http://doi.org/10.1002/hbm.20246.Applying. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J. Variation in susceptibility to rearing influence: an evolutionary argument. Psychol. Inq. 1997;8:182–186. [Google Scholar]
- Belsky J., Bakermans-Kranenburg M.J., Van Ijzendoorn M.H. For better and for worse differential susceptibility to environmental influences. Psychol. Sci. 2007;16(6):300–304. [Google Scholar]
- Belsky J., Pluess M. Beyond risk, resilience, and dysregulation: phenotypic plasticity and human development. Dev. Psychopathol. 2013;25(4 Pt 2):1243–1261. doi: 10.1017/S095457941300059X. http://doi.org/10.1017/S095457941300059X. [DOI] [PubMed] [Google Scholar]
- Biederman J., Hirshfeld-Becker D.R., Rosenbaum J.F., Hérot C., Friedman D., Snidman N. Further evidence of association between behavioral inhibition and social anxiety in children. Am. J. Psychiatry. 2001;158:1673–1679. doi: 10.1176/appi.ajp.158.10.1673. [DOI] [PubMed] [Google Scholar]
- Bliss-Moreau E., Toscano J.E., Bauman M.D., Mason W.a., Amaral D.G. Neonatal amygdala or hippocampus lesions influence responsiveness to objects. Dev. Psychobiol. 2010;52(5):487–503. doi: 10.1002/dev.20451. http://doi.org/10.1002/dev.20451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein M.H., Putnick D.L., Gartstein M.A., Hahn C.-S., Auestad N., O’Connor D.L. Infant temperament: stability by age, gender, birth order, term status, and socioeconomic status. Child Dev. 2015;00(0):1–20. doi: 10.1111/cdev.12367. http://doi.org/10.1111/cdev.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce W.T., Ellis B.J. Biological sensitivity to context: I. An evolutionary—developmental theory of the origins and functions of stress reactivity. Dev. Psychopathol. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Braungart-Rieker J.M., Hill-Soderlund A.L., Karrass J. Fear and anger reactivity trajectories from 4 to 16 months: the roles of temperament, regulation, and maternal sensitivity. Dev. Psychol. 2010;46(4):791–804. doi: 10.1037/a0019673. http://doi.org/10.1037/a0019673. [DOI] [PubMed] [Google Scholar]
- Brooker R.J., Buss K.A., Lemery-Chalfant K., Aksan N., Davidson R.J., Goldsmith H.H. The development of stranger fear in infancy and toddlerhood: normative development, individual differences, antecedents, and outcomes. Dev. Sci. 2013:n/a–n/a. doi: 10.1111/desc.12058. http://doi.org/10.1111/desc.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghy C.A., Stodola D.E., Ruttle P.L., Molloy E.K., Armstrong J.M., Oler J.A. Developmental pathways to amygdala–prefrontal function and internalizing symptoms in adolescence. Nat. Neurosci. 2012;15(12):1736–1744. doi: 10.1038/nn.3257. http://doi.org/10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C., Davis E.P., Hobel C., Sandman C.A. Maternal pregnancy-specific anxiety is associated with child executive function at 6–9 years age. Stress. 2011;14(6):665–676. doi: 10.3109/10253890.2011.623250. http://doi.org/10.3109/10253890.2011.623250.Maternal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C., Davis E.P., Shahbaba B., Pruessner J.C., Head K., Sandman C.A. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc. Natl. Acad. Sci. U.S.A. 2012;109(20) doi: 10.1073/pnas.1201295109. http://doi.org/10.1073/pnas.1201295109/-/DCSupplemental.www.pnas.org/cgi/doi/10.1073/pnas.1201295109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C., Entringer S., Davis E.P., Hobel C.J., Swanson J.M., Wadhwa P.D., Sandman C.a. Impaired executive function mediates the association between maternal pre-pregnancy body mass index and child ADHD symptoms. PLoS ONE. 2012;7(6):e37758. doi: 10.1371/journal.pone.0037758. http://doi.org/10.1371/journal.pone.0037758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell B.M., Bradley R.H. Family & Human Dynamics Research Institute, Arizona State University; Tempe, AZ: 2003. Home Observation for Measurement of the Environment: Administration Manual. [Google Scholar]
