Summary
Uterine natural killer (uNK) cells are the most abundant lymphocyte population in the feto–maternal interface during early gestation, and uNK cells play a significant role in the establishment and maintenance of pregnancy‐related vascularization, as well as in tolerance to the fetus. Tumour necrosis factor‐like weak inducer of apoptosis (TWEAK) and its receptor, fibroblast growth factor‐inducible molecule (Fn14), are involved in preventing local cytotoxicity and counterbalancing the cytotoxic function of uNK cells. Here, we studied the regulation of TWEAK/Fn14‐mediated innate immunity in the uterus using a lipopolysaccharide (LPS)‐induced model of abortion in pregnant mice. Specifically, we detected the expression of TWEAK and Fn14 in the uterus and in uNK cells following LPS treatment. Our results revealed that TWEAK and Fn14 are expressed by uNK cells in pregnant mice; in particular, it appears that the cytokine TWEAK is primarily derived from uNK cells. Interestingly, the down‐regulation of TWEAK in uNK cells and the up‐regulation of the Fn14 receptor in the uterus in LPS‐treated mice may contribute to the disruption of decidual homeostasis by altering uNK cell cytotoxicity – ultimately leading to fetal rejection. In conclusion, the present study strongly suggests that the TWEAK–Fn14 axis in uNK cells is involved in maintaining the tolerance necessary for successful pregnancy.
Keywords: fibroblast growth factor‐inducible molecule, lipopolysaccharide‐induced abortion, pregnant mice, tumour necrosis factor‐like weak inducer of apoptosis, uterine natural killer cell cytotoxicity
Abbreviations
- DBA
Dolichos biflorus agglutinin
- Fn14
fibroblast growth factor‐inducible molecule
- GD
gestational day
- LPS
lipopolysaccharide
- TNF
tumour necrosis factor
- TWEAK
tumour necrosis factor‐like weak inducer of apoptosis
- uNK
uterine natural killer
Introduction
Inflammatory immune responses are often associated with reproductive failures during ovulation, fertilization, implantation and pregnancy. In species with invasive haemochorial placentation, uterine natural killer (uNK) cells contribute to the physiological changes that turn the mesometrial endometrium into a unique stromal environment, called the decidua basalis, through the secretion of cytokines, chemokines, mucins, enzymes and angiogenic growth factors.1, 2, 3 In humans, uNK cells have a CD56bright CD16dim phenotype, and they appear in great numbers during the later secretory phase of the menstrual cycle and early pregnancy.4, 5 Mouse, but not human, uNK cells bind Dolichos biflorus agglutinin (DBA) lectin on their plasma membranes and in numerous cytoplasmic granules, allowing the identification of early uNK cells before their acquisition of cytoplasmic granules.6 The use of DBA lectin staining has mostly replaced Periodic Acid Schiff's staining for the histological recognition of mouse uNK cells.6 Gene expression studies indicate that CD56bright CD16dim uterine and circulating NK cells are functionally distinct.7 Uterine NK cells express normal surface levels of a specific activating receptor as well as killer cell immunoglobulin‐like receptors and the CD94/NKG2A inhibitory receptor.4 Although they may be capable of killing, uNK cells are generally thought to display only limited lytic activity.5 However, the mechanisms underlying the differentiation and secretory activity of uNK cells remain unclear.
Tumour necrosis factor‐like weak inducer of apoptosis (TWEAK) is a cytokine of the tumour necrosis factor (TNF) ligand superfamily that is expressed by many types of leucocytes, including resting and activated monocytes, dendritic cells and circulating NK cells.8 Little is known about the regulation of TWEAK expression in specific cells, although a few studies suggest that the TWEAK protein can be up‐regulated by interferon‐γ or PMA in cultured human peripheral blood monocytes, dendritic cells and NK cells. Like TNF, TWEAK is a type II transmembrane homotrimer that can function as a soluble cytokine with diverse biological roles, including pro‐inflammatory activity, angiogenesis and the regulation of cell survival, proliferation and death.9, 10 TWEAK mediates these effects through its receptor, fibroblast growth factor‐inducible molecule (Fn14), and signals via the nuclear factor‐κB and mitogen‐activated protein kinase pathways. Interestingly, Fn14 is expressed at low levels in normal tissues, whereas it is highly up‐regulated in the contexts of tissue injury and regeneration, suggesting a physiological role for this pathway in coordinating acute inflammation and tissue repair.10 Fn14 is also highly up‐regulated in chronic inflammatory disease, supporting a role for this pathway in promoting end organ pathology in chronic disease settings.11 However, the molecular mechanism by which TWEAK/Fn14 regulates tissue injury has yet to be identified. A previous study reported that the TWEAK/Fn14 pathway controls the innate inflammatory response as well as the transition to T helper type 1‐based adaptive immunity.8 In addition to these important functions, accumulating evidence in humans and mice suggests that TWEAK may play an early role in preventing local cytotoxicity, counterbalancing the cytotoxic functions of uNK cells during gestation in favour of the constructive angiogenic/immunotrophic pathways.12, 13 Uterine NK cells are a transient population of cells; they have a unique immunophenotype and produce a number of cytokines and growth factors. However, the expression profiles of TWEAK and Fn14 in mouse uNK cells, the most abundant lymphocyte population in the feto–maternal interface during early gestation, remain poorly characterized.
Endotoxin is the name given to a group of heat‐stable lipopolysaccharide (LPS) molecules present in the cell walls of Gram‐negative bacteria that possess toxic effects. Endotoxin‐induced pregnancy loss may be mediated by the release of pro‐inflammatory cytokines/growth factors following the activation of lympho‐haematopoietic cells of maternal origin.14, 15 An understanding of the molecular and cellular requirements for pregnancy homeostasis would be extremely useful. However, LPS‐induced changes in the levels and patterns of TWEAK/Fn14 expression in the uterus during the implantation period remain unclear. The present study was designed to determine the early regulation of TWEAK/Fn14‐mediated innate immunity in the uterus in an LPS‐induced model of abortion in pregnant mice.
Materials and methods
Mice
Male and female BALB/c mice were obtained from The Fourth Military Medical University (Xian, China) at the age of 7 weeks and were maintained in our colony for at least 7 days before the experimental matings. One male and two females were housed together for mating. Females that displayed copulation plugs the next morning were dated as gestation day (GD) 0·5 and were isolated from the males. Pregnant animals were anaesthetized and then killed by cervical dislocation on the specific GD noted and their uteri were dissected.
LPS‐induced abortion in mice
The protocol for LPS‐induced abortion was performed as described previously.16 Briefly, a dose of 2 μg LPS (Escherichia coli, 026:B6, Sigma L8274; Sigma, St Louis, MO) in 200 μl PBS was injected intraperitoneally into each mouse at GD 3.
Murine uterus preparation
During early to midpregnancy (GD 0·5, 3, 5 and 9·5) the two uterine horns were separated and then either cut into small fragments for immunohistochemistry and immunofluorescence staining or immediately stored at −80° for later protein extraction. Each immunohistochemistry experiment was repeated at least three times, and a variety of tissue fixation conditions and antibody concentrations were often included in the same experiment to confirm the results. For the Western blots, tissue samples from between five and ten mice were included for all groups.
