Abstract
BACKGROUND/OBJECTIVES
Supplementation with n-3 polyunsaturated fatty acids (PUFAs) has been shown to generally decrease levels of innate immune markers and inflammatory cytokines, but the specific associations between blood levels of PUFAs and those of innate immune markers have not been investigated. Thus, the present study was conducted to test the hypothesis that innate immune markers as well as cytokines are negatively associated with n-3 PUFAs but positively associated with n-6 PUFAs in healthy adults.
MATERIALS/METHODS
One hundred sixty-five healthy Korean adults aged 25-70 years old were included in this cross-sectional study.
RESULTS
Serum levels of n-3 PUFAs, such as 18:3n3, 20:5n3, 22:5n3, and 22:6n3 were negatively correlated with eosinophil and basophil counts and TNF-α, IFN-γ, IL-4, and IL-10 levels. Multivariate analysis also showed that serum levels of n-3 PUFAs were negatively associated with monocyte, eosinophil, and basophil counts and TNF-α, IFN-γ, IL-4, and IL-12 levels. Additionally, the ratio of 20:4n6 to 20:5n3 was positively correlated with eosinophil counts and associated with TNF-α, IFN-γ, and IL-4 levels. However, NK cell activity was not associated with serum fatty acid composition.
CONCLUSIONS
Innate immune markers such as eosinophil, monocyte, and basophil counts were inversely associated with serum levels of n-3 PUFAs, but were positively associated with the 20:4n6/20:5n3 ratio in this population.
Keywords: Cytokine, adult, immune marker, fatty acid, serum
INTRODUCTION
Innate responses involve phagocytic cells (neutrophils, monocytes, and macrophages), cells that secrete inflammatory mediators (basophils and eosinophils), and natural killer (NK) cells, while acquired responses involve proliferation of antigen-specific B and T cells [1]. Both the innate and the acquired immune system use cytokines as messengers both within each system and between the two systems in the regulation of immune responses [2].
Changes in membrane phospholipid fatty acid composition influence immune cell functions in a variety of ways, such as alterations in the physical properties of membranes and the patterns of lipid mediators produced, as well as exerting effects on cell signaling pathways [3]. Epidemiologic studies have shown that inflammatory cytokines are positively associated with blood levels of n-6 polyunsaturated fatty acids (PUFAs) and saturated fatty acids (SFA), but are negatively associated with n-3 PUFAs [4,5,6]. Consistently, clinical trials have found that n-3 PUFA supplementation decreases levels of cytokines such as interleukin (IL)-β, tumor necrosis factor (TNF)-α, and C-reactive protein in healthy adults [7,8].
Although the majority of studies have focused on the association of cytokines with fatty acid composition [9], a few clinical trials have shown that supplementation with docosahexaenoic acid (DHA; 22:6n3) and eicosapentaenoic acid (EPA; 20:5n3) decreases NK cell activity [7,10] and neutrophil counts in healthy adults [11] and patients with hyperlipidemia [12]. However, there has yet to be a study investigating the association between innate immune markers and blood levels of fatty acids. Therefore, the present study undertook to test the hypothesis that innate immune markers as well as cytokines are negatively associated with n-3 PUFAs, but positively associated with n-6 PUFAs, in healthy adults.
MATERIALS AND METHODS
Participants
One hundred sixty-five healthy Korean adults aged 25-70 years old were recruited through poster and newspaper advertisements from September 2012 to September 2013. Participants were excluded if they were pregnant or lactating; had any infectious disease; had chronic diseases such as cardiovascular disease, diabetes mellitus, kidney disease, thyroid disease, or psychiatric disorders; or had taken any medication or supplements regularly during the last 3 months. Additionally, participants were excluded if their white blood cell counts were > 8,000 cells/µL, creatinine level was ≥ 2 times the upper limit of normal, or glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT) levels were ≥ 3 times the upper limit of normal.
This study was approved by the Institutional Review Board of Hanyang University Hospital (HYUH 2012-05-008) and Hanyang University (HYI-13-048-2) and was performed in accordance with the Declaration of Helsinki. Written informed consents were obtained from all participants. This study was also registered with the Clinical Research Information Service as KCT0000536 and KCT0000832.
Anthropometric and clinical measurement
Participants were interviewed to collect sociodemographic data. Height was measured using a stadiometer and weight was measured using an InBody 720 scale (Biospace Corporation, Seoul, Korea). Blood samples were collected after 8 h of overnight fasting, and aliquots of serum were stored at -80℃. Blood chemistry analysis of serum for glucose, total protein, total cholesterol, GOT, and GPT levels was performed using a Hitachi 7150 automated analyzer (Hitachi Ltd, Tokyo Japan) at the Korea Biomedical Laboratory.
GC analysis
Fatty acid composition was measured as previously reported [13]. Briefly, serum was methylated with boron trifluoride methanol (Sigma-Aldrich, St. Louis, MO, USA) at 100℃ for 10 min. Fatty acid methyl esters were extracted with hexane and analyzed by gas chromatography (Shimadzu 2010, Kyoto, Japan) using an SP2560 capillary column (100 m×0.25 mm I.D., 0.2 µm film thickness, Supelco, Bellefonte, PA, USA). Fatty acids were identified by comparison with a standard mixture (GLC-727; Nu-Check Prep, Elysian, MN, USA). The coefficient of variation of a quality control sample was 2.7%.