- Callaghan B.L., Sullivan R.M., Howell B. 2014. The International Society for Developmental Psychobiology Sackler Symposium: Early Adversity and the Maturation of Emotion Circuits—A Cross-Species Analysis. http://doi.org/10.1002/dev.21260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carranza Carnicero J., Perez-Lopez J., Gonzalez Salinas M.D.C., Martinez-Fuentes M. A longitudinal study of temperament in infancy: stability and convergence of measures. Eur. J. Pers. 2000;14:21–37. Retrieved from 〈http://www.ingentaconnect.com/content/jws/per/2000/00000014/00000001/art00367〉. [Google Scholar]
- Casey B.J., Lee F.S. Optimizing treatments for anxiety by age and genetics. Ann. N.Y. Acad. Sci. 2015:n/a–n/a. doi: 10.1111/nyas.12746. http://doi.org/10.1111/nyas.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronis-Tuscano A., Degnan K.A., Pine D.S., Perez-Edgar K., Henderson H.A., Diaz Y. Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48(9):928–935. doi: 10.1097/CHI.0b013e3181ae09df. http://doi.org/10.1097/CHI.0b013e3181ae09df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss J.a., Blackford J.U. Behavioral inhibition and risk for developing social anxiety disorder: a meta-analytic study. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51(10) doi: 10.1016/j.jaac.2012.08.002. 1066.e1-1075.e1. http://doi.org/10.1016/j.jaac.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockenberg S.C., Leerkes E.M. Infant and maternal behavior moderate reactivity to novelty to predict anxious behavior at 2.5 years. Dev. Psychopathol. 2006;18(1):17–34. doi: 10.1017/S0954579406060020. http://doi.org/10.1017/S0954579406060020. [DOI] [PubMed] [Google Scholar]
- Das P., Kemp A.H., Liddell B.J., Brown K.J., Olivieri G., Peduto A. Pathways for fear perception: modulation of amygdala activity by thalamo-cortical systems. NeuroImage. 2005;26(1):141–148. doi: 10.1016/j.neuroimage.2005.01.049. http://doi.org/10.1016/j.neuroimage.2005.01.049. [DOI] [PubMed] [Google Scholar]
- Davis E.P., Glynn L.M., Schetter C.D., Hobel C., Chicz-Demet A., Sandman C.a. Prenatal exposure to maternal depression and cortisol influences infant temperament. J. Am. Acad. Child Adolesc. Psychiatry. 2007;46(6):737–746. doi: 10.1097/chi.0b013e318047b775. http://doi.org/10.1097/chi.0b013e318047b775. [DOI] [PubMed] [Google Scholar]
- Degnan K.A., Almas A.N., Fox N.A. Temperament and the environment in the etiology of childhood anxiety. J. Child Psychol. Psychiatry. 2010;51(4):497–517. doi: 10.1111/j.1469-7610.2010.02228.x. http://doi.org/10.1111/j.1469-7610.2010.02228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degnan K.A., Fox N.A. Behavioral inhibition and anxiety disorders: multiple levels of a resilience process. Dev. Psychopathol. 2007;19(3):729–746. doi: 10.1017/S0954579407000363. http://doi.org/10.1017/S0954579407000363. [DOI] [PubMed] [Google Scholar]
- Delgado M.R., Li J., Schiller D., Phelps E.A. The role of the striatum in aversive learning and aversive prediction errors. Philo. Trans. R. Soc. Lond., Ser. B: Biol. Sci. 2008;363(1511):3787–3800. doi: 10.1098/rstb.2008.0161. http://doi.org/10.1098/rstb.2008.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U.F., Fair D.A., Cohen A.L., Schlaggar B.L., Petersen S.E. A dual-networks architecture of top-down control. Trends in Cogn. Sci. 2008;12(3):99–105. doi: 10.1016/j.tics.2008.01.001. http://doi.org/10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor J.E., Prince S.E., Murty V.P., Kragel P.a., LaBar K.S. Neurobehavioral mechanisms of human fear generalization. NeuroImage. 2011;55(4):1878–1888. doi: 10.1016/j.neuroimage.2011.01.041. http://doi.org/10.1016/j.neuroimage.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. http://doi.org/10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Wager T.D. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Cohen A.L., Power J.D., Dosenbach N.U.F., Church J.A., Miezin F.M. Functional brain networks develop from a “local to distributed” organization. PLoS Comput. Biol. 2009;5(5):e1000381. doi: 10.1371/journal.pcbi.1000381. http://doi.org/10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Dosenbach N.U.F., Church J.A., Cohen A.L., Brahmbhatt S., Miezin F.M. Development of distinct control networks through segregation and integration. Proc. Natl. Acad. Sci. U.S.A. 2007;104(33):13507–13512. http://doi.org/10.1073/pnas.0705843104. [Google Scholar]
