Abstract
Background:
Aspermia caused by exogenous testosterone limit its usage in late-onset hypogonadism (LOH) patients desiring fertility. Saikokaryukotsuboreito (SKRBT) is reported to improve serum testosterone and relieve LOH-related symptoms. However, it is unclear whether SKRBT affects fertility. We aimed to examine the effects of SKRBT on spermatogenesis and fertility in aging male mice.
Methods:
Thirty aging male mice were randomly assigned to three groups. Mice were orally administered with phosphate-buffer solution or SKRBT (300 mg/kg, daily) or received testosterone by subcutaneous injections (10 mg/kg, every 3 days). Thirty days later, each male mouse was mated with two female mice. All animals were sacrificed at the end of 90 days. Intratesticular testosterone (ITT) levels, quality of sperm, expression of synaptonemal complex protein 3 (SYCP3), and fertility were assayed.
Results:
In the SKRBT-treated group, ITT, quality of sperm, and expression of SYCP3 were all improved compared with the control group (ITT: 85.50 ± 12.31 ng/g vs. 74.10 ± 11.45 ng/g, P = 0.027; sperm number: [14.94 ± 4.63] × 106 cells/ml vs. [8.79 ± 4.38] × 106 cells/ml, P = 0.002; sperm motility: 43.16 ± 9.93% vs. 33.51 ± 6.98%, P = 0.015; the number of SYCP3-positive cells/tubule: 77.50 ± 11.01 ng/ml vs. 49.30 ± 8.73 ng/ml, P < 0.001; the expression of SYCP3 protein: 1.23 ± 0.09 vs. 0.84 ± 0.10, P < 0.001), but fertility was not significantly changed (P > 0.05, respectively). In the testosterone-treated group, ITT, quality of sperm, and expression of SYCP3 were markedly lower than the control group (ITT: 59.00 ± 8.67, P = 0.005; sperm number: [4.34 ± 2.45] × 106 cells/ml, P = 0.018; sperm motility: 19.53 ± 7.69%, P = 0.001; the number of SYCP3-positive cells/tubule: 30.00 ± 11.28, P < 0.001; the percentage of SYCP3-positive tubules/section 71.98 ± 8.88%, P = 0.001; the expression of SYCP3 protein: 0.71 ± 0.09, P < 0.001), and fertility was also suppressed (P < 0.05, respectively).
Conclusion:
SKRBT had no adverse effect on fertility potential in aging male mice.
Keywords: Late-onset Hypogonadism, Male Mice, Saikokaryukotsuboreito, Spermatogenesis, Testosterone Synthesis
INTRODUCTION
It has been reported that testicular testosterone synthesis declines with age in males by 1–2%/year after the age of 40 years.[1,2] Age-related decline in testosterone levels in men is associated with many clinical symptoms, such as decreased bone mass and muscle mass, obesity, metabolic disorder, type 2 diabetes, depression, fatigue and sexual dysfunction.[3] The term of late-onset hypogonadism (LOH), which was once described as partial androgen deficiency of the aging male, and ropause and male menopause, was first used by Morales and Lunenfeld in 2002,[4] and was thought to be the best term to describe the nature of these clinical symptoms.[5] The European Male Ageing Study has offered strict diagnostic criteria for LOH: The patient must have three sexual symptoms (lessened sexual thoughts, weakened morning erections, and erectile dysfunction), and the simultaneous presence of reproducibly low serum testosterone (either serum total testosterone level <8 nmol/L, or serum total testosterone of 8–11 nmol/L and free T <220 pmol/L).[3] LOH can affect quality of life and even increase all-cause mortality.[6,7,8]
Although controversial, testosterone replacement therapy (TRT) is still regarded as an effective therapy for LOH and has achieved satisfactory results.[8,9,10,11,12] However, the potential adverse effects of TRT should not be ignored, including erythrocytosis, prostate cancer, dyslipidemia, cardiovascular events, and gynecomastia.[13,14,15,16] Oligozoospermia is also one of the side effects of TRT. It has been proven that intratesticular testosterone (ITT) level is a prerequisite for normal spermatogenesis, and inhibiting ITT will result in azoospermia.[17,18] Exogenous testosterone can suppress the hypothalamic–pituitary–gonadal (HPG) axis and then lead to a decrease in testosterone synthesis, resulting in sterility.[19] Therefore, men who desire future fertility are suggested to avoid TRT.
As a traditional herbal medicine, Saikokaryukotsuboreito(SKRBT) has been widely used in a variety of clinical diseases such as depression, neurosis, anxiety, palpitation, vertigo, and insomnia.[5] SKRBT is also used to treat the symptoms of LOH and has been proven to be effective and safe for aging patients.[20,21] Our previous study indicated that SKRBT can increase the serum testosterone levels by increasing the expression of steroidogenic acute regulatory (StAR), a rate-limiting enzyme of the testosterone synthesis process, and improve sexual function in aging male mice.[5] However, to the best of our knowledge, a few articles have reported if SKRBT affects the reproductive capacity of aging males. To extend on our previous research, we therefore implemented a study to determine whether SKRBT can increase testosterone synthesis without damaging spermatogenesis and fertility.
