Abstract
Background:
Prevention of osteonecrosis (ON) has seldom been addressed. The purpose of this study was to evaluate the effect of resveratrol on preventing steroid-induced ON in rabbits.
Methods:
Seventy-two rabbits were divided into four groups: (1) NEC (ON) group: thirty rabbits were treated with lipopolysaccharide (LPS) once, then with methylprednisolone (MPS) daily for 3 days; (2) PRE (prevention) group: thirty rabbits were given one dose of LPS, then MPS daily for 3 days, and resveratrol on day 0 and daily for 2 weeks; (3) RES (resveratrol) group: six rabbits were given resveratrol for 2 weeks but without LPS/MPS; (4) CON (control) group: six rabbits were given alcohol for 2 weeks but without LPS/MPS. Levels of plasma tissue-type plasminogen activator (t-PA), plasminogen activator inhibitor 1 (PAI-1), thrombomodulin (TM), vascular endothelial growth factor (VEGF), maximum enhancement (ME) by magnetic resonance imaging, and ON incidence were evaluated.
Results:
The PRE group had a lower ON incidence than the NEC group, but with no significant differences at 2 weeks and 12 weeks. The RES and CON groups did not develop ON. TM and VEGF were significantly higher in the NEC group compared with the PRE group at weeks 1, 2, and 4 (TM: 1 week, P = 0.029; 2 weeks, P = 0.005; and 4 weeks, P = 0.047; VEGF: 1 week, P = 0.039; 2 weeks, P = 0.021; 4 weeks, P = 0.014), but the difference disappeared at 12 weeks. The levels of t-PA and PAI-1 were not significantly different between the NEC and PRE groups. The TM, t-PA, PAI-1, and VEGF concentrations in the RES and CON groups did not change over time. Compared to the baseline, ME in the NEC group decreased significantly (P = 0.025) at week 1, increased significantly (P = 0.021) at week 2, and was decreased at week 12. The variance was insignificant in the PRE group.
Conclusions:
Resveratrol may improve blood supply to bone in a rabbit model of ON of the femoral head via anti-inflammatory effects to protect the vascular endothelium and reduce thrombosis.
Keywords: Contrast-enhanced Magnetic Resonance Imaging, Dynamic, Femoral Head Necrosis, Osteonecrosis, Resveratrol
INTRODUCTION
Osteonecrosis of the femoral head (ONFH) is a refractory disease, with 75,000–150,000 cases in China.[1] Injury of the vascular endothelium, abnormal coagulation, dyslipidemia, and abnormal bone metabolism may lead to thromboembolism in the femoral head, ultimately resulting in osteonecrosis (ON).[2] Resveratrol has curative and/or preventive effects on coronary artery disease, diabetes mellitus, renal disease, and cancer.[3,4,5] The main mechanisms include its antioxidative properties, improved lipid metabolism and vascular endothelial function, regulation of cytokine production, and/or inhibition of platelet aggregation and adhesion.[6,7,8,9] Prevention of ON has seldom been addressed. We hypothesized that resveratrol may also have protective or curative properties that would affect steroid-induced ON.
METHODS
Animals
Seventy-two healthy New Zealand white rabbits (aged 28 weeks, weighing 2–3 kg) from Beijing Haidian Experimental Animal Farm were enrolled in this study group. The experimental protocol was approved by the Animal Experiment Ethics Committee of the Peking Union Medical College Hospital. All rabbits underwent an adaptive feeding schedule for 1 week and were weighed before each experiment. The rabbits were randomly divided into four groups: (1) NEC (ON) group: thirty rabbits were treated with lipopolysaccharide (LPS) 10 µg/kg intravenously (i.v.) once, then with methylprednisolone (MPS) 20 mg/kg i.v. at 24, 48, and 72 h after the LPS injection. On the same day, LPS was administered, the rabbits were also treated with 8% alcohol 4 mg/kg intraperitoneally (i.p.) every day for 2 weeks; (2) PRE (prevention) group: thirty rabbits were treated with LPS 10 µg/kg i.v. once, then with (MPS) 20 mg/kg i.v. at 24, 48, and 72 h after the LPS injection. On the same day, that LPS was administered, the rabbits were also treated with resveratrol (dissolved in 8% alcohol) 4 mg/kg i.p. every day for 2 weeks; (3) RES (resveratrol) group: six rabbits were treated with the same volume of normal saline instead of LPS and MPS and were given resveratrol i.p. every day for 2 weeks; and (4) CON (control) group: six rabbits were treated with the same volume of normal saline instead of LPS and MPS and were given 8% alcohol 4 mg/kg i.p. every day for 2 weeks.
Rabbits were sacrificed (NEC group, n = 16; PRE group, n = 16; RES group, n = 3; and CON group, n = 3) at 2 weeks and (NEC group, n = 14; PRE group, n = 14; RES group, n = 3; and CON group, n = 3) at 12 weeks after injection with pentobarbital after the last MPS treatment. Bilateral femoral and humeral bones were harvested and preserved in formalin.