- Fair D.A., Nigg J.T., Iyer S., Bathula D., Mills K.L., Dosenbach N.U.F. Distinct neural signatures detected for ADHD subtypes after controlling for micro-movements in resting state functional connectivity MRI data. Front. Syst. Neurosci. 2012;6(February):80. doi: 10.3389/fnsys.2012.00080. http://doi.org/10.3389/fnsys.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonov V.S., Evans A.C., Botteron K., Almli C.R., McKinstry R.C., Collins D.L. Unbiased average age-appropriate atlases for pediatric studies. NeuroImage. 2011;54(1):313–327. doi: 10.1016/j.neuroimage.2010.07.033. http://doi.org/10.1016/j.neuroimage.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonov V.S., Evans A.C., Mckinstry R.C., Almli C.R., Collins D.L. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. NeuroImage. 2009;47(Suppl. 1):S102. http://doi.org/10.1016/S1053-8119(09)70884-5. [Google Scholar]
- Forman S.D., Cohen J.D., Fitzgerald M., Eddy W.F., Mintun M.A., Noll D.C. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn. Reson. Med. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. http://doi.org/10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fossati P. Neural correlates of emotion processing: from emotional to social brain. Eur. Neuropsychopharmacol. 2012;22(Suppl. 3):S487–S491. doi: 10.1016/j.euroneuro.2012.07.008. http://doi.org/10.1016/j.euroneuro.2012.07.008. (The Journal of the European College of Neuropsychopharmacology) [DOI] [PubMed] [Google Scholar]
- Fox M.D., Raichle M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. http://doi.org/10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox N.A., Pine D.S. Temperament and the emergence of anxiety disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51(2):125–128. doi: 10.1016/j.jaac.2011.10.006. http://doi.org/10.1016/j.jaac.2011.10.006.Temperament. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam L.J., Flannery J., Goff B., Gee D.G., Humphreys K.L., Telzer E. The development of human amygdala functional connectivity at rest from 4 to 23 years: a cross-sectional study. NeuroImage. 2014;95:193–207. doi: 10.1016/j.neuroimage.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Gilmore J.H., Giovanello K.S., Smith J.K., Shen D., Zhu H., Lin W. Temporal and spatial evolution of brain network topology during the first two years of life. PLoS ONE. 2011;6(9):e25278. doi: 10.1371/journal.pone.0025278. http://doi.org/10.1371/journal.pone.0025278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartstein M.A., Bridgett D.J., Rothbart M.K., Robertson C., Iddins E., Ramsay K., Schlect S. A latent growth examination of fear development in infancy: contributions of maternal depression and the risk for toddler anxiety. Dev. Psychol. 2010;46(3):651–668. doi: 10.1037/a0018898. http://doi.org/10.1037/a0018898. [DOI] [PubMed] [Google Scholar]
- Gartstein M.A., Putnam S., Rothbart M.K. Etiology of preschool behavior problems: contributions of temperament attributes in early childhood. Infant Ment. Health J. 2012;33(2):197–211. doi: 10.1002/imhj.21312. http://doi.org/10.1002/imhj. [DOI] [PubMed] [Google Scholar]
- Gartstein M.A., Rothbart M.K. Studying infant temperament via the revised infant behavior questionnaire. Infant Behav. Dev. 2003;26(1):64–86. http://doi.org/10.1016/S0163-6383(02)00169-8. [Google Scholar]
