Summary
The precise mechanisms responsible for immunosenescence still remain to be determined, however, considering the evidence that disruption of the organization of primary and secondary lymphoid organs results in immunodeficiency, we propose that this could be involved in the decline of immune responses with age. Therefore, we investigated the integrity of the splenic microarchitecture in mice of increasing age and its reorganization following immune challenge in young and old mice. Several differences in the anatomy of the spleen with age in both the immune and stromal cells were observed. There is an age‐related increase in the overall size of the white pulp, which occurs primarily within the T‐cell zone and is mirrored by the enlargement of the T‐cell stromal area, concurrent to the distinct boundary between T cells and B cells becoming less defined in older mice. In conjunction, there appears to be a loss of marginal zone macrophages, which is accompanied by an accumulation of fibroblasts in the spleens from older animals. Furthermore, whereas the reorganization of the white pulp is resolved after several days following antigenic challenge in young animals, it remains perturbed in older subjects. All these age‐related changes within the spleen could potentially contribute to the age‐dependent deficiencies in functional immunity.
Keywords: rodent, spleen and lymph nodes, stromal cells
Abbreviations
- FDC
follicular dendritic cells
- FRC
fibroblastic reticular cells
- HRP
horseradish peroxidase
- KLH
keyhole limpet haemocyanin
- MMM
marginal metallophilic macrophages
- MZM
marginal zone macrophages
- PALS
periarterial lymphatic sheaths
- SLO
secondary lymphoid organs
- TNP
trinitrophenyl
Introduction
It is now recognized that the activity and efficiency of the immune system declines with age; a phenomenon referred to as immunosenescence.1, 2, 3 In older individuals this leads to an increased susceptibility to and severity of infections, a decline in response to vaccinations and a higher prevalence of cancer and autoimmune diseases.1, 4, 5 These all contribute to the increased rates of mortality and morbidity that are observed in the elderly.6
Although the exact mechanisms underlying immunosenescence are still unclear, several studies have identified intrinsic defects within many of the cells of the immune system. For instance, various reports have revealed a reduction of receptor diversity in ageing T and B cells, together with alterations in their phenotype and function.2, 7, 8, 9, 10 Others have observed reduced phagocytic ability and reactive oxygen species production in aged macrophages and neutrophils;11, 12 as well as alteration in natural killer cell subsets and impaired cytotoxicity.13, 14 However, given the essential role that the microenvironment plays in the development and activation of many immune cell types,15, 16 changes in the tissue architecture may also contribute towards immunosenescence.17 Indeed, we and others have observed that regression of the thymus is accompanied by significant alterations of the thymic microenvironment,18, 19, 20, 21 and such changes are believed to contribute towards the reduced thymic function that is observed in the aged.22, 23, 24
The spleen is a secondary lymphoid organ that is responsible for initiating immune responses to blood‐borne antigens.25, 26 It is composed of two morphologically and functionally distinct compartments, the red pulp and the white pulp, which have different roles.25, 26 The red pulp is responsible for the removal of old and damaged platelets, old erythrocytes and apoptotic cells mediated by splenic macrophages26, 27 and is separated from the white pulp by the marginal zone. This contains many resident cells that maintain its integrity, including two specific subsets of macrophages – marginal zone macrophages (MZM) and marginal metallophilic macrophages (MMM) – as well as marginal zone B cells and dendritic cells.26, 28 These are supported by a network of specialized cells that line the marginal zone, called fibroblastic reticular cells (FRC).29
The white pulp consists of lymphoid tissue and is further subdivided into a periarterial lymphatic sheath (PALS), where T cells encircle the central arteriole, and B‐cell follicles.25 Embedded within this compartmentalized structure are stromal cells that are uniquely designed to allow effective filtration of the blood as it passes through the zones containing T and B cells, enabling capturing of pathogens, facilitating exchanges between antigen‐presenting cells and pathogen‐specific lymphocytes, and providing necessary signals for survival and differentiation of leucocytes.16 Therefore, the intact microarchitecture of the spleen supports productive lymphocyte–antigen‐presenting cell interactions and is critical for the ability of an organism to produce an efficient and robust immune response.16 Indeed, animals with disordered splenic microanatomy, such as alymphoplasia (aly/aly) mice or those deficient for lymphotoxin, exhibit reduced humoral and cell‐mediated immune responses.16, 30 Moreover, the immune response of these mice is similar to those seen in aged animals; suggesting that disruption of the splenic microanatomy may contribute towards peripheral immunosenescence. However, a detailed analysis of the splenic microarchitecture in mice at varying ages has not been performed. In this study, we examine the splenic microarchitecture in young and old mice together with analysing splenic remodelling in these animals following immunization with a T‐cell‐independent and T‐cell‐dependent antigen.
Materials and methods
Ethics statement
Animal experiments were conducted in accordance with our project licence (PPL 70/7243), which was approved by the Home Office under the Animal Scientific Procedures Act (1986). The project was approved by the local Ethical Review Committee at the Royal Veterinary College.
Mice
Male C57BL/6 mice were purchased from Charles Rivers Laboratory (Margate, UK) and maintained at the Royal Veterinary College, London. Animals were killed by the schedule 1 method and tissues were harvested from 1‐, 6‐, 12‐ and 18‐month‐old mice.