Assessment of immunological status
Serum concentrations of the cytokines TNF-α, interferon-γ (IFN-γ), IL-2, IL-4, IL-10, and IL-12 were measured using Bio-Plex cytokine assay kits and analyzed with Bio-Plex Manager 6.1 software (Bio-Rad Lab., Hercules, CA, USA). Hematology analysis of segmented neutrophil, monocyte, eosinophil, and basophil counts were measured using a Coulter STKS hemocytometer (Beckman Coulter Inc., Fullerton, CA, USA) at the Korea Biomedical Laboratory.
To measure NK cell activity, peripheral blood mononuclear cells (PBMCs) were prepared by density gradient separation. The viability of the cell pellet suspended in phosphate-buffered saline was determined using trypan blue staining. NK cell activity was measured with non-radioactive cytotoxicity assay kits (Promega Inc., Madison, WI, USA). PBMCs (effector cells) were seeded in 96-well plates with K562 cells (target cell; Korean Cell Line Bank, Seoul, Korea). The ratio of effector cells to target cells was 10:1, and each sample was prepared in triplicate. The plates were incubated for 4 hours at 37℃ in 5% CO2 as per the manufacturer's instructions. Absorbance was read at 490 nm using a microplate reader (Bio-Rad Laboratories, Hercules, CA, USA), and the percentage of cytotoxicity was computed according to the following formula: % Cytotoxicity = ((experimental - effector spontaneous - target spontaneous)/(target maximum - target spontaneous)) × 100.
Statistical analysis
All data were analyzed using SPSS 18.0 (SPSS, Inc., Chicago, IL, USA). An independent t-test was used to compare means of normally distributed data. Nominal variables between groups were evaluated using the Chi-square test and Fisher's exact test, and the number of participants and percentage distribution were indicated. The relationships between serum fatty acid composition and immune markers were analyzed using partial correlation analysis after adjusting for age, sex, and BMI. Multivariate analysis of variance of general linear models was also conducted to identify the effects of serum fatty acid composition on immune markers after adjusting for age, sex, and BMI. A P-value < 0.05 was considered statistically significant.
RESULTS
Characteristics of participants
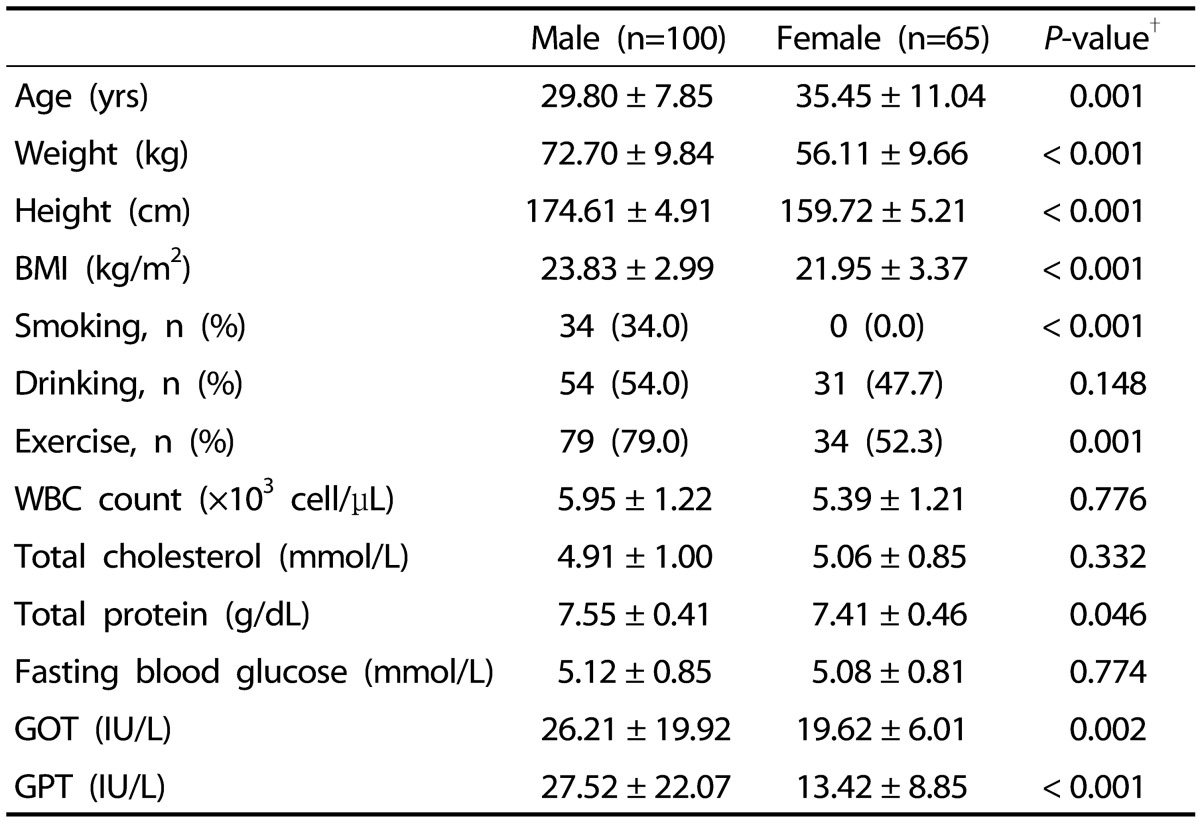
Males in this study were younger; had higher weight, height, BMI, NK cell activity, and blood levels of protein, GOT and GPT; and smoked and exercised more than females (Table 1). There were no significant differences in drinking status, white blood cell counts, or blood levels of cholesterol and glucose.
Table 1. Characteristics of male and female participants.
