Abstract
Previous studies indicate that stress damages oocytes with increased secretion of glucorticoids. However, although injection of female mice with cortisol decreased oocyte competence, exposure of mouse oocytes directly to physiological or stress-induced concentrations of glucorticoids did not affect oocyte maturation and embryo development. This study has explored the mechanisms by which glucocorticoids impair oocyte competence. Female mice were injected with cortisol and the effects of cortisol-injection on oocyte competence, ovarian cell apoptosis and Fas/FasL activation were observed. The results showed that cortisol-injection decreased (a) oocyte developmental potential, (b) the E2/P4 ratio in serum and ovaries, and (c) expression of insulin-like growth factor 1, brain-derived neurotrophic factor and glucocorticoid receptor in mural granulosa cells (MGCs), while increasing levels of (a) cortisol in serum and ovaries, (b) apoptosis in MGCs and cumulus cells (CCs), (c) FasL secretion in ovaries and during oocyte maturation in vitro, and (d) Fas in MGCs, CCs and oocytes. The detrimental effects of cortisol-injection on oocyte competence and apoptosis of MGCs and CCs were significantly relieved when the gld (generalized lymphoproliferative disorder) mice harboring FasL mutations were observed. Together, the results suggested that glucocorticoids impair oocyte competence by triggering apoptosis of ovarian cells via activating the Fas system.
Psychological stress can affect reproduction in women1,2. Restraint of small animals is an effective procedure to mimic human psychological stress3,4. Exposure of mice and rats to restraint stress during gestation impaired function of corpora lutea and decreased pregnancy rates and litter size5,6. However, evidences on the direct effect of psychological stress on the oocyte are limited. Although our recent studies showed that restraint stress of female mice impaired oocyte developmental potential7, the underlying mechanisms are largely unknown.
It is known that stress enhances the activity of the hypothalamus-pituitary-adrenal (HPA) axis and results in increased secretion of glucocorticoids from the adrenal cortex. For example, mice and rats exposed to stress showed significant elevation of serum corticosterone8 and cortisol7,8. However, although injection of female mice with cortisol decreased oocyte developmental potential7, exposure of mouse oocytes directly to physiological or stress-induced concentrations of cortisol7,9 or corticosterone10 during in vitro maturation did not affect nuclear maturation and embryo development. Thus, the mechanism by which glucocorticoids damage the oocyte has yet to be studied.
It is known that Fas signaling can induce apoptosis in various cells and tissues11,12. The presence of Fas/FasL system in ovaries has been reported in different species including mice13, rat14 and human15. Heat stress activated the Fas/FasL system in sertoli cells16. The Fas and caspase-3/8 activity increased significantly in cardiomyocytes undergoing apoptosis after restraint stress of rats17. Activation of the Fas/FasL system was also observed during postovulatory oocyte aging18. Treatment of mice with dexamethasone significantly increased FasL expression in testicular germ cells19. Glucocorticoids induced apoptosis in placenta cells20, testicular germ cells21 and Leydig cells22. Culture of osteocytes and monocytes with glucorticoids induced apoptosis with activation of the Fas/FasL system23,24. Furthermore, restraint stress diminished oocyte developmental potential by inducing apoptosis of ovarian cells25.
We thus hypothesized that glucocorticoids might impair oocyte developmental potential by triggering apoptosis of ovarian cells via activating the Fas system. The objective of this study was to test this hypothesis. Both wild-type mice and the gld (generalized lymphoproliferative disorder) mice that harbor FasL mutations were injected with cortisol, and the effects of cortisol injection on oocyte competence, ovarian cell apoptosis and Fas/FasL activation were observed. Cortisol was used instead of corticosterone because of the following reasons: (1) mouse serum cortisol and corticosterone are closely correlated in dynamics under different physiological or stressful conditions and both can be interchangeably used as indicators for rodent activation of stress8; (2) cortisol exhibits much higher glucocorticoid potency than corticosterone does26; (3) cortisol is cleared less rapidly than corticosterone is27; and (4) results obtained with cortisol will be more referable for studies on human beings because cortisol is the main glucorticoids in human.
Results
Injection of female mice with cortisol decreased oocyte developmental potential while increasing serum and ovarian cortisol levels
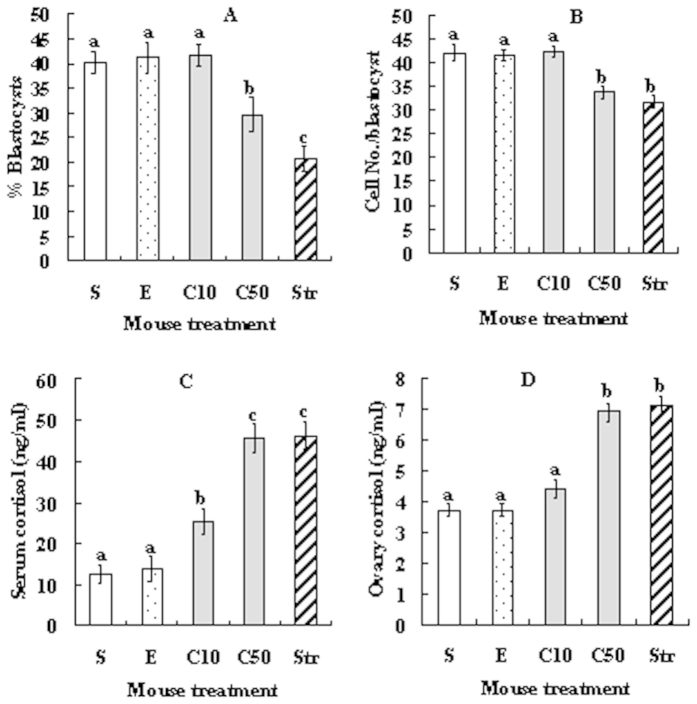
At 24 h after cortisol injection, oocytes at the germinal vesicle (GV) stage were recovered for in vitro maturation while blood and ovaries were collected for cortisol assay. Although rates for oocyte nuclear maturation (ranging from 96% to 98%) and activation (around 97%) did not differ between treatments, blastocyst rates and cell number per blastocyst decreased significantly when the cortisol dosage increased to 50 mg/kg bodyweight (Fig. 1). The blastocyst rate was significantly lower in oocytes from restraint stressed mice than that from mice injected with 50 mg/kg cortisol. Cortisol levels in both serum and ovaries were similar between mice injected with 50 mg/kg cortisol and the stressed mice but were significantly higher than that in control mice injected with saline or ethanol (Fig. 1). The results suggested that cortisol injection decreased oocyte developmental potential while increasing cortisol levels, and the effect was significant only when the injection dosage increased to 50 mg/kg. Although restraint stress and cortisol injection increased cortisol to the same level, the former produced significantly less blastocysts than the latter, suggesting that stress damages oocytes not only by raising glucorticoids but also by other means.
Figure 1. Effects of cortisol injection on blastocyst development of Sr2+-activated oocytes and on cortisol levels in serum and ovarian homogenates.
(A) % Blastocysts; (B) Cell number per blastocyst; (C) Cortisol in serum; and (D) Cortisol in ovary. At 24 h after eCG injection, experimental mice were injected with cortisol at 10 (C10) or 50 (C50) mg/kg body weight, control mice were injected with either saline (S) or ethanol (E), and stressed control (Str) mice were restrained for 24 h. For oocyte maturation, each treatment was repeated 5 times with each replicate containing about 30–35 oocytes from 2 mice. For cortisol assays, each treatment was repeated 3 times with each replicate containing ovarian homogenates or serum from 3 mice. a–c: Values without a common letter above bars differ significantly (P < 0.05).
