Summary
The T‐helper cell type 2‐promoting cytokine interleukin‐33 (IL‐33) has been implicated in asthma pathogenesis. Angiogenesis is a feature of airways remodelling in asthma. We hypothesized that IL‐33 induces airways angiogenesis and expression of angiogenic factors in an established murine surrogate of asthma. In the present study, BALB/c mice were subjected to serial intranasal challenge with IL‐33 alone for up to 70 days. In parallel, ovalbumin (OVA) ‐sensitized mice were subjected to serial intranasal challenge with OVA or normal saline to serve as positive and negative controls, respectively. Immunohistochemical analysis of expression of von Willebrand factor and erythroblast transformation‐specific‐related gene, both blood vessel markers, and angiogenic factors angiogenin, insulin‐like growth factor‐1, endothelin‐1, epidermal growth factor and amphiregulin was performed in lung sections ex vivo. An established in‐house assay was used to test whether IL‐33 was able to induce microvessel formation by human vascular endothelial cells. Results showed that serial intranasal challenge of mice with IL‐33 or OVA resulted in proliferation of peribronchial von Willebrand factor‐positive blood vessels to a degree closely related to the total expression of the angiogenic factors amphiregulin, angiogenin, endothelin‐1, epidermal growth factor and insulin‐like growth factor‐1. IL‐33 also induced microvessel formation by human endothelial cells in a concentration‐dependent fashion in vitro. Our data are consistent with the hypothesis that IL‐33 has the capacity to induce angiogenesis at least partly by increasing local expression of multiple angiogenic factors in an allergen‐independent murine asthma surrogate, and consequently that IL‐33 or its receptor is a potential novel molecular target for asthma therapy.
Keywords: angiogenesis, asthma, interleukin‐33, murine model
Abbreviations
- EGF
epidermal growth factor
- ERG
erythroblast transformation‐specific‐related gene
- IGF‐1
insulin‐like growth factor
- IL‐33
interleukin‐33
- OVA
ovalbumin
- Th2
T helper type 2
- VEGF
vascular endothelial growth factor
- vWF
von Willebrand factor
Introduction
Asthma is a chronic disease affecting more than 300 million people worldwide and its prevalence is still rising.1 It is characterized pathophysiologically by bronchial mucosal inflammation with T helper type 2 (Th2) immune bias and episodic airways obstruction, reflecting airways hyper‐responsiveness.2 Neo‐angiogenesis in the airways mucosa is a distinct feature of asthma pathophysiology that is generally considered to be an important component of airways remodelling, and which may contribute to irreversible airways obstruction. Neo‐angiogenesis may be defined as the emergence of new blood vessels from pre‐existing vasculature and is regulated by a balance of promoting (angiogenic) and counteracting (angiostatic) factors.3, 4 In the course of chronic inflammation, neo‐angiogenic processes are initiated as a result of the dominance of pro‐angiogenic effects, probably involving many growth factors.5 Given that neo‐angiogenesis is a typical feature of remodelling in asthmatic airways, asthma is not only an airways disease, but also a vascular disease.6 Nevertheless, there are relatively few reports addressing its pathogenesis in asthma.
Interleukin‐33 (IL‐33) is a member of the IL‐1 cytokine family and is a ligand of the receptor ST2.7 Recent studies in human subjects and murine asthma surrogates suggest a central role for IL‐33 in driving Th2‐mediated inflammation.8, 9 Direct administration of IL‐33 alone into the airways of naive mice via the intranasal route resulted in elevated expression of Th2 cytokines, eosinophilic inflammation and airways hyper‐responsiveness.10, 11, 12 Deletion or blockade of the IL‐33 receptor (T1/ST2) has been shown to attenuate allergic airways inflammation in animal asthma surrogates.9, 11 Some studies suggest that IL‐33 also promotes angiogenesis and increases vascular permeability, possibly at least partly by promoting endothelial cellular production of nitric oxide and urokinase in human endothelial cells.13, 14
To examine the potential role of IL‐33 to promote angiogenesis in asthma and clarify its mechanisms of action we used a murine surrogate of asthma initiated by direct IL‐33 challenge, which we developed as previously described15 to compare, in parallel, the effects of direct IL‐33 challenge of the airways with those of ‘classical’ ovalbumin (OVA) challenge, necessitating previous IgE anti‐OVA sensitization of the animals as a positive control. In addition, using an in vitro model we investigate whether IL‐33 exerts direct effects on human angiogenesis in vitro.
Materials and methods
Animals
Female BALB/c mice (8–10 weeks old) were obtained from Vital River Laboratories (Beijing, China) and maintained in a pathogen‐free mouse facility located in the Department of Laboratory Animal Sciences, Capital Medical University, Beijing, China. All of the experimental procedures were approved by the Institutional Animal Care and Use Committee.
