Abstract
Background:
Menopausal symptoms and sleep difficulty were physiological processes that were affected by genetic and other factors. This study was to investigate the prevalence of menopausal symptoms and sleep quality in menopausal transition (MT) and postmenopause (PM) women in Taiyuan, Shanxi.
Methods:
A community-based survey of women's menopausal symptoms and sleep quality was conducted between July 2012 and May 2013 at six municipal districts of Taiyuan, Shanxi. A sample of 2429 women aged 40–59 years was divided into four groups: early MT, late MT, early PM, and late PM. Sleep quality in the past 2 weeks before the interview was recorded. The data were analyzed using SPSS 16.0.
Results:
The prevalence of menopausal symptoms was 49.8%. Mild, moderate, and severe symptoms were observed in 28.9%, 18.5%, and 2.5% of participants, respectively. The highest prevalence of menopausal symptoms occurred in the early postmenopausal stage; the subsequences were the late postmenopausal stage and the early MT stage. Interestingly, among the 13 items of modified Kupperman index, the five most common symptoms were fatigue, arthralgia and myalgia, decreased libido, insomnia, and nervousness. Meanwhile, 55% perimenopausal women had poor sleep.
Conclusions:
Menopausal symptoms are common but mild among women in Taiyuan, Shanxi during MT and PM. In these stages, the prevalence of poor sleep is high.
Keywords: Menopausal Symptoms, Menopausal Transition, Postmenopause, Sleep Quality
INTRODUCTION
With the prolongation of human life expectancy, females will spend more than one-third of their lifetime in menopausal transition (MT) and the subsequent postmenopause (PM). This potentially entailed a bothersome series of physical and psychological menopausal symptoms.[1,2] However, most researches concerning menopausal symptoms were focused on Caucasians. It was reported that 68.1–93.7% of Caucasian women in menopause experienced at least one of the typical symptoms mentioned above.[3,4,5,6,7]
At present, data suggested that there were racial differences in the prevalence of menopausal symptoms, with a relatively high prevalence and more serious symptoms in Caucasians. Gold et al.[7] found there was a lower prevalence of menopausal symptoms in the Chinese and Japanese group among five racial sample groups. The study also found the lower prevalence of hot flashes/night sweats in the xanthoderm groups (17.6% and 20.5%, respectively) than in the African Americans, Caucasians, and Hispanics (45.6%, 31.2%, and 35.4%, respectively). The prevalence of other symptoms was also lower among xanthoderms. Other research by Anderson et al.[8] also suggested racial differences in the prevalence of menopausal symptoms. They showed that Japanese and Chinese women had lower prevalence in symptoms except heart pounding and forgetfulness than non-Hispanic Caucasians. Another common symptom-causing discomfort for women undergoing MT and PM was sleep difficulty. According to the epidemiological data, approximately 33–51% of perimenopausal women experienced sleep disturbance in the United Kingdom and the United States.[9,10] The representative research regarding sleep difficulty came from the Study of Women's Health Across the Nation (SWAN). Sleep difficulty was noted in 38% in the community-based survey of 12,603 US women.[11] It was reported that ethnicity has a significant association with sleep difficulty. In the SWAN study of multiethnic groups, Japanese (28.2%) and Chinese (31.6%) women had the lowest prevalence of sleep difficulty compared with the higher prevalence reported in Caucasian (40.3%) and Hispanic (38.0%) women.
Therefore, menopausal symptoms and sleep difficulty were physiological processes that were affected by genetic and other factors. Medical workers in China had gradually focused on this field. However, few investigators assessed menopausal symptoms and sleep quality, and the relevant civil reports were partial and inadequate.[12] We therefore investigated the prevalence of menopausal symptoms and sleep quality among 2429 women in MT and PM in Taiyuan, Shanxi.
METHODS
Design
A cross-sectional study was performed using a convenience sampling technique. Ethical approval for this study was obtained from the local Medical Ethical Committee.
Definition of menopausal status
Menopausal status was defined according to Stages of Reproductive Aging Workshop 2011 and participants were classified into four categories according to their own self-report: early MT, late MT, early PM, and late PM. Early MT was defined as increased variability in menstrual cycle length, and the variation was marked by a persistent difference of ≥7 days in the length of menstrual cycles within ten cycles of the first variable length cycle. Late MT was marked by the occurrence of amenorrhea of ≥60 days before the final menstrual period (FMP) and variability in the menstrual cycle that usually increased during this period. PM was defined as the period from the FMP onward, with early PM lasting for approximately 5 years after the FMP and late PM referring to >5 years after the FMP.[13]
Study population and sampling
The following participants were considered eligible and enrolled into the study: (1) aged 40–59 years; (2) early MT, late MT, early PM, or late PM; (3) an intact uterus and ≥1 ovary; (4) no sex steroid hormone use in the previous 3 months; (5) no pregnant; and (6) exclude hyperthyroidism, hypertension, heart diseases, arthritis, ear disease, cerebrovascular disease, and so on.
