Abstract
Transient global cerebral ischemia (GCI) causes delayed neuronal cell death in the vulnerable hippocampus CA1 subfield, as well as behavioral deficits. Ischemia reperfusion (I/R) produces excessive reactive oxygen species and plays a key role in brain injury. The mitochondrial electron respiratory chain is the main cellular source of free radical generation, and dysfunction of mitochondria has a significant impact on the neuronal cell death in ischemic brain. The aim of the present study is to investigate the potential beneficial effects of methylene blue (MB) in a 4-vessel occlusion (4VO) GCI model on adult male rats. MB was delivered at a dose of 0.5 mg/kg/day for 7 days, through a mini-pump implanted subcutaneously after GCI. We first found that MB significantly improved ischemic neuronal survival in hippocampal CA1 region as measured by cresyl violet staining as well as NeuN staining. We also found that MB has the ability to rescue ischemia-induced decreases of cytochrome c oxidase activity and ATP generation in CA1 region following I/R. Further analysis with labeling of MitoTracker® Red revealed that the depolarization of mitochondrial membrane potential (MMP) was markedly attenuated following MB treatment. In addition, the induction of caspase-3, -8 and -9 activities, and the increased numbers of TUNEL positive cells of CA1 region were significantly reduced by MB application. Correspondingly, Barnes maze tests showed that the deterioration of spatial learning and memory performance following GCI were significantly improved in MB-treatment group compared to ischemic control group. In summary, our study suggests that MB may be a promising therapeutic agent targeting neuronal cell death and cognitive deficits following transient global cerebral ischemia.
Keywords: Global cerebral ischemia, Methylene blue, Mitochondria, Neuroprotection, Functional improvement
Introduction
Cerebral ischemia occurs during cardiac arrest, which affects more than 83% of people over 65 years old [1]. Out-of-hospital cardiac arrest accounts for approximately 450,000 deaths annually, and is responsible for more than one-half of all cardiovascular deaths, with a mortality rate higher than 90% [2,3]. The post-cardiac arrest brain injury is the major cause of its high mortality and serious morbidity. Up to 16% of patients develop clinical brain death, even if they achieve spontaneous circulation return [4]. Cardiac arrest survivors are often complicated with persistent cognitive impairment, such as memory and sensorimotor deficits, reducing their quality of life and impacting a heavy economic burden [5].
The arrest of blood flow reduces the oxygen and glucose supply to the brain, causing ischemic brain injury, especially when hypoxia extends beyond 5 minutes [6]. Computed tomography has shown that the type of pathological change in cardiac arrest brain injury is global brain ischemia rather than focal brain lesions [7]. Microscopy histological examination revealed that the hippocampus has pronounced cell abnormalities even in those patients only suffering from several minutes of hypoxia [8]. Bilateral hippocampal atrophy has been diagnosed by high resolution magnetic resonance after hypoxia lasting 20 minutes [9]. Magnetic brain scan studies also found that the abnormalities mainly occur in the CA1 and CA2 regions of the hippocampus and the amygdala, after a two-day coma [10]. This pattern of regionally selective vulnerability of CA1 has been demonstrated in cardiac arrest patients[11].
The generation of reactive oxygen species (ROS) under ischemia reperfusion has been known as a major contributor to brain damage. The cellular sources of ROS generation include xanthine oxide, NADPH oxidase, cyclooxygenases and lipoxygenases. Our previous studies have shown that inhibition of NADPH oxidase attenuates ROS generation and protects the neurons from ischemic injury [12,13]. More importantly, the mitochondria produce ROS from the electron respiration chain, which is thought to be the main cellular source of ROS generation [14]. The mitochondria are also involved with membrane potential, ATP levels, calcium load, and apoptotic pathways, as well as being a central player in the development of ischemia reperfusion (I/R) injury. Ischemia triggers mitochondrial dysfunction, while re-introduction of oxygen during reperfusion dramatically increases the generation of ROS, such as superoxide and hydrogen peroxide [15].
Methylene blue (MB) has been used clinically for a long time. It has been reported that MB can reduce the formation of amyloid plaques and neurofibrillary tangles and can partially protect mitochondrial function [16,17]. Recent investigations suggest that MB functions as an alternative electron carrier, receiving electrons from NADH and transferring them to cytochrome c, thereby bypassing the complex I/III blockage under pathological situations. This results in the reduction of the infarct volume in rat stroke model, and protection of astrocytes from oxygen glucose deprivation (OGD) injury in vitro with MB treatment [18,19].
There is currently no effective drug or therapy available that protects the brain from global cerebral ischemia (GCI)-induced neuronal impairment. In the current study, MB was administered to a transient GCI rat model to investigate the potential beneficial effects on neuronal injury and behavioral deficits, aiming to find a new method in the rescue of ischemic brain injury following cardiac arrest.
Materials and Methods
Global Cerebral Ischemia Model
The GCI model has been widely used to study the delayed neuronal cell death of vulnerable hippocampus CA1 pyramidal neurons, which occurs 2–4 days after the ischemic reperfusion [20]. Four-vessel occlusion (4VO) ischemic model was performed on male SD rats, 3 months old, as described in our previous study [21]. Briefly, the vertebral arteries were electrocauterized and the common carotid arteries (CCAs) were exposed under anesthesia. Wound clips were used to close the incision and the rats were allowed a 24 h recovery period. After 24 h recovery, the animals were anesthetized using light isoflurane anesthesia and the CCAs were re-exposed and clipped for 15 minutes occlusion. The reperfusion was induced by releasing the clips and restoring the carotid artery blood flow. Rectal temperature was maintained at 37° C using a thermal blanket throughout the experiment. Sham controls underwent the same surgical exposure procedures, except that the arteries were not occluded. All procedures were approved by the Georgia Regent University institutional committee for care and use of animals and were in accordance with National Institutes of Health guidelines. Methylene blue (Fisher scientific, Pittsburgh, PA) was administrated with 7-day release Alzet osmotic mini-pumps (1007D, Durect Corporation, Cupertino, CA) at 0.5mg/kg/d. The mini-pumps were implanted subcutaneously under the upper back skin immediately after ischemia perfusion.
Histology Examination
Rats were anesthetized with chloral hydrate and underwent transcardial perfusion with 0.9% saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer. Brains were removed, postfixed overnight in paraformaldehyde, and cryoprotected with 30% sucrose in 0.1 M PB (pH 7.4) at 4 °C until they sank. Coronal sections (25 μm) were cut on a microtome (Leica RM2155, Nussloch, Germany) and collected through the entire dorsal hippocampus from animals at 10 d after ischemia or sham operation. For histological assessment, brain sections were stained with 0.01% (w/v) cresyl violet for 10 min, followed by graded ethanol dehydration. The stained sections were examined, and images were captured using an AxioVision4Ac microscope system (Carl Zeiss, Germany). For Diaminobenzidine (DAB) staining, sections were blocked with 10% horse serum in PBS for 1 h at room temperature, incubated with anti-NeuN (Millipore, Billerica, MA) overnight at 4°C. The later procedures followed the VECTASTAIN Elite ABC system manufacturer instruction (Vector Laboratories, Burlingame, CA), as described from our previously publication [21]. The NeuN stained hippocampal neurons were observed and images were captured using an Olympus IX70 microscope (Olympus, Japan). The number of CA1 neurons per 250μm length of the medial CA1 pyramidal cell layer was counted bilaterally in four sections per animal. Cell counts from the right and left hippocampus on each of the four sections were averaged to provide mean value, as we reported before [22,23]. Sections were also analyzed for the presence of apoptotic nuclei using the DeadEnd Fluorometric TUNEL System (Promega, Madison, WI, USA) as described. The fluorescein-12-dUTP-labeled fragmented DNA can then be visualized directly by fluorescence microscopy. Nuclei were stained with propidium iodide. Images were collected with a Zeiss LSM 700 confocal microscope (Carl Zeiss, Germany). The quantification of the TUNEL stained nuclei and total nuclei was processed by Image-Pro software and presented as a percentage of total nuclei in the field as previously described [24].