Laboratory assessments
Before LPS injection and at 1, 2, 4, and 12 weeks later, plasma tissue-type plasminogen activator (t-PA), plasminogen activator inhibitor-1 (PAI-1), thrombomodulin (TM), and vascular endothelial growth factor (VEGF) were measured by enzyme-linked immunosorbent assays. The bilateral proximal femurs were evaluated by dynamic contrast-enhanced magnetic resonance imaging (MRI, GE Medical System, Fairfield, CT, USA) with a human knee joint coil and the maximum enhancement (ME) was calculated.[10] Table 1 shows MRI scan sequences and parameters.
Table 1.
MRI scan sequences and parameters
| Parameters | AxT2fs | AxT1 | CorT2fs | CorT1 | CorPD | FSPGRT1 |
|---|---|---|---|---|---|---|
| TE (ms) | 74 | 20 | 74 | 20 | 35.8 | Minimum |
| TR (ms) | 2500 | 420 | 2500 | 420 | 1970 | 18.7 |
| Matrix | 256×192 | 256×192 | 256×192 | 256×192 | 256×192 | 128×64 |
| NEX | 4 | 4 | 4 | 4 | 4 | 2 |
| FOV (cm) | 12 | 12 | 14 | 14 | 14 | 14 |
| Thickness (mm) | 3 | 3 | 2 | 2 | 2 | 2 |
| Spacing | 1 | 1 | 0 | 0 | 0 | 0 |
| ETL | 21 | 2 | 21 | 2 | 10 | – |
| Scan time (min) | 9/1:45 | 9/2:43 | 9/1:45 | 9/2:43 | 9/2:41 | 2/0.05 |
| Bandwidth (kHz) | 31.25 | 31.25 | 31.25 | 31.25 | 16.67 | Flip angle 80 16.67 |
AxT2fs: Axial T2 weighted fat-saturated imaging; AxT1: Axial T1 weighted imaging; CorT2fs: Coronal T2 weighted fat-saturated imaging; CorT1: Coronal T1 weighted imaging; CorPD: Coronal proton density weighted imaging; FSPGRT1: Fast spoiled gradient-recalled T1 weighted imaging; TE: Echo time; TR: Repetition time; NEX: Number of excitations; FOV: Field of view; ETL: Echo train length; –: None.
Histological and immunohistochemical assessment
The proximal third of the femur and humerus were harvested, embedded in paraffin, and sectioned to 6-µm-thick sections. Sections were stained with hematoxylin and eosin (H&E) to evaluate ON. Characteristic histopathological features of ON were defined as a diffuse presence of lacunae or pyknotic nuclei of osteocytes in the trabeculae accompanied by surrounding necrotic bone marrow, adipocyte hypertrophy, and vascular thrombosis. All rabbits that had at least one osteonecrotic lesion in the examined sections were judged to be ON+. Those with no osteonecrotic lesions were deemed ON−. The incidence of ON was defined as the number of ON+ rabbits divided by the total number of rabbits. Immunohistochemical staining was performed using a streptavidin-peroxidase method. Vascular endothelial cells and bone marrow cells containing brown granules and stained darker than the background were defined as positive.[11]
Statistical analysis
Categorical variables were analyzed by Fisher's exact probability test. Numerical variables were expressed as a mean ± standard deviation (SD). Independent samples t-test was used to compare intergroup differences. The paired t-test was applied to compare intragroup differences at the same time point. A P < 0.05 was statistically significant. SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis.
RESULTS
Incidence of osteonecrosis
After 2 weeks of MPS treatment, the ON incidence was 75.0% (12/16) in the NEC group and 43.8% (7/16) in the PRE group but was not significantly different (P = 0.074). One rabbit in the NEC group died after the third MPS injection.
At 12 weeks after MPS administration, the ON incidence was 42.9% (6/14) in the NEC group and 28.6% (4/14) in the PRE group but was not significantly different (P = 0.347). Two rabbits in the NEC group and three in the PRE group died after injection of pentobarbital, MPS, or LPS. There was no ON or death in the RES or CON groups at 2 weeks or 12 weeks after MPS administration.
Laboratory results
At 1 week after MPS injection, the plasma TM concentration was significantly increased in the NEC and PRE groups but then decreased to pre-LPS injection levels. At 1, 2, and 4 weeks after MPS injection, the plasma TM concentration was significantly higher in the NEC group than in the PRE group (P < 0.05). However, at 12 weeks, there was no significant difference between the two groups (P > 0.05) [Table 2].
Table 2.
Plasma TM concentration at different time in NEC group and PRE group (ng/ml), mean ± SD
| Time points | NEC group | PRE group | t | P |
|---|---|---|---|---|
| Baseline | 18.06 ± 3.24 | 17.06 ± 1.48 | 1.004 | 0.330 |
| 1 week | 28.73 ± 3.02 | 24.80 ± 3.18 | 2.451 | 0.029 |
| 2 weeks | 23.15 ± 5.65 | 17.59 ± 2.42 | 3.259 | 0.005 |
| 4 weeks | 23.88 ± 8.51 | 15.41 ± 2.89 | 2.305 | 0.047 |
| 12 weeks | 21.59 ± 7.49 | 16.17 ± 2.40 | 1.552 | 0.185 |
NEC group: Osteonecrosis group; PRE group: Prevention group; TM: Thrombomodulin; SD: Standard deviation.