Antibodies and reagents
Monoclonal antibodies isotype‐ FITC, isotype‐biotin, anti‐CD3‐biotin, anti‐B220‐FITC, IgM‐biotin, anti‐CD157 and isotype controls were all purchased from eBioscience (Hatfield, UK). Rabbit anti‐rat‐biotin was purchased from Jackson ImmunoResearch Laboratories (Newmarket, UK). MOMA‐1‐FITC was obtained from Serotec (Kidlington, UK). Alexa 594 was from Invitrogen Molecular Probes (Paisley, UK). Anti‐Gp38 was obtained from Hybridoma Studies Bank (Iowa, IA). ERTR7 was a generous gift from Professor Graham Anderson (University of Birmingham), ERTR9 was a kind gift from Professor van Ewijk and anti‐FDC‐M2 was generous gift from Professor David Gray (Edinburgh University) and Dr Marie Kosco‐Vilbois (Novimmune). Trinitrophenyl (TNP)–Ficoll, TNP–keyhole limpet haemocyanin (KLH) and TNP–BSA were obtained from Biosearch Technologies Inc. (Bicester, UK). Anti‐mouse IgM‐horseradish peroxidase (HRP) and anti‐mouse IgG‐HRP were purchased from Southern Biotech (Cambridge, UK).
Immunohistological studies
About 7 μm thick tissue sections were cut, air‐dried overnight, fixed in acetone and stored at −20°. Sections were stained using a standard protocol.18 Briefly, double staining was performed by sequentially incubating primary antibodies, which after 45‐min incubations and three washes in PBS were revealed with the appropriate secondary antibody and streptavidin‐Alexa594. Sections were then probed with a second primary antibody that if not directly conjugated to FITC, was recognized by an FITC‐conjugated secondary. These were then mounted with VectaShield mounting medium and viewed on a Leica SP5 confocal microscope (Leica Microsystems Ltd, Milton Keynes, UK). Multiple photographs of each sample were taken and analysed.
Quantification of mean fluorescence intensity
Fluorescent images were analysed using imagej (http://rsb.info.nih.gov/ij/) as previously described.18
Immunization protocol
Mice (either 1 month or 18 months of age) were immunized via an intraperitoneal injection with either TNP–Ficoll, 500 μg (at day 0) or TNP–KLH, 100 μg (at day 0 and day 28).31 Blood was obtained from all mice by cardiac puncture or from the saphenous vein. Tissues were harvested from the young and old mice at time‐points of days 0, 3 and 28 for TNP–Ficoll, and days 0, 14 and 35 for TNP–KLH.
Enzyme‐linked immunosorbent assay
Specific antibody for TNP–Ficoll and TNP–KLH, detected by an ELISA was developed.31 Flat‐bottom 96‐well plates were coated with 5 μg of TNP–BSA in carbonate buffer (pH 9·6) and incubated overnight at 4°. After washing, blocking buffer was added and incubated at room temperature for 2 hr. After further washing, individual serum diluted in PBS 0·5% Tween‐20 was added, incubated for 1 hr and washed. The secondary antibody, either IgM‐HRP or IgG‐HRP was added and incubated for 1 hr. Finally, 3,3′,5,5′‐tetramethylbenzidine substrate purchase from Sigma (Gillingham, UK) was added to each well. The optical density was read at 405 nm.
Statistical analysis
Statistical significance was calculated with a one‐way or two‐way analysis of variance comparing all variables with Bonferroni's multiple comparison post‐test using prism software (graphpad prism 5; GraphPad Software, Inc, La Jolla, CA, USA). P values of 0·05 or less were considered significant.
Results
Disorganization of cellular positioning in the spleens of ageing mice
Immune function declines with age and although disorganization of the splenic architecture can lead to a profound loss of immunocompetence, little is known regarding age‐related changes in the spleen. In this study, the microanatomy of the spleens from mice of different ages was examined. The splenic T‐cell and B‐cell compartments were identified using antibodies to CD3 (in green) and B220 (in red), respectively, and was quantified by imageJ analysis. Using this approach, the T‐cell and B‐cell zones show a clear demarcation in animals at 1 and 6 months of age; however, this becomes increasingly obscured in older mice, demonstrated by an increase in CD3+ B220+ areas, which was significantly greater in size in both 12‐ and 18‐month‐old mice compared with younger animals (Fig. 1a). MZM, identified by the antibody ERTR9 (in red), were clearly observed to be encircling MMM, recognized by MOMA‐1 (in green) in younger mice (Fig. 1b). However, in splenic sections from 12‐month‐old and 18‐month‐old mice, MZM were more difficult to distinguish, and the MMM appeared not to fully surround the marginal zone.
Figure 1.