IL‐33 challenge and OVA challenge
The protocols employed for sensitization and inhalational challenge have previously been described.15 Mice were randomly distributed into three groups including a group serially challenged with 50 μg/dose of OVA as a positive control;16, 17 a group administered saline as a negative control and a group serially challenged with IL‐33.15, 18 The OVA‐challenged mice were first sensitized by intraperitoneal injection of 100 μg/dose OVA (emulsified in Al[OH]3; Sigma‐Aldrich, Beijing, China), on days 0 and 12. These mice were then further intranasally challenged daily on days 18–23 with 50 μg/dose of OVA (OVA50) in 50 μl saline. For IL‐33 challenge, naive mice were challenged intranasally daily from days 18 to 23 with recombinant mouse IL‐33 (ml‐33; R&D Systems, Minneapolis, MN; 100 ng in 50 μl saline = 1·1 × 10−7 m). Then the mice in each group were further challenged intranasally with OVA or IL‐33 every 2 days for a further 30 days. Some of them were observed for a further 17 days after cessation of the challenges. Control mice were intraperitoneally injected with same amount of Al[OH]3 then nasally challenged with normal saline at the corresponding time‐points. Lung tissues were collected on days 20, 24, 36, 48, 54 and 70 (each time‐point n = 5 per group), respectively, 24 hr after the most recent intranasal challenge animals.15
Lung immunohistochemistry
Resected left lung tissue was fixed in 10% neutral‐buffered formalin for 24 hr, then dissected and embedded in paraffin. Immunohistochemistry was used to detect major angiogenic biomarkers in 5‐μm lung sections. Primary monoclonal antibodies against mouse erythroblast transformation‐specific‐related gene (ERG, 1 : 50), angiogenin (1 : 50), insulin‐like growth factor‐1 (IGF‐1, 1 : 400), endothelin‐1 (1 : 600) and epidermal growth factor (EGF, 1 : 100) were purchased from Abcam (Hong Kong, China). Primary monoclonal antibodies against mouse von Willebrand factor (vWF, 1 : 50) and amphiregulin (1 : 100) were purchased from Merck Millipore (Shanghai, China) and Santa Cruz Biotechnology, Inc (Santa Cruz, CA), respectively. The peroxidase/anti‐peroxidase technique was used for detection as previously described.15, 16 Sections were anonymized by coding, then independently analysed by two observers using a Leica DM6000B microscope (Leica, Wetzlar, Germany) connected with a Leica Application Suite Version 3.6. image‐pro plus software (Rockville, MD) was used to delineate and measure the total areas of each section showing positive staining in an objective fashion. For each biomarker three independent experiments were performed. The data were expressed as percentages of the entire areas of the lung sections showing positive staining.
In vitro angiogenesis assay
We used a well established in vitro angiogenesis assay (AngioKit; TCS CellWorks, Buckingham, UK),19, 20 which is based on co‐culture of human umbilical vein endothelial cells over a monolayer of irradiated fibroblasts. Cultures were incubated at 37°C in a humidified atmosphere with 5% CO2. Culture medium with recombinant human IL‐33 (0·1, 1·0 and 10 ng/ml; R&D Systems, Abingdon, UK) was replenished on days 4, 7 and 9. On day 11 cultured cells were fixed and vascular structures were visualized by labelling with mouse anti‐CD31 according to the manufacturer's instructions. Culture medium alone and with recombinant human vascular endothelial growth factor A (VEGF‐A; R&D Systems, 10 ng/ml) served as negative and positive assay controls. The analysis software (AngioSys; TCS CellWorks) segmented the images using a grey‐level threshold tool to select CD31‐labelled cells. The overall numbers of vascular junctions, tubules and tubule length were determined as described previously.19, 20
Statistical analysis
All data are reported as the mean ± SEM and analysed using instat 2.01 software (GraphPad, San Diego, CA). Between‐group comparisons at specific time‐points were performed using two‐way parametric analysis of variance followed by a Bonferroni post test or the Student's unpaired t‐test. Data generated from the angiogenesis assay were analysed using Mann–Whitney test. A P‐value < 0·05 was considered significant.
Results
IL‐33 increased airways vascularity
Compared with saline challenge, IL‐33 induced marked airways angiogenesis as indicated by significantly elevated numbers of peribronchial, vWF+ immunoreactive blood vessels, which appeared as early as day 20, peaked at day 48 and persisted until the end of the experiment (day 70) (Fig. 1). OVA challenge was associated with similar, statistically equivalent changes in vWF+ immunoreactive vessels.
Figure 1.
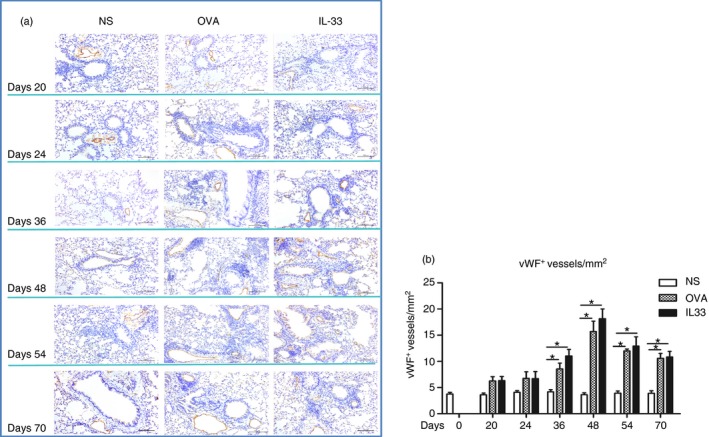
Interleukin‐33 (IL‐33) increased airways von Willebrand factor (vWF) immunoreactivity. (a) Representative photomicrographs of vWF immunoreactive blood vessels in lung sections from saline‐ (NS), ovalbumin‐ (OVA) and IL‐33‐challenged mice at various time‐points as indicated (light microscopy, × 200). (b) Quantitative analysis of numbers of vWF + blood vessels per unit area of lung sections. Data are expressed as mean ± SEM (n = 5 mice in each group at each time‐point). *P < 0·05.