The plasma t-PA and PAI-1 concentrations showed variable changes in the NEC and PRE groups. The t-PA/PAI-1 ratio was lower than that before LPS injection [Tables 3 and 4].
Table 3.
Plasma t-PA concentration at different time in NEC group and PRE group (ng/ml), mean ± SD
| Time points | NEC group | PRE group | t | P |
|---|---|---|---|---|
| Baseline | 40.19 ± 40.78 | 23.84 ± 35.08 | 1.096 | 0.284 |
| 1 week | 29.77 ± 33.10 | 30.52 ± 26.03 | −0.480 | 0.962 |
| 2 weeks | 10.38 ± 10.66 | 28.60 ± 43.03 | −1.482 | 0.151 |
| 4 weeks | 26.35 ± 15.42 | 18.98 ± 35.94 | 0.424 | 0.681 |
| 12 weeks | 4.30 ± 3.74 | 3.52 ± 2.70 | 0.403 | 0.696 |
NEC group: Osteonecrosis group; PRE group: Prevention group; t-PA: Tissue-type plasminogen activator; SD: Standard deviation.
Table 4.
Plasma PAI-1 concentration at different time in NEC group and PRE group (ng/ml), mean ± SD
| Time points | NEC group | PRE group | t | P |
|---|---|---|---|---|
| Baseline | 6.74 ± 3.56 | 7.60 ± 3.70 | −0.599 | 0.555 |
| 1 week | 9.78 ± 4.10 | 5.42 ± 1.48 | 2.668 | 0.019 |
| 2 weeks | 8.20 ± 3.92 | 6.86 ± 2.50 | 1.036 | 0.311 |
| 4 weeks | 6.66 ± 2.34 | 9.86 ± 4.92 | −1.324 | 0.218 |
| 12 weeks | 8.14 ± 1.40 | 6.80 ± 2.48 | 1.082 | 0.307 |
NEC group: Osteonecrosis group; PRE group: Prevention group; PAI-1: Plasminogen activator inhibitor 1; SD: Standard deviation.
Plasma VEGF increased after MPS injection in the NEC and PRE groups and reached a peak at 2 weeks, which then decreased to baseline levels at 12 weeks. The VEGF concentration was significantly higher in the NEC group than in the PRE group at 1, 2, and 4 weeks after MPS injection (P < 0.05), but the difference had disappeared at 12 weeks (P > 0.05) [Table 5].
Table 5.
Plasma VEGF concentration at different time in NEC group and PRE group (ng/ml), mean ± SD
| Time points | NEC group | PRE group | t | P |
|---|---|---|---|---|
| Baseline | 73.73 ± 15.54 | 76.72 ± 16.37 | –0.476 | 0.638 |
| 1 week | 128.82 ± 12.73 | 110.51 ± 18.06 | 2.295 | 0.039 |
| 2 weeks | 155.00 ± 14.23 | 134.13 ± 26.23 | 2.522 | 0.021 |
| 4 weeks | 118.70 ± 7.89 | 101.97 ± 9.86 | 3.056 | 0.014 |
| 12 weeks | 83.92 ± 20.08 | 70.67 ± 11.78 | 1.367 | 0.205 |
NEC group: Osteonecrosis group; PRE group: Prevention group; VEGF: Vascular endothelial growth factor; SD: Standard deviation.
The plasma TM, t-PA, PAI-1, and VEGF concentrations in the RES and CON groups showed no change at the different time points studied [Tables 6–9].
Table 6.
Plasma TM concentration at different time in RES group and CON group (ng/ml), mean ± SD
| Time points | RES group | CON group | t | P |
|---|---|---|---|---|
| Baseline | 18.71 ± 0.41 | 18.31 ± 0.53 | 1.380 | 0.314 |
| 1 week | 19.19 ± 0.56 | 19.16 ± 1.06 | 0.048 | 0.399 |
| 2 weeks | 18.30 ± 1.18 | 17.00 ± 1.73 | 1.419 | 0.643 |
| 4 weeks | 18.75 ± 0.56 | 18.26 ± 0.79 | 1.137 | 0.382 |
| 12 weeks | 18.16 ± 0.97 | 17.63 ± 1.09 | 0.805 | 0.908 |
RES group: Resveratrol group; CON group: Control group; TM: Thrombomodulin; SD: Standard deviation.
Table 9.
Plasma VEGF concentration at different time in RES group and CON group (ng/ml), mean ± SD
| Time points | RES group | CON group | t | P |
|---|---|---|---|---|
| Baseline | 75.25 ± 1.48 | 76.06 ± 3.14 | −0.527 | 0.098 |
| 1 week | 76.80 ± 2.17 | 75.60 ± 4.51 | 0.537 | 0.309 |
| 2 weeks | 77.26 ± 3.14 | 75.20 ± 2.05 | 1.231 | 0.177 |
| 4 weeks | 76.60 ± 2.19 | 74.20 ± 4.76 | 1.023 | 0.240 |
| 12 weeks | 76.06 ± 3.14 | 75.60 ± 5.03 | 0.175 | 0.562 |
RES group: Resveratrol group; CON group: Control group; VEGF: Vascular endothelial growth factor; SD: Standard deviation.
Table 7.
