Summary
T helper type 17 (Th17) and regulatory T (Treg) cells are active players in the establishment of tolerance and defence. These attributes of the immune system enmesh to guarantee the right level of protection. The healthy immune system, on the one hand, recognizes and eliminates dangerous non‐self pathogens and, on the other hand, protects the healthy self. However, there are circumstances where this fine balance is disrupted. In fact, in situations such as in pregnancy, the foreign fetal antigens challenge the maternal immune system and Treg cells will dominate Th17 cells to guarantee fetal survival. In other situations such as autoimmunity, where the Th17 responses are often overwhelming, the immune system shifts towards an inflammatory profile and attacks the healthy tissue from the self. Interestingly, autoimmune patients have meliorating symptoms during pregnancy. This connects with the antagonist role of Th17 and Treg cells, and their specific profiles during these two immune challenging situations. In this review, we put into perspective the Th17/Treg ratio during pregnancy and autoimmunity, as well as in pregnant women with autoimmune conditions. We further review existing systems biology approaches that study specific mechanisms of these immune cells using mathematical modelling and we point out possible future directions of investigation. Understanding what maintains or disrupts the balance between these two opponent yet reciprocal cells in healthy physiological settings, sheds light into the development of innovative pharmacological approaches to fight pregnancy loss and autoimmunity.
Keywords: autoimmunity, defence, mathematical modelling, pregnancy, systems biology, T helper type 17/regulatory T cell ratio, tolerance
Abbreviations
- CNS
conserved non‐coding sequence
- DC
dendritic cell
- FOXP3
forkhead box protein 3
- IL
interleukin
- MS
multiple sclerosis
- PE
pre‐eclampsia
- PlGF
placental growth factor
- PTB
preterm birth
- pTreg cells
peripheral regulatory T cells
- RA
rheumatoid arthritis
- RPL
recurrent pregnancy loss
- sFlt‐1
soluble fms‐like tyrosine kinase receptor‐1
- SLE
systemic lupus erythematodes
- TGF‐β
transforming growth factor‐β
- Th17 cells
T helper 17 cells
- Treg cells
regulatory T cells
- URPL
unexpected recurrent pregnancy loss
Introduction
T helper 17 (Th17) and regulatory T (Treg) cells are part of the complex machinery that constitutes the immune system. The healthy immune system not only recognizes and fights infection but also regulates undesired immune responses against tissue self antigens or harmless non‐self organisms. This requires a fine balance established within the network of molecules and cells that provide an immune response. However, there are circumstances that require a ‘re‐tuning’ of this balance. In fact, in situations such as pregnancy, the immune system faces a dilemma: it has to protect the mother against infection and, at the same time, to accept the semi‐allogeneic fetus.1 This embodies active regulation of inflammatory immune processes occurring during pregnancy to guarantee specific tolerance towards the foreign fetal antigens. The referred regulation process includes a network of various immune cell populations and molecules that are responsible for the immune balance in pregnancy (reviewed in ref. 2). As an example, Th17 and Treg cells form complex and dynamic networks to maintain homeostasis. These cells interact with many other types of cells to orchestrate the desired immune response. In this review, we focus on the interactions between Th17 and Treg cells in two specific situations, namely pregnancy and autoimmunity.
Origin and characteristics of Th17 and Treg cells
T cells are lymphocytes that mature in the thymus3 and have specific receptors, namely the T‐cell receptors.4 These cells are part of the adaptive immune response.5 In particular, Th cells, also known as CD4+ T cells, are activated by antigen‐presenting cells and proceed to clonal expansion and cytokine secretion.6 The cytokine profile secreted at this stage will determine the cell differentiation into any of the several subsets of Th cells and so define the type of immune response.7 These subsets include (but not only) the Th1, Th2, Treg and Th17 cells,8, 9 as illustrated in Fig. 1.
Figure 1.
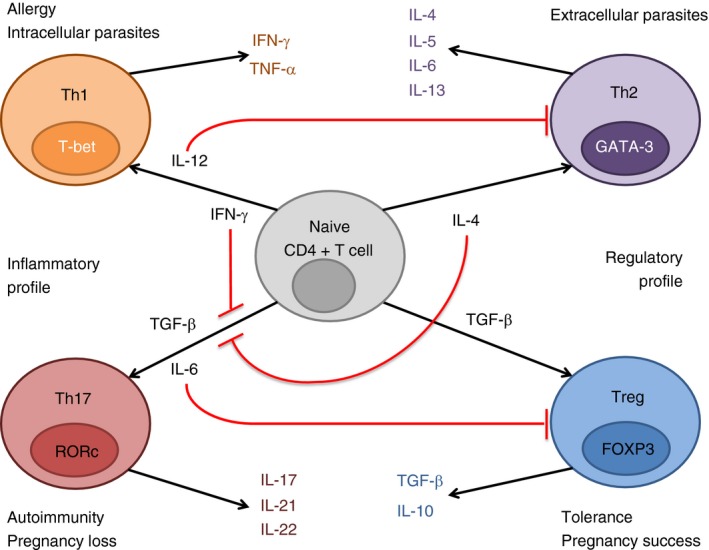
Illustrative scheme of naive T cell differentiation into T helper type 1 (Th1), Th2, Th17 or regulatory T (Treg) cells, depending on the cytokine profile. Interleukin‐12 (IL‐12) and interferon‐γ (IFN‐γ) stimulated naive T cells differentiate to Th1 cells. These cells express IFN‐γ and tumour necrosis factor‐α (TNF‐α), and are responsible for intracellular parasite clearance and allergy conditions. IL‐4 stimulated naive T cells induce a Th2 response. Th2 cells express IL‐4, IL‐5, IL‐6 and IL‐13, and are responsible for clearance of extracellular parasites. Transforming growth factor‐β (TGF‐β) stimulated naive T cells induce a Treg response. Treg cells express TGF‐β and IL‐10 and are responsible for tolerance and pregnancy success. These three cell types express one cytokine responsible for its induction, in a positive feedback mechanism. TGF‐β and IL‐6 stimulated naive T cells induce a Th17 response. Th17 cells express IL‐17, IL‐21 and IL‐22 and are responsible for autoimmunity and pregnancy loss. Th17 cells do not present a positive feedback mechanism, but IFN‐γ or IL‐4 can inhibit the differentiation from naive T cells to Th17. Moreover, IL‐6 and IL‐12 inhibit Treg and Th2 cells, respectively.