METHODS
Animals
C57BL/6 mice were purchased from the Animal Center of Sun Yat-sen University (Guangzhou, China). Males were 24 months of age, weighted 26–30 g, and were housed individually. Females were approximately 3-month-old, weighted 22–25 g, and were housed five per cage. All mice were maintained under a 12 h light-dark cycle at ambient temperature provided with water and food ad libitum. All animal studies were performed in accordance with the guidelines of the Sun Yat-sen University Institutional Animal Care and Use Committee.
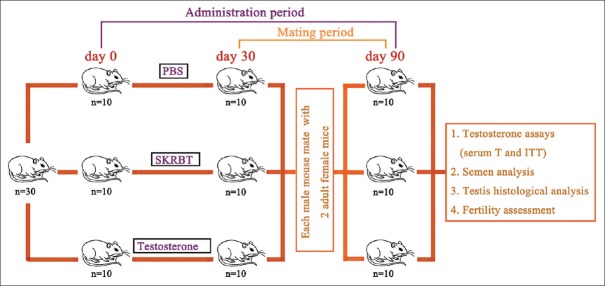
Before the experiment, the blood specimen of each male mouse was collected from the retrobulbar space between 8:00 and 12:00 in the morning for testosterone assessment. Only those with the serum testosterone concentration under 10 ng/ml were selected and randomly assigned to three different groups. Each group contained 10 animals. Group 1 was treated with phosphate-buffer solution 0.5 ml by daily oral gavage as the control group. Group 2 was treated with SKRBT orally at a dosage of 300 mg/kg of body weight every day. Group 3 was injected subcutaneously with testosterone every 3 days at a dose of 10 mg/kg of body weight. All of the drugs were administered until the end of the experiment. At day 30, each aging male mouse was mated with adult female mice. At day 90, all of the male mice were sacrificed and the analysis of serum testosterone, ITT, quality of sperm, and testicular histology was performed. A schematic representation of the experimental design is shown in Figure 1.
Figure 1.
Schematic representation of the experimental design. Phosphate-buffer solution and SKRBT were administered orally at fixed o’clock every day, and testosterone was subcutaneously injected twice every week from the beginning of the test. Each aging male mouse was mated with females at day 30 and was harvested at day 90. PBS: Phosphate-buffer solution; SKRBT: Saikokaryukotsuboreito; T: Testosterone; ITT: Intratesticular testosterone.
Drugs
As depicted previously, SKRBT is composed of 10 crude drugs in fixed proportions: 5.0 g of bupleurum root, 4.0 g of pinelliae tuber, 3.0 g of cinnamon bark, 3.0 g of poria sclerotium, 2.5 g of scutellariae root, 2.5 g of Jujube, 2.5 g of oyster shell, 2.5 g of ginseng, 2.5 g of longgu, 1.0 g of rhubarb, and 0.8 g of ginger.[5] The dried extract powder of SKRBT was manufactured in the dispensary of Traditional Chinese Medicine, the Third Affiliated Hospital of Sun Yat-sen University (Guangzhou, China). The powdered drug was suspended in distilled water and administered orally. Testosterone (Sigma-Aldrich, MO, USA) was administered by subcutaneous injections (1 mg dissolved in 100 μl olive oil, twice weekly).
Testosterone assays
Serum testosterone levels were assessed using a commercially available enzyme-linked immunosorbent assay kit (R & D Systems, MN, USA) according to the manufacturer's instructions.
The ITT was measured according to the following approach: The right testis of each mouse was removed and decapsulated. Two pieces (50 mg each) were picked out from it and were placed separately into two 1.5 ml microfuge tubes containing 1.0 ml Medium 199 (Corning Cellgro, VA, USA). Then, the testis tissue was incubated in duplicate for 2 h at 34°C. After that, these tubes were centrifuged for 5 min at a speed of 10,000 ×g, and then the supernatant was collected and frozen at −80°C for future testosterone assay.
Semen analysis
Sperm was collected from the caudal epididymis of male mice at day 90. The excised epididymides were cut into small pieces and placed in F12 media (1 ml) containing 0.1% bovine serum albumin prewarmed to 34°C and incubated for 15 min to facilitate sperm transmigration from the epididymis. A hemocytometer was used to count the number of sperm. Each specimen was counted twice and averaged. A prewarmed glass slide was loaded with fresh sperm and observed by microscopy to examine sperm motility. Five randomly chosen fields for two samples from each animal were selected to assess sperm motility. The percentage of sperm with forward and progressive activity was counted to assay sperm motility.
Histology and immunohistochemistry analysis
After being harvested, the testes were dissected, fixed by immersion in Bouin's fixative, and embedded in paraffin. Tissues were sectioned serially at 4 μm thickness for all histological and immunohistochemical procedures. To assay spermatogonial proliferation, the number of synaptonemal complex protein 3 (SYCP3, rabbit anti-SCP-3 [Santa Cruz Biotechnology] at 1:500 dilution)-positive cells/tubule in cross-sections of testes and percentages of SYCP3-positive tubules/section in the mice testes were counted.[22] The SYCP3 was detected by DAB (3,3'-diaminobenzidine tetrahydrochloride, Sigma-Aldrich, MO, USA) stain as depicted previously.[23] At least, 100 tubules/testis were counted. All counting was performed on an Olympus microscope (Center Valley, PA, USA).
