Summary
Mucosal‐associated invariant T (MAIT) cells are a novel class of innate‐like T cells, expressing a semi‐invariant T‐cell receptor (TCR) and able to recognize small molecules presented on the non‐polymorphic MHC‐related protein 1. Their intrinsic effector‐memory phenotype, enabling secretion of pro‐inflammatory cytokines, and their relative abundance in humans imply a significant potential to contribute to autoimmune processes. However, as MAIT cells were unknown until recently and specific immunological tools were unavailable, little is known of their roles in disease. Here I review observations from clinical studies and animal models of autoimmune and immune‐mediated diseases including the roles of MAIT cells in systemic lupus erythematosus, rheumatoid arthritis, multiple sclerosis, inflammatory bowel disease and airways diseases. MAIT cell deficiencies are frequently observed in peripheral blood, and at sites of disease such as the airways in asthma. However, MAIT cells have a specific sensitivity to suppression by therapeutic corticosteroids that may confound many of these observations, as may the tendency of the surface marker CD161 to activation‐induced down‐regulation. Nonetheless, the dependence on bacteria for the development of MAIT cells suggests a potentially important protective role linking the influences of early life microbial exposures and subsequent development of autoimmunity. Conversely, MAIT cells could contribute to chronic inflammation either through TCR‐independent activation, or potentially by TCR recognition of as yet undiscovered ligands. Future research will be greatly facilitated by the immunological tools that are now available, including murine genetic models and human and murine specific tetramers.
Keywords: autoimmunity, inflammation, lung, mucosal, T cells
Abbreviations
- 5‐OP‐RU
5‐(2‐oxopropylideneamino)‐6‐d‐ribityllumazine
- CIA
collagen‐induced arthritis
- COPD
chronic obstructive pulmonary disease
- EAE
experimental autoimmune encephalomyelitis
- HLA
human leucocyte antigen
- ICS
inhaled corticosteroids
- IFN
interferon
- IL
interleukin
- iNKT
invariant natural killer T
- MAIT
mucosal‐associated invariant T
- MR1
MHC‐related protein 1
- MS
multiple sclerosis
- PLZF
promyelocytic leukaemia zinc finger protein
- RhA
rheumatoid arthritis
- SLE
systemic lupus erythematosus
- TCR
T‐cell receptor
- Th17
T helper type 17
- TNF
tissue necrosis factor
- XIAP
X‐linked inhibitor of apoptosis
- XLP
X‐linked lymphoproliferative syndrome
Introduction
Mucosal‐associated invariant T (MAIT) cells are a subset of innate‐like T lymphocytes first described in 1999,1 which are abundant in humans and can rapidly express a range of pro‐inflammatory cytokines. Although MAIT cells express an αβ T‐cell receptor (TCR) they differ from conventional T cells in that this receptor has a limited TCR diversity, mostly comprising a semi‐invariant TCR‐α chain associated with a limited repertoire of TCR‐β chains (Box 1). Furthermore MAIT cells are restricted not by MHC, but by the non‐polymorphic class 1b antigen‐presenting molecule MHC‐related protein 1 (MR1).2, 3 Ligands for MAIT cells remained elusive until the recent demonstration by Kjer‐Nielsen et al. that MR1 presented non‐protein antigens, which include precursors and derivatives from highly conserved biosynthetic pathways of riboflavin and folic acid metabolism in bacteria, mycobacteria and yeast.4, 5, 6 To date, only these limited classes of ligands – most importantly the naturally occurring activating ligand 5‐(2‐oxopropylideneamino)‐6‐d‐ribityllumazine (5‐OP‐RU)7 – have been identified for MR1 and it remains to be seen whether other classes of ligand exist.
Box 1. MAIT cell TCR–MR1 recognition.
As with other innate‐like lymphocytes, MAIT cells express a semi‐invariant TCR. Most MAIT cell TCRs comprise a semi‐invariant TCR‐α chain – usually TRAV1‐2‐TRAJ33 (Vα7.2− Jα33 in humans, Vα19− Jα33 in mice),90 although in humans some MAIT cells also use TRAV1‐2‐TRAJ12 or TRAV1‐2‐TRAJ2022) – predominantly associated with the β‐chains TRBV20 (Vβ2) or TRBV6 (Vβ13) in humans1 and TRBV19 (Vβ6) or TRBV13 (Vβ8) in mice.1, 22, 23 These preferential TCR rearrangements arise partly through a process of ‘convergent recombination’91 whereby much of the antigen specificity of MAIT cells is essentially germline‐encoded. This feature, alongside the striking evolutionary conservation of MR1 across over 150 million years of mammalian evolution,3, 92, 93, 94, 95 imply a strong evolutionary pressure maintaining the MAIT cell repertoire, and hence some indispensible role in host defence. This limited diversity of MAIT‐TCR, and the abundance of MAIT cells in humans means that, early in an immune response MAIT cells may markedly outnumber responses from conventional peptide‐specific αβ T cells.14 Abbreviations: MAIT, mucosal‐associated invariant T; MR1, MHC‐related protein 1; TCR, T‐cell receptor; TRAV, TCR‐α chain variable region; TRVB, TCR‐β chain variable region.
Currently, although there is a growing understanding of the role of MAIT cells in host protection from intracellular pathogens8, 9, 10, 11, 12, 13 (Fig. 1), very little is known concerning the roles that these cells play in disease. Several features suggest potential relevance to immune‐mediated pathology. MAIT cells display an intrinsic effector‐memory phenotype – i.e. without the need for prior clonal expansion14 – typically CD45RA− CD45RO+ CD95HiCD62LLo CD44Hi 2, 15, 16, 17 – and can rapidly secrete a range of pro‐inflammatory cytokines including tissue necrosis factor‐α (TNF‐α), interleukin‐17 (IL‐17) and interferon‐γ (IFN‐γ), and also the type 2 cytokine IL‐4 on TCR ligation.15, 17 MAIT cells share some similarities with invariant natural killer T (iNKT) cells, which are implicated in many autoimmune conditions,18, 19 including expression of a semi‐invariant TCR, restriction by non‐classical MHC molecules, and expression of the transcription factor promyelocytic leukaemia zinc finger protein (PLZF)20 although many differences exist, notably the nature of the ligands and the restriction molecule. Other significant features are the remarkable abundance of MAIT cells, which comprise approximately 5% of T cells in peripheral blood11, 17 and 20–40% of liver T cells in humans,15, 21 and their wide tissue distribution in blood, mucosal tissues, liver and joints.15, 19, 22, 23, 24, 25 A peculiarity of MAIT cell biology is that although MR1 expression is ubiquitous,3, 26 it is normally found only at very low levels on the cell surface.26, 27, 28 This has led to speculation that if other classes of MAIT cell ligand exist, they might include autologous molecules whose presentation at the cell surface occurs only when MR1 surface expression is up‐regulated as a signal of cell stress,2, 27, 29 as occurs with other class 1b molecules.14, 30 Inappropriate triggering of such a system would be expected to lead to immune pathology.
Figure 1.