Values are means ± standard deviation or number of participants (percentage distribution), as appropriate. BMI, body mass index; GOT, glutamic oxaloacetic transaminase; GPT, glutamic pyruvic transaminase; exercise, ≥ twice a week.
†P-values were analyzed by independent t-test and Chi-square test.
Correlation between immune markers and serum fatty acid composition
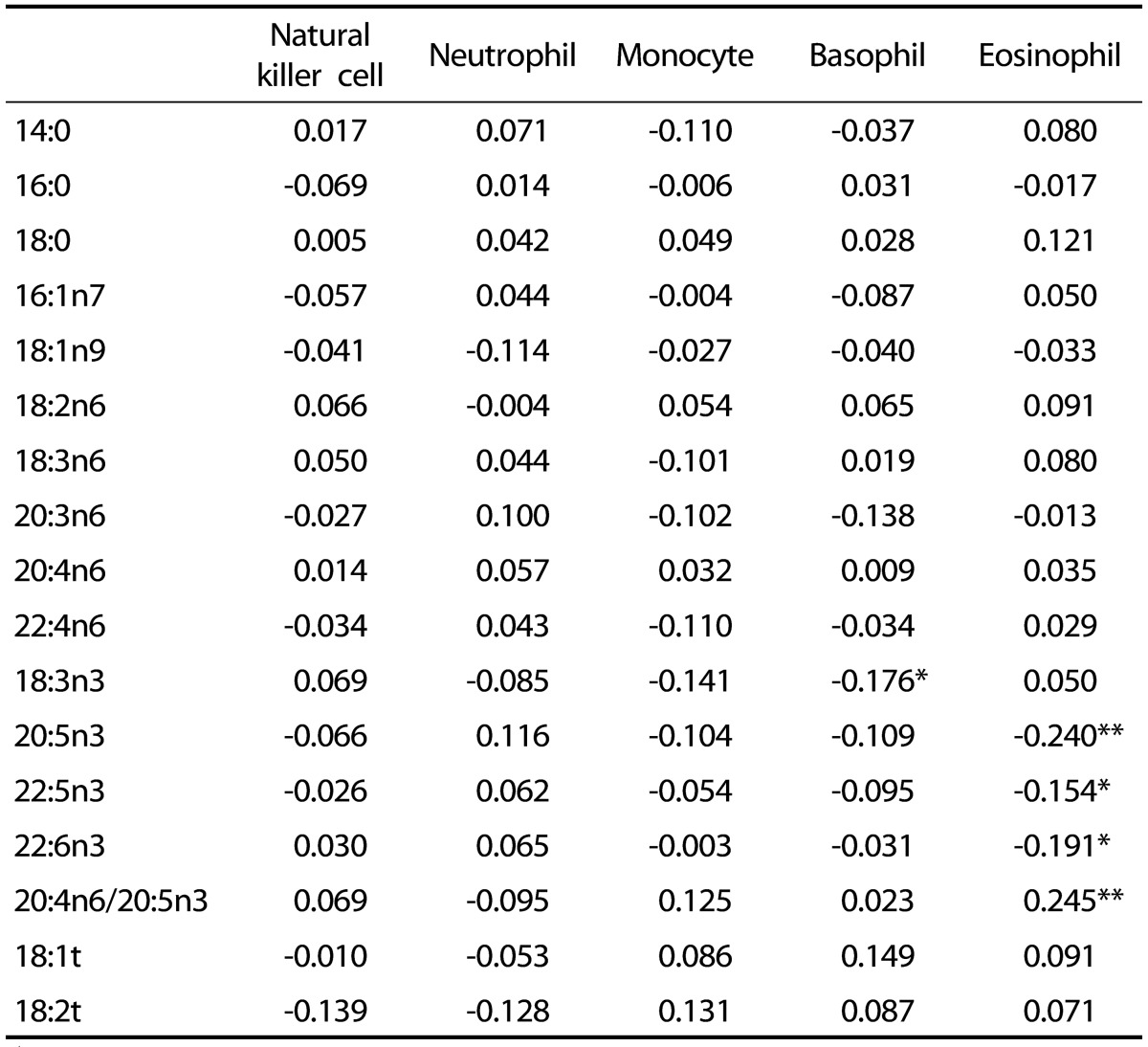
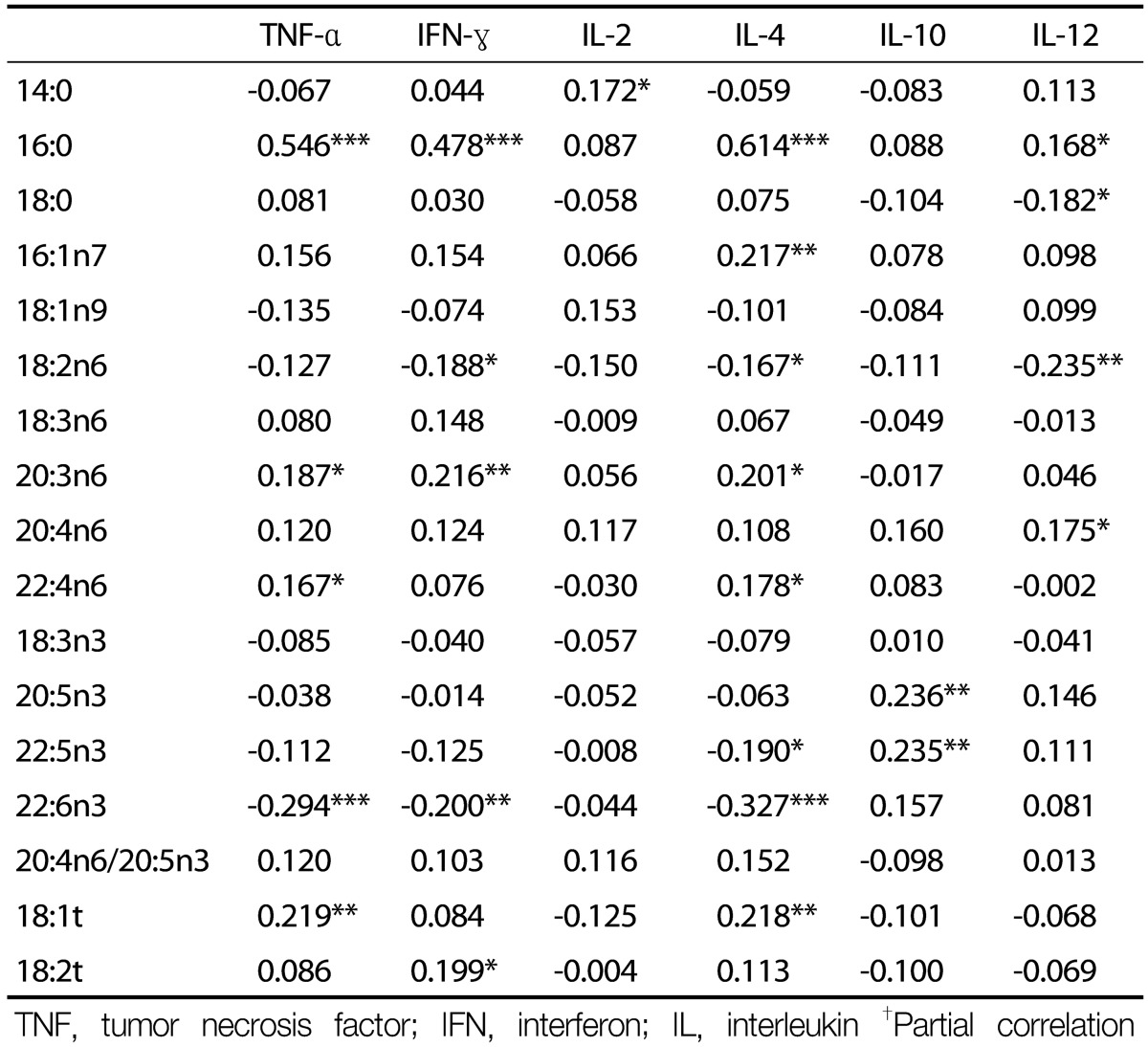
Basophil counts were negatively correlated with 18:3n3, but not with other fatty acids (Table 2). Eosinophil counts were negatively correlated with 20:5n3, 22:5n3, and 22:6n3 levels, but were positively correlated with the 20:4n6/20:5n3 ratio. There were no significant correlations between fatty acid composition and NK cell activity, or neutrophil or monocyte counts. Serum levels of TNF-α were positively correlated with 16:0, 20:3n6, 22:4n6, and 18:1t levels, but were negatively correlated with 22:6n3 levels (Table 3). Serum levels of IFN-γ were positively correlated with 16:0, 20:3n6, and 18:2t levels, but were negatively correlated with 18:2n6 and 22:6n3 levels. Serum levels of IL-2 were positively correlated with 14:0 levels, and levels of IL-10 were positively correlated with 20:5n3 and 22:5n3 levels. Serum levels of IL-4 were positively correlated with 16:0, 16:1n7, 20:3n6, 22:4n6, and 18:1t levels, but were negatively correlated with 18:2n6, 22:5n3, and 22:6n3 levels. Serum levels of IL-12 were positively correlated with 16:0 and 20:4n6 levels, but were negatively correlated with 18:0 and 18:2n6 levels.
Table 2. Correlation coefficients between innate immune markers and serum fatty acid composition†.
†Partial correlation coefficient after adjustment for age, sex, body mass index, smoking, and exercise. *P < 0.05, **P < 0.01, ***P < 0.001.
Table 3. Correlation coefficients between cytokines and serum fatty acid composition†.
TNF, tumor necrosis factor; IFN, interferon; IL, interleukin †Partial correlation coefficient after adjustment for age, sex, body mass index, smoking, and exercise. *P < 0.05, **P < 0.01, ***P < 0.001.