Isolation of uNK cells
The uNK cells were isolated using DBA lectin‐coated magnetic beads, as described previously.17 Briefly, the endometrial tissues were obtained from normal pregnant mice and mice subjected to LPS‐induced abortion on the indicated GD, minced with razor blades, and homogenized in Hanks' balanced salt solution supplemented with 1% BSA and 1000 U DNase type I. After washing by centrifugation with Hanks' solution, the DBA lectin‐conjugated magnetic beads were added to the cell suspension, and the DBA lectin‐reactive uNK cells were isolated using a Magnetic Particle Concentrator (MPC‐2; Dynal, Invitrogen, Waltham, MA) and counted in a Neubauer's chamber. The beads were removed from the uNK cells with 0·1 m N‐acetyl‐d‐galactosamine (Gal‐Nac). The viability of the uNK cells was consistently ≥ 95%, as tested by trypan blue exclusion. The purified uNK cells were cultured in sterile RPMI‐1640 medium supplemented with 10% fetal bovine serum, 0·2 μg/ml interleukin‐15 (IL‐15), and 100 ng/ml CXCL10.
Immunohistochemistry
To examine the localization of uNK cells and TWEAK/Fn14 in the murine uterus, paraffin sections (5 μm) from the endometrium samples were prepared for immunohistochemistry, as previously described.12 Briefly, the sections were incubated with the following reagents: DBA lectin (1 : 1500, Sigma), a rabbit anti‐mouse TWEAK antibody (1 : 100; Abcam, Cambridge, UK) and a rabbit anti‐mouse Fn14 antibody (1 : 75; Abcam). After incubation with the primary antibodies or lectin (16 hr at 4°), the sections were washed and incubated with horseradish peroxidase‐conjugated streptavidin (1 : 1000; Sigma) for DBA lectin staining or horseradish peroxidase‐conjugated goat anti‐rabbit IgG for TWEAK/Fn14 staining (1 : 1000; Vector Laboratories Inc., Burlingame, CA) and visualized with 3,3,‐diaminobenzidine (Sigma) through a hydrogen peroxide reaction. The photomicrographs were collected using an Eclipse 800 imaging system (Nikon, Tokyo, Japan) equipped with Image ProPlus software (Media Cybernetics Inc., Rockville, MD). The number of DBA‐positive uNK cells was counted in three randomly selected fields of view using three sections from each placenta. The fields counted were limited to the endometrial stroma and the metrial gland on the indicated days.
RNA isolation and real‐time PCR analysis
Total RNA was extracted from the uNK cells using TRIzol reagent (Invitrogen), according to the manufacturer's instructions. The total RNA (1 μg) was reversed transcribed into cDNA using random primers and Superscript III (Invitrogen), according to the manufacturer's instructions. Controls without reverse transcriptase were systematically performed to detect genomic DNA contamination. The cDNAs were stored at −20° until further use. The cDNA was amplified with specific primers and Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). 18S rRNA levels were used as an internal normalization control. The sequences of the primers were as follows:
TWEAK, forward 5′‐CTGTCAGGTGCACTTTGATGAG‐3′ (Tm 64·8°) and reverse 5′‐AGCAAGTCCAGCTTCAGGTAGA‐3′ (Tm 64·2°) (product 54 bp); Fn14, forward 5′‐GCGCTCTGAGCCTGACCTTC‐3′ (Tm 68·8°) and reverse 5′‐GGTGGTGAACTTCTCTCTCCTGC‐3′ (Tm 67·4°) (product 86 bp); 18S rRNA, forward 5′‐TTCGGAACTGAGGCCATGAT‐3′ (Tm 66·7°) and reverse 5′‐CGAACCTCCGACTTTCGT‐3′ (Tm 62·9°) (product 151 bp). The following amplification conditions were used: 20 seconds at 95°, followed by 40 cycles of 3 seconds at 95° and 30 seconds at 60°. Quantifications were based on the Ct value, which is the number of cycles necessary to produce a detectable amount of product over background. The difference in the Ct (d) was normalized to the 18S rRNA housekeeping gene and then used to calculate the fold difference in copy number using the formula f = 2(d), where f = the fold difference in the expression of a specific gene and d = the difference in the Ct values between the compared sources of mRNA (corrected for differences in the 18S rRNA levels). We normalized each sample to normal endometrial specimen #1. Melt curves were performed to confirm the purity of the amplified products.
Western blotting analysis
The expression of the TWEAK, Fn14, NKG2D and TNF‐α proteins in the murine uterus and uNK cells during pregnancy was determined using Western blot analysis. Protein homogenates from the mice uteri were isolated, as previously described.18 Briefly, the uterine tissue was rapidly excised and placed in ice‐cold saline until dissection. The uterine tissues were homogenized in RadioImmunoPrecipitation Assay lysis buffer (Sigma) using a pipette. The homogenates were centrifuged (12 000 g for 20 min at 4°) to remove any insoluble material. For the protein homogenates from the uNK cells, the cells were harvested and washed once with cold PBS. The cells were lysed on ice in lysis buffer (50 mm Tris–HCl pH 7·4, 150 mm NaCl, 1 mm EDTA pH 8·0, 1% Triton X‐100, 1% sodium deoxycholate, 0·1% SDS and protease inhibitor cocktail tablets; Roche, Basel, Switzerland) for 20 min. The cell debris was removed by centrifugation at 15 000 g for 5 min at 4°. Protein concentration was determined using the BCA assay kit (Thermo Scientific, Waltham, MA). Then, the uterine tissue or cell lysates were separated on 12% SDS–polyacrylamide gels blotted onto polyvinylidene difluoride membranes and probed with a rabbit polyclonal antibody against mouse TWEAK (1 : 2000; Abcam), a rabbit monoclonal antibody against mouse Fn14 (1 : 3000; Abcam), a rabbit polyclonal antibody against mouse NKG2D (1 : 1000; Abcam), or a rabbit polyclonal antibody against mouse TNF‐α (1 : 1500; Abcam) at 4°. The bands were visualized using horseradish peroxidase‐conjugated goat anti‐rabbit IgG (1 : 20 000; Boster, Wuhan, China) before the ECL protocol (Amersham Biosciences, Piscataway, NJ). The protein bands were recorded using a Konica SRX 101A developer (Konica Minolta Medical Imaging, Wayne, NJ). β‐Actin protein expression was used as an internal standard for all experiments. The results are expressed as ratios (protein of interest/β‐actin) to correct for the loading of each sample.
To determine the role of TWEAK in regulating the expression of the uNK cytotoxic receptor NKG2D and TNF‐α, 106 primary uNK cells isolated from mice with normal pregnancies on GD 9·5 were seeded into each well of a 24‐well plate. Recombinant TWEAK (Abcam) or a TWEAK antibody (Abcam) was added at the indicated final concentration (0, 0·1, 1·0 μg/ml). After 24 hr, the cells were collected and analysed by Western blotting.