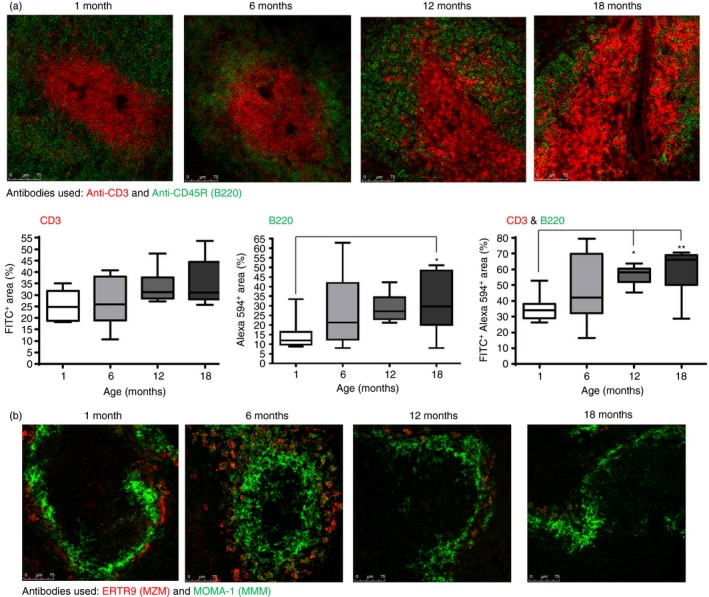
Changes of the splenic microenvironment with increasing age. (a) Splenic sections were stained with antibodies (anti‐CD3, anti‐B220) that recognized T cells (shown in green) and B cells (shown in red), respectively and the level of staining was assessed using imagej and portrayed as a percentage of the total area measured (FITC + Area refers to CD3, Alexa 594+ Area refers to B220 and FITC + Alexa 594+ Area refers to CD3 and B220). The data, displayed as graphs, are shown below the sections. (b) Splenic sections were stained with antibodies ERTR9 and anti‐MOMA‐1, which recognized marginal zone macrophages (MZM; in red) and marginal metallophilic macrophages (MMM; in green), respectively. The distinct demarcation of T and B cells within the splenic white pulp is lost with increasing age, which coincides with alterations in the marginal zone. Magnification ×100. Data representative of four experiments. *P < 0·05; **P < 0·01.
Splenic stromal cell organization is altered with age
As stromal cells provide the foundation for splenic organization, the stromal cells that contribute to this splenic scaffolding were investigated. The arrangement of the T‐cell and B‐cell zone stroma was ascertained by staining sections for gp38 (podoplanin; in red) and CD157 (BP‐3; in green), respectively (Fig. 2a). In young mice, the stromal cells in the T‐cell zone, characterized by gp38 staining, are centred around the PALS. However, with increasing age the T‐cell area becomes significantly larger, as determined by imageJ analysis. In contrast, the B‐cell zone remained unchanged with age. The ER‐TR7 antibody identifies the FRC network which encapsulates the white pulp, but is also present within the PALS.32 This staining pattern can be seen in the spleens of young mice (Fig. 2b); however, this network becomes denser around the marginal zone region with increasing age, implying an accumulation of fibroblast in this area (Fig. 2b). We observed that follicular dendritic cells (FDC), as identified by FDC‐M2 antibody, are arranged in tight clusters within the white pulp from spleens of young mice while in older mice they become scattered and more diffuse (Fig. 2c). Collectively, these observations support the findings that there are age‐related changes in the distribution of cells and the cellular network in the spleens of mice.
Figure 2.
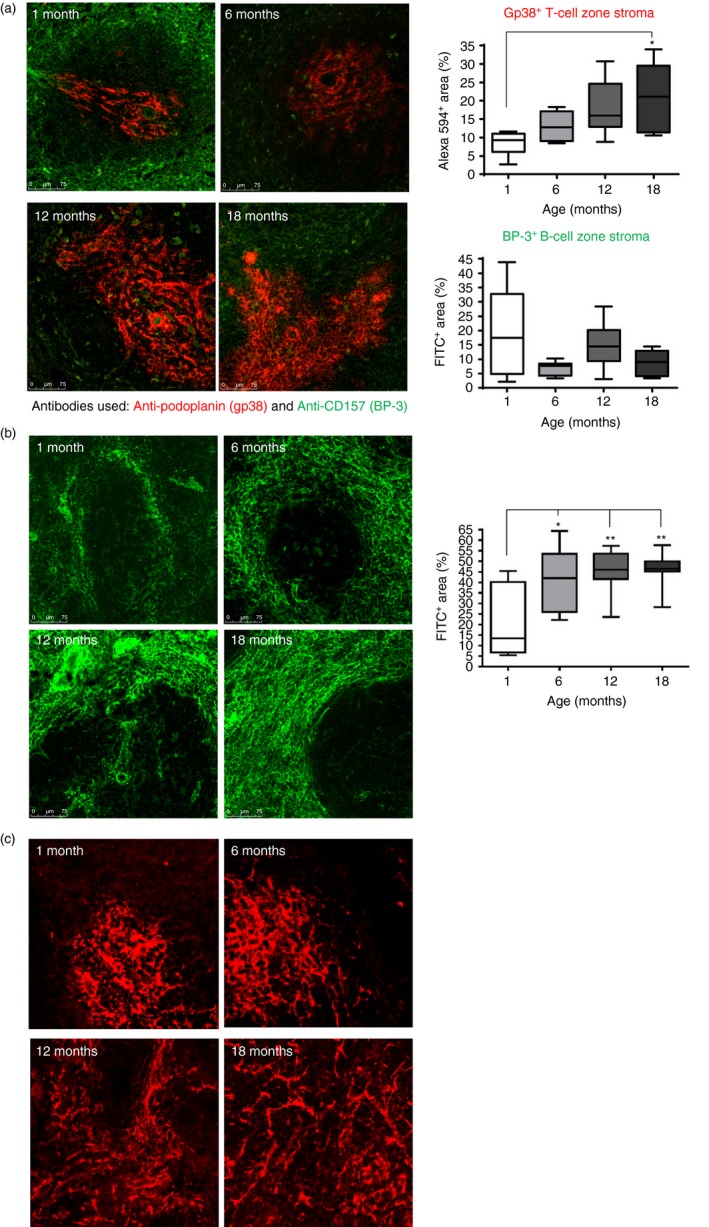
Stromal changes within spleens of young and old mice. (a) Sections were stained with the anti‐gp38 (podoplanin; in red), which detects the T‐cell stroma, and anti‐CD157 (BP‐3), which detects the B‐cell stroma, (shown in green). The level of staining was assessed by imageJ analysis (graphs on the right hand side) and revealed an increase in the T‐cell stromal area. Magnification ×100. (b) Staining for the fibroblastic reticular cell (FRC) with ERTR7 antibody revealed an increased deposition of FDC around the marginal zone in the splenic sections from older mice. Magnification ×100. (c) Staining for follicular dendritic cells (FDC) using anti‐FDC‐M2 revealed that the network of FDC appears more dispersed in the splenic sections from older mice Magnification ×200. Isotype controls revealed no staining (data not shown). Data representative of four experiments. *P < 0·05; **P < 0·01.
