Abstract
ApoA-I mimetics are short synthetic peptides that contain an amphipathic αα-helix and stimulate cholesterol efflux by the ABCA1 transporter in a detergent-like extraction mechanism. We investigated the use of amphipathic peptides with a polypro helix for stimulating cholesterol efflux by ABCA1. Polypro peptides were synthesized with modified prolines, containing either a hydrophobic phenol group (Prop) or a polar N-acetylgalactosamine (Prog) attached to the pyrrolidine ring and were designated as either PP-2, 3, 4, or 5, depending on the number of 3 amino acid repeat units (Prop - Prog - Prop). Based on molecular modeling, these peptides were predicted to be relatively rigid and to bind to a phospholipid bilayer. By CD spectroscopy, PP peptides formed a Type-II polypro helix in an aqueous solution. PP-2 was inactive in promoting cholesterol efflux, but peptides with more than 2 repeat units were active. PP-4 showed a similar Vmax as a much longer amphipathic α-αhelical peptide, containing 37 amino acids, but had a Km that was approximately 20-fold lower. PP peptides were specific in that they did not stimulate cholesterol efflux from cells not expressing ABCA1 and were also non-cytotoxic. Addition of PP-3, 4 and 5 to serum promoted the formation of smaller size HDL species (7 nM) and increased its capacity for ABCA1-dependent cholesterol efflux by approximately 20-35% (p<0.05). Because of their relatively small size and increased potency, amphipathic peptides with a polypro helix may represent an alternative structural motif for the development of apoA-I mimetic peptides.
1. Introduction
There is great interest in the development of synthetic High Density Lipoprotein (HDL) particles as a potential therapy for cardiovascular diseases [1,2,3]. Either full length ApoA-I [4], which contains a tandem array of amphipathic α-helices, or short ApoA-I mimetic peptides [5,6], are used as the protein component of synthetic HDL particles. ApoA-I mimetic peptides are typically designed to form an amphipathic α-helix, so that one side of the helix contains hydrophobic amino acids and faces the lipid core of HDL, whereas the other side contains amino acids with hydrophilic side chains that face toward the aqueous environment. It has been shown in numerous studies that apoA-I mimetic peptides, like the full-length apoA-I protein, can efflux excess cellular cholesterol by the ABCA1 transporter in a detergent-like extraction process [7,8]. These peptides reduce atherosclerosis in various animal models [5,6,9], and also show benefit in several other disease models, particularly inflammatory diseases [10].
Peptides that are enriched in proline can also form helices, namely type I and type II polypro helices [11]. The polypro type II helix, with its peptide bond in the trans-configuration, is more common than the type I polypro helix and is structurally quite different than the α-helix. It forms a left-handed helix with 3 amino acids per turn and has an overall shape of a triangular prism, with a rise per residue of approximately 3.1 Å. In contrast, the α-helix is right-handed, contains 3.6 amino acids per turn and has a rise per residue of only 1.5 Å, which means that for the same number of amino acids a polypro type II helix will be about twice as long an α-helix. Even though the polypro type II helix has no backbone hydrogen bonds to stabilize its secondary structure like the α-helix, it is, nevertheless, very rigid. In fact, polypro peptides are used as “molecular rulers” in FRET type analysis [12]. The reason that polypro peptides are so rigid is because the pyrrolidine side chains ring of proline forms a covalent bond with the amino group on the peptide backbone, which limits the permissible dihedral angles of the peptide bond to a conformation that favors helix formation [11]. As a consequence, polypro helices can be much shorter than α-helices, because they do not require stabilization by backbone hydrogen bonding. A cross section of polypro peptides shows almost a perfect 3-fold symmetry, because their side chains are arranged every 120° unlike α-helices, which instead occur about every 100°. Polypro helices, containing modified prolines with attached side chain groups [13], can potentially form amphipathic helices much longer than the typical limit of 18-20 residues for α-helices, because the relative orientation of their side chains will be maintained throughout the length of the helix.
