Abstract
Ethylene (ET) is a gaseous plant hormone that plays essential roles in biotic and abiotic stress responses in plants. However, the role of ET in cold tolerance varies in different species. This study revealed that low temperature promotes the release of ET in grapevine. The treatment of exogenous 1-aminocyclopropane-1-carboxylate increased the cold tolerance of grapevine. By contrast, the application of the ET biosynthesis inhibitor aminoethoxyvinylglycine reduced the cold tolerance of grapevine. This finding suggested that ET positively affected cold stress responses in grapevine. The expression of VaERF057, an ET signaling downstream gene, was strongly induced by low temperature. The overexpression of VaERF057 also enhanced the cold tolerance of Arabidopsis. Under cold treatment, malondialdehyde content was lower and superoxide dismutase, peroxidase, and catalase activities were higher in transgenic lines than in wild-type plants. RNA-Seq results showed that 32 stress-related genes, such as CBF1-3, were upregulated in VaERF057-overexpressing transgenic line. Yeast one-hybrid results further demonstrated that VaERF057 specifically binds to GCC-box and DRE motifs. Thus, VaERF057 may directly regulate the expression of its target stress-responsive genes by interacting with a GCC-box or a DRE element. Our work confirmed that ET positively regulates cold tolerance in grapevine by modulating the expression of VaERF057.
Ethylene (ET) is a gaseous plant hormone that regulates many aspects of the plant life cycle, including seed germination, leaf senescence, fruit ripening, abscission, and biotic and abiotic stress responses1,2,3. ET biosynthesis is mainly regulated by 1-aminocyclopropane-1-carboxylate synthase (ACS) and 1-aminocyclopropane-1-carboxylate oxidase (ACO) at transcriptional or post-translational levels4,5. In Arabidopsis, ET can be captured by a five-member endoplasmic reticulum (ER)-localized receptor family, including ETHYLENE RESPONSE1 and 2 (ETR1 and ETR2), ETHYLENE RESPONSE SENSOR1 and 2 (ERS1 and ERS2), and ETHYLENE INSENSITIVE4 (EIN4). These receptors remove the block of CONSTITUTIVE TRIPLE RESPONSE1 (CTR1) on EIN26,7,8. EIN2 becomes released and then activates EIN3/EIN3-Like1, which positively regulates the expression of ETHYLENE RESPONSE FACTOR (ERF) transcription factors, such as ERF19,10,11. These ERF transcription factors participate in developmental and stress-related signal pathways by regulating the expression of downstream genes12.
Low temperature is a primary environmental factor that limits plant growth and development, productivity, and geographical distribution13. Plants must adjust to various physiological and biochemical processes in response to cold stress14. Endogenous ET levels altered under cold stress have been observed in many plant species. However, the role of ET in cold tolerance varies in different plant species, even in Arabidopsis grown in different environments. For instance, the cold tolerance of soil-grown Arabidopsis seedlings treated with 1-aminocyclopropane-1-carboxylate (ACC) is enhanced15 but is reduced in vitro; cold tolerance is increased when aminoethoxyvinylglycine (AVG) is applied14. ET positively affects the cold tolerance of tomato (Lycopersicon esculentum)16. By contrast, ET levels are negatively correlated with the cold tolerance of Medicago truncatula17. ET also negatively influences the cold tolerance of tobacco (Nicotiana tabacum), whereas AVG application enhances cold tolerance18. Therefore, the role of ET on cold tolerance is possibly species dependent19. As such, the function of ET in different species under cold stress should be investigated to elucidate the diverse roles of the ET signal transduction pathway in plants during cold stress.
ERF transcription factors, which are located downstream of the ET signal pathway, belong to the APETALA2/ERF (AP2/ERF) family. This family can be divided into AP2, C-repeat binding factor/dehydration-responsive element binding factor (CBF/DREB), ERF, RAV, and other subfamilies on the basis of their sequence similarities and number of AP2/ERF domains20. The ERF subfamily proteins contain single AP2/ERF domain and mainly participate in responses to biotic stresses, such as pathogenesis, by binding to the GCC-box present in the promoter of ET-inducible pathogenesis-related (PR) genes21,22. ERF genes can recognize dehydration-responsive element (DRE) and play an important role in abiotic stress responses23. The ERF overexpression in Arabidopsis, tobacco, and soybean plants positively regulates the expression of several PR genes; as a result, the resistances to bacterial, fungal, or viral pathogens of these plants are enhanced24,25,26. ERF genes also respond to various abiotic stresses. For instance, overexpression in the sense or antisense orientation of TERF2/ERF2 can increase or decrease the freezing tolerance of tomato and tobacco, respectively18. The overexpression of GmERF7 enhances the salt tolerance of transgenic tobacco27; by contrast, the overexpression of OsERF3 decreases the drought tolerance of rice28. Although many ERF genes have been identified in different species, the regulatory pathway of the abiotic stress response of the ERF subfamily remains poorly understood.
Grapevine (Vitis) is a widely cultivated fruit crop worldwide. Low temperature is a crucial environmental factor that negatively affects grapevine productivity and quality. To address this problem, researchers widely investigated the genetic mechanisms of cold acclimation in grapevine. Previous studies mainly focused on the CBF pathway in grapevine. The overexpression of CBF genes, including CBF1−4 of grapevines, induces the expression of downstream genes and thus increases the freezing tolerance of transgenic Arabidopsis or tobacco plants29,30,31. CBF genes belong to the CBF/DREB subfamily of the AP2/ERF family. The AP2/ERF family in grapevine has been subjected to genomic and transcriptomic analyses, and 149 members, including 122 ERF transcription factors, have been identified32. Three ERF members, namely, VpERF1, VpERF2, and VpERF3 from V. pseudoreticulata, are involved in biotic and abiotic stress responses30. Xin et al.33 performed a transcriptomic analysis and identified 16 AP2/ERF transcription factors that respond to low temperature in grapevine. However, the function of ET and its downstream ERF genes under low temperature remains unclear.
This study aimed to obtain insights into the function of ET in the cold tolerance of grapevine. A cold-hardy species (V. amurensis) and a freezing-sensitive cultivar (V. vinifera ‘Muscat Hamburg’) were used to investigate the differences in the ET-related signaling pathway of these two species. ET production under cold treatment was determined in these two grapevine species. The effects of exogenous ET addition and endogenous ET synthesis inhibition on the cold tolerance of grapevine were also evaluated. The function of VaERF057, which belongs to the ERF transcription family and is strongly induced by cold stress and exogenous ET, was analyzed in transgenic Arabidopsis. RNA-Seq was performed with VaERF057-overexpressing Arabidopsis transgenic lines to determine the putative downstream genes regulated by VaERF057. On the basis of our results, we proposed a comprehensive model of ET-dependent signal transduction in grapevine under cold stress.
Results
Low temperature increased ET production in grapevine
The function of ET in cold tolerance varies in different plant species. ET biosynthesis increases or decreases under cold stress depending on the plant species15,16,34,35. To investigate ET production under cold stress in grapevine, we first determined ET biosynthesis in V. amurensis and ‘Muscat Hamburg’ plantlets that were subjected to low temperature (4 °C). A similar evolution of ET production was observed in both species throughout the treatment with low temperature (Fig. 1a). ET production rapidly increased after initiating the cold stress, reached the maximum at 8 h, and then decreased to the original level at 48 h (Fig. 1a). However, the maximum changes in the two grape species were slightly different (1.7-fold in V. amurensis and 2-fold in ‘Muscat Hamburg’). These results indicated that ET biosynthesis in grapevine was remarkably enhanced at the early period of cold stress.
Figure 1.