Caspase Activity Measurement
Hippocampal CA1 regions were quickly separated on an ice pad along the hippocampal fissure, using a standardized microdissection procedure. The separated tissues were homogenized by using a motor-driven Teflon homogenizer in ice-cold homogenization buffer with inhibitors of proteases and enzymes as previously described [21]. Caspase activities for caspase-3, -8, and -9 were measured in the protein homogenate using fluorometric substrates. The following substrates were used for caspase-3, -8, and -9, respectively: Ac-DEVD-AMC, Ac-IETD-AMC, and Ac-LEHD-AMC (AnaSpec, Fremont, CA). The substrates were cleaved proteolytically by the corresponding caspases, and the fluorescence of free AMC was measured. For determination of caspase activities, 100 μg of total protein were incubated for 3 h in caspase buffer (100 mM HEPES, 10% sucrose, 10 mM DTT, 0.1% CHAPS, 1 μg/ml leupeptin, and 1 mM PMSF) with 100 mM substrate. Fluorescence was determined with an excitation wavelength of 380 nm and an emission wavelength of 460 nm for AMC using a Synergy HT Microplate reader (BioTek Instruments Inc, Winooski, VT). Values are expressed as fluorescence of AMC per μg of protein and then were compared with sham group.
Mitochondrial Cytochrome C Oxidase Activity
Cytochrome c oxidase is a part of the cytochrome c oxidase subunit complex, which is located in the inner membrane of the mitochondria and is the terminal electron acceptor in the electron transfer chain, functioning by converting molecular oxygen to water and helping synthesize ATP. The hippocampal CA1 regions from I/R 2 day rats were homogenized using a method as aforementioned. The crude mitochondrial fractions were collected by centrifuging the homogenates at 14000g for 15 min at 4°C. Cytochrome c oxidase activity in the mitochondrial fractions was assessed using activity assay kit (KC310100; BioChain Institute, Hayward, CA). The ability of cytochrome c oxidase to oxidize fully reduced ferrocytochrome c to ferricytochrome c was measured using spectrophotometry. The absorbance of oxidized ferricytochrome c was measured as a loss of absorbance at 550 nm in a 96-well plate reader. The presented value was calculated by the absorbance divided by the mitochondrial lysate protein levels.
Quantification Of Total ATP Levels
ATP concentration was determined using a kit of ENLITEN® rLuciferase/Luciferin reagent (FF2021, Promega, Madison, WI, USA) following the protocol of the manufacturer. Briefly, 30 μg of sample proteins were suspended in 100 μl of reconstituted rL/L reagent buffer containing luciferase, D-luciferin, Tris-acetate buffer (pH 7.75), ethylenediaminetetraacetic acid (EDTA), magnesium acetate, bovine serum albumin (BSA) and dithiothreitol (DTT). Light emission at 10 s intervals from the L/L reaction was measured in a standard microplate luminometer (PE Applied Biosystems). Relative light units (RLU) from “background blank” containing rL/L reagent and the homogenization buffer used to prepare the samples were subtracted from the sample light output in the assay. Values of ATP levels were determined using an ATP standard curve, and data were expressed as folds changes compared with sham control group for graphical depiction.
Mitotracker Red Staining and Confocal Microscopy
MitoTracker® Red CMXRos (M-7512, Life Technologies, NY, USA), a red-fluorescent dye, was used to measure the depolarization of mitochondrial membrane potential (MMP). Briefly, 50 μg of MitoTracker® Red CMXRos were dissolved in high-quality anhydrous dimethylsulfoxide (DMSO) and diluted in saline to a final working solution. In the present study, MitoTracker® Red CMXRos (50 ng/ml in 100 μl of saline) was administered intravenously via tail vein injection. Five min after injection, the animals were deeply anesthetized with isoflurane and perfused transcardially with 0.1 M phosphate-buffered saline (PBS, pH 7.4) followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4). Brains were removed and postfixed in the same fixative overnight and cryoprotected with 30% sucrose in 0.1 M PB (pH 7.4) at 4 °C until they sank. Brain tissues were then embedded in optimal cutting temperature (OCT) compound and frozen coronal sections (20 μm) were cut through the dorsal hippocampus. Thereafter, sections were washed for 4 × 10 min by 0.1% PBS-Triton X-100 and briefly with distilled water. Finally, the sections were mounted and coverslipped in Vectashield mounting medium for fluorescence with 4′, 6-diamidino-2-phenylindole (DAPI) (H-1200; Vector Laboratories, Inc., CA, USA). Three to five sections of each animal (200 μm apart, approximately 1.5–3.3 mm posterior to bregma) were selected for confocal microscopy. Images were acquired on an LSM510 META confocal laser microscope (Carl Zeiss) using 40× objective with the image size set at 1024 × 1024 pixels. The captured images were processed and analyzed by the LSM 510 META software. The fluorescence signals of MitoTracker Red were quantitatively evaluated by using the ImageJ software (National Institutes of Health, Bethesda, MD) and the intensities were normalized as percentage changes compared with those in the sham control group. Data were calculated as mean ± SE from four to five independent animals per group.
Barnes Maze
The Barnes maze test was performed to evaluate the hippocampal dependent spatial learning and memory [25]. The Barnes circular platform maze is a 122 cm diameter circular platform on a 1.0 m stand with 18 evenly spaced 10 cm diameter holes around the circumference with a black box (escape box, 20×15×12 cm) was placed underneath one of the holes. Several visual cues were pasted to the black walls surrounded the platform to facilitate orientation for the rat to use as spatial cues. Testing was performed in a darkened room with bright flood incandescent light (500W, 1000 lux) shining down on the maze surface. Rats were also exposed to noxious auditory stimulus with the use of digital metronome software and two computer speakers facing the platform. Prior to the first trial, animals were subjected to a habituation trial by gently placing the rat in the middle of the platform under a black box and allowing the rat to enter the escape box for one minute before being returned to its home cage. On day 7 after ischemia, 3 days of training trials were performed and each rat was tested one trial per day. The time to enter into the target hole was recorded. The probe trial was performed on day 10 after GCI. The platform and escape box were cleaned with 70% ethanol between each test. Video recordings were made using an FUJINON Lens, Vari-focal, 2.7-13.5mm (FUJIFILM Corporation, Japan). The primary latency and the time spend in quadrants were quantified using ANY-maze video tracking software (Stoelting Co., Wood Dale, IL).
Elevated Plus Maze
Elevated plus maze was used to evaluate exploratory and anxiety-like behavior in a novel environment [26]. The maze consists of two opposing open arms (50 cm × 10 cm × 0.5 cm), an open platform (10 cm × 10 cm) in the center and two opposing closed arms (50 cm × 10 cm × 40 cm). The maze was elevated 50 cm from the floor. At the beginning of the test, the rat was placed on the central platform facing one of the open arms. The cumulative time spent in the open arms, as well as risk assessment behaviors were measured during a 5 min period. An open-arm entry was defined as all four of the paws being placed in the open arm. Risk assessment behavior was defined as a stretch-attend response that the rat stretched its body forward with sniff or obvious scan. The maze was cleaned with 70% ethanol between each test. Videos were recorded and analyzed using Any-maze video tracking system.