Effects of cortisol injection on apoptosis of mural granulosa cells (MGCs) and cumulus cells (CCs)
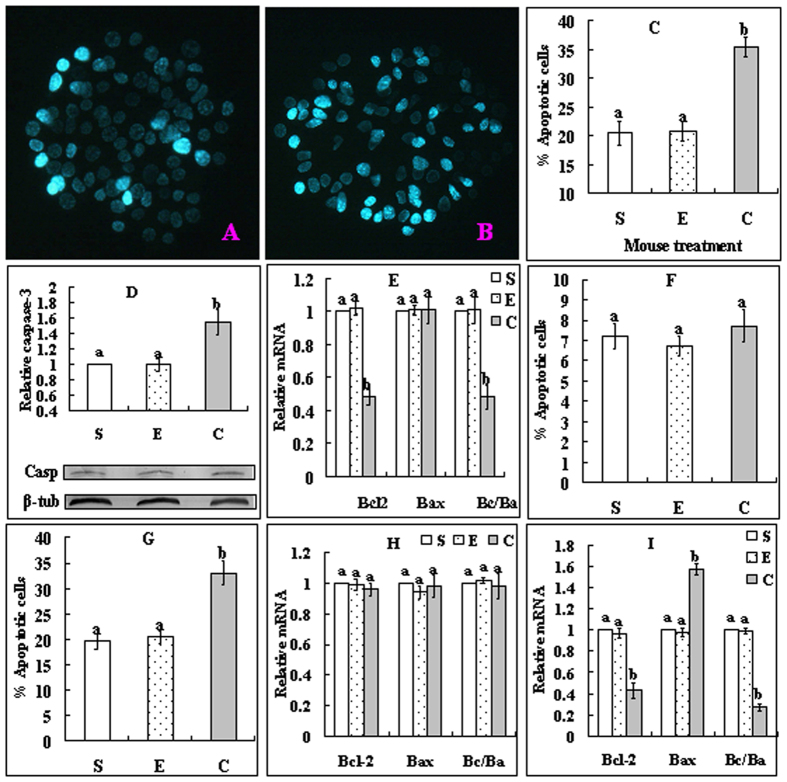
At 24 h after cortisol injection, MGCs and oocytes were collected for assays of apoptotic markers. In MGCs, cortisol injection significantly increased the proportions of apoptotic cells (Fig. 2A–C) and the level of active caspase 3 (Fig. 2D), while decreasing Bcl-2 mRNA expression and the Bcl-2/Bax ratio (Fig. 2E). To observe apoptosis in CCs, while some of the oocytes were used for CCs recovery immediately after collection, others were cultured for 14 h in TCM-199 without serum, growth factors and hormones (SGH) before CCs harvest. Before culture, the percentage of apoptotic CCs did not differ between treatments (Fig. 2F), but after culture, it increased significantly in cortisol-injected mice (Fig. 2G). Before culture, neither Bcl-2 nor Bax mRNA expression differed among treatments (Fig. 2H), but after culture, while the level of Bcl-2 decreased, that of Bax increased significantly, leading to a significant decrease in the Bcl-2/Bax ratio in cortisol-injected mice (Fig. 2I). The results suggested that cortisol injection triggered apoptosis in MGCs, and it initiated an apoptosis program in the CCs during oocyte development in the ovary, which was executed later during oocyte maturation under unfavorable conditions.
Figure 2.
Effects of cortisol injection on apoptosis of MGCs (A–E) and CCs (F–I). Female mice were injected with saline (S), ethanol (E) or 50 mg/kg cortisol (C). A and B are MGCs smears stained with Hoechst 33342 and observed under a fluorescence microscope (Original magnification ×400), which show MGCs from mice injected with saline or cortisol, respectively. The heterochromatin is heavily stained with the Hoechst dye and gives bright fluorescence. Whereas the apoptotic cells show pyknotic nuclei full of heterochromatin, healthy cells show normal nuclei with sparse heterochromatin spots. Graph C shows percentages of apoptotic MGCs after different mouse treatments. Graph D shows levels of active caspase-3 while graph E shows Bcl2 and Bax mRNAs and Bcl2/Bax (Bc/Ba) ratio in MGCs after different mouse treatments. Graphs F and G show percentages of apoptotic CCs while graphs H and I show levels of Bcl2 and Bax mRNAs and Bcl2/Bax ratio in CCs, before and after oocyte culture without SGH, respectively. In all the experiments, each treatment was repeated 3 times. For the MGC experiments, each replicate included MGCs from 2 mice. For CCs Hoechst staining, each replicate contained 60 oocytes from 2 mice. For RT-PCR with CCs, each replicate contained CCs from 200 oocytes from 6–7 mice. a,b: Values without a common letter above their bars differ significantly (P < 0.05) within apoptotic markers.
Effects of cortisol injection on levels of 17β-estradiol (E2), progesterone (P4), FasL and corticotropin-releasing hormone (CRH) in serum and ovarian homogenates
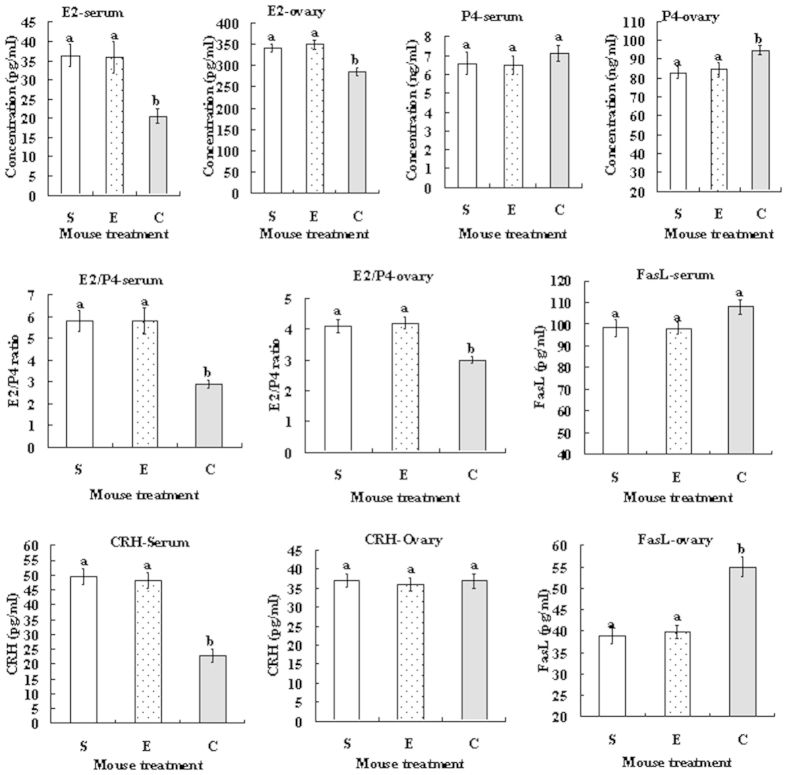
At 24 h after cortisol injection, blood and ovaries were collected for radioimmunoassay for E2 and P4 and for ELISA assay of FasL and CRH. In both serum and ovarian homogenates, whereas the level of E2 decreased, that of P4 increased in cortisol-injected mice compared to that in control mice (Fig. 3). Notably, the E2/P4 ratio in both serum and ovaries decreased significantly in cortisol-injected mice compared to that in control mice. Concentration of FasL in the ovary was significantly higher in cortisol-injected mice than in control mice, although that in serum did not differ significantly among treatments. Cortisol injection decreased the CRH level in serum significantly while having no effect on that in ovarian homogenates.
Figure 3. Effects of cortisol injection on contents of E2, P4, FasL and CRH and E2/P4 ratio in serum and ovarian homogenates.
Female mice were injected with saline (S), ethanol (E) or 50 mg/kg cortisol (C) before assays. Each treatment was repeated 12 times with each replicate including serum or ovarian homogenates from a single (the same) mouse. a,b: Values without a common letter above their bars differ significantly (P < 0.05).