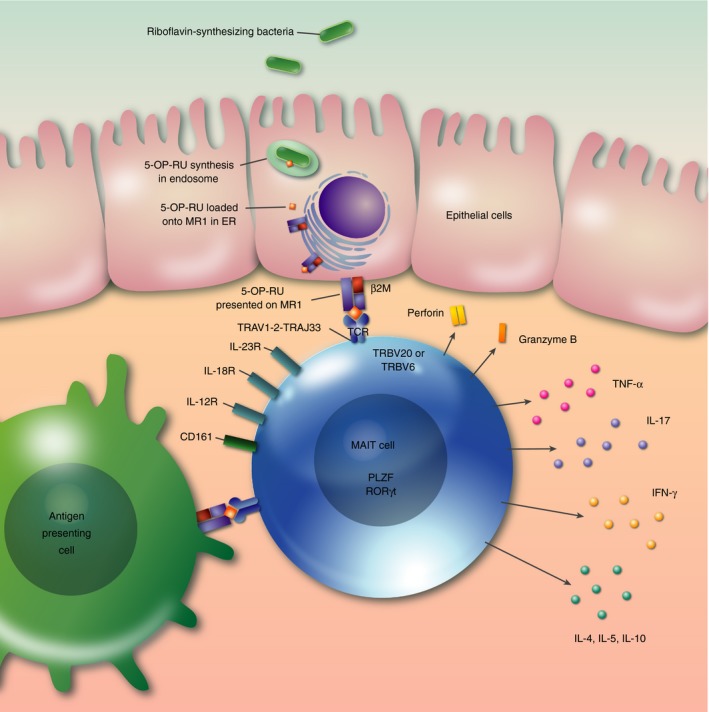
Over view of human MAIT cell biology. Invasive bacteria capable of synthesizing riboflavin enter epithelial cells. Intermediate products of riboflavin synthesis such as 5‐OP‐RU synthesized in the endosome, or potentially delivered by endocytosis, are loaded onto MR1 in the endoplasmic reticulum and transported to the cell surface via the Golgi apparatus. At the surface of infected cells, or antigen‐presenting cells, ligand is presented to the MAIT cell invariant T‐cell receptor, which is predominantly composed of TRAV1‐2‐TRAJ33 α chains assembled with TRBV20 or TRBV6 chains. Activation leads to release of perforin and granzyme B, which may directly lyse infected cells, and pro‐inflammatory cytokines including TNF‐α, IFN‐γ, IL‐17 as well as other cytokines, which may include the type 2 cytokines IL‐4, IL‐5 or the immunoregulatory cytokine IL‐10. MAIT cells typically express the innate lymphocyte transcription factors PLZF and ROR γt and the surface molecules CD161, IL‐12R, IL‐18R and IL‐23R. Abbreviations: 5‐OP‐RU, 5‐(2‐oxopropylideneamino)‐6‐d‐ribityllumazine; β2M, β 2‐microglobulin; CD, cluster of differentiation; ER, endoplasmic reticulum; IFN, interferon; IL, interleukin; MAIT, mucosal‐associated invariant T; MR1, MHC‐related protein 1; PLZF promyelocytic leukaemia zinc finger protein; ROR γt, retinoic acid receptor‐related orphan receptor γ t; TCR, T‐cell receptor; TNF‐α, tumour necrosis factor‐α; TRAV, TCR‐α chain variable region; TRVB, TCR‐β chain variable region.
Nonetheless, to date, data regarding MAIT cells in immune‐mediated disease are scant, at least partly because MAIT cells were unknown until recently and specific immunological tools, such as relevant antibodies, transgenic models16, 24, 31 and specific tetramers for humans7, 22 and mice,17 were unavailable. Furthermore, because of the limited diversity of the MAIT TCR and the non‐polymorphic, non‐human leucocyte antigen (HLA) encoded nature of MR1, it is unlikely that there will be pathological autoreactive MAIT cells leading directly to HLA‐associated diseases meeting the stringent definition of a classic autoimmune disease.32 Instead, in this paper I will review observational data of MAIT frequency and function in human immune‐mediated diseases, alongside mechanistic data from relevant murine models. These studies are summarized in Table 1. I will then discuss the relevance of corticosteroids and receptor down‐regulation to these studies, the potential of MAIT cells to act as non‐specific effectors of inflammation, and speculate on their relevance in early life origins of immune disease and some potential therapeutic implications.
Table 1.
Human and murine studies on MAIT cells in immune‐mediated disease
| Condition | Human | Mouse | Comment | References | ||||
|---|---|---|---|---|---|---|---|---|
| Blood MAIT frequencies | Tissue MAIT frequencies | Confounding by steroids? | Model | MAIT augmentation | MAIT depletion | |||
| Rheumatological diseases | ||||||||
| Systemic lupus erythematosus | ↓ correlating with disease activity | Yes | CIA and CAIA | ↑ severity after adoptive MAIT transfer | ↓ severity in MR1−/− | MAIT cells are likely effector cells | Cho et al.19 | |
| ↓ IFN‐γ‐secreting MAIT | Chiba et al.40 | |||||||
| ↑ PD1 expression | ||||||||
| Rheumatoid arthritis | ↑ correlating with disease activity | ↑ in synovium in disease | Yes | |||||
| Multiple sclerosis | ↓ especially in relapse, improving with remission | Present in inflammatory lesions in MS and CIDP | Yes | EAE | ↓ incidence and severity in Vα19i TG mice | ↑ severity in MR1−/−, with ↑ inflammatory cytokines and ↓ IL‐10 | Murine data suggest MAIT cells protective, but data confounded by ‘MAIT‐like’ cells | Illes et al.47 |
| ↓ pathology by adoptive transfer of Vα19i cells | Miyazaki et al., 49 Croxford et al.50 | |||||||
| Inflammatory bowel disease | ||||||||
| Crohn's | ↓ with MAIT cell activation | ↑ in ileal tissue in disease | Yes | TNBS | ↑ severity after adoptive MAIT | Murine data confounded by non‐ MAIT conventional T cells | Serriari et al.,29 Hiejima et al.54 Hinks55 | |
| Ulcerative colitis | ↓ | Yes | ||||||
| Coeliac disease | ↓ | ↓ in lamina propria | No | Dunne et al.59 | ||||
| Airway diseases | ||||||||
| Asthma | ↓ if taking ICS | ↓ in sputum and bronchial biopsies if taking ICS | Yes | Deficiency might contribute to increased risks of pneumonia. Potential protective role of MAIT cells in early life | Hinks et al.,44 Hinks et al.,45 Unpublished observations | |||
| COPD | ↓ if taking ICS | ↓ in bronchial biopsies if taking ICS | Yes | |||||
| Obesity | ↓ and normalises after bariatric surgery | ↑ in adipose tissue in obesity | Associated with insulin resistance | Magalhaes et al.,82 Carolan et al.83 | ||||
| ↑ IL‐17/IL‐10 profile | ||||||||
CAIA, collagen antibody‐induced arthritis; CIA, collagen‐induced arthritis; CIDP, chronic inflammatory demyelinating polyneuropathy; COPD, chronic obstructive pulmonary disease; DN, double negative; EAE, experimental autoimmune encephalomyelitis; ICS, inhaled corticosteroids; IFN, interferon; MAIT, mucosal‐associated invariant T; MS, multiple sclerosis; PD‐1, programmed cell death protein 1; TG, transgenic; TNBS, 2,4,6‐trinitrobenzenesulphonic acid.
It is important to compare and contrast the biology of MAIT cells in humans and in mice, as differences may explain some discrepancies between human disease and animal models. In both species MAIT cells express orthologous TCR‐α chains, are MR1 restricted and recognize the same antigen. In both species MAIT cells express the master transcription factor PLZF and signature surface markers including CD127 (IL7Rα), CD218 (IL18Rα), CCR9 and CCR6.17 However, by contrast, MAIT cells are at least 10‐fold less abundant in mice than in humans.17, 27 Although some other previously reported species differences may have been artefacts of a specific transgenic murine model,17 other differences include a more limited expression of CD161 in mice17, 24 and, of relevance to this review, a difference in IL‐17 production. Interleukin‐17 is abundantly produced by both mouse and human MAIT cells in response to mitogen, but in humans IL‐17 is not produced in response to TCR triggering.17, 25, 33 In general each section of this review discusses human data from clinical studies first, before addressing murine data where relevant.