ERG is constitutively expressed by endothelial cells and is considered to be another biomarker of endothelial cell numbers and angiogenesis. Immunohistochemical analysis of ERG immunoreactivity similarly confirmed that, compared with saline challenge, both OVA challenge and IL‐33 challenge induced significant expression of ERG, which was first apparent at day 36, peaked at day 48 and persisted until the end of the experiment (day 70) (Fig. 2).
Figure 2.
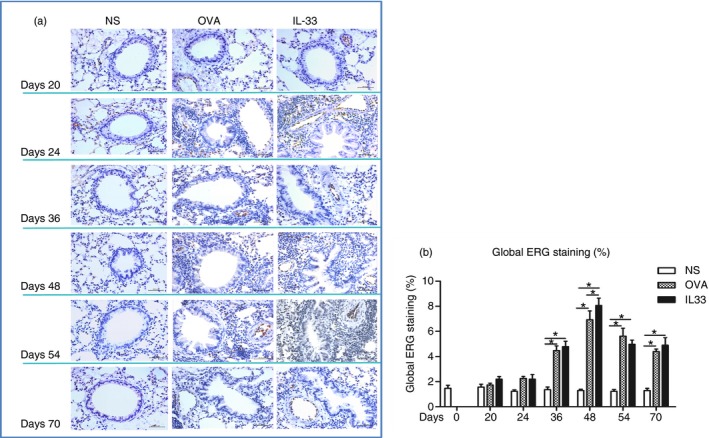
Interleukin‐33 (IL‐33) increased airways erythroblast transformation‐specific‐related gene (ERG) expression. (a) Representative photomicrographs of ERG immunostaining in lung sections from saline‐ (NS), ovalbumin‐ (OVA) and IL‐33‐challenged mice at various time‐points as indicated (light microscopy, × 400). (b) Data are expressed as mean ± SEM (n = 5 mice in each group at each time‐point). *P < 0·05.
IL‐33 induced airways angiogenin and EGF expression
Immunohistochemical analysis showed that compared with control, saline challenge, both OVA challenge and IL‐33 challenge of airways significantly increased the expression of immunoreactivity for lung and parenchymal and peribronchial angiogenin and EGF. Elevated immunoreactivity for angiogenin and EGF was apparent from day 24, peaked at day 48 and persisted until day 70, the end of the experiments (Figs 3 and 4).
Figure 3.
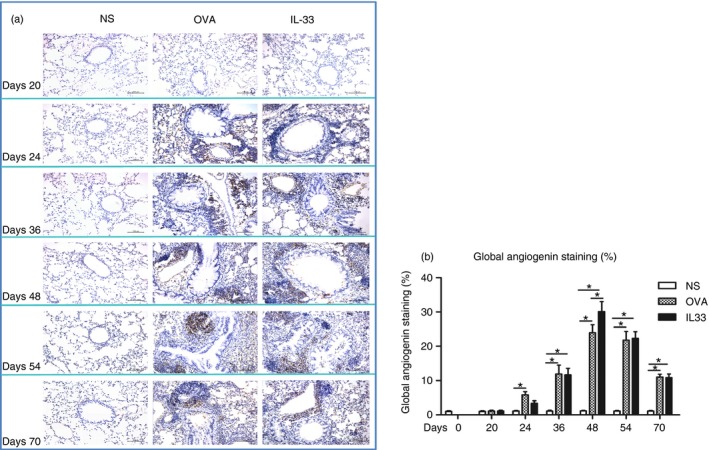
Interleukin‐33 (IL‐33) increased airways angiogenin expression. (a) Representative photomicrographs of angiogenin immunoreactivity in lung sections from saline‐ (NS), ovalbumin‐ (OVA) and IL‐33‐challenged mice at various time‐points as indicated (light microscopy, × 200). (b) Data are expressed as mean ± SEM (n = 5 mice in each group at each time‐point). *P < 0·05.
Figure 4.