Effects of cortisol injection on expression of insulin-like growth factor 1 (Igf1), brain-derived neurotrophic factor (Bdnf) and glucocorticoid receptor (Gr) mRNAs in MGCs
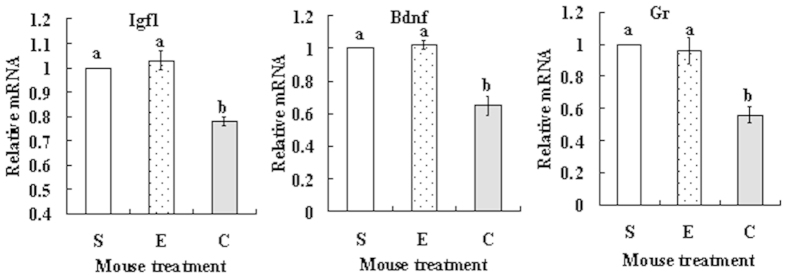
At 24 h after cortisol injection, MGCs were collected for real-time PCR assay. The relative levels of Igf1, Bdnf and Gr mRNAs in MGCs decreased significantly in cortisol-injected mice compared to that in control mice injected with saline or ethanol (Fig. 4).
Figure 4. Effects of cortisol injection on expression of Igf-1, Bdnf and Gr mRNAs in MGCs.
Female mice were injected with saline (S), ethanol (E) or 50 mg/kg cortisol (C) before real-time PCR assay for mRNA expression. Each treatment was repeated 3 times with each replicate including MGCs from 2 mice. a,b: Values without a common letter above their bars differ significantly (P < 0.05).
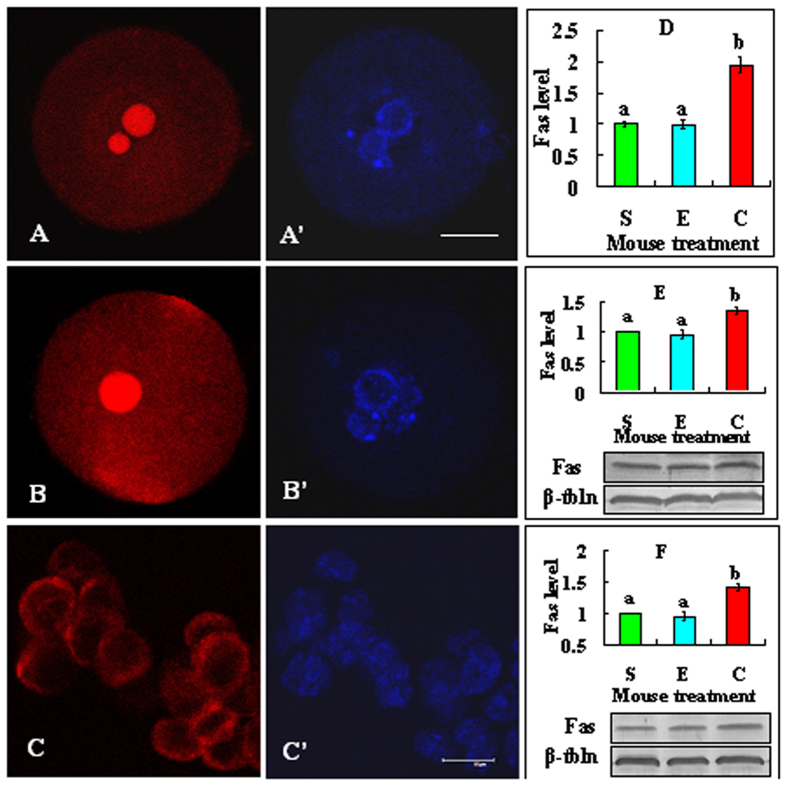
Effects of cortisol injection on Fas expression in oocytes, MGCs and CCs
At 24 h after cortisol injection, oocytes at the GV stage and MGCs were recovered for analysis of Fas expression. After being freed of CCs, the denuded oocytes and some CCs were processed for immunofluorescence microscopy, whereas the MGCs and other CCs recovered were used for western blotting. Although Fas was localized mainly on the cell membrane in CCs, it was distributed not only on the plasma membrane but also throughout the cytoplasm and particularly concentrated in the nucleolus area in the oocyte (Fig. 5A–C). Quantification showed that cortisol injection significantly increased Fas expression in GV oocytes (Fig. 5D), MGCs (Fig. 5E) and CCs (Fig. 5F). Like what we observed in CCs in this study, Fas is usually localized on the plasma membrane in somatic cells28. Different from what we observed in mouse oocytes, however, no Fas expression was observed on the nucleus (GV) in bovine oocytes, although it was distributed at the cellular membrane and its adjacent cytoplasmic region29. Thus, the significance of Fas localization on the GV in mouse oocytes needs further investigations.
Figure 5. Effects of cortisol injection on Fas expression in oocytes, MGCs and CCs.
Micrographs (A–C) are confocal images showing Fas localization in oocytes from saline- or cortisol-injected mice and in CCs, respectively. Images A and A’, B and B’, and C and C’ are the same samples observed under fluorescence after Fas and Hoechst staining, respectively. The bar is 20 μm and 10 μm in the oocyte picture and the CCs picture, respectively. Graph D shows Fas quantification in oocytes from mice injected with saline (S), ethanol (E) or 50 mg/kg cortisol (C). Each treatment was repeated 4 times with each replicate containing 20 oocytes. Graphs E and F show Fas quantification (Western blot analysis) in MGCs and CCs, respectively. Each treatment was repeated 3 times with each replicate including MGCs from 2 mice or CCs from 150 oocytes from 5 mice. a,b: Values without a common letter above their bars differ significantly (P < 0.05).
Oocytes from cortisol-injected mice produced more FasL and were less competent than oocytes from ethanol-injected mice after maturation in TCM-199 without SGH
Oocytes at the GV stage recovered at 24 h after cortisol injection were cultured for maturation in TCM-199 without SGH. At 14 h of maturation culture, oocytes were freed of CCs to observe maturation rate, whereas the conditioned medium (CM) was collected for assay for FasL concentrations. Percentages of oocytes extruding first polar bodies were significantly (P < 0.05) lower in mice injected with cortisol (84.8 ± 3.8%, n = 150) than in control mice injected with saline (96.6 ± 1.0%, n = 150) or ethanol (96.9 ± 1.0%, n = 150). In contrast, concentrations of FasL in CM were significantly (P < 0.05) higher in mice injected with cortisol (172.4 ± 3.3 pg/ml) than in control mice injected with saline (156.4 ± 3.9 pg/ml) or ethanol (155.0 ± 6.2 pg/ml). The results suggested that cortisol-injection impaired oocyte competence by facilitating CCs production of FasL.
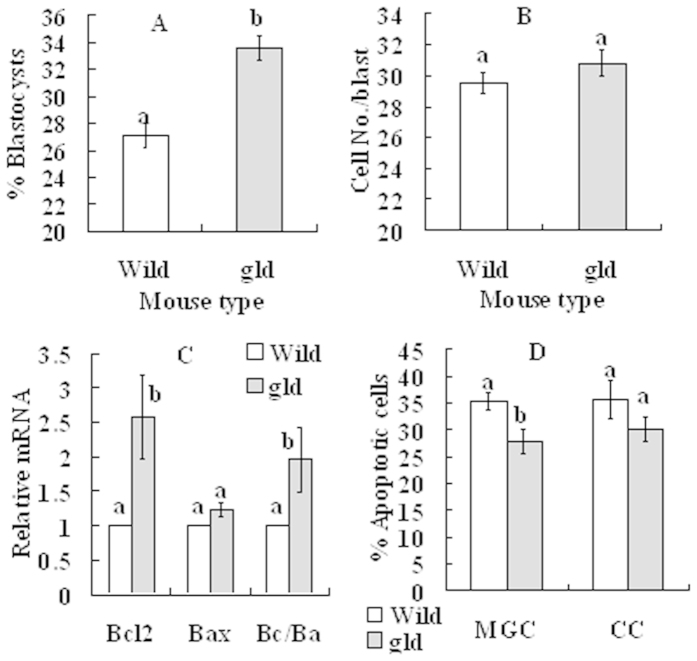
Effects of cortisol injection on oocyte developmental potential and apoptosis of MGCs and CCs in gld mice
Female gld mice and wild-type mice were injected with cortisol at 24 h after eCG injection, and at 24 h after cortisol injection, mice were sacrificed to collect ovaries for further analysis. Blastocyst rates were significantly higher in gld mice than in wild-type mice although the difference in cell number/blastocyst was not significant between mouse strains (Fig. 6A,B). Although levels of Bax mRNA in MGCs did not differ, levels of Bcl2 mRNA and the ratio of Bcl2/Bax mRNAs were significantly higher in gld mice than in wild-type mice (Fig. 6C). Apoptotic percentages of both MGCs and CCs were lower in gld mice than in wild-type mice (Fig. 6D), although the difference in CCs did not reach the level of statistical significance. The results further confirmed that glucocorticoids impaired oocyte developmental potential by causing apoptosis in MGCs and CCs through activating the Fas system.