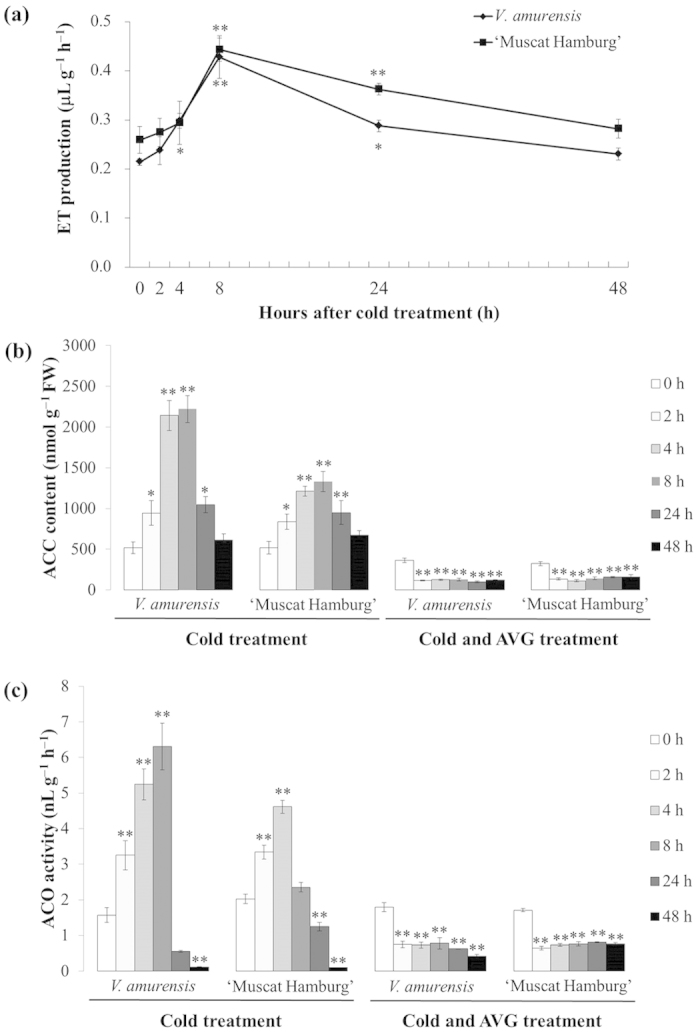
Changes in (a) ET production, (b) ACC content, and (c) ACO activity in V. amurensis and ‘Muscat Hamburg’ under cold stress. Determination was performed at 0, 2, 4, 8, 24, and 48 h under cold stress at 4 °C. For AVG treatment, the plantlets were treated with 100 μM AVG under cold stress, and distilled water was used as control. Data are the mean values ± SE of three biological replicates. **and *indicate significant differences compared with the point of 0 h at P < 0.01 and P < 0.05 (Student’s t-test), respectively.
ACC is a direct product of ACS and its content can be used as an indicator of ACS activity. ACC content in the two grape species rapidly increased, reached the maximum at 8 h after initiating the cold stress, and then decreased (Fig. 1b). The greatest change in ACC content was up to 4.3-fold in V. amurensis and up to 2.6-fold in ‘Muscat Hamburg’. Similar to the changes in ACC content, ACO activity under cold stress first increased and then decreased (Fig. 1c). Moreover, the fold changes in ACO activity were greater in V. amurensis than in ‘Muscat Hamburg’. ACO is an enzyme that catalyzes the last step of the ET biosynthetic pathway by converting ACC into ET. The results of the evolution of both ACC content and ACO activity strongly support the increase in ET production in grapevines during the early stage of cold stress.
Application of ACC and AVG respectively enhanced and reduced the cold tolerance of grapevine
To establish the relationship between ET production and cold tolerance, we measured the semi-lethal temperature (LT50) of grapevine leaves treated with ET precursor (ACC) and ACS inhibitor (AVG). Application of exogenous ACC reduced LT50 value from −9.2 °C to −11.3 °C in V. amurensis and from −7.3 °C to −10.2 °C in ‘Muscat Hamburg’ (Fig. 2). The application of AVG (100 μM) under cold stress largely inhibited ACC biosynthesis and ACO activity in grapevine (Fig. 1b,c), indicating that AVG can inhibit ET production in grapevine under low temperature. By contrast, application of exogenous AVG increased LT50 values to −8.1 °C in V. amurensis and −5.7 °C in ‘Muscat Hamburg’ (Fig. 2). Previous results indicated that an increased ET production resulting from exogenous application of ET precursor enhances cold resistance, whereas a reduced cold tolerance was expected from suppression of ET production both in V. amurensis and ‘Muscat Hamburg’.
Figure 2. Effects of exogenous application of ACC and AVG on the cold tolerance of V. amurensis and ‘Muscat Hamburg’.
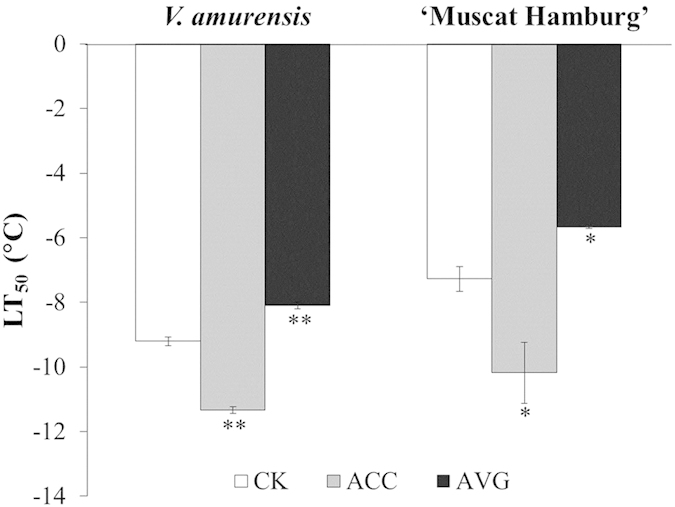
Plantlets of V. amurensis and ‘Muscat Hamburg’ were treated with 100 μM ACC or 100 μM AVG, and distilled water was used as control (CK). LT50 values were calculated after the plants were treated with ACC and AVG for 8 h. Data are the mean values ± SE of three biological replicates. **and *indicate significant differences compared with the CK at P < 0.01 and P < 0.05 (Student’s t-test), respectively.
Isolation and characterization of VaERF057 and VvERF057
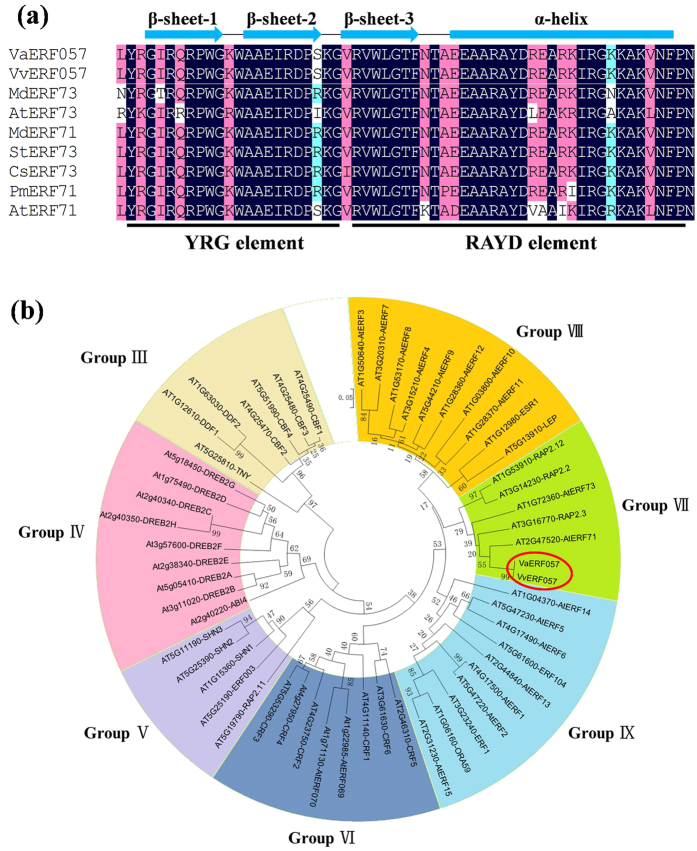
The ERF transcription factors found downstream of the ET signal pathway play an important role in response to abiotic stresses, including salt, drought, and cold stresses18,36. Our previous study revealed that ERF057 is significantly upregulated under cold stress33. We isolated the VaERF057 and VvERF057 genes from V. amurensis and ‘Muscat Hamburg’, respectively. The putative protein sequences of VaERF057 and VvERF057 showed 97.5% identity with proteins containing the same AP2/ERF domain (Fig. S1). Analysis of the conserved AP2/ERF domain of 59 amino acids showed that the AP2/ERF domain shares over 90% identity with its homologs from different plant species (Fig. 3a). Based on the conventional classification37, the AP2/ERF domain is divided into two conserved segments (YRG and RAYD), and the protein formed contains three β-sheets and a single α-helix (Fig. 3a). Phylogenetic analysis further revealed the evolutionary relationship to the ERFs of Arabidopsis, suggesting that VaERF057 and VvERF057 belong to Group VII of the ERF family (Fig. 3b). Given that no significant differences exist between VaERF057 and VvERF057, the functional studies described below mainly focused on VaERF057.
Figure 3. Sequence alignment and phylogenetic analysis of VaERF057 and VvERF057 homologues from different plants.