Western blot analysis
Whole testis tissues of mice were homogenized in liquid nitrogen before protein was isolated and suspended in radioimmunoprecipitation assay lysis buffer (Sigma-Aldrich, MO, USA). The homogenates were centrifuged at 10,000 ×g for 5 min at 4°C. The protein in supernatant was determined using a Bio-Rad DC protein assay (Bio-Rad Laboratories, Hercules, CA, USA). Then, 20 µg of each protein was administered separately to SDS-PAGE and transferred to polyvinylidene difluoride membranes (Millipore, MA, USA). After blocking in Tris-buffered saline with Tween (TBST) containing 5% nonfat dry milk for 1 h, the membranes were incubated with polyclonal primary antibody SYCP3 (1:100,000, Abnova, Taipei, China) overnight at 4°C. After washed 3 times in TBST, membranes were incubated with Horseradish peroxidase-conjugated secondary antibody (1:3000, Vector, CA, USA) for 2 h at room temperature. Bands were visualized using an enhanced chemiluminescence reagent (Amersham Bioscience, Uppsala, Sweden).
Fertility Assessment
We investigated the effects of SKRBT and exogenous testosterone on the fertility of these old male mice using a continuous breeding assay. Each male mouse was paired with two 3-month-old females for 60 days, and the latency to the first litter, total number of litters, and number of pups per litter was assessed in all groups.
Statistical analysis
The SPSS 21.0 package (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA, USA) were used. Quantitative data were expressed as mean ± standard error of the mean. Statistically significant differences between different groups were determined by one-way analysis of variance. A P < 0.05 considered statistically significant.
RESULTS
Changes in testosterone levels after the treatment of Saikokaryukotsuboreito
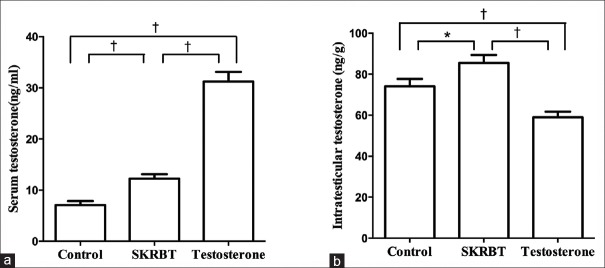
The serum testosterone levels of the SKRBT-treated group and the testosterone-treated group were both significantly higher compared with the control group [the SKRBT-treated group vs. the control group: 12.22 ± 2.87 ng/ml vs. 7.09 ± 2.39 ng/ml, P = 0.002; the testosterone-treated group vs. the control group: 29.46 ± 4.49 ng/ml vs. 7.09 ± 2.39 ng/ml, P < 0.001; F = 120.98; Figure 2a]. These results were in accordance with our former study.[5] Furthermore, the ITTs of the SKRBT-treated group were significantly increased compared with those of the control group [85.50 ± 12.31 ng/g vs. 74.10 ± 11.45 ng/g, P = 0.027; F = 14.81; Figure 2b]. However, the ITT of the testosterone-treated group was significantly lower than that of the control group [59.00 ± 8.67 ng/g vs. 74.10 ± 11.45 ng/g, P = 0.005; F = 14.81; Figure 2b].
Figure 2.
Quantification of testosterone levels after the treatment of Saikokaryukotsuboreito (SKRBT). (a) Shows serum testosterone levels and (b) exhibits the intratesticular testosterone levels. Values represent the mean ± standard error of the mean. *P < 0.05, †P < 0.01; n = 10/group.
Saikokaryukotsuboreito treatment improves sperm number and motility
To determine whether sperm number and motility changed after the treatment of SKRBT, epididymal sperm from the aging male mice were analyzed. Results indicated that there was a significant increase in sperm number and motility in the SKRBT group compared with the control group (sperm number: [14.94 ± 4.63] × 106 cells/ml vs. [8.79 ± 4.38] × 106 cells/ml, P = 0.002; sperm motility: 43.16 ± 9.93% vs. 33.51 ± 6.98%, P = 0.015; F = 18.20) [Figure 2]. We also assessed the effects of exogenous testosterone on sperm quality and found that the sperm number and motility decreased significantly after the administration of testosterone (sperm number: [4.34 ± 2.45] × 106 cells/ml vs. [8.79 ± 4.38] × 106 cells/ml, P = 0.018; sperm motility: 19.53 ± 7.69% vs. 33.51 ± 6.98%, P = 0.015; F = 20.50) [Figure 3].
Figure 3.
Alterations in sperm number and sperm motility after treatment with Saikokaryukotsuboreito (SKRBT). (a) Change in sperm number; (b) Change in sperm motility. Data are shown as mean ± standard error of the mean; *P < 0.05, †P < 0.01; n = 10/group.