Relationship between individual serum fatty acid content and immune markers
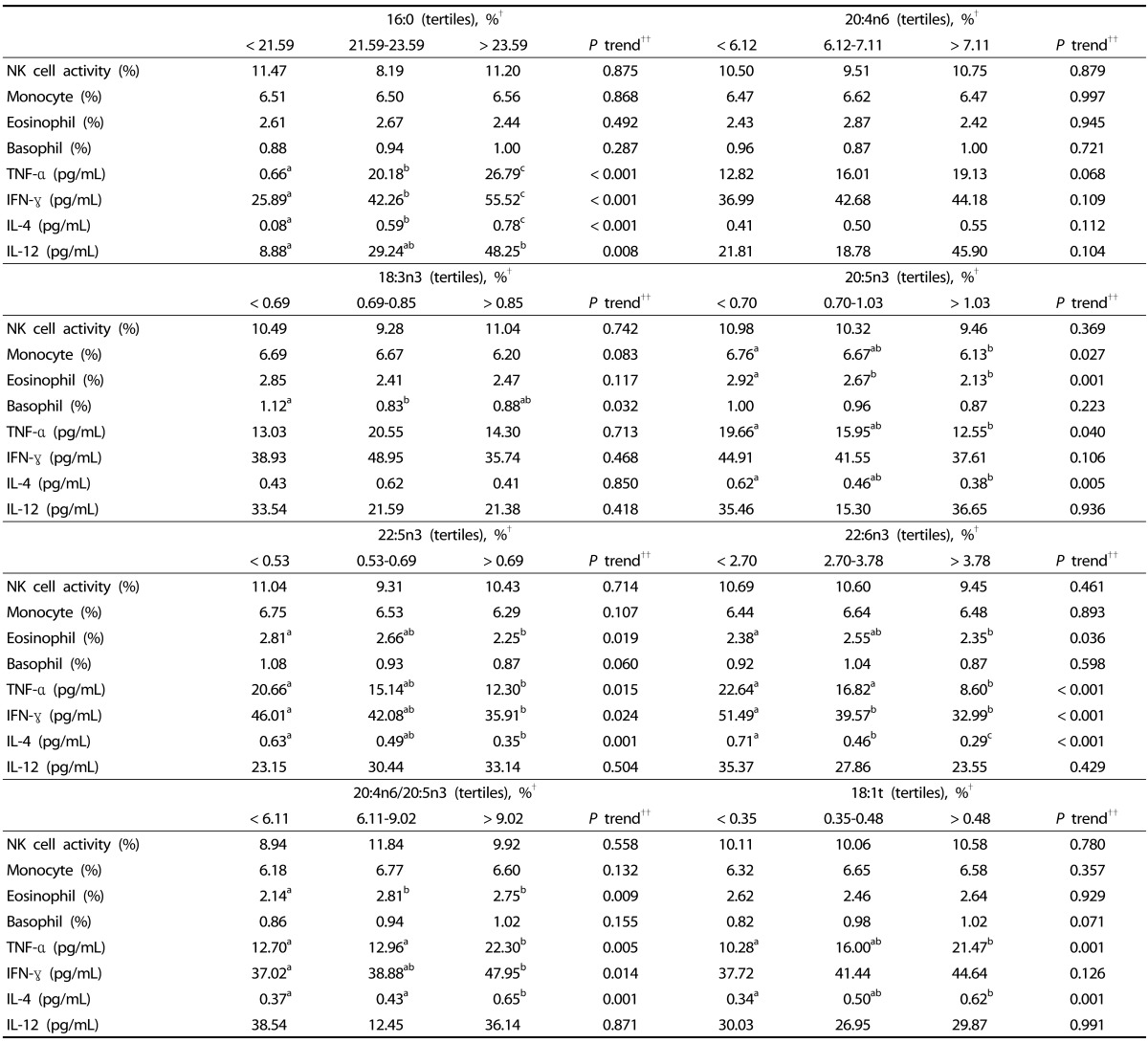
Serum levels of 18:3n3 were negatively associated with basophil counts, and 20:5n3 levels were negatively associated with monocyte and eosinophil counts and TNF-α and IL-4 levels (Table 4). Serum levels of 22:5n3 were negatively associated with eosinophil counts and TNF-α, IFN-γ, and IL-4 levels, and 22:6n3 levels were negatively associated with TNF-α, IFN-γ, and IL-4 levels. The ratio of 20:4n6 to20:5n3 was positively associated with eosinophil counts and TNF-α, IFN-γ, and IL-4 levels. The serum level of 16:0 was positively associated with TNF-α, IFN-γ, IL-4, and IL-12 levels, and 18:1t levels were positively associated with TNF-α and IL-4 levels. However, 20:4n6 levels were not significantly associated with innate immune markers or with cytokine levels.
Table 4. Multivariate analysis of the relationship between individual serum fatty acid content and immune markers.
NK, natural killer; TNF, Tumor necrosis factor; IFN, interferon; IL, interleukin.
†Values with different letters within a row are significantly different with P < 0.05. Mean values and statistics for the association of fatty acids with immune markers were adjusted for age, sex, body mass index, smoking, and exercise.
††P trend was determined by general linear model multivariate analysis.
DISCUSSION
This is the first report to show that serum levels of the n-3 PUFAs, 18:3n3, 20:5n3, 22:5n3, or 22:6n3 are negatively associated with innate immune makers such as eosinophil, basophil, and monocyte counts. Serum levels of n-3 PUFAs were also found to be negatively associated with TNF-α, IFN-γ, IL-4, and IL-10 levels. Moreover, the ratio of 20:4n6 to 20:5n3 was found to be positively associated with eosinophil counts and with TNF-α, IFN-γ, and IL-4 levels [14].
It has been reported that a short-term (48 h) infusion of fish oil-based lipid emulsions significantly inhibits monocyte-endothelium adhesion, transendothelial monocyte migration, and monocyte generation of TNF-α and IL-1 in healthy adults [15]. It has also been reported that supplementation with n-3 PUFAs decreases NK cell activity [7,10] and neutrophil counts in healthy adults [11] and patients with hyperlipidemia [12], and eosinophil counts in asthmatic children [16]. Epidemiologic studies have consistently reported that the risk of asthma is negatively associated with dietary intake of n-3 PUFAs in Americans and Canadians [17], and in Europeans [18]. During chronic allergic inflammatory states such as asthma, basophils produce IL-4 [19], but treatment with EPA and DHA suppresses production of basophilic IL-4 and IL-13 in leukemia cells [20].
During inflammatory conditions, innate immune cells such as eosinophils, basophils, and neutrophils release cytokines such as ILs, TNF-α, or IFN-γ [21]. Epidemiologic studies have shown that plasma levels [22,23] and dietary intake [24,25] of n-3 PUFAs are negatively associated with TNF-α, IL-6, C-reactive protein (CRP), and IFN-γ levels in healthy adults. Clinical trials have consistently shown that supplementation with n-3 PUFAs or DHA significantly reduces IL-2 levels in women [26] and TNF-α levels in men [7], respectively.
