Abstract
Mutations in SCN1A, the gene encoding voltage-gated sodium channel NaV1.1, cause a spectrum of epilepsy disorders that range from genetic epilepsy with febrile seizures plus to catastrophic disorders such as Dravet syndrome. To date, more than 1,250 mutations in SCN1A have been linked to epilepsy. Distinct effects of individual SCN1A mutations on neuronal function are likely to contribute to variation in disease severity and response to treatment in patients. Several model systems have been used to explore seizure genesis in SCN1A epilepsies. In this article we review what has been learned about cellular mechanisms and potential new therapies from these model systems, with a particular emphasis on the novel model system of knockin Drosophila and a look toward the future with expanded use of patient-specific induced pluripotent stem cell-derived neurons.
Keywords: Drosophila, epilepsy, iPSC, model systems, SCN1A
epilepsy is a chronic neurological disorder that is characterized by recurrent seizures. Around 50 million people worldwide have epilepsy and the proportion of the general population with active epilepsy at any given time is between 4 and 10 per 1,000 people (World Health Organization 2012). In the United States, epilepsy is the fourth most common neurological disorder, and it is estimated that 150,000 new cases are diagnosed annually (Hirtz et al. 2007). A population-based study in Minnesota estimated that one of every 26 people will be diagnosed with epilepsy at some point in their lifetime (Hesdorffer et al. 2011).
The causes of epilepsy can be divided into two groups: symptomatic and idiopathic epilepsy. Symptomatic epilepsy, or secondary epilepsy, is usually precipitated by brain injuries, such as trauma, infection, stroke, or tumors. Epilepsy that has no clear cause, which makes up 60% of all epilepsy, is defined as idiopathic or primary epilepsy (World Health Organization 2012).
Over the past decade there has been an increasing appreciation for the importance of genetic factors in causing epilepsy, and single gene mutations have now been associated with a number of epileptic disorders. Not surprisingly, the majority of these mutations occur in genes that encode proteins important in regulating neuronal excitability, including voltage- and ligand-gated ion channels (George 2004; Heron et al. 2007; Liao et al. 2010; Reid et al. 2009; Weckhuysen et al. 2012). Genes encoding voltage-gated sodium channels have emerged as the most frequently identified genes associated with epilepsy (Brunklaus et al. 2014). The SCN1A gene, encoding voltage-gated sodium channel NaV1.1, is a standout in this class, with its more than 1,250 epilepsy-causing mutations (Meng et al. 2015) (http://www.gzneurosci.com/scn1adatabase/). This review focuses on several model systems used to study SCN1A-related epilepsies and how these models have contributed to our understanding of seizure genesis and development of new therapeutics for SCN1A-related epilepsies.
Voltage-gated sodium channels.
Voltage-gated sodium channels open in response to membrane depolarization, allowing sodium ions to enter the neuron. This activity is responsible for generating the fast-rising phase of the action potential and propagation of electrical impulses to synaptic terminals that mediate synaptic transmission (Hodgkin and Huxley 1952). The core component of the sodium channel is the α-subunit. In humans there are nine different α-subunits (NaV1.1–NaV1.9) that are encoded by the genes SCN1A–SCN11A. The NaV1.1, NaV1.2, and NaV1.6 proteins are abundant in adult central nervous system (CNS), and mutations in each of the encoding genes have been associated with epileptic disorders (Brunklaus et al. 2014; Yu and Catterall 2003). The most frequently identified gene associated with genetic epilepsy is SCN1A, which encodes the α-subunit of NaV1.1.
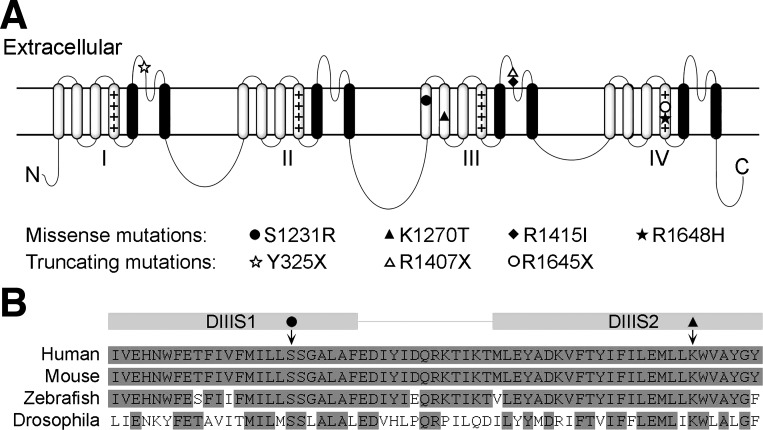
Similar to all α-subunits, the NaV1.1 channel has four homologous domains (DI–DIV) connected by three cytoplasmic loops, with each domain consisting of six transmembrane segments (S1–S6; Fig. 1A). The fourth transmembrane segment (S4) of each domain functions as a voltage sensor. Recent structural studies in bacterial sodium channels report that the outward movement of the voltage sensor, along a groove formed by segments S1–S3, is triggered in response to membrane depolarization. This initiates the transformation of the channel from the closed to the open conformation, permitting sodium ions to flow down their electrochemical gradient (Payandeh et al. 2012; Yarov-Yarovoy et al. 2012). Segments S5 and S6 form the channel pore. The extracellular loops between S5 and S6 are important for establishing the ion-selectivity of the channel, allowing sodium but not potassium or calcium ions to pass through (Payandeh et al. 2011). The cytoplasmic loop between domains DIII and DIV acts as a gate responsible for the inactivation of open sodium channels (Vassilev et al. 1988; West et al. 1992).
Fig. 1.
A: schematic of the structure of a voltage-gated sodium channel α-subunit. Each of the 4 domains (I–IV) contains 6 transmembrane segments (S1–S6, from left to right). Plus sign (+) columns indicate voltage-sensing transmembrane segment S4 in each domain; S5 and S6 form the channel pore (black). The locations of the 7 SCN1A mutations that are referred to in this review are denoted by symbols. B: aligned amino acid sequence from the transmembrane segment S1 to S2 in domain III (DIII) of human, mouse, zebrafish, and fruit fly (Drosophila). The locations of the S1231R (solid circle) and K1270T (solid triangle) mutations are indicated by arrows.
The mutations in the SCN1A gene associated with epilepsy are found throughout the entire NaV1.1 protein. Approximately 600 of these mutations are missense and in-frame deletions, with most of the other mutations resulting in NaV1.1 protein truncation (http://www.gzneurosci.com/scn1adatabase). These mutations cause a wide spectrum of seizure disorders, including genetic epilepsy with febrile seizures plus (GEFS+) and severe myoclonic epilepsy of infancy (SMEI), also known as Dravet syndrome (DS) (Miller and Sotero de Menezes 2014). GEFS+ is characterized by frequent febrile seizures in early childhood that persist beyond 6 yr of age, and affected individuals can also develop a number of other spontaneous seizure phenotypes (Scheffer and Berkovic 1997). Individuals with DS exhibit febrile and afebrile seizures in the first year. In this more severe disorder, developmental delays are also common by the second year of life (Dravet 2011). Seven SCN1A mutations that have been studied in either knockin animal models or induced pluripotent stem cell (iPSC)-derived neurons are discussed in this review (Fig. 1A). These include two missense mutations, K1270T and R1648H, that cause GEFS+; two missense mutations, S1231R and F1415I, that cause DS; and three truncating mutations, Y325X, R1407X, and R1645X, that cause DS. Single amino acid mutations at different locations throughout the channel are likely to give rise to multiple distinct functional deficits, contributing to the varied clinical manifestation and severity of SCN1A epilepsy disorders.
Multiple model systems have been employed to explore the cellular mechanisms of seizure genesis caused by different SCN1A mutations. Combined use of model systems and new technological advancements in genetic engineering are continuing to enhance the ability to identify the mechanisms underlying SCN1A epilepsies and to facilitate development of novel therapies. In this review, we focus on five model systems that have been used in either exploring the cellular mechanism of or searching for new therapeutics for SCN1A epilepsy, including heterologous expression systems, mouse, zebrafish, fruit fly, and iPSC-derived neurons.
Heterologous Expression Systems
Heterologous expression systems were the first models used to investigate the cellular consequences of SCN1A mutations linked to epilepsy. In these systems, cloned sodium channel α-subunits are expressed in a nonexcitable cell, commonly Xenopus oocyte, human embryonic kidney (HEK or tsA201), or Chinese hamster ovary (CHO). These cells provide an excellent environment for analyzing the properties of exogenous sodium channels because they express few, if any, endogenous voltage-gated sodium channels. In addition, expression systems have the advantage of allowing good voltage control during electrophysiological examination, facilitating an accurate biophysical description of channel function. Studies in heterologous systems were the first to demonstrate that some SCN1A missense mutations cause loss-of-function, and others gain-of-function, changes. Forty-four epilepsy-causing missense mutations that have been studied in heterologous expression systems are listed in a SCN1A mutation database (http://www.gzneurosci.com/scn1adatabase). Twenty-seven of these mutations result in no measurable sodium currents or loss of function in sodium channels, and six of the mutations cause gain of function in sodium channels. In addition, there are 11 mutations that cause mixed effects on sodium channel function, with some aspects of channel function being enhanced while others are reduced. Furthermore, they reveal that underlying mechanisms can be highly varied and include alterations in channel number, conductance, and/or gating kinetics (Catterall et al. 2010; Escayg and Goldin 2010).