Plasma t-PA concentration at different time in RES group and CON group (ng/ml), mean± SD
| Time points | RES group | CON group | t | P |
|---|---|---|---|---|
| Baseline | 39.37 ± 1.22 | 34.96 ± 2.85 | 1.317 | 0.230 |
| 1 week | 37.60 ± 1.82 | 37.06 ± 1.23 | 0.550 | 0.181 |
| 2 weeks | 37.96 ± 1.53 | 37.58 ± 1.19 | 0.439 | 0.488 |
| 4 weeks | 38.02 ± 1.61 | 38.46 ± 1.06 | –0.509 | 0.255 |
| 12 weeks | 39.14 ± 1.11 | 39.06 ± 1.18 | 0.110 | 0.923 |
RES group: Resveratrol group; CON group: Control group; t-PA: Tissue-type plasminogen activator; SD: Standard deviation.
Table 8.
Plasma PAI-1 concentration at different time in RES group and CON group (ng/ml), mean ± SD
| Time points | RES group | CON group | t | P |
|---|---|---|---|---|
| Baseline | 7.99 ± 0.58 | 7.80 ± 0.55 | 0.543 | 0.974 |
| 1 week | 7.58 ± 0.99 | 8.24 ± 0.53 | –1.320 | 0.301 |
| 2 weeks | 8.08 ± 0.42 | 7.40 ± 0.49 | 1.463 | 0.432 |
| 4 weeks | 8.28 ± 0.47 | 8.40 ± 0.44 | –0.418 | 0.933 |
| 12 weeks | 7.92 ± 0.58 | 8.04 ± 0.22 | –0.430 | 0.138 |
RES group: Resveratrol group; CON group: Control group; PAI-1: Plasminogen activator inhibitor 1; SD: Standard deviation.
Dynamic contrast-enhanced magnetic resonance imaging results
MRI showed evenly distributed signals in normal bone, and cystic changes in ON bones [Figure 1a and 1b]. In the NEC group, ME was significantly decreased at 1 week after MPS administration, significantly increased at 2 weeks, and decreased at 12 weeks. ME did not change significantly in the PRE group [Figure 2].
Figure 1.
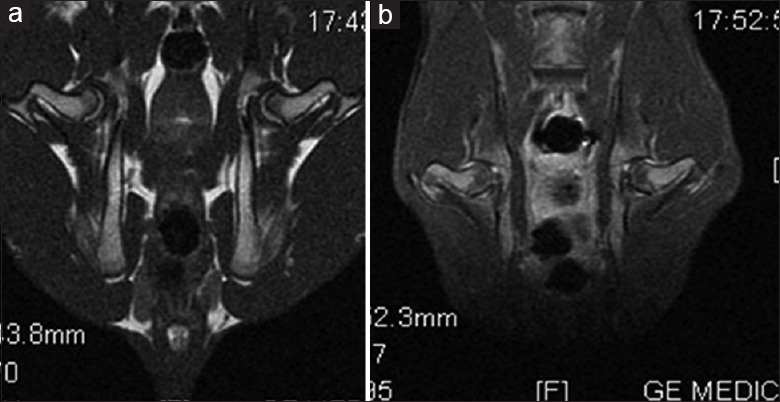
Magnetic resonance imaging appearance of normal and osteonecrosis specimen. (a) Normal femoral bone with evenly distributed signal in both sides. (b) Cystic change in the bilateral femoral head indicated osteonecrosis.
Figure 2.
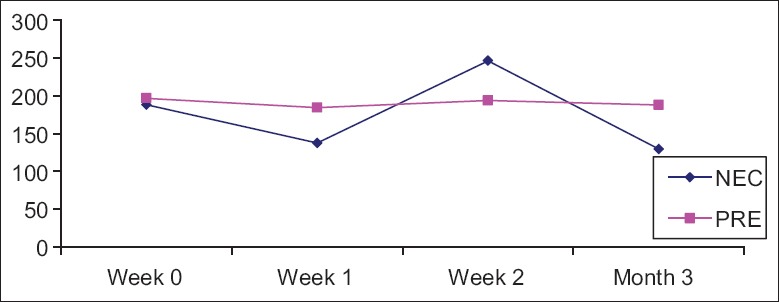
Maximum enhancement results in NEC group and PRE group at different time. NEC group: Osteonecrosis group; PRE group: Prevention group.
Histological results
ON+ specimens with H&E stainings showed that osteocyte lacunae or pyknotic nuclei were present in the trabeculae accompanied by surrounding necrotic bone marrow, adipocyte hypertrophy, and vascular thrombosis. Some specimens also had dead bone, a disappearance of osteoblasts, and/or fibrous tissue and capillary vessel hyperplasia [Figure 3].
Figure 3.
