Abstract
Background:
The possible association between allergy and neoplastic disorders has been the subject of many investigations but no general relationship has been determined. Little attention, however, has been paid to the possible role of allergy in the clinical manifestations of these diseases. In this study, the role of allergy in the susceptibility to uterine leiomyomas and in their growth was investigated. Interaction with ACP1, a genetic polymorphism associated with the growth of leiomyomas, has been also considered.
Methods:
Two hundred and three White woman from the population of Rome hospitalized for symptomatic leiomyomas requiring surgical intervention have been studied. One hundred thirty eight healthy women have been considered as controls. Allergy has been evaluated by prick test. T-test for equality of means, analysis of variance and linear correlation analysis has been performed. The level of statistical significance was set at 0.05.
Results:
The frequency of allergic manifestations in women with leiomyomas does not differ from healthy women. The dimension of leiomyomas is lower in allergic than in non allergic women (p=0.004). The ACP1 *B/*B genotype and allergy cooperate in lowering the dimension of leiomyomas; the proportion of woman with small leiomyomas (<10 percentile) is much higher in allergic women carrying the *B/*B genotype as compared to other women (p<0.001). About 8% of variance of leiomyomas dimension is attributable to the joint effect of ACP1 and allergy.
Conclusion:
Allergic women with high concentration of ACP1 f isoform (*B/*B genotype) are protected from excessive leyomioma growth. If confirmed in other clinical settings, our observation may have practical importance in identifying women at risk of more severe clinical manifestations.
Keywords: Acid phosphatase locus 1, Allergy, Uterine leiomyomas
Introduction
The relationship between allergy and neoplastic disorders has been the subject of many investigations. Although an inverse association between the two conditions has been suspected for a long time, no general relationship has been determined. Inflammatory reactions associated to allergy could favor neoplasia; on the other hand, enhanced immune-surveillance due to allergy could prevent neoplastic diseases (1–5). The studies have been pointed mainly to the risk of cancer; allergy, however, may not have an etiologic role in neoplastic diseases but in presence of the disease could have a role in the clinical manifestations.
Uterine leiomyoma is a common benign neoplasia that may have clinical manifestations due to diffusion and growth of tumor requiring surgical intervention.
In the present study, the possible role of allergy in the susceptibility and in the growth of uterine leiomyomas was investigated. Since previously, an association between acid phosphatase locus 1 (ACP1) genetic polymorphism and dimension of leiomyomas (6) was reported, the joint effects of allergy and ACP1 has been also investigated.
ACP1 shows a polymorphism due the presence of three common alleles *A, *B and *C and correspondingly six genotypes. The enzyme is composed of two isoforms f and s that show different concentrations among genotypes: *B/*B genotype shows the highest concentration of f isoform (7).
Methods
Two hundred and three White women from the population of Rome (Italy) hospitalized consecutively for leiomyoma intervention were recruited from the Division of Gynecology and Obstetrics, Department of Biomedicine and Prevention University of Rome Tor Vergata. One hundred thirty eight women without clinical symptoms randomly selected from the same population were considered as controls for allergic manifestations.
All women gave verbal informed consent for their participation in this study that was approved by the Department. An approval of the ethical committee was not requested. The women were free to withdraw from the study at any time, but none chose to do so.
Subjects were asked if they had allergic disorders including asthma, rhinitis and AEDS (Atopic Eczema/Dermatitis Syndrome). All subjects with allergic symptoms had been evaluated at least once and had at least one positive prick test. Skin prick test was performed with a panel of the local allergens (Lofarma Allergeni SPA, Milan): Dermatofagoides, Poa pratensis (grass), Parietaria officinalis, Olea Europea (olive), Cipressus, milk, albumin, egg white, cat and dog hair.
The main diameter of tumors was considered for our comparisons.
T-test for difference between mean and variance was carried out using commercial software (SPSS). Level of statistical significance was set at 0.05.
