Heart failure (HF) has been described as the inability of the myocardium to deliver oxygen and nutrients to a degree commensurate with the metabolic requirements of the body.1 Myocardial dysfunction induces compensatory neurohumoral mechanisms, including the sympathetic nervous system (SNS), as an attempt to preserve contractile performance. Mediators of the SNS consist predominantly of two catecholamines, namely epinephrine and norepinephrine (NE), released by cardiac sympathetic nerve terminals or secreted directly into the circulation by the adrenal medulla. Effects of these neurotransmitters are mediated through cell surface adrenergic receptors (ARs), members of the G protein-coupled receptor (GPCR) superfamily. Stimulation of the β-AR promotes a conformational change to activate the heterotrimeric G protein Gα and Gβγ subunits, promoting positive inotropic and chronotropic effects culminating in improved myocardial function.2
This functionally beneficial pathway refers exclusively, however, to acute receptor activation; sustained β-AR stimulation, as occurs in most cardiovascular (CV) disease, is characterized by molecular modifications leading to reduced receptor sensitivity and membrane expression. Receptor desensitization and downregulation are regulatory processes thought to moderate persistent agonist stimulation to prevent catecholamine-induced toxicity. The desensitization process is initiated in large part by agonist-dependent phosphorylation of the receptor’s cytoplasmic tail by G protein-coupled receptor kinase 2 (GRK2), a serine/threonine kinase. GRK2 is a cytosolic enzyme that localizes to the plasma membrane following recruitment by active Gβγ subunits. This is followed by recruitment of β-arrestin to the phosphorylated receptor, which prevents recoupling of the dissociated cognate G protein and targets the receptor for internalization and eventual degradation. It is now appreciated that chronic SNS activity and subsequent GRK2-mediated receptor downregulation result in a loss of responsiveness to catecholamines and contribute to further contractile dysfunction and increased patient mortality.2, 3
Pivotal studies have indicated that properties of β-AR signaling appear to be recapitulated in circulating white blood cells. In 1986, Brodde et al reported that, in human subjects, the density of β-ARs on circulating lymphocytes directly correlated with the density and functional responsiveness of β-ARs in solid tissue, specifically right atrial appendages.4 Subsequent data demonstrated that, in addition to β-ARs, there is a direct association between lymphocyte and cardiac GRK2 expression and activity in human HF patients.5 Thus, measurement of lymphocytic GRK2 appeared to provide an accurate assessment of the relative levels of GRK2, as well as β-AR signaling, in the myocardium. This phenomenon has been further examined in end-stage HF patients who have been placed on mechanical circulatory support. Left ventricular assist devices (LVADs) have become a key therapeutic strategy in the management of end-stage HF, and LVAD-induced unloading has been associated with partial normalization of myocardial structure and function, along with partial restoration of β-AR signaling and GRK2 downregulation in cardiomyocytes.6, 7 Interestingly, this restoration in β-AR signaling and GRK2 expression following LVAD implantation is mirrored by the diminished expression of GRK2 in lymphocytes.7 Taken together, this corroborates the potential for using lymphocytes to monitor disease-induced β-AR signaling changes in the heart.
In the present study by Rengo et al,8 the authors have evaluated the prognostic potential of lymphocyte GRK2 levels in predicting outcomes in patients with HF. Lymphocyte GRK2 levels were detected in immunoprecipitated lymphocyte extracts by Western blotting. Selection of participants for the study was based upon the diagnosis of HF of either ischemic or non-ischemic etiology. The study group consisted of 257 patients, and those enrolled fell within either Class II or III of the New York Heart Association (NYHA) functional classification system. Echocardiographic analysis, perhaps the most useful non-invasive tool for diagnosis, revealed that patients exhibited typical characteristics of hemodynamic dysfunction, including reduced mean left ventricular ejection fraction (LVEF). Measurement of the blood concentration of B-type natriuretic peptide was utilized as a complementary diagnostic approach, as elevation in the secretion of this family of hormones is known to occur following cardiac injury. Serum levels of N-terminal pro-B-type natriuretic peptic (NT-proBNP) are commonly measured to assess heart disease severity, and as anticipated, the patients demonstrated significantly elevated serum NT-proBNP levels. Plasma NE levels were also measured, and while this represents a somewhat rudimentary assessment that depends considerably upon the rate of NE reuptake and clearance from the circulation, it provides a useful tool for the estimation of SNS activity.9 These parameters were incorporated into the study to validate the prognostic efficacy of lymphocyte GRK2 level assessment. Patients were followed for an average of 37.5 months; CV and all cause deaths were recorded.