Expression of synaptonemal complex protein 3 increases after the Saikokaryukotsuboreito treatment
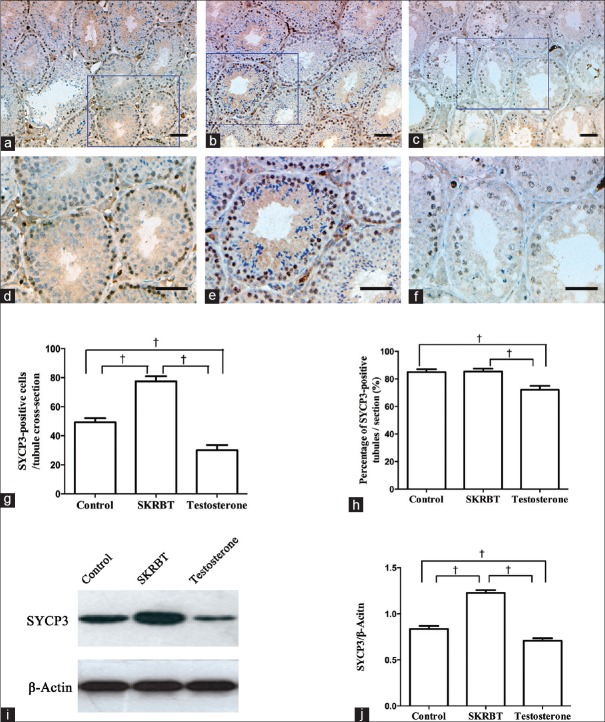
As mentioned above, testosterone is essential for spermatogenesis. To further verify whether SKRBT plays a role in maintaining spermatogenesis, we examined the proliferative capacity of spermatogonial cells in the seminiferous tubules. The expression of SYCP3 was detected to assess the effect of SKRBT on meiosis. IHC analysis after the experiment demonstrated that the distribution of SYCP3 increased in the SKRBT-treated group but decreased in the testosterone-treated group [Figure 4]. According to the results of the quantitative analysis, the number of SYCP3-positive cells/tubule increased in the SKRBT-treated subjects but decreased in the testosterone-treated individuals compared with the controls [the SKRBT-treated group vs. the control group: 77.50 ± 11.01 vs. 49.30 ± 8.73, P < 0.001; the testosterone-treated group vs. the control group: 30.00 ± 11.28 vs. 49.30 ± 8.73, P < 0.001; F = 52.72; Figure 4g]. The differences in the percentage of SYCP3-positive tubules/section were not apparent between the SKRBT-treated group and the control group [85.31 ± 7.05% vs. 84.94 ± 6.86%, P = 0.915; F = 9.85; Figure 4h], but it significantly reduced in the testosterone-treated group [71.98 ± 8.88%, P = 0.001; Figure 4h]. Similarly, the expression of SYCP3 protein was also assessed and was found increased in the SKRBT-treated group, but decreased after testosterone-treatment [the SKRBT-treated group vs. the control group: 1.23 ± 0.09 vs. 0.84 ± 0.10, P < 0.001; the testosterone-treated group vs. the control group: 0.71 ± 0.09 vs. 0.84 ± 0.10, P < 0.001; F = 84.36; Figure 4i and 4j]. These data revealed that the administration of SKRBT can improve the expression of SYCP3 while exogenous testosterone decreases it.
Figure 4.
Effects of Saikokaryukotsuboreito (SKRBT) on spermatogenesis are shown for synaptonemal complex protein 3 expression. IHC detection of germ cells immunopositive for synaptonemal complex protein 3 are shown in (a and d) control group, (b and e) SKRBT group and (c and f) testosterone group. (g) Quantification of immunopositive cells per tubule cross-section is shown. (h) Comparison of percentage of synaptonemal complex protein 3-positive tubules/section between different groups. (i) Western blotting of synaptonemal complex protein 3. (j) The histogram of synaptonemal complex protein 3 expression derived from I. Data are shown as mean ± standard error of the mean; *P < 0.05, †P < 0.01; n = 10/group, bars = 50 μm.
Saikokaryukotsuboreito treatment does not impair the histological structure of testis
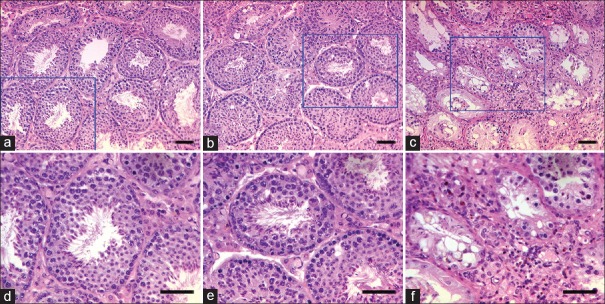
Figure 5 shows the morphological appearance of the seminiferous tubules from the mice in each group. Treatment with SKRBT for 90 days did not have any consequences for testicular histopathology in old male mice compared with the controls [Figure 5a, 5b, 5d, and 5e]. Many mature sperm were seen in the lumen of the control mouse and the SKRBT-treated mouse. However, compared with the control or SKRBT, exogenous testosterone clearly resulted in a markedly damaged seminiferous epithelium, obvious spermatogenic arrest, and the seminiferous tubules were smaller in diameter and exhibited fewer spermatocytes and round spermatids [Figure 5c and 5f]. Seldom mature germ cells were released into the lumen of the testosterone mouse [Figure 5c and 5f].
Figure 5.
Representative light micrographs of seminiferous tubules from control (a and d), Saikokaryukotsuboreito (SKRBT)-treated (b and e), and testosterone-treated (c and f) mice. Normal spermatogenesis is seen in an untreated control mouse (a and d) and in a SKRBT-treated mouse (b and e), and no obvious difference is seen between them. However, in a testosterone-treated mouse (c and f), the structure of the seminiferous epithelium was damaged, the tubule diameter decreased markedly and the spermatogenesis was completely arrested. n = 10/group, bars = 50 μm.