Open Field
Open field was used to detect motor and exploratory behavior [27]. The open field was made of black wood and consisted of a floor (96 cm × 96 cm) with 50-cm walls. The box floor was painted with white lines (6 mm) to form 16 equal squares. During a 5 min observation period, the number of squares crossed, rearing, and grooming was recorded. The field was cleaned with 70% ethanol between each test. Videos were recorded and analyzed using Any-maze video tracking system.
Statistical Analysis
Statistical calculations were performed using the GraphPad Prism V. 4.01 software (GraphPad Software, Inc. La Jolla, CA). The mean±SE were calculated for all samples, and significance was determined by either the Student’s t-test or ANOVA with the Newman-Keuls or Bonferroni post hoc test. A value of P < 0.05 was considered significant.
Results
Treatment With MB Significantly Reduced the Delayed Neuronal Cell Death in Rat Hippocampal CA1 Region Induced by GCI
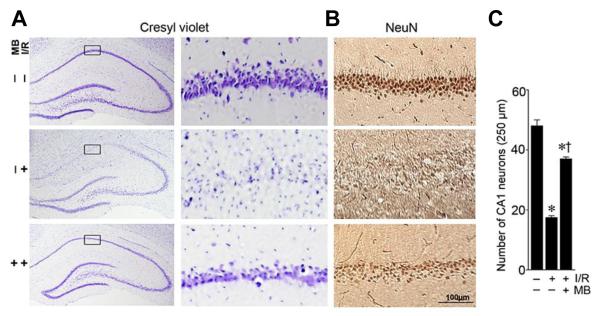
We first evaluated the potential neuroprotective effects of MB treatment against the delayed neuronal cell death induced by I/R following GCI. Histological evaluation by NeuN staining revealed that transient GCI induced a selective neuronal loss in the hippocampal CA1 region of ischemic control rats compared with sham operated animals (middle panel in Fig. 1A,B, and Fig. 1C). As seen in the cresyl violet staining, hippocampal CA1 pyramidal cell layer showed unequivocal signs of condensed, pyknotic and shrunken nuclei 10 days after ischemic reperfusion. By contrast, cresyl violet/NeuN staining and cell-counting study showed that treatment with MB significantly increased the number of surviving neurons in hippocampal CA1 region following ischemic reperfusion (bottom panel in Fig. 1A,B, and Fig. 1C), in comparison with non-treated ischemic control group. These results clearly demonstrate the novel neuroprotective effects of MB against delayed neuronal cell death in the vulnerable hippocampal CA1 region following transient GCI.
Fig.1.
Treatment with MB results in significant neuroprotection in hippocampal CA1 following GCI. A: Cresyl violet staining shows the whole hippocampal overview and the detailed CA1 region. B: High magnification DAB staining with anti-NeuN antibody shows pyramidal neurons in medial hippocampus CA1 region (scale bar: 100μm). C: The number of surviving neurons per 250-μm length of medial CA1 were quantitated and statistically compared. Data are presented as mean ± SE, n= 11 per group. * P<0.05 vs. sham, † P<0.05 vs. ischemic reperfusion (I/R) group.
MB Treatment Improved GCI-induced Mitochondrial Dysfunction in Vulnerable Hippocampal CA1 Region
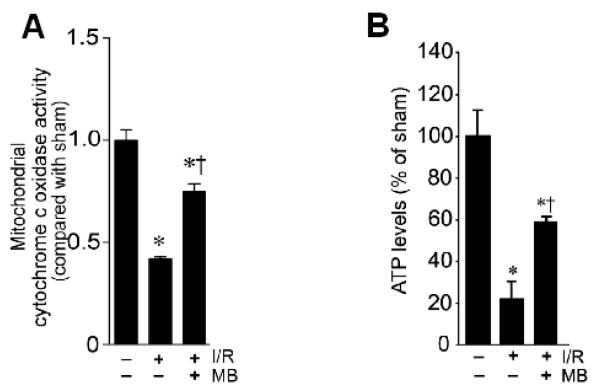
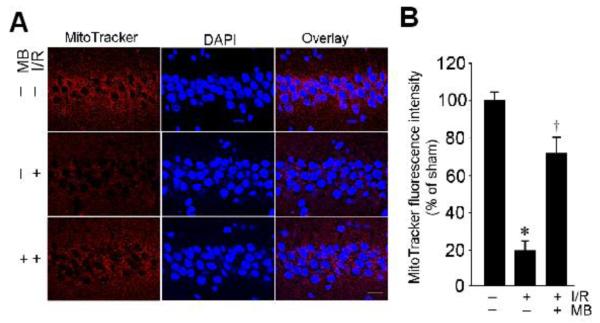
Previous studies suggest that MB has the ability to increase mitochondrial oxidative phosphorylation, prevent electron leaking, and attenuate superoxide production under pathological conditions [28,18]. We then examined whether MB has the properties to improve mitochondrial dysfunction in the vulnerable hippocampal CA1 region following I/R. As shown in Fig. 2A, analysis of mitochondrial cytochrome c oxidase activity demonstrated that the 2-day ischemic reperfusion caused a significant inhibition of mitochondrial cytochrome c oxidase activity in hippocampal CA1 region, indicating the dysfunction of mitochondrial electron transfer chain under I/R following GCI. Interestingly, MB treatment significantly increased the activity of cytochrome c oxidase compared with non-MB-treated ischemic control group (Fig. 2A). In order to further define the role of MB in improving mitochondrial function, we examined ATP concentration in the total CA1 protein samples using a kit of ENLITEN® rLuciferase/Luciferin. Constant with the changes of cytochrome c oxidase activity, results showed that the total ATP levels were significantly attenuated by 2-day ischemic reperfusion in CA1 protein samples, compared with sham control group (Fig. 2B). Importantly, the generation of ATP in CA1 region was significantly elevated by MB in comparison with non-MB-treated ischemic control group (Fig. 2B). Next, mitochondrial membrane potential was further evaluated under confocal microscopy in the hippocampal CA1 neurons by using the fluorescence changes of MitoTracker Red fluorescent dye. In healthy pyramidal neurons of CA1 cell layer in sham control group, mitochondria were labeled with Mitotracker Red with strong fluorescence (Fig. 3A). The intensities of fluorescence signals of MitoTracker Red were quantitatively evaluated and normalized as percentage changes compared with those in the sham control group. As shown in middle row of Fig. 3A&B, in I/R without MB-treated group, the normalized Mitotracker Red fluorescence after 2 d reperfusion was markedly decreased in the cytoplasm of CA1 pyramidal neurons, suggesting a GCI-induced mitochondrial depolarization and potential collapse of MMP. Intriguingly, treatment with MB reversed the GCI-induced change in Mitotracker Red intensity, compared with non-MB-treated control group, indicating a preservation of MMP and the presence of healthy mitochondria. Taken together, MB has the ability to rescue GCI-induced decreases of cytochrome c oxidase activity and ATP generation in CA1 region following I/R, and has the ability to preserve MMP following GCI.
Fig. 2.