Observations from specific diseases
Systemic lupus erythematosus and rheumatoid arthritis
Systemic lupus erythematosus (SLE), an archetypal multi‐system autoimmune disease, is considered a multi‐factorial disorder with evidence of genetic susceptibility, environmental triggers including infections, and dysregulation of innate and adaptive immunity.34, 35 The pro‐inflammatory cytokine IL‐17 is associated with SLE and correlated with disease activity,36, 37 whereas IL‐17‐producing T helper type 17 (Th17) cells are involved in driving local inflammation at sites of disease.38, 39 MAIT cells are potent producers of IL‐17 and due to their similar cytometric profile may in fact have constituted many of the ‘Th17’ populations measured in these studies.40 Local cytokine production by MAIT cells within tissues could contribute to lowering the quantitative threshold of immune signalling, a process believed to interact with genetic predispositions to contribute to the initial pathogenic processes in SLE.35 Furthermore, several non‐MHC loci contribute to genetic predisposition to SLE,35 so potentially polymorphisms in MR1 or other MAIT‐cell related genes could contribute to such predispositions, although to date these have not been described.
An important feature of MAIT cells is that they can be stimulated by cytokines without the need for specific TCR stimulation. Hence, human and murine MAIT cells can produce IFN‐γ 41 or IL‐1719, 40 in response to IL‐12, and IL‐18 or IL‐23, respectively. Given their abundance this may constitute the most important effect of MAIT cells in autoimmunity as they non‐specifically amplify pro‐inflammatory signals within the cytokine milieu, enhancing inflammation in both rheumatological conditions like SLE and other diseases.
Cho et al.19 report a reduced frequency of human MAIT cells in peripheral blood in patients with both SLE and rheumatoid arthritis (RhA), particularly in the CD8+ and double‐negative subsets, which correlated with disease activity scores in both conditions. Furthermore, frequencies of MAIT cells secreting IFN‐γ (though not IL‐17 or IL‐4) were reduced in peripheral blood in SLE, with a similar trend in RhA, attributable to a defect in Ca2+/calcineurin/nuclear factor of activated T cells 1 signalling. This study reported increased expression of the co‐inhibitory molecule programmed cell death protein 1, perhaps a consequence of chronic MAIT‐cell activation leading to T‐cell exhaustion. At the site of disease MAIT cells were increased in synovial tissue in human RhA,19 and so might contribute to maturation and cross‐differentiation of T cells within the tissue microenvironment.35 These findings suggest possible recruitment of MAIT cells to sites of disease.
It should be noted that in this study, as with all the human clinical studies described in this review, MAIT cells were defined by surface phenotype (TCR Vα7.2+ CD161Hi) but not using MAIT cell‐specific tetramers, and are therefore susceptible to confounding by CD161 down‐regulation, as occurs with MAIT cell stimulation,22, 42, 43 which may lead to a failure to detect MAIT cells,42 which are truly present in the clinical samples. Of greater significance here is potential confounding by corticosteroid therapy. As will be discussed later, MAIT cell frequencies are very sensitive to therapeutic corticosteroids, which cause a rapid decrease in MAIT cell frequencies.44, 45 Indeed Cho et al.19 observed decreased MAIT cell frequencies only in patients with SLE or RhA, of which 90% were receiving corticosteroids, but no deficiency in two other autoimmune diseases also studied – ankylosing spondylitis and Behçet's disease – in which no patients were receiving steroids. Although their regression analysis did not detect steroid use as a significant coefficient, this may have been apparent if more non‐steroid‐treated patients were available for analysis or steroid dose had been analysed as a continuous variable. Irrespective of the effects of therapy, in such observational studies it is hard to determine causality: whether changes in MAIT cell frequency are a primary effect or merely reflect a response to cytokines produced by immune dysregulation.35
By contrast with this observed MAIT cell deficiency, a pathogenic role is implied by data from a murine collagen‐induced arthritis (CIA) model. Chiba et al.40 found that MR1−/− mice, which lack MAIT cells, had reduced severity of CIA and of collagen antibody‐induced arthritis, whereas CIA was recapitulated by reconstitution with adoptively transferred MAIT cells. As antigen‐specific responses of other T and B cells were unaffected by the presence of MR1, MAIT cells seem to be acting as effectors rather than initiators of inflammation in this model.
Multiple sclerosis
Autoimmune T‐cell responses by Th1 and Th17 cells against components of myelin play an important pathogenic role in multiple sclerosis (MS) and other demyelinating conditions.46 In humans, MAIT cells were first reported in inflammatory lesions in MS, chronic inflammatory demyelinating polyneuropathy47 and neurological tumours48 using PCR for the canonical Vα7.2− Jα33 TCR. It will be important to verify the specificity of these cells because non‐MAIT cells can express Vα7.2. More recently, using cytometry, frequencies of Vα7.2+ CD161+ MAIT cells have been shown to be decreased in peripheral blood of MS patients, particularly during disease relapse, compared with remission,49 and MAIT cell frequencies increased in paired samples 2–3 months after cessation of corticosteroid therapy. MAIT cells expressed CCR5 and CCR6, necessary for central nervous system invasion, suggesting that they might preferentially migrate to central nervous system lesions, and peripheral MAIT frequencies correlated positively with frequencies of iNKT and natural killer cells. This study sampled peripheral blood only and again these findings, particularly the paired analysis of relapse and post‐relapse remission, will be confounded by the use of corticosteroids, which are central to the management of MS relapse, and which may have long‐lasting suppressive effects on MAIT cell frequencies.
Nonetheless, elegant murine data from experimental autoimmune encephalomyelitis (EAE), a murine model of MS, supports a potentially protective role of MAIT cells in neuro‐inflammation. As there is no specific antibody for the canonical Vα19i TCR chain in mice, and wild‐type mice have very few MR1‐restricted T cells, Croxford et al.50 used transgenic mice over‐expressing Vα19i TCR, in which T cells that express the natural killer marker NK1.1+ are enriched for MAIT cells expressing Vα19− Jα33 TCR with Vβ6 or Vβ8 TCR chains. Incidence and severity of EAE were decreased in Vα19i transgenic mice by clinical and histological scores, and spinal cord lesions had less infiltration by monocytes and CD4+ T cells, with less demyelination. Similarly, EAE was ameliorated in wild‐type mice by adoptive transfer of Vα19i T cells. Lymph node T cells from these mice produced less pro‐inflammatory cytokines and more IL‐10. Conversely, MR1−/− mice, which lack MAIT cells, showed more severe EAE, with earlier onset, and more T‐cell proliferation and pro‐inflammatory cytokine production, with less IL‐10 production. In co‐culture studies Vα19i T cells induced IL‐10 from iNKT cells and conventional T cells, but most potently from CD19+ B cells, and this was, at least partly, by an MR1‐independent mechanism through inducible T‐cell co‐stimulator–B7 related protein‐1 interactions. One important limitation of the methodology used in this and other16, 24, 31 studies is that the Vα19i population includes many MHC‐restricted ‘MAIT‐like’ cells that do not stain with the specific MR1 tetramer or express signature innate cell transcription factors like PLZF.17, 22 Together these findings suggest that MAIT cells are protective against autoimmune demyelinating inflammation by a suppressive effect on other B and T cells, particularly by enhancing IL‐10 secretion and inhibiting Th1 cytokines.
Inflammatory bowel disease and enteropathies
The MAIT cells were shown early on to be present in the human gut lamina propria,1, 2 to express the gut homing integrin α 4/β 7, and have been considered to be preferentially located in mucosal tissue.27 Moreover an important role in gastrointestinal mucosal immunity is suggested by the dependence on commensal gut flora for their development in mice.2, 16 It is therefore likely that MAIT cells play a role in disorders of gastrointestinal mucosal immunity.
Chronic haemorrhagic colitis is a common feature of X‐linked lymphoproliferative syndrome (XLP) type 2.20, 51 XLP‐2 is a rare human genetic immunodeficiency caused by mutations in the X‐linked inhibitor of apoptosis (XIAP) gene, leading to effects including increased apoptosis of iNKT and MAIT cells.20, 51 XLP was therefore the first identified inherited immunodeficiency associated with iNKT or MAIT cell defects, with a 10‐fold decrease in peripheral blood MAIT cell frequencies.20 Colitis occurs in 17% of patients with XLP‐2 and resembles a severe form of inflammatory bowel disease, with accumulations of activated T cells and a mortality of 60%.51 Although XIAP deficiency may have pleiotropic effects on the immune system, including hypogammaglobulinaemia, it seems likely that the colitis is a direct consequence of the MAIT cell deficiency.