Heterologous expression systems also may serve as an efficient system for testing compounds that modulate voltage-gated sodium channels. For example, ranolazine, a U.S. Food and Drug Administration (FDA)-approved drug for chronic angina treatment, was first identified as a selective blocker of persistent currents in mutant NaV1.1 channels expressed in tsA201 cells (Kahlig et al. 2010). In follow-up studies in cultured rat hippocampal neurons, ranolazine was found to reduce neuronal excitability and suppress epileptiform activity evoked by NMDA receptor activation, suggesting it may be useful as an antiepileptic therapy (Kahlig et al. 2014). Furthermore, since there are neuronal cell type-specific differences in the relative expression levels of distinct sodium channel subtypes and isoforms (discussed later in this review), identifying compounds that can differentially modulate different subtype/isoform of sodium channels may be helpful in treating patients with SCN1A-related epilepsies. A study in tsA201 cells demonstrated that two exon 5 splice variants of the NaV1.1 channels respond differentially to phenytoin and lamotrigine, commonly used antiepileptic drugs (Thompson et al. 2011). One of the splice variants, NaV1.1-5N, exhibited enhanced tonic block and use-dependence block by phenytoin and lamotrigine compared with the other splice variant, NaV1.1-5A. Using heterologous expression systems to screen for compounds that differentially modulate varied subtype/isoform of sodium channels may contribute to identifying new therapeutics for SCN1A-related epilepsy.
Studies of sodium channel mutations in heterologous systems also have yielded some observations worth further discussion. First, voltage-gated sodium channels encoded by the same gene can have distinct functional properties when expressed in different cell types (Escayg and Goldin 2010; Ragsdale 2008). For example, cDNA encoding the human NaV1.3 protein produces sodium channels that give rise to sodium currents with both a transient and a persistent component when expressed in HEK cells. This same sequence expressed in CHO cells results in sodium currents with only a transient component (Chen et al. 2000). The mechanism for the difference in persistent currents between cell types is not yet clear. Intracellular signal transduction pathways, such as G protein βγ-subunits, have been shown to modulate persistent component of sodium currents generated by rat NaV1.2 channels expressed in tsA201 cells (Ma et al. 1997; Mantegazza et al. 2005). It is likely that cell type-specific intracellular signal transduction pathways contribute to the difference in persistent currents between cell types.
Expression in Xenopus oocytes of the rat NaV1.1 channels with the mutation homologous to GEFS+-causing R1648H mutation results in sodium currents with accelerated recovery from inactivation, whereas in tsA201 cells, human NaV1.1 channels with this same mutation result in sodium currents with an increased persistent component (Lossin et al. 2002; Spampanato et al. 2001). Furthermore, neither of these findings are predictive of the changes observed in neurons from an R1648H knockin mouse, which exhibit reduced recovery from inactivation and no change in the persistent component (Hedrich et al. 2014; Martin et al. 2010; Tang et al. 2009). These inconsistencies in functional properties of mutant sodium channels in different expression systems and a mouse model are likely due to varied processes involved in regulating expression, trafficking, and/or posttranslational modification of the sodium channels. With this in mind and considering the significant genetic differences between human and other model systems/organisms, it will be illuminating to explore the basis for the differences where they exist between model systems and human cells, as a way to gain additional insight into mechanisms that can regulate channel function. Common mechanisms shared by different model systems are the most likely to also be conserved in humans and, therefore, will be of high value in understanding the pathogenesis of SCN1A mutations in humans.
Mouse
The interneuron hypothesis.
The genetic and physiologic similarities between mice and humans, and the ability to make gene knockouts as well as use homologous recombination to introduce disease-causing mutations, have made mice incredibly useful in modeling human genetic diseases. Because truncating SCN1A mutations cause DS, the first mouse model of DS was generated by deleting the last exon of the Scn1a gene. Studies in this model reveal reduced excitability in inhibitory interneurons in the hippocampus and cerebellum. In contrast, the excitatory pyramidal neurons are not affected (Kalume et al. 2007; Yu et al. 2006). The authors proposed that reduced excitability in GABAergic inhibitory interneurons might lead to epilepsy in DS patients (Yu et al. 2006). It was also found that haploinsufficiency of NaV1.1 impairs excitability of both the parvalbumin-expressing and somatostatin-expressing interneurons in layer V of the neocortex and results in disinhibition of the cortical excitatory pyramidal cells and cortical network (Tai et al. 2014). Consistent with the inhibitory neuron hypothesis, selective deletion of NaV1.1 in inhibitory neurons causes epileptic seizures and premature death in mice, whereas NaV1.1 haploinsufficiency in excitatory neurons does not cause any noticeable abnormalities (Ogiwara et al. 2013). In addition, targeted deletion of NaV1.1 in forebrain GABAergic interneurons or parvalbumin (PV)-positive interneurons is sufficient to cause epileptic seizures in mice (Cheah et al. 2012; Ogiwara et al. 2013). These results are consistent with reduced excitability of inhibitory neurons contributing to seizures in DS patients.
Two additional mouse models based on human mutations have been generated by homologous recombination: R1407X, a DS mutation that results in a truncated α-subunit (Sugawara et al. 2002), and R1648H, a missense mutation identified in GEFS+ (Escayg et al. 2000) (Fig. 1A). In the mouse, both mutations result in reduced excitability in GABAergic inhibitory neurons. In the R1648H knockin mouse, the excitability in the glutamatergic cortical pyramidal neurons is largely normal (Hedrich et al. 2014; Martin et al. 2010; Ogiwara et al. 2007). These findings are consistent with studies from the Scn1a knockout mouse model (Kalume et al. 2007; Tai et al. 2014; Yu et al. 2006), indicating that suppression of inhibitory activity is likely to be a common mechanism contributing to seizures caused by SCN1A mutations. The Scn1a knockout model and the R1648H knockin model also provide direct evidence that reduced sodium current density is the underlying mechanism for decreased excitability in the inhibitory interneurons (Kalume et al. 2007; Martin et al. 2010; Yu et al. 2006).
Genetic background and development-related factors.
Several studies report that the phenotypes caused by Scn1a mutations can be affected by genetic background. Both the Scn1a knockout model and the R1407X knockin model show a mild seizure phenotype on a 129SvJ background and a more severe phenotype on a C57BL/6J background (Ogiwara et al. 2007; Rubinstein et al. 2015; Yu et al. 2006). A recent study systematically examined the cellular alterations caused by a Scn1a deletion in these two genetic backgrounds (Mistry et al. 2014). They reported that F1.Scn1a+/− mice, on a 50% 129S6/SvEvTac and 50% C57BL/6J background, exhibit spontaneous seizures and premature death. The 129.Scn1a+/− mice, an isogenic strain on the 129S6/SvEvTac background, do not have spontaneous seizures or premature death. Consistent with the differences in phenotype severity, sodium current density is reduced in F1.Scn1a+/− GABAergic interneurons but is not affected in 129.Scn1a+/− GABAergic interneurons at postnatal day 21–24 (P21-24).
The reduced sodium current density in GABAergic neurons in the F1.Scn1a+/− mice is consistent with the findings in the first Scn1a knockout mouse model (Yu et al. 2006). In addition, in the F1.Scn1a+/− mice at P14-15, the sodium current density in pyramidal neurons is not affected compared with their wild-type littermates. However, the sodium current density increases significantly in F1.Scn1a+/− pyramidal neurons at P21-24, whereas it stays the same in wild-type pyramidal neurons, which was not observed in the first knockout mice that were only examined at the end of the second week (Yu et al. 2006). Furthermore, the spontaneous action potential firing and evoked firing are both increased in F1.Scn1a+/− pyramidal neurons at P21-24 compared with wild type. Even though the excitability of GABAergic interneurons is not reported in this study, the reduced sodium current density in the F1.Scn1a+/− GABAergic interneurons is likely to cause reduced excitability in these inhibitory neurons, based on the findings in the first knockout mouse model. Taking the deduction one step further, it seems likely that the abnormally reduced excitability in F1.Scn1a+/− GABAergic interneurons results in reduced inhibition of pyramidal neurons and causes increased excitability of pyramidal neurons. This is supported by another study where the impaired excitability of the GABAergic interneurons in the R1648H knockin mice causes hyperexcitability in thalamocortical and hippocampal networks (Hedrich et al. 2014).
The onset of abnormally high excitability of pyramidal neurons during the third week is coincident with the onset of spontaneous seizures and premature death in F1.Scn1a+/− mice. Consistent with this finding, deleting NaV1.1 expression in excitatory neurons ameliorates the epileptic seizures and premature death cause by selective deletion of NaV1.1 in inhibitory neurons (Ogiwara et al. 2013). These data indicate that the excitatory neurons also play an important role in SCN1A mutation-related epileptogenesis. The effects of Scn1a deletion in mice are summarized in Table 1.
Table 1.
Effects of Scn1a deletion in mouse models of Dravet syndrome
| Scn1a+/− (Last exon deletion) | F1.Scn1a+/− (First exon deletion) | 129.Scn1a+/− (First exon deletion) | |
|---|---|---|---|
| Genetic background | C57BL/6J | 129S6/SvEvTac:C57BL/6J (50%:50%) | 129S6/SvEvTac |
| Spontaneous seizures | Beginning at P21 | Beginning at P18 | None (observed from P24 to P184) |
| Premature death | Beginning at P21 | Beginning at P21 | Normal life span |
| Sodium current | |||
| Inhibitory neurons | P13–14: reduced | P21–24: reduced | P21–24: not affected |
| Excitatory neurons | P13–14: not affected | P14–15: not affected | P21–24: increased, but not as large as in F1.Scn1a+/− |
| P21–24: significantly increased | |||
| Excitability | |||
| Inhibitory neurons | P13–14: reduced evoked firing | ||
| Excitatory neurons | P21–24: increased spontaneous action potential firing and evoked firing in both strains; greater increases in F1.Scn1a+/− than in 129.Scn1a+/− | ||
| References | Yu et al. 2006 | Miller and Sotero de Menezes 2014; Mistry et al. 2014 | |
P, postnatal day.