Immunofluorescent staining
To determine whether TWEAK, Fn14, NKG2D and TNF‐α are expressed in uNK cells collected from the LPS‐treated mice, we stained the cells using a commercially available monoclonal antibody and then analysed them using dual reagent immunofluorescent staining. Five‐micrometre paraffin sections mounted on glass slides were rinsed, rehydrated and blocked (1 hr at room temperature; Serum Free Protein Block; Dako, Mississauga, ON, Canada). The following primary antibodies were diluted in a sodium azide‐supplemented antibody diluent with 0·1% Tween (Dako) and used to stain the sections: DBA lectin (1 : 2000; Sigma); rabbit anti‐mouse TWEAK (1 : 100; Abcam); rabbit anti‐mouse Fn14 (1 : 75; Abcam), rabbit anti‐mouse NKG2D (1 : 125; Abcam), and rabbit anti‐mouse TNF‐α (1 : 300; Abcam). The sections were washed and incubated (2 hr at room temperature) with an FITC‐conjugated goat anti‐rabbit secondary antibody (1 : 50; Boster). The sections were then incubated (2 hr at room temperature) with Cy3‐tagged streptavidin (1 : 75), treated with 20 mm l‐lysine (Sigma) to quench the autofluorescence, and finally cover‐slipped with DAPI‐supplemented mounting media (ProLong Gold Anti‐fade Reagent with 4,6‐diamidino‐2‐phenylindole; Invitrogen). Photomicrographs were collected using an AxioCam equipped Zeiss M1 Imager with axiovision software (Carl Zeiss, Toronto, ON, Canada).
To count the number of DBA‐positive uNK cells expressing TWEAK, Fn14, NKG2D, or TNF‐α, the implant sites were examined on GD 0·5, 3, 5 and 9·5 using 21 to 60 fluorescence photomicrographs (200 ×) from randomly selected mesometrial areas. Nuclear DAPI staining was used to estimate total cell number with the aid of Manual Point Count (image proplus 6), as well as the numbers of antibody‐reactive or lectin‐reactive cells.
Statistical analysis
All values are expressed as the arithmetic means of triplicates ± SEM. Significant differences were analysed using a two‐tailed Student's t‐test. Values of P < 0·05 were considered statistically significant.
Results
Distribution of uNK cells in pregnant mice treated with LPS
Mice treated with 2 μg LPS/mouse exhibited complete (100%) embryonic loss, as assessed by an evaluation of the placental units on GD 9·5. The average number of uNK cells in the endometrial stroma and metrial gland were calculated on the indicated days to estimate cell density. DBA lectin staining during pre‐implantation revealed a dynamic change in the number of uNK cells between GD 0·5 and 9·5 of pregnancy. At GD 0·5, which was immediately after copulation, DBA lectin stained a small population of cells that were scattered throughout the stromal region, as well as the endothelial cells of small blood vessels (Fig. 1a,f). In contrast, at GD 3 the number and intensity of DBA lectin‐stained cells had significantly decreased (Fig. 1b,g). At GD 5, the DBA lectin staining increased and primarily stained the endothelial cells of blood vessels, as well as scattered small cells in the stroma. Immunostaining results on GD 9·5 showed an increased accumulation of uNK cells in the mesometrial pole of the implantation sites (Fig. 1c,h). There were no significant differences in the density of uNK cells between the LPS‐induced abortion and normal pregnant control group on days 0·5 and 3 of pregnancy. However, we observed a marked decrease in the number of uNK cells on GD 9·5 in the uterus of the mice subjected to LPS‐induced abortion compared with the control mice (Fig. 1d,i). No positive reaction was observed in the blank control reaction performed with PBS alone (Fig. 1e,j). The mean numbers of uNK cells per mm2 in the superficial endometrial stroma and metrial gland from the normal pregnant mice and the LPS‐treated pregnant mice on the indicated days are shown in Fig. 1(k).
Figure 1.
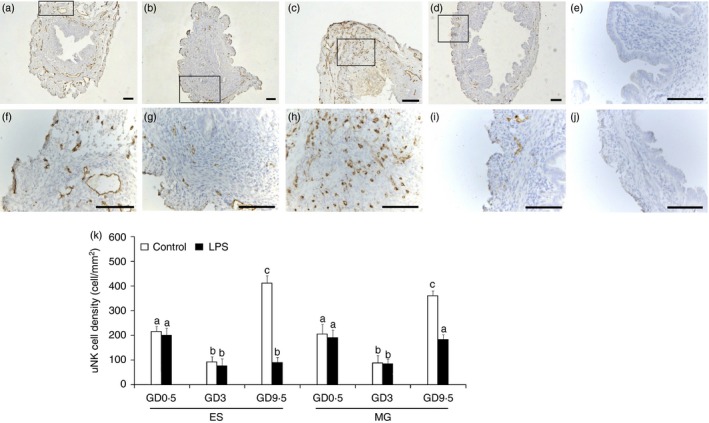
Distribution and density of uterine natural killer (uNK) cells in the mouse uterus during early to mid‐pregnancy. Photomicrographs of Dolichos biflorus agglutinin (DBA) lectin‐stained uterine sections collected from normal pregnant mice on gestational day (GD) 0·5 (a, f), 3 (b, g), and 9·5 (c, h) as well as lipopolysaccharide (LPS) ‐treated pregnant mice on GD 9·5 (d, i). The brown‐yellow stains indicate DBA lectin bound to uNK cells or specific cell components. The boxed areas in a–d are shown at higher magnification in the bottom panels (f–i). A positive reaction was not observed in the blank control reaction performed with PBS alone (e, j). The mean number of uNK cells per mm2 in the superficial endometrial stroma (ES) and metrial gland (MG) from normal pregnant mice and LPS‐treated pregnant mice on the indicated days are shown (k). The data represent the means ± SEM of at least three independent experiments. Columns with different superscript letters are significantly different (P < 0·05). Scale bars = 200 μm.
Expression of TWEAK/Fn14 in the uterus of LPS‐treated pregnant mice
We analysed the localization and expression levels of the TWEAK protein and its receptor, Fn14, in the uterus of normal pregnant mice and mice subjected to LPS‐induced abortion during early pregnancy. TWEAK labelling was first observed on GD 0·5 in the uterus of the normal pregnant mice, and this signal had already begun to decline by GD 3. Immunostaining was visibly stronger on GD 5 compared with GD 3, and this staining peaked by GD 9·5. TWEAK was mainly detected in the luminal epithelium, although it could also be seen scattered within the stroma around the spiral arteries (Fig. 2a). In addition, strong Fn14 staining was observed in the luminal and glandular epithelium in the uterus of normal pregnant mice on GD 0·5, whereas only mild staining was detected from GD 3 to 9·5. Similar results were observed for TWEAK and Fn14 staining in the uterus of mice subjected to LPS‐induced abortion from GD 0·5 to 5 by immunohistochemistry. By GD 9·5, however, very weak TWEAK staining and strong Fn14 staining was observed in the uterus of the LPS‐treated mice compared with the normal pregnant mice (Fig. 2a).
Figure 2.