Crohn's disease is believed to be initiated and driven by CD4+ T cells targeting the commensal gut microbiota, and secreting IL‐12, IL‐17, TNF‐α and IFN‐γ.29 Ulcerative colitis is likely to involve similar mechanisms, but also Th2 cells secreting IL‐5 and IL‐13 and CD1d‐restricted type II NKT cells.52, 53 As with SLE, RhA and MS, in cross‐sectional observational studies there is a deficiency of human MAIT cells in peripheral blood in both Crohn's disease and ulcerative colitis,29, 54 although at least some of this effect is likely to be attributable to current or recent use of therapeutic corticosteroids, whether topical or systemic.55 Other possible factors include selective apoptosis of MAIT cells due to chronic activation‐induced cell death or recruitment to sites of disease. Indeed, consistent with the former possibility, Serriari et al.29 observed increased expression of the activation markers Ki67, NKG2D and BTLA receptor in human blood MAIT cells, which also had enhanced IL‐17 production. Consistent with the latter suggestion these authors also observed greater than fourfold accumulation of CD3+ IL18Rα + Vα7.2+ MAIT cells in biopsies of inflamed tissue from Crohn's disease ileum compared with biopsies from healthy sections.29
It is not clear whether MAIT cells actively contribute to this inflammation. One report suggests that adoptive MAIT transfer is protective in a murine 2,4,6‐trinitrobenzenesulphonic acid colitis model, but this study depended on only an in‐house antibody to Jα33 TCR, and therefore is confounded by non‐MAIT conventional T cells.56 It seems more likely from their known cytokine secretion profile that they would contribute to inflammation at sites of disease. Given the known role of MAIT cells in responding to riboflavin‐synthesizing microbes it is also possible that MAIT cell recruitment and activation is a consequence of the dysbiosis of the gastrointestinal microbiome that occurs in inflammatory bowel disease.57, 58 By contrast, human lamina propria and blood Vα7.2+ CD161+ MAIT cells are deficient in untreated coeliac disease,59 another important autoimmune enteropathy.60 Coeliac disease is driven by exposure to dietary gluten in genetically predisposed people, but it can be associated with compromised mucosal barrier function and bacterial overgrowth and therefore has the potential for activation‐induced MAIT cell death.59
Airways diseases
The role that innate‐like lymphocytes play in airways disease has evoked some controversy.61, 62, 63 Whereas iNKT cells are implicated in murine models of allergic airways disease, they are rare in the human airways;62 in comparison, MAIT cells are fivefold to tenfold more abundant in humans than in mice.27 Asthma is characterized by airways inflammation, classically associated with activated allergen‐specific Th2 cells64 secreting IL‐4, IL‐5 and IL‐13, which orchestrate the function of eosinophils, mast cells and B cells.44, 65 There is increasing recognition of disease heterogeneity and some genetic66, 67 and murine68, 69 data implicating IL‐17 in certain phenotypes of asthma. We conducted a large‐scale bronchoscopy‐based study of 60 patients spanning the spectrum of asthma severity and phenotypes and 24 healthy controls analysing multiple T‐cell subsets in the context of deep clinical and immunological phenotyping.44 We found no evidence of dysregulation of IL‐17‐secreting CD4+ Th17 cells, but observed a striking deficiency of Vα7.2+ CD161+ T cells in blood, sputum and bronchial biopsy samples44, 45 (Fig. 2). This deficiency correlated with disease severity in both blood and sputum and was associated with poor asthma control, poor lung function, longer disease duration and dose of inhaled corticosteroids (ICS). We conducted two open‐label clinical trials of ICS and oral corticosteroids. There was no change in blood or sputum MAIT cell frequencies with a 7‐day moderate dose ICS (twice‐daily Qvar 200 μg), but there was a significant 23% decrease in peripheral blood MAIT cell frequencies (P = 0·03) with just 7 days of systemic corticosteroids (once‐daily prednisolone 20 mg). This effect was specific to MAIT cells; it was not observed with Vα7.2 TCR‐expressing conventional T cells.44, 45
Figure 2.
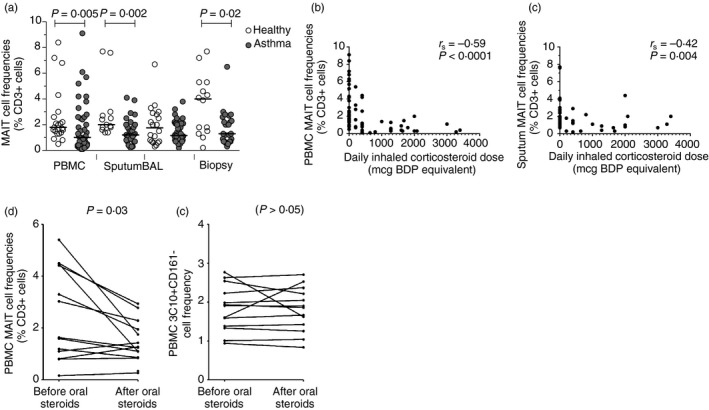
MAIT cells are deficient in asthma and this is related to corticosteroids. Frequencies of Vα7.2+ CD161+ (MAIT) cells as a proportion of total live CD3+ T cells (a) in peripheral blood, sputum, bronchoalveolar‐lavage (BAL) and bronchial biopsies in health and asthma. Horizontal lines represent medians. P‐values are for unpaired t‐tests on log‐transformed data. Frequencies of MAIT cells in (b) peripheral blood and (c) induced sputum correlate inversely with the dose of inhaled corticosteroid. Frequencies of MAIT cells (d), but not conventional T cells (e), were suppressed by 7 days of treatment with 20 mg oral prednisolone in 12 subjects with moderate asthma (23% fall in median frequency, P‐values are for paired t‐tests). Abbreviations: BDP, beclomethasone dipropionate; r s, Spearman's correlation coefficient. Figure adapted from Elsevier Hinks.44
We have since observed a similar deficiency of blood and bronchial biopsy MAIT cells in chronic obstructive pulmonary disease, which is apparent only in individuals receiving therapeutic ICS (Hinks et al., unpublished observations). Together these findings have important implications both for the management of airways diseases and for other conditions. It is remarkable that a systemic deficiency in peripheral blood MAIT cells can be induced even by the small doses of corticosteroid that are absorbed systemically after administration of low‐dose ICS,70 such as the patients with moderate asthma in our study who received a median 400 μg beclomethasone dipropionate daily equivalent. Therefore, it seems likely that much of the observational human data from other diseases discussed in this review are attributable, at least in part, to current or recent corticosteroid therapy.
What might be the consequences of these therapy‐induced deficiencies? MAIT cells are likely to contribute significantly to protection from pulmonary infections. Indeed 27% of individuals with XLP‐2 suffer from recurrent respiratory infections,51 although hypogammaglobulinaemia will also be relevant. Steroid‐induced suppression of airway MAIT cells might underlie the increased risk of pneumonia and invasive pneumococcal disease associated with severe asthma71, 72 and increased pneumonia risk in subjects with chronic obstructive pulmonary disease receiving inhaled fluticasone,73, 74 or contribute to chronic airway colonization with riboflavin‐synthesizing pathogens, notably Haemophilus influenzae in asthma and chronic obstructive pulmonary disease.75
Although MAIT cells are likely to protect against chronic bacterial infections of the airways, they may also play a role in the pathogenesis of allergic disease. Most MAIT cells secrete type 1 cytokines but some clones can produce type 2 cytokines.17, 23, 50 It is possible that in early life during initiating events in the development of allergic and autoimmune disease, such conditions are associated with a skewing towards a type 2 cytokine‐secreting profile in MAIT cells, as occurs with other T‐cell subsets,44, 64 and may occur early in life. Exposure to microbes during early childhood is associated with protection from immune‐mediated diseases.76, 77, 78 One mechanism may be persistent effects on numbers and function of innate lymphocytes, such as the accumulation of iNKT cells, which occurs in the lamina propria and lungs of germ‐free mice, resulting in increased morbidity in models of inflammatory bowel disease and allergic airways inflammation.77, 79 As commensal flora are absolutely required for MAIT cell development, differences in early life exposures would be expected to also impose long‐lasting effects on MAIT cells, which could well play an important role in the tendency towards the initial development of atopy, allergy and autoimmunity.