Figure 6. Effects of cortisol injection on blastocyst development and apoptosis in MGCs and CCs in wild-type mice and gld mice.
(A) Rates of blastocysts; (B) Cell number per blastocyst; (C) Levels of Bcl2 and Bax mRNAs and Bcl2/Bax (Bc/Ba) ratio in MGCs; and D. Percentage of apoptotic MGCs and CCs after Hoechst staining. At 24 h after eCG injection, mice were injected with 50 mg/kg cortisol, and at 24 h after cortisol injection, mice were sacrificed to collect ovaries for further experiments. Percentages of blastocysts and cell number per blastocyst were observed after Sr2+-activation of oocytes. Apoptotic percentages of CCs were observed after oocytes were cultured for 14 h without SGH. For oocyte maturation for embryo development, each treatment was repeated 3 times with each replicate containing about 30–35 oocytes from 2 mice. In experiments with MGCs, each treatment was repeated 4 times with each replicate including MGCs from 3 mice. For Hoechst staining of CCs, each treatment was repeated 3 times with each replicate containing CCs from 30 oocytes from 3 mice. a,b: Values without a common letter above their bars differ significantly (P < 0.05).
Discussion
The present results demonstrated that injecting female mice with cortisol down-regulated Gr mRNA expression in MGCs. Downregulation of GR expression by glucorticoids has been reported in other cell types. For example, whereas long-term adrenalectomy resulted in a large increase in whole-cell GR in rat brain, acute treatment with high dose corticosterone produced a large decrease in whole-cell GR30. Treatment of rats with dexamethasone resulted in a consistent decrease in the accumulation of Gr mRNA in all tissues studied including lung, spleen, brain, liver and kidney31. A progressive decrease in Gr mRNA was observed in the rat hippocampus with increasing doses of corticosterone32. Furthermore, dexamethasone caused a down-regulation of the levels of Gr mRNA and protein in hepatoma tissue culture cells33.
In this study, cortisol injection of female mice significantly increased FasL expression in ovaries and during oocyte maturation in vitro, and it promoted Fas expression in MGCs, CCs and oocytes. The detrimental effects of cortisol-injection on oocyte developmental potential and apoptotic parameters were significantly relieved when gld mice harboring FasL mutations were observed. The results suggested that glucorticoids impaired oocyte competence by activating the Fas signaling and triggering apoptosis in MGCs, CCs and oocytes. That stress induces apoptosis via activating the Fas system has been reported in different tissues and cells. Heat stress of mice activated the Fas/FasL system in sertoli cells16. Restraint stress of rats significantly increased the Fas and caspase-3/8 activity in apoptotic cardiomyocytes17. Treatment of mice with dexamethasone significantly increased FasL expression in testicular germ cells19. Culture with glucorticoids induced apoptosis with activation of the Fas/FasL system in osteocytes and monocytes23,24. Furthermore, GR binding to 2 negative glucorticoids regulatory elements (nGREs) in the FasL promoter reduces activation-induced FasL expression in T cells34. Together with our results that cortisol-injection reduced GR expression in MGCs, the data suggest that glucorticoids activate Fas signaling by down regulating GR expression.
The present results showed that cortisol injection decreased the E2/P4 ratio in serum and ovarian homogenates by reducing E2 while increasing P4 production. Studies have demonstrated that stress activates the HPA axis, and the HPA products affect ovarian function at the hypothalamus and the pituitary gland levels by decreasing the synthesis and release of LH and FSH. However, reports on the direct action of the HPA products on the ovary are few. In vitro studies showed that glucorticoids enhanced FSH-stimulated progesterone synthesis in cultured granulosa cells of rats and cattle35,36,37, and that glucorticoids suppressed P450 aromatase activity and decreased the number of LH receptors in rats36,38, cattle37 and pigs39. In vivo studies are even fewer. In cows, administration of ACTH increased plasma cortisol concentration while reducing 17β-estradiol concentrations in the follicular fluids40. In the protogynous Wrasse, cortisol administration induces sex change from ovary to testis and the plasma 17β-estradiol level was significantly lower in the cortisol treatment group than in the control group41. Furthermore, the level of estrogen is significantly reduced following chronic unpredictable stress or dexamethasone administration of mice supplemented with exogenous gonadotropin42.
The present results indicated that cortisol-injection of female mice reduced the expression of Igf1 and Bdnf mRNAs in MGCs. Short-term treatment of prepubertal mice with dexamethasone decreased growth and the IGF1 expression in the tibial growth plate43. Using cultures of rat osteoblasts, Delany and Canalis44 demonstrated that cortisol decreased Igf1 mRNA by approximately 50% through decreasing gene transcription. Furthermore, studies in the pig have shown that increased glucorticoid concentrations can disrupt ovarian IGF1 synthesis and IGF action both in vitro45 and in vivo46. In rats, both acute and chronic corticosterone administration significantly decreased Bdnf mRNA and protein in the hippocampus47,48. In mice, chronic unpredictable stress, which increased plasma corticosterone levels, significantly decreased BDNF expression in the antral follicles49. Furthermore, the GR-heterozygous mutant mice (GR+/−) with a 50% GR gene dose reduction show significant downregulation of BDNF protein content in their hippocampus50.
There are many reports that IGF1, BDNF and a high E2/P4 ratio are anti-apoptotic. For instance, estrogen, IGF1 and BDNF are all antiapoptotic in the ovary, particularly in granulosa cells51,52. In cattle, the healthy dominant follicles selected for ovulation enjoy a greater availability of IGF and a greater capacity to produce 17β-estradiol than do the subordinate follicles destined to undergo atresia53. In goats, healthy follicles showed significantly higher levels of 17β-estradiol and IGF1 than did the atretic follicles51. The level of progesterone, on the other hand, was higher in atretic follicles than in healthy follicles. The apoptotic percentage of goat granulosa cells cultured in vitro declined significantly in the presence of IGF154. Furthermore, IGF1 exerts a stimulatory effect on aromatase activity and steroidogenesis in cultured rat granulosa cells55,56. In recent years, increasing evidence shows that ovarian BDNF plays an important role in oocyte development and maturation57,58,59. Notably, one study has shown that chronic unpredictable stress decreases the expression of BDNF in mouse ovaries49.
In summary, we have studied the mechanisms by which glucorticoids impair oocyte competence. Both wild-type mice and the gld mice that harbor FasL mutations were injected with cortisol and the effects of cortisol injection on oocyte competence, ovarian cell apoptosis and Fas/FasL activation were observed. The results indicated that cortisol injection increased the level of glucorticoids in blood and ovary (Fig. 7). Within the ovary, the elevation in glucorticoids activated the Fas/FasL system and decreased the levels of growth factors and the E2/P4 ratio by down-regulating the expression of GR. The activation of Fas/FasL and the decrease in growth factors and E2/P4 ratio worked together to induce apoptosis of ovarian cells, leading to a decline in growth factor levels and E2/P4 ratio, and an increase in FasL, in the follicular fluid. The increase in FasL and the decrease in growth factor levels and the E2/P4 ratio in follicular fluid cause apoptosis in both CCs and oocytes, impairing oocyte developmental potential. Furthermore, the apoptotic CCs also produce FasL that affects oocytes by acting on oocyte Fas receptors. The data have the first time up-to-date established the pathways by which glucorticoids diminishes oocyte developmental potential and are important for our understanding the role of glucocorticoids in a broad spectrum of stress-related diseases.
Figure 7. The possible pathways by which cortisol injection of female mice diminishes oocyte developmental competence.