- Gee D.G., Gabard-Durnam L.J., Flannery J., Goff B., Humphreys K.L., Telzer E.H. Early developmental emergence of human amygdala–prefrontal connectivity after maternal deprivation. Proc. Natl. Acad. Sci. U.S.A. 2013;110(39):15638–15643. doi: 10.1073/pnas.1307893110. http://doi.org/10.1073/pnas.1307893110/-/DCSupplemental.www.pnas.org/cgi/doi/10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Humphreys K.L., Flannery J., Goff B., Telzer E.H., Shapiro M.<Et-AL>. A Developmental shift from positive to negative connectivity in human amygdala–prefrontal circuitry. Journal of Neuroscience. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. http://doi.org/10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A.M., Pfeifer J.H., Fisher P.a., Carpenter S., Fair D.a. Early life stress is associated with default system integrity and emotionality during infancy. J. Child Psychol. Psychiatry. 2015:1–11. doi: 10.1111/jcpp.12409. http://doi.org/10.1111/jcpp.12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg B., Gabriels L., Malone D., Rezai A., Friehs G., Okun M. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive–compulsive disorder: worldwide experience. Mol. Psychiatry. 2010;15(1):64–79. doi: 10.1038/mp.2008.55. http://doi.org/10.1038/mp.2008.55.Deep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E., Nelson E.E., Perez-Edgar K., Hardin M.G., Roberson-Nay R., Monk C.S. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. J. Neurosci. 2006;26(24):6399–6405. doi: 10.1523/JNEUROSCI.0666-06.2006. http://doi.org/10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallquist M.N., Hwang K., Luna B. The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. NeuroImage. 2013;82:208–225. doi: 10.1016/j.neuroimage.2013.05.116. http://doi.org/10.1016/j.neuroimage.2013.05.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri A.R., Bookheimer S.Y., Mazziotta J.C. Modulating emotional responses: effects of a neocortical network on the limbic system. NeuroReport. 2000;11(1):43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Hariri A.R., Mattay V.S., Tessitore A., Fera F., Weinberger D.R., James W. Neocortical modulation of the amygdala response to fearful stimuli. Biol. Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. http://doi.org/10.1016/S0002-3223(03)01786-9. [DOI] [PubMed] [Google Scholar]
- Helfinstein S.M., Benson B., Perez-Edgar K., Bar-Haim Y., Detloff A., Pine D.S. Striatal responses to negative monetary outcomes differ between temperamentally inhibited and non-inhibited adolescents. Neuropsychologia. 2011;49(3):479–485. doi: 10.1016/j.neuropsychologia.2010.12.015. http://doi.org/10.1016/j.neuropsychologia.2010.12.015.Striatal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfinstein S.M., Fox N.A., Pine D.S. Approach-withdrawal and the role of the striatum in the temperament of behavioral inhibition. Dev. Psychol. 2012;48(3):815–826. doi: 10.1037/a0026402. http://doi.org/10.1037/a0026402. [DOI] [PubMed] [Google Scholar]
- Herringa R.J., Birn R.M., Ruttle P.L., Burghy C.a., Stodola D.E., Davidson R.J., Essex M.J. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc. Natl. Acad. Sci. U.S.A. 2013;110(47):19119–19124. doi: 10.1073/pnas.1310766110. http://doi.org/10.1073/pnas.1310766110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfeld D., Rosenbaum J., Biederman J., Bolduc E., Faraone S.V., Snidman N. Stable behavioral inhibition and its association with anxiety disorder. J. Am. Acad. Child Adolesc. Psychiatry. 1992;31(1):103–111. doi: 10.1097/00004583-199201000-00016. Retrieved from 〈http://www.sciencedirect.com/science/article/pii/S0890856709644129〉. [DOI] [PubMed] [Google Scholar]
- Hofer M.A. Early relationships as regulators of infant physiology and behavior. Acta Paediatr. 1994;83(s397):9–18. doi: 10.1111/j.1651-2227.1994.tb13260.x. http://doi.org/10.1111/j.1651-2227.1994.tb13260.x. [DOI] [PubMed] [Google Scholar]
- Jensen J., McIntosh A.R., Crawley A.P., Mikulis D.J., Remington G., Kapur S. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron. 2003;40(6):1251–1257. doi: 10.1016/s0896-6273(03)00724-4. http://doi.org/10.1016/S0896-6273(03)00724-4. [DOI] [PubMed] [Google Scholar]
- Kagan J., Snidman N. Early childhood predictors of adult anxiety disorders. Biol. Psychiatry. 1999;46(11):1536–1541. doi: 10.1016/s0006-3223(99)00137-7. http://doi.org/10.1016/S0006-3223(99)00137-7. [DOI] [PubMed] [Google Scholar]