Obesity and type II diabetes
There is growing recognition of the importance of immune components to a wider range of diseases than previously considered, and this produces a potentially broader relevance of MAIT cells. For example, adipose tissue is immunologically active and obesity causes a sterile inflammation80 in which iNKT may play an immunoregulatory role.81, 82 MAIT cells may contribute to this inflammation because in human obesity they are enriched in adipose tissue compared with blood, and have a shift towards higher IL‐17 production and reduced IL‐10 secretion.82, 83 In peripheral blood, MAIT cell frequencies are reduced in obesity and in type 2 diabetes, even below the limit of detection in some severely obese individuals,82 and display a more activated phenotype, with up‐regulated CD25 and IL‐17 production. Both these studies found correlations between MAIT frequencies and insulin resistance, perhaps related to IL‐17 production, and Magalhaes et al. observed some normalization of MAIT cell phenotype and number after bariatric surgery. Hence MAIT cells may act as effectors contributing to the pathology of obesity‐associated insulin resistance. Furthermore, as with inflammatory bowel disease, obesity is associated with dysbiosis,84 which may therefore affect gastrointestinal MAIT cell functions and contribute to the poorly understood mechanistic links between the microbiome and obesity.
Perspective and future directions
Several common themes emerge from these studies, summarized in Fig. 3. Although our knowledge of the emerging field of MAIT cell biology is limited, several factors implicate these cells in immune‐mediated diseases, including their pro‐inflammatory profile,40 their widespread tissue distribution and effector‐memory phenotype. However, many clinical studies to date are confounded by the effect of therapeutic steroids, and others that use a Vα7.2CD161+ definition of MAIT cells may be confounded by the down‐regulation of CD161 which occurs with chronic activation.22, 85 Moreover, most clinical data are only observational, making it hard to determine whether aberrations are primary phenomena, or perhaps represent a response to the effects of a disordered cytokine milieu.35 Indeed, given their remarkable abundance and the potential for TCR‐independent activation of MAIT cells by cytokines such as IL‐12, IL‐18 and IL‐23, the most important effects of MAIT cells in autoimmunity could be non‐specific amplification of inflammatory cytokines leading to increased IFN‐γ 41 and IL‐17.19, 40 Differences between murine models and human studies may be due to such confounding factors in human studies as well as differences between the species.
Figure 3.
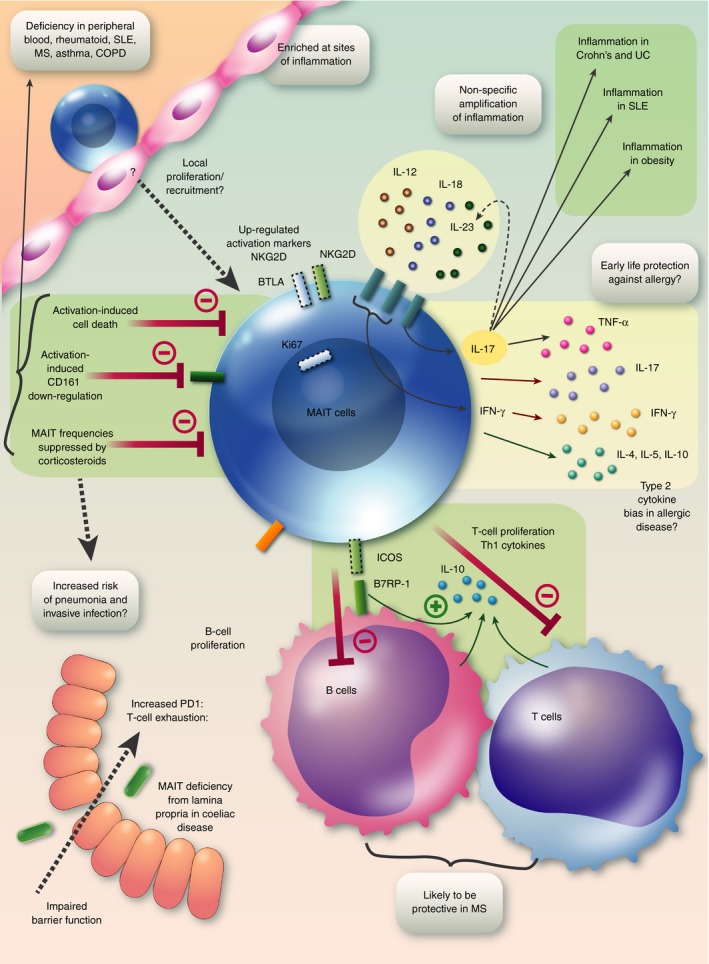
Graphical summary: MAIT cells in immune‐mediated disease. A deficiency of MAIT cells in peripheral blood is probably due to several factors: suppression of MAIT cell frequencies by corticosteroids, activation‐induced cell death and down‐regulation of the popular marker CD161. The consequences of such a deficiency may include increased risk of pneumonia and invasive infections. MAIT cells are enriched at sites of disease, probably as a result of local proliferation and recruitment, and up‐regulate activation markers including NKG2D, BTLA and Ki67. Chronic stimulation may lead to T‐cell exhaustion with PD‐1 up‐regulation. Secretion of pro‐inflammatory cytokines, especially IL‐17 will contribute to inflammation in inflammatory bowel disease, SLE and obesity. Non‐TCR‐mediated secretion of IL‐17 may be driven by IL‐23 and secretion of IFN‐γ can be driven by IL‐12 and IL‐18, allowing MAIT cells to amplify inflammatory signals in a non‐specific manner. It is possible that in allergic disease MAIT cells have a bias towards secretion of type 2 cytokines, whereas in early life microbial exposures may drive MAIT cells to produce a type 1 cytokine response protecting against the development of allergic diseases. MAIT cells may be protective in multiple sclerosis by inducing IL‐10 from T cells and particularly B cells by an MR1‐independent mechanism via ICOS–B7RP‐1 interaction. Abbreviations: B7RP‐1, B7 related protein‐1; COPD, chronic obstructive pulmonary disease; ICOS, inducible T cell co‐stimulator; IFN, interferon; IL, interleukin; MAIT, mucosal‐associated invariant T; MR1, MHC‐related protein 1; MS, multiple sclerosis; PD1, programmed cell death protein 1; SLE, systemic lupus erythematosus; TCR, T‐cell receptor; TNF‐α, tumour necrosis factor‐α; UC, ulcerative colitis.
The following questions should constitute priorities for future research. What other ligands can MAIT cells recognize? Do MAIT cells display significant reactivity to self‐ligands? How can MAIT cells distinguish between colonization and infection, leading to immune tolerance or inflammation? Can a lack of early‐life MAIT cell stimulation contribute to the development of autoimmunity35, 86 and allergy,76 perhaps through a skewing towards a type 2 cytokine profile, or a deficiency of MAIT cells? Do MAIT cells undergo activation‐induced depletion in autoimmune disease?
The tools necessary to answer these questions now exist, including specific human22 and murine17 MR‐1 tetramers, antibodies against MR187 and the MAIT cell TCR,24 and transgenic mice over‐expressing Vα19 T cells.16, 24, 31, 88 The strong evolutionary conservation of MR1 suggests that these tools will soon reveal some critical immune functions for these enigmatic cells. Furthermore, as the MAIT cell ligands are small molecules, this population could be easily targeted with drugs such as MAIT inhibitory ligands,89 making it an exciting time to speculate on the range of clinical applications that will surely emerge.