A statement of power calculation
For a 95% confidence interval (two-sided), a prevalence rate of 0.35 (document retrieval), and allowable error of 0.035, the necessary sample size was estimated to be approximately 713.
Procedure
The study was conducted in six municipal districts of Taiyuan, Shanxi: Jinyuan District, Xinghualing District, Yingze District, Xiaodian District, Wanbailin, District and Jiancaoping District. The household survey was performed from July 2012 to May 2013. A multistage random sampling strategy was used for subject collection. The six municipal districts were involved as clusters in the sampling process. Three community health stations were randomly selected in each district. Then, one neighborhood committee was randomly selected from each community health station. According to the order of the neighborhood list, 52 women aged 40–59 years were selected from each neighborhood committee. Ultimately, 2429 subjects were invited to participate in the cross-sectional questionnaire study. Interviewers received uniform training. Questionnaires were filled out by participates themselves. The face-to-face interview was used to fill out the survey questionnaire if the participant could not read or write. Of 2429 participants, 2308 (95%) women returned complete questionnaires.
Measures
The contents used in the questionnaire included demographic characteristics, age, marital status, education, economics, menstruation status, the modified Kupperman index (KI), and the Pittsburgh Sleep Quality Index (PSQI). The questionnaire also included the physical characteristics of the study participants such as parity, height, waist-hip ratio, systolic/diastolic pressure and pulse rate, the status of smoke, alcohol intake, and exercise. All the participants were asked to answer the question about psychological and somatic symptoms over the past 2 weeks.
Menopausal symptoms
Menopausal symptoms were evaluated using the 13-item modified KI. The modified version added urinary infection and decreased libido to the original KI. It contained the following 13 components: hot flashes (with or without sweating), paresthesia, insomnia (alteration in sleep pattern), nervousness (irritability), melancholia, vertigo, arthralgia and myalgia, headache, palpitation, formication, low sex drive (decreased libido), fatigue, and urinary tract infection. Scores for each item of the modified KI ranges from 0 to 3 (0 = no symptoms, 1 = mild, 2 = moderate, 3 = severe). The weighting factors were the same as those used in the original KI. The weighting factors for urinary infection and decreased libido were two points. Total scores ranged from 0 to 63. Perimenopausal symptoms were categorized into four groups: none (0–6), mild (7–15), moderate (16–30), and severe (>30).[14] Higher total KI indicated more severe menopausal symptoms.[15] The modified KI had good feasibility, reliability, and validity for identifying symptoms of menopause when used to screen women in China.[16,17]
Sleep quality
The 19-item PSQI was used to assess global sleep quality in the previous 2 weeks. It contained seven components: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction during the previous month. Each component was scored on a scale of 0–3 (higher scores indicate worse sleep) and the global score ranged from 0 to 21 (a high PSQI indicates poor sleep quality). Liu et al. reported that the global PSQI had a diagnostic sensitivity of 98.3% and specificity of 90.2% in China. Poor sleepers were defined by a global PSQI of ≥5, and good sleepers were defined by scores <5.[18]
Statistical analysis
Data were presented as a mean ± standard deviation (SD). SPSS version 16.0 software package (SPSS Inc., Chicago, Illinois, USA) was used for data analysis. Categorical variables were compared with a Chi-square test. Continuous variables were assessed using the two-sample t-test for independent samples. The related factors on the menopausal symptoms and sleep quality were analyzed using univariate logistic regression and multivariate stepwise logistic regression analysis. P < 0.05 was considered significant.
RESULTS
Participant baseline characteristics
This study enrolled 2429 participants and 2308 (95%) women returned complete questionnaires. According to STRAW 2011 criteria, 38.9%, 31.5%, 18.8%, and 10.8% of the sample were in early MT, late MT, early PM, and late PM, respectively. Baseline characteristics for the 2308 women are depicted in Table 1. Most women were married (94.8%) and had a moderate or good level of education (63.3% secondary education and 22.2% college and graduate). Most participants had a median family income (77.1%). Most women had a moderate physical condition, and they do not have the habit of smoke or alcohol.
Table 1.
