Abstract
Background:
There are limited data on longer-term outcomes (>5 years) for patients with unprotected left main coronary artery (ULMCA) disease who underwent percutaneous coronary intervention (PCI) in the drug-eluting stents (DES) era. This study aimed at comparing the long-term (>5 years) outcomes of patients with ULMCA disease underwent PCI with DES and coronary artery bypass grafting (CABG) and the predictors of adverse events.
Methods:
All consecutive patients with ULMCA disease treated with DES implantation versus CABG in our center, between January 2003 and July 2009, were screened for analyzing. A propensity score analysis was carried out to adjust for potential confounding between the two groups.
Results:
Nine hundred and twenty-two patients with ULMCA disease were enrolled for the analyses (DES = 465 vs. CABG = 457). During the median follow-up of 7.1 years (interquartile range 5.3–8.2 years), no difference was found between PCI and CABG in the occurrence of death (P = 0.282) and the composite endpoint of cardiac death, myocardial infarction (MI) and stroke (P = 0.294). Rates of major adverse cardiac and cerebrovascular events were significantly higher in the PCI group (P = 0.014) in large part because of the significantly higher rate of repeat revascularization (P < 0.001). PCI was correlated with the lower occurrence of stroke (P = 0.004). Multivariate analysis showed ejection fraction (EF) (P = 0.012), creatinine (P = 0.016), and prior stroke (P = 0.031) were independent predictors of the composite endpoint of cardiac death, MI, and stroke in the DES group, while age (P = 0.026) and EF (P = 0.002) were independent predictors in the CABG group.
Conclusions:
During a median follow-up of 7.1 years, there was no difference in the rate of death between PCI with DES implantation and CABG in ULMCA lesions in the patient cohort. CABG group was observed to have significantly lower rates of repeat revascularization but higher stroke rates compared with PCI. EF, creatinine, and prior stroke were independent predictors of the composite endpoint of cardiac death, MI, and stroke in the DES group, while age and EF were independent predictors in the CABG group.
Keywords: Coronary Artery Bypass Grafting, Drug-eluting Stents, Percutaneous Coronary Intervention, Unprotected Left Main Coronary Artery
INTRODUCTION
A significant lesion in left main (LM) coronary artery is regarded as the most prognostically important coronary lesion because it puts 70% of the left ventricular myocardium at risk.[1] The 12-year survival of medically treated patients with unprotected left main coronary artery (ULMCA) disease is unsatisfactory as it ranges from 35% to 49% depending on the number of other coronary vessels involved.[2] According to current guidelines, coronary artery bypass grafting (CABG) was considered the standard treatment for ULMCA disease as studies had shown its survival benefit over medical treatment.[3,4] The introduction of drug-eluting stents (DES) and advances in catheter techniques have led to increasing acceptance of percutaneous coronary intervention (PCI) as a viable alternative to CABG for ULMCA disease.[5,6] Randomized controlled trials and observational studies with up to 5 years follow-up have consistently shown significantly increased need for repeat revascularization with PCI versus CABG in patients with LM coronary disease, but no difference in mortality or combined rates of death and myocardial infarction (MI).[7,8]
However, there are limited data on longer-term outcomes (>5 years) for patients with ULMCA disease who underwent PCI in the DES era. Bypass grafts are anastomosed to coronary arteries distal to the lesions, thus rendering the complexity of the lesion inconsequential and providing an adequate buffer against the development of new lesions in the future. The patency of the left internal mammal artery at 10–15 years was reported to be as high as approximately 90%.[9] Limited duration of follow-up (usually <5 years) might incompletely depict the advantages of CABG, which initially accrue with time but which may also eventually be eroded by progressive vein graft failure. Whether there was some difference in terms of mortality between PCI with DES and CABG in longer follow-up (>5 years) remains uncertain. Therefore, this study aimed at comparing the long-term real-world outcomes of consecutive patients with ULMCA disease underwent PCI with DES and CABG.
METHODS
Patients and procedure
ULMCA disease was defined as LM coronary artery stenosis ≥50% and no bypass grafts to the left anterior descending or left circumflex coronary artery. Eligible patients with de novo ULMCA disease who received DES implantation or underwent CABG between January 2003 and July 2009 in Beijing Anzhen Hospital were consecutively enrolled. The follow-up period extended through August 01, 2013, to ensure that all patients had at least 4 years and approximately up to 10 years of follow-up information. Patients with prior stents implanted at the LM coronary artery were excluded. Patients with age >80 years old when the procedures were operated, prior CABG, concomitant valvular or aortic surgery, or cardiogenic shock were excluded. Those ST-elevation myocardial infarction (STEMI)/non-STEMI patients who underwent primary PCI or urgent CABG were excluded. A total of 922 patients was finally analyzed (DES, n = 465; CABG, n = 457).