Fertility does not change after Saikokaryukotsuboreito administration
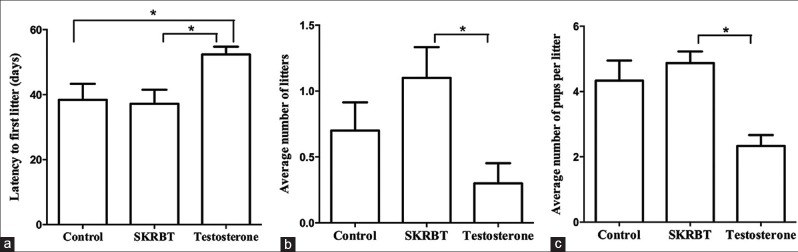
Fertility was also assessed in these aging male mice. As shown in Figure 6, there was no difference between the control group and the SKRBT-treated group with regard to the latency to the first litter [P = 0.835; Figure 6a], but the testosterone-treated group had a longer latency to the first litter than the other two groups [the testosterone-treated group vs. the control group: 52.40 ± 7.52 days vs. 38.40 ± 15.54 days, P = 0.021; the testosterone-treated group vs. the SKRBT-treated group: 52.40 ± 7.52 days vs. 37.20 ± 13.69 days, P = 0.013; F = 4.41; Figure 6a]. There were more litters and a greater number of pups per litter in the SKRBT-treated group than in the control group, but the differences were not significant [total number of litters: 1.10 ± 0.74 vs. 0.70 ± 0.68, F = 3.89, P = 0.174; number of pups per litter: 4.88 ± 0.99 vs. 4.33 ± 1.51, F = 5.26, P = 0.402; Figure 6b and 6c]. However, the aging male mice in the testosterone-treated group sired fewer litters and had fewer offspring per litter than the mice in the control group during the 90 days continuous breeding study [number of pups per litter: 2.33 ± 0.58 vs. 4.33 ± 1.51, F = 5.26, P = 0.029; Figure 6b and Figure 6c].
Figure 6.
Effect of Saikokaryukotsuboreito (SKRBT) on the fertility of aging male mice. Aging males were paired with 2-month-old females for a 60 days continuous breeding study. (a) The latency to the first litter after pairing with the females; (b) The average number of litters in a 60-day period; (c) The average number of pups per litter. *P <s 0.05; n = 10/group.
DISCUSSION
We have previously proven that treatments of SKRBT could increase the serum testosterone concentration of aging male mice. This study further verifies that SKRBT can also increase the ITT and demonstrates for the first time that SKRBT can elevate the quality of sperm and improve spermatogenesis without impairing the fertility of aging male mice.
In recent years, studies on adopting herbal medicines in the treatment of LOH-related symptoms have emerged. A previous study has documented that Pueraria mirifica, a Thai herbal plant, is useful to prevent osteoporosis in elderly hypogonadism subjects without influences on reproductive organs.[24] Eurycoma longifolia Jack, also known as “Malaysian ginseng,” is proven to be useful as a supplement in increasing serum testosterone concentration and overcoming the symptoms of LOH.[25] As an herbal medicine, SKRBT has also been explored for the treatment of LOH-related symptoms. A clinic trial has reported that SKRBT is a safe and effective treatment for patients with LOH.[21] An animal experiment has verified that SKRBT can improve serum testosterone levels by inhibiting aromatase inhibitory activity.[26] SKRBT has also been proven to possess the function of relieving some LOH-related symptoms such as preventing and curing soft tissue calcification,[27] anti-hypertension,[28] reducing blood lipid,[29] and preventing atherosclerosis.[30]
However, articles have seldom investigated the mechanism of action governing herbal medicine in LOH. The findings in our previous study provided preliminary evidence that SKRBT may increase serum testosterone levels by enhancing the expression of StAR and improve the sexual functions of aging male mice.[5] To the best of our knowledge, the effect of SKRBT on spermatogenesis has not been elucidated. In our current study, we selected naturally aging male mice as an animal model of LOH to make further assessment of the effect of SKRBT on the fertility of aging male mice. Our previous research shows no obvious dose-dependent effect was seen, so we think that SKRBT has an optimal dose for treatment.[5] According to our previous results, the effect at the dose of 300 mg/kg seemed most noticeable.[5] Therefore, we treated these mice at the dosage of 300 mg/kg in the present study. To compare SKRBT with TRT, which is regarded as the first-line treatment for LOH, we also assigned a testosterone-treated group.