(a) Analysis of the conserved AP2/ERF domain of ERF057 homologues from different plants. Three β-sheets and one α-helix of the AP2/ERF domain are marked above their corresponding sequences. The YRG and RAYD elements are indicated below the consensus sequence. The GenBank accession numbers are as follows: Malus domestica MdERF73 (XP_008369034), MdERF71 (XP_008386662), Arabidopsis thaliana AtERF73 (AT1G72360), ATERF71 (AT2G47520), Solanum tuberosum StERF73 (XP_006359865), Cucumis sativus CsERF73 (XP_004152238), and Prunus mume PmERF71 (XP_008235129). (b) Phylogenetic tree of VaERF057 and VvERF057 with ERFs from Arabidopsis. The Arabidopsis ERF sequences were downloaded from TAIR. The protein sequences were aligned using Clustal W2, and phylogenetic tree was generated by MEGA6.0 software using the neighbor-joining method.
Increased expression levels of VaERF057 and VvERF057 were dependent on enhanced ET production under cold stress
The expression patterns of VaERF057 and VvERF057 in response to cold, ACC and AVG were analyzed by quantitative real time RT-PCR (qRT-PCR). Transcript levels of VaERF057 and VvERF057 rapidly increased after initiating cold stress and peaked at 12 h (6.2-fold) and 48 h (12.8-fold), respectively (Fig. 4a). Expression of VaERF057 and VvERF057 also rapidly increased in the plants after ACC application (Fig. 4b). The expression of VaERF057 increased by 8.2-fold at 2 h and VvERF057 increased by 15.3-fold at 4 h. The effects of cold stress on the expression of VaERF057 and VvERF057 were totally inhibited when the plants were subjected with AVG under cold condition (Fig. 4a,c). These results suggested that the response of VaERF057 and VvERF057 possibly relies on the increased ET release during cold stress.
Figure 4.

Expression analyses of VaERF057 and VvERF057 under (a) cold, (b) ACC, and (c) cold + AVG treatment. Malate dehydrogenase gene (MDH, GenBank accession number: EC921711) and β-actin (GenBank accession number: EC969944) were used as internal controls. Each reaction was replicated three times for each biological sample, using a total of three biological replicates. The expression level of VaERF057 and VvERF057 was normalized to that of the internal controls. For each treatment, gene expression at 0 h was set at 1.0 and the expression levels in other time points were calculated accordingly. The relative expression and standard errors for each sample were calculated using Biogazelle qbasePLUS. Error bars represent the standard deviations of nine PCR replicates of three biological replicates.
Overexpression of VaERF057 enhanced cold tolerance of transgenic Arabidopsis by reducing malondialdehyde (MDA) content and by increasing superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) activities
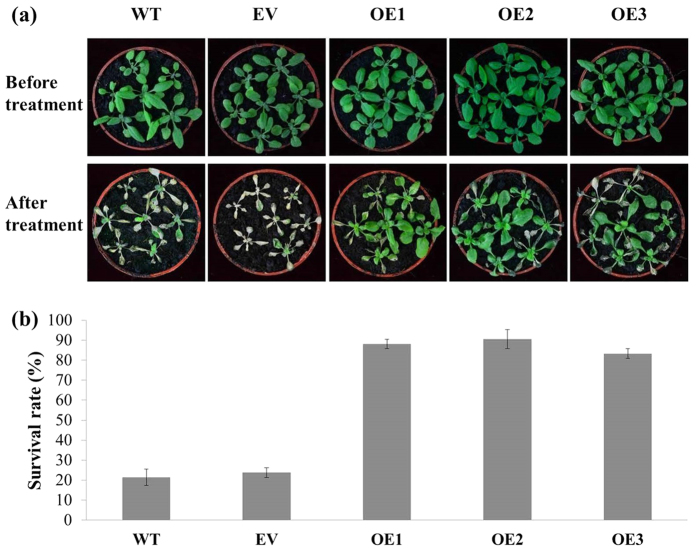
To further investigate the possible role of VaERF057 in abiotic stress responses, we generated 10 T3 homozygous transgenic Arabidopsis lines, which were confirmed by genomic DNA PCR and qRT-PCR (data not shown). Three representative transgenic lines (OE1, OE2, and OE3) displaying high VaERF057 expression levels were used in subsequent experiments. VaERF057-overexpressing Arabidopsis plants displayed significantly improved cold tolerance (Fig. 5a). The average survival rate of the VaERF057-overexpressing plants was over 85% after cold stress at −11 °C, whereas that of the wild-type (WT) and empty vector (EV) plants demonstrated only 21.4% and 23.8% survival rate, respectively (Fig. 5b).
Figure 5. VaERF057 overexpression conferred enhanced cold tolerance in Arabidopsis.
(a) Phenotypes of the overexpression lines (OE1, OE2, and OE3) and controls [wild type (WT) and empty vector (EV)] after cold treatment. (b) Survival rate of Arabidopsis after cold treatment.
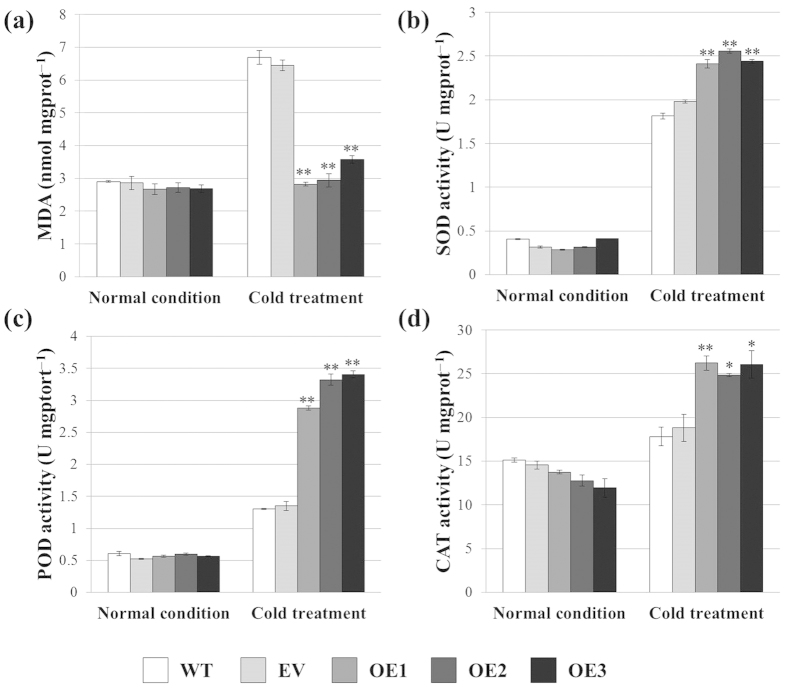
To further investigate the physiological changes under cold treatment in the VaERF057-overexpressing Arabidopsis, we determined the four important physiological indexes, namely, MDA, SOD, POD, and CAT, which reflect plant cold tolerance. No significant differences were observed among transgenic lines and the controls (WT and EV) under normal conditions (Fig. 6). However, under cold stress, MDA content was significantly lower in transgenic plants than in WT and EV plants (Fig. 6a), whereas SOD, POD, and CAT activities were significantly increased in the transgenic plants compared with that in the WT and EV plants (Fig. 6b–d). These results suggested that the tissues of the VaERF057-overexpressing plants were less injured as reflected in their low MDA index, and this phenomenon should increase SOD, POD, and CAT activities to improve tolerance of Arabidopsis to cold stress.
Figure 6.
Comparison of the (a) MDA content, (b) SOD, (c) POD, and (d) CAT activities of the overexpression lines (OE1, OE2, and OE3) and controls [wild type (WT) and empty vector (EV)] exposed to cold stress. Data are mean values ± SE of three biological replicates. **and *indicate significant differences compared with the WT at P < 0.01 and P < 0.05 (Student’s t-test), respectively.
Transcriptome analysis of the VaERF057-overexpressing Arabidopsis revealed altered expression of genes involved in biotic and abiotic stress responses
To determine the possible direct or indirect targets of VaERF057, we performed whole transcriptome sequencing (RNA-Seq) of two biological replicates of WT and three transgenic Arabidopsis lines mixture (OE1, OE2, and OE3) under normal conditions. A total of 140 significantly differentially expressed genes demonstrating changes of more than 2-fold were found in the transgenic lines compared with the WT plants. Among these altered genes, 89 were upregulated and 51 were downregulated (Table S2). To confirm the RNA-Seq results, we verified the 23 significantly differentially expressed genes, including 16 upregulated and 7 downregulated genes by using qRT-PCR (Fig. S3). All of these verified genes showed similar changes in expression as revealed by RNA-Seq and qRT-PCR, indicating the reliability of the transcription profile analysis.