The decision to perform CABG or PCI was dependent on patient comorbidities, physician's choice, and/or patient preference. Coronary angioplasty and stent implantation was performed according to the operator's criteria following the center's usual practice. The choice of sirolimus-, paclitaxel-, or zotarolimus-eluting stents was at the discretion of the physician (zotarolimus-eluting stents became available for clinical use in our center in September 2006). CABG was performed with standard bypass techniques.[10] The internal thoracic artery was preferentially used for revascularization of the left anterior descending artery. Revascularization was considered complete when all vessels >1.5 mm in diameter with diameter stenoses ≥50% were treated.
Before stent implantation, all patients received aspirin according to their physicians’ normal procedures and either clopidogrel 75 mg/d for 3 days before the procedure or a preprocedural loading dose of clopidogrel ≥300 mg/d. Patients were continued on clopidogrel for at least 1 year (75 mg/d) and aspirin indefinitely (100 mg/d) after the procedure.
All patients provided informed consent for both the procedure and subsequent data collection and analysis for research purposes. The study was in accordance with the Declaration of Helsinki and was approved by the local ethics committee (the Ethics Committee of Beijing Anzhen Hospital, Capital Medical University). The local ethics committee approved the use of the data for this study. There was no industry involvement in the design, conduct, financial support, or analysis of the study.
Clinical definitions and follow-up
Clinical follow-up was performed at 1 month, 6 months, 1 year, and then annually thereafter. All follow-up data were collected by outpatient or telephone interview and angiographic follow-up. Angiographic follow-up was recommended in the DES group from 8 to 12 months after the procedure or whenever clinically indicated. For patients who underwent CABG, angiographic follow-up was recommended if there were ischemic symptoms or signs during follow-up. Angiographic follow-up was not mandatory. All outcomes of interest were confirmed by source documentation and were adjudicated by the local events committee at Beijing Anzhen Hospital, Capital Medical University.
The endpoints of the study were death; cardiac death; repeat revascularization; MI; stroke; the composite of cardiac death, MI, or stroke (major adverse cardiac and cerebrovascular events [MACCE], the composite of cardiac death, MI, stroke, or repeat revascularization). Any death due to proximate cardiac cause (e.g., MI, low-output failure, and fatal arrhythmia), unwitnessed death and death of unknown cause, and all procedure-related deaths, including those related to concomitant treatment, will be classified as cardiac death.[11] Periprocedural MI (<7 days after intervention) was defined as elevated serum creatinine kinase-MB isoenzyme 5 times the upper limit of normal after CABG and 3 times the upper limit of normal after PCI.[11] MI after the periprocedural period was defined as any typical increase and decrease of biochemical markers of myocardial necrosis with 1 of the following: cardiac symptoms, development of Q waves on electrocardiography, or electrocardiographic changes indicative of ischemia. Cardiac enzymes were not measured routinely unless there was clinical suspicion of myocardial ischemia. Stroke, as indicated by neurologic deficits, was confirmed by a neurologist based on imaging studies. Repeat revascularization included PCI and CABG. All events were based on clinical diagnoses assigned by the patients’ physicians and were centrally adjudicated by an independent group of clinicians in Beijing Anzhen Hospital. In the cumulative analysis of endpoints, events were counted only once, whichever occurred first.
Statistical analysis
Continuous variables were presented as a mean ± standard deviation or medians (interquartile range [IQR]) and compared between the study group with independent sample Student's t-test or Mann–Whitney U-test, dependent on whether the data followed a normal distribution. Categorical variables were reported as counts and percentages, and differences between the two groups were assessed by means of the Chi-square test. Time to the primary endpoint will be evaluated according to Kaplan–Meier method, and the log-rank test will be applied to compare the incidence of the endpoint between patients underwent PCI and CABG.
Given the nonrandomized nature of the study, a propensity score analysis was carried out. This analysis included a number of variables, such as age, gender, left ventricular ejection fraction (EF), European System for Cardiac Operative Risk Evaluation, indications for PCI or CABG, prior peripheral vascular disease, hypertension, diabetes, dyslipidemia, smoking history, prior MI, family history, prior stroke, prior PCI, chronic total occlusion, LM coronary artery lesion location, and extent of disease vessel.
The logistic model by which the propensity score was estimated showed good predictive value (C-statistic = 0.753, 95% confidence interval [CI]: 0.722–0.785) and calibration characteristics by the Hosmer–Lemeshow test (P = 0.36). The score was then incorporated into subsequent Cox proportional hazard models as a covariate. Cox proportional hazards models were used to compare risks of adverse events between patients underwent PCI with DES and CABG. Cox proportional hazard models were tested with CABG as the reference category.
All statistical analyses were performed with the Statistical Package for Social Sciences version 17.0 system for Windows (SPSS Inc., USA). A P < 0.05 was considered statistically significant.