In this study, we used modified prolines to synthesize novel amphipathic polypro peptides. To form the hydrophobic base of the triangular prism shape of the polypro peptide, we used two modified proline residues (Prop), containing a hydrophobic phenol group covalently attached to the pyrrolidine ring. The hydrophilic “apex” of the peptide was designed to contain a modified proline residue with a polar N-Acetylgalactosamine (GalNAc) sugar attached to its side chain (Prog). We hypothesized that short peptides based on a repeating unit of these 3 modified amino acids (Prop - Prog - Prop) would form polypro helices and be effective in promoting cholesterol efflux by the ABCA1 transporter, because of their relative rigidity and amphipathic structure.
2. Materials and methods
2.1 Peptide synthesis and modification
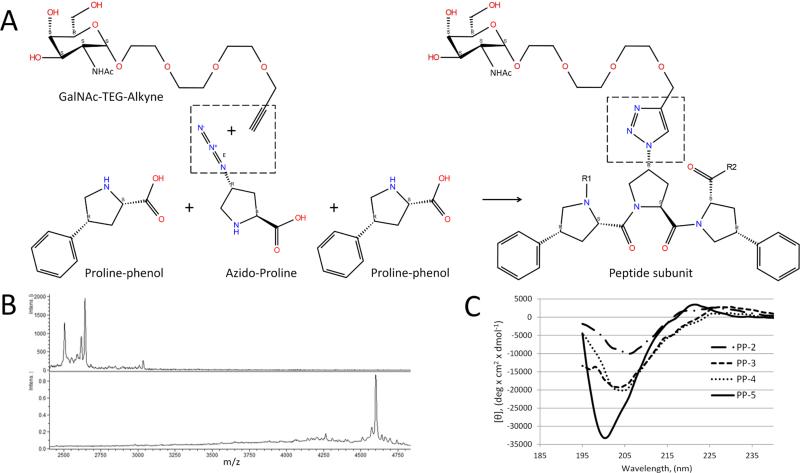
Peptides were synthesized by a solid-phase procedure, using FMOC–protected amino acids on a Biosearch 9600 peptide synthesizer (Bioresearch, Japan), as described in Fig. 1. Trans-Fmoc-4-azido-L-proline (Proa) from IRIS BIOTECH, GMBH (Germany) and Fmoc - (2S, 4R) - 4 - benzyl -pyrrolidine - 2 - carboxylic acid (Prop) from AnaSpec, Inc. (Fremont, CA) were used to form polypro peptides with a variable number of the (Prop – Proa -Prop)n repeat units and were abbreviated as PP-n, with n designating the number of repeat units (Fig. 1A). After cleavage from resin with trifluoroacetic acid, the peptides were lyophilized and α-GalNAc was attached via a PEG trimer linkage to the azido group of Proa residues by the following click chemistry reaction: 3 mM peptides (PP-2, PP-3, PP-4, PP-5) and α-GalNAc-TEG-Alkyne (IRIS BIOTECH, GMBH) at a concentration of 9 mM, 13.5 mM, 18 mM and 22.5 mM respectively (alkyne/azide molar ratio was 1.5) were solubilized in dimethylformamide (Sigma-Aldrich, Saint-Louis, MO) and then added to the reaction mixture of 70% dimethylformamide with 4.5 mM CuSO4 and 9mM sodium ascorbate. The reaction was heated in a microwave (CEM Corp., USA) at 50°C with 25W power for 2 hours. The reaction was monitored by taking 2ul of the final peptide product and placing it on an Anchorchip target and allowed to dry at 45°C. It was overlaid with 2ul of a saturated solution of α-cyano-4-hydroxycinnamic acid in 50% ACN, 2.5% TFA and analyzed in a MALDI AutoFlex III (Bruker Daltonic, Billerica, MA), using the linear detector in positive mode between 2,000 and 20,000 Da.
Fig.1. Synthesis and post synthetic modification of polypro peptides.