In this experimental study, we found that SKRBT could not only raise the serum testosterone levels but also increase the testosterone concentration in testicular tissue. ITT is an absolute prerequisite for maintaining normal spermatogenesis.[31,32] Normally, ITT levels are roughly 50–100 times higher than serum testosterone levels.[33] Exogenous testosterone therapies can suppress the HPG axis and then decrease the ITT production to such a degree that spermatogenesisis dramatically decreased.[32] Complete inhibition of ITT can even result in azoospermia.[17,18] As shown in Figure 2, ITT increased after the administration of SKRBT, which indicated that SKRBT might promotes permatogenesis. We also observed the serum testosterone levels and ITT of mice in the testosterone-treated group and found that although the serum testosterone concentration increased in these mice, the ITT decreased significantly. These data suggested that unlike exogenous testosterone, SKRBT seemed not to suppress the HPG axis and could increase ITT. We proceeded with the analysis of sperm in the epididymis and observed that the sperm number and motility increased significantly after the administration of SKRBT, but decreased significantly after testosterone exposure. Combined with our previous data,[5] the present results indicate that SKRBT can improve testosterone synthesis of Leydig cells by up-regulating the expression of StAR and then elevate ITT, which may ultimately lead to an increase in semen quality in the epididymis via enhancing the number and motility of sperm.
In previous studies, testosterone has been proven to be critical for the completion of meiosis and spermatogenesis in rodents.[34] To determine whether the administration of SKRBT affected spermatogenesis, we thereby detected the expression of a meiotic marker, SYCP3, by immunofluorescence staining. After the experiment, we observed that the SYCP3 expression was significantly increased in the SKRBT-treated group, but dramatically decreased in the testosterone-treated group when compared with the control group. Our results indicate that SKRBT could increase meiotic spermatogenesis while exogenous testosterone suppressed it. Hematoxylin and eosin analysis revealed that the administration of SKRBT did not affect the testicular tissue, but exogenous testosterone destroyed the normal structure of seminiferous epithelium.
To evaluate whether SKRBT impaired fertility, we performed a mating study in these aging male mice. The number of new-born mice was recorded every day during the process. According to the results, no difference was seen in the latency to first litter, average number of litters and average number of pups per litter between the SKRBT treated group and the control group. However, these items were statistically lower in the testosterone-treated group compared to the SKRBT-treated group. Our data indicate that SKRBT could ameliorate spermatogenesis and would not impair fertility. Our results also show that exogenous testosterone could not only decrease the semen quality, but also harm fertility which is consistent with the previously reported studies.[19,33]
Our study had several limitations. Further studies are needed to explore whether SKRBT affects the release of gonadotropin-releasing hormone, luteinizing hormone and follicle-stimulating hormone. Another limitation is that the number of samples in this study is relatively small, which may influence the results of statistical tests. In addition, we should declare that this study focused on the medicine effects on the fertility of aging male mice and should not be generalized completely to other conditions. Although as mentioned above, exogenous testosterone can suppress the HPG axis and lead to infertility, it could really improve sperm quality when combined with tamoxifen, an estrogen receptor modulator, in the treatment of idiopathic oligozoospermia.[35] Whether exogenous testosterone can be used for male infertility is still controversial, and the clear causes of male infertility are difficult to determine in most patients. Therefore, some experts suggest that empirical drug therapy, including androgens, for male infertility requires further research to generate related evidence.[36]
In conclusion, SKRBT has a physiological role in improving serum testosterone level and ITT simultaneously, which enables SKRBT to increase testosterone in vivo while not impairing fertility. Thus, this study provides the groundwork for SKRBT administration, which would shed new light on therapy for LOH patients desiring future fertility. Further studies are still needed to evaluate its long-term use, and to test its effects on other organs.
Financial support and sponsorship
This work was supported by a grant from Medical Scientific Research Foundation of Guangdong Province, China (No. B2013104).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We are grateful to Alissa Davis (Department of Epidemiology and Biostatistics, Indiana University-Bloomington, USA) for language revisions.