Regulatory T cells are classified as CD4+ T cells expressing the transcription factor Forkhead box protein 3 (FOXP3)10 and generate in the thymus (referred as tTreg cells) and in the periphery (referred as pTreg cells).11 These immune cells express specific anti‐inflammatory cytokines such as interleukin‐10 (IL‐10) and transforming growth factor‐β (TGF‐β), which dampen an excessive effector immune response.12 This effector response arises from the fight against bacterial and pathogenic infections, which is partly mediated by Th17 cells (reviewed in ref. 13). Th17 cells are also a lineage of CD4+ T cells and are characterized by expressing the transcription factor retinoic acid receptor‐related orphan receptors and the cytokine IL‐17,14 among others. However, Th17 and Treg cells present a certain level of plasticity, meaning that distinct milieus account for distinct cell fate.15 In particular, the Th17 cells have different fates in different inflammatory frameworks16, 17 and, interestingly, Th17 cells that present plasticity towards Th1 have higher survival rate and less senescence than Th1 cells.18 Moreover, under some circumstances, Treg cells are able to transdifferentiate into Th17 cells19 and vice versa.20 Notwithstanding, diminishing the Treg and/or augmenting the Th17 dynamic profiles in humans can: (i) antecede gestational problems, such as preterm birth (PTB),21 pre‐eclampsia (PE)22 or recurrent pregnancy loss (RPL)23 and (ii) ensue the onset of autoimmune diseases, such as multiple sclerosis (MS)24 and rheumatoid arthritis (RA).25
Role of Th17/Treg balance in pregnancy
At the onset of pregnancy, the semen also modulates the maternal immune response to promote a fetus‐friendly environment, so contributing to a healthy gestation.26, 27 This immune‐modulatory stimulus will signal to the maternal immune response,28 activating a network of cells [human and murine macrophages and dendritic cells (DCs), as well as murine granulocytes,29 CD4+ CD25+ Treg cells,30 human lymphocytes31 and FOXP3− Treg cells32] and molecules (human TGF‐β 1 and IL‐10,32 IL‐8 and IL‐2 receptor,33 among others) that will dampen the inflammatory immune response of the mother and allow the growth and development of the semi‐allogeneic fetus.32 However, this immune tolerance towards the fetus must not compromise the capacity to fight infections and diseases. This tightly regulated mechanism between tolerance and defence encompasses specific kinetic profiles for Treg cells present at the feto–maternal interface.34 These profiles will determine the pregnancy fate. Specifically, the fine balance between Treg and Th17 cells mediates maternal tolerance to the fetus.35, 36 In fact, in humans, specific Treg subsets such as CD4+ CD25bright and CD4+ CD25+ Treg cells increase in the first trimester of pregnancy.37, 38 This last Treg subset peaks in the second trimester and decreases until reaching a low level, measured post‐partum.38 In the last stage of pregnancy, fetal Treg cells decrease to allow labour initiation.39 Controversially, other Treg subtypes, such as CD4dim CD25high Foxp3+ Treg cells have been reported in humans to start decreasing already in the second trimester.40 In addition, only sustained and not transient Foxp3+ Treg cell expression allows the efficient suppression of the maternal effector immune response towards the fetus.41 Later in pregnancy, the hormonal and cytokine setting at the feto–maternal interface is thought to recruit the immune cells necessary to establish a favourable environment for the human42, 43 and murine44 pregnancy to proceed. Specifically, murine DCs have the ability to signal not only the production of Treg cells but also of IL‐17‐producing cells, effectively protecting against specific invading pathogens and, meanwhile, allowing a successful pregnancy.45 However, this shift in the immune response during pregnancy has a systemic impact. In fact, the human microbiota communities might oscillate when there is a change in the immune response, such as in the case of pregnancy.46
In evolutionary terms, mammals have developed mechanisms of maternal tolerance towards the fetus. To shed light on this phenomenon, Samstein and colleagues studied specific evolutionary traits involved in pTreg cell differentiation during pregnancy.11 This study hypothesizes that a conserved non‐coding sequence (CNS1), enhancer of pTreg but not essential for thymus Treg cell generation, plays an important role in feto–maternal tolerance. The authors showed, in a murine model, that pTreg cells recognize paternal antigens and suppress a maternal effector T‐cell response. CNS1‐deficient mice are unable to induce pTreg cells, therefore maternal effector T cells can enter the placenta, so inducing spontaneous abortions. Interestingly, and according to the same study, CNS1 is present in the genome of specific placental mammals, but not in specific non‐placental mammals or non‐mammals. Hence, the authors suggest that CNS1 is an essential factor for pTreg expression in placental mammals.
Next, we review the role of the tandem Th17/Treg in specific pregnancy complications, namely PTB, PE and (unexpected) RPL.
Preterm birth
Preterm birth is defined as birth before 37 weeks of gestation47 and is a global problem that, in 2010, affected 11·1% of all live births.48 It ensues short‐ and long‐term complications; it is a risk factor in > 50% of neonatal deaths48 and is reported to be responsible for 35% of effective neonatal death.49 Long‐term complications such as metabolic bone disease of prematurity50 or respiratory complications51 are associated with PTB.
At the cellular level, Treg cells are involved in murine fetal acceptance28, 52 and Koucky et al.21 report that, in humans, low levels of circulating Treg cells, together with short cervical length, correlate with PTB. Moreover, IL‐6 is elevated in such situations, in humans and mice.53 This suggests that the Th17/Treg balance differs between term and PTB. Accepted strategies to diminish the risk of PTB, encompass the vaginal administration of the hormone progesterone.54 Interestingly, Areia et al.55 show that in healthy pregnant women, Treg cells express the receptor for progesterone, the membrane progesterone receptor α, suggesting that the protective effect of progesterone on PTB situations might act by activating Treg cell proliferation.
Pre‐eclampsia
Pre‐eclampsia is a hypertensive disorder that manifests during pregnancy. It affects 2–8% of all pregnancies56 and is involved in 40% of births before the 35th week.57 PE manifests during the second half of pregnancy, through high blood pressure and high proteinuria levels;58 however, an impaired trophoblast invasion during the early stage of pregnancy has been proposed to cause PE.57 As a consequence, the blood supply to the fetus diminishes.59 To increase PE diagnosis accuracy, Maynard et al.60 proposed that excessive placental soluble fms‐like tyrosine kinase receptor‐1 (sFlt‐1) and placental growth factor (PlGF) correlate with PE condition. Stepan et al.61 suggested an algorithm to test if a woman is prone to PE based on measured sFlt‐1/PlGF ratios. Women with an abnormal sFlt‐1/PlGF ratio can have an increased risk of PE. Interestingly, the inflammatory immune response observed in PE patients is, in fact, an exacerbation of the normal inflammatory response preceding birth. Indeed, several studies suggest that the immune system plays an important role at the onset of PE. Vargas‐Rojas et al. tested the T‐cell composition in umbilical cord blood from healthy and pre‐eclamptic pregnant women. The authors observed a shift towards inflammation in the Th1/Th2 and Th17/Treg balances, where the Th1 and Th17 populations remained constant, but the Th2 and Treg populations decreased.22 In contrast, other authors point out that maternal cytotoxic T lymphocytes increase in patients with PE.62, 63 Perez‐Sepulveda et al. suggest that, in PE patients, immature DCs undergo maturation and initiate a pro‐inflammatory response, whereas in a normal pregnancy, DC maturation is blocked, so rendering an immune response dominated by Treg and Th2 cells.57 Besides T cells, decidual natural killer cells play a critical role in the pathogenesis of PE. This unique immune cell population contributes to the remodelling of the spiral arteries and a failure in this process was associated with PE development. Hiby et al.59 found that the interaction between maternal killer immunoglobulin receptors expressed by natural killer cells and MHC molecules expressed on trophoblasts determines placental development. The same research group revealed that specific combinations of HLA‐C and activating killer immunoglobulin receptors not only increase the risk for PE but also augment the risk for other pregnancy complications.64 In addition, reduced numbers of CD56++ CD16− non‐activated natural killer cells and diminished Treg cell frequencies were detected in the cord blood of babies from women with PE, underlining the importance of both immune cell populations for successful pregnancy outcome.65
In short, women with PE have normal levels of Th17 populations, but decreased levels of Treg populations so altering the Th17/Treg ratio.