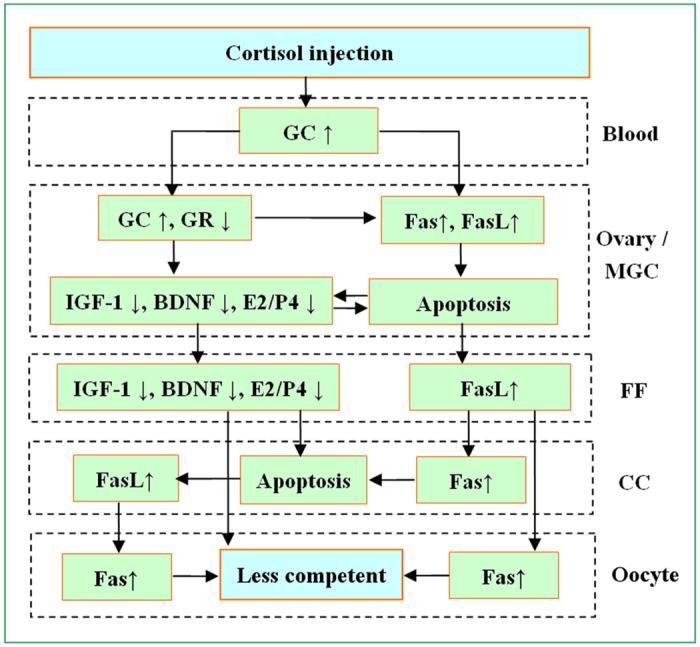
Cortisol injection increases the level of glucorticoids (GC) in blood and ovary. Within the ovary, the GC elevation activates the Fas/FasL system and decreases levels of growth factors and the E2/P4 ratio by down-regulating the expression of GR. The activation of Fas/FasL and the decrease in growth factors and E2/P4 ratio work together to induce apoptosis of ovarian cells, leading to a decline in growth factor levels and E2/P4 ratio but an increase in FasL in the follicular fluid (FF). The increase in FasL and the decrease in growth factor levels and the E2/P4 ratio in FF cause apoptosis in both CCs and oocytes, impairing oocyte developmental potential. Furthermore, the apoptotic CCs also produce FasL that affects oocytes by acting on their Fas receptors.
Methods
The experimental procedures used for animal care and handling were approved by the Animal Care and Use Committee of the Shandong Agricultural University P. R. China (Permit number: SDAUA-2001-001). The methods were carried out in accordance with the approved guidelines. Unless otherwise specified, all chemicals and reagents used were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Mice and treatment
Mice of the Kunming breed, which were used in most of the experiments, were bred in this laboratory. The gld mice harboring a germline mutation F273L in FasL with a C57BL/6J genomic background and the wild-type C57BL/6J mice were obtained from the Key Laboratory of Stem Cell Biology, Shanghai Institute for Biological Sciences, China. Mice were raised in rooms with a constant temperature between 22 and 25 °C and a photoperiod of 14 h light and 10 h dark with lights off at 20:00. Female mice, 8–10 weeks after birth, were injected with equine chorionic gonadotropin (eCG, 10 IU i.p.), and at 24 h after eCG injection, mice were injected with cortisol or subjected to restraint stress for 24 h.
Cortisol injection: Cortisol dissolved in 50% alcohol in saline was administrated intraperitoneally. Cortisol powder was first dissolved in absolute alcohol, and the stock solution of cortisol was then diluted with the same volume of saline before injection. To avoid oocyte damage by alcohol, the amount of solution injected was strictly controlled at less than 0.2 ml/animal. To this end, the amounts of cortisol powder to be dissolved in absolute alcohol were carefully calculated for different dosages. For example, to inject mice weighing 30 g with a dose of 50 mg/kg, a stock solution was prepared by dissolving 15 mg of cortisol powder in 1 ml of absolute alcohol. The 1-ml stock solution was then diluted with 1 ml of saline, with the resulting solution therefore containing 7.5 mg/ml (i.e., 1.5 mg/0.2 ml) of cortisol.
Restraint procedure: For restraint treatment, an individual mouse was put in a microcage constructed by the authors11, which was placed in an ordinary home cage. The microcage offered the same photoperiod and controlled temperature as in the large home cage for the unstressed animals. While in the microcage, mice could move back and forth to some extent and could take food and water freely, but they could not turn around.
Recovery of ovaries, oocytes and mural granulosa cells (MGCs)
At 24 h after cortisol injection or restraint stress (48 h after eCG injection), mice were killed by decollation to collect ovaries for the recovery of oocytes and MGCs. The large follicles on the ovary were ruptured in M2 medium to release oocytes. Only oocytes with more than three layers of unexpanded cumulus cells (CCs), containing oocytes larger than 70 μm in diameter, and with a homogenous cytoplasm were used for experiments. The MGCs sheets released into M2 medium at puncture of follicles were collected for further use. CCs were freed from oocytes mechanically by pipetting oocytes in M2 medium. Both MGCs and CCs were pelleted by centrifugation at 500 × g for 5 min at room temperature. The pellets were then resuspended in Trizol (Invitrogen, Australia Pty. Ltd.) or RIPA lysis buffer for use in quantitative real-time PCR and western blot analysis, respectively.
Oocyte maturation in vitro
The recovered oocytes were washed three times in M2 medium and once in maturation medium. The oocytes were then cultured in groups (30–35 oocytes) in 100 μl drops of maturation medium at 37.5 °C in a humidified atmosphere of 5% CO2 in air. The maturation medium was TCM-199 (Gibco) supplemented with 10% (v/v) fetal calf serum (Gibco), 1 μg/ml of 17β-estradiol, 24.2 mg/L of sodium pyruvate, 0.05 IU/ml of FSH, 0.05 IU/ml of LH, and 10 ng/ml of epidermal growth factor (EGF). In the maturation medium used to test CCs apoptosis, serum, growth factor and hormone (SGH) were omitted, and 24.2 mg/L of sodium pyruvate and 0.3 mg/ml of polyvinyl alcohol was supplemented.
Oocyte activation and embryo culture
At 24 h of maturation culture, oocytes were stripped of their CCs by pipetting in M2 containing 0.1% hyaluronidase. After being washed twice in M2 and once in the activating medium (Ca2+-free CZB medium supplemented with 10 mM SrCl2 and 5 μg/ml cytochalasin B), the oocytes were incubated in the activating medium for 6 h at 37.5 °C in a humidified atmosphere with 5% CO2 in air. At the end of activation treatment, the oocytes were examined with a microscope for the evidence of activation. Oocytes were considered activated when each contained one or two well-developed pronuclei.
Activated oocytes were cultured for 4 days in regular CZB (about 30 oocytes per 100 μl drop) at 37.5 °C under humidified atmosphere with 5% CO2 in air. Glucose (5.5mM) was added to CZB when embryos were cultured beyond 3- or 4-cell stages. At the end of culture, embryo development was examined, and some of the blastocysts were stained with Hoechst 33342 for cell number counting.
Blood serum preparation and ovarian homogenization
Mice were killed by decollation, and trunk blood was collected into ice-cooled centrifugal tubes and centrifuged (1700 × g, 10 min, 4 °C) to separate serum. The serum collected was stored at −80 °C until hormone assay. For ovarian homogenization, the ovaries were snap-frozen in liquid nitrogen immediately after removal from the mice. The frozen ovaries were weighed and transferred to an electrical homogenizer (ULTRA TURRAX IKA T18 basic) with the proper amount of homogenization solutions. Homogenization was performed while cooling on ice. Following homogenization, the homogenates were centrifuged (15000 × g, 10 min, 4 °C), and the supernatant was collected for immediate use or stored at −80 °C until use.
Hormone assays
Ovaries were homogenized in PBS (800 μl per 100 mg of ovarian tissue). Radioimmunoassay was conducted by the Central Hospital of Tai-An City using commercial kits from Jiuding Biomedical Techniques Co. Ltd. The minimum levels of detection for assays of 17β-estradiol, progesterone, and cortisol were 1 pg/ml, 0.1 ng/ml, and 0.15 ng/ml, respectively. The intra- and inter-assay coefficients of variation were, respectively, 7.7% and 8.9% for 17β-estradiol, 7.2% and 8.9% for progesterone, and less than 10% for cortisol.