Consistent with our results, previous studies found that pro-inflammatory cytokine levels are positively correlated with blood levels of 20:4n6, but are inversely correlated with 18:2n6 levels [4,27]. Unlike EPA, membrane 20:4n6 is a pro-inflammatory eicosanoid that increases pro-inflammatory cytokine levels [28]. In addition, treatment with 20:4n6 increases the expression of inflammatory cytokines such as IL-1α and TNF-α in human osteoblasts [29]. Dietary intake of fish results in EPA replacing 20:4n6 in cell membranes and leads to decreased formation of 20:4n6-derived eicosanoids, such as prostaglandin E2 and leukotriene B4 [30]. Although 18:2n6 is partly converted into 20:4n6 through desaturation and elongation, 18:2n6 itself is essential for cell growth and signaling [31]. Plasma levels of 18:2n6 were inversely associated with levels of IFN-γ and CRP in healthy adults [4]. In the Nurses' Health Study and Health Professionals Follow-up study, intake of 18:2n6 was negatively associated with levels of soluble TNF receptor 1 and 2, suggesting that 18:2n6 did not inhibit the anti-inflammatory actions of n-3 PUFAs, but was rather associated with anti-inflammatory activity [32,33].
The present study also showed that pro-inflammatory cytokines are positively correlated with serum levels of 14:0 and 16:0, and are negatively correlated with serum levels of 18:0. Perreault et al. [4] consistently found that plasma levels of 14:0 and 16:0 were positively associated, but 18:0 was negatively associated, with CRP and IFN-γ in healthy Canadians. Dietary intake of SFAs such as 14:0 and 16:0 was shown to activate Toll-like receptors (TLRs) -2 and -4, which activated NFκB and increased expression of IL-6 and TNF-α, while the longer-chain SFA, 18:0, was shown not to do so [34]. Additionally, serum levels of the trans fatty acid, 18:1t, were positively associated with TNF-α and IL-4 levels in the present study. In cross-sectional studies [35,36] and a clinical trial [37], blood levels or intake of trans fatty acids were positively associated with levels of inflammatory cytokines such as CRP, TNF-α, and IL-6. Accumulation of trans fatty acids in endothelial cell membranes resulted in altered hyper-adhesiveness to blood leukocytes and increased release of cytokines [38]. Serum levels of 16:1n7 were also positively correlated with IL-4 levels in the present study. Although the dietary intake and blood levels of 16:1n7 were negligible, a previous study consistently found that erythrocyte levels of 16:1n7 were positively correlated with CRP levels [39].
This study has a few limitations. First, the cross-sectional study design does not establish a cause-effect relationship between blood fatty acid profile and immune markers. Second, a number of potential confounders were adjusted for in the statistical analysis; however, there is the possibility that there are other factors present that affect immune markers. Finally, fatty acid composition was measured in serum, but not in immune cells.
The present study suggests that innate immune markers such as eosinophil, monocyte, and basophil counts are inversely associated with serum levels of n-3 PUFAs in Korean adults. A clinical trial is necessary to determine whether consumption of n-3 PUFAs modulates the innate immune system.
Footnotes
This work was supported by a grant from the Korea Research Foundation (2015R1D1A1A09060823) funded by the Korean government.
References
- 1.Delves PJ, Roitt IM. The immune system. First of two parts. N Engl J Med. 2000;343:37–49. doi: 10.1056/NEJM200007063430107. [DOI] [PubMed] [Google Scholar]
- 2.Mire-Sluis AR. Cytokines: from technology to therapeutics. Trends Biotechnol. 1999;17:319–325. doi: 10.1016/s0167-7799(99)01330-x. [DOI] [PubMed] [Google Scholar]
- 3.Calder PC. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim Biophys Acta. 2015;1851:469–484. doi: 10.1016/j.bbalip.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Perreault M, Roke K, Badawi A, Nielsen DE, Abdelmagid SA, El-Sohemy A, Ma DW, Mutch DM. Plasma levels of 14:0, 16:0, 16:1n-7, and 20:3n-6 are positively associated, but 18:0 and 18:2n-6 are inversely associated with markers of inflammation in young healthy adults. Lipids. 2014;49:255–263. doi: 10.1007/s11745-013-3874-3. [DOI] [PubMed] [Google Scholar]