The cell-specific effect of Scn1a mutations.
All of these studies using mouse models have reported differential effects of the Scn1a mutations on inhibitory neurons vs. excitatory neurons; however, the underlying mechanisms are not clear. One hypothesis is that the inhibitory neurons express a higher percentage of the mutant NaV1.1 channels than excitatory neurons. The sodium current densities in inhibitory neurons in the first Scn1a+/− mouse model and the F1.Scn1a+/− mouse model are reduced by 53% and 43%, respectively, consistent with NaV1.1 being the major sodium channel subtype in these inhibitory neurons (Mistry et al. 2014; Yu et al. 2006). This interpretation is supported by detection of high levels of NaV1.1 expression in inhibitory neurons (Ogiwara et al. 2007, 2013).
There also is evidence indicating that NaV1.1 expression level in pyramidal cells is lower than in GABAergic inhibitory neurons. Homozygous Scn1a gene deletion in GABAergic neurons results in ∼42% reduction of NaV1.1 expression level in P12.5 mouse brain, based on Western blot analysis (Ogiwara et al. 2013). Assuming pyramidal neurons express the remaining 58% of the NaV1.1 protein in P12.5 mouse brain and assuming pyramidal neurons comprise ∼70–90% of neurons in mammal brains (Jinno and Kosaka 2010; Markram et al. 2004), we can deduce that the average NaV1.1 expression level is lower in pyramidal compared with interneurons. However, an average lower level of NaV1.1 expression in pyramidal neurons does not translate into a lower percentage of NaV1.1 compared with all sodium channels, because the total sodium current density is also lower in pyramidal neurons, ∼37–65% of that in inhibitory neurons (Martin et al. 2010; Mistry et al. 2014; Yu et al. 2006). Therefore, it is not clear if the percentage of mutant NaV1.1 is lower in pyramidal neurons than in inhibitory neurons.
Even assuming that NaV1.1 is expressed at a low percentage among all types of sodium channels in pyramidal neurons, this alone cannot explain the increased excitability of pyramidal neurons observed in F1.Scn1a+/− and 129.Scn1a+/− mice at P21-24 (Mistry et al. 2014). It is likely that compensatory upregulation of other subtypes of sodium channels also plays a role in altering the excitability of the pyramidal neurons.
Tasks and tools.
Approximately half of the epilepsy-causing SCN1A mutations are nonsense or frame-shift mutations that result in truncated NaV1.1 (http://www.gzneurosci.com/scn1adatabase/). On the basis of findings in the SCN1A knockout mouse models, it is reasonable to suggest that these two types of SCN1A mutations cause loss of function in NaV1.1 and reduced excitability in inhibitory interneurons. However, in addition to nonsense and frame-shift mutations, there are also ∼600 missense mutations and in-frame deletions that are associated with SCN1A epilepsies (http://www.gzneurosci.com/scn1adatabase/). It is unlikely they all cause loss of function of NaV1.1 in inhibitory interneurons given their highly varied locations, amino acid alterations, and reports of SCN1A mutations causing both gain- and loss-of-function alterations in heterologous expression systems (Catterall et al. 2010; Escayg and Goldin 2010; Meng et al. 2015).
Advances in CRISPR (clustered regularly interspaced short palindromic repeats)-mediated genome editing have made it easier to produce transgenic mice (Harms et al. 2014; Singh et al. 2015; Yang et al. 2014). It still, however, requires a significant investment of resources, both in development of the line carrying a mutation of interest and in housing and maintaining the resulting colony. Therefore, other genetically tractable model organisms in which targeted mutations can be efficiently made have proved to be useful tools for exploring the effects of SCN1A mutations or searching for new therapeutics.
Zebrafish
Zebrafish is a simple and genetically tractable vertebrate that retains close homology with higher vertebrates. With the advantage of a behavior-based high-throughput assay, it has emerged in the past decade as a new platform for identification of seizure suppressing mutations and drug studies (Baraban et al. 2005, 2013; Hortopan et al. 2010). Adding a common convulsant agent, pentylenetetrazole (PTZ), to the water induces behavioral changes that resemble clonus-like convulsions in zebrafish larvae, and extracellular recordings reveal ictal and interictal-like electrographic discharges in the optic tectum. These epileptiform discharges are suppressed by the commonly used antiepileptic drugs (AEDs) valproate and diazepam (Baraban et al. 2005). Besides responding to these AEDs, there is additional evidence that zebrafish exhibit similar responses to pharmacological agents as mouse (Leclercq et al. 2015). Furthermore, with its relatively short generation time (3–4 mo) and large clutch sizes of 50–200 embryos from a pair of adults every week, zebrafish has the potential to be a powerful and efficient system for testing of novel pharmaceuticals.
More recently, a mutant Zebrafish strain that harbors a loss-of-function mutation in the sodium channel gene scn1Lab was discovered in a chemical mutagenesis screen (Schoonheim et al. 2010). Although this strain is a homozygous mutant, the scn1Lab zebrafish line is considered analogous to the Scn1a+/− knockout mouse model of DS given the genome duplication in zebrafish and the presence of an additional NaV1.1 homolog (scn1Laa) (Baraban et al. 2013; Novak et al. 2006). The scn1Lab zebrafish exhibits elevated levels of swim activity and unprovoked seizure-like behavior starting at 4 days postfertilization (dpf) and dies prematurely between 10 and 12 dpf. The seizure-like behavior consists of whole body convulsions and rapid undirected movement. Forebrain extracellular field recordings from scn1Lab mutants show frequent brief interictal-like bursts and large-amplitude, long-duration, ictal-like events starting at 3 dpf that progressively become more prominent between 4 and 7 dpf. These behavioral and electrophysiological changes in the scn1Lab zebrafish resemble many of the characteristics of DS. In addition, a ketogenic diet and several antiepileptic drugs that are effective for DS patients were shown to attenuate mutant seizure activity in the mutant fish (Baraban et al. 2013). These data indicate that zebrafish exhibit similar responses to pharmacological agents as humans.
A high-throughput phenotype-based screen of 320 compounds identified clemizole as a potential novel inhibitor for convulsive behavior and electrographic seizures. Clemizole is an H1 antihistamine compound that is approved by the FDA. Since four other H1 antihistamines did not suppress convulsive behavior in scn1Lab mutants, the mechanism underlying seizure inhibition is unlikely to be antihistamine related (Baraban et al. 2013). A recent study reports that clemizole is a potent inhibitor of transient receptor potential channel 5 (TRPC5), one of the seven mammalian TRPC proteins that are nonselective cation channels (Richter et al. 2014). Knocking out TRPC5 in mice significantly reduces pilocarpine-induced seizures (Phelan et al. 2013). These findings suggest that the seizure inhibition effects of clemizole in scn1Lab mutants may be mediated by TRPC5-related mechanisms, and this channel may be a potential new therapeutic target for epilepsy caused by SCN1A haploinsufficiency.
Introducing specific disease-causing mutations into the zebrafish germline has been difficult and has limited the ability to create more genetically precise zebrafish models (Brookfield et al. 2012). The first application of homologous recombination in zebrafish was recently reported, but whether this procedure will allow for efficient targeting of the sodium channel gene remains to be seen (Zu et al. 2013). However, with the recent advance of CRISPR-mediated genome editing, it is now possible to more rapidly and efficiently generate zebrafish models with targeted mutations (Hruscha and Schmid 2015; Hwang et al. 2013). Combined with the behavior-based high-throughput assay, this genetically tractable model system is likely to further contribute to our understanding of SCN1A-related epilepsy and facilitate identification of novel therapeutics.
Fruit Fly
History and background.
Drosophila, also known as fruit flies, are a common organism for modeling human genetic disease. Drosophila models have traditionally employed forward genetic screens to isolate mutants that mimic some phenotypic aspect of a human disease and map to a homologous gene linked to that disease. In Drosophila, para is the only gene that encodes for voltage-gated sodium channels; however, alternative splicing of the para transcript produces a variety of sodium channels with distinct biophysical properties (Lin et al. 2009; O'Dowd et al. 1995; O'Dowd and Smith 1996; Thackeray and Ganetzky 1994, 1995). Para sodium channel structure is virtually identical to that of the vertebrate sodium channels. Regions that are critical for channel function show high conservation with the human NaV1.1 channels at the amino acid level, including S4 voltage sensors and the cytoplasmic segment between domains III and IV (Loughney et al. 1989). The amino acid sequence of the transmembrane segments (S1–S6) is 50–80% identical between the human NaV1.1 channels and Drosophila para sodium channels. The amino acid sequence of S1 and S2 in domain III, where the S1231R and K1270T mutations were knocked into the para gene, is illustrated as an example in Fig. 1B. The amino acid sequence of the para sodium channels is less conserved with human NaV1.1 channel compared with that of vertebrates, such as mouse and zebrafish. This raises the question of whether Drosophila will be useful in modeling epilepsy that is caused by SCN1A mutations.