The authors first sought to evaluate markers that correlated with patient mortality. NYHA class, LVEF, serum NT-proBNP and plasma NE were among the factors significantly associated with CV mortality in these patients. As observed previously, mean lymphocyte GRK2 protein levels were significantly elevated in HF patients compared with healthy controls. Similar to the established markers of heart disease, elevated lymphocyte GRK2 levels were also significantly associated with patient mortality; analysis revealed a significant difference in lymphocyte GRK2 levels between survivors and non-survivors with an average of 1.17±0.62 D.U. and 1.66±0.71 D.U., respectively. Furthermore, lymphocyte GRK2 expression significantly correlated with age and LVEF, along with serum NE and NT-proBNP levels. Stratification of age, LVEF, lymphocyte GRK2 and NT-proBNP data generally depicted a significant, consistent increase along the quartiles of these four factors. Finally, NT-proBNP and lymphocyte GRK2 levels appeared to have the greatest impact on CV-related and all-cause death.
The prognostic values of NT-proBNP and lymphocyte GRK2 were further evaluated by decision curve analysis to investigate the individual and combined clinical net benefit profiles of these markers. While typical prediction models provide the probability of an event on the basis of a set of prognostic factors, they do not incorporate clinical consequences and do not inform clinical practice. The authors have employed a model that is suitable for evaluating alternative clinical prognostic strategies and has advantages over other commonly used measures and techniques.10 Interestingly, the two partial models, evaluating either NT-proBNP or lymphocyte GRK2 independently, show very similar net benefit profiles, suggesting the prognostic power of lymphocyte GRK2 level to be equally effective as NT-proBNP. Furthermore, the full model combining these markers demonstrates a clinical net benefit over the utilization of each marker independently. These data present convincing evidence that assessment of lymphocyte GRK2 levels may provide a valuable addition to currently available diagnostic tests for patients with HF.
Inhibitors of the adrenergic and angiotensin signaling pathways have become the standard of care for heart disease patients amongst all degrees of severity.11 Interestingly, the use of β-blockers did not significantly correlate with lymphocyte levels of GRK2, nor did it effectively predict mortality in these patients. While more effective markers may have overshadowed the prognostic value of the use of these medications, it is possible these data also identify inefficient protection against CV-related mortality. This potential absence in protection suggests the need for the development of novel therapies for the treatment of HF. As a chief component in the β-AR downregulation process, GRK2 represents an attractive target for the amelioration of aberrant SNS activity in the setting of HF. Preclinical studies from these authors and others have demonstrated that GRK2 inhibition by either peptides12, 13 or small molecules14, 15 can improve cardiac function and reverse pathological remodeling in animal models of HF.
The manuscript by Rengo et al8 is the first to show the prognostic capacity of lymphocyte GRK2 levels to independently predict mortality in HF patients. Moreover, it has demonstrated strong prognostic efficacy equivalent to, and synergistic with, the evaluation of NT-proBNP levels. These findings provide further evidence advocating for the measurement of lymphocyte GRK2 to indirectly evaluate adrenergic activity in the myocardium and approximate heart disease severity (Figure). Future studies will help to further determine its efficacy in the evaluation and guidance of treatment strategies in these patient populations. As the authors have suggested, assessment of a larger population, inclusive of patients in additional disease classes, will ultimately be necessary to confirm the findings presented in their manuscript. Furthermore, with proof of concept established, development of a high-throughput assay to assess lymphocyte GRK2 expression will be necessary prior to translation into the clinic. These results present strong evidence that lymphocyte GRK2 levels provide independent and synergistic prognosis with existing HF biomarkers and future studies will further establish its clinical prognostic efficacy.