Baseline characteristics of 2308 respondent women
| Characteristics | Number (%) |
|---|---|
| Age (years) | |
| 40–44 | 755 (32.7) |
| 45–49 | 730 (31.6) |
| 50–54 | 574 (24.9) |
| 55–59 | 249 (10.8) |
| Marital | |
| Single | 23 (1.0) |
| Married | 2187 (94.8) |
| Divorced | 63 (2.7) |
| Widowed | 35 (1.5) |
| Education | |
| None | 69 (3.0) |
| Primary | 266 (11.5) |
| Secondary | 1461 (63.3) |
| College and graduate | 512 (22.2) |
| Average household monthly income (RMB) | |
| <2000 | 528 (22.9) |
| 2000–3000 | 1006 (43.6) |
| >3000–4000 | 481 (20.8) |
| >4000 | 293 (12.7) |
| Parity | |
| 0 | 55 (2.4) |
| 1 | 1886 (81.7) |
| 2 | 289 (12.5) |
| 3 | 59 (2.6) |
| >3 | 19 (0.8) |
| Exercise | |
| None | 787 (34.1) |
| 1–3 times/month | 478 (20.7) |
| 1–3 times/week | 376 (16.3) |
| 4–5 times/week | 302 (13.1) |
| Everyday | 365 (15.8) |
| Smoking habits (cigarettes/day) | |
| Nonsmokers | 2238 (97.0) |
| 1–10 | 42 (1.8) |
| 11–20 | 18 (0.8) |
| ≥21 | 10 (0.4) |
| Alcohol consumption | |
| Never | 1537 (66.6) |
| Prior | 522 (22.6) |
| Active | 249 (10.8) |
| Height (cm) | |
| ≤160 | 318 (13.8) |
| 161–165 | 1092 (47.3) |
| 166–170 | 695 (30.1) |
| >170 | 203 (8.8) |
| WHR | |
| <0.85 | 988 (42.8) |
| ≥0.85 | 1320 (57.2) |
| Systolic pressure (mmHg) | |
| 90–99 | 141 (6.1) |
| 100–109 | 212 (9.2) |
| 110–119 | 829 (35.9) |
| 120–129 | 662 (28.7) |
| 130–139 | 464 (20.1) |
| Diastolic pressure (mmHg) | |
| 60–69 | 273 (11.8) |
| 70–79 | 1539 (66.7) |
| 80–89 | 496 (21.5) |
| Pulse rate (beats/min) | |
| 60–69 | 155 (6.7) |
| 70–79 | 1595 (69.1) |
| 80–89 | 505 (21.9) |
| 90–100 | 53 (2.3) |
WHR: Waist-hip ratio.
Menopausal symptoms of different menopausal status and multivariate analysis
Of the 2308 participants, 50.2% had no symptoms and 49.8% had varying degrees of menopausal symptoms. The prevalence of mild, moderate, and severe symptoms was 28.9%, 18.5%, and 2.5%, respectively [Table 2]. Menopausal symptom severity was mainly mild and moderate, with fewer participants experiencing severe symptoms. Table 2 also showed the prevalence of menopausal symptoms among women in early MT, late MT, early PM, or late PM. Among the four menopausal statuses, women in early PM had the highest prevalence of menopausal symptoms, followed by those in late PM, late MT, and early MT (P < 0.001). The prevalence of symptoms differed significantly among the four menopausal statuses [Figure 1].
Table 2.
Prevalence of menopausal symptoms
| Symptoms | None n (%) | Mild n (%) | Moderate n (%) | Severe n (%) | Total n |
|---|---|---|---|---|---|
| Early MT | 615 (68.5) | 197 (21.9) | 84 (9.4) | 2 (0.2) | 898 |
| Late MT | 329 (45.3) | 221 (30.4) | 153 (21.1) | 23 (3.2) | 726 |
| Early PM | 130 (29.9) | 162 (37.2) | 124 (28.5) | 19 (4.4) | 435 |
| Late PM | 84 (33.7) | 86 (34.5) | 66 (26.5) | 13 (5.2) | 249 |
| Total | 1158 (50.2) | 666 (28.9) | 427 (18.5) | 57 (2.5) | 2308 |
MT: Menopausal transition; PM: Postmenopause.
Figure 1.
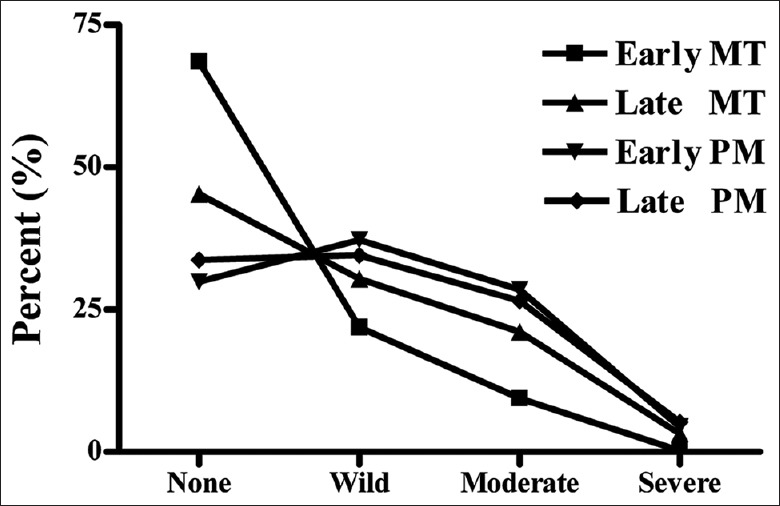
Prevalence of menopausal symptoms during different menopausal statuses. MT: Menopausal transition; PM: Postmenopause.