- Kim M.J., Loucks R.a., Palmer A.L., Brown A.C., Solomon K.M., Marchante A.N., Whalen P.J. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav. Brain Res. 2011;223(2):403–410. doi: 10.1016/j.bbr.2011.04.025. http://doi.org/10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanska G., Gross J.N., Lin M.-H., Nichols K.E. Guilt in young children: development, determinants, and relations with a broader system of standards. Child Dev. 2002;73(2):461–482. doi: 10.1111/1467-8624.00418. http://doi.org/10.1111/1467-8624.00418. [DOI] [PubMed] [Google Scholar]
- Lancaster J.L., Glass T.G., Lankipalli B.R., Downs H., Mayberg H., Fox P.T. A modality-independent approach to spatial normalization of tomographic images of the human brain. Hum. Brain Mapp. 1995;3(1995):209–223. [Google Scholar]
- Landers M., Sullivan R.M. The development and neurobiology of infant attachment and fear. Dev. Neurosci. 2012;34(2–3):101–114. doi: 10.1159/000336732. http://doi.org/10.1159/000336732.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppanen J.M., Nelson C.A. Early development of fear processing. Curr. Dir. Psychol. Sci. 2012;21(3):200–204. http://doi.org/10.1177/0963721411435841. [Google Scholar]
- Linnman C., Rougemont-Bücking A., Beucke J.C., Zeffiro T.a., Milad M.R. Unconditioned responses and functional fear networks in human classical conditioning. Behav. Brain Res. 2011;221(1):237–245. doi: 10.1016/j.bbr.2011.02.045. http://doi.org/10.1016/j.bbr.2011.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo-gil J., Tamis-lemonda C.S. Family resources and parenting quality: links to children's cognitive development across the first 3 years. Child Dev. 2008;79(4):1065–1085. doi: 10.1111/j.1467-8624.2008.01176.x. [DOI] [PubMed] [Google Scholar]
- Maltbie E., Bhatt K., Paniagua B., Smith R.G., Graves M.M., Mosconi M.W. Asymmetric bias in user guided segmentations of brain structures. NeuroImage. 2012;59(2):1315–1323. doi: 10.1016/j.neuroimage.2011.08.025. http://doi.org/10.1016/j.neuroimage.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. http://doi.org/10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Menon V. second ed. vol. 2. Elsevier; Acadenuc Press: 2015. pp. 597–611. (Salience Network. In Brain Mapping: An Encyclopedic Reference). http://doi.org/10.1016/B978-0-12-397025-1.00052-X. [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. http://doi.org/10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelon P., Snyder A.Z., Buckner R.L., McAvoy M., Zacks J.M. Neural correlates of incongruous visual information an event-related fMRI study. NeuroImage. 2003;19(4):1612–1626. doi: 10.1016/s1053-8119(03)00111-3. http://doi.org/10.1016/S1053-8119(03)00111-3. [DOI] [PubMed] [Google Scholar]
- Miezin F.M., Maccotta L., Ollinger J.M., Petersen S.E., Buckner R.L. Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. NeuroImage. 2000;11:735–759. doi: 10.1006/nimg.2000.0568. http://doi.org/10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- Milad M.R., Quirk G.J. Fear extinction as a model for translational neuroscience: ten years of progress. Annu. Rev. Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. http://doi.org/10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad M.R., Quirk G.J., Pitman R.K., Orr S.P., Fischl B., Rauch S.L. A role for the human dorsal anterior cingulate cortex in fear expression. Biol. Psychiatry. 2007;62(10):1191–1194. doi: 10.1016/j.biopsych.2007.04.032. http://doi.org/10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Milad M.R., Rauch S.L. Obsessive–compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn. Sci. 2012;16(1):43–51. doi: 10.1016/j.tics.2011.11.003. http://doi.org/10.1016/j.tics.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S., Roth T.L., Sullivan R.M. Rodent model of infant attachment learning and stress. Dev. Psychobiol. 2010;52(7):651–660. doi: 10.1002/dev.20482. http://doi.org/10.1002/dev.20482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S., Sullivan R.M. Maternal presence serves as a switch between learning fear and attraction in infancy. Nat. Neurosci. 2006;9(8):1004–1006. doi: 10.1038/nn1733. Retrieved from 〈http://www.nature.com/neuro/journal/v9/n8/abs/nn1733.html〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S., Wilson D.A., Levine S., Sullivan R.M. Dual circuitry for odor–shock conditioning during infancy: corticosterone switches between fear and attraction via amygdala. J. Neurosci. 2006;26(25):6737–6748. doi: 10.1523/JNEUROSCI.0499-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C.A., Dolgin K.G. The generalized discrimination of facial expressions by seven-month-old infants nelson and dolgin. Child Dev. 1985;56:58–61. [PubMed] [Google Scholar]