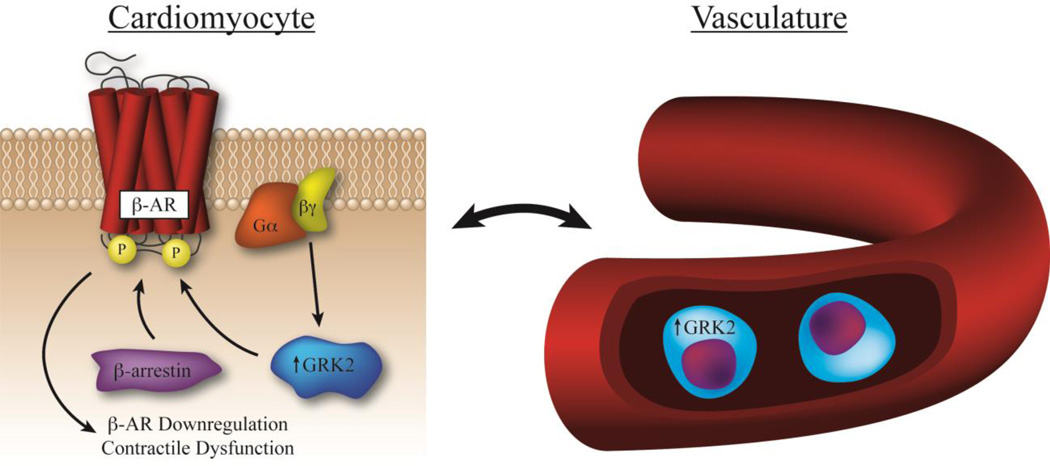
Figure. Lymphocyte GRK2 expression correlates with myocardial GRK2 levels and β-AR dysfunction.
In the setting of HF, chronic SNS activity results in enhanced myocardial GRK2 levels and activity, contributing to further hemodynamic dysfunction and increased patient mortality. β-AR downregulation is initiated by GRK2-mediated receptor phosphorylation following recruitment by activated Gβγ subunits. β-arrestin-mediated desensitization prevents the receptor from recoupling with its cognate G protein and targets it for internalization and degradation. GRK2 expression in lymphocytes directly correlates with levels of this kinase in cardiomyocytes, and can be used to assess disease severity and predict patient mortality.
Acknowledgments
Sources of Funding
This work was supported by National Institutes of Health grants R01 HL129772, R01 HL132551 and P01 HL069779 (BCB), and a Predoctoral Fellowship from the Pharmaceutical Research and Manufacturers of America Foundation (JGT).
Footnotes
Disclosures
None.