Footnotes
Edited by: Li-Shao Guo
REFERENCES
- 1.Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: Longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589–98. doi: 10.1210/jcem.87.2.8201. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 2.Huhtaniemi I. Late-onset hypogonadism: Current concepts and controversies of pathogenesis, diagnosis and treatment. Asian J Androl. 2014;16:192–202. doi: 10.4103/1008-682X.122336. doi: 10.4103/1008-1682X.122336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363:123–35. doi: 10.1056/NEJMoa0911101. doi: 10.1056/NEJMoa0911101. [DOI] [PubMed] [Google Scholar]
- 4.Morales A, Lunenfeld B. International Society for the Study of the Aging Male. Investigation, treatment and monitoring of late-onset hypogonadism in males. Official recommendations of ISSAM. International Society for the Study of the Aging Male. Aging Male. 2002;5:74–86. doi: 10.1080/tam.5.2.74.86. [PubMed] [Google Scholar]
- 5.Zang ZJ, Ji SY, Dong W, Zhang YN, Zhang EH, Bin Z. A herbal medicine, Saikokaryukotsuboreito, improves serum testosterone levels and affects sexual behavior in old male mice. Aging Male. 2015;18:106–11. doi: 10.3109/13685538.2014.963042. doi: 10.3109/13685538.2014.963042. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman JM. Mortality associated to late-onset hypogonadism: Reasons not to treat with testosterone? J Clin Endocrinol Metab. 2014;99:1161–3. doi: 10.1210/jc.2014-1103. doi: 10.1210/jc.2014-1103. [DOI] [PubMed] [Google Scholar]
- 7.Pye SR, Huhtaniemi IT, Finn JD, Lee DM, O’Neill TW, Tajar A, et al. Late-onset hypogonadism and mortality in aging men. J Clin Endocrinol Metab. 2014;99:1357–66. doi: 10.1210/jc.2013-2052. doi: 10.1210/jc.2013-2052. [DOI] [PubMed] [Google Scholar]
- 8.Srinivas-Shankar U, Roberts SA, Connolly MJ, O’Connell MD, Adams JE, Oldham JA, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: A randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639–50. doi: 10.1210/jc.2009-1251. doi: 10.1210/jc.2009-1251. [DOI] [PubMed] [Google Scholar]
- 9.Corona G, Isidori AM, Buvat J, Aversa A, Rastrelli G, Hackett G, et al. Testosterone supplementation and sexual function: A meta-analysis study. J Sex Med. 2014;11:1577–92. doi: 10.1111/jsm.12536. doi: 10.1111/jsm.12536. [DOI] [PubMed] [Google Scholar]
- 10.Liao CH, Wu YN, Lin FY, Tsai WK, Liu SP, Chiang HS. Testosterone replacement therapy can increase circulating endothelial progenitor cell number in men with late onset hypogonadism. Andrology. 2013;1:563–9. doi: 10.1111/j.2047-2927.2013.00086.x. doi: 10.1111/j.2047-2927.2013.00086.x. [DOI] [PubMed] [Google Scholar]
- 11.Ruige JB, Ouwens DM, Kaufman JM. Beneficial and adverse effects of testosterone on the cardiovascular system in men. J Clin Endocrinol Metab. 2013;98:4300–10. doi: 10.1210/jc.2013-1970. doi: 10.1210/jc.2013-1970. [DOI] [PubMed] [Google Scholar]
- 12.Wylie K, Froggatt N. Late onset hypogonadism, sexuality and fertility. Hum Fertil (Camb) 2010;13:126–33. doi: 10.3109/14647273.2010.501890. doi: 10.3109/14647273.2010.501890. [DOI] [PubMed] [Google Scholar]
- 13.Calof OM, Singh AB, Lee ML, Kenny AM, Urban RJ, Tenover JL, et al. Adverse events associated with testosterone replacement in middle-aged and older men: A meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci. 2005;60:1451–7. doi: 10.1093/gerona/60.11.1451. doi: 10.1093/gerona/60.11.1451. [DOI] [PubMed] [Google Scholar]
- 14.Coviello AD, Kaplan B, Lakshman KM, Chen T, Singh AB, Bhasin S. Effects of graded doses of testosterone on erythropoiesis in healthy young and older men. J Clin Endocrinol Metab. 2008;93:914–9. doi: 10.1210/jc.2007-1692. doi: 10.1210/jc.2007-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernández-Balsells MM, Murad MH, Lane M, Lampropulos JF, Albuquerque F, Mullan RJ, et al. Clinical review 1: Adverse effects of testosterone therapy in adult men: A systematic review and meta-analysis. J Clin Endocrinol Metab. 2010;95:2560–75. doi: 10.1210/jc.2009-2575. doi: 10.1210/jc.2009-2575. [DOI] [PubMed] [Google Scholar]
- 16.Weikert C, Pischon T, Weikert S. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:1865. doi: 10.1056/NEJMc1009326. doi: 10.1056/NEJMc1009326. [DOI] [PubMed] [Google Scholar]
- 17.McLachlan RI, O’Donnell L, Meachem SJ, Stanton PG, De K, Pratis K, et al. Hormonal regulation of spermatogenesis in primates and man: Insights for development of the male hormonal contraceptive. J Androl. 2002;23:149–62. doi: 10.1002/j.1939-4640.2002.tb02607.x. [PubMed] [Google Scholar]
- 18.Weinbauer GF, Nieschlag E. Gonadotrophin-releasing hormone analogue-induced manipulation of testicular function in the monkey. Hum Reprod. 1993;8(Suppl2):45–50. doi: 10.1093/humrep/8.suppl_2.45. doi: 10.1093/humrep/8.suppl_2.45. [DOI] [PubMed] [Google Scholar]