- 5.Grenon SM, Conte MS, Nosova E, Alley H, Chong K, Harris WS, Vittinghoff E, Owens CD. Association between n-3 polyunsaturated fatty acid content of red blood cells and inflammatory biomarkers in patients with peripheral artery disease. J Vasc Surg. 2013;58:1283–1290. doi: 10.1016/j.jvs.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steffen BT, Steffen LM, Tracy R, Siscovick D, Jacobs D, Liu K, He K, Hanson NQ, Nettleton JA, Tsai MY. Ethnicity, plasma phospholipid fatty acid composition and inflammatory/endothelial activation biomarkers in the Multi-Ethnic Study of Atherosclerosis (MESA) Eur J Clin Nutr. 2012;66:600–605. doi: 10.1038/ejcn.2011.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelley DS, Taylor PC, Nelson GJ, Schmidt PC, Ferretti A, Erickson KL, Yu R, Chandra RK, Mackey BE. Docosahexaenoic acid ingestion inhibits natural killer cell activity and production of inflammatory mediators in young healthy men. Lipids. 1999;34:317–324. doi: 10.1007/s11745-999-0369-5. [DOI] [PubMed] [Google Scholar]
- 8.Browning LM, Krebs JD, Moore CS, Mishra GD, Mishra GD, Mishra GD, O'Connell MA, Jebb SA. The impact of long chain n-3 polyunsaturated fatty acid supplementation on inflammation, insulin sensitivity and CVD risk in a group of overweight women with an inflammatory phenotype. Diabetes Obes Metab. 2007;9:70–80. doi: 10.1111/j.1463-1326.2006.00576.x. [DOI] [PubMed] [Google Scholar]
- 9.Kang JX, Weylandt KH. Modulation of inflammatory cytokines by omega-3 fatty acids. Subcell Biochem. 2008;49:133–143. doi: 10.1007/978-1-4020-8831-5_5. [DOI] [PubMed] [Google Scholar]
- 10.Rasmussen LB, Kiens B, Pedersen BK, Richter EA. Effect of diet and plasma fatty acid composition on immune status in elderly men. Am J Clin Nutr. 1994;59:572–577. doi: 10.1093/ajcn/59.3.572. [DOI] [PubMed] [Google Scholar]
- 11.Rees D, Miles EA, Banerjee T, Wells SJ, Roynette CE, Wahle KW, Calder PC. Dose-related effects of eicosapentaenoic acid on innate immune function in healthy humans: a comparison of young and older men. Am J Clin Nutr. 2006;83:331–342. doi: 10.1093/ajcn/83.2.331. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto T, Kajikawa Y, Otani S, Yamada Y, Takemoto S, Hirota M, Ikeda M, Iwagaki H, Saito S, Fujiwara T. Protective effect of eicosapentaenoic acid on insulin resistance in hyperlipidemic patients and on the postoperative course of cardiac surgery patients: the possible involvement of adiponectin. Acta Med Okayama. 2014;68:349–361. doi: 10.18926/AMO/53024. [DOI] [PubMed] [Google Scholar]
- 13.Harris WS, von Schacky C, Park Y. Chapter 19. Standardizing methods for assessing omega-3 fatty acid biostatus. In: McNamara RK, editor. The Omega-3 Fatty Acid Deficiency Syndrome: Opportunities for Disease Prevention. New York (NY): Nova Science Publishers, Inc.; 2013. pp. 385–398. [Google Scholar]
- 14.Cho E. Association between serum fatty acid composition and innate immune markers in healthy adults [master's thesis] Seoul: Hanyang University; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayer K, Meyer S, Reinholz-Muhly M, Maus U, Merfels M, Lohmeyer J, Grimminger F, Seeger W. Short-time infusion of fish oil-based lipid emulsions, approved for parenteral nutrition, reduces monocyte proinflammatory cytokine generation and adhesive interaction with endothelium in humans. J Immunol. 2003;171:4837–4843. doi: 10.4049/jimmunol.171.9.4837. [DOI] [PubMed] [Google Scholar]
- 16.Hodge L, Salome CM, Hughes JM, Liu-Brennan D, Rimmer J, Allman M, Pang D, Armour C, Woolcock AJ. Effect of dietary intake of omega-3 and omega-6 fatty acids on severity of asthma in children. Eur Respir J. 1998;11:361–365. doi: 10.1183/09031936.98.11020361. [DOI] [PubMed] [Google Scholar]
- 17.Burns JS, Dockery DW, Neas LM, Schwartz J, Coull BA, Raizenne M, Speizer FE. Low dietary nutrient intakes and respiratory health in adolescents. Chest. 2007;132:238–245. doi: 10.1378/chest.07-0038. [DOI] [PubMed] [Google Scholar]
- 18.Kompauer I, Demmelmair H, Koletzko B, Bolte G, Linseisen J, Heinrich J. Association of fatty acids in serum phospholipids with lung function and bronchial hyperresponsiveness in adults. Eur J Epidemiol. 2008;23:175–190. doi: 10.1007/s10654-007-9218-y. [DOI] [PubMed] [Google Scholar]
- 19.Falcone FH, Haas H, Gibbs BF. The human basophil: a new appreciation of its role in immune responses. Blood. 2000;96:4028–4038. [PubMed] [Google Scholar]
- 20.Jin M, Park S, Park BK, Choi JJ, Yoon SJ, Yang M, Pyo MY. Eicosapentaenoic acid and docosahexaenoic acid suppress Th2 cytokine expression in RBL-2H3 basophilic leukemia cells. J Med Food. 2014;17:198–205. doi: 10.1089/jmf.2013.2935. [DOI] [PubMed] [Google Scholar]
- 21.Bochner BS, Luscinskas FW, Gimbrone MA, Jr, Newman W, Sterbinsky SA, Derse-Anthony CP, Klunk D, Schleimer RP. Adhesion of human basophils, eosinophils, and neutrophils to interleukin 1-activated human vascular endothelial cells: contributions of endothelial cell adhesion molecules. J Exp Med. 1991;173:1553–1557. doi: 10.1084/jem.173.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani F, Martin A, Andres-Lacueva C, Senin U, Guralnik JM. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab. 2006;91:439–446. doi: 10.1210/jc.2005-1303. [DOI] [PubMed] [Google Scholar]