Splenic remodelling in young and aged mice following an immune response to a T‐independent and T‐dependent antigen
Changes in the splenic microanatomy are often seen during an immune challenge and are believed to be necessary to facilitate an efficient immune response.16 Given our observations showing disorganization of the splenic architecture in older mice, we therefore examined the ability of immune responses to alter the microanatomy of this secondary lymphoid organ by challenging young (1 month of age) and older (18 months of age) mice with either a T‐independent antigen (TNP–Ficoll) or T‐dependent antigen (TNP–KLH) and subsequently examined the splenic structure.
Both young and older mice demonstrated an increase in IgM serum responses during the time course following immunization with TNP–Ficoll (Fig. 3a). However, basal levels of IgM in older mice appeared significantly higher before challenge, which continued until day 7, when induced IgM production peaked in both cohorts. Both young and older mice show disorganization of T and B cells within the white pulp at day 3 after immunization, which appears to be resolved in the spleens of young mice at day 28, but is still apparent in older animals (Fig. 3b). Examination of the splenic marginal zone microarchitecture, in particular MZM and MMM, reveals alterations in the spleens of immunized young mice, notable being the appearance of enhanced clustering of MMM around the marginal zone, and the infiltration into the white pulp. In contrast, such reorganization, as judged by the pattern of MZM and MMM staining, appears less prominent within the spleens of immunized old mice (Fig. 3b).
Figure 3.
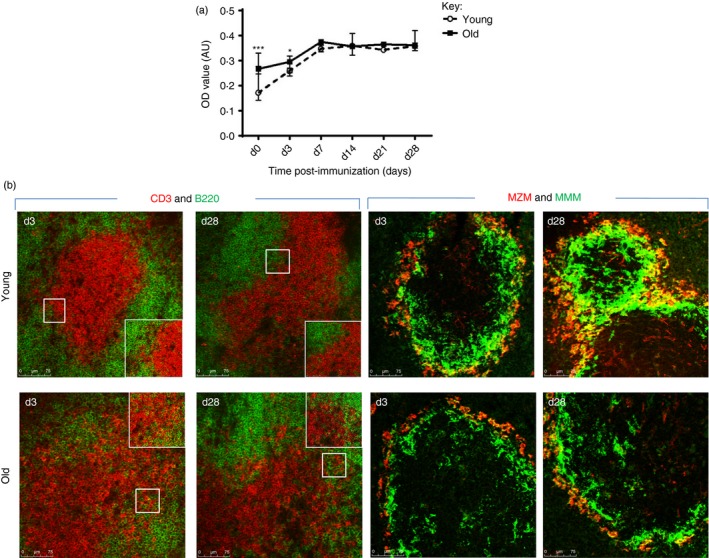
Architectural changes in the splenic microenvironment following immunization with a T‐independent antigen. (a) Blood was taken from young (1 month) and old (18 months) mice at days 0, 3, 7, 14, 21 and 28 after immunization with trintriphenyl (TNP) –Ficoll and the specific anti‐IgM TNP–Ficoll response was determined by ELISA. Both young and old mice show a similar increase in the IgM response after immunization, although the level of the anti‐IgM TNP–Ficoll antibody is initially higher at days 0 and 3 in older animals. Six mice were used for each time‐point. *P < 0·05; ***P < 0·001. Data representative of three experiments. (b) Splenic sections of young and old mice were stained to detect T (red) and B (green) cells and marginal zone macrophages (MZM; red) and marginal metallophilic macrophages (MMM; green). Following immunization, the distinct demarcation of the T‐ and B‐cell zone of both age groups is disrupted (day 3), which is resolved by day 28 in young mice, but is still disorganized in the spleen of old mice. Magnification ×100. Insert of an area highlighted by a white box is shown representing a higher magnification (×200). In the spleen of young mice MMM and MZM encircle the marginal zone, which is incomplete, despite the appearance of MZM, in old animals. Magnification ×100. Isotype controls revealed no staining (data not shown). Data representative of four experiments.
Following challenge with TNP–KLH, IgG increased in both cohorts of mice and similar to the TNP–Ficoll IgM response, older mice exhibited a higher basal level of IgG (Fig. 4). The response peaked at day 7 after immunization and then plateaued; however, after another immunization at day 28, the IgG level rose further in both age cohorts. Examination of the white pulp area from both age groups 14 days post‐immunization revealed a greater degree of disorganization of T and B cells in the spleens of old mice, which is also apparent 8 days after secondary immunization; indicating a failure of the spleens in older mice to undergo resolution following antigenic challenge. Similarly, the MZM and MMM appeared not to fully encircle the marginal zone in older mice.
Figure 4.