Univariate logistic regression showed that age, education, family income status, parity, regular exercise, and menopausal status were significantly correlated with the menopausal symptom. Of the six predictor variables, three variables entered the final multivariate logistic regression model [Table 3]. This result indicated that the three final variables – age, exercise, and menopausal status were significantly and independently related with the menopausal symptom.
Table 3.
Related factors of menopausal symptoms in MT and PM women
| Variables | B | SE | Wald | OR | 95% CI | P |
|---|---|---|---|---|---|---|
| Age | 0.379 | 0.062 | 37.266 | 1.461 | 1.294–1.605 | 0.000 |
| Exercise | −0.182 | 0.072 | 6.441 | 0.833 | 0.724–0.959 | 0.011 |
| Menopausal status | 0.295 | 0.043 | 47.112 | 1.343 | 1.234–1.460 | 0.000 |
OR: Odds ratio; CI: Confidence interval; SE: Standard error; MT: Menopausal transition; PM: Postmenopause.
Prevalence of the 13 Modified Kupperman index items
Each modified KI item score ranged from 0 to 3 (0 = no symptoms, 1 = mild, 2 = moderate, and 3 = severe). Figure 2 shows the prevalence of menopausal symptoms and the proportion of women experiencing each symptom. Symptom prevalence varied from 15% to 49%. The five most common symptoms were fatigue (49%), arthralgia and myalgia (46%), decreased libido (42%), insomnia (40%), and nervousness (39%). Paresthesia (15%) had the lowest prevalence. The five most severe symptoms were hot flashes (4.0%), decreased libido (2.7%), arthralgia and myalgia (2.4%), insomnia (2.0%), and urinary tract infection (1.5%).
Figure 2.
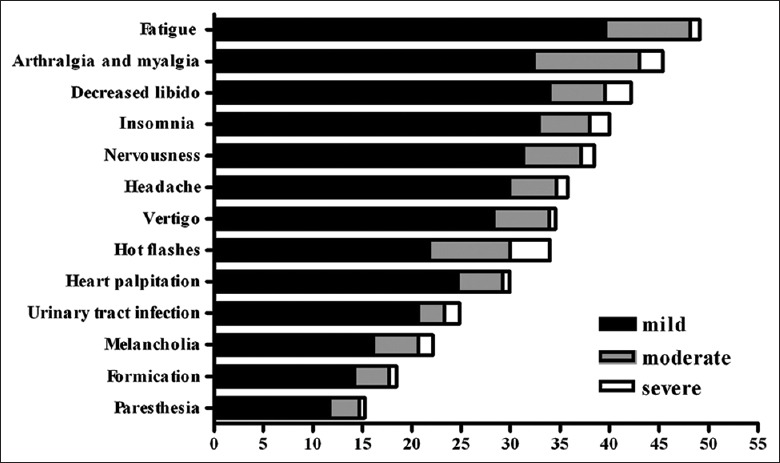
Prevalence of 13 items of modified Kupperman index.
Sleep quality and multivariate analysis of poor sleep
The mean PSQI of the 2308-participant cohort was 5.97 ± 4.30 (range: 0–20) [Table 4]. In a comparison of global PSQI across the four menopausal statuses, the PSQI was lowest in early MT, indicating the best sleep quality. Late MT was associated with good sleep quality. Scores were highest in early PM and late PM, indicating the worst sleep quality (P < 0.001). The sleep quality in the four menopausal statuses was shown in Table 4. We found significant differences in subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, and use of sleep medication but not in daytime dysfunction (P < 0.001). Women in early MT had better sleep quality, shorter sleep latency, lesser sleep disturbance, and lesser sleep medication use than the other menopausal statuses. Participants were divided into poor sleepers and good sleepers according to their global PSQI (≥5 and <5). Of all women 55% (n = 1270) were confirmed as poor sleepers and 45% (n = 1038) were classified as good sleepers [Table 5]. Poor sleepers had a PSQI ranging from 5 to 20, with a mean of 7.8 ± 3.2, which was significantly higher than that of good sleepers (2.9 ± 1.2, P < 0.001).
Table 4.