Effects of MB on mitochondrial cytochrome c oxidase activity and total ATP levels after GCI. A: Total protein samples from CA1 region at 2 day reperfusion were subjected to activity analysis for mitochondrial cytochrome c oxidase. B: ATP assay in total protein samples from CA1 region indicates that MB treatment significantly increases total ATP levels compared with control group at ischemic reperfusion day 2. N= 4-5 in each group. *P<0.05 versus sham control, †P< 0.05 vs. ischemic control group without MB treatment.
Fig. 3.
MB attenuates depolarization of mitochondrial membrane potential (MMP) induced by transient global ischemic reperfusion. A: Confocal analysis for MitoTracker® Red CMXRos (red), DAPI (blue) and merged images in the hippocampal CA1 region (magnification: X40, scale bar: 20 mm). There was marked loss of MitoTracker Red staining at 2 day reperfusion compared with sham group. The loss of MitoTracker Red was prevented by treatment with MB. B: The fluorescence intensity of MitoTracker Red was measured and normalized as percentage changes compared with sham control group. MMP became hyperpolarized in ischemic group at 2d reperfusion without MB treatment, which was significantly recovered in the MB-treated groups. Data represent mean ± SE (n = 4-5). *P<0.05 versus sham control, †P< 0.05 vs. ischemic control group without MB treatment.
Treatment with MB Significantly Inhibited the Activities of Caspase-3,-8,-9 and Decreased Apoptotic Neuronal Death in Rat Hippocampal CA1 Region Induced by GCI
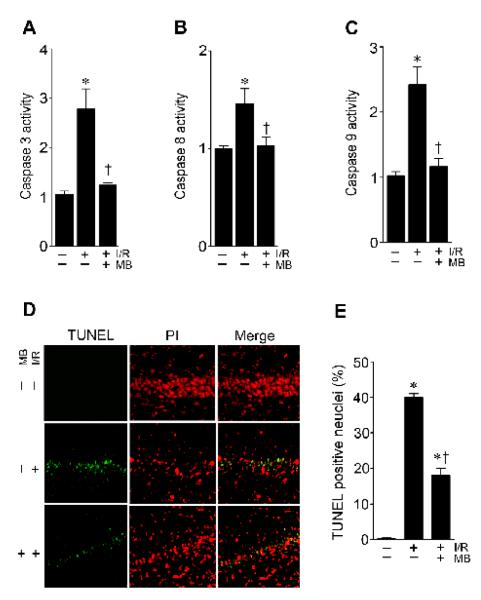
It is well accepted that the activation of caspase-3 cleaved by caspase-9 requires cytochrome c released following mitochondrial dysfunction [29], and that caspase 8 activation also plays a causal role in caspase 3 activation and apoptotic cell death [30]. Therefore, we evaluated whether the neuroprotective properties of MB involve an inhibition of caspase-3 activity, because one major putative consequence of mitochondrial dysfunction and subsequent release of cytochrome c is the activation of caspase-3. Caspase-3 activity was measured by a fluorometric substrates assay using hippocampal CA1 homogenates. As shown in Fig. 4A, 2-day of I/R evoked a 3-fold increase in caspase-3 activity. Importantly, the increased caspase-3 protease activity was dramatically prevented in MB-treated animals. Furthermore, caspase-9 and caspase-8 activities were also highly induced following 2-day ischemic reperfusion, and were effectively reduced by MB treatment (Fig. 4B&C). In order to analyze GCI-induced apoptotic cell death of hippocampal CA1 neurons following caspases activation, coronal brain sections were also subjected to TUNEL staining following ischemic reperfusion. As shown in Fig. 4D&E, in the non-MB-treated ischemic control animals, most of the hippocampal CA1 neurons were positive for TUNEL staining. However, immunofluorescence staining and quantitative analyses indicated that the number of TUNEL-positive cells in the pyramidal cell layer was significantly attenuated in the MB-treated animals compared with the ischemic control animals (Fig. 4D&E). These data clearly indicated the ability of MB to inhibit the intrinsic apoptotic pathway following GCI.
Fig. 4.
Effects of MB on caspase-3, -8 and -9 activities and the apoptotic neuronal death in rat hippocampal CA1 region. A-C: Caspase-3, -8 and -9 activities were measured by a fluorometric substrates assay using hippocampal CA1 homogenates. D-E: TUNEL staining and the cell-counting study showed a significant decrease in TUNEL-positive cells in the hippocampal CA1 region in the MB-treated rats compared with the non-treated animals. Data are presented as mean ± SE, n= 4–5 per group. * P<0.05 vs. sham control group, † P<0.05 vs. I/R group.
Treatment with MB Significantly Attenuated Learning and Memory Deficits Induced by GCI
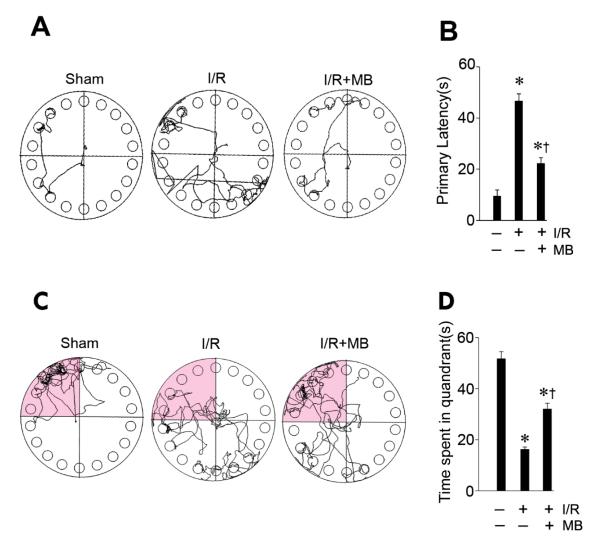
Spatial memory is the part of memory responsible for recording information about one’s environment and its spatial orientation. The hippocampus plays an important role in the processing of spatial locations, especially within CA1 region [31]. The Barnes maze is designed to test spatial memory and long term memory, and it has been shown that rodents with hippocampal damage showed impaired performance in the Barnes maze [32]. To investigate whether the beneficial effects of MB that reduced apoptotic neuronal cell death also led to functional improvement, we then measured the spatial learning ability in the Barnes maze. All animals were subjected to training trials at day 7, 8, and 9 after GCI and the probe trials were performed on day 10 after GCI. As shown in Fig. 5A&B, non-MB-treated ischemic control animals required more time to find the black escape box, compared to sham control rats. By contrast, MB-treated animals showed significantly decreased escape latencies to find the escape box on the last training trials. In the probe test on day 10 after GCI, the MB-treated animals and sham-operated animals spent significantly longer times in the target quadrant, respectively (Fig. 5C&D). However, animals in the non-MB-treated ischemic control group spent less time in the target quadrant where the escape box had been. Therefore, treatment of MB has the ability to significantly attenuate learning and memory impairment following the neuronal injury of hippocampal CA1 induced by GCI.
Fig. 5.
MB significantly attenuated learning and memory deficits induced by GCI. A-B: Barnes maze task was performed to test the spatial learning ability of the sham, ischemic control, MB-treated animals, as shown by the latency to find the black escape box at day 9 after GCI. C-D: Probe test in the quadrant where the escape box had been on day 10 after cerebral ischemia. The representative tracks in the quadrant zone are shown and data were statistically compared. Data are presented as mean ± SE, n= 11 per group. . * P<0.05 vs. sham control group, † P<0.05 vs. I/R group.