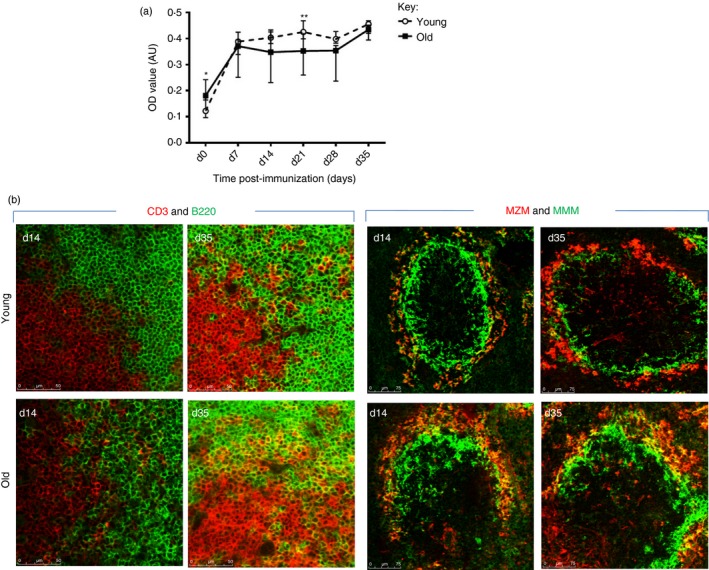
Alterations in the splenic microenvironment following immunization with a T‐cell‐dependent antigen. (a) Young (1 month) and old (18 months) mice were immunized with trinitrophenyl–keyhole limpet haemocyanin (TNP–KLH) at day 0 and day 28, and blood was taken from young (1 month) and old (18 months) mice at days; 0, 7, 14, 21, 28 and 35 after immunization. Level of anti‐IgG TNP–KLH antibody increases after immunization in both age groups; young mice show a higher level at day 21. Although older mice showed a higher level before immunization. Six mice were used for each time‐point. *P < 0·05; **P < 0·01. Data representative of three experiments. (b) Splenic sections of young and old mice were stained with anti‐CD3 (green) and anti‐B220 (red) and revealed disruption of the T‐cell and B‐cell zone in both age groups, although it was more apparent in the sections from older mice. Sections stained with marginal zone macrophages (MZM; red) and marginal metallophilic macrophages (MMM; green) reveals that in the spleens of young mice MMM and MZM encircle the marginal zone, which is incomplete, despite the appearance of MZM, in old animals. Magnification ×100. Isotype controls revealed no staining (data not shown). Data representative of four experiments.
Discussion
Immunosenescence is characterized by an age‐related decline in immune function and, although the precise mechanism is not known, evidence points to an accumulation of both intrinsic and extrinsic defects.1, 2, 3, 4, 5 The microenvironment plays an essential role in supporting and facilitating the development and activation of immune cells15, 25 and although many have observed significant alterations of the thymic architecture in the aged,33 much less is known about the impact of age on the structure and integrity of secondary lymphoid organs (SLO). In this study we aimed to address this deficiency and identified clear age‐related alterations of the splenic architecture.
We observed that the delineation between the T‐cell and B‐cell zones became less distinct with increasing age, and T cells appeared to be present in B‐cell regions. This loss of the demarcation between the T‐cell and B‐cell area is also seen in the SLO from various mouse models that exhibit altered chemokine expression.16, 26 Primarily, the chemokine CXCL13 is required for the organization and maintenance of the B‐cell area,34 whereas CCL19 and CCL21 are needed to perform a similar role in the T‐cell region.35 Moreover, these chemokines play an instrumental role in orchestrating immune responses in SLO.16, 26 Interestingly, recent studies have shown altered patterning of CXCL1336, 37 and CCL2137 expression in the aged mouse spleen together with reduced splenic protein content of CCL21 and CCL19.37 Hence, these reports could offer an explanation for the observed dissolution between the T‐cell and B‐cell boundaries. Furthermore, in adoptive transfer experiments, the dysregulation of these chemokines may account for the impaired recruitment of young T cells37 and B cells36 in the spleens of aged mice. Hence, a consequence of lymphocytes being out of position is the inability to forge key physical interactions16, 26 with the likely scenario of generating reduced immune responses within the aged SLO. Moreover, these studies highlight that the microenvironment in ageing SLO can potentially contribute towards peripheral immunosenescence.17
The stromal network represents key components to the functional activity of SLO and we noted that the stromal network undergoes dramatic changes with age. In particular, the enlargement of the T‐cell zone stroma, which may account for the loss of the distinct boundary between the T‐cell and B‐cell areas. Similarly, the microarchitecture of the marginal zone region appears to be disrupted in the aged spleen, as judged by the organization and distribution of MZM and MMM. This could be attributed to the disappearance of MZM and MMM or the loss of the antibody epitope that identifies these cell types. The alteration of the marginal zone region could be caused by changes in CCL19, CCL21 or CXCL13 expression as these chemokines have been shown to play a key role in the organization of this area.26 Lymphoid tissue inducer cells may also regulate the organization of the marginal zone as they control chemokine expression levels by lymphotoxin‐β receptor signalling.38 Disruption of the marginal zone sinus‐lining may also account for the altered distribution of MZM and MMM. Indeed, the marginal zone sinus‐lining cells. as detected by the expression of mucosal addressin cell adhesion molecule 1, has been shown to be less intact in the aged spleen.39, 40 Furthermore, sphingosine‐1‐phosphate receptor 3 deficient mice display a disorganized marginal zone region and exhibit a reduced T‐independent response;41 highlighting the importance of an intact region. Moreover, this region is not only important as the entry and exit point of leucocyte trafficking, but also the site where antigens are sequestered. Hence, the age‐associated disorganization of the marginal zone could impact on the ability of the spleen to mount an immune response, as shown by the recent report showing reduced binding of dextran, administered intravenously, within the marginal zone of spleen from old mice in comparison to young.39