The first para mutation, a temperature-sensitive conditional mutant (parats1), was identified by forward genetic screen and is characterized by impaired nerve conduction and heat-induced paralysis (Siddiqi and Benzer 1976; Suzuki et al. 1971). Screens for additional temperature-sensitive mutants identified a number of new para alleles that cause heat-induced paralysis. However, unlike the epilepsy-causing sodium channel mutations in humans, these do not exhibit temperature-sensitive seizure phenotypes (Siddiqi and Benzer 1976). More recently, one of the bang-sensitive (BS) mutants, bang senseless (parabss1), was mapped to the para mutation L1699F (Parker et al. 2011). This BS mutant exhibits seizure-like activity following mechanical shock and has a lower threshold for electric shock-induced seizures (Ganetzky and Wu 1982; Jan and Jan 1978; Parker et al. 2011). However, the mutation paraL1699F does not correspond to the location of known epilepsy-causing SCN1A mutations in human sodium channels, and it is not clear whether this mutation causes temperature-sensitive seizure phenotypes in Drosophila.
Forward genetic screens have in rare cases resulted in mutations that are homologous to mutations found in human genetic diseases. For example, the paraG1517R mutation, which is homologous to the SCN4AG1306V mutation identified in paramyotonia congenetia, confers a dominant cold-sensitive paralytic phenotype. Paramyotonia congenetia is an autosomal dominant disorder in which myotonia can be elicited by exercise or cold temperatures (Lindsay et al. 2008). This suggests there may be conservation of genotype-to-phenotype mapping of human sodium channel diseases in Drosophila.
Knockin Drosophila models carrying human SCN1A mutations.
The two initial epilepsy-causing SCN1A mutations examined in Drosophila are located in the transmembrane segments S1 and S2 of domain III in NaV1.1 and para sodium channels (Fig. 1). The SCN1AK1270T mutation was identified in a GEFS+ family with 27 affected individuals (Abou-Khalil et al. 2001). The SCN1AS1231R mutation is a missense mutation identified in a DS patient who began experiencing hemiclonic seizures at 7 mo of age (Fujiwara et al. 2003). Three knockin Drosophila lines were generated. Two mutant lines, one carrying SCN1AS1231R mutation (DS) and one carrying SCN1AK1270T mutation (GEFS+) at the homologous sites in the para gene, were generated by ends-out homologous recombination (Staber et al. 2011). A third line with wild-type substitutions was generated using the same procedure to serve as the control.
In contrast to the para mutants identified in forward genetic screens, both the DS and GEFS+ lines exhibited heat-induced seizures (Schutte et al. 2014; Sun et al. 2012). Although these are clearly not identical to epileptic events in humans, they are defined as heat-induced seizures on the basis of loss of standing posture followed by continuous movement of the legs, wings, or abdomen in the fallen flies following immersion of the test vial in a heated water bath. In addition, the DS flies are more sensitive to the heat-induced seizure than the GEFS+ flies, consistent with greater severity of DS over GEFS+ in humans. The control flies do not seize under the same condition (Schutte et al. 2014; Sun et al. 2012). Flies that are compound heterozygous for both the DS and GEFS+ mutations (ParaDS/GEFS+) are less sensitive to heat-induced seizures than the DS and GEFS+ homozygotes. This suggests that the underlying mechanisms of these two mutations are different and likely to be in opposite directions.
Both gain- and loss-of function mutations result in reduced inhibition of GABAergic neurons.
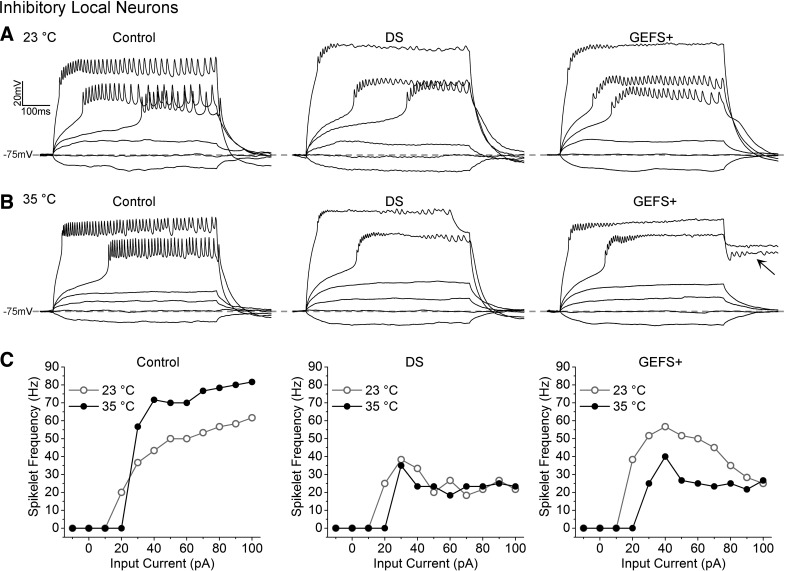
Examination of sodium currents in a population of GABAergic local neurons (LNs) in adult fly antennal lobes reveals that the GEFS+ mutation lowers the threshold to elicit sodium current and causes a hyperpolarizing shift in the voltage dependence of persistence sodium current deactivation. As a result, the sodium currents are active over a wider voltage range, and this GEFS+ alteration is defined as a gain-of-function mutation at the sodium current level (Sun et al. 2012). On the other hand, the DS mutation reduces sodium current magnitude, increases the threshold to elicit sodium current, and causes a depolarizing shift in the voltage-dependent deactivation of the persistent sodium current. Therefore, the DS mutation is defined as a loss-of-function mutation at the sodium current level (Schutte et al. 2014). These two mutations provided a unique opportunity to examine how loss-of function vs. gain-of-function mutations on the sodium current level affect neuronal excitability and contribute to seizure genesis.
The DS LNs have reduced evoked firing frequency compared with control LNs at both room temperature and elevated temperature (Fig. 2), consistent with the findings in mice where the reduced sodium currents cause decreased excitability of GABAergic interneurons. The effects of the GEFS+ mutations are temperature dependent. The maximal firing frequency of GEFS+ LN is not different from that of control LNs at room temperature, but it is significantly reduced at elevated temperature, opposite to the increased firing frequency in control LNs. The decreased firing frequency of GEFS+ LNs likely results from the depolarizing shift in the voltage-dependent deactivation of the persistent sodium currents, which impairs membrane repolarization in the GEFS+ LNs at high temperature (Fig. 2B). These data demonstrate that both loss-of-function and gain-of-function mutations in a single sodium channel gene can cause reduced excitability in GABAergic neurons and contribute to heat-induced seizure phenotypes.
Fig. 2.
A and B: representative trains of evoked spikelets recorded from control, Dravet syndrome (DS), and genetic epilepsy with febrile seizures plus (GEFS+) local neurons (LNs) at 23 and 35°C. Arrow indicates the poststimulus depolarization in GEFS+ LNs at 35°C. C: spikelet frequency plotted as a function of injected current for the LNs shown above. [Modified from Sun et al. 2012 and Schutte et al. 2014 with permission.]
GEFS+ and DS mutations differentially affect firing in excitatory and inhibitory neurons.
In mouse there are at least nine voltage-gated sodium channels (Yu and Catterall 2003). It is likely that the ratio of mutant NaV1.1 channels to all other wild-type sodium channels is different in inhibitory vs. excitatory neurons, which may contribute to the differential effects of Scn1a mutations in inhibitory vs. excitatory neurons. In Drosophila, whereas there are a variety of sodium channel variants produced via alternative splicing, para is the only sodium channel gene (Lin et al. 2009; O'Dowd et al. 1995; Thackeray and Ganetzky 1994, 1995). The GEFS+ and DS mutations are both in a constitutively expressed exon, and therefore, all of the sodium channels in the GEFS+ and DS homozygous lines carry their engineered mutations. This provides a unique opportunity to investigate whether there are factors besides differential expression of the mutant sodium channels contributing to cell-specific effects of the SCN1A mutations in inhibitory vs. excitatory neurons. The firing properties of a population of excitatory motor neurons in GEFS+ and DS flies were examined. The motor neurons (MNs) selected for recording were located at the medial aspect of the T1 and T2 region in the 2-day-old adult thoracic ganglia and were visually identified by D42-GAL4-driven expression of green fluorescent protein (stock no. 8816; Bloomington Drosphila Stock Center, Indiana University, Bloomington, IN).
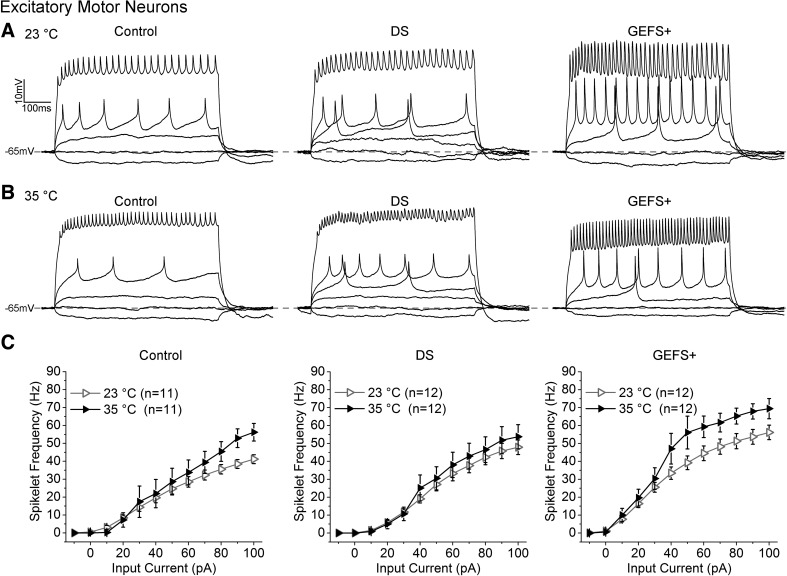
In DS MNs, the firing frequency is indistinguishable from that of control at both 23 and 35°C (Fig. 3, A and B). The evoked firing frequency increases as the input current increases at both the room temperature and high temperature. The average firing frequency shows an increase at high temperature compared with room temperature (Fig. 3C). In GEFS+ MNs, the firing frequency is significantly higher than control at room temperature, and it increases further at elevated temperature (Fig. 3, A and B). In addition, there are no sustained depolarizations in the GEFS+ MNs at elevated temperature as seen in LNs (Figs. 2B and 3B). These data demonstrate that the DS and GEFS+ mutation do not reduce repetitive firing in the MNs as they do in the LNs. Instead, the firing frequency is not affected in DS MNs and is increased in GEFS+ MNs.