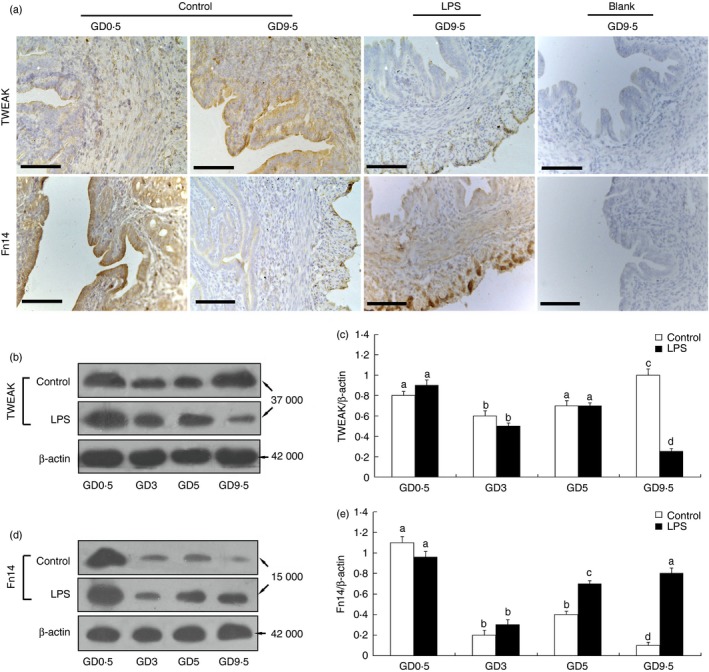
Location and expression of the tumour necrosis factor‐like weak inducer of apoptosis (TWEAK) and fibroblast growth factor‐inducible molecule (Fn14) proteins in the mouse endometrium during early to mid‐pregnancy. (a) Representative microphotographs of immunohistochemical staining for TWEAK and Fn14 in the mouse endometrium from lipopolysaccharide (LPS) ‐treated and untreated mice at the indicated days of gestation. A positive reaction was not observed in the endometrium from early pregnancy stained with PBS as blank control (a). Scale bars = 200 μm. The levels of TWEAK and Fn14 protein in the mouse endometrium at the indicated days of pregnancy were analysed by Western blotting. Representative blots are shown in the left panel (b, d), and the histograms in the right panel (c, e) show the results of densitometric analyses of TWEAK or Fn14 expression (normalized to β‐actin to correct for protein loading). The data represent means ± SEM from at least three independent experiments. Columns with different superscript letters are significantly different (P < 0·05).
Quantitative differences in TWEAK and Fn14 expression were assessed by Western blot analysis. As shown in Fig. 2, the TWEAK and Fn14 proteins were mainly detected as 37 000 and 15 000 MW bands, respectively (Fig. 2b,d). Densitometric analysis confirmed that the protein levels of both TWEAK (Fig. 2c) and Fn14 (Fig. 2e) in the uteri of control and LPS‐treated mice from GD 0·5 to 9·5 showed the same trends as the histological assessments.
Co‐localization of TWEAK/Fn14 and uNK cells in the LPS‐treated mice
To determine whether TWEAK and Fn14 are expressed by mouse uNK cells, we stained the cells with a commercially available monoclonal antibody and analysed them using dual reagent immunofluorescence detection. A large percentage of uNK cells in the uterus of the control pregnant mice that were TWEAK‐positive could be detected as early as GD 0·5, followed by a decline on GD 3 and a peak at GD 9·5 (Fig. 3a,b). The percentage of Fn14‐positive uNK cells in the control uteri peaked on GD 0·5 followed by a decrease in the percentage of positive cells from GD 3 to 9·5 (Fig. 3c,d). Similar expression profiles for TWEAK and Fn14 were observed from GD 0·5 to 3 in the uNK cells from the mice subjected to LPS‐induced abortion. Interestingly, in the LPS‐induced abortion group, only a very small percentage of TWEAK‐positive uNK cells was observed on GD 5 and GD 9·5, whereas there was a marked increase in the percentage of Fn14‐positive uNK cells on GD 5 and GD 9·5 compared with the control group (Fig. 3). Immunofluorescence staining showed that the changes in the numbers of TWEAK‐positive and Fn14‐positive uNK cells in the uteri from control and abortion‐induced mice were consistent with TWEAK and Fn14 protein expression in the uterus as well as changes in the number of uNK cells, as determined by Western blotting (Fig. 2) and histological (Fig. 1) analyses, respectively.
Figure 3.
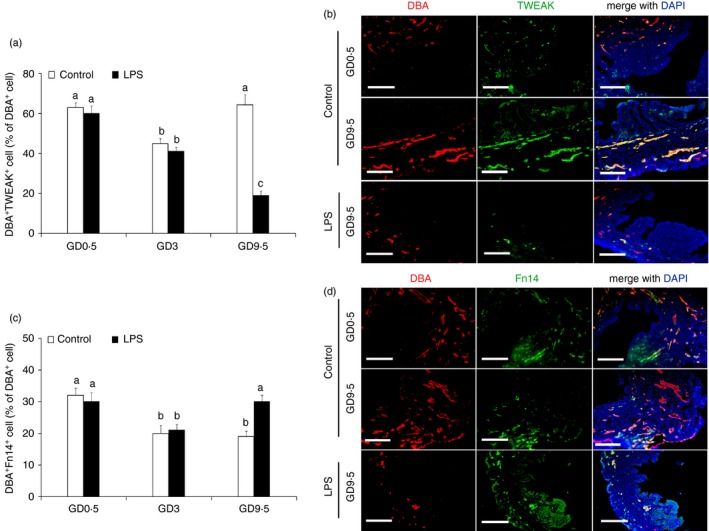
Co‐localization of tumour necrosis factor‐like weak inducer of apoptosis (TWEAK) and fibroblast growth factor‐inducible molecule (Fn14) with uterine natural killer (uNK) cells from lipopolysaccharide (LPS) ‐treated and untreated mice at the indicated gestation days. The percentage of TWEAK‐positive (a) and Fn14‐positive cells (c) of the Dolichos biflorus agglutinin (DBA) ‐positive uNK cells from LPS‐treated and untreated mice at the indicated gestation days. Representative fluorescence photomicrographs show the co‐localization of TWEAK (b) and Fn14 (d) in the uNK cells in the endometrium of a pregnant mouse. Mouse uNK cells in the 4% PFA‐fixed, paraffin‐embedded tissues were labelled with DBA lectin and rabbit anti‐mouse TWEAK or rabbit anti‐mouse Fn14 antibodies, and then stained with Cy3‐tagged streptavidin for the uNK cells or an FITC‐conjugated goat anti‐rabbit secondary antibody for TWEAK or Fn14. Nuclei were labelled with DAPI. Scale bars = 200 μm.
Expression of TWEAK/Fn14 in uNK cells isolated from mice
Viable and highly pure mouse uNK cells were effectively isolated using DBA lectin‐conjugated magnetic beads (Fig. 4a,b). To confirm the increased TWEAK/Fn14 production by uNK cells, uNK cell suspensions isolated from pregnant and LPS‐induced abortion mice from GD 0·5 to GD 9·5 were analysed using quantitative real‐time RT‐PCR and Western blot analysis. Consistent with the co‐localization of TWEAK/Fn14 immunofluorescence observed in uNK cells (Fig. 3), quantitative real‐time PCR analysis confirmed strong TWEAK and Fn14 mRNA expression in GD 0·5 uNK cells, which was followed by a decrease in TWEAK and Fn14 expression on GD 5 and GD 3, respectively, in the uNK cells from both the control and LPS‐treated mice. On GD 9·5, the levels of TWEAK mRNA increased significantly (Fig. 4c), whereas Fn14 mRNA was expressed at a low level in the uNK cells from the control mice (Fig. 4d). By contrast, compared with the control mice, significantly decreased TWEAK mRNA levels (Fig. 4c) and increased Fn14 mRNA levels (Fig. 4d) were detected in the uNK cells isolated at GD 9·5 from mice subjected to LPS‐induced abortion, approximately the time at which fetal loss occurs. Overall, the expression profiles of the TWEAK and Fn14 proteins were consistent with their mRNA expression levels (Fig. 4e–h). A densitometric analysis of the Western blotting results revealed similar levels of TWEAK and Fn14 proteins in the uNK cells from both the control and LPS‐treated mice on GD 3 and GD 5, whereas decreased TWEAK and increased Fn14 protein expression was observed in uNK cells from the LPS‐treated mice compared with the normal pregnancy group on GD 9·5 (Fig. 4f,h).