Disclosures
The author declares no financial conflicts of interest.
Acknowledgements
The author was supported by a Wellcome Trust Postdoctoral Research Fellowship (104553/Z/14/z). I am grateful to James McCluskey for critical review of the manuscript.
Senior author: Dr Timothy SC Hinks
References
- 1. Tilloy F, Treiner E, Park SH, Garcia C, Lemonnier F, de la Salle H et al An invariant T cell receptor α chain defines a novel TAP‐independent major histocompatibility complex class Ib‐restricted α/β T cell subpopulation in mammals. J Exp Med 1999; 189:1907–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F et al Selection of evolutionarily conserved mucosal‐associated invariant T cells by MR1. Nature 2003; 422:164–9. [DOI] [PubMed] [Google Scholar]
- 3. Hinks TS. MR1 (in Mouse and Man) In: Ratcliffe M, ed. Encyclopedia of Immunobiology. Oxford: Elsevier, 2016: 3750. [Google Scholar]
- 4. Kjer‐Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L et al MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 2012; 491:717–23. [DOI] [PubMed] [Google Scholar]
- 5. Patel O, Kjer‐Nielsen L, Le Nours J, Eckle SB, Birkinshaw R, Beddoe T et al Recognition of vitamin B metabolites by mucosal‐associated invariant T cells. Nat Commun 2013; 4:2142. [DOI] [PubMed] [Google Scholar]
- 6. Eckle SB, Corbett AJ, Keller AN, Chen Z, Godfrey DI, Liu L et al Recognition of vitamin B precursors and byproducts by mucosal associated invariant T cells. J Biol Chem 2015; 290:30204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Corbett AJ, Eckle SB, Birkinshaw RW, Liu L, Patel O, Mahony J et al T‐cell activation by transitory neo‐antigens derived from distinct microbial pathways. Nature 2014; 509:361–5. [DOI] [PubMed] [Google Scholar]
- 8. Meierovics A, Yankelevich WJ, Cowley SC. MAIT cells are critical for optimal mucosal immune responses during in vivo pulmonary bacterial infection. Proc Natl Acad Sci USA 2013; 110:E3119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gold MC, Eid T, Smyk‐Pearson S, Eberling Y, Swarbrick GM, Langley SM et al Human thymic MR1‐restricted MAIT cells are innate pathogen‐reactive effectors that adapt following thymic egress. Mucosal Immunol 2013; 6:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Le Bourhis L, Dusseaux M, Bohineust A, Bessoles S, Martin E, Premel V et al MAIT cells detect and efficiently lyse bacterially‐infected epithelial cells. PLoS Pathog 2013; 9:e1003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Le Bourhis L, Martin E, Peguillet I, Guilhot A, Fourx N, Core M et al Antimicrobial activity of mucosal‐associated invariant T cells. Nat Immunol 2010; 11:701–8. [DOI] [PubMed] [Google Scholar]
- 12. Chua WJ, Truscott SM, Eickhoff CS, Blazevic A, Hoft DF, Hansen TH. Polyclonal mucosa‐associated invariant T cells have unique innate functions in bacterial infection. Infect Immun 2012; 80:3256–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Georgel P, Radosavljevic M, Macquin C, Bahram S. The non‐conventional MHC class I MR1 molecule controls infection by Klebsiella pneumoniae in mice. Mol Immunol 2011; 48:769–75. [DOI] [PubMed] [Google Scholar]
- 14. Godfrey DI, Uldrich AP, McCluskey J, Rossjohn J, Moody DB. The burgeoning family of unconventional T cells. Nat Immunol 2015; 16:1114–23. [DOI] [PubMed] [Google Scholar]
- 15. Dusseaux M, Martin E, Serriari N, Peguillet I, Premel V, Louis D et al Human MAIT cells are xenobiotic‐resistant, tissue‐targeted, CD161hi IL‐17‐secreting T cells. Blood 2011; 117:1250–9. [DOI] [PubMed] [Google Scholar]
- 16. Seach N, Guerri L, Le Bourhis L, Mburu Y, Cui Y, Bessoles S et al Double‐positive thymocytes select mucosal‐associated invariant T cells. J Immunol 2013; 191:6002–9. [DOI] [PubMed] [Google Scholar]
- 17. Rahimpour A, Koay HF, Enders A, Clanchy R, Eckle SB, Meehan B et al Identification of phenotypically and functionally heterogeneous mouse mucosal‐associated invariant T cells using MR1 tetramers. J Exp Med 2015; 212:1095–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rossjohn J, Pellicci DG, Patel O, Gapin L, Godfrey DI. Recognition of CD1d‐restricted antigens by natural killer T cells. Nat Rev Immunol 2012; 12:845–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cho YN, Kee SJ, Kim TJ, Jin HM, Kim MJ, Jung HJ et al Mucosal‐associated invariant T cell deficiency in systemic lupus erythematosus. J Immunol 2014; 193:3891–901. [DOI] [PubMed] [Google Scholar]
- 20. Gerart S, Siberil S, Martin E, Lenoir C, Aguilar C, Picard C et al Human iNKT and MAIT cells exhibit a PLZF‐dependent pro‐apoptotic propensity that is counterbalanced by XIAP. Blood 2013; 121:614–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Billerbeck E, Kang YH, Walker L, Lockstone H, Grafmueller S, Fleming V et al Analysis of CD161 expression on human CD8+ T cells defines a distinct functional subset with tissue‐homing properties. Proc Natl Acad Sci USA 2010; 107:3006–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reantragoon R, Corbett AJ, Sakala IG, Gherardin NA, Furness JB, Chen Z et al Antigen‐loaded MR1 tetramers define T cell receptor heterogeneity in mucosal‐associated invariant T cells. J Exp Med 2013; 210:2305–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lepore M, Kalinicenko A, Colone A, Paleja B, Singhal A, Tschumi A et al Parallel T‐cell cloning and deep sequencing of human MAIT cells reveal stable oligoclonal TCRβ repertoire. Nat Commun 2014; 5:3866. [DOI] [PubMed] [Google Scholar]
- 24. Martin E, Treiner E, Duban L, Guerri L, Laude H, Toly C et al Stepwise development of MAIT cells in mouse and human. PLoS Biol 2009; 7:e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tang XZ, Jo J, Tan AT, Sandalova E, Chia A, Tan KC et al IL‐7 licenses activation of human liver intrasinusoidal mucosal‐associated invariant T cells. J Immunol 2013; 190:3142–52. [DOI] [PubMed] [Google Scholar]
- 26. Miley MJ, Truscott SM, Yu YY, Gilfillan S, Fremont DH, Hansen TH et al Biochemical features of the MHC‐related protein 1 consistent with an immunological function. J Immunol 2003; 170:6090–8. [DOI] [PubMed] [Google Scholar]
- 27. Treiner E, Duban L, Moura IC, Hansen T, Gilfillan S, Lantz O. Mucosal‐associated invariant T (MAIT) cells: an evolutionarily conserved T cell subset. Microbes Infect 2005; 7:552–9. [DOI] [PubMed] [Google Scholar]
- 28. Huang S, Gilfillan S, Kim S, Thompson B, Wang X, Sant AJ et al MR1 uses an endocytic pathway to activate mucosal‐associated invariant T cells. J Exp Med 2008; 205:1201–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Serriari NE, Eoche M, Lamotte L, Lion J, Fumery M, Marcelo P et al Innate mucosal‐associated invariant T (MAIT) cells are activated in inflammatory bowel diseases. Clin Exp Immunol 2014; 176:266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Braud VM, Allan DS, McMichael AJ. Functions of nonclassical MHC and non‐MHC‐encoded class I molecules. Curr Opin Immunol 1999; 11:100–8. [DOI] [PubMed] [Google Scholar]
- 31. Kawachi I, Maldonado J, Strader C, Gilfillan S. MR1‐restricted Vα19i mucosal‐associated invariant T cells are innate T cells in the gut lamina propria that provide a rapid and diverse cytokine response. J Immunol 2006; 176:1618–27. [DOI] [PubMed] [Google Scholar]