RESULTS
From January 2003 to July 2009, 922 patients with ULMCA disease in Beijing Anzhen Hospital were finally enrolled for the analyses; 465 patients were treated with PCI using DES and 457 patients were treated with CABG.
Baseline clinical and procedure characteristics were shown in Table 1. Overall, the CABG patients had a higher-risk clinical and angiographic profile than PCI patients. Complete revascularization was achieved in 298 PCI patients (64.1%) and in 320 CABG patients (70.0%; P = 0.055). Among CABG patients, 85.3% underwent revascularization of the left anterior descending artery with an arterial conduit, and 92.3% were off-pump CABG.
Table 1.
Baseline clinical and procedural characteristics
| Variables | PCI (n = 465) | CABG (n = 457) | P |
|---|---|---|---|
| Age, mean (range), years | 62 (54–70) | 64 (57–70) | 0.052 |
| Male, n (%) | 367 (78.9) | 377 (82.5) | 0.170 |
| Diabetes mellitus, n (%) | 143 (30.8) | 131 (28.7) | 0.488 |
| Smoking history, n (%) | 230 (49.5) | 205 (44.9) | 0.161 |
| Hypertension, n (%) | 286 (61.5) | 269 (58.9) | 0.412 |
| Family history, n (%) | 41 (8.8) | 33 (7.2) | 0.372 |
| Dyslipidemia, n (%) | 231 (49.7) | 158 (34.6) | <0.001 |
| Prior stroke, n (%) | 45 (9.7) | 42 (9.2) | 0.800 |
| Prior myocardial infarction, n (%) | 66 (14.2) | 99 (21.7) | 0.003 |
| Prior PVD, n (%) | 24 (5.2) | 34 (7.4) | 0.154 |
| Prior PCI, n (%) | 68 (14.6) | 31 (6.8) | <0.001 |
| EuroSCORE, mean (range) | 5 (3–6) | 5 (3–6) | 0.290 |
| LVEF, mean (range), % | 64 (59–70) | 62 (54–68) | <0.001 |
| Serum creatinine, mean (range), μmol/L | 79.6 (70.0–93.7) | 84.0 (70.7–97.2) | 0.012 |
| Indications for revascularization, n (%) | |||
| NSTEMI | 19 (4.1) | 16 (3.5) | 0.001 |
| STEMI | 55 (11.8) | 41 (9.0) | |
| Stable angina | 52 (11.2) | 80 (17.5) | |
| Unstable angina | 323 (69.5) | 318 (69.6) | |
| Silent ischemia | 16 (3.4) | 2 (0.4) | |
| Involved location, n (%) | |||
| Ostium | 98 (21.1) | 59 (12.9) | 0.004 |
| Body | 51 (11.0) | 51 (11.2) | |
| Distal bifurcation | 316 (68.0) | 347 (75.9) | |
| Extent of diseased vessel, n (%) | |||
| LM only | 31 (6.7) | 12 (2.6) | <0.001 |
| LM plus single-vessel disease | 96 (20.6) | 33 (7.2) | |
| LM plus double-vessel disease | 174 (37.4) | 105 (23.0) | |
| LM plus triple-vessel disease | 164 (35.3) | 307 (67.2) | |
| Chronic total occlusion, n (%) | 75 (16.1) | 184 (40.3) | <0.001 |
| Complete revascularization, n (%) | 298 (64.1) | 320 (70.0) | 0.055 |
| Number of stents (range) | 2 (1–4) | – | |
| Off-pump CABG | – | 422 (92.3) | |
| Vessel bypassed | – | 3 (3–3) | |
| Left internal thoracic use, n (%) | – | 390 (85.3) |
PCI: Percutaneous coronary intervention; CABG: Coronary artery bypass grafting; PVD: Peripheral vascular disease; LVEF: Left ventricular ejection fraction; NSTEMI: Non-ST-elevation myocardial infarction; STEMI: ST-elevation myocardial infarction; LM: Left main. EuroSCORE: European System for Cardiac Operative Risk Evaluation. Complete revascularization: All lesions occupying >50% diameter of a segment with a reference diameter of ≥1.50 mm were treated.
The median follow-up was 7.1 years (IQR 5.3–8.2 years) in the overall patients. Complete follow-up was obtained in 93.2% of the overall cohort (93.8% for the PCI group, and 92.6% for the CABG group; P = 0.47). Angiographic follow-up was performed in 254 patients of the DES group and in 58 of the CABG group (55% vs. 16%, P < 0.001). During overall follow-up, 113 patients (12.3%) died, of whom 69 (7.5%) died of a cardiovascular cause. A total of 59 (6.4%) suffered an MI, and 57 (6.2%) suffered a stroke. Repeat revascularization was performed in 167 (18.1%).