Fig. 3.
A and B: representative trains of evoked spikelets recorded from control, DS, and GEFS+ motor neurons (MNs) at 23 and 35°C. C: average spikelet frequency plotted as a function of injected current for control, DS, and GEFS+ MNs. Symbols and error bars represent means ± SE from the number (n) of MNs indicated.
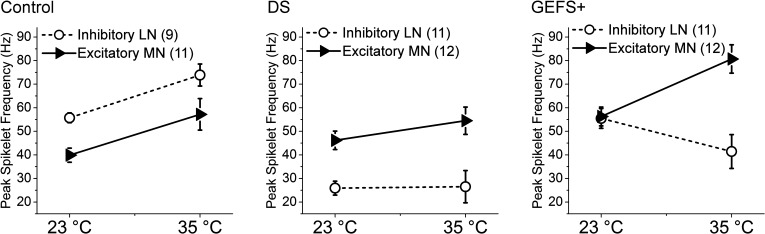
The differential effects of both the GEFS+ and DS mutations on LNs vs. MNs are most clearly illustrated when the peak firing frequency of LNs and MNs is compared within each genotype (Fig. 4). In control, the LNs have a higher firing frequency than the MNs at 23°C. Assuming the excitability of LNs and MNs reflects balanced neuronal inhibition and excitation, this balance is not disturbed by temperature elevation since there is a coordinated increase in excitability in both control LNs and MNs at 35°C. In contrast, the DS mutation causes a constitutive reduction in excitability in LNs at both room and elevated temperature, whereas the excitability of the MNs is indistinguishable from control (Fig. 4). This results in a clear imbalance between neuronal inhibition and excitation. These data indicate that other mechanisms besides differential ratio of the mutant sodium channels to wild-type sodium channels can contribute to the cell-specific effects, such as differential expression of SCN1A isoforms and/or other ion channels in different types of neurons.
Fig. 4.
Comparison of peak firing frequency of LNs vs. MNs at 23 and 35°C in control, DS, and GEFS+ flies. Symbols and error bars represent means ± SE from the number (n) of neurons indicated.
Studies have shown that para mRNA is subject to extensive alternative splicing, allowing a variety of sodium channel variants with different biophysical properties to be produced from one common transcript (Lin et al. 2009; Marley and Baines 2011; Olson et al. 2008; Thackeray and Ganetzky 1994, 1995). One possibility is that although the DS mutation is in a constitutively expressed exon, its effects on sodium channel function may vary depending on the interaction between the mutation site and different alternatively spliced exons. In addition, alternative splicing can be activity dependent, meaning that the activity of neurons can affect alternative splicing and result in the expression of a different combination of sodium channel isoforms in those neurons (Lin et al. 2012). Therefore, it is possible that aberrant neuronal activities caused by SCN1A mutations may cause further alterations in alternative splicing of sodium channels, and even other ion channels.
The GEFS+ mutation, like DS, also exhibits differential effects in LNs vs. MNs. At room temperature, the maximal firing frequency in GEFS+ LNs is not significantly different from that in the control LNs, but GEFS+ MNs are more excitable than control MNs (Fig. 4). The increased excitability in excitatory neurons is consistent with the findings in F1.Scn1a+/− mice at 3 wk of age (Mistry et al. 2014). There are also unique temperature-dependent alterations in GEFS+ LNs and MNs. When the temperature is elevated, the excitability decreases in the LNs but increases in the MNs, indicating that reduced inhibitory activity and increased excitatory activity both contribute to seizure genesis at elevated temperature. In addition, there are poststimulus depolarizations in GEFS+ LNs but not the MNs (Figs. 2B and 3B). The mechanism underlying the poststimulus depolarizations in GEFS+ LNs is due to a temperature-dependent hyperpolarizing shift of the persistent sodium current deactivation threshold in GEFS+ LNs when the temperature is elevated (Sun et al. 2012). The increased firing frequency and absence of poststimulus depolarizations at elevated temperature in GEFS+ MNs are consistent with the expression of sodium channel variants with different biophysical properties in MNs and LNs. However, it also is possible that the different expression levels of other ion channels than in LNs, such as augmented potassium currents, overcome the persistent sodium currents and repolarize the membrane potential at elevated temperature.
These data from GEFS+ and DS mutations identify many cellular mechanisms similar to those in mice that are likely to contribute to seizure genesis in the SCN1A epilepsies. The studies in the GEFS+ mutation also show that gain of function in sodium channels may reduce excitability in inhibitory neurons and cause seizures. In addition, the cellular mechanisms can be further complicated by temperature-dependent effects of the mutation on sodium channel function and neuronal excitability.
Identifying new therapies for SCN1A-related seizure disorders.
With the use of a behavior-based assay, the serotonin precursor 5-hydroxytryptophan (5-HTP) was identified as a seizure suppressor for the DS mutants (Schutte et al. 2014). DS flies fed on food containing 5-HTP for 3 days showed a dose-dependent reduction in total seizure time during a 2-min heating period, whereas 50 mM 5-HTP in food resulted in ∼65% reduction in seizure time. The seizure activity returns to the untreated level 3 days after 5-HTP is removed from the diet. These data demonstrate that 5-HTP reversibly suppresses seizure activity in DS flies (Schutte et al. 2014). In addition, acute increases in serotonin through injection of serotonin in the dorsal vessel reduces the total seizure time by ∼27% with 1 μM (40 ± 3 s) and 10 μM serotonin (39 ± 3s) compared with DS flies injected with saline only (53 ± 3s) at 1–3 h postinjection. The reduction is considerably smaller compared with the ∼65% reduction caused by 3-day 5-HTP treatment, indicating that in addition to direct modulation, 5-HTP/serotonin may regulate seizure sensitivity of DS via other pathways, such as transcriptional regulation.
The rescuing effect of serotonin on seizure activity in DS flies is consistent with the outcome of two clinical studies that used the serotonin reuptake inhibitors fluoxetine and citalopram to treat refractory epilepsies (Albano et al. 2006; Favale et al. 1995, 2003). All participating patients with refractory epilepsies showed reduced seizure frequency after receiving fluoxetine or citalopram as an add-on treatment. These studies, along with the rescuing effect of serotonin in DS flies, suggest serotonin pathway could be further exploited as a therapeutic target for DS.
Patient-Specific iPSC-Derived Neurons
Although animal models continue to serve as invaluable assets in studying SCN1A-related epilepsy, there are intrinsic limitations such as genetic and developmental differences between human and each model organism. Because of the difficulty of examining living human brain tissue, patient-specific induced pluripotent stem cell (iPSC)-derived neurons are emerging as valuable substitutes for studying cellular mechanisms of various neurological disorders, including SCN1A-related epilepsy. Three recent studies have reported results regarding excitability in iPSC-derived neurons from DS patients. iPSC-derived neurons from a DS patient carrying a SCN1A mutation that causes truncation in the fourth homologous domain of the NaV1.1 (R1645X) decreased excitability in SCN1A-expressing neurons. The majority of these neurons were GABAergic, consistent with the findings in the DS mouse models (Higurashi et al. 2013). In contrast, two other studies reported increased excitability in iPSC-derived neurons carrying SCN1A mutations. Liu et al. (2013b) found that a deletion of exon 14, which affects transmembrane segments 2–4 in domain II, and a truncation mutation (Y325X) within the pore loop of domain I confer increased sodium currents and hyperexcitability in both bipolar and pyramidal iPSC-derived neurons. Similarly, a missense DS-causing mutation (F1415I) resulted in increased excitability in glutamatergic neurons (Jiao et al. 2013). It was proposed that compensatory overexpression of other α-subunits in response to loss of NaV1.1 may underlie the increased sodium currents in neurons derived from DS patient-specific iPSCs (Liu et al. 2013b).
The studies in F1.Scn1+/− mice demonstrate an abnormal development-dependent increase in sodium currents in excitatory pyramidal neurons (Mistry et al. 2014). Whereas there is no difference in sodium current density between wild-type and Scn1a+/− pyramidal neurons at P14-15, the sodium current density is significantly increased in Scn1a+/− pyramidal neurons compared with controls at P21-24. It is possible that the variety of alterations observed in different iPSC-derived neurons reflect changes associated with a specific developmental stage. However, it is not currently possible to accurately correlate the developmental stages of iPSC-derived neurons in culture with the developmental stages in vivo. This makes it difficult to map the development-associated epileptogenesis events in SCN1A-related epilepsy in chronological order using iPSC-derived neurons. In addition, electrophysiological analysis of neuronal excitability at elevated temperatures may reveal differential effects of temperature between these DS mutant sodium channels as demonstrated in the GEFS+ knockin flies.