- Nigg J.T. Temperament and developmental psychopathology. J. Child Psychol. Psychiatry. 2006;47(3–4):395–422. doi: 10.1111/j.1469-7610.2006.01612.x. http://doi.org/10.1111/j.1469-7610.2006.01612.x. [DOI] [PubMed] [Google Scholar]
- Obradović J., Boyce W.T. Individual differences in behavioral, physiological, and genetic sensitivities to contexts: implications for development and adaptation. Dev. Neurosci. 2009;31(4):300–308. doi: 10.1159/000216541. Retrieved from 〈http://www.karger.com/Article/Abstract/216541〉. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. The cognitive control of emotion. Trends Cogn. Sci. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. http://doi.org/10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Parade S.H., Leerkes E.M. The reliability and validity of the Infant Behavior Questionnaire-Revised. Infant Behav. Dev. 2008;31(4):637–646. doi: 10.1016/j.infbeh.2008.07.009. http://doi.org/10.1016/j.infbeh.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltola M.J., Leppänen J.M., Mäki S., Hietanen J.K. Emergence of enhanced attention to fearful faces between 5 and 7 months of age. Soc. Cogn. Affect. Neurosci. 2009;4(2):134–142. doi: 10.1093/scan/nsn046. http://doi.org/10.1093/scan/nsn046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltola M.J., Leppänen J.M., Vogel-farley V.K., Hietanen J.K., Nelson C.A. Fearful faces but not fearful eyes alone delay attention disengagement in 7-month-old infants. Emotion. 2009;9(4):560–565. doi: 10.1037/a0015806. http://doi.org/10.1037/a0015806.Fearful. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K., Fox N.A. Temperament and anxiety disorders. Child Adolesc. Psychiatr. Clin. N. Am. 2005;14(4):681–706. doi: 10.1016/j.chc.2005.05.008. http://doi.org/10.1016/j.chc.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Perlman S.B., Pelphrey K.A. Developing connections for affective regulation: age-related changes in emotional brain connectivity. J. Exp. Child Psychol. 2011;108(3):607–620. doi: 10.1016/j.jecp.2010.08.006. http://doi.org/10.1016/j.jecp.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nat. Rev. Neurosci. 2008;9:148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Phelps E.A., Connor K.J.O., Gatenby J.C., Gore J.C., Davis M. Activation of the left amygdala to a cognitive representation of fear. Nat. Neurosci. 2001;4(4):437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Phelps E.A., Delgado M.R., Nearing K.I., Ledoux J.E. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phelps E.A., LeDoux J.E. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48(2):175–187. doi: 10.1016/j.neuron.2005.09.025. http://doi.org/10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pohlack S.T., Nees F., Ruttorf M., Schad L.R., Flor H. Activation of the ventral striatum during aversive contextual conditioning in humans. Biol. Psychol. 2012;91(1):74–80. doi: 10.1016/j.biopsycho.2012.04.004. http://doi.org/10.1016/j.biopsycho.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. http://doi.org/10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Cohen A.L., Nelson S.M., Wig G.S., Barnes K.A., Church J.A. Functional network organization of the human brain. Neuron. 2011;72(4):665–678. doi: 10.1016/j.neuron.2011.09.006. http://doi.org/10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Fair D.A., Schlaggar B.L., Petersen S.E. The development of human functional brain networks. Neuron. 2010;67(5):735–748. doi: 10.1016/j.neuron.2010.08.017. http://doi.org/10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Schlaggar B.L., Petersen S.E. Recent progress and outstanding issues in motion correction in resting state fMRI. NeuroImage. 