(Unexplained) recurrent pregnancy loss
Recurrent pregnancy loss is defined as the loss of three or more consecutive pregnancies before the 20th week of pregnancy.66 RPL affects couples in 1%,67 60% of which remain to be explained.68 Uterus anatomic abnormalities, parental chromosomal incompatibility or poor blood supply can associate with an elevated rate of miscarriage. However, there are situations of RPL where no epidemiological or pathological diagnosis associates with miscarriage.69 These are referred to as unexplained RPL (URPL). Some authors suggest that the condition of URPL can be due to maternal immune rejection of the fetus.70 In fact, URPL incidence increases when Th17 populations increase and Treg populations decrease,23 which suggests overwhelming pro‐inflammatory responses towards the fetus. Moreover, elevated presence of IL‐6 correlates with higher abortion levels in humans and mice.71
Other authors tested if ovarian ageing increases the rate of URPL, but the results indicate that the former does not affect the levels of the latter.72
In conclusion, the Th17/Treg ratio during a healthy pregnancy shifts in favour of the Treg cells. However, in specific pregnancy disorders (PTB: decrease of Treg levels; PE: increase of Th17 levels; URPL: increase of Th17 levels and a decrease of Treg levels), the balance between the Th17/Treg is disturbed and inflammatory immune responses are insufficiently regulated.
Role of Th17/Treg balance in autoimmune disorders
When mechanisms underlying tolerance induction fail, autoreactive immune cells survive, are activated and attack self tissue structures. These undesired immune responses can be directed towards specific organs or multiple organ systems and will result in a phenomenon called autoimmunity. Autoimmune disorders are often characterized by Th1 or Th2 dominance. However, other Th subsets like Th17 and Treg cells were suggested to be critically involved in disease severity and progression. More precisely, the ratio between Th17 and Treg cells seems to be fundamental for disease outcome. Here we will discuss the participation of both, Th17 and Treg cells, in some of the most common autoimmune diseases with a specific focus on their balance.
Systemic lupus erythematodes
Systemic lupus erythematodes (SLE) is a severe chronic autoimmune disorder affecting multiple organ systems. The disease is characterized by elevated levels of autoantibodies, an activation of complement factors and abnormal Th responses. Reportedly, patients with SLE suffer from a dysregulated balance of Th1 and Th2 responses and there is accumulating evidence that an altered Th17/Treg ratio contributes to disease activity and its progression. It has been shown that patients with SLE have significantly diminished Treg levels73, 74 and an impaired Treg cell suppressive activity,74, 75 when compared with healthy controls. This leads to stronger T effector cell responses defined by increased proliferation rates and elevated cytokine levels74 as well as to augmented autoantibody levels,76 all processes resulting in an increase of disease severity. The potential of Treg cells to positively influence disease activity was confirmed in a study by Scalapino et al. The authors showed that adoptive transfer of in vitro expanded Treg cells ameliorated disease progression in a murine lupus‐prone model.77 Along with a reduced number of Treg cells, elevated Th17 levels have been reported in the same patient pool. Thereby augmented Th17 numbers positively correlated with disease activity.78, 79, 80, 81, 82, 83, 84 Underlying the significance of an imbalance in the ratio between both Th subsets, Ma et al.85 observed that Th17 or Treg cells alone were not correlated to SLE development; however, their ratio was significantly altered in SLE patients and this strongly correlated with disease severity. Hence, it can be assumed that, in this specific situation, the diminution in the Treg pool ‐ accompanied by an increase in the number of Th17 cells ‐ follows Treg cell differentiation into Th17 cells.
Rheumatoid arthritis
Rheumatoid arthritis is a chronic autoimmune disorder affecting the synovial joints of the body. It was identified as a disease with a Th1‐dominant profile; however, disease pathology seems additionally to be influenced by an altered Th17/Treg balance. Patients suffering from RA display increased Th17 and reduced Treg levels.25, 86 Moreover, they produce more pro‐inflammatory cytokines that are involved in the differentiation and maintenance of pathogenic Th17 cells.87 Komatsu et al.19 observed that under arthritic conditions Treg cells lose their Foxp3 expression and undergo transdifferentiation into Th17 cells; a mechanism that seems to be IL‐6 dependent. Pathogenic Th17 cells then accumulate in inflamed joints and contribute to the progression of the disease.19 Interestingly, treatment strategies tested in rodent models resulting in a shift of the Th17/Treg ratio in favour of immune suppression improved disease activity in the joints.88, 89, 90 In addition to the significant influence of surrounding cytokines on Treg to Th17 cell differentiation, DCs may impact Th17/Treg cell ratio. It has been proposed that monocyte‐derived DCs from patients with RA produce high levels of IL‐6 and IL‐23, so favouring Th17 cell generation. Furthermore, the same DCs had an impaired ability to induce Treg cells.91 Molecules implicated in Th17/Treg plasticity in RA may belong to the signal transducer and activator of transcription pathway, as suggested by different studies.92, 93 In contrast to most studies, Ju et al. found no alterations in Th17 and Treg levels when compared with healthy controls.92, 93 For that reason, more research is needed to finally confirm a significant contribution of Th17 cells for disease aggravation and of Treg cells for disease amelioration.
Multiple sclerosis
Multiple sclerosis is an inflammatory, demyelinating disease of the central nervous system. The disorder was reported to be associated with a reduced number and functionality of Treg cells,94 although studies differ in their findings regarding various Treg subsets. For instance, Fletcher et al.24 showed that patients with MS revealed a normal frequency of the CD4+ CD25+ CD127low Foxp3+ Treg subset, but had a deficit in the relative frequency and the suppressive function of the CD4+ CD25+ CD127low Foxp3+ CD39+ Treg subset. The impaired ability of Treg cells obtained from patients with MS in suppressing T effector cells seems to be associated with an enhanced IL‐6 receptor expression, as well as IL‐6 production.95 Moreover, Th17 cell populations are augmented in MS96 and they express higher basal levels of activation markers and other markers involved in cell adhesion to the endothelium.97 Nyirenda et al.98 suggested that infections leading to an activation of the TLR2 on the surface of Treg cells may promote a shift into a more Th17‐like phenotype. Interestingly, the Th17/Treg balance fluctuates during disease and is responsible for disease activity and progression.99 Although relapses are associated with increased Th17 numbers, elevated levels of Treg cells can be observed during the remitting phases. Thereby, fluctuations in the numbers of both Th populations seem to be influenced by microRNA clusters,100, 101 suggesting miRNAs as targets for MS treatment. Additionally, vitamin A supplementation was proposed to up‐regulate Treg markers and may therefore be suitable to reduce disease activity in patients with MS.102 Other therapeutic interventions shown to reduce IL‐17 and IL‐23 levels,103 decrease peripheral and central Th17 responses and enhance Treg frequency and IL‐10 production104 are promising in MS therapy.
In conclusion, autoimmune disorders are often characterized by a disturbed Th17/Treg balance that results in increased levels of pathogenic Th17 cells associated with reduced Treg numbers and activity. Hence, therapies altering the Th17/Treg ratio in favour of the Treg cells may have a great benefit for the patients. Figure 2 qualitatively illustrates how the Th17/Treg ratio can differ between a healthy pregnancy or self tolerance situations and conditions of autoimmunity and pregnancy complications.
Figure 2.