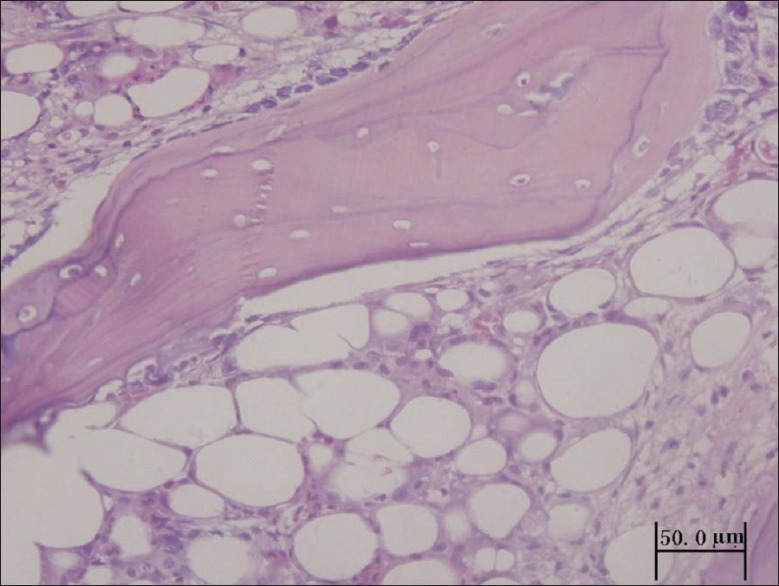
ON+ specimens (hematoxylin and eosin staining). There were lots of osteocyte lacunae in the trabeculae, disappearance of osteoblast, adipocyte hypertrophy, and tissue repair.
Tissue factor (TF) and VEGF staining were positive (++) or strongly positive (+++) in the ON+ specimens [Figure 4a and 4b] but were negative (−), weakly positive (+), or positive (++) in ON− specimens [Figure 4c and 4d]. TF and VEGF staining were positive (++) or strongly positive (+++) in endothelial and bone marrow cells of the NEC group but weakly positive (+) or positive (++) in the PRE group.
Figure 4.
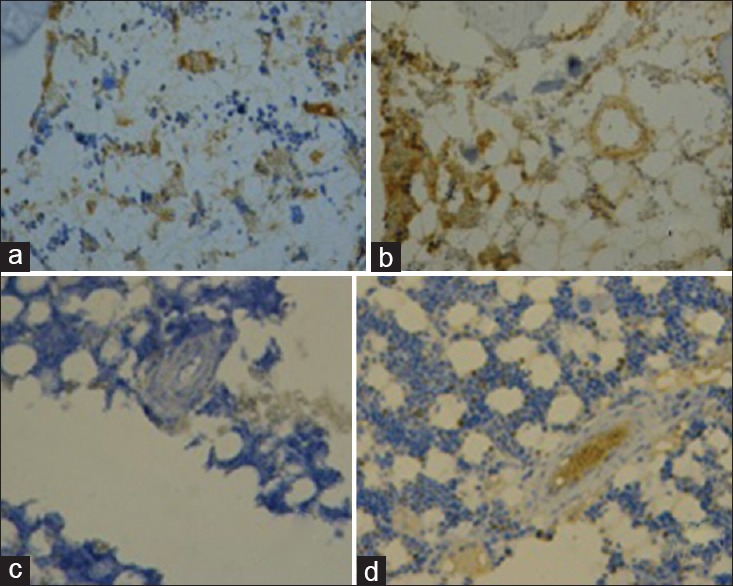
Immunohistochemical staining in the ON+ and ON- specimens. (a) Tissue factor staining in ON+ specimens (×200). Bone marrow laden with multiple brown granules indicated positive (++). (b) Vascular endothelial growth factor staining in ON+ specimens (×80). Multiple brown granules laden in bone marrow and endothelial cells indicated strongly positive (+++). (c) Tissue factor staining in ON- specimens (×200). Negative immunohistochemical staining presented with only background staining. (d) Vascular endothelial growth factor staining in ON- specimens (×80). Bone marrow and vascular endothelial cells stained background only indicated negative (-). ON: Osteonecrosis.
DISCUSSION
Steroid-associated ONFH is the main cause of nontraumatic ON. Numerous studies have attempted to establish an animal model of steroid-associated ON. A previous study showed that a small dose of LPS combined with high-dose MPS produced a high rate of ON with a low mortality rate.[12] In the current study, we modified the method of Qin et al.[12] by administering a single i.v. injection of LPS, then three doses of MPS. The ON incidence was 75.0% at 2 weeks in the NEC group, with a mortality rate of 6.3% (1/16), suggesting that this method successfully induced steroid-associated ON. Resveratrol was diluted in 8% alcohol, and Con groups received an i.p. injection of 8% alcohol alone. Considering the small volume and low concentration of alcohol and that ON did not occur in the RES or CON groups, we believe that ON was induced by the LPS/MPS combination and that the alcohol had no effect.