To gain insight into the functions of these genes, we performed Gene Ontology (GO) term enrichment analysis by using agriGO v1.2 in default parameters38. The most significantly enriched biological processes among these significantly differentially expressed genes in transgenic Arabidopsis are involved in responding to stimuli (including biotic and abiotic stimuli, cold, heat, water deprivation, hypoxia, wounding, pathogen, and osmotic and oxidative stresses), metabolic processes, biological regulation, and signal transduction (Fig. S2).
Among the significantly differentially expressed genes, 39 genes are involved in cold, drought, salt, hypoxia, and pathogen stresses; 32 of these genes were upregulated and 7 were downregulated (Table 1). Many key genes involved in these stress tolerances were identified. For instance, CBF1, CBF2, and CBF3, which encode a small family of transcriptional activators that play an important role in freezing tolerance and cold acclimation in Arabidopsis39,40, were upregulated by 3.9-, 2.4-, and 4.5-fold, respectively. NCED3 (upregulated by 2.7-fold) is a key gene in the ABA biosynthesis pathway and enhances plant tolerance to abiotic stresses by regulating endogenous ABA biosynthesis41,42. These results showed that VaERF057 overexpression can regulate expression of many genes involved in biotic and abiotic stress response. The enhanced cold tolerance of the VaERF057-overexpressing Arabidopsis suggested that VaERF057 directly or indirectly regulates expression of these stress-responsive genes.
Table 1. Significant differentially expressed genes involved in cold, drought, salt, hypoxia, and pathogen stresses in transgenic Arabidopsis.
| AGI locus | Symbol | Fold change | FDR | Response to abiotic or biotic stress* |
||||
|---|---|---|---|---|---|---|---|---|
| Cold | Drought | Salt | Hypoxia | Pathogen | ||||
| Upregulated genes | ||||||||
| AT5G39890 | PCO2 | 8.05 | 0.0056 | YES | ||||
| AT5G54470 | BBX29 | 5.62 | 0.0056 | YES | ||||
| AT2G44840 | ATERF13 | 4.81 | 0.0360 | YES | YES | |||
| AT5G47230 | ERF5 | 4.52 | 0.0056 | YES | ||||
| AT4G25480 | CBF3 | 4.47 | 0.0056 | YES | YES | YES | ||
| AT2G16060 | GLB1 | 4.36 | 0.0056 | YES | ||||
| AT4G25490 | CBF1 | 3.87 | 0.0273 | YES | YES | |||
| AT5G27420 | CNI1 | 3.57 | 0.0056 | YES | YES | |||
| AT2G20880 | ERF053 | 3.46 | 0.0056 | YES | YES | |||
| AT2G38470 | WRKY33 | 3.42 | 0.0056 | YES | YES | YES | YES | |
| AT1G76650 | CML38 | 3.35 | 0.0056 | YES | ||||
| AT2G40140 | CZF1 | 3.21 | 0.0056 | YES | YES | |||
| AT5G54490 | PBP1 | 3.18 | 0.0056 | YES | YES | |||
| AT1G02450 | NIMIN1 | 3.13 | 0.0498 | YES | YES | |||
| AT3G02550 | LBD41 | 3.13 | 0.0056 | YES | ||||
| AT1G27730 | STZ | 3.12 | 0.0056 | YES | YES | YES | ||
| AT3G14440 | NCED3 | 2.69 | 0.0056 | YES | YES | YES | ||
| AT1G66090 | – | 2.62 | 0.0056 | YES | ||||
| AT5G54610 | ANK | 2.56 | 0.0056 | YES | ||||
| AT1G09350 | AtGolS3 | 2.51 | 0.0391 | YES | ||||
| AT3G56710 | SIB1 | 2.49 | 0.0056 | YES | ||||
| AT5G57220 | CYP81F2 | 2.46 | 0.0102 | YES | YES | |||
| AT5G15120 | PCO1 | 2.45 | 0.0441 | YES | ||||
| AT3G55980 | SZF1 | 2.42 | 0.0056 | YES | ||||
| AT5G60900 | RLK1 | 2.37 | 0.0056 | YES | ||||
| AT4G25470 | CBF2 | 2.36 | 0.0418 | YES | ||||
| AT3G61890 | ATHB-12 | 2.35 | 0.0214 | YES | YES | YES | ||
| AT5G61600 | ERF104 | 2.27 | 0.0056 | YES | ||||
| AT2G33380 | RD20 | 2.16 | 0.0056 | YES | YES | YES | YES | |
| AT5G20830 | SUS1 | 2.08 | 0.0056 | YES | YES | YES | ||
| AT3G56400 | WRKY70 | 2.06 | 0.0056 | YES | YES | YES | ||
| AT1G32920 | – | 2.01 | 0.0056 | YES | ||||
| Downregulated genes | ||||||||
| AT1G35140 | PHI-1 | −7.12 | 0.0056 | YES | ||||
| AT2G26020 | PDF1.2b | −6.61 | 0.0214 | YES | ||||
| AT1G21910 | DREB26 | −3.74 | 0.0056 | YES | ||||
| AT2G14610 | PR1 | −3.49 | 0.0056 | YES | YES | |||
| AT4G16260 | – | −2.84 | 0.0056 | YES | YES | |||
| AT5G57560 | TCH4 | −2.66 | 0.0056 | YES | ||||
| AT3G05727 | – | −2.03 | 0.0056 | YES | ||||
*The annotation of all genes was retrieved from TAIR 10.
VaERF057 demonstrated binding activity to GCC-box and DRE motifs
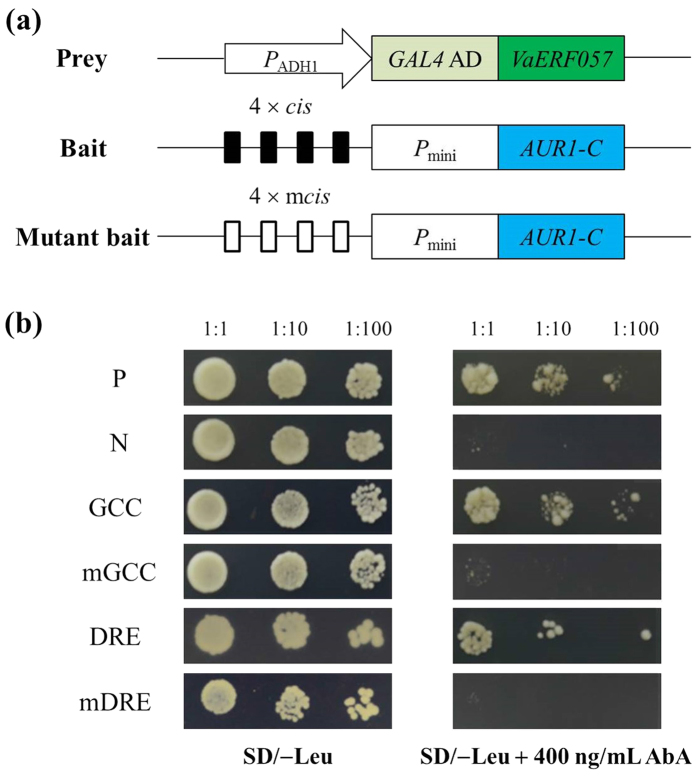
Previous studies have shown that some ERF proteins can bind to GCC-box or DRE motif24,25,43. Especially, AtERF71/HRE2, the closest homologue of VaERF057 in Arabidopsis, can bind to both GCC-box and DRE motifs43. To determine whether VaERF057 can also bind to GCC-box and DRE motifs, we cloned four tandem copies of GCC-box, DRE, or their mutants into pAbAi vector, which was interacted with the pGADT7 activation domain vector harboring VaERF057 by using Y1H system. Figure 7 shows that VaERF057 can specifically bind to both GCC-box and DRE motifs but failed to bind to all of the mutated GCC-box and DRE motifs. Among the 140 significantly differentially expressed genes, 24 genes contain GCC-box and 54 genes contain at least one DRE core motif upstream of their 1 kb promoter region (Table S2). These results suggested that VaERF057 regulates the expression of target genes by binding to GCC-box and DRE motifs in their promoter regions.
Figure 7. Analyses of the binding activity of VaERF057 to GCC-box and DRE motifs.
(a) Diagram of prey and bait vectors. VaERF057 protein was fused with the GAL4 AD in a pGADT7-AD vector to generate the prey (pGAD-VaERF057). Four tandem copies of the GCC-box, DRE, or their corresponding mutants were cloned into the pAbAi vector and used as baits. (b) Positively co-transformed yeast cells were determined by spotting serial dilutions (1:1, 1:10, and 1:100) of yeast on SD/−Leu medium supplemented with 400 ng ml−1 AbA. P: positive control (pGAD-p53 + p53-AbAi); N: negative control (pGADT7-AD + p53-AbAi).