- 32. Rose NR, Bona C. Defining criteria for autoimmune diseases (Witebsky's postulates revisited). Immunol Today 1993; 14:426–30. [DOI] [PubMed] [Google Scholar]
- 33. Walker LJ, Kang YH, Smith MO, Tharmalingham H, Ramamurthy N, Fleming VM et al Human MAIT and CD8αα cells develop from a pool of type‐17 precommitted CD8+ T cells. Blood 2012; 119:422–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lisnevskaia L, Murphy G, Isenberg D. Systemic lupus erythematosus. Lancet 2014; 384:1878–88. [DOI] [PubMed] [Google Scholar]
- 35. Wahren‐Herlenius M, Dorner T. Immunopathogenic mechanisms of systemic autoimmune disease. Lancet 2013; 382:819–31. [DOI] [PubMed] [Google Scholar]
- 36. Crispin JC, Oukka M, Bayliss G, Cohen RA, Van Beek CA, Stilman IE et al Expanded double negative T cells in patients with systemic lupus erythematosus produce IL‐17 and infiltrate the kidneys. J Immunol 2008; 181:8761–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wong CK, Lit LC, Tam LS, Li EK, Wong PT, Lam CW. Hyperproduction of IL‐23 and IL‐17 in patients with systemic lupus erythematosus: implications for Th17‐mediated inflammation in auto‐immunity. Clin Immunol 2008; 127:385–93. [DOI] [PubMed] [Google Scholar]
- 38. Yang J, Chu Y, Yang X, Gao D, Zhu L, Yang X et al Th17 and natural Treg cell population dynamics in systemic lupus erythematosus. Arthritis Rheum 2009; 60:1472–83. [DOI] [PubMed] [Google Scholar]
- 39. Kwan BC, Tam LS, Lai KB, Lai FM, Li EK, Wang G et al The gene expression of type 17 T‐helper cell‐related cytokines in the urinary sediment of patients with systemic lupus erythematosus. Rheumatology (Oxford) 2009; 48:1491–7. [DOI] [PubMed] [Google Scholar]
- 40. Chiba A, Tajima R, Tomi C, Miyazaki Y, Yamamura T, Miyake S. Mucosal‐associated invariant T cells promote inflammation and exacerbate disease in murine models of arthritis. Arthritis Rheum 2012; 64:153–61. [DOI] [PubMed] [Google Scholar]
- 41. Ussher JE, Bilton M, Attwod E, Shadwell J, Richardson R, de Lara C et al CD161 CD8 T cells, including the MAIT cell subset, are specifically activated by IL‐12 + IL‐18 in a TCR‐independent manner. Eur J Immunol 2014; 44:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fernandez CS, Amarasena T, Kelleher AD, Rossjohn J, McCluskey J, Godfrey DI et al MAIT cells are depleted early but retain functional cytokine expression in HIV infection. Immunol Cell Biol 2015; 93:177–88. [DOI] [PubMed] [Google Scholar]
- 43. Leeansyah E, Ganesh A, Quigley MF, Sonnerborg A, Andersson J, Hunt PW et al Activation, exhaustion, and persistent decline of the antimicrobial MR1‐restricted MAIT‐cell population in chronic HIV‐1 infection. Blood 2013; 121:1124–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hinks TS, Zhou X, Staples KJ, Dimitrov BD, Manta A, Petrossian T et al Innate and adaptive T cells in asthmatic patients: relationship to severity and disease mechanisms. J Allergy Clin Immunol 2015; 136:323–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hinks T, Zhou X, Staples K, Dimitrov BD, Manta A, Petrossian T et al Multidimensional endotypes of asthma: topological data analysis of cross‐sectional clinical, pathological, and immunological data. Lancet 2015; 385(Suppl 1):S42. [DOI] [PubMed] [Google Scholar]
- 46. Goverman J. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol 2009; 9:393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Illes Z, Shimamura M, Newcombe J, Oka N, Yamamura T. Accumulation of Vα7.2‐Jα33 invariant T cells in human autoimmune inflammatory lesions in the nervous system. Int Immunol 2004; 16:223–30. [DOI] [PubMed] [Google Scholar]
- 48. Peterfalvi A, Gomori E, Magyarlaki T, Pal J, Banati M, Javorhazy A et al Invariant Vα7.2‐Jα33 TCR is expressed in human kidney and brain tumors indicating infiltration by mucosal‐associated invariant T (MAIT) cells. Int Immunol 2008; 20:1517–25. [DOI] [PubMed] [Google Scholar]
- 49. Miyazaki Y, Miyake S, Chiba A, Lantz O, Yamamura T. Mucosal‐associated invariant T cells regulate Th1 response in multiple sclerosis. Int Immunol 2011; 23:529–35. [DOI] [PubMed] [Google Scholar]
- 50. Croxford JL, Miyake S, Huang YY, Shimamura M, Yamamura T. Invariant Vα19i T cells regulate autoimmune inflammation. Nat Immunol 2006; 7:987–94. [DOI] [PubMed] [Google Scholar]
- 51. Pachlopnik Schmid J, Canioni D, Moshous D, Touzot F, Mahlaoui N, Hauck F et al Clinical similarities and differences of patients with X‐linked lymphoproliferative syndrome type 1 (XLP‐1/SAP deficiency) versus type 2 (XLP‐2/XIAP deficiency). Blood 2011; 117:1522–9. [DOI] [PubMed] [Google Scholar]
- 52. Fuss IJ, Heller F, Boirivant M, Leon F, Yoshida M, Fichtner‐Feigl S et al Nonclassical CD1d‐restricted NK T cells that produce IL‐13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest 2004; 113:1490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fuss IJ, Strober W. The role of IL‐13 and NK T cells in experimental and human ulcerative colitis. Mucosal Immunol 2008; 1(Suppl 1):S31–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hiejima E, Kawai T, Nakase H, Tsuruyama T, Morimoto T, Yasumi T et al Reduced numbers and proapoptotic features of mucosal‐associated invariant T cells as a characteristic finding in patients with inflammatory bowel disease. Inflamm Bowel Dis 2015; 21:1529–40. [DOI] [PubMed] [Google Scholar]
- 55. Hinks TS. Reduced numbers and proapoptotic features of mucosal‐associated invariant T cells as a characteristic finding in patients with inflammatory bowel disease. Inflamm Bowel Dis 2015; 21:E30. [DOI] [PubMed] [Google Scholar]
- 56. Ruijing X, Mengjun W, Xiaoling Z, Shu P, Mei W, Yingcheng Z et al Jα33+ MAIT cells play a protective role in TNBS induced intestinal inflammation. Hepatogastroenterology 2012; 59:762–7. [DOI] [PubMed] [Google Scholar]
- 57. Rajilic‐Stojanovic M, Shanahan F, Guarner F, de Vos WM. Phylogenetic analysis of dysbiosis in ulcerative colitis during remission. Inflamm Bowel Dis 2013; 19:481–8. [DOI] [PubMed] [Google Scholar]
- 58. Andoh A, Kuzuoka H, Tsujikawa T, Nakamura S, Hirai F, Suzuki Y et al Multicenter analysis of fecal microbiota profiles in Japanese patients with Crohn's disease. J Gastroenterol 2012; 47:1298–307. [DOI] [PubMed] [Google Scholar]
- 59. Dunne MR, Elliott L, Hussey S, Mahmud N, Kelly J, Doherty DG et al Persistent changes in circulating and intestinal γδ T cell subsets, invariant natural killer T cells and mucosal‐associated invariant T cells in children and adults with coeliac disease. PLoS ONE 2013; 8:e76008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sollid LM, Jabri B. Is celiac disease an autoimmune disorder? Curr Opin Immunol 2005; 17:595–600. [DOI] [PubMed] [Google Scholar]
- 61. Umetsu DT, Dekruyff RH. Immune dysregulation in asthma. Curr Opin Immunol 2006; 18:727–32. [DOI] [PubMed] [Google Scholar]
- 62. Vijayanand P, Seumois G, Pickard C, Powell RM, Angco G, Sammut D et al Invariant natural killer T cells in asthma and chronic obstructive pulmonary disease. N Engl J Med 2007; 356:1410–22. [DOI] [PubMed] [Google Scholar]