A. Each peptide repeat unit contained three amino (two Proline-Phenols and one Azido-Proline). After peptide synthesis, GalNAc-PEG-alkyne molecules were attached to Azido-Prolines by a “click chemistry” reaction (highlighted by dashed square). B. MALDI-TOF MS spectrum of PP-15 peptide before (top panel) and after (bottom panel) addition of GalNAc-PEG. C. CD spectra of polypro peptides. The relative proportion of polypro type II helical content can be observed by peak heights at approximately 198 and 218 nM.
2.2 Circular Dichroism (CD) spectroscopy
Peptides at a concentration of 0.1 mg/mL in 0.015 M sodium phosphate buffer, pH 7.4 were loaded into a quartz cuvette (d = 0.2 cm path length) and the CD spectra from 185 to 240 nm were recorded on a Jasco J715 spectropolarimeter at 24°C. The polypro type II helix was identified by its characteristic CD spectrum, which has a nadir at 198 nm and a peak at 218 nm [14].
2.3 In Vitro Cholesterol Efflux Assay
Cholesterol efflux studies were performed as previously described [6]. Briefly, BHK-mock (control) and ABCA1-transfected BHK cells were incubated with 1 mCi/ml of [3H]-cholesterol in DMEM. After 24 hours, the media was replaced with DMEM containing the peptides or PBS vehicle control. After 18 hours of incubation, radioactive counts in media and cell lysates were measured by liquid scintillation counting on a Perkin Elmer MicroBeta 1450 scintillation counter. Results are expressed as the percentage of total counts appearing in the media.
Cholesterol efflux to LDL-depleted plasma spiked with the polypro peptides was performed as described above but only with 4 hours of incubation. LDL-depleted plasma was prepared by PEG precipitation [15] and tested at a final concentration of 1% (vol:vol).
2.4 Plasma HDL Remodeling
Remodeling of plasma HDL was assessed by adding 50 uL of 5 mg/ml of peptides or 50 uL of PBS as a vehicle control to 450 uL of pooled human plasma. Samples were incubated at 37°C for 1 hour in an orbital shaker at 300 rpm. HDL subclasses were separated by size by native PAGE, using 10-well Tris-Borate-EDTA gradient (3-25%) acrylamide gels (Jule, Inc.,USA)[16]. Proteins were transferred onto PVDF membrane and incubated overnight with anti-human apoA-I-HRP conjugate antibody (Meridian Life Science,USA). Images were acquired on an ΑIpha Innotech Chemi Imager 5500.
2.5 Molecular dynamic modeling
PP-5 structure was modelled using UCSF Chimera software (v.1.10.2, Regents of the University of California, USA) [17], with ϕ, ψ torsional angles of − 75°, 145° for the peptide's secondary structure [11]. The peptide was relaxed in an aqueous environment by all atom simulation, using Desmond Molecular Dynamics System (version 4.3, DE Shaw Research, USA, 2015)[18]. The finalized structure was oriented against a POPC lipid bilayer and energy minimization was performed for 25 nanosec, using the same software application. The results were visualized using Maestro software (v.10.2.011, Schrödinger, New York, NY, 2015.) Initial GSG-10 peptide structure was modeled as an α-helix (ϕ, ψ= − 60°, − 45°), using UCSF Chimera software followed by relaxation in an aqueous environment as described above.