ACP1 genotype:
ACP1 genotype was determined by DNA analysis as previously described (6). In brief, DNA was extracted from blood collected in EDTA. Polymerase chain reaction was set up in 30 μl containing 0.1 μmol/l of primers, 0.1 mmoli/l deoxynucleotide triphosphate, 1.5 mmol/l MgCl2, 0.5 IU of Taq polymerase, 1x Ampli Taq buffer and 50 ng DNA template. After an initial denaturation at 94°C for 2 min, the amplification was followed by 35 cycles at 94°C (45 s), 54°C (45 s), 72°C (45 s) and finally 72°C for 5 min. The oligonucleotide primers used for amplification are reported in table 1. A 341-Bp segment including exons 3 and 4 was amplified using primers 263 and 264. A 299 Bp segment including the exon 6 was amplified using primers 267 and 268. The 341 Bp segment was cleaved by CfoI restriction enzyme and then electrophoresed in agarose gel. The 299 bp was digested by Taq1 restriction enzyme generating two fragments for the ACP1 *A allele but not for *B and *C alleles. The digestion of 341 bp Amplicon created two fragments for the ACP1 *A and ACP1 *B alleles whereas the ACP1 *C allele was not cut.
Table 1.
Primers used for ACP 1 polymorphism analysis (modified from ref 6)
| Primer | Nucleotide sequence 5′-3′ |
|---|---|
| 263 | AGGCCAACCTGAACTCCTCT |
| 264 | CCTGTCTTGCTTTATGGGCT |
| 267 | TTCAGAAGACCCTAGCAGAGATG |
| 268 | TGGCAAAACCTGCATAACAA |
Results
Table 2 shows some demographic and clinical data of the sample. The proportions of allergic manifestations in women with leiomyomas and in controls were very similar suggesting the absence of a role of allergy in the susceptibility to leiomyomas (Table 3).
Table 2.
Demographic and clinical data of women
| Variable | Mean ± SE |
|---|---|
| Age (year) | 44.06±0.64 |
| BMI (kg/m 2 ) | 25.22±0.30 |
|
| |
| Proportion (%) | |
|
| |
| Smoker | 26.8% |
| Women who had pregnancies | 68.6% |
| Women who had abortions | 34.5% |
| Treatment with estro- progestins | 58.9% |
| Diameter of leiomyomas | |
| <10 centile | 10.8% |
| 10–90 centile | 79.0% |
| >90 centile | 10.2% |
| Localization | |
| Intramucosal | 18.7% |
| Submucosal | 76.0% |
| Both localizations | 5.3% |
| Symptoms | |
| Pain | 72.4% |
| Bleeding | 84.6% |
| Surgery | |
| Total abdominal hysterectomy | 87.2% |
| Myomectomy | 12.8% |
Table 3.
Frequency of allergic manifestations in women with uterine leiomyomas and control group
| % of allergic | Total (n) | |
|---|---|---|
| Uterine leiomyomas | 38.4% | 203 |
| Control | 37.8% | 138 |
In table 4, the dimension of leiomyomas in allergic women is compared to dimension in non allergic women. A highly significant difference was observed, the dimension of leiomyomas is lower in allergic women than in non allergic ones. This indicates that allergy exerts a protection against the growth of the tumor.
Table 4.
Diameter of leiomyomas in allergic and non allergic women
T-test for equality of means, p=0.004,
Mean±SE
No statistically significant differences were observed in the dimension of leiomyomas in relation to treatment with estroprogestins (5.35 cm vs. 5.67 cm: p=0.366).
Table 5 shows the diameter of leiomyomas in *B/*B and in other ACP1 genotypes. As reported in a previous study (6), in women with ACP1 *B/*B genotype the dimension of leiomyomas is lower than in other ACP1 genotypes. This confirms the previously reported observation (6) of a protective action of this genotype against the growth of tumor.
Table 5.
Diameter of leiomyomas in ACP 1 * B/ * B and in other ACP 1 genotypes
| Diameter (cm) | Subjects (n) | |
|---|---|---|
| ACP 1 non * B/ * B genotypes | 6.05±0.24 * | 118 |
| ACP 1 * B/ * B genotypes | 4.80±0.26 * | 85 |
Student t for equality of means, p<0.001,
Mean±SE
Also, a three way contingency table analysis by a log linear model considering ACP1, dimension of leiomyomas and allergy was carried out. The results indicate the absence of epistatic interaction, i.e. ACP1 does not affect the action of allergy and vice versa allergy does not affect the action of ACP1. On the contrary, a highly significant cooperative effect of ACP1 and allergy on the dimension of tumors was observed (p=0.005, data not shown) suggesting that allergy and ACP1 act independently on the growth of the tumor.