Treatment with MB Significantly Improved the GCI-injured Performances in Elevated Plus Maze and Open Field
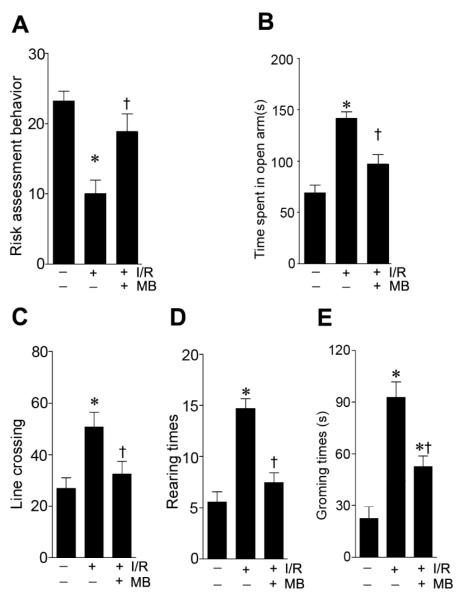
Elevated plus maze was performed to accesses exploratory and anxiety-like behavior in rodent models of brain disorders. As shown in Fig. 6A, ischemic animals showed decreased risk assessment behavior compared with sham operated animals, whereas MB-treated showed better risk assessment behavior. In the open arm entries tests, rats with GCI spent more time in open arms than sham operated rats (Fig. 6B), while MB-treated animals spent less time in the open arms than non-MB-treated ischemic control group. The Open field task test was also used to measure the locomotor activity in rodents. The data showed that rats with GCI displayed significant higher locomotor activity with increased amount of line crossings, rearing time and grooming time than those in sham operated rats. Notably, these GCI-induced hyperactivities were effectively alleviated in the MB-treated animals compared with non-MB-treated ischemic control group.
Fig. 6.
Effects of MB treatment on performances in the elevated plus maze test and open field test. A-B: The number of risk assessment behavior (A), and time spent in open arms (B) were recorded and statistically compared. An open-arm entry was defined as all four of the paws being placed in the open arm. Risk assessment behavior was defined as a stretch-attend response where t the rat stretched its body forward with sniff or obvious scan. C-E: In the open field test, the number of lines crossed, rearing, and grooming in the open field were recorded and statistically analyzed. Data are presented as mean ± SE, n= 11 per group. * P<0.05 vs. sham control group, † P<0.05 vs. I/R group.
Discussion
In the present study, we firstly demonstrated that MB has the ability to protect the vulnerable hippocampal pyramidal neurons against ischemic insult following transient GCI, as confirmed by both cresyl violet staining and NeuN staining. We observed that MB treatment significantly rescued ischemia-induced decreases of cytochrome c oxidase activity and ATP generation in CA1 region. We also found that MB preserved mitochondrial membrane potential following GCI, as examined by the MitoTracker® Red staining. Concomitantly, MB-treated animals showed significantly attenuated activation of caspase-3, -8 and -9 in response to ischemic insult following GCI. Consistent with the inhibitory effects on caspase activation was the gross reduction in the amounts of apoptotic cells, as shown by TUNEL staining, in the vulnerable hippocampal CA1 region following GCI. Importantly, treatment with MB significantly attenuated the learning and memory deficits that were seriously injured by GCI (as evaluated in the Barnes maze test). In addition, treatment with MB significantly improved the exploratory and anxiety-like behavior in Elevated plus maze, as well as the locomotor activity measured in Open field. Taken together, these research findings clearly supported the neuroprotective and functional improvement effects of MB in the model of GCI.
More than half of the cardiac arrest survivors have permanent brain damage [33,34]. But clinically, computed tomographic (CT) images are usually normal when taken immediately after a cardiac arrest, while after three days, the patients with poor outcomes show brain swelling and inversion of the gray white densities [35]. This type of delayed neuronal death following GCI after cardiac arrest is often characterized with selectively vulnerable neuronal cell death, in the hippocampus CA1 region. Neurons are more susceptible than glial cells in response to injury following ischemic reperfusion because they have higher energy demands and produce glutamate. On the other hand, the vulnerability of hippocampus can be affected by sex differences, with males having a greater volume and more neurons in all subregions than females, and males experiencing a greater loss of neurons in CA1 and the dentate gyrus than females [36]. In the present study, only male rats were included to minimize the variations of sex influence on brain injury. The 4-vessel occlusion rat model was adopted in the present study, wherein the delayed brain injury of the hippocampus is severe in CA1 subfield, and the CA3/DG cell layers are relatively resistance to neuronal loss. This well used GCI model mimics the brain damage patterns in patients with cardiac arrest.
Numerous factors contribute to the CA1 susceptibility in ischemic insult, such as the density of NMDA receptors [37]; MAP-2, NR1 and NR2B subunits binding [38]; ATP-sensitive potassium channel [39]; and Na+-K+ ATPase activity [40]. Interestingly, the mitochondrial function in the hippocampus regions also shows differences before and after cerebral ischemia. The succinate dehydrogenase level is higher in CA1, and after ischemia its activity reduction is relatively lower in CA1 [41] , suggesting CA1 region may have a high level of aerobic ATP production. In that case, if CA1 loses respiratory chain capacity, more ROS generation is expected. Our data demonstrates that in the CA1 region another electron transfer chain complex, cytochrome c oxidase activity was significantly reduced after I/R, adding to the evidence of electron respiratory chain disruption following cerebral ischemia. The mitochondrial released cytochrome c may also respond to the vulnerability of CA1 neurons. Immunohistochemistry study showed that cytosolic cytochrome c-positive cells were present exclusively in the CA1 region as early as 2 hr after ischemia, with a significant number of TUNEL positive cells [42]. These findings suggest that the mitochondrial dysfunction in CA1 region plays an important role in the pathology of neuronal cell death following cardiac arrest.
Mitochondria produce 90% of the cellular energy by oxidation phosphorylation. This process consumes 85% of the oxygen of the cell during ATP generation. Under normal conditions 0.4-4.0% of oxygen is converted to superoxide radical, through mitochondrial electron transfer chain complex I, III and IV. During ischemia, the arrest of the blood supply causes a reduction of oxygen and glucose to the brain and disrupts the mitochondria oxidative phosphorylation and ATP production. The reduction of ATP level limits the ion pumps in neuron membrane, inducing mitochondria damage. The generation of ROS combines with calcium overload, may also increase the mitochondrial permeability transition (MPT) pore opening, abolishing transmembrane potential, and inducing more superoxide generation [43]. The opening of the inner membrane permeability transition pore and the rupture of the outer membrane cause the cytochrome c release from mitochondria to the cytosol. With apoptosis inducing factor (AIF) involved, cytochrome c induces the mitochondrial apoptotic caspase pathway. When the blood supply is re-established, the sudden increase of oxygen in tissue will cause the dysfunctional mitochondria to generate a burst of ROS, which amplifies the oxidative stress injury to the brain [44]. The mitochondrial induced apoptotic neuronal cell death has been demonstrated in cerebral I/R brain injury[45]. Accumulating evidence suggests that mitochondria play a central role in ROS generation and neuronal cell death [46,14]. Our present results showed the complex IV activity was significantly reduced after I/R, indicating the mitochondrial dysfunction of in CA1 region. Complex IV consumes 95% of the O2 that reaches the cell. Complex I and III are involved in the production of superoxide, by non-specific transfer of electrons to O2. The electrons stalling, caused by GCI upstream of complex IV, would definitely enhance the superoxide overproduction.