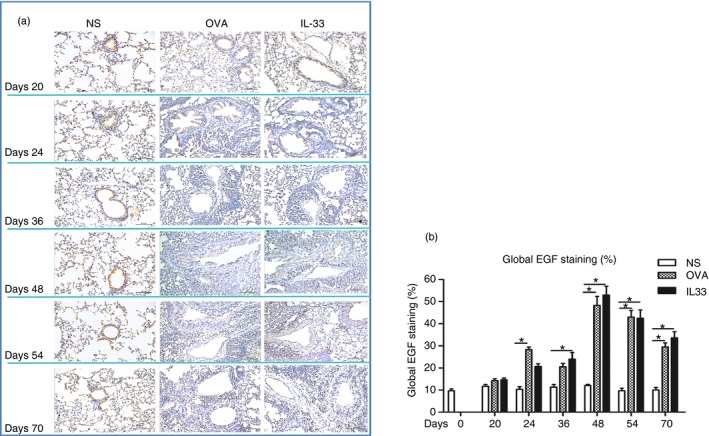
Interleukin‐33 (IL‐33) increased airways epidermal growth factor (EGF) expression. (a) Representative photomicrographs of EGF immunoreactivity in lung sections from saline‐ (NS), ovalbumin‐ (OVA) and IL‐33‐challenged mice at various time‐points as indicated (light microscopy, × 200). (b) Data are expressed as mean ± SEM (n = 5 mice in each group at each time‐point). *P < 0·05.
IL‐33 induced elevated airways IGF‐1, endothelin‐1 and amphiregulin expression
Immunoanalysis showed that IL‐33, compared with saline challenge of the airways, significantly increased expression of lung parenchymal and peribronchial IGF‐1, endothelin‐1 and amphiregulin immunoreactivity from day 20 (endothelin‐1) or day 24 (IGF‐1 and amphiregulin), which persisted until the end of the experiment (Figs 5, 6 and 7). OVA challenge again induced elevated expression of these growth factors with temporal courses statistically equivalent to those induced by IL‐33 challenge.
Figure 5.
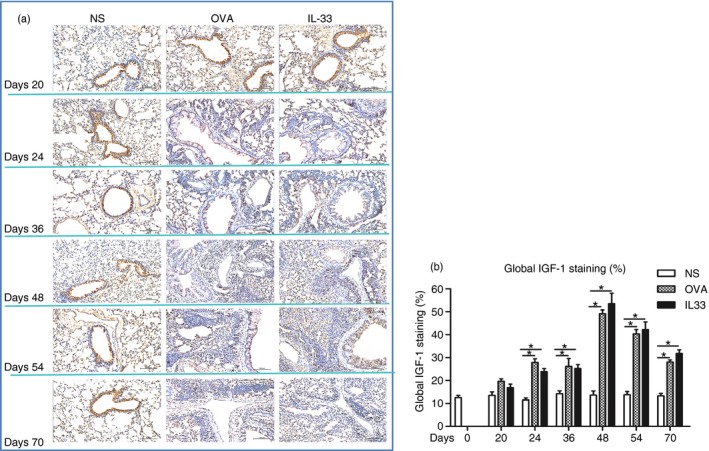
Interleukin‐33 (IL‐33) increased airways insulin‐like growth factor 1 (IGF‐1) expression. (a) Representative photomicrographs of IGF‐1 immunoreactivity in lung sections from saline‐ (NS), ovalbumin‐ (OVA) and IL‐33‐challenged mice at various time‐points as indicated (light microscopy, × 200). (b) Data are expressed as mean ± SEM (n = 5 mice in each group at each time‐point). *P < 0·05.
Figure 6.
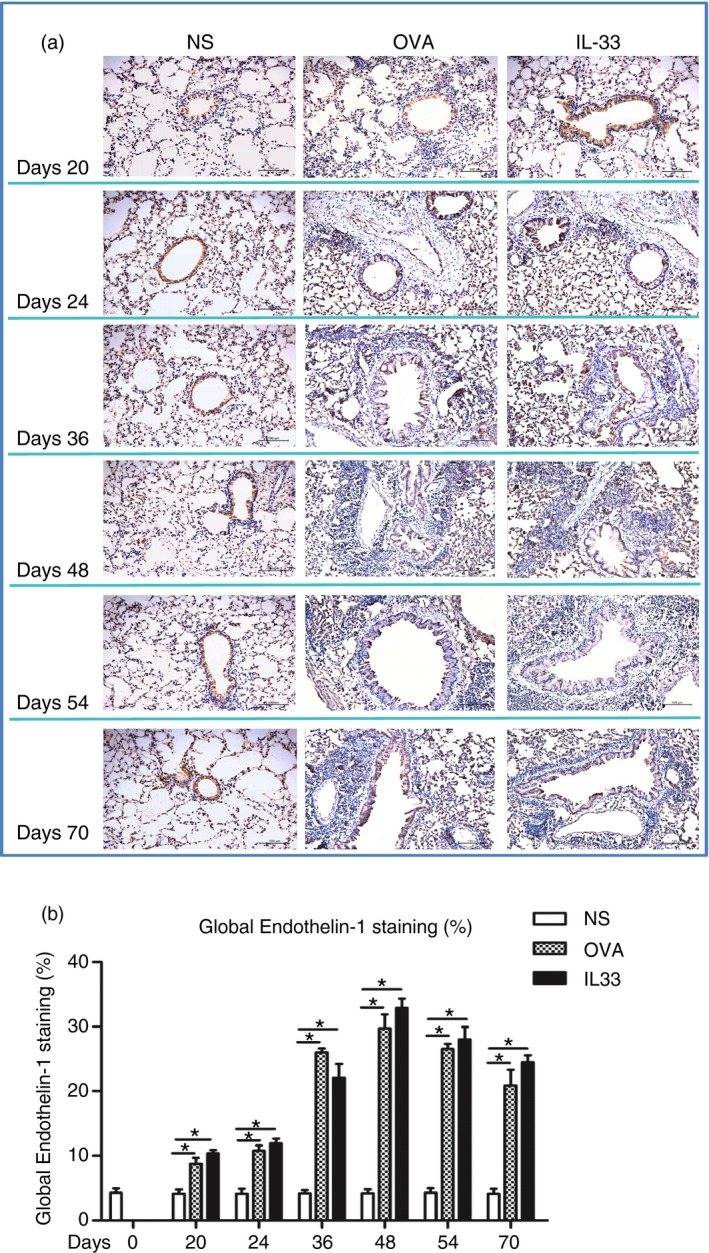
Interleukin‐33 (IL‐33) increased airways endothelin‐1 expression. (a) Representative photomicrographs of endothelin‐1 immunoreactivity in lung sections from saline‐ (NS), ovalbumin‐ (OVA) and IL‐33‐challenged mice at various time‐points as indicated (light microscopy, × 200). (b) Data are expressed as mean ± SEM (n = 5 mice in each group at each time‐point). *P < 0·05.