2015;105:536–551. doi: 10.1016/j.neuroimage.2014.10.044. http://doi.org/10.1016/j.neuroimage.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S., Young C.B., Duan X., Chen T., Supekar K., Menon V. Amygdala subregional structure and intrinsic functional connectivity predicts individual differences in anxiety during early childhood. Biol. Psychiatry. 2014;75(11):892–900. doi: 10.1016/j.biopsych.2013.10.006. http://doi.org/10.1016/j.biopsych.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S., Young C.B., Supekar K., Uddin L.Q., Menon V. Immature integration and segregation of emotion-related brain circuitry in young children. Proc. Natl. Acad. Sci. U.S.A. 2012;109(20):7941–7946. doi: 10.1073/pnas.1120408109. http://doi.org/10.1073/pnas.1120408109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A., Anh T.T., Li Y., Chen H., Rifkin-Graboi A., Broekman B.F.P. Prenatal maternal depression alters amygdala functional connectivity in 6-month-old infants. Transl. Psychiatry. 2015;5(November (2014)):e508. doi: 10.1038/tp.2015.3. http://doi.org/10.1038/tp.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak C.A., Angstadt M., Welsh R.C., Kenndy A.E., Lyubkin M., Martis B., Phan K.L. Altered amygdala resting-state functional connectivity in post-traumatic stress disorder. Front. Psychiatry. 2011;2(November):62. doi: 10.3389/fpsyt.2011.00062. http://doi.org/10.3389/fpsyt.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff L.S. Center for epidemiologic studies depression scale (CESD) keywords: background: developer(s): copyright: reliability: assessment: scale items: references. Health (San Francisco) 1977;51:4–6. [Google Scholar]
- Raper J., Wallen K., Sanchez M.M., Stephens S.B.Z., Henry A., Villareal T., Bachevalier J. Sex-dependent role of the amygdala in the development of emotional and neuroendocrine reactivity to threatening stimuli in infant and juvenile rhesus monkeys. Horm. Behav. 2013;63(4):646–658. doi: 10.1016/j.yhbeh.2013.01.010. http://doi.org/10.1016/j.yhbeh.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart M.K., Bates J.E. Temperament. In: Damon W., Lerner R., Eisenberg N., editors. sixth ed. vol. 3. Wiley; New York, NY: 2006. pp. 99–166. (Handbook of Child Psychology: Social, Emotional, and Personality Development). [Google Scholar]
- Roy A.K., Fudge J.L., Kelly A.M.C., Perry J.S.a., Daniele T., Carlisi C. Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2013;52(3) doi: 10.1016/j.jaac.2012.12.010. 290.e2-299.e2. http://doi.org/10.1016/j.jaac.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A.K., Shehzad Z., Margulies D.S., Kelly A.M.C., Uddin L.Q., Gotimer K. Functional connectivity of the human amygdala using resting state fMRI. NeuroImage. 2009;45(2):614–626. doi: 10.1016/j.neuroimage.2008.11.030. http://doi.org/10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman D.C., Fusi S. Emotion, cognition, and mental state representation in amygdala and prefrontal cortex. Annu. Rev. Neurosci. 2011;33:173–202. doi: 10.1146/annurev.neuro.051508.135256. http://doi.org/10.1146/annurev.neuro.051508.135256.Emotion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D., Delgado M.R. Overlapping neural systems mediating extinction, reversal and regulation of fear. Trends Cogn. Sci. 2010;14(6):268–276. doi: 10.1016/j.tics.2010.04.002. http://doi.org/10.1016/j.tics.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz C.E., Snidman N., Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. J. Am. Acad. Child Adolesc. Psychiatry. 1999;38(8):1008–1015. doi: 10.1097/00004583-199908000-00017. http://doi.org/10.1097/00004583-199908000-00017. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H.<Et-AL>. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. http://doi.org/10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon D., Kaufmann C., Müsch K., Kischkel E., Kathmann N. Fronto-striato-limbic hyperactivation in obsessive–compulsive disorder during individually tailored symptom provocation. Psychophysiology. 2010;47(4):728–738. doi: 10.1111/j.1469-8986.2010.00980.x. http://doi.org/10.1111/j.1469-8986.2010.00980.x. [DOI] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. http://doi.org/10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Bannister P., Beckmann C., Brady M. FSL: new tools for functional and structural brain image analysis. NeuroImage. 2001;13 Retrieved from 〈http://c3s2i.free.fr/cv/fsl.pdf〉. (2001) [Google Scholar]