Figure 4.
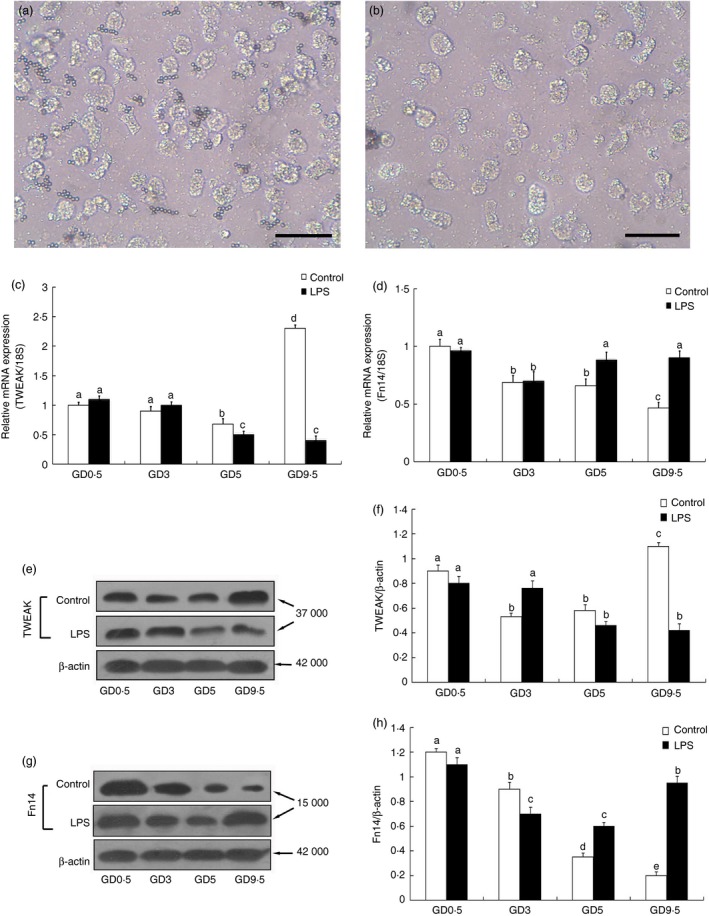
Expression of tumour necrosis factor‐like weak inducer of apoptosis (TWEAK) and fibroblast growth factor‐inducible molecule (Fn14) by uterine natural killer (uNK) cells isolated from mice during early to mid‐pregnancy. Uterine NK cells were isolated using Dolichos biflorus agglutinin (DBA) lectin‐coated magnetic beads. Differential interference contrast images of viable mouse uterine leucocytes attached to the DBA lectin‐coated magnetic beads (a) and after they were detached by the addition of 0·1 m Gal‐NAc (b). Scale bars = 50 μm. The expression of TWEAK and Fn14 in the uNK cells on the indicated days of pregnancy in response to the LPS treatment was analysed by quantitative real‐time RT‐PCR (c, d) and Western blot analysis (e–h). Representative blots are shown in the left panel (e, g) and the histograms in the right panel (f, h) show the results of densitometric analysis of TWEAK and Fn14 expression (normalized to β‐actin to correct for protein loading). The data represent the means ± SEM from at least three independent experiments. Columns with different superscript letters are significantly different (P < 0·05).
Expression of NKG2D and TNF‐α in GD 9·5 uNK cells from the LPS‐treated mice
Next, we tested the hypothesis that decreased TWEAK levels in uNK cells led to increased cytotoxicity in uNK cells in LPS‐treated mice. The uNK cytotoxic receptor, NKG2D, was expressed in uNK cells in both the control and LPS‐treated mice (Fig. 5a). However, the percentage of NKG2D‐positive cells on GD 9·5 was significantly higher in the LPS‐treated group compared with the controls (Fig. 5b). To further quantify the expression of NKG2D in the uNK cell populations, we assessed NKG2D expression in isolated GD 9·5 LPS‐treated uNK cells by Western blotting. Western blot analysis revealed that NKG2D protein expression in the GD 9·5 uNK cells from the LPS‐treated mice was significantly higher than in the gestational age‐matched control mice (P < 0·05; Fig. 5e).
Figure 5.
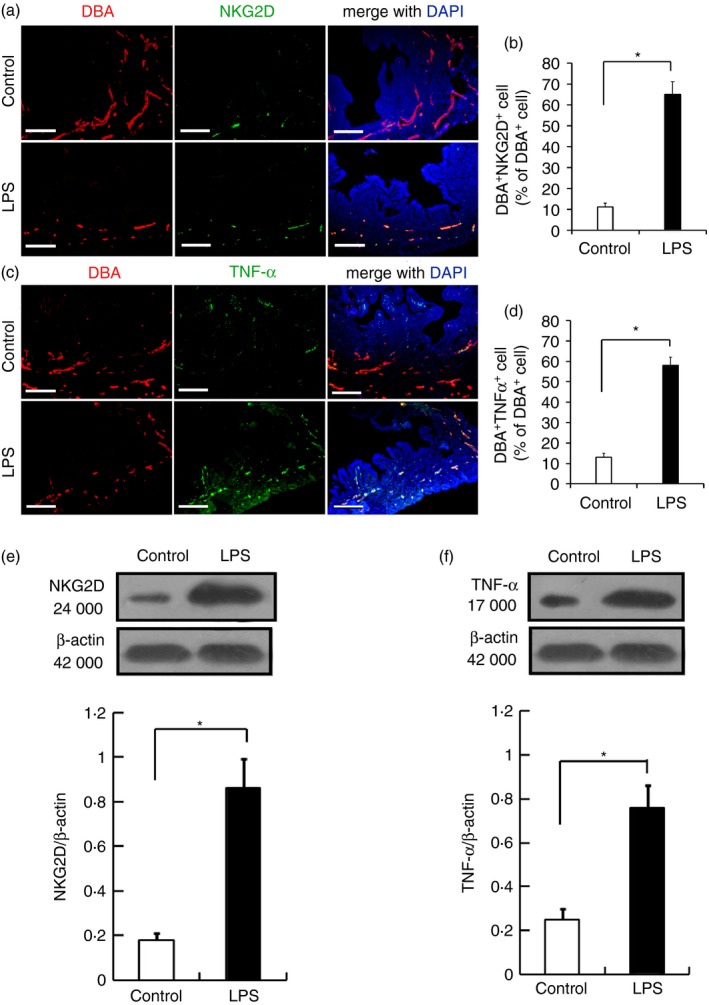
Expression of NKG2D and tumour necrosis factor‐α (TNF‐α) by gestational day (GD) 9·5 uterine natural killer (uNK) cells from the lipopolysaccharide (LPS) ‐treated and untreated pregnant mice. Representative fluorescence photomicrographs show co‐localization of NKG2D (a) and TNF‐α (c) with GD 9·5 uNK cells from LPS‐treated and untreated pregnant mice. The percentage of NKG2D‐positive (b) and TNF‐α‐positive cells (d) among the Dolichos biflorus agglutinin (DBA) ‐positive uNK cells from the LPS‐treated and untreated pregnant mice on GD 9·5 are shown. Mouse uNK cells in the 4% PFA‐fixed, paraffin‐embedded tissue were labelled with DBA lectin and rabbit anti‐mouse NKG2D or rabbit anti‐mouse TNF‐α antibodies, and then stained with Cy3‐tagged streptavidin for the uNK cells or an FITC‐conjugated goat anti‐rabbit secondary antibody for NKG2D or TNF‐α. Nuclei were labelled with DAPI. Scale bars = 200 μm. The levels of NKG2D and TNF‐α protein in uNK cells isolated from the GD 9·5 mouse endometrium were analysed by Western blotting. Representative blots are shown in the upper panel, and the histograms in the lower panel show the results of densitometric analyses of NKG2D (e) and TNF‐α (f) (normalized to β‐actin to correct for protein loading). The data represent the means ± SEM from at least three independent experiments. Asterisks indicate differences that are statistically significant (P < 0·05).