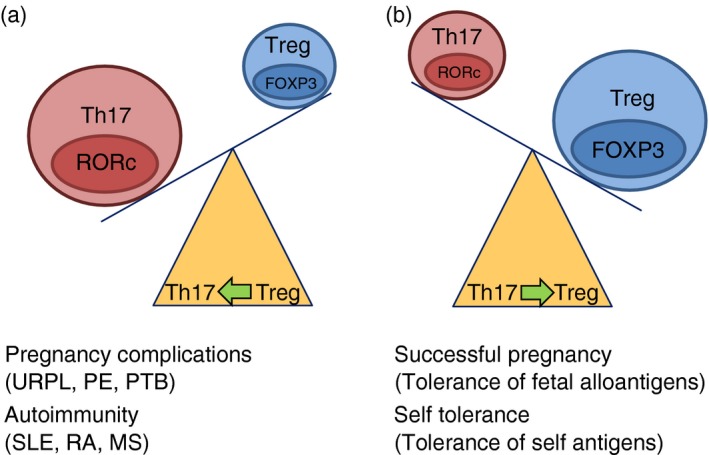
Illustrative description of the T helper type 17 (Th17)/regulatory T (Treg) balance in (a) pregnancy complications and autoimmunity and (b) a healthy pregnancy and self tolerance. Abbreviations: MS, multiple sclerosis, PE, pre‐eclampsia, PTB, preterm birth; RA, rheumatoid arthritis; SLE, systemic lupus erythemsatosus; URPL, unexpected recurrent pregnancy loss.
Influence of Th17 and Treg cells on autoimmune disease activity during pregnancy
Many women suffering from autoimmune diseases experience alterations in their disease activity when they become pregnant. In addition, autoreactive immune responses may interfere with fetal well‐being in pregnant autoimmune patients. Strong hormonal and immunological changes take place during pregnancy. Autoimmune diseases dominated by inflammatory immune responses such as RA and MS reportedly improve during late pregnancy. As discussed above, patients with MS or RA suffer from a disturbed Th17/Treg ratio with increased Th17 levels and diminished Treg cell numbers, which have been implicated in disease activity and progression. Normal pregnancy is characterized by an increased Treg/Th17 ratio and this may positively influence disease outcome. Indeed, two studies suggested an association between the number of Treg cells and an improvement of RA disease activity in pregnancy.105, 106 Another study investigated fluctuations of Th17 and Treg cells in patients with MS during pregnancy. Based on their results the authors concluded that disease amelioration in patients with MS during their third trimester could not be related to a decrease in the number of circulating Th17 cells or to an increase in Treg cell levels.107 In contrast, Sánchez‐Ramón et al.108 proposed an association between pregnancy‐induced Treg cell expansion and MS amelioration. However, Treg cells from pregnant women with MS seem to have an impaired suppressive capacity when compared with Treg cells from healthy pregnant women.94 Hence, although there is accumulating evidence of a disturbed Th17/Treg balance in RA and MS, it remains to be clarified whether pregnancy‐induced changes in the Th17/Treg ratio may be responsible for disease amelioration.
Systems biology approaches to understand the Th17/Treg balance
Systems biology is a scientific approach to tackle medical questions using mathematical modelling.
Mathematical models have been successfully used to answer a broad spectrum of medical questions. In the specific field of immunology, the pair Th17/Treg has been modelled by Hong et al.,109 by combining different experimental published observations in a mathematical model that shows how polarized signals can control the different T‐cell phenotypes.
Busse et al.110 studied the spatiotemporal dynamics of IL‐2 using mathematical modelling and experiments to show that this cytokine switches between two functions, working as a digital toggle switch in T‐cell proliferation and as an analogue amplifier for the IL‐2 uptake capacity of Treg cells.
Naldi et al.111 proposed a logical model of the regulatory network and signalling pathways involved in Th cell differentiation, which paves the way to explore novel in silico approaches to a better understanding of Th cell differentiation. Other authors also investigated the differentiation of Th cells by analysing the dynamic properties of the molecular network controlling the differentiation of Th cells to Th1, Th2, Th17 or Treg cells.112
However, to the best of our knowledge, mathematical modelling has not yet been applied to understand the Th17/Treg balance in pregnancy and in autoimmunity. This sophisticated immune process can be antagonistic, and a deeper understanding of the mechanisms leading to the polarization to Treg or Th17 will pave the way to new biopharmaceutical approaches that fight disorders of the immune system leading to pregnancy loss or autoimmunity.113
The investigation of the dynamic profiles of the Th17/Treg ratio in a natural, physiological condition such as pregnancy can help our understanding of how to recover homeostasis in the setting of autoimmune diseases.
It is therefore paramount to understand how the immune balance is established and what disrupts it, using an innovative methodology that combines mathematical modelling with experimental data.
Discussion
Pregnancy and autoimmunity are challenging situations for the immune system. Treg and Th17 cells play a dominant role in both, although with opposing profiles. In fact, Treg activation ensures pregnancy success and lower Treg numbers are associated with higher risk of pregnancy loss. In parallel, Th17 cells are important players in the development and progression of autoimmune diseases such as collagen‐induced arthritis and its antagonist partner, Treg cells, dampen the strength of this inflammatory response and hence diminish the symptoms of autoimmunity.114
Strikingly, the immune cellular phenotype that regulates inflammation during pregnancy, also hinders the development of autoimmunity, such as RA or MS.115
The role of Treg cells in pregnancy has been extensively studied34, 44, 116, 117 and reviewed.28, 118, 119 Treg numbers increase during pregnancy and decrease in the case of spontaneous abortion.37, 120 In fact, abrogation of the protective effect of Treg cells culminates in pregnancy loss in a gestational murine model.121 Moreover, higher levels of inflammatory markers, such as IL‐6 or TGF‐β, have been measured in many women suffering from PTB115 and higher levels of Th17 cells have been measured in women with RPL compared with normal pregnancy situations.23 Complementing this, the symptoms of autoimmune diseases such as MS and RA diminish during a normal pregnancy, which is explained by the immune regulatory mechanisms that dampen an overwhelming inflammatory immune response against the semi‐allogeneic fetus. It is therefore fundamental to understand the regulatory mechanisms ensuing during pregnancy, and this will pave the way to develop new medical and pharmaceutical strategies to fight autoimmune diseases.
Within this review, we put into perspective the role of Th17/Treg balance in pregnancy and autoimmunity. We further review this paradigm in autoimmunity during pregnancy and we inspect the literature that includes mathematical modelling approaches to understanding specific aspects of the Th17 and/or Treg cell profiles.
Disclosures
The authors declare that there are no conflicts of interest.
Acknowledgements
The authors thank A.C. Zenclussen for critically reading the manuscript. ASF designed the study and wrote the paper; AS wrote the paper.