3. Results
3.1. Molecular dynamic modeling of the polyproline peptides
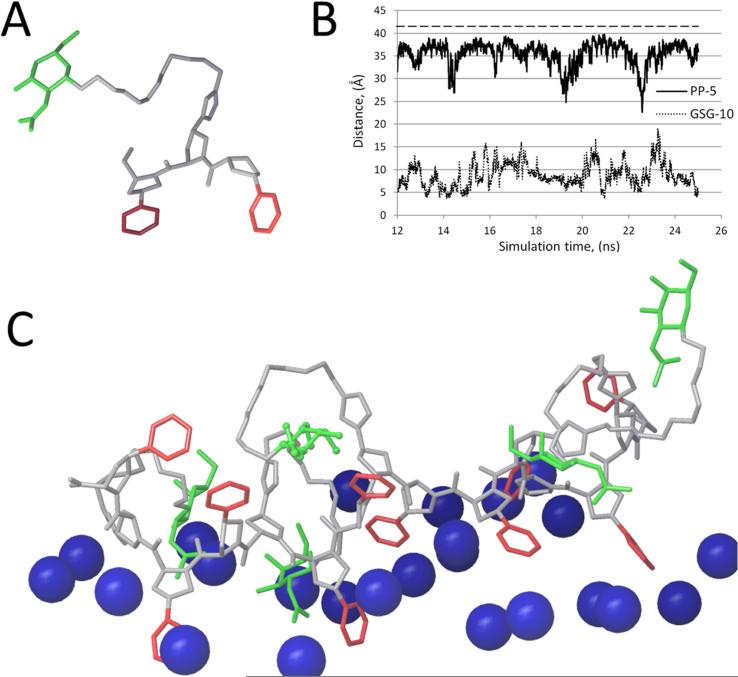
Before peptide synthesis, we first performed molecular dynamic modeling to predict the possible behavior of the polypro peptide PP-5, containing 5 trimeric repeat units (Prop - Prog - Prop)5 in an aqueous solvent and when associated with a phospholipid bilayer. A structural model for one turn of the PP-5 peptide is shown in Fig.2A. Each side chain radiates off the central peptide backbone at 120 °, so that the two hydrophobic phenyl rings attached to the modified Prop residues form a hydrophobic base and the polar Pro residue with the attached polar GalNac side chain (Prog) is at the top. The stability of a hypothetical PP-5 peptide forced into a polypro Type-II configuration was compared to a theoretical GSG-10 peptide, containing 10 repeat units of Gly-Ser-Gly, which were initially arranged in an α-helical conformation. The distance between α-carbon atoms in the first and the last peptide bonds were plotted against the simulation time (Fig. 2B). The starting distance for a “perfect” helix for both peptides was calculated to be approximately 42 Å. After a 12 nanosec stabilization period, the PP-5 peptide had an average length of 35.5 Å, with a 7.3% coefficient of variation throughout the simulation, whereas the average distance for the GSG-10 peptide was only 8.8 Å, with a 29.7% coefficient of variation. These data are consistent with the PP-5 peptide largely maintaining its initial polypro type-II helical structure, whereas the GSG-10 peptide collapsed over time into a random coil.
Fig.2. Molecular Dynamic modeling of the peptides.
A. Prop - Prog - Prop subunit model. Phenyl rings on Prop are highlighted in red and GalNac-PEG-alkyne attached to Prog is highlighted in green. B. Molecular dynamic simulation of PP-5 peptide (solid line) and GSG-10 peptide (dotted line). The distance from the first peptide bond to the last over simulation time is plotted. Dashed line represents the distance for the “perfect” helix for both peptides. C. Molecular dynamics simulation of PP-5 associated with a POPC membrane. Blue spheres represent the head groups of phospholipids. Color scheme for the proline residues is the same as above.
When bound to a phospholipid bilayer, the PP-5 peptide was predicted to peripherally associate with the membrane, with most of the hydrophobic phenyl groups of the modified Prop residues inserting into the lipid bilayer (Fig. 2C). The position of the polar GalNAc residues were more variable but usually faced toward the aqueous water phase. Based on these simulations, the PP-5 peptide appeared to potentially have the proper structural features for promoting cholesterol efflux by the ABCA1 transporter [8].
3.2. Polyproline peptides biophysical properties
A total of 4 polypro peptides (PP-2, PP-3, PP-4 and PP-5), containing 2, 3, 4 and 5 of the trimer repeat unit (Prop - Prog - Prop), were synthesized as described in Fig. 1A. The complete synthesis of each peptide and the attachment of GalNAc groups were confirmed by determining the final MW of the peptides by MALDI-TOF analysis (Fig.1B). The secondary structure of the synthesized peptides in aqueous solution was determined by CD spectroscopy (Fig. 1C). Based on their CD spectra, all of the peptides were found to form polypro Type-II helices, and the degree of helicity increased with peptide length.