PSQI score of menopausal status (Mean ± SD)
| PSQI | Early MT | Late MT | Early PM | Late PM | Total |
|---|---|---|---|---|---|
| Global PSQI | 4.90 ± 2.90 | 5.65 ± 3.58 | 6.39 ± 3.88 | 6.58 ± 3.76 | 5.60 ± 3.48 |
| Subjective Sleep quality | 0.90 ± 0.67 | 1.10 ± 0.77 | 1.21 ± 0.76 | 1.25 ± 0.72 | 1.06 ± 0.74 |
| Sleep latency | 0.71 ± 0.77 | 0.89 ± 0.82 | 1.07 ± 0.92 | 1.23 ± 0.98 | 0.89 ± 0.86 |
| Sleep duration | 0.91 ± 0.88 | 0.94 ± 0.93 | 1.12 ± 1.00 | 1.12 ± 0.98 | 0.98 ± 0.94 |
| Sleep efficiency | 0.27 ± 0.64 | 0.37 ± 0.79 | 0.50 ± 0.93 | 0.40 ± 0.81 | 0.36 ± 0.77 |
| Sleep disturbances | 0.81 ± 0.57 | 0.94 ± 0.61 | 1.04 ± 0.60 | 1.04 ± 0.63 | 0.92 ± 0.60 |
| Use of sleeping medication | 0.12 ± 0.44 | 0.21 ± 0.58 | 0.24 ± 0.65 | 0.33 ± 0.73 | 0.19 ± 0.57 |
| Daytime dysfunction | 1.20 ± 0.72 | 1.21 ± 0.75 | 1.22 ± 0.77 | 1.21 ± 0.68 | 1.21 ± 0.74 |
MT: Menopausal transition; PM: Postmenopause; PSQI: Pittsburgh Sleep Quality Index.
Table 5.
Sleep quality of menopausal status
| Status | Good, n (%) | Bad, n (%) | Total (n) |
|---|---|---|---|
| Early MT | 467 (52.0) | 431 (48) | 898 |
| Late MT | 323 (44.5) | 403 (55.5) | 726 |
| Early PM | 166 (38.2) | 269 (61.8) | 435 |
| Late PM | 82 (32.9) | 167 (67.1) | 249 |
| Total | 1038 (45.0) | 1270 (55.0) | 2308 |
MT: Menopausal transition; PM: Postmenopause.
Univariate logistic regression showed that age, marital, family income status, regular exercise, menopausal status were significantly correlated with sleep quality. Of the five predictor variables, three variables entered the final multivariate logistic regression model [Table 6]. This result indicated that the three final variables, which including age, marital, and menopausal statuses, were significantly and independently related with sleep quality.
Table 6.
Related factors of sleep quality in MT and PM women
| Variables | B | SE | Wald | OR | 95% CI | P |
|---|---|---|---|---|---|---|
| Age | 0.207 | 0.061 | 11.636 | 1.230 | 1.092–1.385 | 0.001 |
| Marital | 0.184 | 0.080 | 5.293 | 1.203 | 1.028–1.407 | 0.021 |
| Menopausal status | 0.154 | 0.042 | 13.157 | 1.166 | 1.073–1.267 | 0.000 |
OR: Odds ratio; CI: Confidence interval; SE: Standard error; MT: Menopausal transition; PM: Postmenopause.
DISCUSSION
It has been reported that 68.1–93.7% of menopausal Caucasian women were bothered by at least one of the typical menopausal symptoms mentioned above.[3,4,5,6] In our study, 49.8% of participants experienced various degrees of menopausal symptoms, and the prevalence of mild, moderate, and severe symptoms was 28.9%, 18.5%, and 2.5%, respectively. The prevalence was lower than that reported in the foreign literature, which may be attributed to racial differences. Most previously available information was obtained from Caucasian women, and the sample size of the xanthoderm race was small. Even in the study of SWAN, as a representative study, the sample size of Chinese individuals was only 542. This small sample size may not accurately reflect the health of perimenopausal women in China.
Our study showed that the menopausal symptom severity was mostly mild and moderate, with fewer severe symptoms. This was similar to the findings of Shea.[19] Jokinen et al.[20] also found that only 2% of younger women (42–46 years) and 11% of older women (52–56 years) experienced severe climacteric symptoms. However, a relatively high and severe prevalence of menopausal symptoms among xanthoderm women was reported in the SWAN study. This may be attributed to research methods, evaluation methods, and the target population and population age selection.
Our study showed that among the four menopausal statuses, women in early PM had the highest prevalence of menopausal symptoms, followed by those in late PM, late MT, and early MT. In terms of menopausal symptoms and age distribution, our findings were consistent with those in the literature. Avis et al.[21] analyzed hot flashes, night sweats, and other symptoms among 15,642 perimenopausal women and obtained similar results. They found that menopausal symptoms at this stage were most frequent and severe because of fluctuations and drop in estrogen levels. Symptoms usually lasted for 2–3 years, and for ≥5 years in some cases. In our study, we also found that the prevalence of menopausal symptoms was highest during early PM, whereas fewer clinical symptoms were experienced during early MT.