References
- 1. Chatterjee P, Chiasson VL, Bounds KR, Mitchell BM. Regulation of the anti‐inflammatory cytokines interleukin‐4 and interleukin‐10 during pregnancy. Front Immunol 2014; 5:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schjenken JMT, Paul JW, Clifton VL, Smith R. Mechanisms of maternal immune tolerance during pregnancy In: Zheng J, ed. Recent Advances in Research on the Human Placenta. Shanghai, China: InTechOpen, 2012:211–242. [Google Scholar]
- 3. McClory S, Hughes T, Freud AG, Briercheck EL, Martin C, Trimboli AJ et al Evidence for a stepwise program of extrathymic T cell development within the human tonsil. J Clin Invest 2012; 122:1403–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Valor L, Teijeiro R, Aristimuño C, Faure F, Alonso B, Andrés Cd et al Estradiol‐dependent perforin expression by human regulatory T‐cells. Eur J Clin Invest 2011; 41:357–64. [DOI] [PubMed] [Google Scholar]
- 5. Aluvihare VR, Betz AG. The role of regulatory T cells in alloantigen tolerance. Immunol Rev 2006; 212:330–43. [DOI] [PubMed] [Google Scholar]
- 6. Li W, Li B, Fan W, Geng L, Li X, Li L et al CTLA4 Ig gene transfer alleviates abortion in mice by expanding CD4+CD25+ regulatory T cells and inducing indoleamine 2,3‐dioxygenase. J Reprod Immunol 2009; 80:1–11. [DOI] [PubMed] [Google Scholar]
- 7. Jimeno R, Leceta J, Garin M, Ortiz AM, Mellado M, Rodriguez‐Frade JM et al Th17 polarization of memory Th cells in early arthritis: the vasoactive intestinal peptide effect. J Leukoc Biol 2015; 98:257–69. [DOI] [PubMed] [Google Scholar]
- 8. Saito S, Nakashima A, Shima T, Ito M. Th1/Th2/Th17 and regulatory T‐cell paradigm in pregnancy. Am J Reprod Immunol 2010; 63:601–10. [DOI] [PubMed] [Google Scholar]
- 9. Li X, Wang B, Li Y, Wang L, Zhao X, Zhou X et al The Th1/Th2/Th17/Treg paradigm induced by stachydrine hydrochloride reduces uterine bleeding in RU486‐induced abortion mice. J Ethnopharmacol 2013; 145:241–53. [DOI] [PubMed] [Google Scholar]
- 10. Nie H, Zheng Y, Li R, Guo TB, He D, Fang L et al Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF‐α in rheumatoid arthritis. Nat Med 2013; 19:322–8. [DOI] [PubMed] [Google Scholar]
- 11. Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal–fetal conflict. Cell 2012; 150:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jutel M, Akdis M, Budak F, Aebischer‐Casaulta C, Wrzyszcz M, Blaser K et al IL‐10 and TGF‐β cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur J Immunol 2003; 33:1205–14. [DOI] [PubMed] [Google Scholar]
- 13. Kimura A, Kishimoto T. IL‐6: regulator of Treg/Th17 balance. Eur J Immunol 2010; 40:1830–5. [DOI] [PubMed] [Google Scholar]
- 14. Wichner K, Stauss D, Kampfrath B, Kruger K, Muller G, Rehm A et al Dysregulated development of IL‐17‐and IL‐21‐expressing follicular helper T cells and increased germinal center formation in the absence of RORγt. FASEB J 2015; 30:761–4. [DOI] [PubMed] [Google Scholar]
- 15. Kleinewietfeld M, Hafler DA. The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin Immunol 2013; 25:305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ et al Fate mapping of IL‐17‐producing T cells in inflammatory responses. Nat Immunol 2011; 12:255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wan YY, Flavell RA. Regulatory T‐cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature 2007; 445:766–70. [DOI] [PubMed] [Google Scholar]
- 18. Muranski P, Borman ZA, Kerkar SP, Klebanoff CA, Ji Y, Sanchez‐Perez L et al Th17 cells are long lived and retain a stem cell‐like molecular signature. Immunity 2011; 35:972–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh‐hora M, Kodama T et al Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med 2014; 20:62–8. [DOI] [PubMed] [Google Scholar]
- 20. Bellemore SM, Nikoopour E, Schwartz JA, Krougly O, Lee‐Chan E, Singh B. Preventative role of interleukin‐17 producing regulatory T helper type 17 (T 17) cells in type 1 diabetes in non‐obese diabetic mice. Clin Exp Immunol 2015; 182:261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koucky M, Malickova K, Cindrova‐Davies T, Germanova A, Parizek A, Kalousova M et al Low levels of circulating T‐regulatory lymphocytes and short cervical length are associated with preterm labor. J Reprod Immunol 2014; 106:110–7. [DOI] [PubMed] [Google Scholar]
- 22. Vargas‐Rojas MI, Solleiro‐Villavicencio H, Soto V. Th1, Th2, Th17 and Treg levels in umbilical cord blood in preeclampsia. J Matern Fetal Neonatal Med 2015; 29:1642–5. [DOI] [PubMed] [Google Scholar]
- 23. Wang WJ, Hao CF, Yi L, Yin GJ, Bao SH, Qiu LH et al Increased prevalence of T helper 17 (Th17) cells in peripheral blood and decidua in unexplained recurrent spontaneous abortion patients. J Reprod Immunol 2010; 84:164–70. [DOI] [PubMed] [Google Scholar]
- 24. Fletcher JM, Lonergan R, Costelloe L, Kinsella K, Moran B, O'Farrelly C et al CD39+Foxp3+ regulatory T cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J Immunol 2009; 183:7602–10. [DOI] [PubMed] [Google Scholar]
- 25. Wang W, Shao S, Jiao Z, Guo M, Xu H, Wang S. The Th17/Treg imbalance and cytokine environment in peripheral blood of patients with rheumatoid arthritis. Rheumatol Int 2012; 32:887–93. [DOI] [PubMed] [Google Scholar]
- 26. Schumacher A, Zenclussen AC. The paternal contribution to fetal tolerance. Adv Exp Med Biol 2015; 868:211–25. [DOI] [PubMed] [Google Scholar]
- 27. Schjenken JE, Robertson SA. Seminal fluid signalling in the female reproductive tract: implications for reproductive success and offspring health. Adv Exp Med Biol 2015; 868:127–58. [DOI] [PubMed] [Google Scholar]
- 28. Schumacher A, Zenclussen AC. Regulatory T cells: regulators of life. Am J Reprod Immunol 2014; 72:158–70. [DOI] [PubMed] [Google Scholar]
- 29. De M, Choudhuri R, Wood GW. Determination of the number and distribution of macrophages, lymphocytes, and granulocytes in the mouse uterus from mating through implantation. J Leukoc Biol 1991; 50:252–62. [DOI] [PubMed] [Google Scholar]
- 30. Robertson SA, Guerin LR, Bromfield JJ, Branson KM, Ahlström AC, Care AS. Seminal fluid drives expansion of the CD4+CD25+ T regulatory cell pool and induces tolerance to paternal alloantigens in mice. Biol Reprod 2009; 80:1036–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sharkey DJ, Macpherson AM, Tremellen KP, Robertson SA. Seminal plasma differentially regulates inflammatory cytokine gene expression in human cervical and vaginal epithelial cells. Mol Hum Reprod 2007; 13:491–501. [DOI] [PubMed] [Google Scholar]