3.3. Polyproline peptides promote cholesterol efflux
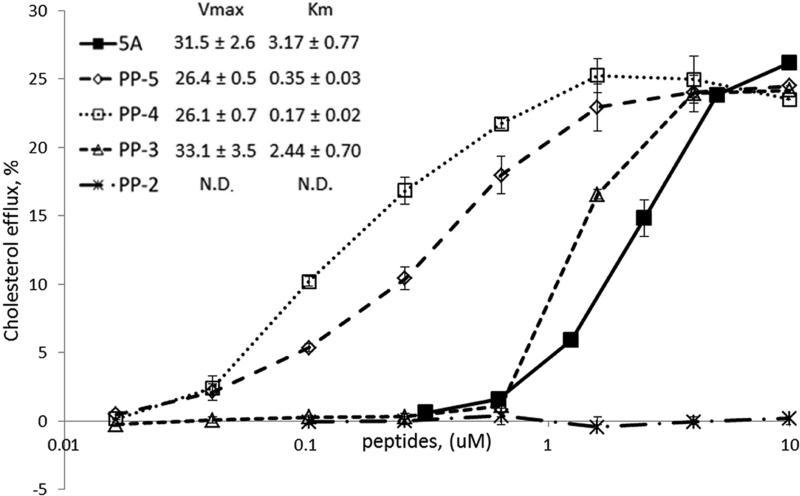
We next tested the ability of the polypro peptides to stimulate cholesterol efflux from BHK cells transfected with the ABCA1 transporter (Fig. 3). As a comparator, we also tested the 5A peptide [19]. 5A is a bi-helical peptide of 37 amino acids. It contains two amphipathic α-helices linked by proline and has been shown to promote ABCA1-dependent cholesterol efflux and reduce atherosclerosis in mice [6]. The PP-2 peptide was inactive, but the PP-3 peptide was equivalent or better than the much longer 5A peptide in stimulating cholesterol efflux. PP-4 was the most potent peptide. It had a similar apparent Vmax as the 5A peptide for cholesterol efflux but its apparent Km was approximately 20-fold lower than the 5A peptide. Interestingly, the longer PP-5 peptide was less effective than the PP-4 peptide. None of the peptides show non-specific cholesterol efflux when tested in BHK cells not expressing the ABCA1 transporter (Supplement Fig. 1) nor did they appear to be cytotoxic, as measured by erythrocyte hemolysis (Supplement Fig. 2).
Fig.3. In vitro cholesterol efflux assay.
Cholesterol efflux from ABCA1-BHK cells to peptides at the indicated concentration on the X-axis plotted, using a logarithmic scale. Results are expressed as the mean ± 1 SD of triplicates. N.D. (not determined).
3.4. Polyproline peptides increase plasma cholesterol efflux capacity
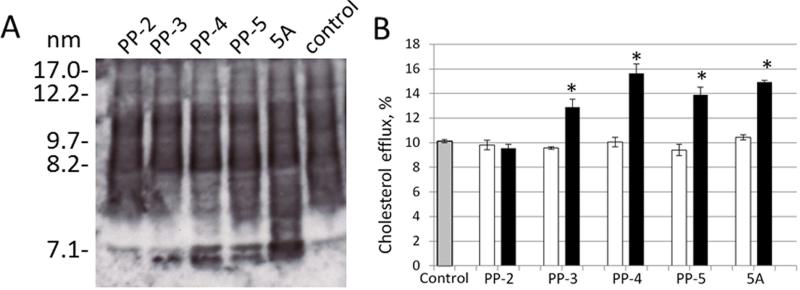
We next tested the effect of the polypro peptides on HDL subclass distribution and on ex vivo plasma cholesterol efflux (Fig. 4). It has been previously shown that 5A and other apoA-I mimetic peptides can remodel existing plasma HDL particles in serum by causing the displacement of apoA-I and the formation of small pre-β HDL size particles [5,9]. The generation of pre-β HDL in serum leads to an increase in ABCA1-dependent cholesterol efflux and has been used as a marker for synthetic HDL function [4]. Like the 5A peptide, the polypro peptides, particularly the longer ones (PP-3, PP-4, PP-5), caused HDL remodeling and the formation of small pre-β like HDL of approximately 7 nM in diameter (Fig. 4A). At the 1 uM dose, the effect of the addition of PP-3, PP-4 and PP-5 on increasing cholesterol efflux to serum from ABCA1 expressing BHK cells was similar to the 5A peptide (Fig. 4B).
