A correlation between increases in the intracellular Ca2+ concentration of vascular endothelial cells and release of endothelium-dependent relaxing factors was first reported decades ago by Lückhoff et al.1. In agreement with these formative observations, subsequent studies demonstrated that Ca2+ sensitive biosynthetic pathways, such as endothelial nitric oxide synthase (eNOS), as well as small and intermediate conductance Ca2+-activated K+ channels (KCa2.3 and KCa3.1) are primary drivers of endothelium-dependent vasodilation. As endothelial dysfunction and loss of vasodilatory capacity is a common hallmark of cardiovascular diseases, the underlying Ca2+ signaling mechanisms are of considerable interest. Technological advances, including the development of high affinity fluorescent Ca2+ indicator dyes, transgenic mice expressing genetically encoded Ca2+ indicator (GECI) proteins selectively in the endothelium2, high-speed, high-resolution confocal Ca2+ imaging, and total internal reflection fluorescent (TIRF) microscopy allow the Ca2+ signals controlling endothelium-dependent vasodilation to be probed in ever increasing detail. Application of these methods has revealed that Ca2+ mobilization pathways in the endothelium are unexpectedly complex, dynamic, and diverse. For example, spreading intracellular and intercellular Ca2+ waves are stimulated by the well-characterized endothelium-dependent vasodilator acetylcholine in a number of vascular beds2. These propagating Ca2+ events may be critically important for conducted vasodilatory responses3. Another type of Ca2+ signal was reported by Ledoux et al., who demonstrated that in mouse mesenteric arteries, acetylcholine enhances localized release of Ca2+ from the endoplasmic reticulum (ER) through inositol trisphosphate receptors (IP3R)4. These subcellular Ca2+ signals, referred to as “Ca2+ pulsars”, are localized to membrane domains that project through the internal elastic lamina separating the endothelium from underlying vascular smooth muscle cells. Ca2+ pulsar sites co-localize with areas densely expressing KCa3.1 channels, suggesting that Ca2+ pulsars may be a fundamental signal driving the activity of these channels to cause endothelium-dependent smooth muscle hyperpolarization (EDH) and vasodilation. Further studies linked localized, transient influx of Ca2+ though members of the transient receptor potential (TRP) cation channel superfamily5 with endothelium-dependent vasodilation. These events, called “sparklets” represent Ca2+ influx through a single TRP channel or clusters of channels present on the plasma membrane and can be detected using confocal6 or TIRF microscopy7. Sonkusare et al. elegantly showed that stimulated activity of vanilloid (V) TRPV4 sparklets causes KCa2.3- and KCa3.1-dependent dilation of mouse mesenteric arteries6. Studies from our laboratory show that ankyrin (A)-type TRPA1 sparklets promote dilation of mouse and rat cerebral pial arteries in response to electrophilic compounds and endogenously generated reactive oxygen species8, 9, and the dietary compound carvacrol, found in oregano, stimulates TRPV3 sparklets in the endothelium of mouse cerebral parenchymal arterioles to cause EDH-dependent dilation10. Taken together, these studies show that multiple Ca2+-dependent pathways have significant impact on arterial tone and endothelial cell function in diverse vascular beds, but very little is known about the integration and spatial and temporal regulation of Ca2+ signaling modalities in the intact endothelial syncytium. Exciting new findings by Francis et al.11, published in the current issue of Circulation Research, boldly address this critical issue.
Working with coronary arteries from swine, Francis et al. showed that under basal (unstimulated) conditions, spontaneous Ca2+ signaling in the endothelium is inherently dynamic and complex. Ca2+ signaling events were recorded from a broad field of the endothelium (~120 cells) of opened arteries pinned in the en face configuration. Approximately 35% of the endothelial cells in the recording field displayed spontaneous Ca2+ signaling activity that was heterogeneous in terms of amplitude, duration and spatial spread. However, average full field Ca2+ fluorescence remained virtually unchanged over the entire recording period (10 minutes). A pharmacological approach was used to show that this activity was almost entirely due to release of intracellular Ca2+ from the ER through IP3R and was not affected by blockade of ryanodine receptors. Further, dynamic Ca2+ signals were almost entirely quenched under these conditions by inhibition of phospholipase C (PLC). Thus, it appears that basal endothelial cell Ca2+ signaling in this vascular bed is dependent on tonic production of IP3 by a PLC-dependent pathway. The functional consequences of basal Ca2+ signals were explored in companion with wire myography experiments showing that blockade of eNOS, KCa2.3, or KCa3.1 channels caused an elevation in isometric tension of endothelium-intact coronary artery rings, and these responses were similarly linked to IP3R and PLC. These data suggest that dynamic IP3R-mediated Ca2+ signals (and not global changes in intracellular Ca2+ concentration) are critically important for endothelium-dependent regulation of basal tone in swine coronary arteries. This concept was extended in experiments employing the potent coronary artery endothelium-dependent vasodilator substance P. Strikingly, the concentration dependence of substance P-induced relaxation of coronary artery rings was shown to be identical to that of the effect of the compound on the frequency of transient Ca2+ signaling events, primarily due to increased activity of basally activity sites. Higher concentrations of substance P further elevated mean Ca2+ signal amplitude, spatial spread, and event duration, but these effects appear to be unrelated to endothelium-dependent relaxation. Further, the data show that changes in global intracellular Ca2+ in the endothelium are not correlated with the vasorelaxant effects of substance P. This observation is consistent with a prior report from this group showing that vasodilation of cerebral arteries in response to activation of TRPA1 channels with an electrophilic compound was due to elevation in local Ca2+ signaling activity and not global changes in endothelial cell Ca2+ levels12. The main conclusion that can be drawn from these observations is that tissue level changes in endothelial cell Ca2+ concentration do not adequately describe the Ca2+ signaling pathways that underlie endothelium-dependent vasodilation and that frequency modulation of dynamic Ca2+ signals is the primary driver of this response.