Figure 7.
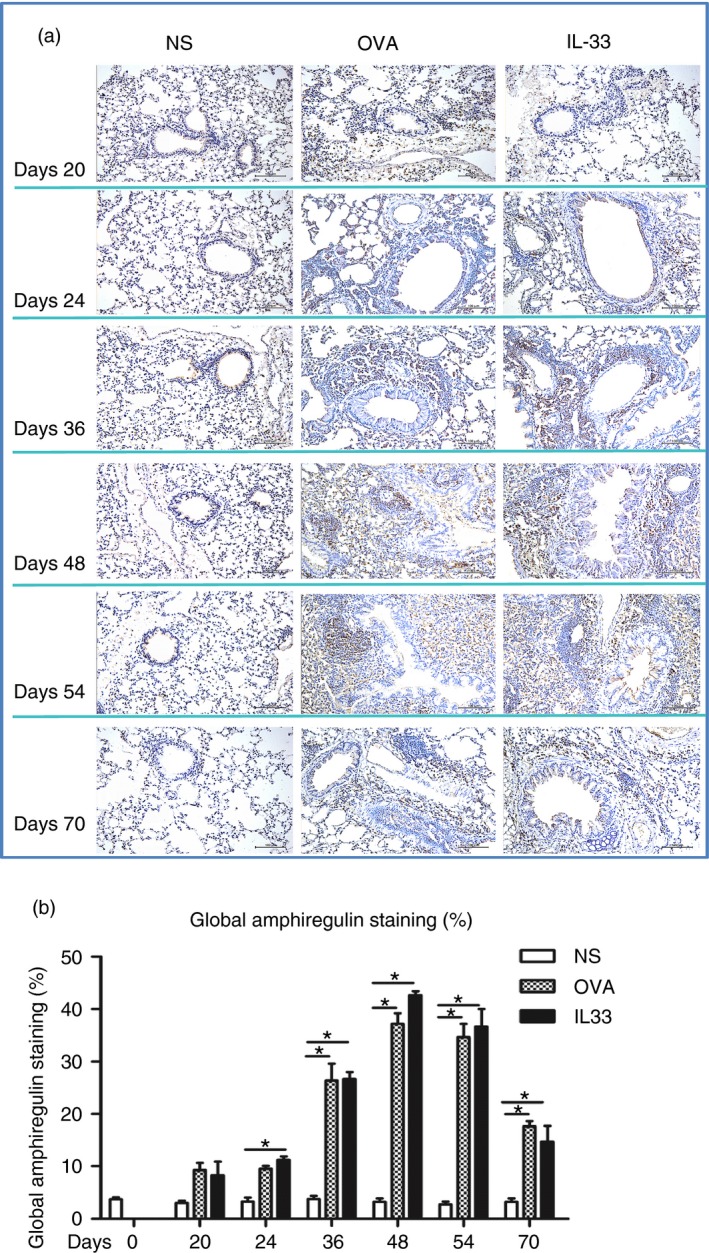
Interleukin‐33 (IL‐33) increased airways amphiregulin expression. (a) Representative photomicrographs of amphiregulin immunoreactivity in lung sections from saline‐ (NS), ovalbumin‐ (OVA) and IL‐33‐challenged mice at various time‐points as indicated (light microscopy, × 200). (b) Data are expressed as mean ± SEM (n = 5 mice in each group at each time‐point). *P < 0·05.
IL‐33 induced human angiogenesis in vitro
In an in vitro angiogenesis assay, IL‐33 induced statistically significant increases in the numbers, lengthenings and branching of microvessels produced by human umbilical vein endothelial cells in a concentration‐dependent fashion (Fig. 8). The effects of IL‐33 at a concentration of 10 ng/ml were equivalent to those of VEGF at the same concentration, which was employed as a positive control.