The suggestion that the aged spleen has a reduced ability to capture antigen could also be extended to FDC. Using FDC‐M2 antibody, we observed that the FDC in young mice were arranged in tight clusters, whereas in old mice their organization appeared diffuse and scattered. Interestingly, FDC‐M2 labelling has been shown to specifically reflect deposition of immune complexes associated with complement activation.42 Additionally, the authors observed that old FDC, as assessed by flow cytometry, have lower levels of FcγRII – a key molecule involved in capturing immune complexes and also demonstrated that such cells from aged animals exhibit reduced co‐stimulatory activity.43
Immune responses are often accompanied by remodelling of secondary lymphoid tissue, which subsequently resolved.16 Our results of immunizing young and old mice revealed that although the spleen from young animals showed resolution after immunization, the spleen from the older cohort remained disorganized. The higher basal level of antibody reactivity that was observed in old mice may be due to the age‐associated increase in autoantibody production.44 Interestingly, studies have shown that some parasitic45, 46 and viral47, 48 infections can directly cause significant disruption of the splenic architecture, resulting in the inability of the host to mount a robust immune response, suggesting that this may be a potential mechanism used by pathogens to reduce immune efficacy and increase pathogenesis. Hence, the age‐associated disruption of the splenic architecture may reflect the accumulation of remodelling due to the continual antigenic challenge of the host, which over time might impact on the structural integrity of SLO. A consequence of such continual remodelling is the likely increase in fibrosis that may also account for the altered patterning of FRC, as judged by ERTR7 staining, in the aged spleen. These cells have been shown to be a target for viral infections altering their functions49 and in some instances displaying immunosuppressive properties.48 Furthermore, aged stromal cells may also contribute to the ‘inflamm‐ageing’ process itself,1 as a recent study showed that splenic stromal cells from ageing mice produce higher amounts of interleukin‐6 in comparison to young animals.50 Interestingly, the production of pro‐inflammatory cytokines along with the secretion of growth factors, chemokines and proteases represent important characteristics of senescent cells. Such cells are referred to as having a senescence‐associated secretory phenotype51 and their accumulation, which occurs with age, has been postulated to cause alteration in tissue architecture, impair tissue homeostasis; contributing towards pathophysiology.51, 52 Furthermore, the expression of p16, which promotes cellular senescence, has been shown to be up‐regulated in the spleens of ageing mice.53 Hence the ageing process itself may contribute towards the alteration of tissue architecture.
Corroboratively, the studies highlighting splenic remodelling and the efficacy of the immune response,46, 47, 48 indicate a correlation between the structural integrity of the splenic microenvironment and immune competency.16 As previously mentioned, adoptive transfer experiments demonstrated altered trafficking of young lymphocytes within the aged spleen.36, 37 Moreover, in adoptive transfer experiments, young anti‐virus‐specific transgenic CD8 T cells fail to clonally expand in aged spleen independently of antigen‐presenting activity.54 In contrast, it was recently demonstrated that the inability of aged mice to develop transmissible spongiform encephalopathy was the result of disruption of the splenic marginal zone microarchitecture, which led to reduced accumulation of the transmissible spongiform encephalopathy agent in the spleen; thereby limiting disease progression.40
In summary, our study shows that the splenic architecture undergoes age‐associated changes, which is consistent with similar observations that have been reported in rats55, 56 and humans, and suggests that such alterations might impact on immune activity and therefore contribute towards peripheral immunosenescence and may also offer a potential target for rejuvenating the immune system.
Disclosures
The authors have no financial or commercial conflicts of interest.
Author contributions
DA, LH, YN, ETC and RL performed the experiments. DA, RL and DBP designed the experiments. DA, LH, RL and DBP wrote the paper.
Acknowledgements
DA is supported by Research into Ageing (AgeUK) and by a grant (BB/D013550/1) from the Biotechnology and Biological Sciences Research Council (BBSRC).