Discussion
This study explored the function of ET and the roles of its downstream gene VaERF057 in grapevine during cold stress. Our results demonstrated that cold stress enhanced the production of ET and the expression of ET signal pathway downstream genes, such as VaERF057 and VvERF057, in grapevine. Enhanced ET synthesis is essential in cold tolerance both in cold-hardy (V. amurensis) or cold-sensitive (V. vinifera ‘Muscat Hamburg’) grapevine.
Our findings are consistent with previous results15,16,18,35 wherein an increase of ET level positively regulates the cold tolerance of grapevine. In addition, ET biosynthesis is required for the accumulation of antifreeze proteins in winter rye (Secale cereale) during cold acclimation44. ET has also been proposed to protect mitochondrial activity in Arabidopsis under cold stress45. However, an opposite conclusion was reached in other studies on several plant species, including Arabidopsis14, M. truncatula17, winter wheat (Triticum aestivum)34, and dwarf bean (Phaseolus vulgaris)46. Especially in Arabidopsis, Catala et al.15 reported that ET production increases under cold treatment and that ET positively regulates cold tolerance, whereas Shi et al.14 obtained the opposite results. These different results can be largely caused by the different growth conditions employed in these studies. Catala et al.15 used soil-grown plants, whereas Shi et al.14 used seedlings grown on Murashige and Skoog (MS) medium in Petri dishes, implying a growth condition of high relative humidity. Indeed, ET production is inhibited in response to cold stress under high relative humidity47,48. In our research, the grapevine plantlets were grown on half-strength MS medium in a conical flask covered with a sealing film that allows good aeration. The relative humidity in our study was higher than the relative humidity the soil-grown plants were exposed to but lower than that to which the plants grown on MS medium in Petri dishes were exposed. Therefore, a critical value of relative humidity possibly inhibits ET production under cold stress. The function of ET in cold stress in different species exposed to different conditions still requires further investigation.
ERF transcription factors, which are located downstream of the ET signal pathway, were reported to respond to various biotic and abiotic stresses in several species. In Arabidopsis, AtERF1−5 genes were differentially regulated by ET and by abiotic stresses, such as cold, drought, wounding, high salinity, or pathogen. AtERF1, AtERF2, and AtERF5 function as activators, whereas AtERF3 and AtERF4 act as repressors by downregulating not only the basal transcription levels of a reporter gene but also the transactivation activity of other transcription factors49,50. Many other ERFs were identified as stress-responsive through detection of their expression under various stress conditions, although functional studies on ERF genes remain limited. Transcript analysis in ‘Cabernet Sauvignon’ grapevines revealed 13 ET–responsive transcripts upregulated and 7 downregulated after cold treatment, whereas ERF057 wasn’t included51. This study isolated the Group VII ERF genes (VaERF057 and VvERF057) from grapevine, and these genes can be induced by cold stress and exogenous ET. AVG can also totally inhibit the inductive effect of cold stress on the expression of these genes. Low temperature promoted the release of ET, and the ET increased the expression of VaERF057 and VvERF057. Therefore, the enhanced expression of VaERF057 under cold stress was possibly directly induced by ET, the release of which was promoted by low temperature (Fig. 8).
Figure 8. Model of ET-dependent signal transduction through VaERF057 under cold stress in grapevine.
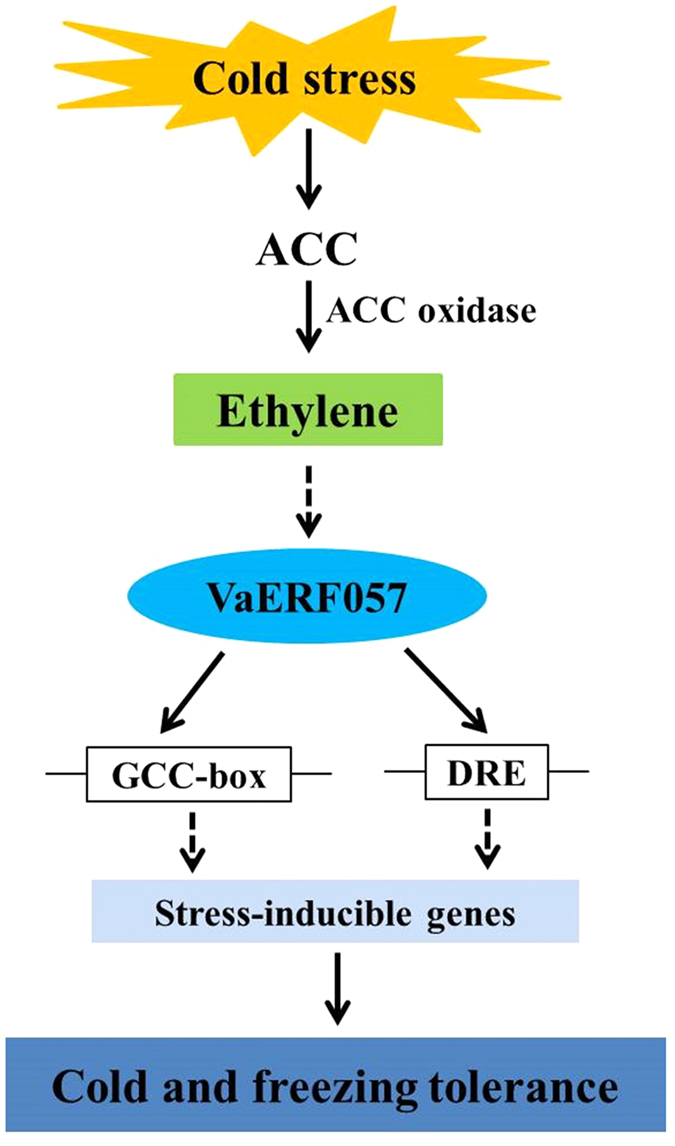
The model indicates that the cold stress-induced increase in ET level activates the ET signaling pathway and upregulates VaERF057 expression, resulting in transcriptional activation of stress-inducible genes and in enhanced cold tolerance of grapevine.
As transcription factors, the AP2/ERFs play important roles in stress response by regulating the expression of downstream stress-related genes. For instance, overexpression of CBF genes in plants enhances cold tolerance by regulating the expression of cold-responsive genes52,53,54. TERF2/ERF2 overexpression can also increase cold tolerance in rice, tomato, and tobacco by activating expression of cold-related genes18,55. In the present study, VaERF057 overexpression enhanced cold tolerance in transgenic Arabidopsis. These physiological adjustments confer cold tolerance to the transgenic Arabidopsis. Moreover, RNA-Seq analysis showed that VaERF057 overexpression in Arabidopsis regulated the expression of the cold-induced regulatory genes, including AtCBF1−3, WRKY33, WRKY70, NCED3, and ERF5 under normal condition. An increasing number of evidence indicates that ERF transcription factors play critical regulatory roles in response to stress by interacting with GCC-box or DRE motif to activate expression of targeted stress-responsive genes24,56,57. AtERF71/HRE2 is the closest homologue of VaERF057 in Arabidopsis. Binding of AtERF71/HRE2 to GCC-box and DRE motifs was detected by electrophoretic mobility shift assay (EMSA), fluorescence measurement and surface plasmon resonance spectroscopy (BIAcore) experiments43. In this study, we provided evidence that VaERF057 can bind to both GCC-box and DRE motifs by Y1H system. Our results indicate that VaERF057 in Arabidopsis possibly directly upregulates expression of its targeted genes by interacting with these cis-elements. The accumulated expression of VaERF057 and VvERF057 was also found in other stress conditions, such as drought and high salinity (data not shown). Further studies should be conducted to overexpress or to knock down VaERF057 and VvERF057 in grapevine and thus elucidate their functions in stress response.
In summary, low temperature can promote the release of ET and endogenous ET can enhance the cold tolerance of grapevine. We isolated a group VII stress-responsive ERF gene (VaERF057), which is located downstream of the ET signal pathway and is implicated as a positive regulator of cold tolerance. VaERF057 overexpression enhanced the cold tolerance of Arabidopsis. This finding indicated that VaERF057, when induced by ET, may function as a key regulator that improves the cold tolerance of grapevine. VaERF057 may directly upregulate the expression of its target stress-responsive genes by interacting with GCC-box or DRE/CRT element in grapevine. On the basis of our results, we proposed a comprehensive model of ET-dependent signal transduction in grapevine under cold stress (Fig. 8). Further studies should be performed to determine other components related to VaERF057 and to gain a clear-cut silhouette of the major hub in the network.