- 63. Djukanovic R, Gadola SD. Virus infection, asthma, and chronic obstructive pulmonary disease. N Engl J Med 2008; 359:2062–4. [DOI] [PubMed] [Google Scholar]
- 64. Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM et al Predominant TH2‐like bronchoalveolar T‐lymphocyte population in atopic asthma. N Engl J Med 1992; 326:298–304. [DOI] [PubMed] [Google Scholar]
- 65. Hinks TS, Brown T, Lau LCK, Rupani H, Barber C, Elliott S et al Multidimensional endotyping in severe asthma reveals inflammatory heterogeneity in MMPs and YKL‐40. J Allergy Clin Immunol 2016; Feb 3. doi: 10.1016/j.jaci.2015.11.020. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chen J, Deng Y, Zhao J, Lou Z, Peng W, Yang J et al The polymorphism of IL‐17 G‐152A was associated with childhood asthma and bacterial colonization of the hypopharynx in bronchiolitis. J Clin Immunol 2010; 30:539–45. [DOI] [PubMed] [Google Scholar]
- 67. Lluis A, Schedel M, Liu J, Illi S, Depner M, von Mutius E et al Asthma‐associated polymorphisms in 17q21 influence cord blood ORMDL3 and GSDMA gene expression and IL‐17 secretion. J Allergy Clin Immunol 2011; 127:1587–94.e6. [DOI] [PubMed] [Google Scholar]
- 68. Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH et al A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 2005; 6:1133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schnyder‐Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V et al Interleukin‐17 is a negative regulator of established allergic asthma. J Exp Med 2006; 203:2715–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bernstein DI, Allen DB. Evaluation of tests of hypothalamic–pituitary–adrenal axis function used to measure effects of inhaled corticosteroids. Ann Allergy Asthma Immunol 2007; 98:118–27. [DOI] [PubMed] [Google Scholar]
- 71. Talbot TR, Hartert TV, Mitchel E, Halasa NB, Arbogast PG, Poehling KA et al Asthma as a risk factor for invasive pneumococcal disease. N Engl J Med 2005; 352:2082–90. [DOI] [PubMed] [Google Scholar]
- 72. Klemets P, Lyytikainen O, Ruutu P, Ollgren J, Kaijalainen T, Leinonen M et al Risk of invasive pneumococcal infections among working age adults with asthma. Thorax 2010; 65:698–702. [DOI] [PubMed] [Google Scholar]
- 73. Crim C, Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C et al Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. Eur Respir J 2009; 34:641–7. [DOI] [PubMed] [Google Scholar]
- 74. Calverley PM, Anderson JA, Celli B et al Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007; 356:775–89. [DOI] [PubMed] [Google Scholar]
- 75. Wilson R, Allegra L, Huchon G, Izquierdo JL, Jones P, Schaberg T et al Short‐term and long‐term outcomes of moxifloxacin compared to standard antibiotic treatment in acute exacerbations of chronic bronchitis. Chest 2004; 125:953–64. [DOI] [PubMed] [Google Scholar]
- 76. Strachan DP. Family size, infection and atopy: the first decade of the “hygiene hypothesis”. Thorax 2000; 55(Suppl 1):S2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A et al Microbial exposure during early life has persistent effects on natural killer T cell function. Science 2012; 336:489–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Teo SM, Mok D, Pham K, Kusel M, Serralha M, Troy N et al The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe 2015; 17:704–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Herbst T, Sichelstiel A, Schar C, Yadava K, Burki K, Cahenzli J et al Dysregulation of allergic airway inflammation in the absence of microbial colonization. Am J Respir Crit Care Med 2011; 184:198–205. [DOI] [PubMed] [Google Scholar]
- 80. O'Shea D, Corrigan M, Dunne MR, Jackson R, Woods C, Gaoatswe G et al Changes in human dendritic cell number and function in severe obesity may contribute to increased susceptibility to viral infection. Int J Obes (Lond) 2013; 37:1510–3. [DOI] [PubMed] [Google Scholar]
- 81. Lynch L, Nowak M, Varghese B, Clark J, Hogan AE, Toxavidis V et al Adipose tissue invariant NKT cells protect against diet‐induced obesity and metabolic disorder through regulatory cytokine production. Immunity 2012; 37:574–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Magalhaes I, Pingris K, Poitou C, Bessoles S, venteclef N, Kiaf B et al Mucosal‐associated invariant T cell alterations in obese and type 2 diabetic patients. J Clin Invest 2015; 125:1752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Carolan E, Tobin LM, Mangan BA, Corrigan M, Gaoatswe G, Byrne G et al Altered distribution and increased IL‐17 production by mucosal‐associated invariant T cells in adult and childhood obesity. J Immunol 2015; 194:5775–80. [DOI] [PubMed] [Google Scholar]
- 84. Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL et al Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013; 341:1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Birkinshaw RW, Kjer‐Nielsen L, Eckle SB, McCluskey J, Rossjohn J. MAITs, MR1 and vitamin B metabolites. Curr Opin Immunol 2014; 26:7–13. [DOI] [PubMed] [Google Scholar]
- 86. Scher JU, Abramson SB. The microbiome and rheumatoid arthritis. Nat Rev Rheumatol 2011; 7:569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Huang S, Gilfillan S, Cella M, Miley MJ, Lantz O, Lybarger L et al Evidence for MR1 antigen presentation to mucosal‐associated invariant T cells. J Biol Chem 2005; 280:21183–93. [DOI] [PubMed] [Google Scholar]
- 88. Cui Y, Franciszkiewicz K, Mburu YK, Mondot S, Le Bourhis L, Premel V et al Mucosal‐associated invariant T cell‐rich congenic mouse strain allows functional evaluation. J Clin Invest 2015; 125:4171–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gold MC, Napier RJ, Lewinsohn DM. MR1‐restricted mucosal associated invariant T (MAIT) cells in the immune response to Mycobacterium tuberculosis . Immunol Rev 2015; 264:154–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Porcelli S, Yockey CE, Brenner MB, Balk SP. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4–8– α/β T cells demonstrates preferential use of several V β genes and an invariant TCR α chain. J Exp Med 1993; 178:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Greenaway HY, Ng B, Price DA, Douek DC, Davenport MP, Venturi V. NKT and MAIT invariant TCRα sequences can be produced efficiently by VJ gene recombination. Immunobiology 2013; 218:213–24. [DOI] [PubMed] [Google Scholar]
- 92. Riegert P, Wanner V, Bahram S. Genomics, isoforms, expression, and phylogeny of the MHC class I‐related MR1 gene. J Immunol 1998; 161:4066–77. [PubMed] [Google Scholar]
- 93. Yamaguchi H, Hirai M, Kurosawa Y, Hashimoto K. A highly conserved major histocompatibility complex class I‐related gene in mammals. Biochem Biophys Res Commun 1997; 238:697–702. [DOI] [PubMed] [Google Scholar]
- 94. Tsukamoto K, Deakin JE, Graves JA, Hashimoto K. Exceptionally high conservation of the MHC class I‐related gene, MR1, among mammals. Immunogenetics 2013; 65:115–24. [DOI] [PubMed] [Google Scholar]
- 95. Mikkelsen TS, Wakefield MJ, Aken B, Amemiya CT, Chang JL, Duke S et al Genome of the marsupial Monodelphis domestica reveals innovation in non‐coding sequences. Nature 2007; 447:167–77. [DOI] [PubMed] [Google Scholar]