- Sripada R.K., King A.P., Garfinkel S.N., Wang X., Sripada C.S., Welsh R.C., Liberzon I. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J. Psychiatry Neurosci. 2012;37(4):241–249. doi: 10.1503/jpn.110069. http://doi.org/10.1503/jpn.110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein J.L., Wiedholz L.M., Bassett D.S., Weinberger D.R., Zink C.F., Mattay V.S., Meyer-Lindenberg A. A validated network of effective amygdala connectivity. NeuroImage. 2007;36(3):736–745. doi: 10.1016/j.neuroimage.2007.03.022. http://doi.org/10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Supekar K., Menon V. Developmental maturation of dynamic causal control signals in higher-order cognition: a neurocognitive network model. PLoS Comput. Biol. 2012;8(2):e1002374. doi: 10.1371/journal.pcbi.1002374. http://doi.org/10.1371/journal.pcbi.1002374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach P., Tournoux J. Georg Thieme Verlag; Stuttgart, Germany: 1988. Co-planar Stereotaxic Altas of the Human Brain. [Google Scholar]
- Tottenham N. The importance of early experiences for neuro-affective development. In: Pine D.S., Andersen S.L., editors. Current Topics in Behavioral Neuroscience, Volume 16: The Neurobiology of Childhood. Springer-Verlag; Berlin: 2014. http://doi.org/10.1007/7854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursache A., Blair C., Stifter C., Voegtline K. Emotional reactivity and regulation in infancy interact to predict executive functioning in early childhood. Dev. Psychol. 2013;49(1):127–137. doi: 10.1037/a0027728. http://doi.org/10.1037/a0027728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marle H.J.F., Hermans E.J., Qin S., Fernández G. Enhanced resting-state connectivity of amygdala in the immediate aftermath of acute psychological stress. NeuroImage. 2010;53(1):348–354. doi: 10.1016/j.neuroimage.2010.05.070. http://doi.org/10.1016/j.neuroimage.2010.05.070. [DOI] [PubMed] [Google Scholar]
- van Wingen G.A., Geuze E., Vermetten E., Fernández G. Perceived threat predicts the neural sequelae of combat stress. Mol. Psychiatry. 2011;16(6):664–671. doi: 10.1038/mp.2010.132. http://doi.org/10.1038/mp.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vértes P.E., Bullmore E.T. Annual research review: growth connectomics—the organization and reorganization of brain networks during normal and abnormal development. J. Child Psychol. Psychiatry. 2015;56(3):299–320. doi: 10.1111/jcpp.12365. http://doi.org/10.1111/jcpp.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Vachet C., Rumple A., Gouttard S., Ouziel C., Perrot E. Multi-atlas segmentation of subcortical brain structures via the AutoSeg software pipeline. Front. Neuroinf. 2014;8(February):1–11. doi: 10.3389/fninf.2014.00007. http://doi.org/10.3389/fninf.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters E., Matas L., Sroufe L.A. Reactions to an approaching stranger: description, validation, and functional significance of wariness. Child Dev. 1975;46:348–356. [PubMed] [Google Scholar]
- White L., McDermott J., Degnan K.A., Henderson H.A., Fox N.A. Behavioral inhibition and anxiety: the moderating roles of inhibitory control and attention shifting. J. Abnorm. Child Psychol. 2011;39(5):735–747. doi: 10.1007/s10802-011-9490-x. http://doi.org/10.1007/s10802-011-9490-x.Behavioral. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich P.a., Piven J., Hazlett H.C., Smith R.G., Ho S., Gee J.C., Gerig G. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. NeuroImage. 2006;31(3):1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. http://doi.org/10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]