Next, we quantified the expression of TNF‐α in mice subjected to LPS‐induced abortion using dual reagent histochemistry and Western blot analysis. Immunohistological staining of TNF‐α revealed different intensity distributions in the uterus samples from the control and LPS‐treated mice on GD 9·5 (Fig. 5c). A higher density of TNF‐α‐positive uNK cells was observed in the LPS‐treated mice compared with the control mice (Fig. 5d). Western blot analysis revealed that the expression of TNF‐α protein was significantly increased in uNK cells from the LPS‐treated mice compared with the control mice (P < 0·05; Fig. 5f).
Expression of NKG2D and TNF‐α in GD 9·5 uNK cells treated with TWEAK or its antibody
As the NKG2D and TNF‐α proteins were expressed on isolated GD 9·5 LPS‐treated uNK cells at significantly higher levels than in gestational age‐matched control mice, we next determined whether there was a direct relationship between variations in TWEAK, NKG2D and TNF‐α expression in the uNK cells. As shown in Fig. 6, Western blot analysis did not detect any significant differences in NKG2D and TNF‐α expression in GD 9·5 uNK cells from control mice treated with any concentration of recombinant TWEAK protein (Fig. 6a). In contrast, neutralization of TWEAK activity using a TWEAK‐specific antibody resulted in a significant dose‐dependent up‐regulation of NKG2D and TNF‐α expression in the GD 9·5 uNK cells isolated from normal pregnant mice (Fig. 6b).
Figure 6.
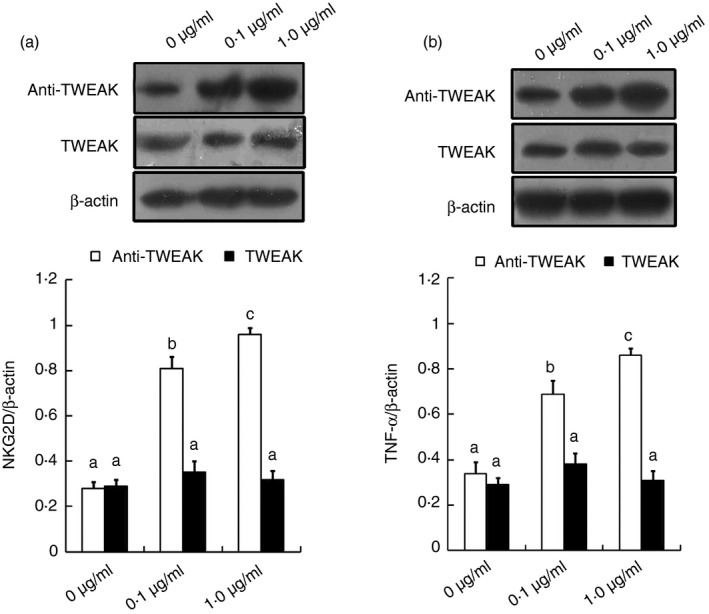
Expression of NKG2D and tumour necrosis factor‐α (TNF‐α) in gestational day (GD) 9·5 uterine natural killer (uNK) cells treated with tumour necrosis factor‐like weak inducer of apoptosis (TWEAK) or a TWEAK‐specific antibody. Uterine NK cells were isolated from a GD 9·5 normal pregnant mouse and incubated with recombinant TWEAK or a TWEAK‐specific antibody at the indicated final concentrations for 24 hr. The levels of NKG2D (a) and TNF‐α (b) protein in the uNK cells were analysed by Western blotting. Representative blots are shown in the upper panel and the histograms in the lower panel show the results of densitometric analyses of NKG2D or TNF‐α expression (normalized to β‐actin to correct for protein loading). The data represent the means ± SEM from at least three independent experiments. Columns with different superscript letters are significantly different (P < 0·05).
Discussion
In the current study, we demonstrated that LPS induces alterations in the expression of TWEAK/Fn14 and increases cytotoxicity in mouse uterine DBA‐positive leucocytes, resulting in a loss of balance between immunity and tolerance at the feto–maternal interface. Furthermore, these findings demonstrate, for the first time, that TWEAK and its receptor, Fn14, are expressed by uterine DBA‐positive leucocytes in pregnant mice; in particular, the cytokine TWEAK is primarily derived from uNK cells and the neutralization of this cytokine led to increased expression of NKG2D by uNK cells. Therefore, we suggest that a TWEAK autocrine feedback loop may play a critical role in regulating uNK cell cytotoxicity and contribute to the maintenance of tolerance.
Uterine NK cells are the major lymphocyte population at the feto–maternal interface. These cells possess a unique receptor phenotype with latent cytotoxicity and produce various cytokines that are essential for survival of the fetus. Previous studies have demonstrated that although uNK cells are beneficial in regulating normal pregnancy, they can be transformed into detrimental cells in response to bacterial and viral infections.19, 20, 21 The LPS‐treated pregnant mice exhibited a significant decrease in the number of uNK cells, as well as a significant increase in the number of uterine pro‐inflammatory neutrophils.14 TWEAK has been shown to play an important role in the regulation of innate immune cells, such as macrophages and NK cells.8 Accumulating evidence in humans and mice suggests that TWEAK may play an early role in preventing local cytotoxicity counterbalancing the cytotoxic function of uNK cells and favouring the constructive angiogenic/immunotrophic pathways.12, 13 However, the expression of TWEAK/Fn14 by uNK cells had not been described.