References
- 1.Braunwald E, Ross J, Jr, Sonnenblick EH. Mechanisms of contraction of the normal and failing heart. N Engl J Med. 1967;277:1012–1022. doi: 10.1056/NEJM196711092771907. concl. [DOI] [PubMed] [Google Scholar]
- 2.Lymperopoulos A, Rengo G, Koch WJ. Adrenergic nervous system in heart failure: Pathophysiology and therapy. Circ Res. 2013;113:739–753. doi: 10.1161/CIRCRESAHA.113.300308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belmonte SL, Blaxall BC. G protein coupled receptor kinases as therapeutic targets in cardiovascular disease. Circ Res. 2011;109:309–319. doi: 10.1161/CIRCRESAHA.110.231233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brodde OE, Kretsch R, Ikezono K, Zerkowski HR, Reidemeister JC. Human beta-adrenoceptors: Relation of myocardial and lymphocyte beta-adrenoceptor density. Science. 1986;231:1584–1585. doi: 10.1126/science.3006250. [DOI] [PubMed] [Google Scholar]
- 5.Iaccarino G, Barbato E, Cipolletta E, De Amicis V, Margulies KB, Leosco D, Trimarco B, Koch WJ. Elevated myocardial and lymphocyte grk2 expression and activity in human heart failure. Eur Heart J. 2005;26:1752–1758. doi: 10.1093/eurheartj/ehi429. [DOI] [PubMed] [Google Scholar]
- 6.Blaxall BC, Tschannen-Moran BM, Milano CA, Koch WJ. Differential gene expression and genomic patient stratification following left ventricular assist device support. J Am Coll Cardiol. 2003;41:1096–1106. doi: 10.1016/s0735-1097(03)00043-3. [DOI] [PubMed] [Google Scholar]
- 7.Hata JA, Williams ML, Schroder JN, Lima B, Keys JR, Blaxall BC, Petrofski JA, Jakoi A, Milano CA, Koch WJ. Lymphocyte levels of grk2 (betaark1) mirror changes in the lvad-supported failing human heart: Lower grk2 associated with improved beta-adrenergic signaling after mechanical unloading. J Card Fail. 2006;12:360–368. doi: 10.1016/j.cardfail.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Rengo G, Pagano G, Perrone-Filardi P, Femminella GD, Parisi V, Cannavo A, Liccardo D, Komici K, Gambino G, D'Amico ML, de Lucia C, Paolillo S, Trimarco B, Vitale DF, Ferrara N, Koch WJ, Leosco D. Prognostic value of lymphocyte g protein-coupled receptor kinase-2 protein levels in patients with heart failure. Circ Res. 2016 doi: 10.1161/CIRCRESAHA.115.308207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Task Force for the D, Treatment of A, Chronic Heart Failure of the European Society of C. Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P Guidelines ESCCfP. Esc guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the european society of cardiology. Developed in collaboration with the heart failure association (hfa) of the esc. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 10.Vickers AJ, Elkin EB. Decision curve analysis: A novel method for evaluating prediction models. Med Decis Making. 2006;26:565–574. doi: 10.1177/0272989X06295361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McAlister FA, Wiebe N, Ezekowitz JA, Leung AA, Armstrong PW. Meta-analysis: Beta-blocker dose, heart rate reduction, and death in patients with heart failure. Ann Intern Med. 2009;150:784–794. doi: 10.7326/0003-4819-150-11-200906020-00006. [DOI] [PubMed] [Google Scholar]
- 12.Raake PW, Schlegel P, Ksienzyk J, Reinkober J, Barthelmes J, Schinkel S, Pleger S, Mier W, Haberkorn U, Koch WJ, Katus HA, Most P, Muller OJ. Aav6.Betaarkct cardiac gene therapy ameliorates cardiac function and normalizes the catecholaminergic axis in a clinically relevant large animal heart failure model. Eur Heart J. 2013;34:1437–1447. doi: 10.1093/eurheartj/ehr447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rengo G, Lymperopoulos A, Zincarelli C, Donniacuo M, Soltys S, Rabinowitz JE, Koch WJ. Myocardial adeno-associated virus serotype 6-betaarkct gene therapy improves cardiac function and normalizes the neurohormonal axis in chronic heart failure. Circulation. 2009;119:89–98. doi: 10.1161/CIRCULATIONAHA.108.803999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamal FA, Mickelsen DM, Wegman KM, Travers JG, Moalem J, Hammes SR, Smrcka AV, Blaxall BC. Simultaneous adrenal and cardiac g-protein-coupled receptor-gbetagamma inhibition halts heart failure progression. J Am Coll Cardiol. 2014;63:2549–2557. doi: 10.1016/j.jacc.2014.02.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schumacher SM, Gao E, Zhu W, Chen X, Chuprun JK, Feldman AM, Tesmer JJ, Koch WJ. Paroxetine-mediated grk2 inhibition reverses cardiac dysfunction and remodeling after myocardial infarction. Sci Transl Med. 2015;7:277ra231. doi: 10.1126/scitranslmed.aaa0154. [DOI] [PMC free article] [PubMed] [Google Scholar]