A defining strength of the present study lies in the use of a custom-made plug-in for the NIH ImageJ image analysis software suite called “LC_Pro”13, 14. This plug-in was originally developed by the authors and allows automated analysis of dynamic Ca2+ signaling events recorded using fluorescent microscopy. The algorithm is designed to detect and track sites of dynamic changes in Ca2+ that rise above statistical noise and automatically define regions of interest (ROI). Amplitude (F/F0), kinetics, and spatial spread are determined for all events occurring within each ROI, rapidly providing a biophysical fingerprint of discreet Ca2+ signals. In addition to the removal of potential investigator bias, LC_Pro allows rapid high-throughput analysis of complex dynamic Ca2+ signaling events occurring within all endothelial cells in a broad imaging field, allowing the large-scale statistical evaluation of these events. Findings enabled by this powerful tool presented in the current study and recent reports from other investigators strongly argue that “Ca2+ signaling” in the endothelium could be more accurately described as “definitive Ca2+ patterning” set by multiple interacting parts. Efforts directed at defining the principal components involved in shaping these patterns will help to build useful bed-specific vascular models to predict the overall influence of the endothelium. One hope is that distinctive pattern shifts will predict specific endothelial functions and dysfunctions.
As with all groundbreaking studies, the novel insights provided by Francis et al. raise many new questions. For example, the findings indicate that under basal conditions the majority of Ca2+ signaling events result from Ca2+ release from the ER through IP3R that is dependent on PLC activity. However, the physiological basis for IP3 generation and spontaneous Ca2+ signaling activity under these conditions and the particular PLC isoform(s) responsible are not known. In addition, all of the Ca2+ imaging experiments were performed in the absence of physiological levels of flow and shear stress, factors that strongly stimulate endothelium-dependent vasodilation. Studies to determine the effects of shear stress on endothelial cell Ca2+ dynamics may require the use of advanced endothelial cell-specific GECI mice, such as Cx40-GCaMP5-mCherry mice created by Dr. Michael Kotlikoff and colleagues as part of the CHROMus resource development program (http://chromus.vet.cornell.edu/). These mice will allow high spatial and temporal resolution of discreet signal components under the most physiologic conditions possible. It is also critically important to have a better understanding of the intracellular organization of IP3R clusters along the endothelial cell axis that likely promote the propagation of Ca2+ waves in the endothelium. The organization of TRP channel domains that regulate Ca2+ influx in the endothelium is also unresolved. It is possible that the distribution and organization of these structures differs between vascular beds with important functional consequences. Complete description of this architecture in the intact endothelium will require the use of advanced imaging techniques, such as super-resolution microscopy. Finally, it remains undetermined how chronic pathological conditions alter Ca2+ dynamics in endothelial cells. Expanding Ca2+ events that occur during supraphysiological levels of stimulation (i.e., high concentrations of substance P) may underlie a switch to a more pathologic, or refractory, signaling pattern. In addition, current evidence suggests that hypoxia increases expression of IP3R in cerebellar neurons15. Thus, it is possible that chronic hypoxia caused by coronary artery disease may increase expression or disrupt the architecture of IP3R in coronary arteries, leading to alteration in intracellular Ca2+ dynamics in endothelial cells and dysfunction. The work by Francis et al. provides an excellent starting point for new studies using advanced analytical techniques to address these important issues.
Figure 1.