Methods
Plant materials and treatments
Micropropagated V. amurensis and V. vinifera ‘Muscat Hamburg’ plantlets were grown on half-strength Murashige and Skoog (1/2 MS, pH 5.8) solid medium containing 30 g L−1 of sucrose and 0.7% agar in conical flasks (120 mL) in a growth chamber at 26 °C (Fig. S3). The average photosynthetic photon flux was 100 μmol m−2 s−1 with a 16 h light and 8 h dark cycle. Six-week-old plantlets were subjected to cold stress or supplied with plant growth regulators. For cold treatment, whole plants in conical flasks were placed in an illuminated incubation chamber at 4 °C. For treatments with plant growth regulators, plantlet leaves were sprayed with 100 μ μM 1-aminocyclopropane-1-carboxylate (ACC, an ethylene (ET) precursor) or 100 μM aminoethoxyvinylglycine (AVG, an ACC synthase inhibitor), whereas distilled water was used as control. The shoot apex with one well developed leaf was harvested at appropriate times. To reduce the differences between individuals, we collected the samples from three independent replicate plantlets at a single time point, with three biological replicates per treatment. After the collection, all samples were immediately frozen in liquid nitrogen and then stored at −70 °C.
Seeds of Arabidopsis thaliana (ecotype Columbia-0, Col-0) were surface-sterilized with 6% sodium hypochlorite for 5 min followed by three washes with sterile water and then plated on 1/2 MS solid medium containing 0.7% agar. One-week-old seedlings were transferred from plates into pots filled with peat moss in a greenhouse under controlled environmental conditions, at 22 °C, under 50–60% relative humidity, and under 130 μmol photons m−2 s−1 with a 16 h/8 h light/dark cycle.
Determination of ET production
Six-week-old plants grown in conical flasks (120 ml) under standard conditions (26 °C) or exposed to low temperature (4 °C) were maintained open for 1 h. The conical flasks were subsequently sealed with rubber stoppers to collect ET produced within 1 hour at 0, 2, 4, 8, 24, and 48 h. Gas (1 ml) was withdrawn from each conical flask using a gas-tight syringe and left for 5 min to equilibrate. The gas samples were subsequently injected into a gas chromatograph (Agilent 7890A) equipped with an HP-5 column and a flame ionization detector. The detector and injector were operated at 180 °C and 120 °C, respectively, whereas the oven temperature was set at 110 °C. The carrier gas was nitrogen at a flow rate of 40 ml min−1. ET production (μl g−1 FW h−1) was determined by comparison with an ET standard. All measurements were obtained in triplicate from three independent samples.
Determination of ACC content
Determination of ACC content was performed according to the method described by Zhao et al.17 with some minor modifications. In brief, the tissues were ground with a mortar and pestle in 2 ml of 80% ethanol until homogenized. After centrifugation at 12,857 × g for 10 min, the supernatant liquid was dried using a centrifugal dryer. The residue was dissolved in 1 ml of deionized water, and 0.3 ml of sample to be assayed was added in a 7 ml vial in an ice bath. Subsequently, 100 μl of HgCl2 (10 mM) was added and then the vial was sealed with a rubber serum cap. Afterwards, 100 μl of cold mixtures of 5% NaOCl and saturated NaOH (2:1, v/v) was injected into the vial and the mixture was kept on ice for 30 min. After shaking vigorously, ET production was determined as described above. The efficiency of conversion of ACC into ET was calculated by adding authentic ACC (1 mM) as an internal standard. ACC content was calculated from the quantity of ET liberated and from the conversion efficiency.
Determination of ACO activity
ACO activity was detected according to the method described by Zhao et al.17 with some minor modifications. Briefly, the tissues were ground with a mortar and pestle in extraction buffer containing 100 mM Tris (pH 7.2), 30 mM sodium ascorbate, 10% (w/v) glycerol, and 5% (w/v) polyvinylpolypyrrolidone (PVPP). After centrifugation at 18,514 g for 30 min at 4 °C, the supernatant liquid was used for enzyme activity analysis. Crude extract (200 μl) was then mixed with 1.7 ml of extraction buffer (without PVPP), 100 μl of FeSO4 (1 M), and 100 μl of ACC (20 mM) in a 7-ml capped vial. After incubation at 30 °C for 1 h, ET production was determined as described above.
Electrolyte leakage assay
Electrolyte leakage was measured to verify the effect of exogenous ACC and AVG treatment on cold tolerance of grapevine. After treatment with ACC (100 μM) and AVG (100 μM) for 8 h, leaf discs were obtained from V. amurensis and ‘Muscat Hamburg’ plantlets. The 10 ml tubes containing five leaf discs were placed in a low-temperature illuminated incubation chamber at 0 °C for an hour. The temperature was subsequently reduced at 2 °C h−1. The tubes were then removed at −6 °C, −8 °C, −10 °C, and −12 °C for V. amurensis and at −4 °C, −6 °C, −8 °C, and −10 °C for ‘Muscat Hamburg’. The leaf discs were thawed overnight at 4 °C in the dark, and 3 ml of deionized water was added into each tube. After shaking at 200 rpm for 2 h at 25 °C, the electrical conductivity of the samples was first measured. The samples were then autoclaved at 121 °C for 20 min, and the final conductivity was measured. Relative electric conductivity is the ratio of initial conductivity before autoclaving to the final conductivity after autoclaving. The semi-lethal temperature (LT50) was calculated by logistic equation. Each point was repeated for five times.
Cloning and sequence analyses of VaERF057 and VvERF057
To clone the ERF057 gene (GSVIVT01028050001) of V. amurensis and ‘Muscat Hamburg’, we designed a pair of primers (ERF057-ORF-F/ERF057-ORF-R) based on the sequence of V. vinifera ‘Pinot Noir’ (PN40024) genomes (Table S1). The open reading frame (ORF) sequences of VaERF057 and VvERF057 were amplified by polymerase chain reaction (PCR). An analysis of the VaERF057 and VvERF057 protein structures was performed using Smart (http://smart.embl-heidelberg.de/). Sequence alignments of the AP2/ERF domain with the other ERFs from different plant species was done by DNAMAN 7.0. Phylogenetic analysis was performed using MEGA6.0 software by using the neighbor-joining method, and the internal branch support was estimated using 1000 bootstrap replicates.
Gene expression analysis by quantitative real time RT-PCR (qRT-PCR)
Total RNA was extracted from the collected samples by using Column Plant RNAOUT 2.0 Kit (Tiandz, Beijing, China) according to the manufacturer’s instructions. RNase-free DNase I (Promega, USA) was used to degrade any DNA present in the extracted RNA. cDNA was synthesized using SuperScript III Reverse Transcriptase (Invitrogen, USA) with Oligo-dT according to the manufacturer’s instructions. qRT-PCR was performed in an ABI SteopOneplusTM Real-Time PCR System (Applied Biosystems, USA) using FastStart Universal SYBR Green Master (Roche, Shanghai, China). Each reaction was replicated three times for each biological sample, using a total of three biological replicates. The relative expression and standard errors were calculated using Biogazelle qbasePLUS as described in our previous study58.
Generation of overexpression transgenic Arabidopsis
The full-length ORF of VaERF057 containing the restriction sites of Kpn I and Xba I was ligated into pCAMBIA 1301s driven by the CaMV 35S promoter and transformed into Arabidopsis using the floral dip method. The positively transformed plants were selected by screening successive generations on hygromycin B (50 mg L−1) and confirmed by genomic DNA PCR and qRT-PCR. Three T3 homozygous transgenic lines were selected for further analyses.
Stress treatments of transgenic Arabidopsis
Three-week-old WT and transgenic Arabidopsis were subjected under cold tolerance assays. To evaluate cold tolerance, we placed the plants in a growth chamber set at −1 °C for 8 h in the dark. The chamber was then cooled at a rate of 1 °C h−1 until −11 °C was reached. After exposure to −11 °C for 3 h, the plants were thawed at 4 °C for 12 h in the dark and then transferred into standard condition. The survival rate was scored 3 days later. Each sample contained 14 seedlings, and each experiment was performed in three biological replicates.
Physiological analysis of the cold stress response of the Arabidopsis plants
For physiological analysis, 3-week-old seedlings of WT and transgenic Arabidopsis plants were treated at 4 °C (cold stress) for 4 days, and the plants grown under normal condition served as control. Superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) activities, along with malondialdehyde (MDA) contents, were measured according to the protocol described by Shin et al.59. Each sample was pooled from three seedlings, and each experiment was performed in three biological replicates.