In H&E-stained ON+ specimens, lacunae and/or pyknotic nuclei were observed in osteocytes in the trabeculae. This appearance was accompanied by surrounding necrotic bone marrow, adipocyte hypertrophy, and vascular thrombosis. Some rabbits also had a repair response such as fibrous tissue and capillary vessel hyperplasia.[12,13] All of these characteristics are similar to those of ON in humans, indicating this model will be useful for medical research to prevent steroid-associated ON. The incidence of ON in the NEC group at 12 weeks was lower than that at 2 weeks, which could be related to the repair process.[14]
Although the mechanism of ONFH is not fully understood, a consensus was recently reached to accept that endothelial cell injury, intravascular thrombosis, and vascularization insufficiency contribute to its development.[15] TM is a glycoprotein expressed mainly on endothelial cell membrane and is an excellent biomarker of endothelial cell injury. In addition to protein C, it has a role in counteracting hypercoagulation and hypofibrinolysis after endothelial cell injury, thereby maintaining a balance between coagulation and fibrinolysis. In this study, after 1 week of i.v. MPS, the TM level was significantly increased in the NEC and PRE groups. However, TM levels were significantly lower in the PRE group than in the NEC group at 1, 2, and 4 weeks, indicating a reduction of LPS/MPS-induced endothelial cell injury. Possible mechanisms include inhibiting the release of tumor necrosis factor-α (TNF-α) and liposomes, stabilizing the liposomal membrane and prohibiting cyclooxygenase and nuclear factor-κB (NF-κB) activation, thereby reducing cytokine formation and tissue damage.[16,17,18]
TF is a cell membrane glycoprotein and a type 2 cytokine receptor and is the main factor in initiating the coagulation cascade as well as being a biomarker of endothelial cell injury. TF is up-regulated after endothelial damage and initiates the coagulation process. Resveratrol dose-dependently inhibited endothelium and monocyte NF-κB transcription to reduce TF production. Resveratrol was also shown to reduce LPS-, interleukin-1β-, and/or TNF-α-induced TF mRNA expression.[8,9,19] Furthermore, resveratrol treatment protected cultured endothelial cells against increases in the expression of TNF-α and attenuated inflammatory injury of the vascular wall.[20] Resveratrol treatment also significantly enhanced baboon arterial endothelial cell proliferation and attenuated the TNF-α-induced impairment of proliferation at optimal doses of 1–50 µmol/L. The authors of that study recommended resveratrol should be considered a candidate drug for the prevention and treatment of inflammatory vascular diseases.[21] In this study, TF expression was positive (++) or strongly positive (+++) in the NEC group but weakly positive (+) or positive (++) in the PRE group, indicating that resveratrol reduced TF production and thrombosis formation.
t-PA and PAI-1 levels are controlled by a dynamic balance, and disturbance of the balance leads to thrombosis formation. ON was significantly associated with elevated levels of PAI-1 activity and elevated PAI-1/t-PA ratios. In the sera of patients with ONFH, the levels of PAI-1 were increased, whereas t-PA levels were significantly decreased. The stimulation of PAI-1 and the inhibition of t-PA inhibited the fibrinolytic system leading to increased coagulation.[22,23,24] In a triple-blind, randomized, placebo-controlled, 1-year follow-up trial, resveratrol prevented an increase in PAI-1 and inhibited atherothrombotic signals in peripheral blood mononuclear cells.[23] In the current study, the t-PA and PAI-1 levels fluctuated in the NEC and PRE groups, but the t-PA/PAI-1 ratio was consistently lower than at baseline. There was no significant difference between the NEC and PRE groups, indicating an absence of sufficient fibrinolysis and a tendency toward thrombosis development. It also suggested that a single resveratrol dose of 4 mg/kg had a limited effect on t-PA and PAI-1 levels. Increasing the dosage of resveratrol might improve its function, but whether it could reduce the risk of thrombosis needs further study.
Dynamic contrast-enhanced MRI can reflect the extent of vascularized tissue. ME declined in the presence of insufficient vascularization and poor tissue perfusion.[25] In this study, ME was significantly reduced at week 1 in the NEC group but did not vary significantly in the PRE group, indicating that resveratrol improved vascularization and blood supply. VEGF is an important signaling protein for angiogenesis under hypoxic conditions.[15] Tissue ischemia or hypoxia-induced local increases in VEGF expression to promote neovascularization and relieve local tissue ischemia or hypoxia, indicating that VEGF is an important signaling protein for physiologic and pathologic angiogenesis under hypoxic conditions.[26,27,28] Consistent with ME changes by dynamic contrast-enhanced MRI, plasma VEGF concentrations in the PRE group were significantly lower than in the NEC group at weeks 1, 2, and 4. In addition, VEGF staining was positive or strongly positive in the NEC group but weakly positive or positive in the PRE group, further indicating that resveratrol protected the endothelium from MPS-induced damage and suggests it should improve local blood flow and reduce tissue hypoxia.
This study used serology, dynamic enhanced MRI, and immunohistochemical studies to show that resveratrol improved local blood flow through its anti-inflammatory effect, protection of endothelium, and reduction of thrombosis formation, although there was no significant difference in the incidence of ON between the NEC and PRE groups at week 2 and week 12. The reasons for this might be explained by: (1) a repair reaction in ON bone; (2) the lack of clarity as to whether the timing and dosage of resveratrol applied in this study are appropriate and therefore requires further study. Increasing the dosage of icaritin further reduced the incidence of steroid-associated ON.[15,29] Resveratrol and icaritin are both polyphenols, although resveratrol has a stronger bioactivity. Therefore, increasing the resveratrol dosage might further reduce the incidence of ON; (3) the number of rabbits in this study was relatively small, and some rabbits died in some groups, further reducing the group number. The difference in ON incidence between the NEC and PRE groups might be found to be exaggerated if more rabbits were included in this study. This study demonstrated that the effects of resveratrol on reducing the incidence of ON require further research.