The technology for making patient-specific iPSC-derived neurons is evolving rapidly, and multiple protocols have been developed to make neurons from patients with SCN1A epilepsy (Liu et al. 2013a; Maroof et al. 2013; Nicholas et al. 2013; Stover et al. 2013; Zhang et al. 2013). The neurons derived from different protocol vary widely in neuronal types generated, and there is little quantitative information about the degree and rate of functional maturation. Detailed characterization of differentiation protocols that support highly reproducible patterns of morphological and functional development of iPSC-derived neurons is an essential step in effectively using this model system to identify cellular defects associated with specific disease-causing mutations. In addition, examination of neurons derived from the same mutant iPSC line using more than one protocol will be extremely helpful for understanding the effects of specific differentiation conditions on neuronal excitability.
In addition to their potential to contribute to the knowledge of the cellular mechanism underlying SCN1A-related epilepsy, patient-specific iPSC-derived neurons will also be useful as a platform for drug discovery and transplant treatment. In a recent study, human iPSC-derived maturing GABAergic interneurons were injected into the brains of mice with temporal lobe epilepsy (TLE), where they integrated into the host tissue and were capable of inducing inhibitory postsynaptic responses in host hippocampal neurons. Additionally, the transplanted cells suppressed seizures and behavioral abnormalities in TLE mice (Cunningham et al. 2014). Although there is still a long way to go before iPSC-derived neurons can be used to treat patients, these early findings show promise in developing new therapeutics for patients with refractory epilepsies.
Conclusions and Future Directions
The results from multiple animal models and human iPSC-derived neurons have shown the following:
SCN1A mutations can cause both gain and loss of function at the channel levels, as shown in both heterologous expression systems and in knockin flies.
Both gain-of-function and loss-of-function mutations at the sodium channel level can result in reduced excitability in inhibitory neurons, as demonstrated by the GEFS+ and DS knockin flies.
The same mutation can have differential effects on different types of neurons, as shown in mouse models and knockin flies. Furthermore, it is likely that cell-specific patterns of NaV1.1 channel expression, different splicing variants, and/or other ion channels are important in determining how an SCN1A mutation affects neuronal excitability.
The same type of mutation, such as SCN1A truncations, may have different effects on neuronal excitability depending on development stage, as demonstrated in F1.Scn1a+/− mice (Mistry et al. 2014; Yu et al. 2006).
Temperature can influence the function of SCN1A mutant channels and in turn alter neuronal excitability, as best demonstrated in the GEFS+ knockin flies. The effects of temperature on sodium channel function and neuronal excitability should be further investigated for other SCN1A mutations in Drosophila and the other model systems.
Several compounds and pathways identified in the heterologous expression system, zebrafish, and fly models provide novel targets for developing new treatments for SCN1A-related epilepsy.
A few studies have reported altered sleep regulation, one of the comorbidities of SCN1A-related epilepsy, in the R1648H knockin mouse model and the GEFS+ knockin Drosophila. However, little is known regarding the underlying mechanisms (Papale et al. 2013; Petruccelli et al. 2015). Additional studies addressing comorbidities caused by SCN1A mutations will be important in helping us understand the full spectrum of disorders caused by SCN1A mutations.
The recent advance in the CRISPR/Cas9 gene editing system makes it possible to more rapidly and efficiently generate targeted mutations in animal model systems and human iPSC lines (Bassett et al. 2013; Cong et al. 2013; Ran et al. 2013; Sung et al. 2014; Wang et al. 2013). This provides an opportunity to generate the same SCN1A mutations in different model systems and to examine different mutations in an isogenic background in iPSC-derived neurons. Because each model system has its own advantages and limitations, combining the findings from multiple model systems and identifying the common mechanisms will be important in unraveling the complicated cellular mechanisms contributing to the SCN1A-related epilepsies and in identifying new treatments.
GRANTS
This study was supported by National Institute of Neurological Disorders and Stroke Grant R01NS0830009 (to D. K. O'Dowd), Howard Hughes Medical Institute Professor Program 52007056 (to D. K. O'Dowd), and University of California, Irvine California Institute for Regenerative Medicine Postdoctoral Fellowship TG2-01152 (to R. J. Schutte).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.S.S., R.J.S., and D.K.O. conception and design of research; S.S.S. and E.V.B. performed experiments; S.S.S. and E.V.B. analyzed data; S.S.S., E.V.B., and D.K.O. interpreted results of experiments; S.S.S. prepared figures; S.S.S. and R.J.S. drafted manuscript; S.S.S., R.J.S., and D.K.O. edited and revised manuscript; S.S.S., R.J.S., and D.K.O. approved final version of manuscript.
REFERENCES
- Abou-Khalil B, Ge Q, Desai R, Ryther R, Bazyk A, Bailey R, Haines JL, Sutcliffe JS, George AL. Partial and generalized epilepsy with febrile seizures plus and a novel SCN1A mutation. Neurology 57: 2265–2272, 2001. [DOI] [PubMed] [Google Scholar]
- Albano C, Cupello A, Mainardi P, Scarrone S, Favale E. Successful treatment of epilepsy with serotonin reuptake inhibitors: proposed mechanism. Neurochem Res 31: 509–514, 2006. [DOI] [PubMed] [Google Scholar]
- Baraban SC, Dinday MT, Hortopan GA. Drug screening in Scn1a zebrafish mutant identifies clemizole as a potential Dravet syndrome treatment. Nat Commun 4: 2410, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraban SC, Taylor MR, Castro PA, Baier H. Pentylenetetrazole induced changes in zebrafish behavior, neural activity and c-fos expression. Neuroscience 131: 759–768, 2005. [DOI] [PubMed] [Google Scholar]
- Bassett AR, Tibbit C, Ponting CP, Liu JL. Highly efficient targeted mutagenesis of Drosophila with the CRISPR/Cas9 system. Cell Rep 4: 220–228, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookfield R, Dafhnis-Calas F, Xu Z, Brown W. Unsuccessful attempt at gene-editing by homologous recombination in the zebrafish germ line using the approach of “Rong and Golic”. Transgenic Res 21: 1125–1136, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunklaus A, Ellis R, Reavey E, Semsarian C, Zuberi SM. Genotype phenotype associations across the voltage-gated sodium channel family. J Med Genet 51: 650–658, 2014. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Kalume F, Oakley JC. NaV1.1 channels and epilepsy. J Physiol 588: 1849–1859, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah CS, Yu FH, Westenbroek RE, Kalume FK, Oakley JC, Potter GB, Rubenstein JL, Catterall WA. Specific deletion of NaV1.1 sodium channels in inhibitory interneurons causes seizures and premature death in a mouse model of Dravet syndrome. Proc Natl Acad Sci USA 109: 14646–14651, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Dale TJ, Romanos MA, Whitaker WR, Xie XM, Clare JJ. Cloning, distribution and functional analysis of the type III sodium channel from human brain. Eur J Neurosci 12: 4281–4289, 2000. [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science 339: 819–823, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham M, Cho JH, Leung A, Savvidis G, Ahn S, Moon M, Lee PK, Han JJ, Azimi N, Kim KS, Bolshakov VY, Chung S. hPSC-derived maturing GABAergic interneurons ameliorate seizures and abnormal behavior in epileptic mice. Cell Stem Cell 15: 559–573, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dravet C. The core Dravet syndrome phenotype. Epilepsia 52, Suppl 2: 3–9, 2011. [DOI] [PubMed] [Google Scholar]
- Escayg A, MacDonald BT, Meisler MH, Baulac S, Huberfeld G, An-Gourfinkel I, Brice A, LeGuern E, Moulard B, Chaigne D, Buresi C, Malafosse A. Mutations of SCN1A, encoding a neuronal sodium channel, in two families with GEFS+2. Nat Genet 24: 343–345, 2000. [DOI] [PubMed] [Google Scholar]
- Escayg A, Goldin AL. Sodium channel SCN1A and epilepsy: mutations and mechanisms. Epilepsia 51: 1650–1658, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favale E, Audenino D, Cocito L, Albano C. The anticonvulsant effect of citalopram as an indirect evidence of serotonergic impairment in human epileptogenesis. Seizure 12: 316–318, 2003. [DOI] [PubMed] [Google Scholar]
- Favale E, Rubino V, Mainardi P, Lunardi G, Albano C. Anticonvulsant effect of fluoxetine in humans. Neurology 45: 1926–1927, 1995. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Sugawara T, Mazaki-Miyazaki E, Takahashi Y, Fukushima K, Watanabe M, Hara K, Morikawa T, Yagi K, Yamakawa K, Inoue Y. Mutations of sodium channel alpha subunit type 1 (SCN1A) in intractable childhood epilepsies with frequent generalized tonic-clonic seizures. Brain 126: 531–546, 2003. [DOI] [PubMed] [Google Scholar]