References
- 1. Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology 2007; 120:435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Haynes L, Swain SL. Why aging T cells fail: implications for vaccination. Immunity 2006; 24:663–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pawelec G, Akbar A, Caruso C, Solana R, Grubeck‐Loebenstein B, Wikby A. Human immunosenescence: is it infectious? Immunol Rev 2005; 205:257–68. [DOI] [PubMed] [Google Scholar]
- 4. Chen WH, Kozlovsky BF, Effros RB, Grubeck‐Loebenstein B, Edelman R, Sztein MB. Vaccination in the elderly: an immunological perspective. Trends Immunol 2009; 30:351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gardner ID. The effect of aging on susceptibility to infection. Rev Infect Dis 1980; 2:801–10. [DOI] [PubMed] [Google Scholar]
- 6. McElhaney JE, Zhou X, Talbot HK, Soethout E, Bleackley RC, Granville DJ et al The unmet need in the elderly: how immunosenescence, CMV infection, co‐morbidities and frailty are a challenge for the development of more effective influenza vaccines. Vaccine 2012; 30:2060–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Akbar AN, Henson SM. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat Rev Immunol 2011; 11:289–95. [DOI] [PubMed] [Google Scholar]
- 8. Goronzy JJ, Weyand CM. T cell development and receptor diversity during aging. Curr Opin Immunol 2005; 17:468–75. [DOI] [PubMed] [Google Scholar]
- 9. Cancro MP, Hao Y, Scholz JL, Riley RL, Frasca D, Dunn‐Walters DK et al B cells and aging: molecules and mechanisms. Trends Immunol 2009; 30:313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 2006; 24:1159–69. [DOI] [PubMed] [Google Scholar]
- 11. Kovacs EJ, Palmer JL, Fortin CF, Fulop T Jr, Goldstein DR, Linton PJ. Aging and innate immunity in the mouse: impact of intrinsic and extrinsic factors. Trends Immunol 2009; 30:319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shaw AC, Joshi S, Greenwood H, Panda A, Lord JM. Aging of the innate immune system. Curr Opin Immunol 2010; 22:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hayhoe RP, Henson SM, Akbar AN, Palmer DB. Variation of human natural killer cell phenotypes with age: identification of a unique KLRG1‐negative subset. Hum Immunol 2010; 71:676–81. [DOI] [PubMed] [Google Scholar]
- 14. Gayoso I, Sanchez‐Correa B, Campos C, Alonso C, Pera A, Casado JG et al Immunosenescence of human natural killer cells. J Innate Immun 2011; 3:337–43. [DOI] [PubMed] [Google Scholar]
- 15. Anderson G, Jenkinson EJ. Lymphostromal interactions in thymic development and function. Nat Rev Immunol 2001; 1:31–40. [DOI] [PubMed] [Google Scholar]
- 16. Junt T, Scandella E, Ludewig B. Form follows function: lymphoid tissue microarchitecture in antimicrobial immune defence. Nat Rev Immunol 2008; 8:764–75. [DOI] [PubMed] [Google Scholar]
- 17. Su DM, Aw D, Palmer DB. Immunosenescence: a product of the environment? Curr Opin Immunol 2013; 25:498–503. [DOI] [PubMed] [Google Scholar]
- 18. Aw D, Silva AB, Maddick M, von Zglinicki T, Palmer DB. Architectural changes in the thymus of aging mice. Aging Cell 2008; 7:158–67. [DOI] [PubMed] [Google Scholar]
- 19. Aw D, Taylor‐Brown F, Cooper K, Palmer DB. Phenotypical and morphological changes in the thymic microenvironment from ageing mice. Biogerontology 2009; 10:311–22. [DOI] [PubMed] [Google Scholar]
- 20. Gray DH, Seach N, Ueno T, Milton MK, Liston A, Lew AM et al Developmental kinetics, turnover, and stimulatory capacity of thymic epithelial cells. Blood 2006; 108:3777–85. [DOI] [PubMed] [Google Scholar]
- 21. Steinmann GG, Klaus B, Muller‐Hermelink HK. The involution of the ageing human thymic epithelium is independent of puberty. A morphometric study. Scand J Immunol 1985; 22:563–75. [DOI] [PubMed] [Google Scholar]
- 22. Andrew D, Aspinall R. Age‐associated thymic atrophy is linked to a decline in IL‐7 production. Exp Gerontol 2002; 37:455–63. [DOI] [PubMed] [Google Scholar]
- 23. Zhu X, Gui J, Dohkan J, Cheng L, Barnes PF, Su DM. Lymphohematopoietic progenitors do not have a synchronized defect with age‐related thymic involution. Aging Cell 2007; 6:663–72. [DOI] [PubMed] [Google Scholar]
- 24. Gui J, Zhu X, Dohkan J, Cheng L, Barnes PF, Su DM. The aged thymus shows normal recruitment of lymphohematopoietic progenitors but has defects in thymic epithelial cells. Int Immunol 2007; 19:1201–11. [DOI] [PubMed] [Google Scholar]
- 25. Cesta MF. Normal structure, function, and histology of mucosa‐associated lymphoid tissue. Toxicol Pathol 2006; 34:599–608. [DOI] [PubMed] [Google Scholar]
- 26. Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol 2005; 5:606–16. [DOI] [PubMed] [Google Scholar]
- 27. Brendolan A, Rosado MM, Carsetti R, Selleri L, Dear TN. Development and function of the mammalian spleen. BioEssays 2007; 29:166–77. [DOI] [PubMed] [Google Scholar]
- 28. Martin F, Kearney JF. Marginal‐zone B cells. Nat Rev Immunol 2002; 2:323–35. [DOI] [PubMed] [Google Scholar]
- 29. Balogh P, Horvath G, Szakal AK. Immunoarchitecture of distinct reticular fibroblastic domains in the white pulp of mouse spleen. J Histochem Cytochem 2004; 52:1287–98. [DOI] [PubMed] [Google Scholar]
- 30. Karrer U, Althage A, Odermatt B, Hengartner H, Zinkernagel RM. Immunodeficiency of alymphoplasia mice (aly/aly) in vivo: structural defect of secondary lymphoid organs and functional B cell defect. Eur J Immunol 2000; 30:2799–807. [DOI] [PubMed] [Google Scholar]
- 31. Gaspal FM, McConnell FM, Kim MY, Gray D, Kosco‐Vilbois MH, Raykundalia CR et al The generation of thymus‐independent germinal centers depends on CD40 but not on CD154, the T cell‐derived CD40‐ligand. Eur J Immunol 2006; 36:1665–73. [DOI] [PubMed] [Google Scholar]