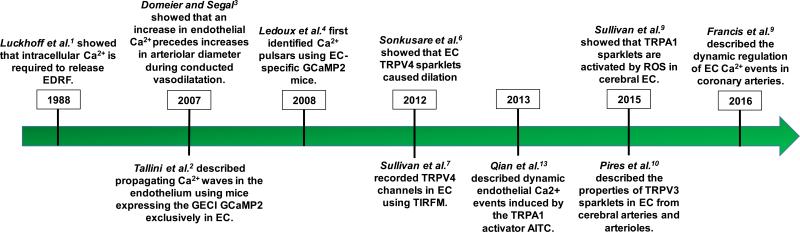
Timeline of discoveries regarding intracellular Ca2+ signaling in endothelial cells. EDRF: endothelium-derived relaxing factor; GECI: genetically-encoded Ca2+ indicator; EC: endothelial cells; TRPV: transient receptor potential vanillin; TRPA: transient receptor potential ankyrin; AITC: allyl isothiocyanate; ROS: reactive oxygen species.
ACKNOWLEDGEMENTS
This work was supported by the National Heart, Lung, and Blood Institute Grant R01HL091905 to SE and the American Heart Association Grant 15POST24720002 to PWP.
Footnotes
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Luckhoff A, Pohl U, Mulsch A, Busse R. Differential role of extra- and intracellular calcium in the release of edrf and prostacyclin from cultured endothelial cells. Br J Pharmacol. 1988;95:189–196. doi: 10.1111/j.1476-5381.1988.tb16564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tallini YN, Brekke JF, Shui B, Doran R, Hwang SM, Nakai J, Salama G, Segal SS, Kotlikoff MI. Propagated endothelial ca2+ waves and arteriolar dilation in vivo: Measurements in cx40bac gcamp2 transgenic mice. Circulation research. 2007;101:1300–1309. doi: 10.1161/CIRCRESAHA.107.149484. [DOI] [PubMed] [Google Scholar]
- 3.Domeier TL, Segal SS. Electromechanical and pharmacomechanical signalling pathways for conducted vasodilatation along endothelium of hamster feed arteries. The Journal of physiology. 2007;579:175–186. doi: 10.1113/jphysiol.2006.124529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ledoux J, Taylor MS, Bonev AD, Hannah RM, Solodushko V, Shui B, Tallini Y, Kotlikoff MI, Nelson MT. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc Natl Acad Sci U S A. 2008;105:9627–9632. doi: 10.1073/pnas.0801963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Earley S, Brayden JE. Transient receptor potential channels in the vasculature. Physiological reviews. 2015;95:645–690. doi: 10.1152/physrev.00026.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT. Elementary ca2+ signals through endothelial trpv4 channels regulate vascular function. Science. 2012;336:597–601. doi: 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sullivan MN, Francis M, Pitts NL, Taylor MS, Earley S. Optical recording reveals novel properties of gsk1016790a-induced vanilloid transient receptor potential channel trpv4 activity in primary human endothelial cells. Molecular pharmacology. 2012;82:464–472. doi: 10.1124/mol.112.078584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Earley S, Gonzales AL, Crnich R. Endothelium-dependent cerebral artery dilation mediated by trpa1 and ca2+-activated k+ channels. Circulation research. 2009;104:987–994. doi: 10.1161/CIRCRESAHA.108.189530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sullivan MN, Gonzales AL, Pires PW, Bruhl A, Leo MD, Li W, Oulidi A, Boop FA, Feng Y, Jaggar JH, Welsh DG, Earley S. Localized trpa1 channel ca2+ signals stimulated by reactive oxygen species promote cerebral artery dilation. Science signaling. 2015;8:ra2. doi: 10.1126/scisignal.2005659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pires PW, Earley S. A trpc3 signalling complex promotes cerebral artery remodelling during hypertension. Cardiovascular research. 2016;109:4–5. doi: 10.1093/cvr/cvv261. [DOI] [PubMed] [Google Scholar]
- 11.Francis CM, Waldrup JR, Qian X, Solodushko V, Meriwether J, Taylor MS. Functional tuning of intrinsic endothelial ca2+ dynamics in swine coronary arteries. Circ Res. 2016;118:xxx–xxx. doi: 10.1161/CIRCRESAHA.115.308141. in this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qian X, Francis M, Solodushko V, Earley S, Taylor MS. Recruitment of dynamic endothelial ca2+ signals by the trpa1 channel activator aitc in rat cerebral arteries. Microcirculation. 2013;20:138–148. doi: 10.1111/micc.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francis M, Waldrup J, Qian X, Taylor MS. Automated analysis of dynamic ca2+ signals in image sequences. J Vis Exp. 2014 doi: 10.3791/51560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francis M, Qian X, Charbel C, Ledoux J, Parker JC, Taylor MS. Automated region of interest analysis of dynamic ca(2)+ signals in image sequences. American journal of physiology. Cell physiology. 2012;303:C236–243. doi: 10.1152/ajpcell.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jurkovicova D, Kopacek J, Stefanik P, Kubovcakova L, Zahradnikova A, Jr., Zahradnikova A, Pastorekova S, Krizanova O. Hypoxia modulates gene expression of ip3 receptors in rodent cerebellum. Pflugers Archiv : European journal of physiology. 2007;454:415–425. doi: 10.1007/s00424-007-0214-6. [DOI] [PubMed] [Google Scholar]