RNA-Seq analysis
RNA-Seq analysis was performed using 3-week-old WT and transgenic Arabidopsis mixture under normal condition. Total RNA was extracted using Column Plant RNAOUT 2.0 Kit (Tiandz, Beijing, China) as described above, and mRNA sequencing libraries were constructed using the TruSeq RNA Sample Preparation Kit (Illumina, USA). Sequencing was performed on an Illumina HiSeq3000 platform. Materials of the libraries were pooled from six WT plants and three transgenic Arabidopsis lines (OE1, OE2, and OE3). The RNA-Seq experiments were performed in two biological replicates. The total reads were mapped to the TAIR10 Arabidopsis genome reference by using the TopHat software60. The number of sequencing reads generated from each sample was converted into fragments per kilobase of transcript per million fragments mapped. Significantly differentially expressed transcripts between WT and transgenic lines were detected from two biological replicates by using Cuffdiff with default Benjamini–Hochberg correction for multiple-testing, based on a False Discovery Rate (FDR) <0.05. Functional annotations of genes and AGI symbols were retrieved from TAIR10 datasets. RNA-Seq data were submitted to NCBI and can be accessed under the GEO accession number GSE75456.
Yeast one-hybrid assay
Yeast one-hybrid assay was performed using the Matchmaker Gold Yeast One-Hybrid Library Screening System (Clontech) to examine the ability of VaERF057 to bind with GCC-box and DRE motifs. The ORF of VaERF057 was fused in-frame with the GAL4 activation domain (AD) in a pGADT7-AD vector to generate the prey plasmid (pGAD-VaERF057). A synthesized DNA fragment harboring four tandem copies of the GCC-box, DRE or their mutants, and mGCC or mDRE was cloned into the pAbAi vector to construct the baits. The BstBI-cut bait vectors were transformed into the Y1HGold yeast strain. After selecting the transformants on SD/−Ura plates and determining the minimal inhibitory concentration of Aureobasidin A (AbA) for the bait strains, we introduced pGAD-VaERF057 into the Y1HGold strain. Positively co-transformed yeast cells were determined by spotting serial dilutions (1:1, 1:10, and 1:100) of yeast on SD/−Leu medium supplemented with 400 ng ml−1 AbA and cultured at 30 °C for 3 days. Positive (pGAD-p53 + p53-AbAi) and negative (pGADT7-AD + p53-AbAi) controls were processed in the same manner.
Statistical analysis
The reported experimental data represent at least three independent biological repeats. Where appropriate, results are reported as mean values ± standard errors. Mean comparisons were calculated by Student’s t-test, and P-values and data sizes were indicated in the figure legends.
Additional Information
How to cite this article: Sun, X. et al. Ethylene positively regulates cold tolerance in grapevine by modulating the expression of ETHYLENE RESPONSE FACTOR 057. Sci. Rep. 6, 24066; doi: 10.1038/srep24066 (2016).
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NSFC Accession No. 31130047, 31471857), Grape Breeding Project of Ningxia (NXNYYZ201502), National Key Technology R&D Program of the Ministry of Science and Technology during the Twelfth Five-year Plan Period (2013BAD02B04-1) and Youth Innovation Promotion Association of CAS (2015281).
Footnotes
Author Contributions H.X., S.L. and X.S. designed and oversaw the research. X.S., T.Z. and X.R. performed the experiments and took care of the plants. L.F. S.K.K. and L.C. participated in the sequence analysis and helped to modify the manuscript. X.S., H.X. and Y.W. analysed the data. X.S., H.X. and S.L. wrote the manuscript, made the figures and finalized the tables. All authors read and approved the final manuscript.
References
- Chen H. et al. ETHYLENE INSENSITIVE3 and ETHYLENE INSENSITIVE3-LIKE1 repress SALICYLIC ACID INDUCTION DEFICIENT2 expression to negatively regulate plant innate immunity in Arabidopsis. Plant Cell 21, 2527–2540 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C. C. et al. Ethylene responses in rice roots and coleoptiles are differentially regulated by a carotenoid isomerase-mediated abscisic acid pathway. Plant Cell 27, 1061–1081 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick M. D. & Chang C. Ethylene signaling: new levels of complexity and regulation. Curr. Opin. Plant Biol. 11, 479–485 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argueso C. T., Hansen M. & Kieber J. J. Regulation of ethylene biosynthesis. J. Plant Growth Regul. 26, 92–105 (2007). [Google Scholar]
- Lyzenga W. J., Booth J. K. & Stone S. L. The Arabidopsis RING-type E3 ligase XBAT32 mediates the proteasomal degradation of the ethylene biosynthetic enzyme, 1-aminocyclopropane-1-carboxylate synthase 7. Plant J. 71, 23–34 (2012). [DOI] [PubMed] [Google Scholar]
- Wang K. L., Li H. & Ecker J. R. Ethylene biosynthesis and signaling networks. Plant Cell 14 Suppl, S131–151 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker A. B. & Kende H. Ethylene: a gaseous signal molecule in plants. Annu. Rev. Cell Dev. Biol. 16, 1–18 (2000). [DOI] [PubMed] [Google Scholar]
- Ji Y. & Guo H. From endoplasmic reticulum (ER) to nucleus: EIN2 bridges the gap in ethylene signaling. Mol. Plant 6, 11–14 (2013). [DOI] [PubMed] [Google Scholar]
- An F. et al. Ethylene-induced stabilization of ETHYLENE INSENSITIVE3 and EIN3-LIKE1 is mediated by proteasomal degradation of EIN3 binding F-box 1 and 2 that requires EIN2 in Arabidopsis. Plant Cell 22, 2384–2401 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchante C., Alonso J. M. & Stepanova A. N. Ethylene signaling: simple ligand, complex regulation. Curr. Opin. Plant Biol. 16, 554–560 (2013). [DOI] [PubMed] [Google Scholar]
- Qiao H. et al. Processing and subcellular trafficking of ER-tethered EIN2 control response to ethylene gas. Science 338, 390–393 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrocal-Lobo M., Molina A. & Solano R. Constitutive expression of ETHYLENE-RESPONSE-FACTOR1 in Arabidopsis confers resistance to several necrotrophic fungi. Plant J. 29, 23–32 (2002). [DOI] [PubMed] [Google Scholar]
- Thomashow M. F. PLANT COLD ACCLIMATION: Freezing Tolerance Genes and Regulatory Mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 571–599 (1999). [DOI] [PubMed] [Google Scholar]
- Shi Y. et al. Ethylene signaling negatively regulates freezing tolerance by repressing expression of CBF and type-A ARR genes in Arabidopsis. Plant Cell 24, 2578–2595 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catala R. et al. The Arabidopsis 14-3-3 protein RARE COLD INDUCIBLE 1A links low-temperature response and ethylene biosynthesis to regulate freezing tolerance and cold acclimation. Plant Cell 26, 3326–3342 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciardi J. A., Deikman J. & Orzolek M. D. Increased ethylene synthesis enhances chilling tolerance in tomato. Physiol. Plantarum 101, 333–340 (1997). [Google Scholar]
- Zhao M., Liu W., Xia X., Wang T. & Zhang W. H. Cold acclimation-induced freezing tolerance of Medicago truncatula seedlings is negatively regulated by ethylene. Physiol. Plantarum 152, 115–129 (2014). [DOI] [PubMed] [Google Scholar]
- Zhang Z. & Huang R. Enhanced tolerance to freezing in tobacco and tomato overexpressing transcription factor TERF2/LeERF2 is modulated by ethylene biosynthesis. Plant Mol. Biol. 73, 241–249 (2010). [DOI] [PubMed] [Google Scholar]
- Kazan K. Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci. 20, 219–229 (2015). [DOI] [PubMed] [Google Scholar]
- Sakuma Y. et al. DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem. Bioph. Res. Co. 290, 998–1009 (2002). [DOI] [PubMed] [Google Scholar]
- Sharma M. K. et al. Identification, phylogeny, and transcript profiling of ERF family genes during development and abiotic stress treatments in tomato. Mol. Genet. Genomics 284, 455–475 (2010). [DOI] [PubMed] [Google Scholar]
- Ohme-Takagi M. & Shinshi H. Ethylene-inducible DNA binding proteins that interact with an ethylene-responsive element. Plant Cell 7, 173–182 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z. S. et al. Isolation and molecular characterization of the Triticum aestivum L. ethylene-responsive factor 1 (TaERF1) that increases multiple stress tolerance. Plant Mol. Biol. 65, 719–732 (2007). [DOI] [PubMed] [Google Scholar]