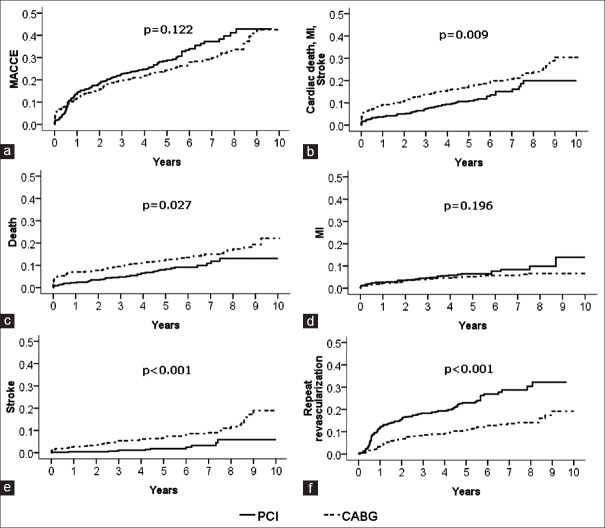
The crude relative risks are presented in Figure 1 and Table 2. The occurrences of death (PCI 13.0% vs. CABG 22.1%, P = 0.009) and stroke (PCI 5.8% vs. CABG 18.9%, P < 0.001) were significantly higher with the CABG group, whereas the rate of repeat revascularization (PCI 32.3% vs. CABG 19.2%, P < 0.001) was significantly higher in the PCI group. There was a higher trend toward higher rate of MI with the PCI group (PCI 13.9% vs. CABG 6.7%, P = 0.196). No significant difference was observed in the rate of MACCE.
Figure 1.
Kaplan–Meier incidence curves of clinical outcome in patients with unprotected left main coronary artery disease underwent percutaneous coronary intervention with drug-eluting stents and coronary artery bypass grafting. (a) Major adverse cardiac and cerebrovascular events; (b) cardiac death, myocardial infarction and stroke; (c) death; (d) myocardial infarction; (e) stroke; (f) repeat revascularization.
Table 2.
Clinical outcomes of patients with unprotected left main coronary artery disease underwent revascularization
| Outcomes | Incidence of adverse events | P | HR (95% CI) | |
|---|---|---|---|---|
| PCI (%) | CABG (%) | |||
| Unadjusted | ||||
| MACCE | 42.9 | 42.5 | 0.122 | 1.204 (0.951–1.523) |
| Cardiac death/MI/stroke | 19.9 | 30.4 | 0.009 | 0.651 (0.469–0.902) |
| Death | 13.0 | 22.1 | 0.027 | 0.647 (0.439–0.954) |
| Cardiac death | 7.3 | 10.1 | 0.059 | 0.627 (0.384–1.022) |
| MI | 13.9 | 6.7 | 0.196 | 1.407 (0.836–2.366) |
| Stroke | 5.8 | 18.9 | <0.001 | 0.298 (0.153–0.581) |
| Repeat revascularization | 32.3 | 19.2 | <0.001 | 2.256 (1.633–3.115) |
| Propensity score-adjusted | ||||
| MACCE | 49.8 | 39.0 | 0.014 | 1.394 (1.070–1.817) |
| Cardiac death/MI/stroke | 22.9 | 27.0 | 0.294 | 0.825 (0.576–1.182) |
| Death | 15.7 | 19.4 | 0.282 | 0.791 (0.516–1.212) |
| Cardiac death | 7.4 | 8.0 | 0.737 | 0.912 (0.531–1.565) |
| MI | 11.3 | 7.0 | 0.082 | 1.664 (0.937–2.954) |
| Stroke | 6.5 | 17.5 | 0.004 | 0.348 (0.171–0.707) |
| Repeat revascularization | 36.8 | 17.6 | <0.001 | 2.368 (1.653–3.392) |
PCI: Percutaneous coronary intervention; CABG: Coronary artery bypass grafting; HR: Hazard ratio; MACCE: Major adverse cardiac and cerebrovascular events, the composite of cardiac death, myocardial infarction, stroke or repeat revascularization; MI: Myocardial infarction; CI: Confidence interval.