The PRE group had a lower ON incidence than the NEC group, but with no significant differences at 2 weeks and 12 weeks. The RES and CON groups did not develop ON. TM and VEGF were significantly higher in the NEC group compared with the PRE group at weeks 1, 2, and 4, but the difference disappeared at 12 weeks. Resveratrol may improve blood supply to bone in a rabbit model of ON of the femoral head via anti-inflammatory effects to protect the vascular endothelium and reduce thrombosis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We thank Dr. Qian-Yu Zhuang from Department of Orthopaedic Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College for English editing.
Footnotes
Edited by: Xiu-Yuan Hao and Xin Chen
REFERENCES
- 1.Wei S. Etiology, pathology and pathogenesis of osteonecrosis. Chin J Gen Pract. 2006;2:75–7. [Google Scholar]
- 2.Assouline-Dayan Y, Chang C, Greenspan A, Shoenfeld Y, Gershwin ME. Pathogenesis and natural history of osteonecrosis. Semin Arthritis Rheum. 2002;32:94–124. [PubMed] [Google Scholar]
- 3.Ogas T, Kondratyuk TP, Pezzuto JM. Resveratrol analogs: Promising chemopreventive agents. Ann N Y Acad Sci. 2013;1290:21–9. doi: 10.1111/nyas.12196. doi: 10.1111/nyas.12196. [DOI] [PubMed] [Google Scholar]
- 4.Cottart CH, Nivet-Antoine V, Beaudeux JL. Review of recent data on the metabolism, biological effects, and toxicity of resveratrol in humans. Mol Nutr Food Res. 2014;58:7–21. doi: 10.1002/mnfr.201200589. doi: 10.1002/mnfr.201200589. [DOI] [PubMed] [Google Scholar]
- 5.Zhao K. Biological features and effects of resveratrol. Chin J Pathophysiol. 2012;28:1709–11. [Google Scholar]
- 6.Harikumar KB, Aggarwal BB. Resveratrol: A multitargeted agent for age-associated chronic diseases. Cell Cycle. 2008;7:1020–35. doi: 10.4161/cc.7.8.5740. [DOI] [PubMed] [Google Scholar]
- 7.Zhong C, Liu XH, Chang J, Yu JM, Sun X. Inhibitory effect of resveratrol dimerized derivatives on nitric oxide production in lipopolysaccharide-induced RAW 264.7 cells. Bioorg Med Chem Lett. 2013;23:4413–8. doi: 10.1016/j.bmcl.2013.05.058. doi: 10.1016/j.bmcl.2013.05.058. [DOI] [PubMed] [Google Scholar]
- 8.Andrade JM, Paraíso AF, de Oliveira MV, Martins AM, Neto JF, Guimarães AL, et al. Resveratrol attenuates hepatic steatosis in high-fat fed mice by decreasing lipogenesis and inflammation. Nutrition. 2014;30:915–9. doi: 10.1016/j.nut.2013.11.016. doi: 10.1016/j.nut.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Yi L, Xiang Z, Zhong J, Zhang H, Sun T. Resveratrol attenuates spinal cord injury-induced inflammatory damage in rat lungs. Int J Clin Exp Pathol. 2015;8:1237–46. [PMC free article] [PubMed] [Google Scholar]
- 10.Qin L, Zhang G, Sheng H, Yeung K, Yeung H, Chan C, et al. Multiple bioimaging modalities in evaluation of an experimental osteonecrosis model induced by a combination of lipopolysaccharide and methylprednisolone (in Chinese) Chin J Reparat Reconstruct Surg. 2008;22:258–64. [PubMed] [Google Scholar]
- 11.Zhang D, Zhang J, Wang P, He J, Liu Y. Expression and clinical significance of C-erbB2, PCNA, ER, PR in 126 cases of breast carcinoma. Mod Oncol. 2009;17:1874–7. [Google Scholar]
- 12.Qin L, Zhang G, Sheng H, Yeung KW, Yeung HY, Chan CW, et al. Multiple bioimaging modalities in evaluation of an experimental osteonecrosis induced by a combination of lipopolysaccharide and methylprednisolone. Bone. 2006;39:863–71. doi: 10.1016/j.bone.2006.04.018. doi: 10.1016/j.bone.2006.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu X, Yang S, Duan D, Zhang Y, Wang J. Experimental osteonecrosis induced by a combination of low-dose lipopolysaccharide and high-dose methylprednisolone in rabbits. Joint Bone Spine. 2008;75:573–8. doi: 10.1016/j.jbspin.2007.11.004. doi: 10.1016/j.jbspin.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Wang KZ, Wang CS, Wu YG, Chen H. Changes of vessel in steroid-induced osteonecrosis of femoral head: Experimental study of rabbits (in Chinese) Natl Med J China. 2006;86:2024–7. [PubMed] [Google Scholar]
- 15.Zhang G, Qin L, Sheng H, Wang XL, Wang YX, Yeung DK, et al. Anovel semisynthesized small molecule icaritin reduces incidence of steroid-associated osteonecrosis with inhibition of both thrombosis and lipid-deposition in a dose-dependent manner. Bone. 2009;44:345–56. doi: 10.1016/j.bone.2008.10.035. doi: 10.1016/j.bone.2008.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mobasheri A, Shakibaei M. Osteogenic effects of resveratrol in vitro: Potential for the prevention and treatment of osteoporosis. Ann N Y Acad Sci. 2013;1290:59–66. doi: 10.1111/nyas.12145. doi: 10.1111/nyas.12145. [DOI] [PubMed] [Google Scholar]