- Wang L., Qin L., Liu W., Zhang D. & Wang Y. A novel ethylene-responsive factor from Tamarix hispida, ThERF1, is a GCC-box- and DRE-motif binding protein that negatively modulates abiotic stress tolerance in Arabidopsis. Physiol. Plantarum 152, 84–97 (2014). [DOI] [PubMed] [Google Scholar]
- Dong L. et al. Overexpression of GmERF5, a new member of the soybean EAR motif-containing ERF transcription factor, enhances resistance to Phytophthora sojae in soybean. J. Exp Bot. 66, 2635–2647 (2015). [DOI] [PubMed] [Google Scholar]
- Zuo K. J. et al. Over-expression GbERF2 transcription factor in tobacco enhances brown spots disease resistance by activating expression of downstream genes. Gene 391, 80–90 (2007). [DOI] [PubMed] [Google Scholar]
- Zhai Y. et al. Isolation and molecular characterization of GmERF7, a soybean ethylene-response factor that increases salt stress tolerance in tobacco. Gene 513, 174–183 (2013). [DOI] [PubMed] [Google Scholar]
- Zhang H. et al. EAR motif mutation of rice OsERF3 alters the regulation of ethylene biosynthesis and drought tolerance. Planta 237, 1443–1451 (2013). [DOI] [PubMed] [Google Scholar]
- Xiao H., Tattersall E. A., Siddiqua M. K., Cramer G. R. & Nassuth A. CBF4 is a unique member of the CBF transcription factor family of Vitis vinifera and Vitis riparia. Plant Cell Environ. 31, 1–10 (2008). [DOI] [PubMed] [Google Scholar]
- Zhu Z. et al. Three ERF transcription factors from Chinese wild grapevine Vitis pseudoreticulata participate in different biotic and abiotic stress-responsive pathways. J. Plant Physiol. 170, 923–933 (2013). [DOI] [PubMed] [Google Scholar]
- Xiao H., Siddiqua M., Braybrook S. & Nassuth A. Three grape CBF/DREB1 genes respond to low temperature, drought and abscisic acid. Plant Cell Environ. 29, 1410–1421 (2006). [DOI] [PubMed] [Google Scholar]
- Licausi F. et al. Genomic and transcriptomic analysis of the AP2/ERF superfamily in Vitis vinifera. BMC Genomics 11, 719 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H. et al. Genome wide transcriptional profile analysis of Vitis amurensis and Vitis vinifera in response to cold stress. PloS ONE 8, e58740 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machackova I., Hanisova A. & Krekule J. Levels of Ethylene, Acc, Macc, Aba and Proline as Indicators of Cold Hardening and Frost-Resistance in Winter-Wheat. Physiol. Plantarum 76, 603–607 (1989). [Google Scholar]
- Chu C. & Lee T. M. The Relationship between Ethylene Biosynthesis and Chilling Tolerance in Seedlings of Rice (Oryza-Sativa-L). Bot. Bull. Acad. Sinica 30, 263–273 (1989). [Google Scholar]
- Makhloufi E. et al. Isolation and molecular characterization of ERF1, an ethylene response factor gene from durum wheat (Triticum turgidum L. subsp. durum), potentially involved in salt-stress responses. J. Exp Bot. 65, 6359–6371 (2014). [DOI] [PubMed] [Google Scholar]
- Mazarei M., Puthoff D. P., Hart J. K., Rodermel S. R. & Baum T. J. Identification and characterization of a soybean ethylene-responsive element-binding protein gene whose mRNA expression changes during soybean cyst nematode infection. Mol. Plant Microbe In. 15, 577–586 (2002). [DOI] [PubMed] [Google Scholar]
- Du Z., Zhou X., Ling Y., Zhang Z. & Su Z. agriGO: a GO analysis toolkit for the agricultural community. Nucleic Acids Res. 38, W64–70 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnusamy V., Zhu J. & Zhu J. K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 12, 444–451 (2007). [DOI] [PubMed] [Google Scholar]
- Novillo F., Medina J. & Salinas J. Arabidopsis CBF1 and CBF3 have a different function than CBF2 in cold acclimation and define different gene classes in the CBF regulon. P. Natl. Acad. Sci. USA 104, 21002–21007 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg Z. & Blumwald E. Hormone balance and abiotic stress tolerance in crop plants. Curr. Opin. Plant Biol. 14, 290–295 (2011). [DOI] [PubMed] [Google Scholar]
- Barrero J. M. et al. Both abscisic acid (ABA)-dependent and ABA-independent pathways govern the induction of NCED3, AAO3 and ABA1 in response to salt stress. Plant Cell Environ. 29, 2000–2008 (2006). [DOI] [PubMed] [Google Scholar]
- Lee S. Y. et al. Arabidopsis AtERF71/HRE2 functions as transcriptional activator via cis-acting GCC box or DRE/CRT element and is involved in root development through regulation of root cell expansion. Plant Cell Rep. 34, 223–231 (2015). [DOI] [PubMed] [Google Scholar]
- Yu X. M., Griffith M. & Wiseman S. B. Ethylene induces antifreeze activity in winter rye leaves. Plant Physiol. 126, 1232–1240 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Huang J., Liang X. & Bi Y. Involvement of hydrogen peroxide, calcium, and ethylene in the induction of the alternative pathway in chilling-stressed Arabidopsis callus. Planta 235, 53–67 (2012). [DOI] [PubMed] [Google Scholar]
- Field R. J. The Role of 1-Aminocyclopropane-1-Carboxylic Acid in the Control of Low-Temperature Induced Ethylene Production in Leaf Tissue of Phaseolus-Vulgaris L. Ann. Bot. 54, 61–67 (1984). [Google Scholar]
- Guye M. G., Vigh L. & Wilson J. M. Chilling-Induced Ethylene Production in Relation to Chill-Sensitivity in Phaseolus Spp. J. Exp. Bot. 38, 680–690 (1987). [Google Scholar]
- Janowiak F. & Dorffling K. Chilling-induced changes in the contents of 1-aminocyclopropane-1-carboxylic acid (ACC) and its N-malonyl conjugate (MACC) in seedlings of two maize inbreds differing in chilling tolerance. J. Plant Physiol. 147, 257–262 (1995). [Google Scholar]
- Fujimoto S. Y., Ohta M., Usui A., Shinshi H. & Ohme-Takagi M. Arabidopsis ethylene-responsive element binding factors act as transcriptional activators or repressors of GCC box-mediated gene expression. Plant Cell 12, 393–404 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M. C., Liao P. M., Kuo W. W. & Lin T. P. The Arabidopsis ETHYLENE RESPONSE FACTOR1 regulates abiotic stress-responsive gene expression by binding to different cis-acting elements in response to different stress signals. Plant Physiol. 162, 1566–1582 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall E. A. et al. Transcript abundance profiles reveal larger and more complex responses of grapevine to chilling compared to osmotic and salinity stress. Funct. Integr. Genomics 7, 317–333 (2007). [DOI] [PubMed] [Google Scholar]
- Ito Y. et al. Functional analysis of rice DREB1/CBF-type transcription factors involved in cold-responsive gene expression in transgenic rice. Plant Cell Physiol. 47, 141–153 (2006). [DOI] [PubMed] [Google Scholar]
- Kidokoro S. et al. Soybean DREB1/CBF-type transcription factors function in heat and drought as well as cold stress-responsive gene expression. Plant J. 81, 505–518 (2015). [DOI] [PubMed] [Google Scholar]
- Zhang X. et al. Freezing-sensitive tomato has a functional CBF cold response pathway, but a CBF regulon that differs from that of freezing-tolerant Arabidopsis. Plant J. 39, 905–919 (2004). [DOI] [PubMed] [Google Scholar]
- Tian Y. et al. Overexpression of ethylene response factor TERF2 confers cold tolerance in rice seedlings. Transgenic Res. 20, 857–866 (2011). [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K. & Shinozaki K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 57, 781–803 (2006). [DOI] [PubMed] [Google Scholar]
- Wu L., Zhang Z., Zhang H., Wang X. C. & Huang R. Transcriptional modulation of ethylene response factor protein JERF3 in the oxidative stress response enhances tolerance of tobacco seedlings to salt, drought, and freezing. Plant Physiol. 148, 1953–1963 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X. et al. Identification of cold-inducible microRNAs in grapevine. Front. Plant Sci. 6, 595 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin L. J., Lo J. C. & Yeh K. C. Copper chaperone antioxidant protein1 is essential for copper homeostasis. Plant Physiol. 159, 1099–1110 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C., Pachter L. & Salzberg S. L. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25, 1105–1111 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.