- Ganetzky B, Wu CF. Indirect suppression involving behavioral mutants with altered nerve excitability in Drosophila melanogaster. Genetics 100: 597–614, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George ALJ. Inherited channelopathies associated with epilepsy. Epilepsy Curr 4: 65–70, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms DW, Quadros RM, Seruggia D, Ohtsuka M, Takahashi G, Montoliu L, Gurumurthy CB. Mouse genome editing using the CRISPR/Cas system. Curr Protoc Hum Genet 83: 15.7.1–15.7.27, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich UB, Liautard C, Kirschenbaum D, Pofahl M, Lavigne J, Liu Y, Theiss S, Slotta J, Escayg A, Dihne M, Beck H, Mantegazza M, Lerche H. Impaired action potential initiation in GABAergic interneurons causes hyperexcitable networks in an epileptic mouse model carrying a human NaV1.1 mutation. J Neurosci 34: 14874–14889, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron SE, Scheffer IE, Berkovic SF, Dibbens LM, Mulley JC. Channelopathies in idiopathic epilepsy. Neurotherapeutics 4: 295–304, 2007. [DOI] [PubMed] [Google Scholar]
- Hesdorffer DC, Logroscino G, Benn EK, Katri N, Cascino G, Hauser WA. Estimating risk for developing epilepsy: a population-based study in Rochester, Minnesota. Neurology 76: 23–27, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higurashi N, Uchida T, Lossin C, Misumi Y, Okada Y, Akamatsu W, Imaizumi Y, Zhang B, Nabeshima K, Mori MX, Katsurabayashi S, Shirasaka Y, Okano H, Hirose S. A human Dravet syndrome model from patient induced pluripotent stem cells. Mol Brain 6: 19, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the “common” neurologic disorders? Neurology 68: 326–337, 2007. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Neurophysiol 117: 500–544, 1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortopan GA, Dinday MT, Baraban SC. Zebrafish as a model for studying genetic aspects of epilepsy. Dis Model Mech 3: 144–148, 2010. [DOI] [PubMed] [Google Scholar]
- Hruscha A, Schmid B. Generation of zebrafish models by CRISPR/Cas9 genome editing. Methods Mol Biol 1254: 341–350, 2015. [DOI] [PubMed] [Google Scholar]
- Hwang WY, Fu Y, Reyon D, Maeder ML, Tsai SQ, Sander JD, Peterson RT, Yeh JR, Joung JK. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol 31: 227–229, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan YN, Jan LY. Genetic dissection of short-term and long-term facilitation at the Drosophila neuromuscular junction. Proc Natl Acad Sci USA 75: 515–519, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao J, Yang Y, Shi Y, Chen J, Gao R, Fan Y, Yao H, Liao W, Sun XF, Gao S. Modeling Dravet syndrome using induced pluripotent stem cells (iPSCs) and directly converted neurons. Hum Mol Genet 22: 4241–4252, 2013. [DOI] [PubMed] [Google Scholar]
- Jinno S, Kosaka T. Stereological estimation of numerical densities of glutamatergic principal neurons in the mouse hippocampus. Hippocampus 20: 829–840, 2010. [DOI] [PubMed] [Google Scholar]
- Kahlig KM, Hirakawa R, Liu L, George AL, Belardinelli L, Rajamani S. Ranolazine reduces neuronal excitability by interacting with inactivated states of brain sodium channels. Mol Pharmacol 85: 162–174, 2014. [DOI] [PubMed] [Google Scholar]
- Kahlig KM, Lepist I, Leung K, Rajamani S, George AL. Ranolazine selectively blocks persistent current evoked by epilepsy-associated NaV1.1 mutations. Br J Pharmacol 161: 1414–1426, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalume F, Yu FH, Westenbroek RE, Scheuer T, Catterall WA. Reduced sodium current in Purkinje neurons from NaV1.1 mutant mice: implications for ataxia in severe myoclonic epilepsy in infancy. J Neurosci 27: 11065–11074, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq K, Afrikanova T, Langlois M, De Prins A, Buenafe OE, Rospo CC, Van Eeckhaut A, de Witte PA, Crawford AD, Smolders I, Esguerra CV, Kaminski RM. Cross-species pharmacological characterization of the allylglycine seizure model in mice and larval zebrafish. Epilepsy Behav 45: 53–63, 2015. [DOI] [PubMed] [Google Scholar]
- Liao Y, Anttonen AK, Liukkonen E, Gaily E, Maljevic S, Schubert S, Bellan-Koch A, Petrou S, Ahonen VE, Lerche H, Lehesjoki AE. SCN2A mutation associated with neonatal epilepsy, late-onset episodic ataxia, myoclonus, and pain. Neurology 75: 1454–1458, 2010. [DOI] [PubMed] [Google Scholar]
- Lin WH, Gunay C, Marley R, Prinz AA, Baines RA. Activity-dependent alternative splicing increases persistent sodium current and promotes seizure. J Neurosci 32: 7267–7277, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WH, Wright DE, Muraro NI, Baines RA. Alternative splicing in the voltage-gated sodium channel DmNav regulates activation, inactivation, and persistent current. J Neurophysiol 102: 1994–2006, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay HA, Baines R, ffrench-Constant R, Lilley K, Jacobs HT, O'Dell KM. The dominant cold-sensitive Out-cold mutants of Drosophila melanogaster have novel missense mutations in the voltage-gated sodium channel gene paralytic. Genetics 180: 873–884, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liu H, Sauvey C, Yao L, Zarnowska ED, Zhang SC. Directed differentiation of forebrain GABA interneurons from human pluripotent stem cells. Nat Protoc 8: 1670–1679, 2013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lopez-Santiago LF, Yuan Y, Jones JM, Zhang H, O'Malley HA, Patino GA, O'Brien JE, Rusconi R, Gupta A, Thompson RC, Natowicz MR, Meisler MH, Isom LL, Parent JM. Dravet syndrome patient-derived neurons suggest a novel epilepsy mechanism. Ann Neurol 74: 128–139, 2013b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lossin C, Wang DW, Rhodes TH, Vanoye CG, George AL. Molecular basis of an inherited epilepsy. Neuron 34: 877–884, 2002. [DOI] [PubMed] [Google Scholar]
- Loughney K, Kreber R, Ganetzky B. Molecular analysis of the para locus, a sodium channel gene in Drosophila. Cell 58: 1143–1154, 1989. [DOI] [PubMed] [Google Scholar]
- Ma JY, Catterall WA, Scheuer T. Persistent sodium currents through brain sodium channels induced by G protein betagamma subunits. Neuron 19: 443–452, 1997. [DOI] [PubMed] [Google Scholar]
- Mantegazza M, Yu FH, Powell AJ, Clare JJ, Catterall WA, Scheuer T. Molecular determinants for modulation of persistent sodium current by G-protein betagamma subunits. J Neurosci 25: 3341–3349, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci 5: 793–807, 2004. [DOI] [PubMed] [Google Scholar]
- Marley R, Baines RA. Increased persistent Na+ current contributes to seizure in the slamdance bang-sensitive Drosophila mutant. J Neurophysiol 106: 18–29, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroof AM, Keros S, Tyson JA, Ying SW, Ganat YM, Merkle FT, Liu B, Goulburn A, Stanley EG, Elefanty AG, Widmer HR, Eggan K, Goldstein PA, Anderson SA, Studer L. Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells. Cell Stem Cell 12: 559–572, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MS, Dutt K, Papale LA, Dube CM, Dutton SB, de Haan G, Shankar A, Tufik S, Meisler MH, Baram TZ, Goldin AL, Escayg A. Altered function of the SCN1A voltage-gated sodium channel leads to gamma-aminobutyric acid-ergic (GABAergic) interneuron abnormalities. J Biol Chem 285: 9823–9834, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H, Xu HQ, Yu L, Lin GW, He N, Su T, Shi YW, Li B, Wang J, Liu XR, Tang B, Long YS, Yi YH, Liao WP. The SCN1A mutation database: updating information and analysis of the relationships among genotype, functional alteration, and phenotype. Hum Mutat 36: 573–580, 2015. [DOI] [PubMed] [Google Scholar]
- Miller IO, Sotero de Menezes MA. SCN1A-related seizure disorders. In: GeneReviews (Online), edited by Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJ, Bird TD, Fong CT, Mefford HC, Smith RJ, Stephens K. Seattle, WA: University of Washington, Seattle; [updated 2014 May 15]. [Google Scholar]
- Mistry AM, Thompson CH, Miller AR, Vanoye CG, George AL, Kearney JA. Strain- and age-dependent hippocampal neuron sodium currents correlate with epilepsy severity in Dravet syndrome mice. Neurobiol Dis 65: 1–11, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas CR, Chen J, Tang Y, Southwell DG, Chalmers N, Vogt D, Arnold CM, Chen YJ, Stanley EG, Elefanty AG, Sasai Y, Alvarez-Buylla A, Rubenstein JL, Kriegstein AR. Functional maturation of hPSC-derived forebrain interneurons requires an extended timeline and mimics human neural development. Cell Stem Cell 12: 573–586, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak AE, Taylor AD, Pineda RH, Lasda EL, Wright MA, Ribera AB. Embryonic and larval expression of zebrafish voltage-gated sodium channel alpha-subunit genes. Dev Dyn 235: 1962–1973, 2006. [DOI] [PubMed] [Google Scholar]
- O'Dowd DK, Gee JR, Smith MA. Sodium current density correlates with expression of specific alternatively spliced sodium channel mRNAs in single neurons. J Neurosci 15: 4005–4012, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dowd DK, Smith MA. Single-cell analysis of gene expression in the nervous system. Measurements at the edge of chaos. Mol Neurobiol 13: 199–211, 1996. [DOI] [PubMed] [Google Scholar]