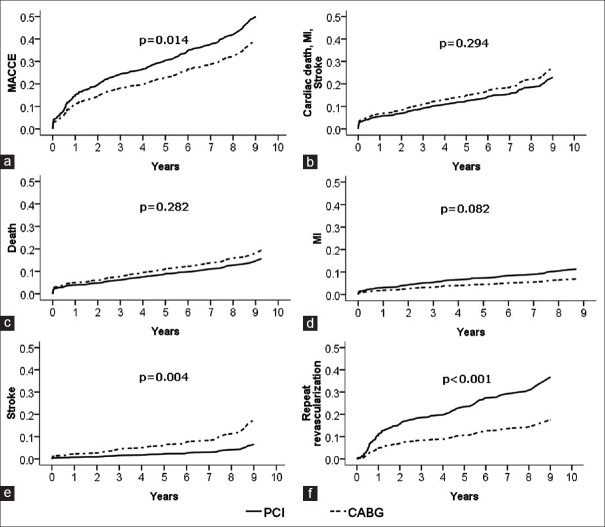
The propensity score-adjusted results are presented in Figure 2 and Table 2. In propensity-matching methods, there was no significant difference in rates of death, MI, and a composite of serious outcomes (cardiac death, MI, or stroke) between the 2 groups. Rates of MACCE were significantly higher in the PCI group (P = 0.014), in large part because of an increased rate of repeat revascularization (P < 0.001). However, stroke was still significantly more likely to occur with CABG (P = 0.004).
Figure 2.
Propensity score-adjusted incidence curves of clinical outcome in patients with unprotected left main coronary artery disease underwent percutaneous coronary intervention with drug-eluting stents and coronary artery bypass grafting. (a) Major adverse cardiac and cerebrovascular events; (b) cardiac death, myocardial infarction and stroke; (c) death; (d) myocardial infarction; (e) stroke; (f) repeat revascularization.
Multivariate analysis showed EF (P = 0.012), creatinine (P = 0.016), and prior stroke (P = 0.031) were independent predictors of the composite endpoint of cardiac death, MI, and stroke in the DES group [Table 3]. Multivariate analysis showed age (P = 0.026) and EF (P = 0.002) were independent predictors of the composite endpoint of cardiac death, MI, and stroke in the CABG group [Table 4].
Table 3.
Predictors of cardiac death/MI/stroke in ULMCA patients underwent PCI according to multivariate analysis
| Variables | P | HR | 95% CI for HR | |
|---|---|---|---|---|
| Lower | Upper | |||
| Age | 0.618 | 1.007 | 0.980 | 1.034 |
| EF | 0.012 | 0.971 | 0.949 | 0.994 |
| Serum creatinine | 0.016 | 1.004 | 1.001 | 1.008 |
| Prior stroke | 0.031 | 2.087 | 1.070 | 4.070 |
| Extent of diseased vessel | 0.279 | 1.198 | 0.864 | 1.662 |
| Chronic total occlusion | 0.358 | 1.360 | 0.706 | 2.620 |
CI: Confidence interval; HR: Hazard ratio; EF: Ejection fraction; ULMCA: Unprotected left main coronary artery; CABG: Coronary artery bypass grafting; MI: Myocardial infarction; PCI: Percutaneous coronary intervention.
Table 4.
Predictors of cardiac death/MI/stroke in ULMCA patients underwent CABG according to multivariate analysis
| Variables | P | HR | 95% CI for HR | |
|---|---|---|---|---|
| Lower | Upper | |||
| Age | 0.026 | 1.030 | 1.004 | 1.056 |
| EF | 0.002 | 0.975 | 0.959 | 0.991 |
| Serum creatinine | 0.059 | 1.007 | 1.000 | 1.014 |
| Extent of diseased vessel | 0.075 | 1.391 | 0.967 | 1.999 |
| Prior stroke | 0.158 | 1.504 | 0.854 | 2.651 |
| Chronic total occlusion | 0.573 | 1.122 | 0.751 | 1.677 |
CI: Confidence interval; HR: Hazard ratio; EF: Ejection fraction; ULMCA: Unprotected left main coronary artery; CABG: Coronary artery bypass grafting; MI: Myocardial infarction.
DISCUSSION
In this study of consecutive patients with unprotected LMCA disease during a median follow-up of 7.1 years, we found that rates of death, MI, and the composite of cardiac death, MI, or stroke were similar between the DES and the CABG group. DES group was observed to have significantly lower stroke rates but higher rates of repeat revascularization compared with CABG.