Assessment of cell apoptosis by Hoechst 33342 staining
Hoechst staining was used to assess MGCs and CCs apoptosis because previous studies have shown that TUNEL and Hoechst staining are comparable methods for detecting apoptosis60. The MGCs obtained as described above and CCs released from 60–80 oocytes were dispersed by repeatedly pipetting using a thin pipette. The dispersed cells were centrifuged (200 × g) for 5 min at room temperature. The pellets were resuspended in 50 μl of M2 medium containing 10 μg/ml of Hoechst 33342 and stained in the dark for 5 min. The stained cells were resuspended in M2 and centrifuged at 200 × g twice (5 min each). Finally, a 5-μl drop of suspension was smeared on a slide and examined under a Leica DMLB fluorescence microscope (400×). Six to eight fields were randomly observed on each smear, and the percentages of apoptotic cells were calculated double blindly by 2 investigators.
Western blotting
MGCs from 2 mice and CCs from about 150 oocytes from 5 mice were placed in a 1.5 ml microfuge tube containing 20 μl sample buffer (20 mM Hepes, 100 mM KCl, 5 mM MgCl2, 2 mM DTT, 0.3 mM phenylmethyl sulfonyl fluoride, 3 μg/mL leupetin, pH 7.5) and frozen at −80 °C. For running the gel, 5μl of 5 × SDS-PAGE loading buffer was added to each tube, and the tubes were heated to 100 °C for 5 min. Total proteins were separated on a 15% polyacrylamide gel by SDS-PAGE and transferred electrophoretically onto PVDF membranes. After being washed in TBST (150 mM NaCl, 2 mM KCl, 25 mM Tris, 0.05% Tween 20, pH 7.4) and blocked with TBST containing 3% BSA for 1 h at 37 °C, the membranes were incubated at 4 °C overnight with rabbit anti-active Caspase 3 (Casp3) polyclonal antibodies (1:1000, ab13847, Abcam Co. Ltd.) or rabbit anti-Fas (1:1000, ab82419, Abcam Co. Ltd.) and mouse anti-β-tubulin monoclonal antibodies (1:1000, 05-661, Merck Millipore). Then, the membranes were washed in TBST and incubated for 1 h at 37 °C with alkaline phosphatase-conjugated goat anti-rabbit IgG (1:1000, cw0111, Kangweishiji Biotechnology Co. Ltd., Beijing, China) and goat anti-mouse IgG (1:1000, cw0110, Kangweishiji Biotechnology Co. Ltd., Beijing, China). Finally, signals were detected by a BCIP/NBT alkaline phosphatase color development kit (Beyotime Institute of Biotechnology, Haimen City, China). The relative quantities of proteins were determined with Image J software by analyzing the sum density of each protein band image. The relative quantity values of active-Casp3 and Fas in control mice injected with saline were set as one and the other values were expressed relative to this quantity.
Quantitative real-time PCR
Ovarian homogenization was performed using Trizol reagent (0.5 ml per ovary). MGCs from two mice and CCs from about 200 oocytes were treated with Trizol reagent for RNA isolation. The RNA isolated was resuspended in diethylpyrocarbonate-treated MilliQ water (DEPC-dH2O). The purified RNA was dissolved in DEPC -dH2O and spectroscopically quantified at 260 nm. Purity and integrity of the RNA was assessed by determination of the A260/A280 ratio (1.8–2.0) and electrophoresis in 1% agarose.
Reverse transcription was performed in a total volume of 20 μl using Transcriptor Reverse Transcriptase (Roche). Briefly, 2 μl of each RNA sample were mixed in a 0.2 ml reaction tube with 1 μl Oligo dT18 (Fermentas), and 10 μl of DEPC-dH2O, and the mixture was incubated in a PCR instrument at 65 °C for 10 min. As soon as the incubation ended, the reaction tube was cooled on ice for 2 min and then centrifuged (200 × g, 4 °C) for a few seconds. Then, 4 μl of 5 × RT buffer, 0.5 μl RNase inhibitor (Roche), 2 μl dNTP (Fermentas) and 0.5 μl Transcriptor Reverse Transcriptase were added to the reaction tube. The mixture was then incubated at 55 °C for 30 min, followed by incubation at 85 °C for 5 min before storage at −20 °C until use.
Gene-specific primers for real-time RT-PCR are listed in Table 1. Quantification of mRNA was conducted using the Mx3005P real-time PCR instrument (Stratagene, Valencia, CA). Amplification reactions were performed in a 10 μl reaction volume containing 1 μl of cDNA, 5 μl of 2 × SYBR Green Master Mix (Agilent), 0.15 μl of ROX (reference dye), 3.25 μl of RNase-free water, and 0.3 μl each of forward and reverse gene-specific primers (10 μM). Cycle amplification conditions comprised an initial denaturation step at 95 °C for 3 min followed by 40 cycles at 95 °C for 20 sec and 60 °C for 20 sec. Immediately after amplification, PCR products were analyzed by sequencing, dissociation curve analysis, and gel electrophoresis to determine specificity of the reaction. Gene expression was normalized to the glyceraldehyde 3-phosphate dehydrogenase (gapdh) internal control. All values were then expressed relative to the calibrator samples using the 2−(ΔΔCT) method.
Table 1. Oligonucleotide primer sequences used for real-time PCR in this study.
| cDNA | Oligonucleotide sequences (5′—3′) | Amplified product size (bp) |
|---|---|---|
| Bcl2 | F: TTCGGGATGGAGTAAACTGG | 157 |
| R: TGGATCCAAGGCTCTAGGTG | ||
| Bax | F: TGCAGAGGATGATTGCTGAC | 183 |
| R: GATCAGCTCGGGCACTTTAG | ||
| Igf1 | F: GGACCAGAGACCCTTTGCGGGG | 210 |
| R: GGCTGCTTTTGTAGGCTTCAGTGG | ||
| Bdnf | F: GCCTCCTCTACTCTTTCTG | 255 |
| R: GGATTACACTTGGTCTCGT | ||
| GR | F: AGTCAAGGTTTCTGCGT | 233 |
| R: CCATCACTTTTGTTTCG | ||
| Gapdh | F: AAGGTGGTGAAGCAGGCAT | 244 |
| R: GGTCCAGGGTTTCTTACTCCT |
F, forward; R, reverse.
Enzyme-linked immunosorbent assay (ELISA)
ELISA for CRH and FasL was performed using CRH Elisa kits (E03P0031) and FasL Elisa kits (E03F0051) purchased from Shanghai BlueGene Biological Technology Co., Ltd. The OD value at 450 nm was read using a microplate reader (BioTek-ELx808, BioTek Instruments, Inc.), and the results were calculated according to the standard curve.
Immunofluorescence
All the procedures were performed at room temperature unless otherwise specified. Cumulus-free oocytes were washed 3 times in M2 medium between treatments. Oocytes were (i) fixed with 3.7% paraformaldehyde in PHEM buffer (60 mM Pipes, 25 mM Hepes, 10 mM EGTA and 4 mM MgSO4, pH 7.0) for 30 min, followed by treatment with 0.25% protease for 2 seconds to remove zona pellucida; (ii) permeabilized with 0.1% Triton X-100 in PHEM for 5 min; (iii) blocked in PHEM containing 3% BSA for 1 h; (iv) incubated at 4 °C overnight with rabbit anti-Fas (1:100, ab82419, Abcam) in 3% BSA in M2; (v) incubated for 1 h with Cy3-conjugated goat-anti-rabbit IgG (1:1000, 111-165-144, Jackson ImmunoResearch) in 3% BSA in M2; (vi) incubated for 10 min with 10 μg/ml Hoechst 33342 in M2. Negative control samples with the primary antibody omitted were also processed. The stained oocytes were mounted on glass slides and observed with a Leica laser scanning confocal microscope (TCS, SP2; Leica Microsystems). Fluorescence was detected with bandpass emission filters (Hoechst 33342, 420–480 nm; Cy3, 560–605 nm), and the captured signals for Hoechst and Cy3 were recorded as blue and red, respectively. The relative content of Fas was quantified by measuring the fluorescence intensities. For each experimental series, all high-resolution z-stack images were acquired with identical settings. The relative intensities were measured on the raw images using Image-Pro Plus software (Media Cybernetics Inc., Silver Spring, MD) under fixed thresholds across all slides. The average relative fluorescence of oocytes from the saline-injected mice was set to one, and the averages of oocytes from other treatments were expressed relative to this value.