- 32. Bajenoff M, Glaichenhaus N, Germain RN. Fibroblastic reticular cells guide T lymphocyte entry into and migration within the splenic T cell zone. J Immunol 2008; 181:3947–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Palmer DB. The effect of age on thymic function. Front Immunol 2013; 4:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ansel KM, Ngo VN, Hyman PL, Luther SA, Förster R, Sedgwick JD et al A chemokine‐driven positive feedback loop organizes lymphoid follicles. Nature 2000; 406:309–14. [DOI] [PubMed] [Google Scholar]
- 35. Gunn MD, Kyuwa S, Tam C, Kakiuchi T, Matsuzawa A, Williams LT et al Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J Exp Med 1999; 189:451–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wols HA, Johnson KM, Ippolito JA, Birjandi SZ, Su Y, Le PT et al Migration of immature and mature B cells in the aged microenvironment. Immunology 2010; 129:278–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lefebvre JS, Maue AC, Eaton SM, Lanthier PA, Tighe M, Haynes L. The aged microenvironment contributes to the age‐related functional defects of CD4 T cells in mice. Aging Cell 2012; 11:732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Withers DR, Kim MY, Bekiaris V, Rossi SW, Jenkinson WE, Gaspal F et al The role of lymphoid tissue inducer cells in splenic white pulp development. Eur J Immunol 2007; 37:3240–5. [DOI] [PubMed] [Google Scholar]
- 39. Birjandi SZ, Ippolito JA, Ramadorai AK, Witte PL. Alterations in marginal zone macrophages and marginal zone B cells in old mice. J Immunol 2011; 186:3441–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brown KL, Gossner A, Mok S, Mabbott NA. The effects of host age on the transport of complement‐bound complexes to the spleen and the pathogenesis of intravenous scrapie infection. J Virol 2012; 86:25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Girkontaite I, Sakk V, Wagner M, Borggrefe T, Tedford K, Chun J et al The sphingosine‐1‐phosphate (S1P) lysophospholipid receptor S1P3 regulates MAdCAM‐1+ endothelial cells in splenic marginal sinus organization. J Exp Med 2004; 200:1491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Taylor PR, Pickering MC, Kosco‐Vilbois MH, Walport MJ, Botto M, Gordon S et al The follicular dendritic cell restricted epitope, FDC‐M2, is complement C4; localization of immune complexes in mouse tissues. Eur J Immunol 2002; 32:1888–96. [DOI] [PubMed] [Google Scholar]
- 43. Aydar Y, Balogh P, Tew JG, Szakal AK. Follicular dendritic cells in aging, a “bottle‐neck” in the humoral immune response. Ageing Res Rev 2004; 3:15–29. [DOI] [PubMed] [Google Scholar]
- 44. Zhao KS, Wang YF, Gueret R, Weksler ME. Dysregulation of the humoral immune response in old mice. Int Immunol 1995; 7:929–34. [DOI] [PubMed] [Google Scholar]
- 45. Cadman ET, Abdallah AY, Voisine C, Sponaas AM, Corran P, Lamb T et al Alterations of splenic architecture in malaria are induced independently of Toll‐like receptors 2, 4, and 9 or MyD88 and may affect antibody affinity. Infect Immun 2008; 76:3924–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Martin‐Jaular L, Ferrer M, Calvo M, Rosanas‐Urgell A, Kalko S, Graewe S et al Strain‐specific spleen remodelling in Plasmodium yoelii infections in Balb/c mice facilitates adherence and spleen macrophage‐clearance escape. Cell Microbiol 2011; 13:109–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Benedict CA, De Trez C, Schneider K, Ha S, Patterson G, Ware CF. Specific remodeling of splenic architecture by cytomegalovirus. PLoS Pathog 2006; 2:e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mueller SN, Matloubian M, Clemens DM, Sharpe AH, Freeman GJ, Gangappa S et al Viral targeting of fibroblastic reticular cells contributes to immunosuppression and persistence during chronic infection. Proc Natl Acad Sci USA 2007; 104:15430–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fletcher AL, Acton SE, Knoblich K. Lymph node fibroblastic reticular cells in health and disease. Nat Rev Immunol 2015; 15:350–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Park J, Miyakawa T, Shiokawa A, Nakajima‐Adachi H, Tanokura M, Hachimura S. Splenic stromal cells from aged mice produce higher levels of IL‐6 compared to young mice. Mediators Inflamm 2014; 2014:826987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Campisi J, Andersen JK, Kapahi P, Melov S. Cellular senescence: a link between cancer and age‐related degenerative disease? Semin Cancer Biol 2011; 21:354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. van Deursen JM. The role of senescent cells in ageing. Nature 2014; 509:439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al‐Regaiey K, Su L et al Ink4a/Arf expression is a biomarker of aging. J Clin Investig 2004; 114:1299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jiang J, Bennett AJ, Fisher E, Williams‐Bey Y, Shen H, Murasko DM. Limited expansion of virus‐specific CD8 T cells in the aged environment. Mech Ageing Dev 2009; 130:713–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cheung HT, Nadakavukaren MJ. Age‐dependent changes in the cellularity and ultrastructure of the spleen of Fischer F344 rats. Mech Ageing Dev 1983; 22:23–33. [DOI] [PubMed] [Google Scholar]
- 56. Pahlavani MA, Richardson A, Cheung HT. Age‐dependent changes of the mesenteric lymph node of Fischer F344 rats: morphological and histometric analysis. Mech Ageing Dev 1987; 39:137–46. [DOI] [PubMed] [Google Scholar]