Fig.4. HDL remodeling.
A. HDL size subfracation analysis after native PAGE before and after addition of 1 uM peptides. Antibodies against human ApoA-I in conjugation with HRP were used to visualize subclass size distribution. B. In vitro cholesterol efflux assay using LDL-depleted human pooled plasma before (grey column) and after addition of either 0.1 uM (white columns) or 1 uM (black columns) peptides. Results are expressed as the mean ± 1 SD of triplicates. * indicates (P<0.05) compared to control.
Discussion
The ABCA1 transporter is induced in cholesterol loaded cells, such as those in atherosclerotic plaques, and promotes the efflux of excess cellular cholesterol to HDL [7,8]. The exact molecular mechanism for cholesterol efflux is still not fully understood, but it is thought that ABCA1 flips phospholipids to the outer membrane leaflet and creates small membrane microdomains that form outward evaginations [20]. It has been shown that apoA-I, the main protein component of HDL, particularly when in a relatively lipid poor state, such as pre-β HDL, can bind to these lipid microdomains and solubilize them, thereby releasing cholesterol and forming larger HDL subspecies. This is thought to occur by a detergent-like extraction process, whereby the amphipathic helices of apoA-I insert into the lipid microdomain created by ABCA1 and then later reorganizes to stabilize the nascent discoidal shaped HDL particle that forms by this process [20].
ApoA-I has 10 amphipathic helices, but small synthetic peptides containing only a few or even one amphipathic α helix can also promote cholesterol efflux by the ABCA1 transporter [21]. These peptides can be made with either L or D amino acids, indicating that this is not a stereoselective process and that either right-handed or left-handed amphipathic α-helices can promote cholesterol efflux [22]. The same peptides, however, made with a mixture of L and D amino acids, which interfere with backbone hydrogen bonding and do not form a helix, are inactive[22]. Amphipathic peptides that efflux cholesterol are typically at least 18 residues or longer, because much shorter peptides cannot form stable helices. Adding salt bridges [2] or hydrocarbon staples [23] to stabilize helix formation has been shown to increase the ability of these peptides to efflux cholesterol by ABCA1.
In this study, we show that an amphipathic polypro helical peptide as short as 9 amino acids, PP-3, was comparable in cholesterol efflux to the much longer 5A peptide containing 37 amino acids. The PP-4 peptide containing 4 trimeric repeats (12 amino acids) had an apparent Km that was approximately 20-fold lower in molar units than the 5A peptide. Interestingly, PP-5, which by CD spectroscopy had the most polypro type-II helical content (Fig. 2C), was less effective in cholesterol efflux than PP-4. It may be that this peptide has exceeded the optimum length for cholesterol efflux by ABCA1. When in a perfect helical conformation PP-5 would be expected to have a peptide length of 46.5 Å, which is considerably longer than the predicted length of the typical α-helix found on ApoA-I, which has 18 residues and is about 27 Å in length. It may be that when an amphipathic peptide is too long, it is less effective in solubilizing the lipid domain created by ABCA1 and or cannot as readily stabilize the nascent HDL particle structure, particularly if it is inflexible. Similarly, peptides that are too short, such as PP-2, may also be less efficient in both of these processes and or may have too low of an affinity for lipid. Although the dihedral angles of polypro peptides are constrained, it has been shown that polypro peptides containing 6 or less residues do not form stable helices [24,25], and thus short polypro peptides would be expected to have less lipid affinity. When added to plasma the polypro peptides were similar in potency to 5A in stimulating cholesterol efflux by ABCA1 (Fig. 4). This suggests that although they are more potent when tested by themselves (Fig. 3), polypro peptides are comparable to 5A in causing the remodeling of endogenous HDL and the formation of pre-β HDL (Fig. 4), which is the primary HDL particle in plasma that causes ABCA1-dependent cholesterol efflux.