We administered LPS to pregnant mice to study how TWEAK and Fn14 expression in the uterus was altered by systemic infection of inflammation. Consistent with earlier studies,14 we did observe markedly decreased numbers of uNK cells in the LPS‐treated mice. Interestingly, we found that both TWEAK and its receptor, Fn14, were expressed by uNK cells in the control pregnant mice and in the LPS‐induced abortion group. In particular, the kinetics of TWEAK expression in uterus was consistent with the kinetics in the uNK cells from both the LPS‐treated and control mice, suggesting that the cytokine TWEAK is primarily produced by uNK cells. It is interesting to note that LPS treatment resulted in a distinct expression pattern for TWEAK/Fn14 by GD 9·5 in uNK cells compared with control mice. In contrast to the control group, significant down‐regulation of TWEAK and the up‐regulation of Fn14 in GD 9·5 uNK cells were observed following LPS treatment in this study, suggesting that expression changes in the TWEAK–Fn14 axis may play a role in fetal loss. Another interesting aspect of our study was the decreased expression of TWEAK in the uterus of LPS‐treated mice, not only because they have fewer uNK cells but also because they contain a smaller percentage of TWEAK‐producing uNK cells compared with untreated controls. Although a variety of cell types, including NK cells, macrophages and dendritic cells, have previously been shown to express TWEAK and Fn14,8 our report is the first to examine their expression and function in uNK cells. Our finding that uNK cells express both TWEAK and Fn14 suggests that the ability to produce the autocrine cytokine TWEAK may be important for uNK cell function. A number of in vitro and in vivo studies have suggested that the TWEAK/Fn14 pathway may be a mechanism regulating the abrogation of excessive infection‐induced inflammation within tissues, such as the kidney, lung, liver and muscle.9, 10, 22 Previous studies in humans have indicated that the ratio of IL‐18 to TWEAK mRNA levels is often associated with pregnancy failure.23 Therefore, TWEAK appears to be a modulator that prevents endometrial uNK cell cytotoxicity induced by IL‐18 over‐expression. Indeed, combined endometrial IL‐18 over‐expression and TWEAK inhibition increased the number of cytotoxic receptors on uNK cells, leading to the production of massive amounts of pro‐inflammatory cytokines.12 Furthermore, a previous study by our group indicated that the Fn14 receptor is a modulator of ovarian steroid‐related regulation of goat endometrial epithelial cell IL‐18 expression.24 Therefore, the inverse expression of TWEAK and Fn14 in uNK cells from LPS‐treated mice could alter the receptor phenotype on uNK cells, contributing to fetal loss. Although TWEAK and Fn14 are bona fide partners, Fn14‐independent TWEAK activities and alternative TWEAK receptors have also been postulated.25 Furthermore, Fn14 signalling events that are ligand‐ or TWEAK‐independent have also been described.26 Ligand‐independent signalling has been observed for other members of the TNF receptor superfamily under conditions of high receptor expression, in which receptor self‐association and clustering might occur. Indeed, ectopic over‐expression of Fn14 alone is sufficient to induce a variety of cellular responses.27 Hence, there may be physiological or pathophysiological conditions in which highly up‐regulated Fn14 receptor expression can signal and produce biological responses independently of TWEAK activity. Although it remains a formal possibility that a yet to be identified ligand other than TWEAK can bind to and signal through Fn14, a more intriguing scenario is that it is evolutionarily advantageous for Fn14 to retain some ability to trigger ligand‐independent signalling under certain biological conditions.
Uterine NK cells have a unique phenotype and maintain a dynamic balance between activating and inhibitory receptors. The presence or absence of appropriate inhibitory receptors is a deciding factor in the recognition of fetal trophoblast cells by maternal uNK cells.28 Increased expression of activating receptors has also been associated with fetal loss in humans.29, 30 NKG2D is a molecule that activates NK cells and promotes their subsequent killing activity, and it can be further induced in response to cellular transformation, stress or infection.31 Our results showed an increase in the expression of NKG2D receptors on GD 9·5 uNK cells following LPS treatment compared with normal pregnant controls. This aberrant expression could have impaired the function of uNK cells at the feto–maternal interface. Interestingly, a small percentage of TWEAK‐positive uNK cells and reduced TWEAK protein expression were detected in both the uNK cells and uterus of the LPS‐treated mice on GD 9·5 compared with the control mice. An earlier study demonstrated that TWEAK appears to be a modulator of the endometrial IL‐18‐induced cytotoxic activity of uNK cells.12 In this study, the addition of an exogenous TWEAK antibody caused a dose‐dependent enhancement in the expression of NKG2D and TNF‐α in uNK cells isolated from normal pregnant mice, whereas no statistically significant differences could be detected for these two molecules in response to treatment with a recombinant TWEAK protein at any of the tested concentrations. Combined with the above findings of decreased expression of TWEAK in uNK cells following LPS treatment, our results clearly indicate that endogenous TWEAK is probably critical for regulating uNK cell cytotoxicity in this LPS‐induced mouse model of abortion. However, a more precise analysis of uNK cells in TWEAK−/− or Fn14−/− mice will be required to confirm a key role for endogenous TWEAK in uNK cell cytotoxicity.
In addition to affecting the expression of the cytotoxic NK cell receptor NKG2D on uNK cells, LPS also increased the expression of TNF‐α, a crucial stimulator of the innate inflammatory response, in uNK cells in this study. The increased levels of TNF‐α created an imbalance in the tightly controlled tolerant microenvironment at the feto–maternal interface. This finding is in agreement with previous studies demonstrating that uNK cell‐produced TNF‐α causes placental cell death in response to LPS.32, 33 NKG2D‐positive uNK cells were identified as the source of TNF‐α production, which leads to fetal resorption in mice, as previously reported by Thaxton et al.34 In addition, Fn14 appears to be expressed at low levels in a variety of cell types, and its expression is readily up‐regulated by growth factors and has been shown to be induced within injured tissues.10 The expression of Fn14 in the lower genital tract has previously been demonstrated to play a role in amplifying inflammation during infection.35 In this study, we detected a significant down‐regulation in the expression of TWEAK and an up‐regulation of Fn14 in GD 9·5 uNK cells following LPS treatment. Decreased levels of TWEAK can lead to increased numbers of TNF‐α‐producing NKG2D‐positive uNK cells, and up‐regulated Fn14 expression in uNK cells may further amplify inflammation at the feto–maternal interface. Overall, this in vitro study further identified a critical role for endogenous TWEAK in regulating TNF‐α expression by uNK cells.
In conclusion, our results suggest that down‐regulation of TWEAK and up‐regulation of the Fn14 receptor in the uteri of LPS‐treated mice contribute to a disruption of decidual homeostasis by altering uNK cell cytotoxicity, ultimately leading to fetal rejection. Taken together, the present study strongly suggests that the TWEAK–Fn14 axis functions in uNK cells to maintain the tolerance necessary for successful pregnancy.
Disclosures
The authors declared no financial or commercial conflicts of interest.
Ethics statement
All the procedures were in strict accordance with the PR China legislation on the guidelines of the Institutional Animal Care and Use Committee (IACUC) established by the Institute for Experimental Animals of Northwest A&F University and was approved by the university committee for animal experiments. The protocol involving animals in the study entitled “Endogenous TWEAK is critical for regulating the function of mouse uterine NK cells in an immunological model of pregnancy loss” was approved by the IACUC with the project 153 number: 2014BAD23B11.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No.31572588), and the Fundamental Research Funds for the Central Universities of China (Grant No. ZD2013008).