- 23.Maes M, Christophe A, Bosmans E, Lin A, Neels H. In humans, serum polyunsaturated fatty acid levels predict the response of proinflammatory cytokines to psychologic stress. Biol Psychiatry. 2000;47:910–920. doi: 10.1016/s0006-3223(99)00268-1. [DOI] [PubMed] [Google Scholar]
- 24.Zampelas A, Panagiotakos DB, Pitsavos C, Das UN, Chrysohoou C, Skoumas Y, Stefanadis C. Fish consumption among healthy adults is associated with decreased levels of inflammatory markers related to cardiovascular disease: the ATTICA study. J Am Coll Cardiol. 2005;46:120–124. doi: 10.1016/j.jacc.2005.03.048. [DOI] [PubMed] [Google Scholar]
- 25.Niu K, Hozawa A, Kuriyama S, Ohmori-Matsuda K, Shimazu T, Nakaya N, Fujita K, Tsuji I, Nagatomi R. Dietary long-chain n-3 fatty acids of marine origin and serum C-reactive protein concentrations are associated in a population with a diet rich in marine products. Am J Clin Nutr. 2006;84:223–229. doi: 10.1093/ajcn/84.1.223. [DOI] [PubMed] [Google Scholar]
- 26.Meydani SN, Endres S, Woods MM, Goldin BR, Soo C, Morrill-Labrode A, Dinarello CA, Gorbach SL. Oral (n-3) fatty acid supplementation suppresses cytokine production and lymphocyte proliferation: comparison between young and older women. J Nutr. 1991;121:547–555. doi: 10.1093/jn/121.4.547. [DOI] [PubMed] [Google Scholar]
- 27.Petersson H, Lind L, Hulthe J, Elmgren A, Cederholm T, Risérus U. Relationships between serum fatty acid composition and multiple markers of inflammation and endothelial function in an elderly population. Atherosclerosis. 2009;203:298–303. doi: 10.1016/j.atherosclerosis.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 28.Calder PC, Ahluwalia N, Brouns F, Buetler T, Clement K, Cunningham K, Esposito K, Jönsson LS, Kolb H, Lansink M, Marcos A, Margioris A, Matusheski N, Nordmann H, O'Brien J, Pugliese G, Rizkalla S, Schalkwijk C, Tuomilehto J, Wärnberg J, Watzl B, Winklhofer-Roob BM. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr. 2011;106(Suppl 3):S5–S78. doi: 10.1017/S0007114511005460. [DOI] [PubMed] [Google Scholar]
- 29.Priante G, Bordin L, Musacchio E, Clari G, Baggio B. Fatty acids and cytokine mRNA expression in human osteoblastic cells: a specific effect of arachidonic acid. Clin Sci (Lond) 2002;102:403–409. [PubMed] [Google Scholar]
- 30.Ferretti A, Nelson GJ, Schmidt PC, Kelley DS, Bartolini G, Flanagan VP. Increased dietary arachidonic acid enhances the synthesis of vasoactive eicosanoids in humans. Lipids. 1997;32:435–439. doi: 10.1007/s11745-997-0057-5. [DOI] [PubMed] [Google Scholar]
- 31.Innis SM. Perinatal biochemistry and physiology of long-chain polyunsaturated fatty acids. J Pediatr. 2003;143(Suppl):S1–S8. doi: 10.1067/s0022-3476(03)00396-2. [DOI] [PubMed] [Google Scholar]
- 32.Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Willett WC, Rimm EB. Habitual dietary intake of n-3 and n-6 fatty acids in relation to inflammatory markers among US men and women. Circulation. 2003;108:155–160. doi: 10.1161/01.CIR.0000079224.46084.C2. [DOI] [PubMed] [Google Scholar]
- 33.Fritsche KL. Too much linoleic acid promotes inflammation-doesn't it? Prostaglandins Leukot Essent Fatty Acids. 2008;79:173–175. doi: 10.1016/j.plefa.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 34.Lee JY, Zhao L, Hwang DH. Modulation of pattern recognition receptor-mediated inflammation and risk of chronic diseases by dietary fatty acids. Nutr Rev. 2010;68:38–61. doi: 10.1111/j.1753-4887.2009.00259.x. [DOI] [PubMed] [Google Scholar]
- 35.Mozaffarian D, Rimm EB, King IB, Lawler RL, McDonald GB, Levy WC. trans fatty acids and systemic inflammation in heart failure. Am J Clin Nutr. 2004;80:1521–1525. doi: 10.1093/ajcn/80.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopez-Garcia E, Schulze MB, Meigs JB, Manson JE, Rifai N, Stampfer MJ, Willett WC, Hu FB. Consumption of trans fatty acids is related to plasma biomarkers of inflammation and endothelial dysfunction. J Nutr. 2005;135:562–566. doi: 10.1093/jn/135.3.562. [DOI] [PubMed] [Google Scholar]
- 37.Bendsen NT, Stender S, Szecsi PB, Pedersen SB, Basu S, Hellgren LI, Newman JW, Larsen TM, Haugaard SB, Astrup A. Effect of industrially produced trans fat on markers of systemic inflammation: evidence from a randomized trial in women. J Lipid Res. 2011;52:1821–1828. doi: 10.1194/jlr.M014738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Caterina R, Liao JK, Libby P. Fatty acid modulation of endothelial activation. Am J Clin Nutr. 2000;71:213S–223S. doi: 10.1093/ajcn/71.1.213S. [DOI] [PubMed] [Google Scholar]
- 39.Zong G, Ye X, Sun L, Li H, Yu Z, Hu FB, Sun Q, Lin X. Associations of erythrocyte palmitoleic acid with adipokines, inflammatory markers, and the metabolic syndrome in middle-aged and older Chinese. Am J Clin Nutr. 2012;96:970–976. doi: 10.3945/ajcn.112.040204. [DOI] [PMC free article] [PubMed] [Google Scholar]