Figure 8.
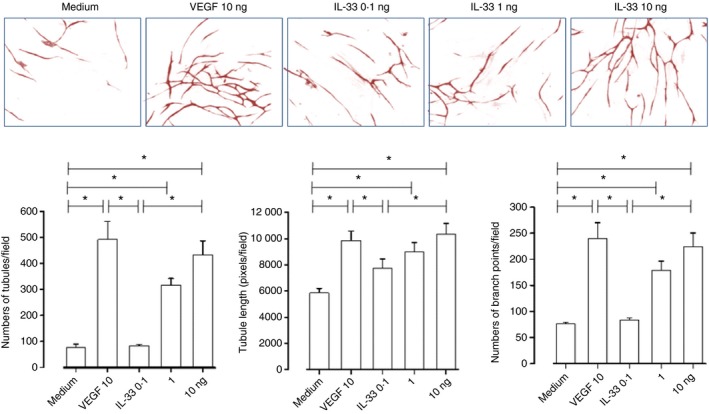
Interleukin‐33 (IL‐33) induced angiogenesis in vitro. Top panel: representative light photomicrographs (4 × original magnification) show formation of primitive vascular tubule structures by human vascular endothelial cells after 11 days of culture with medium control, vascular endothelial growth factor (VEGF) (10 ng/ml) and IL‐33 (0·1, 1·0 and 10 ng/ml). Bottom panel: computer‐assisted quantification of mean total numbers of tubules, mean total tubule lengths and mean numbers of branch points. Bars show the mean ± SEM of three separate experiments performed in duplicate. *P < 0·05.
Discussion
Angiogenesis, a feature of airways remodelling in asthma, can be defined as the emergence of new blood vessels from pre‐existing vasculature. Although the pathophysiological significance of this change still needs to be further explored, increased blood flow and permeability in the asthmatic lung might contribute to oedema and local inflammation because neo‐angiogenesis provides the prerequisites for extravasation of inflammatory cells into local tissue. Some studies have shown that vascularity is associated with asthma severity.1, 3, 4, 5, 6 Previous investigations have also suggested that angiogenesis in asthmatics is closely related to the local expression of angiogenic factors.6, 19, 21 Both IL‐33 and its ST2 receptor are expressed in the endothelial cells of human veins and arteries.22 In human endothelial cells, IL‐33 induced inflammatory activation, as shown by increased vascular permeability, increased production of inflammatory cytokines and the stimulation of angiogenesis.13, 23 The data from the present study confirm that IL‐33 has the capacity to induce angiogenesis in vivo and to increase local expression of multiple angiogenic growth factors in the epithelium and sub‐mucosal tissues of the airways in an established IL‐33‐induced allergen‐independent murine surrogate of asthma. These changes were comparable to those induced by conventional OVA challenge of IgE‐sensitized animals, a surrogate of allergic asthma, and significantly more marked with some analytes, particularly at the peak time‐point of expression at day 48. Furthermore, using an in vitro model of human angiogenesis, we have shown that IL‐33 affects concentration‐dependent microvessel formation by endothelial cells in a manner comparable to that of VEGF, a well‐established angiogenic agent.
Angiogenesis is a complex process, probably regulated by a variety of both discrete and interacting local mechanisms. ERG, expressed throughout the life of the endothelium, appears to contribute to angiogenesis by regulating vascular homeostasis, vascular integrity and endothelial cell growth.24, 25 Inhibition of ERG in human umbilical vein endothelial cells causes loss of cell–cell contact and attenuation of microvessel formation.26 Angiogenin also appears to play a fundamental, if ill‐defined, role in vascular growth.27, 28 Amphiregulin is a potent mitogen for several types of cell, including epithelial cells, vascular smooth muscle cells and endothelial cells acting via the EGF receptor.29 In addition, both innate and adaptive immune cells are able to express this molecule, suggesting that amphiregulin might play a role in orchestrating immunity, inflammation and tissue repair.30 Interestingly, IL‐33 can induce type 2 innate lymphoid cells to produce amphiregulin and Th2‐type cytokines in vitro.31 Endothelin‐1 is a potent vasoconstrictor, which may also cause vascular remodelling, cellular proliferation, extracellular matrix production and capillary permeability.32, 33 As a multi‐functional vascular protein, vWF has been implicated in the regulation of angiogenesis in many diseases from cancer to atherosclerosis.34 EGF promotes cellular proliferation and is involved in embryogenesis, angiogenesis and cellular differentiation.35 IGF‐1 plays an important role in the physiology of endothelial cell structure and function by promoting migration, microvessel formation and production of VEGF and the vasodilator nitric oxide.36
Because angiogenesis is such a complex process, the question of how IL‐33 regulates angiogenesis directly or indirectly through its effects on the expression of these angiogenic factors in asthma and other diseases remains to be clarified. Recent studies indicate that IL‐33 might contribute to the pathogenesis of angiogenesis‐dependent and inflammatory vascular diseases by promoting the production of endothelial nitric oxide and a range of additional mediators including IL‐6, IL‐8, monocyte chemoattractant protein‐1, vascular cell adhesion molecule‐1, intercellular adhesion molecule‐1 and endothelial E‐selectin.13, 23, 37 Interleukin‐33 is constitutively expressed in the nuclei of vascular endothelial cells in various human tissues, with the apparent exception of tumour vessels.38 In line with these studies, we here show that IL‐33 directly promotes angiogenesis in vitro, and in vivo in the setting of asthma‐like inflammation of the airways, directly and/or indirectly by up‐regulating a range of pro‐angiogenic factors. Whatever the case, IL‐33 may be a key molecular target for the inhibition of neo‐angiogenesis and its consequences in the setting of asthma.