Currently, there are no effective clinical interventions for the delayed brain injury following cardiac arrest. The therapeutic hypothermia treatment has been tested but the protective effect still remains uncertain [47,48]. Targeting mitochondrial protection and searching for an effective pharmacological compound which can block the free radical production and enhance mitochondrial function seems to be a promising strategy in the protection of the cardiac arrest-induced brain injury [49]. The neuroprotective effects of MB have been demonstrated both in vivo models of Alzheimer’s disease[17], Parkinson’s disease [50], stroke[51], as well as optic neuropathy [52]; and in vitro hypoxic injury of astrocyte and hepatocytes [19,53]. MB can easily pass the blood brain barrier and accumulate in the brain tissue where it concentrates in the mitochondrial matrix [54]. The most remarkable effect of MB is to transfer electrons to oxygen or alternate electron acceptors. This electron transfer resembles the activity of mitochondrial electron transfer complexes [55]. Recent studies have found that MB, at low dose level, prevents the effect of rotenone on mitochondrial complex I-III inhibition, and reduces the free radical production [18]. The MB electron shunt effect indicates that MB directly accepts electrons from NADH, NADPH, and FADH2 [56]. In a piglet model of extended cardiac arrest, MB significantly reduced myocardial damage [57], and protected the brain [58]. In a rat model of 2-VO brain injury, MB reduced cytochrome c oxidase activity and improved memory impairment [59]. The memory retention impaired by the inhibition of cytochrome oxidase, can be completely restored by administration of MB [60]. These recent investigations suggested MB protects neuron from I/R insult. Most recent studies show that MB is able to enhance brain function, with mechanisms involving increased autophagy and mitophagy in a rat ischemic stroke model [61,62]. It will be very interesting to further investigate the relationship between mitochondrial function and cognitive behavior, which may be helpful in the understanding of the cognitive improvement effects of MB in brain ischemia.
In conclusion, the results of the present study demonstrate the neuroprotective and behavioral improvement actions of MB against ischemic brain damage following GCI, and a potential mechanism associated with the preservation of mitochondrial cytochrome c oxidase activity and ATP generation, and the preservation of mitochondrial membrane potential. The observations also demonstrate that MB was capable of preventing intrinsic (caspase-9-dependent) and extrinsic (caspase-8-dependent) signaling pathways, both leading to the inactivation of the final executioner, caspase-3, a molecular mechanism underlying the beneficial effects of MB in GCI. Taken together, these results suggest that MB may have promising clinical efficacy in reducing ischemic brain damage resulting from cardiac arrest or other types of brain injury.
Acknowledgements
This research was supported by Research Grant (NS086929) from the National Institutes of Neurological Disorders and Stroke, National Institutes of Health.
Footnotes
Conflict of Interest The authors declare that there is no conflict of interest in the current study.
References
- 1.Zheng ZJ, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104(18):2158–2163. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]
- 2.Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, Rea T, Lowe R, Brown T, Dreyer J, Davis D, Idris A, Stiell I, Resuscitation Outcomes Consortium I Regional variation in out-of-hospital cardiac arrest incidence and outcome. Jama. 2008;300(12):1423–1431. doi: 10.1001/jama.300.12.1423. doi:10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callans DJ. Out-of-hospital cardiac arrest--the solution is shocking. The New England journal of medicine. 2004;351(7):632–634. doi: 10.1056/NEJMp048174. doi:10.1056/NEJMp048174. [DOI] [PubMed] [Google Scholar]
- 4.Adrie C, Haouache H, Saleh M, Memain N, Laurent I, Thuong M, Darques L, Guerrini P, Monchi M. An underrecognized source of organ donors: patients with brain death after successfully resuscitated cardiac arrest. Intensive care medicine. 2008;34(1):132–137. doi: 10.1007/s00134-007-0885-7. doi:10.1007/s00134-007-0885-7. [DOI] [PubMed] [Google Scholar]
- 5.Burke DT, Shah MK, Dorvlo AS, Al-Adawi S. Rehabilitation outcomes of cardiac and non-cardiac anoxic brain injury: a single institution experience. Brain injury. 2005;19(9):675–680. doi: 10.1080/02699050400024953. doi:10.1080/02699050400024953. [DOI] [PubMed] [Google Scholar]
- 6.Safar P. Cerebral resuscitation after cardiac arrest: a review. Circulation. 1986;74(6 Pt 2):IV138–153. [PubMed] [Google Scholar]
- 7.Sulzgruber P, Kliegel A, Wandaller C, Uray T, Losert H, Laggner AN, Sterz F, Kliegel M. Survivors of cardiac arrest with good neurological outcome show considerable impairments of memory functioning. Resuscitation. 2015;88:120–125. doi: 10.1016/j.resuscitation.2014.11.009. doi:10.1016/j.resuscitation.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Cummings JL, Tomiyasu U, Read S, Benson DF. Amnesia with hippocampal lesions after cardiopulmonary arrest. Neurology. 1984;34(5):679–681. doi: 10.1212/wnl.34.5.679. [DOI] [PubMed] [Google Scholar]
- 9.Gadian DG, Aicardi J, Watkins KE, Porter DA, Mishkin M, Vargha-Khadem F. Developmental amnesia associated with early hypoxic-ischaemic injury. Brain : a journal of neurology. 2000;123(Pt 3):499–507. doi: 10.1093/brain/123.3.499. [DOI] [PubMed] [Google Scholar]
- 10.Kartsounis LD, Rudge P, Stevens JM. Bilateral lesions of CA1 and CA2 fields of the hippocampus are sufficient to cause a severe amnesic syndrome in humans. Journal of neurology, neurosurgery, and psychiatry. 1995;59(1):95–98. doi: 10.1136/jnnp.59.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng T, Graham DI, Adams JH, Ford I. Changes in the hippocampus and the cerebellum resulting from hypoxic insults: frequency and distribution. Acta neuropathologica. 1989;78(4):438–443. doi: 10.1007/BF00688181. [DOI] [PubMed] [Google Scholar]