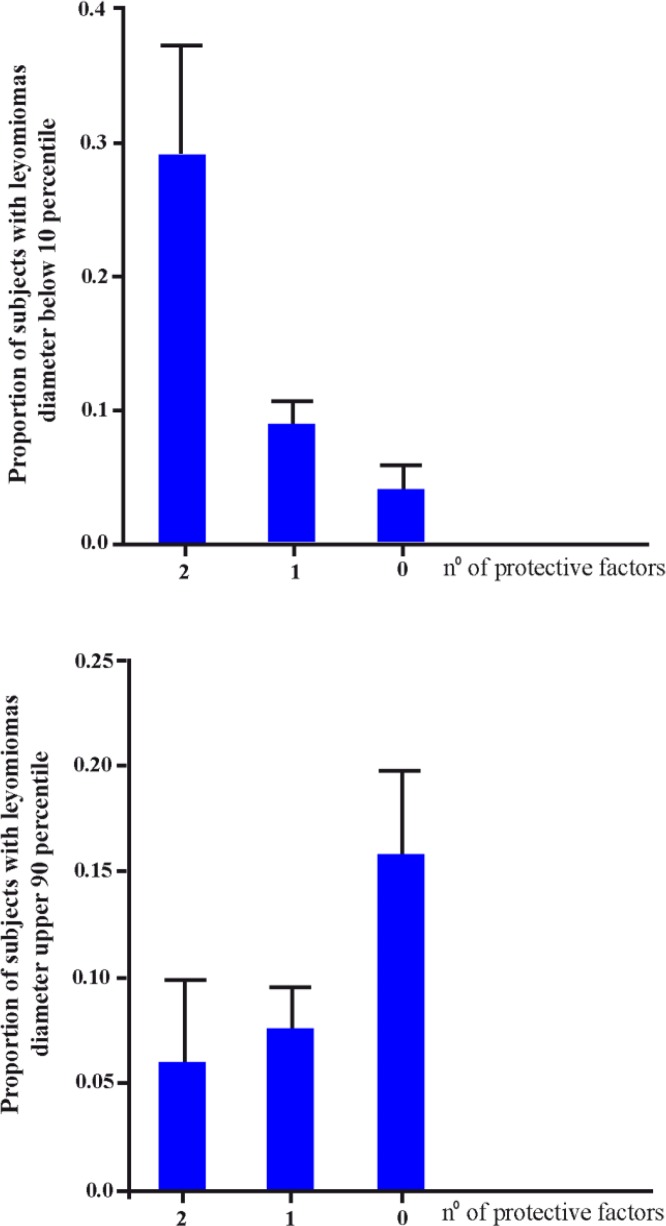
Table 6 shows the distribution of mean diameter of leiomyomas in relation to the joint ACP1-allergy phenotype. A highly significant difference was observed among the joint phenotypes. Assuming allergy and ACP1 *B/*B genotype as protective factors, there was a highly significant correlation between the number of factors and the diameter of leiomyomas. The relationship between the number of protective factors and the dimension of leiomyomas is depicted in figure 1. The proportion of women with small leiomyomas (<10 percentile) is much higher in those with two protective factors as compared to those with only one or no protective factor. On the contrary, the proportion of women with large leiomyomas (>90 percentile) is higher in those with no protective factors than in those with one or two factors. Statistical analysis suggests that about 8% of variance in dimension of leiomyomas is related to joint ACP1-allergy phenotype.
Table 6.
Distribution of mean diameter of leiomyomas in relation to the joint ACP 1 -allergy phenotype
| Diameter (cm) | Subjects (n) | |
|---|---|---|
| Allergic * B/ * B | 4.29±0.40 * | 34 |
| Non allergic * B/ * B | 5.14±0.33 * | 51 |
| Allergic non * B/ * B | 5.24±0.38 * | 42 |
| Non allergic non * B/ * B | 6.50±0.31 * | 76 |
Variance analysis p<0.001, Linear correlation p<0.001, Deviation from linearity p=0.430,
Mean±SE
Figure 1.