Disclosures
The authors declare no conflicts of interest.
Acknowledgements
We acknowledge financial support from the National Natural Science Foundation of China (81373177, 81471594) and the Scientific Research Common Programme of the Beijing Municipal Commission of Education (KM201410025006).
References
- 1. Keglowich LF, Borger P. The three A's in asthma – airway smooth muscle, airway remodeling & angiogenesis. Open Respir Med J 2015; 9:70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bosse Y, Pare PD, Seow CY. Airway wall remodeling in asthma: from the epithelial layer to the adventitia. Curr Allergy Asthma Rep 2008; 8:357–66. [DOI] [PubMed] [Google Scholar]
- 3. Erle DJ, Sheppard D. The cell biology of asthma. J Cell Biol 2014; 205:621–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Meyer N, Akdis CA. Vascular endothelial growth factor as a key inducer of angiogenesis in the asthmatic airways. Curr Allergy Asthma Rep 2013; 13:1–9. [DOI] [PubMed] [Google Scholar]
- 5. Liekens S, De Clercq E, Neyts J. Angiogenesis: regulators and clinical applications. Biochem Pharmacol 2001; 61:253–70. [DOI] [PubMed] [Google Scholar]
- 6. Harkness LM, Ashton AW, Burgess JK. Asthma is not only an airway disease, but also a vascular disease. Pharmacol Ther 2015; 148:17–33. [DOI] [PubMed] [Google Scholar]
- 7. Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK et al IL‐33, an interleukin‐1‐like cytokine that signals via the IL‐1 receptor‐related protein ST2 and induces T helper type 2‐associated cytokines. Immunity 2005; 23:479–90. [DOI] [PubMed] [Google Scholar]
- 8. Prefontaine D, Nadigel J, Chouiali F, Audusseau S, Semlali A, Chakir J et al Increased IL‐33 expression by epithelial cells in bronchial asthma. J Allergy Clin Immunol 2010; 125:752–4. [DOI] [PubMed] [Google Scholar]
- 9. Kearley J, Buckland KF, Mathie SA, Lloyd CM. Resolution of allergic inflammation and airway hyperreactivity is dependent upon disruption of the T1/ST2‐IL‐33 pathway. Am J Respir Crit Care Med 2009; 179:772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kurowska‐Stolarska M, Stolarski B, Kewin P, Murphy G, Corrigan CJ, Ying S et al IL‐33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J Immunol 2009; 183:6469–77. [DOI] [PubMed] [Google Scholar]
- 11. Kurowska‐Stolarska M, Kewin P, Murphy G, Russo RC, Stolarski B, Garcia CC et al IL‐33 induces antigen‐specific IL‐5+ T cells and promotes allergic‐induced airway inflammation independent of IL‐4. J Immunol 2008; 181:4780–90. [DOI] [PubMed] [Google Scholar]
- 12. Kondo Y, Yoshimoto T, Yasuda K, Futatsugi‐Yumikura S, Morimoto M, Hayashi N et al Administration of IL‐33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int Immunol 2008; 20:791–800. [DOI] [PubMed] [Google Scholar]
- 13. Choi YS, Choi HJ, Min JK, Pyun BJ, Maeng YS, Park H et al Interleukin‐33 induces angiogenesis and vascular permeability through ST2/TRAF6‐mediated endothelial nitric oxide production. Blood 2009; 114:3117–26. [DOI] [PubMed] [Google Scholar]
- 14. Stojkovic S, Kaun C, Heinz M, Krychtiuk KA, Rauscher S, Lemberger CE et al Interleukin‐33 induces urokinase in human endothelial cells–possible impact on angiogenesis. J Thromb Haemost 2014; 12:948–57. [DOI] [PubMed] [Google Scholar]
- 15. Li Y, Wang W, Huang P, Zhang Q, Yao X, Wang J et al Distinct sustained structural and functional effects of interleukin‐33 and interleukin‐25 on the airways in a murine asthma surrogate. Immunology 2015; 145:508–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yao XJ, Huang KW, Li Y, Zhang Q, Wang JJ, Wang W et al Direct comparison of the dynamics of IL‐25‐ and ‘allergen’‐induced airways inflammation, remodelling and hypersensitivity in a murine asthma model. Clin Exp Allergy 2014; 44:765–77. [DOI] [PubMed] [Google Scholar]
- 17. McMillan SJ, Lloyd CM. Prolonged allergen challenge in mice leads to persistent airway remodelling. Clin Exp Allergy 2004; 34:497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL‐33‐responsive lineage‐ CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol 2012; 188:1503–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Corrigan CJ, Wang W, Meng Q, Fang C, Wu H, Reay V et al T‐helper cell type 2 (Th2) memory T cell‐potentiating cytokine IL‐25 has the potential to promote angiogenesis in asthma. Proc Natl Acad Sci USA 2011; 108:1579–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang W, Fan YQ, Lv Z, Yao XJ, Huang KW, Meng Q et al Interleukin‐25 promotes basic fibroblast growth factor expression by human endothelial cells through interaction with IL‐17RB, but not IL‐17RA. Clin Exp Allergy 2012; 42:1604–14. [DOI] [PubMed] [Google Scholar]