- Ogiwara I, Iwasato T, Miyamoto H, Iwata R, Yamagata T, Mazaki E, Yanagawa Y, Tamamaki N, Hensch TK, Itohara S, Yamakawa K. NaV1.1 haploinsufficiency in excitatory neurons ameliorates seizure-associated sudden death in a mouse model of Dravet syndrome. Hum Mol Genet 22: 4784–4804, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogiwara I, Miyamoto H, Morita N, Atapour N, Mazaki E, Inoue I, Takeuchi T, Itohara S, Yanagawa Y, Obata K, Furuichi T, Hensch TK, Yamakawa K. NaV1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J Neurosci 27: 5903–5914, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson RO, Liu Z, Nomura Y, Song W, Dong K. Molecular and functional characterization of voltage-gated sodium channel variants from Drosophila melanogaster. Insect Biochem Mol Biol 38: 604–610, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papale LA, Makinson CD, Christopher Ehlen J, Tufik S, Decker MJ, Paul KN, Escayg A. Altered sleep regulation in a mouse model of SCN1A-derived genetic epilepsy with febrile seizures plus (GEFS+). Epilepsia 54: 625–634, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker L, Padilla M, Du Y, Dong K, Tanouye MA. Drosophila as a model for epilepsy: bss is a gain-of-function mutation in the para sodium channel gene that leads to seizures. Genetics 187: 523–534, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payandeh J, Gamal El-Din TM, Scheuer T, Zheng N, Catterall WA. Crystal structure of a voltage-gated sodium channel in two potentially inactivated states. Nature 486: 135–139, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payandeh J, Scheuer T, Zheng N, Catterall WA. The crystal structure of a voltage-gated sodium channel. Nature 475: 353–358, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruccelli E, Lansdon P, Kitamoto T. Exaggerated nighttime sleep and defective sleep homeostasis in a Drosophila knock-in model of human epilepsy. PLoS One 10: e0137758, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan KD, Shwe UT, Abramowitz J, Wu H, Rhee SW, Howell MD, Gottschall PE, Freichel M, Flockerzi V, Birnbaumer L, Zheng F. Canonical transient receptor channel 5 (TRPC5) and TRPC1/4 contribute to seizure and excitotoxicity by distinct cellular mechanisms. Mol Pharmacol 83: 429–438, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragsdale DS. How do mutant NaV1.1 sodium channels cause epilepsy? Brain Res Rev 58: 149–159, 2008. [DOI] [PubMed] [Google Scholar]
- Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc 8: 2281–2308, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid CA, Berkovic SF, Petrou S. Mechanisms of human inherited epilepsies. Prog Neurobiol 87: 41–57, 2009. [DOI] [PubMed] [Google Scholar]
- Richter JM, Schaefer M, Hill K. Clemizole hydrochloride is a novel and potent inhibitor of transient receptor potential channel TRPC5. Mol Pharmacol 86: 514–521, 2014. [DOI] [PubMed] [Google Scholar]
- Rubinstein M, Westenbroek RE, Yu FH, Jones CJ, Scheuer T, Catterall WA. Genetic background modulates impaired excitability of inhibitory neurons in a mouse model of Dravet syndrome. Neurobiol Dis 73: 106–117, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer IE, Berkovic SF. Generalized epilepsy with febrile seizures plus. A genetic disorder with heterogeneous clinical phenotypes. Brain 120: 479–490, 1997. [DOI] [PubMed] [Google Scholar]
- Schoonheim PJ, Arrenberg AB, Del Bene F, Baier H. Optogenetic localization and genetic perturbation of saccade-generating neurons in zebrafish. J Neurosci 30: 7111–7120, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte RJ, Schutte SS, Algara J, Barragan EV, Gilligan J, Staber C, Savva YA, Smith MA, Reenan R, O'Dowd DK. Knock-in model of Dravet syndrome reveals a constitutive and conditional reduction in sodium current. J Neurophysiol 112: 903–912, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi O, Benzer S. Neurophysiological defects in temperature-sensitive paralytic mutants of Drosophila melanogaster. Proc Natl Acad Sci USA 73: 3253–3257, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Schimenti JC, Bolcun-Filas E. A mouse geneticist's practical guide to CRISPR applications. Genetics 199: 1–15, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spampanato J, Escayg A, Meisler MH, Goldin AL. Functional effects of two voltage-gated sodium channel mutations that cause generalized epilepsy with febrile seizures plus type 2. J Neurosci 21: 7481–7490, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staber CJ, Gell S, Jepson JE, Reenan RA. Perturbing A-to-I RNA editing using genetics and homologous recombination. Methods Mol Biol 718: 41–73, 2011. [DOI] [PubMed] [Google Scholar]
- Stover AE, Brick DJ, Nethercott HE, Banuelos MG, Sun L, O'Dowd DK, Schwartz PH. Process-based expansion and neural differentiation of human pluripotent stem cells for transplantation and disease modeling. J Neurosci Res 91: 1247–1262, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara T, Mazaki-Miyazaki E, Fukushima K, Shimomura J, Fujiwara T, Hamano S, Inoue Y, Yamakawa K. Frequent mutations of SCN1A in severe myoclonic epilepsy in infancy. Neurology 58: 1122–1124, 2002. [DOI] [PubMed] [Google Scholar]
- Sun L, Gilligan J, Staber C, Schutte RJ, Nguyen V, O'Dowd DK, Reenan R. A knock-in model of human epilepsy in Drosophila reveals a novel cellular mechanism associated with heat-induced seizure. J Neurosci 32: 14145–14155, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung YH, Kim JM, Kim HT, Lee J, Jeon J, Jin Y, Choi JH, Ban YH, Ha SJ, Kim CH, Lee HW, Kim JS. Highly efficient gene knockout in mice and zebrafish with RNA-guided endonucleases. Genome Res 24: 125–131, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki DT, Grigliatti T, Williamson R. Temperature-sensitive mutations in Drosophila melanogaster. VII. A mutation (para-ts) causing reversible adult paralysis. Proc Natl Acad Sci USA 68: 890–893, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai C, Abe Y, Westenbroek RE, Scheuer T, Catterall WA. Impaired excitability of somatostatin- and parvalbumin-expressing cortical interneurons in a mouse model of Dravet syndrome. Proc Natl Acad Sci USA 111: E3139–E3148, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang B, Dutt K, Papale L, Rusconi R, Shankar A, Hunter J, Tufik S, Yu FH, Catterall WA, Mantegazza M, Goldin AL, Escayg A. A BAC transgenic mouse model reveals neuron subtype-specific effects of a generalized epilepsy with febrile seizures plus (GEFS+) mutation. Neurobiol Dis 35: 91–102, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thackeray JR, Ganetzky B. Developmentally regulated alternative splicing generates a complex array of Drosophila para sodium channel isoforms. J Neurosci 14: 2569–2578, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thackeray JR, Ganetzky B. Conserved alternative splicing patterns and splicing signals in the Drosophila sodium channel gene para. Genetics 141: 203–214, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CH, Kahlig KM, George AL. SCN1A splice variants exhibit divergent sensitivity to commonly used antiepileptic drugs. Epilepsia 52: 1000–1009, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev PM, Scheuer T, Catterall WA. Identification of an intracellular peptide segment involved in sodium channel inactivation. Science 241: 1658–1661, 1988. [DOI] [PubMed] [Google Scholar]
- Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, Jaenisch R. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153: 910–918, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weckhuysen S, Mandelstam S, Suls A, Audenaert D, Deconinck T, Claes LR, Deprez L, Smets K, Hristova D, Yordanova I, Jordanova A, Ceulemans B, Jansen A, Hasaerts D, Roelens F, Lagae L, Yendle S, Stanley T, Heron SE, Mulley JC, Berkovic SF, Scheffer IE, de Jonghe P. KCNQ2 encephalopathy: emerging phenotype of a neonatal epileptic encephalopathy. Ann Neurol 71: 15–25, 2012. [DOI] [PubMed] [Google Scholar]
- West JW, Patton DE, Scheuer T, Wang Y, Goldin AL, Catterall WA. A cluster of hydrophobic amino acid residues required for fast Na+-channel inactivation. Proc Natl Acad Sci USA 89: 10910–10914, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Epilepsy fact sheet no. 999. October 2012 (Online) http://www.who.int/mediacentre/factsheets/fs999/en/index.html [updated February 2016]. [Google Scholar]
- Yang H, Wang H, Jaenisch R. Generating genetically modified mice using CRISPR/Cas-mediated genome engineering. Nat Protoc 9: 1956–1968, 2014. [DOI] [PubMed] [Google Scholar]
- Yarov-Yarovoy V, DeCaen PG, Westenbroek RE, Pan CY, Scheuer T, Baker D, Catterall WA. Structural basis for gating charge movement in the voltage sensor of a sodium channel. Proc Natl Acad Sci USA 109: E93–E102, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FH, Catterall WA. Overview of the voltage-gated sodium channel family. Genome Biol 4: 207, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FH, Mantegazza M, Westenbroek RE, Robbins CA, Kalume F, Burton KA, Spain WJ, McKnight GS, Scheuer T, Catterall WA. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat Neurosci 9: 1142–1149, 2006. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Pak C, Han Y, Ahlenius H, Zhang Z, Chanda S, Marro S, Patzke C, Acuna C, Covy J, Xu W, Yang N, Danko T, Chen L, Wernig M, Sudhof TC. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron 78: 785–798, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu Y, Tong X, Wang Z, Liu D, Pan R, Li Z, Hu Y, Luo Z, Huang P, Wu Q, Zhu Z, Zhang B, Lin S. TALEN-mediated precise genome modification by homologous recombination in zebrafish. Nat Methods 10: 329–331, 2013. [DOI] [PubMed] [Google Scholar]