- 32. Balandya E, Wieland‐Alter W, Sanders K, Lahey T. Human seminal plasma fosters CD4+ regulatory T‐cell phenotype and transforming growth factor‐β1 expression. Am J Reprod Immunol 2012; 68:322–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Srivastava MD, Lippes J, Srivastava BI. Cytokines of the human reproductive tract. Am J Reprod Immunol 1996; 36:157–66. [DOI] [PubMed] [Google Scholar]
- 34. Thuere C, Zenclussen ML, Schumacher A, Langwisch S, Schulte‐Wrede U, Teles A et al Kinetics of regulatory T cells during murine pregnancy. Am J Reprod Immunol 2007; 58:514–23. [DOI] [PubMed] [Google Scholar]
- 35. Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol 2004; 5:266–71. [DOI] [PubMed] [Google Scholar]
- 36. Lee JH, Ulrich B, Cho J, Park J, Kim CH. Progesterone promotes differentiation of human cord blood fetal T cells into T regulatory cells but suppresses their differentiation into Th17 cells. J Immunol 2011; 187:1778–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod 2004; 10:347–53. [DOI] [PubMed] [Google Scholar]
- 38. Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T‐cell subset. Immunology 2004; 112:38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Steinborn A, Engst M, Haensch GM, Mahnke K, Schmitt E, Meuer S et al Small for gestational age (SGA) neonates show reduced suppressive activity of their regulatory T cells. Clin Immunol 2010; 134:188–97. [DOI] [PubMed] [Google Scholar]
- 40. Mjösberg J, Svensson J, Johansson E, Hellström L, Casas R, Jenmalm MC et al Systemic reduction of functionally suppressive CD4dimCD25highFoxp3+ Tregs in human second trimester pregnancy is induced by progesterone and 17β‐estradiol. J Immunol 2009; 183:759–69. [DOI] [PubMed] [Google Scholar]
- 41. Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature 2012; 490:102–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schumacher A, Brachwitz N, Sohr S, Engeland K, Langwisch S, Dolaptchieva M et al Human chorionic gonadotropin attracts regulatory T cells into the fetal–maternal interface during early human pregnancy. J Immunol 2009; 182:5488–97. [DOI] [PubMed] [Google Scholar]
- 43. Schumacher A, Heinze K, Witte J, Poloski E, Linzke N, Woidacki K et al Human chorionic gonadotropin as a central regulator of pregnancy immune tolerance. J Immunol 2013; 190:2650–8. [DOI] [PubMed] [Google Scholar]
- 44. Teles A, Schumacher A, Kuhnle MC, Linzke N, Thuere C, Reichardt P et al Control of uterine microenvironment by foxp3+ cells facilitates embryo implantation. Front Immunol 2013; 4:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Martínez FF, Knubel CP, Sánchez MC, Cervi L, Motrán CC. Pregnancy‐specific glycoprotein 1a activates dendritic cells to provide signals for Th17‐, Th2‐, and Treg‐cell polarization. Eur J Immunol 2012; 42:1573–84. [DOI] [PubMed] [Google Scholar]
- 46. DiGiulio DB, Callahan BJ, McMurdie PJ, Costello EK, Lyell DJ, Robaczewska A et al Temporal and spatial variation of the human microbiota during pregnancy. Proc Natl Acad Sci U S A 2015; 112:11060–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Steer P. The epidemiology of preterm labour. BJOG 2005; 112(Suppl. 1):1–3. [DOI] [PubMed] [Google Scholar]
- 48. Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller AB et al Born too soon: the global epidemiology of 15 million preterm births. Reprod Health 2013; 10(Suppl. 1):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wardlaw T, You D, Hug L, Amouzou A, Newby H. UNICEF report: enormous progress in child survival but greater focus on newborns urgently needed. Reprod Health 2014; 11:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Isojima T, Kushima R, Goishi K, Tsuchida S, Watanabe T, Takahashi N et al Mineral status of premature infants in early life and linear growth at age three. Pediatr Int 2015; 57:864–9. [DOI] [PubMed] [Google Scholar]
- 51. Pryhuber GS, Maitre NL, Ballard RA, Cifelli D, Davis SD, Ellenberg JH et al Prematurity and respiratory outcome program (PROP): study protocol of a prospective multicenter study of respiratory outcomes of preterm infants in the United States. BMC Pediatr 2015; 15:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B et al Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 1998; 281:1191–3. [DOI] [PubMed] [Google Scholar]
- 53. Prins JR, Gomez‐Lopez N, Robertson SA. Interleukin‐6 in pregnancy and gestational disorders. J Reprod Immunol 2012; 95:1–14. [DOI] [PubMed] [Google Scholar]
- 54. Cabrera‐Garcia L, Cruz‐Melguizo S, Ruiz‐Antoran B, Torres F, Velasco A, Martinez‐Payo C et al Evaluation of two treatment strategies for the prevention of preterm birth in women identified as at risk by ultrasound (PESAPRO Trial): study protocol for a randomized controlled trial. Trials 2015; 16:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Areia A, Vale‐Pereira S, Alves V, Rodrigues‐Santos P, Moura P, Mota‐Pinto A. Membrane progesterone receptors in human regulatory T cells: a reality in pregnancy. BJOG 2015; 122:1544–50. [DOI] [PubMed] [Google Scholar]
- 56. Organization WH . WHO Recommendations for Prevention and Treatment of Pre‐Eclampsia and Eclampsia. WHO Guidelines Approved by the Guidelines Review Committee. Geneva: Organization WH, 2011. [Google Scholar]
- 57. Perez‐Sepulveda A, Torres MJ, Khoury M, Illanes SE. Innate immune system and preeclampsia. Front Immunol 2014; 5:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre‐eclampsia. Lancet 2010; 376:631–44. [DOI] [PubMed] [Google Scholar]
- 59. Hiby SE, Walker JJ, O'Shaughnessy KM, Redman CW, Carrington M, Trowsdale J et al Combinations of maternal KIR and fetal HLA‐C genes influence the risk of preeclampsia and reproductive success. J Exp Med 2004; 200:957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S et al Excess placental soluble fms‐like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 2003; 111:649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Stepan H, Herraiz I, Schlembach D, Verlohren S, Brennecke S, Chantraine F et al Implementation of the sFlt‐1/PlGF ratio for prediction and diagnosis of pre‐eclampsia in singleton pregnancy: implications for clinical practice. Ultrasound Obstet Gynecol 2015; 45:241–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Molvarec A, Shiozaki A, Ito M, Toldi G, Stenczer B, Szarka A et al Increased prevalence of peripheral blood granulysin‐producing cytotoxic T lymphocytes in preeclampsia. J Reprod Immunol 2011; 91:56–63. [DOI] [PubMed] [Google Scholar]
- 63. de Groot CJ, van der Mast BJ, Visser W, De Kuiper P, Weimar W, Van Besouw NM. Preeclampsia is associated with increased cytotoxic T‐cell capacity to paternal antigens. Am J Obstet Gynecol 2010; 203:496.e1–6. [DOI] [PubMed] [Google Scholar]