Menopausal symptoms vary according to race. Gold et al.[7] have shown that the prevalence of symptoms in Chinese and Japanese women was the lowest, vasomotor symptoms were most prominent in African Americans and that the prevalence of sleep difficulties was highest in Caucasians. In our study, the prevalence of each symptom varied from 15% to 49%. The five most common health problems were included fatigue (49%), arthralgia and myalgia (46%), decreased libido (42%), insomnia (40%), and nervousness (39%). However, the prevalence of paresthesia is the lowest (15%). These results indicated that somatic symptoms (muscle and joint pain, fatigue), urogenital tract symptoms (decreased libido), and neuropsychiatric symptoms (insomnia, mood changes) were the main symptoms experienced by perimenopausal women. The prevalence of vasomotor symptoms (hot flashes and night sweats) was not high, which is similar to results reported domestically. Zhang et al.[22] had also suggested that menopausal symptoms in Chinese women mainly manifest as bone and joint pain, memory loss, and easy fatigue, whereas Caucasian women generally experience the typical symptoms known as “climacteric syndrome” such as hot flashes and night sweats.
The difference in menopausal symptoms between Eastern and Western women might be related to race. In a study similar to ours, Kasuga et al.[23] administered a cross-sectional questionnaire to 1069 women aged 40–60 years in 2004 and found the five most prevalent symptoms: limb weakness (88.2%), shoulder stiffness (85.4%), forgetfulness (80.9%), and nervousness (75.9%). On the other hand, menopausal symptoms were found to be closely related to economic conditions, health status, lifestyle, environment, culture, diet and other social factors.[20,24,25,26,27] Therefore, differences between Eastern and Western women in the characteristics of menopausal symptoms may be related to differences in economic status, stress, and other factors. Chedraui et al.[26] also found that muscle and joint pain (77%), depression (74.6%), sexual problems (69.6%), and sleep disorders (45.6%) were the most common symptoms in perimenopausal women in Ecuador. Our study found that the prevalence of hot flashes and sweating was 35%, ranking eighth among 13 symptoms. The prevalence of vasomotor symptoms varied widely among different races. The SWAN study[8] showed that the prevalence of hot flashes among African Americans, Caucasians, Chinese, and Japanese was 45.6%, 31.2%, 20.5%, and 17.6%, respectively. A study conducted in Hawaii also showed that Europeans and Americans were more vulnerable to hot flashes and night sweats than Japanese women.[28] Based on the results of this study, age, exercise, and menopausal status were significantly and independently related with sleep quality.
Sleep disorder is another common problem among perimenopausal and postmenopausal women. The international community generally used the PSQI for evaluation of subjective sleep quality and polysomnography and sleep electroencephalography to evaluate objective sleep quality. Kloss et al.[29] showed that 48% of perimenopausal women had sleep disorders, which was considerably higher than the average prevalence of adult sleep disorders.[30] The study reported that average prevalence of adult sleep disorders was 30–77%. Therefore, sleep disorders have become an important concern affecting menopausal and postmenopausal women's health, and research in this area has recently attracted the attention of domestic and foreign scholars.
Research on sleep disorders in menopausal and postmenopausal women in China had been inadequate. In our study, 55% of all women (n = 1270) were confirmed as poor sleepers, similar to the findings reported by Bromberger, Hsu and Lin.[10,31] Kravitz et al.[11] investigated the sleep quality of 12,603 perimenopausal women including Caucasian, African American, Chinese, Japanese, and Hispanic women. Their results showed that the prevalence of difficulty sleeping in Japanese women was the lowest (28%) and in Caucasian women was the highest (40%). Therefore, ethnicity may contribute to differences in the prevalence of sleep disorders.[32] However, our study showed a higher prevalence of sleep disorders than that reported in Western research, which requires further study. Based on the results of this study, age, marital, and menopausal statuses were significantly and independently related with sleep quality.
In summary, the performance and related factors of menopausal symptoms are various; it is difficult to find an appropriate method to investigate the menopausal symptoms and sleep quality during MT and PM comprehensively. Therefore, some limitations are existed in our study inevitably. One limitation is that we have only reported subject sleep quality lacking of polysomnographic data considering of a large sample of participants and expensive cost of polysomnography. Another limitation is that it was inappropriate to investigate the relationship between KI and PSQI score because insomnia was 1 item of KI, it reflected sleep quality partly, which was confounded with PSQI score in some degree. The third limitation is that our study will be much better if we have a larger sample number and a longer observation time. We will do further research after we obtain more support.