- 19.Moss JL, Crosnoe LE, Kim ED. Effect of rejuvenation hormones on spermatogenesis. Fertil Steril. 2013;99:1814–20. doi: 10.1016/j.fertnstert.2013.04.003. doi: 10.1016/j.fertnstert.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Amano T, Imao T, Takemae K. Clinical efficacy of Japanese traditional herbal medicine (Kampo) in patients with late-onset hypogonadism. Aging Male. 2010;13:166–73. doi: 10.3109/13685530903536684. doi: 10.3109/13685530903536684. [DOI] [PubMed] [Google Scholar]
- 21.Tsujimura A, Takada S, Matsuoka Y, Nakayama J, Takao T, Miyagawa Y, et al. Clinical trial of treatment with Saikokaryukotsuboreito for eugonadal patients with late-inset hypogonadism-related symptoms. Aging Male. 2008;11:95–9. doi: 10.1080/13685530802172529. doi: 10.1080/13685530802172529. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Eto K, Honmyou A, Nakao K, Kiyonari H, Abé S. Neuregulins are essential for spermatogonial proliferation and meiotic initiation in neonatal mouse testis. Development. 2011;138:3159–68. doi: 10.1242/dev.062380. doi: 10.1242/dev.062380. [DOI] [PubMed] [Google Scholar]
- 23.Varghese F, Bukhari AB, Malhotra R, De A. IHC profiler: An open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS One. 2014;9:e96801. doi: 10.1371/journal.pone.0096801. doi: 10.1371/journal.pone.0096801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urasopon N, Hamada Y, Asaoka K, Cherdshewasart W, Malaivijitnond S. Pueraria mirifica, a phytoestrogen-rich herb, prevents bone loss in orchidectomized rats. Maturitas. 2007;56:322–31. doi: 10.1016/j.maturitas.2006.09.007. doi: 10.1016/j.maturitas.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Tambi MI, Imran MK, Henkel RR. Standardised water-soluble extract of Eurycoma longifolia, Tongkat ali, as testosterone booster for managing men with late-onset hypogonadism? Andrologia. 2012;44(Suppl1):226–30. doi: 10.1111/j.1439-0272.2011.01168.x. doi: 10.1111/j.1439-0272.2011.01168.x. [DOI] [PubMed] [Google Scholar]
- 26.Michihara S, Shin N, Watanabe S, Morimoto Y, Okubo T, Norimoto H. A Kampo formula, Saikokaryukotsuboreito, improves serum testosterone levels of castrated mice and its possible mechanism. Aging Male. 2013;16:17–21. doi: 10.3109/13685538.2012.755507. doi: 10.3109/13685538.2012.755507. [DOI] [PubMed] [Google Scholar]
- 27.Hidaka S, Okamoto Y, Abe K, Miyazaki K, Suekawa M, Liu SY. Inhibitory effects of 1-hydroxyethylidene-1,1-bisphosphonate and Chinese traditional (Kampo) medicines on calcification of the heart and tongue in DBA/2NCrj mice. Am J Chin Med. 1996;24:65–75. doi: 10.1142/S0192415X96000098. doi: 10.1142/S0192415X96000098. [DOI] [PubMed] [Google Scholar]
- 28.Okano H, Ohkubo C. Anti-pressor effect of a Chinese-Japanese herbal medicine, Saiko-ka-ryukotsu-borei-to on hemodynamics in rabbits. In Vivo. 1999;13:333–7. [PubMed] [Google Scholar]
- 29.Nomura S, Hattori N, Sakakibara I, Fukuhara S. Effects of Saiko-ka-ryukotsu-borei-to in patients with hyperlipidemia. Phytomedicine. 2001;8:165–73. doi: 10.1078/0944-7113-00035. doi: 10.1078/0944-7113-00035. [DOI] [PubMed] [Google Scholar]
- 30.Kim DW, Chung HJ, Nose K, Maruyama I, Tani T. Preventive effects of a traditional Chinese formulation, Chaihu-jia-Longgu-Muli-tang, on intimal thickening of carotid artery injured by balloon endothelial denudation in rats. J Pharm Pharmacol. 2002;54:571–5. doi: 10.1211/0022357021778691. doi: 10.1211/0022357021778691. [DOI] [PubMed] [Google Scholar]
- 31.Sharpe RM, Donachie K, Cooper I. Re-evaluation of the intratesticular level of testosterone required for quantitative maintenance of spermatogenesis in the rat. J Endocrinol. 1988;117:19–26. doi: 10.1677/joe.0.1170019. doi: 10.1677/joe.0.1170019. [DOI] [PubMed] [Google Scholar]
- 32.Zirkin BR, Santulli R, Awoniyi CA, Ewing LL. Maintenance of advanced spermatogenic cells in the adult rat testis: Quantitative relationship to testosterone concentration within the testis. Endocrinology. 1989;124:3043–9. doi: 10.1210/endo-124-6-3043. doi: 10.1210/endo-124-6-3043. [DOI] [PubMed] [Google Scholar]
- 33.Kim ED, Crosnoe L, Bar-Chama N, Khera M, Lipshultz LI. The treatment of hypogonadism in men of reproductive age. Fertil Steril. 2013;99:718–24. doi: 10.1016/j.fertnstert.2012.10.052. doi: 10.1016/j.fertnstert.2012.10.052. [DOI] [PubMed] [Google Scholar]
- 34.McLachlan RI, O’Donnell L, Meachem SJ, Stanton PG, de Kretser DM, Pratis K, et al. Identification of specific sites of hormonal regulation in spermatogenesis in rats, monkeys, and man. Recent Prog Horm Res. 2002;57:149–79. doi: 10.1210/rp.57.1.149. doi: 10.1210/rp.57.1.149. [DOI] [PubMed] [Google Scholar]
- 35.Adamopoulos DA, Nicopoulou S, Kapolla N, Karamertzanis M, Andreou E. The combination of testosterone undecanoate with tamoxifen citrate enhances the effects of each agent given independently on seminal parameters in men with idiopathic oligozoospermia. Fertil Steril. 1997;67:756–62. doi: 10.1016/s0015-0282(97)81379-9. doi: 10.1016/S0015-0282(97)81379.9. [DOI] [PubMed] [Google Scholar]
- 36.Li HJ. More attention should be paid to the treatment of male infertility with drugs – Testosterone: To use it or not? Asian J Androl. 2014;16:270–3. doi: 10.4103/1008-682X.122343. doi: 10.4103/1008-682X.122343. [DOI] [PMC free article] [PubMed] [Google Scholar]