- 64. Hiby SE, Apps R, Sharkey AM, Farrell LE, Gardner L, Mulder A et al Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA‐C2. J Clin Invest 2010; 120:4102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Loewendorf AI, Nguyen TA, Yesayan MN, Kahn DA. Preeclampsia is characterized by fetal NK cell activation and a reduction in regulatory T cells. Am J Reprod Immunol 2015; 74:258–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Stirrat GM. Recurrent miscarriage. Lancet 1990; 336:673–5. [DOI] [PubMed] [Google Scholar]
- 67. McNamee K, Dawood F, Farquharson RG. Thrombophilia and early pregnancy loss. Best Pract Res Clin Obstet Gynaecol 2012; 26:91–102. [DOI] [PubMed] [Google Scholar]
- 68. Jaslow CR, Carney JL, Kutteh WH. Diagnostic factors identified in 1020 women with two versus three or more recurrent pregnancy losses. Fertil Steril 2010; 93:1234–43. [DOI] [PubMed] [Google Scholar]
- 69. Saravelos SH, Li TC. Unexplained recurrent miscarriage: how can we explain it? Hum Reprod 2012; 27:1882–6. [DOI] [PubMed] [Google Scholar]
- 70. Wang WJ, Hao CF, Qu QL, Wang X, Qiu LH, Lin QD. The deregulation of regulatory T cells on interleukin‐17‐producing T helper cells in patients with unexplained early recurrent miscarriage. Hum Reprod 2010; 25:2591–6. [DOI] [PubMed] [Google Scholar]
- 71. Zenclussen AC, Blois S, Stumpo R, Olmos S, Arias K, Malan Borel I et al Murine abortion is associated with enhanced interleukin‐6 levels at the feto–maternal interface. Cytokine 2003; 24:150–60. [DOI] [PubMed] [Google Scholar]
- 72. Yuan X, Lin HY, Wang Q, Li TC. Is premature ovarian ageing a cause of unexplained recurrent miscarriage? J Obstet Gynaecol 2012; 32:464–6. [DOI] [PubMed] [Google Scholar]
- 73. Crispin JC, Martínez A, Alcocer‐Varela J. Quantification of regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun 2003; 21:273–6. [DOI] [PubMed] [Google Scholar]
- 74. Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol 2007; 178:2579–88. [DOI] [PubMed] [Google Scholar]
- 75. Bonelli M, Savitskaya A, Dalwigk KV, Steiner CW, Aletaha D, Smolen JS et al Quantitative and qualitative deficiencies of regulatory T cells in patients with systemic lupus erythematosus (SLE). Int Immunol 2008; 20:861–8. [DOI] [PubMed] [Google Scholar]
- 76. Hsu W‐T, Suen J‐L, Chiang B‐L. The role of CD4CD25 T cells in autoantibody production in murine lupus. Clin Exp Immunol 2006; 145:513–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Scalapino KJ, Tang Q, Bluestone JA, Bonyhadi ML, Daikh DI. Suppression of disease in New Zealand Black/New Zealand White lupus‐prone mice by adoptive transfer of ex vivo expanded regulatory T cells. J Immunol 2006; 177:1451–9. [DOI] [PubMed] [Google Scholar]
- 78. Kleczynska W, Jakiela B, Plutecka H, Milewski M, Sanak M, Musial J. Imbalance between Th17 and regulatory T‐cells in systemic lupus erythematosus. Folia Histochem Cytobiol 2011; 49:646–53. [DOI] [PubMed] [Google Scholar]
- 79. Liu M‐F, Wang C‐R. Increased Th17 cells in flow cytometer‐sorted CD45RO‐positive memory CD4 T cells from patients with systemic lupus erythematosus. Lupus Sci Med 2014; 1:e000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mengya Z, Hanyou M, Dong L, Xiaohong L, Lihua Z. Th17/Treg imbalance induced by increased incidence of atherosclerosis in patients with systemic lupus erythematosus (SLE). Clin Rheumatol 2013; 32:1045–52. [DOI] [PubMed] [Google Scholar]
- 81. Szmyrka‐Kaczmarek M, Kosmaczewska A, Ciszak L, Szteblich A, Wiland P. Peripheral blood Th17/Treg imbalance in patients with low‐active systemic lupus erythematosus. Postȩpy Hig Med Dośw (Online) 2014; 68:893–8. [DOI] [PubMed] [Google Scholar]
- 82. Szodoray P, Nakken B, Barath S, Csipo I, Nagy G, El‐Hage F et al Altered Th17 cells and Th17/regulatory T‐cell ratios indicate the subsequent conversion from undifferentiated connective tissue disease to definitive systemic autoimmune disorders. Hum Immunol 2013; 74:1510–8. [DOI] [PubMed] [Google Scholar]
- 83. Talaat RM, Mohamed SF, Bassyouni IH, Raouf AA. Th1/Th2/Th17/Treg cytokine imbalance in systemic lupus erythematosus (SLE) patients: correlation with disease activity. Cytokine 2015; 72:146–53. [DOI] [PubMed] [Google Scholar]
- 84. Xing Q, Wang B, Su H, Cui J, Li J. Elevated Th17 cells are accompanied by FoxP3+ Treg cells decrease in patients with lupus nephritis. Rheumatol Int 2012; 32:949–58. [DOI] [PubMed] [Google Scholar]
- 85. Ma J, Yu J, Tao X, Cai L, Wang J, Zheng SG. The imbalance between regulatory and IL‐17‐secreting CD4+ T cells in lupus patients. Clin Rheumatol 2010; 29:1251–8. [DOI] [PubMed] [Google Scholar]
- 86. Niu Q, Cai B, Huang Z‐C, Shi Y‐Y, Wang L‐L. Disturbed Th17/Treg balance in patients with rheumatoid arthritis. Rheumatol Int 2012; 32:2731–6. [DOI] [PubMed] [Google Scholar]
- 87. Dong L, Wang X, Tan J, Li H, Qian W, Chen J et al Decreased expression of microRNA‐21 correlates with the imbalance of Th17 and Treg cells in patients with rheumatoid arthritis. J Cell Mol Med 2014; 18:2213–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Astry B, Venkatesha SH, Laurence A, Christensen‐Quick A, Garzino‐Demo A, Frieman MB et al Celastrol, a Chinese herbal compound, controls autoimmune inflammation by altering the balance of pathogenic and regulatory T cells in the target organ. Clin Immunol 2015; 157:228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tong L, Nanjundaiah SM, Venkatesha SH, Astry B, Yu H, Moudgil KD. Pristimerin, a naturally occurring triterpenoid, protects against autoimmune arthritis by modulating the cellular and soluble immune mediators of inflammation and tissue damage. Clin Immunol 2014; 155:220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Son H‐J, Lee J, Lee S‐Y, Kim E‐K, Park M‐J, Kim K‐W et al Metformin attenuates experimental autoimmune arthritis through reciprocal regulation of Th17/Treg balance and osteoclastogenesis. Mediators Inflamm 2014; 2014:973986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Estrada‐Capetillo L, Hernández‐Castro B, Monsiváis‐Urenda A, Alvarez‐Quiroga C, Layseca‐Espinosa E, Abud‐Mendoza C et al Induction of Th17 lymphocytes and Treg cells by monocyte‐derived dendritic cells in patients with rheumatoid arthritis and systemic lupus erythematosus. Clin Dev Immunol 2013; 2013:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ju JH, Heo Y‐J, Cho M‐L, Jhun J‐Y, Park J‐S, Lee S‐Y et al Modulation of STAT‐3 in rheumatoid synovial T cells suppresses Th17 differentiation and increases the proportion of Treg cells. Arthritis Rheum 2012; 64:3543–52. [DOI] [PubMed] [Google Scholar]
- 93. Moon S‐J, Park J‐S, Woo Y‐J, Lim M‐A, Kim S‐M, Lee S‐Y et al Rebamipide suppresses collagen‐induced arthritis through reciprocal regulation of Th17/Treg cell differentiation and heme oxygenase 1 induction. Arthritis Rheumatol 2014; 66:874–85. [DOI] [PubMed] [Google Scholar]