Practice guidelines.[3,4] have assigned a Class I recommendation to CABG surgery to improve survival in patients with ULMCA disease as studies had shown its survival benefit over medical treatment.[12] Guidelines have recently assigned a Class IIa recommendation to PCI to improve survival in selected patients with ULMCA disease, yet no trials have directly compared PCI with medicine therapy for patients with ULMCA disease. The recommendation was based on the reasoning that because CABG confers a survival advantage over medicine therapy for ULMCA disease and PCI is equivalent to CABG, then PCI confers a survival advantage over medicine therapy for ULMCA disease. Studies with up to 5 years follow-up have shown no difference in mortality or combined rates of death and MI between PCI with DES and CABG.[7,8] However, as CABG showed the survival benefit over 10 years,[12] the importance of confirming that these results remain durable with longer-term follow-up (at least 5 years) is vital. Limited data for >5 years follow-up of patients with ULMCA disease in the DES era suffered from small sample and lacking of CABG as reference.[13,14]
In our cohort with a median follow-up of 7.1 years, PCI showed an even slightly lower rate of all-cause mortality compared to CABG (PCI 15.7% vs. CABG 19.4%, P = 0.282). This lower trend of mortality with PCI for ULMCA was not uncommon. Wu et al. reported, there was a trend toward lower mortality with DES versus CABG for patients with ULMCA in propensity score-adjusted (hazard ratio [HR] 0.34, 95% CI: 0.12–1.03, P = 0.06) analyses.[15] Athappan et al. reported a meta-analysis comprising 14203 patients with ULMCA underwent PCI with DES or CABG, which had shown a lower trend of 5 years all-cause mortality with PCI compared to CABG (odds ratio 0.79, 95% CI: 0.57–1.08).[16] The 3-year result of SYNTAX LM subgroup had shown significantly lower incidence of death in those undergoing PCI with low or intermediate SYNTAX scores (survival advantage over medic P = 0.03), whereas slightly higher rate of death in PCI group with high SYNTAX score (>32) (PCI 13.4% vs. CABG 7.6%, P = 0.10).[17] Farooq et al. developed SYNTAX score II which contained eight predictors included the presence of ULMCA disease and could well predict 4-year mortality in patients with complex coronary artery disease.[18] In the SYNTAX score II nomogram, the presence of ULMCA disease drove mortality predictions in favor of PCI, requiring higher anatomical SYNTAX scores among PCI patients to achieve similarity in long-term prognosis between CABG and PCI.[18] From these results above we speculated that diffusion of disease is the limiting factor for PCI, more than the LM location itself. In terms of the occurrence of the composite endpoint of death, MI, and stroke, some studies even showed a significant reduction with PCI group for patients with ULMCA disease compared to CABG.[7,19]
The advantage of CABG in reducing the need for repeat revascularization was once again confirmed even during the median 7-year follow-up period. The lower need for revascularization in CABG suggests that at least “ first-generation” DESs (exclusively used in this preliminary phase of our experience from January 2003 to August 2006) are still an imperfect solution that is unable to completely eliminate restenosis in complex settings such as bifurcational lesions and multivessel disease. However, it might be fair to point out that significantly higher rate of angiographic follow-up with PCI group (55% vs. 16%, P < 0.001) is certainly likely to increase revascularization rates after PCI. Routine angiographic follow-up after LM coronary artery stenting has never been shown to improve outcomes as many repeat revascularization were angiographically rather than clinically driven. Moreover, the double occurrence of repeat revascularization in the PCI group (PCI 36.8% vs. CABG 17.6%) did not translate into any disparity of mortality between PCI and CABG.
Although several reports had shown the beneficial impact of off-pump CABG on neurological outcomes,[20] our cohort in which off-pump CABG accounted for 92.3% demonstrated CABG was still associated with the significantly higher rate of stroke compared to the DES group even during the median 7-year follow-up period. This trend was in accordance with several meta-analysis with large sample comparing PCI and CABG for ULMCA patients with up to 5 years follow-up.[16,21] Embolic dislodgement of atherosclerotic plaques during surgical aortic manipulations has been recognized as a major source of stroke. However, we could find that the difference in stroke rate was not so obvious within the 1st year, and the disparity became wider in the longer follow-up in our study [Figure 1], implying there were other reasons in addition to the aortic manipulation during the CABG procedure. The high incidence of postoperative atrial fibrillation after CABG (approximately 30%) might be accounted for this disparity.[22] It was reported postoperative atrial fibrillation is associated with a long-term risk for stroke and death following CABG.[23]
Our study showed age was the independent predictor of cardiac death/MI/stroke in patients underwent CABG but not PCI, while creatinine was the independent predictor of cardiac death/MI/stroke in patients underwent PCI but not CABG. This was in accordance with the result of SYNTAX score II nomogram.[18] Farooq et al. reported that 4-year mortality significantly increased per 10 years old in CABG group (HR 1.88, 95% CI: 1.34–2.64) but not in the PCI group (HR 1.29, 95% CI: 0.97–1.71), showing significant interaction (P = 0.095).[18]
Our study suffers the most important limitations of nonrandomized and observational studies. Although propensity score analyses are known to be a valuable method for taking into account the potential confounding factors attributable to between-groups imbalances, it is not possible to control for all variables. It is important to realize, however, that randomization eliminates clinical judgment in the patient selection and therefore carries a potential for being misleading as a predictor of outcomes in actual clinical practice. The second limitation was a lack of subgroup analysis according to SYNTAX score. The third limitation was that our study excluded patients of over 80 years old, and those underwent primary PCI.