Data analysis
At least three replicates were used for each treatment. Percentage data were arcsine transformed and analyzed with one-way ANOVA when each measure contained more than two groups or with independent-sample t test when each measure had only two groups. A Duncan multiple comparison test was used to locate differences during ANOVA. The software used was Statistics Package for Social Sciences (SPSS 20, SPSS, Inc.). Data were expressed as means ± SEM, and P < 0.05 was considered significant.
Additional Information
How to cite this article: Yuan, H.-J. et al. Glucocorticoids impair oocyte developmental potential by triggering apoptosis of ovarian cells via activating the Fas system. Sci. Rep. 6, 24036; doi: 10.1038/srep24036 (2016).
Acknowledgments
This study was supported by grants from the National Basic Research Program of China (Nos. 2014CB138503 and 2012CB944403) and the China National Natural Science Foundation (No. 31272444 and 30972096).
Footnotes
Author Contributions H.J.Y., X.H., N.H., G.L.W., S.G., J.L. and M.G. conducted the experiments; H.J.Y. and J.H.T. analyzed the data; J.H.T. designed the experiments and wrote the manuscript. All authors reviewed the manuscript.
References
- Kee B. S., Jung B. J. & Lee S. H. A study on psychological strain in IVF patients. J Assist Reprod Genet 17, 445–448 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neggers Y., Goldenberg R., Cliver S. & Hauth J. The relationship between psychosocial profile, health practices, and pregnancy outcomes. Acta Obstet Gynecol Scand 85, 277–285 (2006). [DOI] [PubMed] [Google Scholar]
- Pare´ W. P. & Glavin G. B. Restraint stress in biomedical research: a review. Neurosci Biobehav Rev 10, 339–370 (1986). [DOI] [PubMed] [Google Scholar]
- Glavin G. B., Pare´ W. P., Sandbak T., Bakke H. K. & Murison R. Restraint stress in biomedical research: an update. Neurosci Biobehav Rev 18, 223–249 (1994). [DOI] [PubMed] [Google Scholar]
- Wiebold J. L., Stanfield P. H., Becker W. C. & Hillers J. K. The effect of restraint stress in early pregnancy in mice. J Reprod Fertil 78, 185–192 (1986). [DOI] [PubMed] [Google Scholar]
- Sugino N. et al. Effects of restraint stress on luteal function in rats during mid-pregnancy. J Reprod Fertil 101, 23–26 (1994). [DOI] [PubMed] [Google Scholar]
- Zhang S. Y. et al. Maternal restraint stress diminishes the developmental potential of oocytes. Biol Reprod 84, 672–681 (2011). [DOI] [PubMed] [Google Scholar]
- Gong S. et al. Dynamics and correlation of serum cortisol and corticosterone under different physiological or stressful conditions in mice. PLos One 10, e0117503 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen C. Y. Effect of glucocorticoids on spontaneous and follicle-stimulating hormone-induced oocyte maturation in mouse oocytes during culture. J Steroid Biochem Mol Biol 85, 423–427 (2003). [DOI] [PubMed] [Google Scholar]
- Gonzalez R., Ruiz-Leon Y., Gomendio M. & Roldan E. R. The effect of glucocorticoids on mouse oocyte in vitro maturation and subsequent fertilization and embryo development. Toxicol In Vitro 24, 108–115 (2010). [DOI] [PubMed] [Google Scholar]
- Ju S. T. et al. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature 373(6513), 444–448 (1995). [DOI] [PubMed] [Google Scholar]
- Dhein J., Walczak H., Bäumler C., Debatin K. M. & Krammer P. H. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95). Nature 373(6513), 438–441 (1995). [DOI] [PubMed] [Google Scholar]
- Dharma S. J., Kelkar R. L. & Nandedkar T. D. Fas and Fas ligand protein and mRNA in normal and atretic mouse ovarian follicles. Reproduction 126, 783–789 (2003). [DOI] [PubMed] [Google Scholar]
- Guan S. et al. Effects of gonadotropin on Fas and/or FasL expression and proliferation in rat ovary. Theriogenology 83, 21–29 (2015). [DOI] [PubMed] [Google Scholar]
- José de los Santos M., Anderson D. J., Racowsky C. & Hill J. A. Presence of Fas-Fas ligand system and bcl-2 gene products in cells and fluids from gonadotropin-stimulated human ovaries. Biol Reprod 63, 1811–1816 (2000). [DOI] [PubMed] [Google Scholar]
- Guo X. et al. Baicalin protects sertoli cells from heat stress-induced apoptosis via activation of the Fas/FasL pathway and Hsp72 expression. Reprod Toxicol 57, 196–203 (2015). [DOI] [PubMed] [Google Scholar]
- Gao X. et al. HSP70 inhibits stress-induced cardiomyocyte apoptosis by competitively binding to FAF1. Cell Stress Chaperones 20, 653–661 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J. et al. Cumulus cells accelerate oocyte aging by releasing soluble Fas ligand in mice. Sci Rep 5, 8683 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorsandi L. S., Hashemitabar M., Orazizadeh M. & Albughobeish N. Dexamethasone effects on fas ligand expression in mouse testicular germ cells. Pak J Biol Sci 11, 2231–2236 (2008). [DOI] [PubMed] [Google Scholar]
- Waddell B. H., Hisheh S., Dharmarajan A. M. & Burton P. J. Apoptosis in rat placenta is zone-dependent and stimulated by glucocorticoids. Biol Reprod 63, 1913–1917 (2000). [DOI] [PubMed] [Google Scholar]
- Yazawa H., Sasagawa I. & Nakada T. Apoptosis of testicular germ cells induced by exogenous glucocorticoid in rats. Hum Reprod 15, 1917–1920 (2000). [DOI] [PubMed] [Google Scholar]
- Gao H. B. et al. Mechanisms of glucocorticoid-induced Leydig cell apoptosis. Mol Cell Endocrinol 199, 153–163 (2003). [DOI] [PubMed] [Google Scholar]
- Kogianni G. et al. Fas/CD95 is associated with glucocorticoid-induced osteocyte apoptosis. Life Sci 75, 2879–2895 (2004). [DOI] [PubMed] [Google Scholar]
- Schmidt M. et al. Role of the CD95/CD95 ligand system in glucocorticoid-induced monocyte apoptosis. J Immunol 166, 1344–1351 (2001). [DOI] [PubMed] [Google Scholar]
- Liang B. et al. Restraint stress impairs oocyte developmental potential in mice: Role of CRH-induced apoptosis of ovarian cells. Biol Reprod 89, 64 (2013). [DOI] [PubMed] [Google Scholar]
- Magiakou M. A. & Chrousos G. P. In Current Therapy in Endocrinology and Metabolism, 5th ed (ed. Bardwin C. W.) Ch. Corticosteroid therapy, nonendocrine disease and corticosteroid withdrawal, 120–124 (Philadelphia: Mosby Yearbook, 1994). [PubMed] [Google Scholar]
- Colby H. D. & Kitay I. Sex and substrate effects on hepatic corticosteroid metabolism in the rat. Endocrinology 90, 473–478 (1972). [DOI] [PubMed] [Google Scholar]
- Reis-Sobreiro M., Gajate C. & Mollinedo F. Involvement of mitochondria and recruitment of Fas/CD95 signaling in lipid rafts in resveratrol-mediated antimyeloma and antileukemia actions. Oncogene 28, 3221–3234 (2009). [DOI] [PubMed] [Google Scholar]
- Rubio Pomar F. J. et al. Role of Fas-mediated apoptosis and follicle-stimulating hormone on the developmental capacity of bovine cumulus oocyte complexes in vitro. Biol Reprod 71, 790–796 (2004). [DOI] [PubMed] [Google Scholar]