- 21. Salvato G. Quantitative and morphological analysis of the vascular bed in bronchial biopsy specimens from asthmatic and non‐asthmatic subjects. Thorax 2001; 56:902–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bartunek J, Delrue L, Van Durme F, Muller O, Casselman F, De Wiest B et al Nonmyocardial production of ST2 protein in human hypertrophy and failure is related to diastolic load. J Am Coll Cardiol 2008; 52:2166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aoki S, Hayakawa M, Ozaki H, Takezako N, Obata H, Ibaraki N et al ST2 gene expression is proliferation‐dependent and its ligand, IL‐33, induces inflammatory reaction in endothelial cells. Mol Cell Biochem 2010; 335:75–81. [DOI] [PubMed] [Google Scholar]
- 24. Birdsey GM, Shah AV, Dufton N, Reynolds LE, Osuna Almagro L, Yang Y et al The endothelial transcription factor ERG promotes vascular stability and growth through Wnt/beta‐catenin signaling. Dev Cell 2015; 32:82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Adamo P, Ladomery MR. The oncogene ERG: a key factor in prostate cancer. Oncogene 2016; 35:403–14. [DOI] [PubMed] [Google Scholar]
- 26. Birdsey GM, Dryden NH, Amsellem V, Gebhardt F, Sahnan K, Haskard DO et al Transcription factor Erg regulates angiogenesis and endothelial apoptosis through VE‐cadherin. Blood 2008; 111:3498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Palgan K, Bartuzi Z. Angiogenesis in bronchial asthma. Int J Immunopathol Pharmacol 2015; 28:415–20. [DOI] [PubMed] [Google Scholar]
- 28. Kishimoto K, Liu S, Tsuji T, Olson KA, Hu GF. Endogenous angiogenin in endothelial cells is a general requirement for cell proliferation and angiogenesis. Oncogene 2005; 24:445–56. [DOI] [PubMed] [Google Scholar]
- 29. Busser B, Sancey L, Brambilla E, Coll JL, Hurbin A. The multiple roles of amphiregulin in human cancer. Biochim Biophys Acta 2011; 2:119–31. [DOI] [PubMed] [Google Scholar]
- 30. Zaiss DM, Gause WC, Osborne LC, Artis D. Emerging functions of amphiregulin in orchestrating immunity, inflammation, and tissue repair. Immunity 2015; 42:216–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska‐Owsiak D, Wang X et al A role for IL‐25 and IL‐33‐driven type‐2 innate lymphoid cells in atopic dermatitis. J Exp Med 2013; 210:2939–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pala S, Atilgan R, Ozkan ZS, Kavak SB, Ilhan N, Akpolat N et al Effect of varying doses of tamoxifen on ovarian histopathology, serum VEGF, and endothelin 1 levels in ovarian hyperstimulation syndrome: an experimental study. Drug Des Devel Ther 2015; 9:1761–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Maguire JJ, Davenport AP. Endothelin receptors and their antagonists. Semin Nephrol 2015; 35:125–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Starke RD, Ferraro F, Paschalaki KE, Dryden NH, McKinnon TA, Sutton RE et al Endothelial von Willebrand factor regulates angiogenesis. Blood 2011; 117:1071–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schreier B, Gekle M, Grossmann C. Role of epidermal growth factor receptor in vascular structure and function. Curr Opin Nephrol Hypertens 2014; 23:113–21. [DOI] [PubMed] [Google Scholar]
- 36. Bach LA. Endothelial cells and the IGF system. J Mol Endocrinol 2015; 54:14–0215. [DOI] [PubMed] [Google Scholar]
- 37. Demyanets S, Konya V, Kastl SP, Kaun C, Rauscher S, Niessner A et al Interleukin‐33 induces expression of adhesion molecules and inflammatory activation in human endothelial cells and in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol 2011; 31:2080–9. [DOI] [PubMed] [Google Scholar]
- 38. Kuchler AM, Pollheimer J, Balogh J, Sponheim J, Manley L, Sorensen DR et al Nuclear interleukin‐33 is generally expressed in resting endothelium but rapidly lost upon angiogenic or proinflammatory activation. Am J Pathol 2008; 173:1229–42. [DOI] [PMC free article] [PubMed] [Google Scholar]