In conclusion, during a median follow-up of 7.1 years (IQR 5.3–8.2 years), there was still no difference in the rate of death between PCI with DES implantation and CABG in ULMCA lesions in this patients cohort. CABG group was observed to have significantly lower rates of repeat revascularization but higher stroke rates compared with PCI. EF, creatinine, and prior stroke were independent predictors of the composite endpoint of cardiac death, MI, and stroke in the DES group, while age and EF were independent predictors in the CABG group.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Min Chen
REFERENCES
- 1.Leaman DM, Brower RW, Meester GT, Serruys P, van den Bran M. Coronary artery atherosclerosis: Severity of the disease, severity of angina pectoris and compromised left ventricular function. Circulation. 1981;63:285–99. doi: 10.1161/01.cir.63.2.285. doi: 10.1161/01.CIR.63.2.285. [DOI] [PubMed] [Google Scholar]
- 2.Emond M, Mock MB, Davis KB, Fisher LD, Holmes DR, Jr, Chaitman BR, et al. Long-term survival of medically treated patients in the coronary artery surgery study (CASS) registry. Circulation. 1994;90:2645–57. doi: 10.1161/01.cir.90.6.2645. doi: 10.1161/01.CIR.90.6.2645. [DOI] [PubMed] [Google Scholar]
- 3.Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011. ACCF/AHA/SCAI guideline for percutaneous coronary intervention: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:e574–651. doi: 10.1161/CIR.0b013e31823ba622. doi: 10.1161/CIR.0b013e31823ba622. [DOI] [PubMed] [Google Scholar]
- 4.Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS); European Association for Percutaneous Cardiovascular Interventions (EAPCI) Wijns W, Kolh P, Danchin N, Di Mario C. Guidelines on myocardial revascularization. Eur Heart J. 2010;31:2501–55. doi: 10.1093/eurheartj/ehu278. doi: 10.1093/eurheartj/ehq277. [DOI] [PubMed] [Google Scholar]
- 5.Park DW, Seung KB, Kim YH, Lee JY, Kim WJ, Kang SJ, et al. Long-term safety and efficacy of stenting versus coronary artery bypass grafting for unprotected left main coronary artery disease: 5-year results from the MAIN-COMPARE (Revascularization for Unprotected Left Main Coronary Artery Stenosis: Comparison of Percutaneous Coronary Angioplasty Versus Surgical Revascularization) registry. J Am Coll Cardiol. 2010;56:117–24. doi: 10.1016/j.jacc.2010.04.004. doi: 10.1016/j.jacc.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Park SJ, Kim YH, Park DW, Yun SC, Ahn JM, Song HG, et al. Randomized trial of stents versus bypass surgery for left main coronary artery disease. N Engl J Med. 2011;364:1718–27. doi: 10.1056/NEJMoa1100452. doi: 10.1056/NEJMoa1100452. [DOI] [PubMed] [Google Scholar]
- 7.Chieffo A, Magni V, Latib A, Maisano F, Ielasi A, Montorfano M, et al. 5-year outcomes following percutaneous coronary intervention with drug-eluting stent implantation versus coronary artery bypass graft for unprotected left main coronary artery lesions the Milan experience. JACC Cardiovasc Interv. 2010;3:595–601. doi: 10.1016/j.jcin.2010.03.014. doi: 10.1016/j.jcin.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Mohr FW, Morice MC, Kappetein AP, Feldman TE, Ståhle E, Colombo A, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet. 2013;381:629–38. doi: 10.1016/S0140-6736(13)60141-5. doi: 10.1016/S0140.6736(13)60141.5. [DOI] [PubMed] [Google Scholar]
- 9.Tatoulis J, Buxton BF, Fuller JA. Patencies of 2127 arterial to coronary conduits over 15 years. Ann Thorac Surg. 2004;77:93–101. doi: 10.1016/s0003-4975(03)01331-6. doi: 10.1016/S0003.4975(03)01331.6. [DOI] [PubMed] [Google Scholar]
- 10.Eagle KA, Guyton RA, Davidoff R, Edwards FH, Ewy GA, Gardner TJ, et al. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: Summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery) J Am Coll Cardiol. 2004;44:e213–310. doi: 10.1016/j.jacc.2004.07.021. doi: 10.1016/j.jacc.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 11.Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. Clinical end points in coronary stent trials: A case for standardized definitions. Circulation. 2007;115:2344–51. doi: 10.1161/CIRCULATIONAHA.106.685313. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 12.Caracciolo EA, Davis KB, Sopko G, Kaiser GC, Corley SD, Schaff H, et al. Comparison of surgical and medical group survival in patients with left main coronary artery disease. Long-term CASS experience. Circulation. 1995;91:2325–34. doi: 10.1161/01.cir.91.9.2325. doi: 10.1161/01.CIR.91.9.2325. [DOI] [PubMed] [Google Scholar]