The relationship between the number of protective factors (allergy, ACP1 *B/*B genotype) and the diameter of uterine leyomiomas
Discussion
The data suggest that allergy and ACP1 *B/*B genotype exert a protective action against the growth of leiomyomas and that such effects are cooperative. It is interesting to note that allergy and ACP1 (6) are not involved in the etiology of leiomyoma, but in presence of the tumor, these factors influence its growth and in turn clinical manifestations.
Enhanced immunologic surveillance due to allergy could contribute to the control of leiomyomas growth. Such mechanism has been suggested in studies showing a negative association between some types of cancer and allergy.
Concerning the effect of ACP1 in a previous study, a mechanism involving platelet growth factors (PDGF) was suggested. This factor favors the growth of leiomyomas (8) and on the other hand, ACP1 dephosphorylates PDGF receptors decreasing its activity as the growth factor (9). *B/*B genotype shows the highest concentration of f isoform and is negatively correlated to the dimension of leiomyomas (6).
ACP1 is also able to dephosphorylate a negatively regulating phosphorylation site in ZAP70 tyrosine-kinase (7). This event leads to increased activation of the kinase and enhanced signaling from the T-cell antigen receptor. Therefore, an immune mechanism could also be involved in the association between ACP1 and leiomyomas dimension. Enhanced immunologic surveillance due to high f isoform, increased activation of ZAP70 and enhanced signaling from the T-cell antigen receptors could contribute to controlling the growth of leiomyomas.
Conclusion
Allergy and ACP1 *B/*B genotype cooperate in lowering the dimension of leiomyomas; about 8% of leiomyomas growth could be attributable to the joint effect of allergy and ACP1.
If confirmed in other clinical settings, our observation may have practical importance in identifying women at risk of severe manifestations requiring surgical treatment. On the other hand, a pharmacologically negative modulation of ACP1 activity could improve the clinical course of the disease.
Footnotes
Conflict of Interest
Authors declare no conflict of interest.
References
- 1. Engkilde K, Thyssen JP, Menne T, Johansen JD. Association between cancer and contact allergy: a linkage study. BMJ Open. 2011; 1 (1): e000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roncarolo F, Infante-Rivard C. Asthma and risk of brain cancer in children. Cancer Causes Control. 2012; 23 (4): 617– 23. [DOI] [PubMed] [Google Scholar]
- 3. Rittmeyer D, Lorentz A. Relationship between allergy and cancer: an overview. Int Arch Allergy Immunol. 2012; 159 (3): 216– 25. [DOI] [PubMed] [Google Scholar]
- 4. Stott-Miller M, Chen C, Doody DR, Carter JL, Galloway DA, Madeleine MM, et al. A history of allergies is associated with reduced risk of oral squamous cell carcinoma. Cancer Causes Control. 2012; 23 (12): 1911– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chae YK, Neagu S, Kim J, Smyrlis A, Gooptu M, Tester W. Association between common allergic symptoms and cancer in the NHANES III female cohort. PLoS One. 2012; 7 (9): e42896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ammendola ML, Pietropolli A, Lista F, Saccucci P, Piccione E, Bottini E, et al. Is there an association between uterine leiomyomas and acid phosphatase locus 1 polymorphism? Am J Obstet Gynecol. 2009; 200 (1): 110.e1– 5. [DOI] [PubMed] [Google Scholar]
- 7. Bottini N, Bottini E, Gloria-Bottini F, Mustelin T. Low-molecular-weight protein tyrosine phosphatase and human disease: in search of biochemical mechanisms. Arch Immunol Ther Exp (Warsz). 2002; 50 (2): 95– 104. [PubMed] [Google Scholar]
- 8. Parker WH. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril. 2007; 87 (4): 725– 36. [DOI] [PubMed] [Google Scholar]
- 9. Stefani M, Caselli A, Bucciantini M, Pazzagli L, Dolfi F, Camici G, et al. Dephosphorylation of tyrosine phosphorylated synthetic peptides by rat liver phosphotyrosine protein phosphatase isoenzymes. FEBS Lett. 1993; 326 (1–3): 131– 4. [DOI] [PubMed] [Google Scholar]