References
- 1. Croy BA, van den Heuvel MJ, Borzychowski AM, Tayade C. Uterine natural killer cells: a specialized differentiation regulated by ovarian hormones. Immunol Rev 2006; 214:161–85. [DOI] [PubMed] [Google Scholar]
- 2. Karimi K, Solano ME, Ashkar AA, Ho H, Steidle EM, McVev Neufeld KA et al Regulation of pregnancy maintenance and fetal survival in mice by CD27 (low) mature NK cells. J Mol Med (Berl) 2012; 90:1047–57. [DOI] [PubMed] [Google Scholar]
- 3. Fu B, Li X, Sun R, Tong X, Ling B, Tian Z et al Natural killer cells promote immune tolerance by regulating inflammatory TH17 cells at the human maternal–fetal interface. Proc Natl Acad Sci U S A 2013; 110:E231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hofmann AP, Gerber SA, Croy BA. Uterine natural killer cells pace early development of mouse decidua basalis. Mol Hum Reprod 2014; 20:66–76. [DOI] [PubMed] [Google Scholar]
- 5. Tayade C, Hilchie D, He H, Fang Y, Moons L, Carmeliet P et al Genetic deletion of placenta growth factor in mice alters uterine NK cells. J Immunol 2007; 178:4267–75. [DOI] [PubMed] [Google Scholar]
- 6. Croy BA, Zhang J, Tayade C, Colucci F, Yadi H, Yamada AT et al Analysis of uterine natural killer cells in mice. Methods Mol Biol 2010; 612:465–503. [DOI] [PubMed] [Google Scholar]
- 7. Bell E. TWEAK and TNF: Yin and Yang in innate immunity. Nat Rev Immunol 2006; 6:91. [Google Scholar]
- 8. Maecker H, Varfolomeev E, Kischkel F, Lawrence D, LeBlanc H, Lee W et al TWEAK attenuates the transition from innate to adaptive immunity. Cell 2005; 123:931–44. [DOI] [PubMed] [Google Scholar]
- 9. Bertin D, Stephan D, Khrestchatisky M, Desplat‐Jégo S. Is TWEAK a Biomarker for Autoimmune/Chronic Inflammatory Diseases? Front Immunol 2013; 4:489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burkly LC, Michaelson JS, Zheng TS. TWEAK/Fn14 pathway: an immunological switch for shaping tissue responses. Immunol Rev 2011; 244:99–114. [DOI] [PubMed] [Google Scholar]
- 11. Gurunathan S, Winkles JA, Ghosh S, Hayden MS. Regulation of fibroblast growth factor‐inducible 14 (Fn14) expression levels via ligand‐independent lysosomal degradation. J Biol Chem 2014; 289:12976–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Petitbarat M, Rahmati M, Serazin V, Dubanchet SM, Orvan C, Wainer C et al TWEAK appears as a modulator of endometrial IL‐18 related cytotoxic activity of uterine natural killers. PLoS One 2011; 6:e14497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ledee N, Petitbarat M, Rahmati M, Dubanchet S, Chaouat G, Sandra O et al New pre‐conception immune biomarkers for clinical practice: interleukin‐18, interleukin‐15 and TWEAK on the endometrial side, G‐CSF on the follicular side. J Reprod Immunol 2011; 88:118–23. [DOI] [PubMed] [Google Scholar]
- 14. Zhao H, Kalish F, Schulz S, Yang Y, Wong RJ, Stevenson DK. Unique roles of infiltrating myeloid cells in the murine uterus during early to midpregnancy. J Immunol 2015; 194:3713–22. [DOI] [PubMed] [Google Scholar]
- 15. Wang X, Zhao Y, Zhong X. Protective effects of baicalin on decidua cells of LPS‐induced mice abortion. J Immunol Res 2014; 2014:859812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chaouat G, Petitbarat M, Bulla R, Dubanchet S, Valdivia K, Ledee N et al Early regulators in abortion and implications for a preeclampsia model. J Reprod Immunol 2009; 82:131–40. [DOI] [PubMed] [Google Scholar]
- 17. Bizinotto MC, Tamashiro WM, Gabriel DL, Yamade AT. Uterine natural killer cells are immunogenic in syngeneic male mice. J Reprod Immunol 2008; 79:18–25. [DOI] [PubMed] [Google Scholar]
- 18. Aisemberg J, Vercelli CA, Bariani MV, Billi SC, Wolfson ML, Franchi AM. Progesterone is essential for protecting against LPS‐induced pregnancy loss. LIF as a potential mediator of the anti‐inflammatory effect of progesterone. PLoS One 2013; 8:e56161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tripathi S, Chabtini L, Dakle PJ, Smith B, Akiba H, Yagita H et al Effect of TIM‐3 blockade on the immunophenotype and cytokine profile of murine uterine NK cells. PLoS One 2015; 10:e0123439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mariee NG, Tuckerman E, Laird S, Li TC. The correlation of autoantibodies and uNK cells in women with reproductive failure. J Reprod Immunol 2012; 95:59–66. [DOI] [PubMed] [Google Scholar]
- 21. Chen Y, Wang Y, Zhuang Y, Zhou F, Huang L. Mifepristone increases the cytotoxicity of uterine natural killer cells by acting as a glucocorticoid antagonist via ERK activation. PLoS One 2012; 7:e36413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Enwere EK, Lacasse EC, Adam NJ, Korneluk RG. Role of the TWEAK‐Fn14‐cIAP1‐NF‐κB signaling axis in the regulation of myogenesis and muscle homeostasis. Front Immunol 2014; 5:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lei MZ, Qin LJ, Zhao DD, Wang AH, Zhao XJ, Jin YP et al Tumor necrosis factor‐like weak inducer of apoptosis regulates the phenotype and cytotoxic activity of goat uterine natural killer cells. J Anim Sci 2015; 93:589–97. [DOI] [PubMed] [Google Scholar]
- 24. Lei MZ, Qin LJ, Wang AH, Jin YP, Zhao XJ, Qi XF. Fn14 receptor appears as a modulator of ovarian steroid‐related regulation of goat endometrial epithelial cell IL‐18 expression. Am J Reprod Immunol 2015; 73:428–36. [DOI] [PubMed] [Google Scholar]
- 25. Wajant H. The TWEAK‐Fn14 system as a potential drug target. Br J Pharmacol 2013; 170:748–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Burkly LC, Michaelson JS, Hahm K, Jakubowski A, Zheng TS. TWEAKing tissue remodeling by a multifunctional cytokine: role of TWEAK/Fn14 pathway in health and disease. Cytokine 2007; 40:1–16. [DOI] [PubMed] [Google Scholar]
- 27. Burkly LC. TWEAK/Fn14 axis: the current paradigm of tissue injury‐inducible function in the midst of complexities. Semin Immunol 2014; 26:229–36. [DOI] [PubMed] [Google Scholar]
- 28. Rahim MM, Tu MM, Mahmoud AB, Wight A, Abou‐Samra E, Lima PD et al Ly49 receptors: innate and adaptive immune paradigms. Front Immunol 2014; 5:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Varla‐Leftherioti M, Spyropoulou‐Vlachou M, Niokou D, Keramitsoglou T, Darlamitsou C, Papadimitropoulos M et al Natural killer (NK) cell receptors' repertoire in couples with recurrent spontaneous abortions. Am J Reprod Immunol 2003; 49:183–91. [DOI] [PubMed] [Google Scholar]
- 30. Varla‐Leftherioti M, Spyropoulou‐Vlachou M, Keramitsoglou T, Papadimitropoulos M, Tsekoura C, Graphou O et al Lack of the appropriate natural killer cell inhibitory receptors in women with spontaneous abortion. Hum Immunol 2005; 66:65–71. [DOI] [PubMed] [Google Scholar]
- 31. Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol 2003; 3:781–90. [DOI] [PubMed] [Google Scholar]
- 32. Murphy SP, Fast LD, Hanna NN, Sharma S. Uterine NK cells mediate inflammation‐induced fetal demise in IL‐10‐null mice. J Immunol 2005; 175:4084–90. [DOI] [PubMed] [Google Scholar]
- 33. Murphy SP, Hanna NN, Fast LD, Shaw SK, Berg G, Padbury JF et al Evidence for participation of uterine natural killer cells in the mechanisms responsible for spontaneous preterm labor and delivery. Am J Obset Gynecol 2009; 200: 308. e1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thaxton JE, Nevers T, Lippe EO, Blosi SM, Saito S, Sharma S. NKG2D blockade inhibits poly (I:C)‐triggered fetal loss in wild type but not in IL‐10–/– mice. J Immunol 2013; 190:3639–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Han ES, Mekasha S, Ingalls RR. Fibroblast growth factor‐inducible 14 (Fn14) is expressed in the lower genital tract and may play a role in amplifying inflammation during infection. J Reprod Immunol 2010; 84:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]