- 13.Ielasi A, Latib A, Chieffo A, Takagi K, Mussardo M, Davidavicius G, et al. Very long-term outcomes following drug-eluting stent implantation for unprotected left main coronary artery stenosis: A single center experience. Rev Esp Cardiol (Engl Ed) 2013;66:24–33. doi: 10.1016/j.recesp.2012.06.026. doi: 10.1016/j.recesp. 2012.06.026. [DOI] [PubMed] [Google Scholar]
- 14.Kubo S, Kadota K, Shimada T, Ozaki M, Ichinohe T, Eguchi H, et al. Seven-year clinical outcomes of unprotected left main coronary artery stenting with drug-eluting stent and bare-metal stent. Circ J. 2013;77:2497–504. doi: 10.1253/circj.cj-13-0032. doi: 10.1253/circj.CJ.13.0032. [DOI] [PubMed] [Google Scholar]
- 15.Wu X, Chen Y, Liu H, Teirstein PS, Kirtane AJ, Ge C, et al. Comparison of long-term (4-year) outcomes of patients with unprotected left main coronary artery narrowing treated with drug-eluting stents versus coronary-artery bypass grafting. Am J Cardiol. 2010;105:1728–34. doi: 10.1016/j.amjcard.2010.01.353. doi: 10.1016/j.amjcard.2010.01.353. [DOI] [PubMed] [Google Scholar]
- 16.Athappan G, Patvardhan E, Tuzcu ME, Ellis S, Whitlow P, Kapadia SR. Left main coronary artery stenosis: A meta-analysis of drug-eluting stents versus coronary artery bypass grafting. JACC Cardiovasc Interv. 2013;6:1219–30. doi: 10.1016/j.jcin.2013.07.008. doi: 10.1016/j.jcin.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Morice MC, Serruys PW, Kappetein AP, Feldman TE, Ståhle E, Colombo A, et al. Outcomes in patients with de novo left main disease treated with either percutaneous coronary intervention using paclitaxel-eluting stents or coronary artery bypass graft treatment in the synergy between percutaneous coronary intervention with TAXUS and cardiac surgery (SYNTAX) trial. Circulation. 2010;121:2645–53. doi: 10.1161/CIRCULATIONAHA.109.899211. doi: 10.1161/CIRCULATIONAHA.109.899211. [DOI] [PubMed] [Google Scholar]
- 18.Farooq V, van Klaveren D, Steyerberg EW, Meliga E, Vergouwe Y, Chieffo A, et al. Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: Development and validation of SYNTAX score II. Lancet. 2013;381:639–50. doi: 10.1016/S0140-6736(13)60108-7. doi: 10.1016/S0140.6736(13)60108.7. [DOI] [PubMed] [Google Scholar]
- 19.Chieffo A, Morici N, Maisano F, Meliga E, Vergouwe Y, Chieffo A, et al. Percutaneous treatment with drug-eluting stent implantation versus bypass surgery for unprotected left main stenosis: A single-center experience. Circulation. 2006;113:2542–7. doi: 10.1161/CIRCULATIONAHA.105.595694. doi: 10.1161/CIRCULATIONAHA.105.595694.20. [DOI] [PubMed] [Google Scholar]
- 20.Hannan EL, Wu C, Smith CR, Higgins RS, Carlson RE, Culliford AT, et al. Off-pump versus on-pump coronary artery bypass graft surgery: Differences in short-term outcomes and in long-term mortality and need for subsequent revascularization. Circulation. 2007;116:1145–52. doi: 10.1161/CIRCULATIONAHA.106.675595. doi: 10.1161/CIRCULATIONAHA.106.675595. [DOI] [PubMed] [Google Scholar]
- 21.Alam M, Huang HD, Shahzad SA, Kar B, Virani SS, Rogers PA, et al. Percutaneous coronary intervention vs. coronary artery bypass graft surgery for unprotected left main coronary artery disease in the drug-eluting stents era. An aggregate data meta-analysis of 11,148 patients. Circ J. 2013;77:372–82. doi: 10.1253/circj.cj-12-0747. doi: 10.1253/circj.CJ.12.0747. [DOI] [PubMed] [Google Scholar]
- 22.Echahidi N, Pibarot P, O’Hara G, Mathieu P. Mechanisms, prevention, and treatment of atrial fibrillation after cardiac surgery. J Am Coll Cardiol. 2008;51:793–801. doi: 10.1016/j.jacc.2007.10.043. doi: 10.1016/j.jacc.2007.10.043.23. [DOI] [PubMed] [Google Scholar]
- 23.Horwich P, Buth KJ, Legare JF. New onset postoperative atrial fibrillation is associated with a long-term risk for stroke and death following cardiac surgery. J Card Surg. 2013;28:8–13. doi: 10.1111/jocs.12033. doi: 10.1111/jocs.12033. [DOI] [PubMed] [Google Scholar]