Financial support and sponsorship
The research was supported by grants from the scientific and technological projects of Shanxi Provincial Health Office, China (No. 200918).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yi Cui
REFERENCES
- 1.Makara-Studzinska M, Krys-Noszczyka K, Jakiel G. The influence of selected socio-demographic variables on symptoms occurring during the menopause. Prz Menopauzalny. 2015;14:20–6. doi: 10.5114/pm.2015.48637. doi: 10.5114/pm.2015.48637; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abedi P, Nikkhah P, Najar S. Effect of pedometer-based walking on depression, anxiety and insomnia among postmenopausal women. Climacteric. 2015;18:841–5. doi: 10.3109/13697137.2015.1065246. doi: 10.3109/13697137.2015.1065246. [DOI] [PubMed] [Google Scholar]
- 3.Brown WJ, Mishra GD, Dobson A. Changes in physical symptoms during the menopause transition. Int J Behav Med. 2002;9:53–67. doi: 10.1207/s15327558ijbm0901_04. doi: 10.1207/S15327558IJBM0901_04. [DOI] [PubMed] [Google Scholar]
- 4.Islam MR, Gartoulla P, Bell RJ, Fradkin P, Davis SR. Prevalence of menopausal symptoms in Asian midlife women: A systematic review. Climacteric. 2015;18:157–76. doi: 10.3109/13697137.2014.937689. doi: 10.3109/13697137.2014.937689. [DOI] [PubMed] [Google Scholar]
- 5.AlDughaither A, AlMutairy H, AlAteeq M. Menopausal symptoms and quality of life among Saudi women visiting primary care clinics in Riyadh, Saudi Arabia. Int J Womens Health. 2015;7:645–53. doi: 10.2147/IJWH.S84709. doi: 10.2147/IJWH.S84709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Donato P, Giulini NA, Bacchi Modena A, Cicchetti G, Comitini G, Gentile G, et al. Factors associated with climacteric symptoms in women around menopause attending menopause clinics in Italy. Maturitas. 2005;52:181–9. doi: 10.1016/j.maturitas.2005.01.008. doi: 10.1016/j.maturitas.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Gold EB, Sternfeld B, Kelsey JL, Brown C, Mouton C, Reame N, et al. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40-55 years of age. Am J Epidemiol. 2000;152:463–73. doi: 10.1093/aje/152.5.463. doi: 10.1093/aje/152.5.463. [DOI] [PubMed] [Google Scholar]
- 8.Anderson D, Yoshizawa T, Gollschewski S, Atogami F, Courtney M. Menopause in Australia and Japan: Effects of country of residence on menopausal status and menopausal symptoms. Climacteric. 2004;7:165–74. doi: 10.1080/13697130410001713760. doi: 10.1080/13697130410001713760. [DOI] [PubMed] [Google Scholar]
- 9.Stewart R, Besset A, Bebbington P, Brugha T, Lindesay J, Jenkins R, et al. Insomnia comorbidity and impact and hypnotic use by age group in a national survey population aged 16 to 74 years. Sleep. 2006;29:1391–7. doi: 10.1093/sleep/29.11.1391. doi: 10.1186/s12888-015-0397-x. [DOI] [PubMed] [Google Scholar]
- 10.Kravitz HM, Joffe H. Sleep during the perimenopause: A SWAN story. Obstet Gynecol Clin North Am. 2011;38:567–86. doi: 10.1016/j.ogc.2011.06.002. doi: 10.1016/j.ogc.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kravitz HM, Ganz PA, Bromberger J, Powell LH, Sutton-Tyrrell K, Meyer PM. Sleep difficulty in women at midlife: A community survey of sleep and the menopausal transition. Menopause. 2003;10:19–28. doi: 10.1097/00042192-200310010-00005. doi: 10.1097/00042192-200310010-00005. [DOI] [PubMed] [Google Scholar]
- 12.Zhang S, Wang J, Zhou H, Rao K, Deng X. The investigation and analysis of the factors related with the menopausal age of women in Beijing area. Beijing Med J. 2002;24:177–80. doi: 10.3969/j.issn.0253-9713.2002.03.011. [Google Scholar]
- 13.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, et al. Executive summary of the Stages of Reproductive Aging Workshop +10: Addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97:1159–68. doi: 10.1210/jc.2011-3362. doi: 10.1210/jc.2011-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeyi C. Chinese Obstetrics and Gynecology. People's Republic of China: People's Medical Publishing House. 2005:2537. [Google Scholar]
- 15.Smolinski D, Wollner D, Orlowski J, Curcio J, Nevels J, Kim LS. A pilot study to examine a combination botanical for the treatment of menopausal symptoms. J Altern Complement Med. 2005;11:483–9. doi: 10.1089/acm.2005.11.483. doi: 10.1089/acm.2005.11.483. [DOI] [PubMed] [Google Scholar]