- 17.Ye J, Liu Z, Wei J, Lu L, Huang Y, Luo L, et al. Protective effect of SIRT1 on toxicity of microglial-derived factors induced by LPS to PC12 cells via the p53-caspase-3-dependent apoptotic pathway. Neurosci Lett. 2013;553:72–7. doi: 10.1016/j.neulet.2013.08.020. doi: 10.1016/j.neulet.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 18.Kwon YJ. Resveratrol has anabolic effects on disc degeneration in a rabbit model. J Korean Med Sci. 2013;28:939–45. doi: 10.3346/jkms.2013.28.6.939. doi: 10.3346/jkms.2013.28.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lakota K, Mrak-Poljsak K, Rozman B, Sodin-Semrl S. Increased responsiveness of human coronary artery endothelial cells in inflammation and coagulation. Mediators Inflamm 2009. 2009 doi: 10.1155/2009/146872. 146872. doi: 10.1155/2009/146872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng X, Zhu S, Chang S, Cao Y, Dong J, Li J, et al. Protective effects of chronic resveratrol treatment on vascular inflammatory injury in steptozotocin-induced type 2 diabetic rats: Role of NF-kappa B signaling. Eur J Pharmacol. 2013;720:147–57. [PubMed] [Google Scholar]
- 21.Xiao J, Song J, Hodara V, Ford A, Wang XL, Shi Q, et al. Protective effects of resveratrol on TNF-a-induced endothelial cytotoxicity in baboon femoral arterial endothelial cells. J Diabetes Res 2013. 2013:185172. doi: 10.1155/2013/185172. doi: 110.1155/2013/185172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan X, Cai D, Wu Y, Liu B, Rong L, Chen Z, et al. Comparative analysis of serum proteomes: Discovery of proteins associated with osteonecrosis of the femoral head. Transl Res. 2006;148:114–9. doi: 10.1016/j.trsl.2006.05.001. doi: 10.1016/j.trsl.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Tomé-Carneiro J, Gonzálvez M, Larrosa M, Yáñez-Gascón MJ, García-Almagro FJ, Ruiz-Ros JA, et al. Grape resveratrol increases serum adiponectin and downregulates inflammatory genes in peripheral blood mononuclear cells: A triple-blind, placebo-controlled, one-year clinical trial in patients with stable coronary artery disease. Cardiovasc Drugs Ther. 2013;27:37–48. doi: 10.1007/s10557-012-6427-8. doi: 10.1007/s10557-012-6427-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zagotta I, Dimova EY, Funcke JB, Wabitsch M, Kietzmann T, Fischer-Posovszky P. Resveratrol suppresses PAI-1 gene expression in a human in vitro model of inflamed adipose tissue. Oxid Med Cell Longev 2013. 2013 doi: 10.1155/2013/793525. 793525. doi: 10.1155/2013/793525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheng H, Zhang G, Wang YX, Yeung DK, Griffith JF, Leung KS, et al. Functional perfusion MRI predicts later occurrence of steroid-associated osteonecrosis: An experimental study in rabbits. J Orthop Res. 2009;27:742–7. doi: 10.1002/jor.20765. doi: 10.1002/jor.20765. [DOI] [PubMed] [Google Scholar]
- 26.Zhang G, Sheng H, He YX, Xie XH, Wang YX, Lee KM, et al. Continuous occurrence of both insufficient neovascularization and elevated vascular permeability in rabbit proximal femur during inadequate repair of steroid-associated osteonecrotic lesions. Arthritis Rheum. 2009;60:2966–77. doi: 10.1002/art.24847. doi: 10.1002/art.24847. [DOI] [PubMed] [Google Scholar]
- 27.Cullberg KB, Olholm J, Paulsen SK, Foldager CB, Lind M, Richelsen B, et al. Resveratrol has inhibitory effects on the hypoxia-induced inflammation and angiogenesis in human adipose tissue in vitro. Eur J Pharm Sci. 2013;49:251–7. doi: 10.1016/j.ejps.2013.02.014. doi: 10.1016/j.ejps.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 28.Zhang M, Li W, Yu L, Wu S. The suppressive effect of resveratrol on HIF-1a and VEGF expression after warm ischemia and reperfusion in rat liver. PLoS One. 2014;9:e109589. doi: 10.1371/journal.pone.0109589. doi: 10.1371/journal.pone.0109589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang G, Wang XL, Sheng H, Xie XH, He YX, Yao XS, et al. Constitutional flavonoids derived from Epimedium dose-dependently reduce incidence of steroid-associated osteonecrosis not via direct action by themselves on potential cellular targets. PLoS One. 2009;4:e6419. doi: 10.1371/journal.pone.0006419. doi: 10.1371/journal.pone.0006419. [DOI] [PMC free article] [PubMed] [Google Scholar]