- 12.Raz L, Zhang QG, Zhou CF, Han D, Gulati P, Yang LC, Yang F, Wang RM, Brann DW. Role of Rac1 GTPase in NADPH oxidase activation and cognitive impairment following cerebral ischemia in the rat. PloS one. 2010;5(9):e12606. doi: 10.1371/journal.pone.0012606. doi:10.1371/journal.pone.0012606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu Q, Wainwright MS, Harris VA, Aggarwal S, Hou Y, Rau T, Poulsen DJ, Black SM. Increased NADPH oxidase-derived superoxide is involved in the neuronal cell death induced by hypoxia-ischemia in neonatal hippocampal slice cultures. Free radical biology & medicine. 2012;53(5):1139–1151. doi: 10.1016/j.freeradbiomed.2012.06.012. doi:10.1016/j.freeradbiomed.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanderson TH, Reynolds CA, Kumar R, Przyklenk K, Huttemann M. Molecular mechanisms of ischemia-reperfusion injury in brain: pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Molecular neurobiology. 2013;47(1):9–23. doi: 10.1007/s12035-012-8344-z. doi:10.1007/s12035-012-8344-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ambrosio G, Flaherty JT, Duilio C, Tritto I, Santoro G, Elia PP. Oxygen radicals generated at reflow induce peroxidation of membrane lipids in reperfused hearts. The Journal of clinical investigation. 1991;87(6):2056–2066. doi: 10.1172/JCI115236. doi:10.1172/JCI115236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oz M, Lorke DE, Petroianu GA. Methylene blue and Alzheimer’s disease. Biochemical pharmacology. 2009;78(8):927–932. doi: 10.1016/j.bcp.2009.04.034. doi:10.1016/j.bcp.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 17.Atamna H, Kumar R. Protective role of methylene blue in Alzheimer’s disease via mitochondria and cytochrome c oxidase. Journal of Alzheimer’s disease : JAD. 2010;20(Suppl 2):S439–452. doi: 10.3233/JAD-2010-100414. doi:10.3233/JAD-2010-100414. [DOI] [PubMed] [Google Scholar]
- 18.Wen Y, Li W, Poteet EC, Xie L, Tan C, Yan LJ, Ju X, Liu R, Qian H, Marvin MA, Goldberg MS, She H, Mao Z, Simpkins JW, Yang SH. Alternative mitochondrial electron transfer as a novel strategy for neuroprotection. The Journal of biological chemistry. 2011;286(18):16504–16515. doi: 10.1074/jbc.M110.208447. doi:10.1074/jbc.M110.208447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roy Choudhury G, Winters A, Rich RM, Ryou MG, Gryczynski Z, Yuan F, Yang SH, Liu R. Methylene Blue Protects Astrocytes against Glucose Oxygen Deprivation by Improving Cellular Respiration. PloS one. 2015;10(4):e0123096. doi: 10.1371/journal.pone.0123096. doi:10.1371/journal.pone.0123096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Annals of neurology. 1982;11(5):491–498. doi: 10.1002/ana.410110509. doi:10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- 21.Zhang QG, Raz L, Wang R, Han D, De Sevilla L, Yang F, Vadlamudi RK, Brann DW. Estrogen attenuates ischemic oxidative damage via an estrogen receptor alpha-mediated inhibition of NADPH oxidase activation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(44):13823–13836. doi: 10.1523/JNEUROSCI.3574-09.2009. doi:10.1523/JNEUROSCI.3574-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang QG, Wang R, Han D, Dong Y, Brann DW. Role of Rac1 GTPase in JNK signaling and delayed neuronal cell death following global cerebral ischemia. Brain research. 2009;1265:138–147. doi: 10.1016/j.brainres.2009.01.033. doi:10.1016/j.brainres.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang P, Chen C, Wang R, Chechneva OV, Chung SH, Rao MS, Pleasure DE, Liu Y, Zhang Q, Deng W. hESC-derived Olig2+ progenitors generate a subtype of astroglia with protective effects against ischaemic brain injury. Nature communications. 2013;4:2196. doi: 10.1038/ncomms3196. doi:10.1038/ncomms3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu Q, Rau TF, Harris V, Johnson M, Poulsen DJ, Black SM. Increased p38 mitogen-activated protein kinase signaling is involved in the oxidative stress associated with oxygen and glucose deprivation in neonatal hippocampal slice cultures. Eur J Neurosci. 2011;34(7):1093–1101. doi: 10.1111/j.1460-9568.2011.07786.x. doi:10.1111/j.1460-9568.2011.07786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. Journal of comparative and physiological psychology. 1979;93(1):74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- 26.Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neuroscience and biobehavioral reviews. 2005;29(8):1193–1205. doi: 10.1016/j.neubiorev.2005.04.017. doi:10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 27.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. European journal of pharmacology. 2003;463(1-3):3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- 28.Poteet E, Winters A, Yan LJ, Shufelt K, Green KN, Simpkins JW, Wen Y, Yang SH. Neuroprotective actions of methylene blue and its derivatives. PloS one. 2012;7(10):e48279. doi: 10.1371/journal.pone.0048279. doi:10.1371/journal.pone.0048279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91(4):479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 30.Chandra D, Choy G, Deng X, Bhatia B, Daniel P, Tang DG. Association of active caspase 8 with the mitochondrial membrane during apoptosis: potential roles in cleaving BAP31 and caspase 3 and mediating mitochondrion-endoplasmic reticulum cross talk in etoposide-induced cell death. Molecular and cellular biology. 2004;24(15):6592–6607. doi: 10.1128/MCB.24.15.6592-6607.2004. doi:10.1128/MCB.24.15.6592-6607.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodrich-Hunsaker NJ, Hunsaker MR, Kesner RP. The interactions and dissociations of the dorsal hippocampus subregions: how the dentate gyrus, CA3, and CA1 process spatial information. Behavioral neuroscience. 2008;122(1):16–26. doi: 10.1037/0735-7044.122.1.16. doi:10.1037/0735-7044.122.1.16. [DOI] [PubMed] [Google Scholar]
- 32.Fox GB, Fan L, LeVasseur RA, Faden AI. Effect of traumatic brain injury on mouse spatial and nonspatial learning in the Barnes circular maze. Journal of neurotrauma. 1998;15(12):1037–1046. doi: 10.1089/neu.1998.15.1037. [DOI] [PubMed] [Google Scholar]
- 33.Pusswald G, Fertl E, Faltl M, Auff E. Neurological rehabilitation of severely disabled cardiac arrest survivors. Part II. Life situation of patients and families after treatment. Resuscitation. 2000;47(3):241–248. doi: 10.1016/s0300-9572(00)00240-9. [DOI] [PubMed] [Google Scholar]
- 34.Herlitz J, Andersson E, Bang A, Engdahl J, Holmberg M, lindqvist J, Karlson BW, Waagstein L. Experiences from treatment of out-of-hospital cardiac arrest during 17 years in Goteborg. European heart journal. 2000;21(15):1251–1258. doi: 10.1053/euhj.2000.2150. doi:10.1053/euhj.2000.2150. [DOI] [PubMed] [Google Scholar]
- 35.Zingler VC, Krumm B, Bertsch T, Fassbender K, Pohlmann-Eden B. Early prediction of neurological outcome after cardiopulmonary resuscitation: a multimodal approach combining neurobiochemical and electrophysiological investigations may provide high prognostic certainty in patients after cardiac arrest. European neurology. 2003;49(2):79–84. doi: 10.1159/000068503. doi:68503. [DOI] [PubMed] [Google Scholar]
- 36.Madeira MD, Paula-Barbosa M, Cadete-Leite A, Tavares MA. Unbiased estimate of hippocampal granule cell numbers in hypothyroid and in sex-age-matched control rats. Journal fur Hirnforschung. 1988;29(6):643–650. [PubMed] [Google Scholar]