- 16.Tao M, Shao H, Li C, Teng Y. Correlation between the modified Kupperman index and the Menopause Rating Scale in Chinese women. Patient Prefer Adherence. 2013;7:223–9. doi: 10.2147/PPA.S42852. doi: 10.2147/PPA.S42852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang XY, Yang HY, Nie GN, Wen ZH, Wu DR, Zhang CL, et al. Study on the reliability and validity of the Chinese Menopause Rating Scale (CMRS) (in Chinese) Chin J Epidemiol. 2008;29:882–6. doi: 10.3321/j.issn: 0254.6450.2008.09.008. [PubMed] [Google Scholar]
- 18.Liu X, Tang M, Lei H. Reliability and validity of the Pittsburgh Sleep Quality Index (in Chinese) Chin J Psychiatry. 1996;29:103–7. doi: 10.1007/BF02951625. [Google Scholar]
- 19.Shea JL. Chinese women's symptoms: Relation to menopause, age and related attitudes. Climacteric. 2006;9:30–9. doi: 10.1080/13697130500499914. doi: 10.1080/13697130500499914. [DOI] [PubMed] [Google Scholar]
- 20.Jokinen K, Rautava P, Mäkinen J, Ojanlatva A, Sundell J, Helenius H. Experience of climacteric symptoms among 42-46 and 52-56-year-old women. Maturitas. 2003;46:199–205. doi: 10.1016/s0378-5122(03)00216-0. doi: 10.1016/S0378-5122(03)00216-0. [DOI] [PubMed] [Google Scholar]
- 21.Avis NE, Brockwell S, Colvin A. A universal menopausal syndrome? Am J Med. 2005;118(Suppl 12B):37–46. doi: 10.1016/j.amjmed.2005.09.057. doi: 10.1016/j.amjmed.2005.09.057. [DOI] [PubMed] [Google Scholar]
- 22.Zhang QX, Yang DZ, Wang WJ, Liang XY, Xie MQ, Wang LA, et al. The prevalence of climacteric symptoms in Guangzhou with report of 1090 women (in Chinese) Maternal &Child Health Care Chin. 2004;19:95–8. doi: 10.3969/j.issn.1001-4411.2004.21.057. [Google Scholar]
- 23.Kasuga M, Makita K, Ishitani K, Takamatsu K, Watanabe K, Plotnikoff GA, et al. Relation between climacteric symptoms and ovarian hypofunction in middle-aged and older Japanese women. Menopause. 2004;11(6 Pt 1):631–8. doi: 10.1097/01.gme.0000119984.87302.30. doi: 10.1097/01.GME.0000119984.8730;2.30. [DOI] [PubMed] [Google Scholar]
- 24.Reynolds RF, Obermeyer CM. Age at natural menopause in Spain and the United States: Results from the DAMES project. Am J Hum Biol. 2005;17:331–40. doi: 10.1002/ajhb.20121. doi: 10.1002/ajhb.20121. [DOI] [PubMed] [Google Scholar]
- 25.Karaçam Z, Seker SE. Factors associated with menopausal symptoms and their relationship with the quality of life among Turkish women. Maturitas. 2007;58:75–82. doi: 10.1016/j.maturitas.2007.06.004. doi: 10.1016/j.maturitas.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Chedraui P, Aguirre W, Hidalgo L, Fayad L. Assessing menopausal symptoms among healthy middle aged women with the Menopause Rating Scale. Maturitas. 2007;57:271–8. doi: 10.1016/j.maturitas.2007.01.009. doi: 10.1016/j.maturitas.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Lu J, Liu J, Eden J. The experience of menopausal symptoms by Arabic women in Sydney. Climacteric. 2007;10:72–9. doi: 10.1080/13697130601159649. doi: 10.1080/13697130601159649. [DOI] [PubMed] [Google Scholar]
- 28.Sievert LL, Morrison L, Brown DE, Reza AM. Vasomotor symptoms among Japanese-American and European-American women living in Hilo, Hawaii. Menopause. 2007;14:261–9. doi: 10.1097/01.gme.0000233496.13088.24. doi: 10.1097/01.gme.0000233496.13088.24. [DOI] [PubMed] [Google Scholar]
- 29.Kloss JD, Tweedy K, Gilrain K. Psychological factors associated with sleep disturbance among perimenopausal women. Behav Sleep Med. 2004;2:177–90. doi: 10.1207/s15402010bsm0204_1. doi: 10.1207/s15402010bsm0204_1. [DOI] [PubMed] [Google Scholar]
- 30.Polo-Kantola P, Erkkola R. Sleep and the menopause. J Br Menopause Soc. 2004;10:145–50. doi: 10.1258/1362180042721076. doi: 10.1258/1362180042721076. [DOI] [PubMed] [Google Scholar]
- 31.Hsu HC, Lin MH. Exploring quality of sleep and its related factors among menopausal women. J Nurs Res. 2005;13:153–64. doi: 10.1097/01.jnr.0000387536.60760.4e. doi: 10.1097/01.JNR.000038753;6.60760.4e. [DOI] [PubMed] [Google Scholar]
- 32.Tomfohr LM, Schweizer CA, Dimsdale JE, Loredo JS. Psychometric characteristics of the Pittsburgh Sleep Quality Index in English speaking non-Hispanic whites and English and Spanish speaking Hispanics of Mexican descent. J Clin Sleep Med. 2013;9:61–6. doi: 10.5664/jcsm.2342. doi: 10.5664/jcsm.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]