- Spencer R. L., Kalman B. A., Cotter C. S. & Deak T. Discrimination between changes in glucocorticoid receptor expression and activation in rat brain using western blot analysis. Brain Res 868, 275–286 (2000). [DOI] [PubMed] [Google Scholar]
- Kalinyak J. E., Dorin R. I., Hoffman A. R. & Perlman A. J. Tissue-specific regulation of glucocorticoid receptor mRNA by dexamethasone. J Biol Chem 262, 10441–10444 (1987). [PubMed] [Google Scholar]
- Hügin-Flores M. E., Steimer T., Aubert M. L. & Schulz P. Mineralo- and glucocorticoid receptor mrnas are differently regulated by corticosterone in the rat hippocampus and anterior pituitary. Neuroendocrinology 79, 174–184 (2004). [DOI] [PubMed] [Google Scholar]
- Dong Y., Poellinger L., Gustafsson J. A. & Okret S. Regulation of glucocorticoid receptor expression: evidence for transcriptional and posttranslational mechanisms. Mol Endocrinol 2, 1256–1264 (1988). [DOI] [PubMed] [Google Scholar]
- Baumann S. et al. Glucocorticoids inhibit activation-induced cell death (AICD) via direct DNA-dependent repression of the CD95 ligand gene by a glucocorticoid receptor dimer. Blood 106, 617–625 (2005). [DOI] [PubMed] [Google Scholar]
- Adashi E. Y., Jones P. B. & Hsueh A. J. Synergistic effect of glucocorticoids on the stimulation of progesterone production by follicle-stimulating hormone in cultured rat granulosa cells. Endocrinology 109, 1888–1894 (1981). [DOI] [PubMed] [Google Scholar]
- Schoonmaker J. N. & Erickson G. F. Glucocorticoid modulation of follicle-stimulating hormone-mediated granulosa cell differentiation. Endocrinology 113, 1356–1363 (1983). [DOI] [PubMed] [Google Scholar]
- Kawate N., Inaba T. & Mori J. Effects of cortisol on the amounts of estradiol-17β and progesterone secreted and the number of luteinizing hormone receptors in cultured bovine granulosa cells. Anim Reprod Sci 32, 15–25 (1993). [Google Scholar]
- Hsueh A. J. & Erickson G. F. Glucocorticoid inhibition of FSH-induced estrogen production in cultured rat granulosa cells. Steroids 32, 639–648 (1978). [DOI] [PubMed] [Google Scholar]
- Danisova A., Sebokova E. & Kolena J. Effect of corticosteroids on estradiol and testosterone secretion by granulosa cells in culture. Exp Clin Endocrinol 89, 165–173 (1987). [PubMed] [Google Scholar]
- Biran D., Braw-Tal R., Gendelman M., Lavon Y. & Roth Z. ACTH administration during formation of preovulatory follicles impairs steroidogenesis and angiogenesis in association with ovulation failure in lactating cows. Domest Anim Endocrinol 53, 52–59 (2015). [DOI] [PubMed] [Google Scholar]
- Nozu R. & Nakamura M. Cortisol administration induces sex change from ovary to testis in the protogynous Wrasse, Halichoeres trimaculatus. Sex Dev 9, 118–124 (2015). [DOI] [PubMed] [Google Scholar]
- Nie L. N. & Liu Y. S. Study of the non-gonadotropin dependent effect of chronic unpredictable stress and glucorticoid on ovarian development and endocrine E2 function. A Master thesis, Anhui Medical University, Hefei, P. R. China (2012).
- Smink J. J. et al. Short-term glucocorticoid treatment of prepubertal mice decreases growth and IGF-I expression in the growth plate. J Endocrinol 177, 381–388 (2003). [DOI] [PubMed] [Google Scholar]
- Delany A. M. & Canalis E. Transcriptional repression of insulin-like growth factor I by glucocorticoids in rat bone cells. Endocrinology 136, 4776–4781 (1995). [DOI] [PubMed] [Google Scholar]
- Viveiros M. & Liptrap R. M. Glucocorticoid influence on porcine granulosa cell IGF-I and steroid hormone production in vitro. Theriogenology 51, 1027–1043 (1999). [DOI] [PubMed] [Google Scholar]
- Viveiros M. M. & Liptrap R. M. ACTH treatment disrupts ovarian IGF-I and steroid hormone production. J Endocrinol 164, 255–264 (2000). [DOI] [PubMed] [Google Scholar]
- Schaaf M. J., de Jong J., de Kloet E. R. & Vreugdenhil E. Downregulation of BDNF mRNA and protein in the rat hippocampus by corticosterone. Brain Res 813, 112–120 (1998). [DOI] [PubMed] [Google Scholar]
- Jacobsen J. P. & Mørk A. Chronic corticosterone decreases brain-derived neurotrophic factor (BDNF) mRNA and protein in the hippocampus, but not in the frontal cortex, of the rat. Brain Res 1110, 221–225 (2006). [DOI] [PubMed] [Google Scholar]
- Wu L. M. et al. Chronic unpredictable stress decreases expression of brain-derived neurotrophic factor (BDNF) in mouse ovaries: relationship to oocytes developmental potential. PLos One 7, e52331 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridder S. et al. Mice with genetically altered glucocorticoid receptor expression show altered sensitivity for stress-induced depressive reactions. J Neurosci 25, 6243–6250 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. S. et al. Apoptosis in granulosa cells during follicular atresia: relationship with steroids and insulin-like growth factors. Cell Res 14, 341–346 (2004). [DOI] [PubMed] [Google Scholar]
- Bencomo E. et al. Apoptosis of cultured granulosa-lutein cells is reduced by insulin-like growth factor I and may correlate with embryo fragmentation and pregnancy rate. Fertil Steril 85, 474–480 (2006). [DOI] [PubMed] [Google Scholar]
- Quirk S. M., Cowan R. G., Harman R. M., Hu C. L. & Porter D. A. Ovarian follicular growth and atresia: the relationship between cell proliferation and survival. J Anim Sci 82 (suppl), E40–E52 (2004). [DOI] [PubMed] [Google Scholar]
- Yu Y. et al. The effect of follicle-stimulating hormone on follicular development, granulosa cell apoptosis and steroidogenesis and its mediation by insulin-like growth factor-I in the goat ovary. Theriogenology 60, 1691–1704 (2003). [DOI] [PubMed] [Google Scholar]
- Adashi E. Y., Resnick C. E., Brodie A. M., Svoboda M. E. & Van Wyk J. J. Somatomedin-C-mediated potentiation of follicle-stimulating hormone-induced aromatase activity of cultured rat granulosa cells. Endocrinology 117, 2313–2320 (1985). [DOI] [PubMed] [Google Scholar]
- Adashi E. Y., Resnick C. E., Svoboda M. E. & Van Wyk J. J. Somatomedin-C synergizes with follicle-stimulating hormone in the acquisition of progestin biosynthetic capacity by cultured rat granulosa cells. Endocrinology 116, 2135–2142 (1985). [DOI] [PubMed] [Google Scholar]
- Martins da Silva S. J. et al. BDNF promotes bovine oocyte cytoplasmic competence for embryo development. Reproduction 129, 423–434 (2005). [DOI] [PubMed] [Google Scholar]
- Kawamura K. et al. Ovarian BDNF promotes the development of oocytes into preimplantation embryos. Proc Natl Acad Sci USA 102, 9206–9211 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. et al. Effects of combined epidermal growth factor, brain-derived neurotrophic factor and insulin-like growth factor-1 on human oocyte maturation and early fertilized and cloned embryo development. Hum Reprod 27, 2146–2159 (2012). [DOI] [PubMed] [Google Scholar]
- Eldadah B. A., Ren R. F. & Faden A. I. Ribozyme-mediated inhibition of caspase-3 protects cerebellar granule cells from apoptosis induced by serum-potassium deprivation. J Neurosci 20, 179–186 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]