- 37.Monaghan DT, Cotman CW. Distribution of N-methyl-D-aspartate-sensitive L-[3H]glutamate-binding sites in rat brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1985;5(11):2909–2919. doi: 10.1523/JNEUROSCI.05-11-02909.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Butler TR, Self RL, Smith KJ, Sharrett-Field LJ, Berry JN, Littleton JM, Pauly JR, Mulholland PJ, Prendergast MA. Selective vulnerability of hippocampal cornu ammonis 1 pyramidal cells to excitotoxic insult is associated with the expression of polyamine-sensitive N-methyl-D-asparate-type glutamate receptors. Neuroscience. 2010;165(2):525–534. doi: 10.1016/j.neuroscience.2009.10.018. doi:10.1016/j.neuroscience.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mourre C, Ben Ari, Bernardi H, Fosset M, Lazdunski M. Antidiabetic sulfonylureas: localization of binding sites in the brain and effects on the hyperpolarization induced by anoxia in hippocampal slices. Brain research. 1989;486(1):159–164. doi: 10.1016/0006-8993(89)91288-2. [DOI] [PubMed] [Google Scholar]
- 40.Haglund MM, Stahl WL, Kunkel DD, Schwartzkroin PA. Developmental and regional differences in the localization of Na, K-ATPase activity in the rabbit hippocampus. Brain research. 1985;343(1):198–203. doi: 10.1016/0006-8993(85)91180-1. [DOI] [PubMed] [Google Scholar]
- 41.Kuroiwa T, Terakado M, Yamaguchi T, Endo S, Ueki M, Okeda R. The pyramidal cell layer of sector CA 1 shows the lowest hippocampal succinate dehydrogenase activity in normal and postischemic gerbils. Neuroscience letters. 1996;206(2-3):117–120. doi: 10.1016/s0304-3940(96)12439-3. [DOI] [PubMed] [Google Scholar]
- 42.Sugawara T, Fujimura M, Morita-Fujimura Y, Kawase M, Chan PH. Mitochondrial release of cytochrome c corresponds to the selective vulnerability of hippocampal CA1 neurons in rats after transient global cerebral ischemia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19(22):RC39. doi: 10.1523/JNEUROSCI.19-22-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. American journal of physiology Cell physiology. 2004;287(4):C817–833. doi: 10.1152/ajpcell.00139.2004. doi:10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 44.Kalogeris T, Bao Y, Korthuis RJ. Mitochondrial reactive oxygen species: a double edged sword in ischemia/reperfusion vs preconditioning. Redox biology. 2014;2:702–714. doi: 10.1016/j.redox.2014.05.006. doi:10.1016/j.redox.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chan PH. Mitochondria and neuronal death/survival signaling pathways in cerebral ischemia. Neurochemical research. 2004;29(11):1943–1949. doi: 10.1007/s11064-004-6869-x. [DOI] [PubMed] [Google Scholar]
- 46.Chen H, Yoshioka H, Kim GS, Jung JE, Okami N, Sakata H, Maier CM, Narasimhan P, Goeders CE, Chan PH. Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxidants & redox signaling. 2011;14(8):1505–1517. doi: 10.1089/ars.2010.3576. doi:10.1089/ars.2010.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Young GB. Clinical practice. Neurologic prognosis after cardiac arrest. The New England journal of medicine. 2009;361(6):605–611. doi: 10.1056/NEJMcp0903466. doi:10.1056/NEJMcp0903466. [DOI] [PubMed] [Google Scholar]
- 48.Colbourne F, Sutherland G, Corbett D. Postischemic hypothermia. A critical appraisal with implications for clinical treatment. Molecular neurobiology. 1997;14(3):171–201. doi: 10.1007/BF02740655. doi:10.1007/BF02740655. [DOI] [PubMed] [Google Scholar]
- 49.Gonzalez-Lima F, Barksdale BR, Rojas JC. Mitochondrial respiration as a target for neuroprotection and cognitive enhancement. Biochemical pharmacology. 2014;88(4):584–593. doi: 10.1016/j.bcp.2013.11.010. doi:10.1016/j.bcp.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 50.Ohlow MJ, Moosmann B. Phenothiazine: the seven lives of pharmacology’s first lead structure. Drug discovery today. 2011;16(3-4):119–131. doi: 10.1016/j.drudis.2011.01.001. doi:10.1016/j.drudis.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 51.Rojas JC, Simola N, Kermath BA, Kane JR, Schallert T, Gonzalez-Lima F. Striatal neuroprotection with methylene blue. Neuroscience. 2009;163(3):877–889. doi: 10.1016/j.neuroscience.2009.07.012. doi:10.1016/j.neuroscience.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rojas JC, John JM, Lee J, Gonzalez-Lima F. Methylene blue provides behavioral and metabolic neuroprotection against optic neuropathy. Neurotoxicity research. 2009;15(3):260–273. doi: 10.1007/s12640-009-9027-z. doi:10.1007/s12640-009-9027-z. [DOI] [PubMed] [Google Scholar]
- 53.Muratsubaki H, Yajima N, Yoneda H, Enomoto K, Tezuka T. Methylene blue protection against hypoxic injury in primary cultures of rat hepatocyte monolayers. Cell biochemistry and function. 2008;26(2):275–278. doi: 10.1002/cbf.1429. doi:10.1002/cbf.1429. [DOI] [PubMed] [Google Scholar]
- 54.Gabrielli D, Belisle E, Severino D, Kowaltowski AJ, Baptista MS. Binding, aggregation and photochemical properties of methylene blue in mitochondrial suspensions. Photochemistry and photobiology. 2004;79(3):227–232. doi: 10.1562/be-03-27.1. [DOI] [PubMed] [Google Scholar]
- 55.Scott A, Hunter FE., Jr. Support of thyroxine-induced swelling of liver mitochondria by generation of high energy intermediates at any one of three sites in electron transport. The Journal of biological chemistry. 1966;241(5):1060–1066. [PubMed] [Google Scholar]
- 56.Atamna H, Nguyen A, Schultz C, Boyle K, Newberry J, Kato H, Ames BN. Methylene blue delays cellular senescence and enhances key mitochondrial biochemical pathways. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2008;22(3):703–712. doi: 10.1096/fj.07-9610com. doi:10.1096/fj.07-9610com. [DOI] [PubMed] [Google Scholar]
- 57.Miclescu A, Basu S, Wiklund L. Methylene blue added to a hypertonic hyperoncotic solution increases short-term survival in experimental cardiac arrest. Critical care medicine. 2006;34(11):2806–2813. doi: 10.1097/01.CCM.0000242517.23324.27. doi:10.1097/01.CCM.0000242517.23324.27. [DOI] [PubMed] [Google Scholar]
- 58.Miclescu A, Sharma HS, Martijn C, Wiklund L. Methylene blue protects the cortical blood-brain barrier against ischemia/reperfusion-induced disruptions. Critical care medicine. 2010;38(11):2199–2206. doi: 10.1097/CCM.0b013e3181f26b0c. doi:10.1097/CCM.0b013e3181f26b0c. [DOI] [PubMed] [Google Scholar]
- 59.de la Torre JC, Cada A, Nelson N, Davis G, Sutherland RJ, Gonzalez-Lima F. Reduced cytochrome oxidase and memory dysfunction after chronic brain ischemia in aged rats. Neuroscience letters. 1997;223(3):165–168. doi: 10.1016/s0304-3940(97)13421-8. [DOI] [PubMed] [Google Scholar]
- 60.Callaway NL, Riha PD, Wrubel KM, McCollum D, Gonzalez-Lima F. Methylene blue restores spatial memory retention impaired by an inhibitor of cytochrome oxidase in rats. Neuroscience letters. 2002;332(2):83–86. doi: 10.1016/s0304-3940(02)00827-3. [DOI] [PubMed] [Google Scholar]
- 61.Di Y, He YL, Zhao T, Huang X, Wu KW, Liu SH, Zhao YQ, Fan M, Wu LY, Zhu LL. Methylene Blue Reduces Acute Cerebral Ischemic Injury via the Induction of Mitophagy. Molecular medicine. 2015 doi: 10.2119/molmed.2015.00038. doi:10.2119/molmed.2015.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang Z, Watts LT, Huang S, Shen Q, Rodriguez P, Chen C, Zhou C, Duong TQ. The Effects of Methylene Blue on Autophagy and Apoptosis in MRI-Defined Normal Tissue, Ischemic Penumbra and Ischemic Core. PloS one. 2015;10(6):e0131929. doi: 10.1371/journal.pone.0131929. doi:10.1371/journal.pone.0131929. [DOI] [PMC free article] [PubMed] [Google Scholar]