- 94. Viglietta V, Baecher‐Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med 2004; 199:971–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Trinschek B, Luessi F, Gross CC, Wiendl H, Jonuleit H. Interferon‐β therapy of multiple sclerosis patients improves the responsiveness of T cells for immune suppression by regulatory T cells. Int J Mol Sci 2015; 16:16330–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Matusevicius D, Kivisäkk P, He B, Kostulas N, Ozenci V, Fredrikson S et al Interleukin‐17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler 1999; 5:101–4. [DOI] [PubMed] [Google Scholar]
- 97. Brucklacher‐Waldert V, Stuerner K, Kolster M, Wolthausen J, Tolosa E. Phenotypical and functional characterization of T helper 17 cells in multiple sclerosis. Brain 2009; 132:3329–41. [DOI] [PubMed] [Google Scholar]
- 98. Nyirenda MH, Morandi E, Vinkemeier U, Constantin‐Teodosiu D, Drinkwater S, Mee M et al TLR2 stimulation regulates the balance between regulatory T cell and Th17 function: a novel mechanism of reduced regulatory T cell function in multiple sclerosis. J Immunol 2015; 194:5761–74. [DOI] [PubMed] [Google Scholar]
- 99. Jamshidian A, Shaygannejad V, Pourazar A, Zarkesh‐Esfahani S‐H, Gharagozloo M. Biased Treg/Th17 balance away from regulatory toward inflammatory phenotype in relapsed multiple sclerosis and its correlation with severity of symptoms. J Neuroimmunol 2013; 262:106–12. [DOI] [PubMed] [Google Scholar]
- 100. Naghavian R, Ghaedi K, Kiani‐Esfahani A, Ganjalikhani‐Hakemi M, Etemadifar M, Nasr‐Esfahani MH, et al miR‐141 and miR‐200a, Revelation of new possible players in modulation of Th17/Treg differentiation and pathogenesis of multiple sclerosis. PLoS ONE 2015; 10:e0124555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wang T, Sun X, Zhao J, Zhang J, Zhu H, Li C et al Regulatory T cells in rheumatoid arthritis showed increased plasticity toward Th17 but retained suppressive function in peripheral blood. Ann Rheum Dis 2015; 74:1293–301. [DOI] [PubMed] [Google Scholar]
- 102. Saboor‐Yaraghi AA, Harirchian MH, Mohammadzadeh Honarvar N, Bitarafan S, Abdolahi M, Siassi F et al The effect of vitamin A supplementation on FoxP3 and TGF‐β gene expression in avonex‐treated multiple sclerosis patients. J Mol Neurosci 2015; 56:608–12. [DOI] [PubMed] [Google Scholar]
- 103. Kürtüncü M, Tüzün E, Türkoğlu R, Petek‐Balcı B, Içöz S, Pehlivan M et al Effect of short‐term interferon‐β treatment on cytokines in multiple sclerosis: significant modulation of IL‐17 and IL‐23. Cytokine 2012; 59:400–2. [DOI] [PubMed] [Google Scholar]
- 104. Álvarez‐Sánchez N, Cruz‐Chamorro I, López‐González A, Utrilla JC, Fernández‐Santos JM, Martínez‐López A et al Melatonin controls experimental autoimmune encephalomyelitis by altering the T effector/regulatory balance. Brain Behav Immun 2015; 50:101–14. [DOI] [PubMed] [Google Scholar]
- 105. Förger F, Marcoli N, Gadola S, Möller B, Villiger PM, Østensen M. Pregnancy induces numerical and functional changes of CD4+CD25high regulatory T cells in patients with rheumatoid arthritis. Ann Rheum Dis 2008; 67:984–90. [DOI] [PubMed] [Google Scholar]
- 106. Munoz‐Suano A, Kallikourdis M, Sarris M, Betz AG. Regulatory T cells protect from autoimmune arthritis during pregnancy. J Autoimmun 2012; 38:J103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Neuteboom RF, Verbraak E, Wierenga‐Wolf AF, van Meurs M, Steegers EA, Groot CJd et al Pregnancy‐induced fluctuations in functional T‐cell subsets in multiple sclerosis patients. Mult Scler 2010; 16:1073–8. [DOI] [PubMed] [Google Scholar]
- 108. Sánchez‐Ramón S, Navarro AJ, Aristimuño C, Rodríguez‐Mahou M, Bellón JM, Fernández‐Cruz E et al Pregnancy‐induced expansion of regulatory T‐lymphocytes may mediate protection to multiple sclerosis activity. Immunol Lett 2005; 96:195–201. [DOI] [PubMed] [Google Scholar]
- 109. Hong T, Xing J, Li L, Tyson JJ. A mathematical model for the reciprocal differentiation of T helper 17 cells and induced regulatory T cells. PLoS Comput Biol 2011; 7:e1002122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Busse D, de la Rosa M, Hobiger K, Thurley K, Flossdorf M, Scheffold A et al Competing feedback loops shape IL‐2 signaling between helper and regulatory T lymphocytes in cellular microenvironments. Proc Natl Acad Sci U S A 2010; 107:3058–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Naldi A, Carneiro J, Chaouiya C, Thieffry D. Diversity and plasticity of Th cell types predicted from regulatory network modelling. PLoS Comput Biol 2010; 6:e1000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Mendoza L, Pardo F. A robust model to describe the differentiation of T‐helper cells. Theory Biosci 2010; 129:283–93. [DOI] [PubMed] [Google Scholar]
- 113. Lee SK, Kim JY, Hur SE, Kim CJ, Na BJ, Lee M et al An imbalance in interleukin‐17‐producing T and Foxp3+ regulatory T cells in women with idiopathic recurrent pregnancy loss. Hum Reprod 2011; 26:2964–71. [DOI] [PubMed] [Google Scholar]
- 114. Afzali B, Lombardi G, Lechler RI, Lord GM. The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clin Exp Immunol 2007; 148:32–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Tsur A, Hughes GC, Shoenfeld Y, Carp H. Interdisciplinary exchange of ideas: progestagens for autoimmunity, biologics for pregnancy complications. Immunol Res 2015; 61:31–4. [DOI] [PubMed] [Google Scholar]
- 116. Schumacher A, Poloski E, Spörke D, Zenclussen AC. Luteinizing hormone contributes to fetal tolerance by regulating adaptive immune responses. Am J Reprod Immunol 2014; 71:434–40. [DOI] [PubMed] [Google Scholar]
- 117. Sasaki Y, Darmochwal‐Kolarz D, Suzuki D, Sakai M, Ito M, Shima T et al Proportion of peripheral blood and decidual CD4+ CD25bright regulatory T cells in pre‐eclampsia. Clin Exp Immunol 2007; 149:139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Teles A, Zenclussen AC, Schumacher A. Regulatory T cells are baby's best friends. Am J Reprod Immunol 2013; 69:331–9. [DOI] [PubMed] [Google Scholar]
- 119. Lee SK, Kim JY, Lee M, Gilman‐Sachs A, Kwak‐Kim J. Th17 and regulatory T cells in women with recurrent pregnancy loss. Am J Reprod Immunol 2012; 67:311–8. [DOI] [PubMed] [Google Scholar]
- 120. Zenclussen AC, Sollwedel A, Bertoja AZ, Gerlof K, Zenclussen ML, Woiciechowsky C et al Heme oxygenase as a therapeutic target in immunological pregnancy complications. Int Immunopharmacol 2005; 5:41–51. [DOI] [PubMed] [Google Scholar]
- 121. Wafula PO, Teles A, Schumacher A, Pohl K, Yagita H, Volk HD et al PD‐1 but not CTLA‐4 blockage abrogates the protective effect of regulatory T cells in a pregnancy murine model. Am J Reprod Immunol 2009; 62:283–92. [DOI] [PubMed] [Google Scholar]