Like the polypro peptides in this study, the hydrophobic face of apoA-I mimetic peptides are often designed to have phenylalanine residues because of the high lipid affinity of the phenyl side chains. The polar face of apoA-I mimetic peptides like the full length apoA-I protein typically have a mixture of positively and negatively charged amino acids and form what are called Type A amphipathic α-helices [26]. Because ionic interactions between charged groups on peptides with either the charged head groups of lipids or charged groups on other membrane proteins has been proposed to contribute to the cytotoxicity [19,27], we instead used polar sugars on the hydrophilic face of our polypro peptides. Despite the high potency of polypro peptides in promoting cholesterol efflux, they did not cause non-specific cholesterol efflux from control cells not expressing ABCA1 (Supplement Fig. 1), which typically occurs with non-specific and cytotoxic apoA-I mimetic peptides [19]. They also showed no signs of cytotoxicity on red blood cells (Supplement Fig. 2), which are very susceptible to any type of cell membrane damage. This may be analogous to the situation of how low molecular weight detergents made with sugars, such as octylglucoside, are less membrane damaging and preserve membrane protein function better than ionic detergents, such as sodium dodecyl sulfate [28,29]. Any type of polar sugar would probably suffice for making the polypro amphipathic peptides, although different type of sugars could possibly be chosen to target specific cells for cholesterol efflux, such as mannose for targeting the mannose receptor on macrophages [30]. In this study, we chose to use N-acetyl glucosamine to potentially target the asialoglycoprotein receptor for facilitating hepatic uptake [31], which is the usual final fate of cholesterol that is removed by HDL. α
Supplementary Material
Highlights.
Amphipathic peptides synthesized with modified prolines formed a polyproline helix.
Polypro peptides effluxed cholesterol via ABCA1 transporter with high efficiency.
Addition of polypro peptides to plasma caused the formation of small size HDL.
Addition of polypro peptides to plasma increased its cholesterol efflux capacity.
Acknowledgments
This work was supported by the Intramural Research Program, National Heart, Lung, and Blood Institute, National Institutes of Health.
Abbreviations
- ABCA1
ATP-binding cassette transporter A1
- BHK
baby hamster kidney cell line
- HDL
high density lipoproteins
- LDL
low density lipoproteins
- PEG
polyethylene glycol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Van Lenten BJ, Wagner AC, Anantharamaiah GM, et al. Apolipoprotein A-I mimetic peptides. Current atherosclerosis reports. 2009;11:52–57. doi: 10.1007/s11883-009-0008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bielicki JK, Zhang H, Cortez Y, et al. A new HDL mimetic peptide that stimulates cellular cholesterol efflux with high efficiency greatly reduces atherosclerosis in mice. Journal of Lipid Research. 2010;51:1496–1503. doi: 10.1194/jlr.M003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Remaley AT, Amar M, Sviridov D. HDL-replacement therapy: Mechanism of action, types of agents and potential clinical indications. Expert Review of Cardiovascular Therapy. 2008;6:1203–1215. doi: 10.1586/14779072.6.9.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diditchenko S, Gille A, Pragst I, et al. Novel formulation of a reconstituted high-density lipoprotein (CSL112) dramatically enhances ABCA1-dependent cholesterol efflux. Arterioscler Thromb Vasc Biol. 2013;33:2202–2211. doi: 10.1161/ATVBAHA.113.301981. [DOI] [PubMed] [Google Scholar]
- 5.Schwendeman A, Sviridov DO, Yuan W, et al. The effect of phospholipid composition of reconstituted HDL on its cholesterol efflux and anti- inflammatory properties. J Lipid Res. 2015;56:1727–1737. doi: 10.1194/jlr.M060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amar MJ, D'Souza W, Turner S, et al. 5A apolipoprotein mimetic peptide promotes cholesterol efflux and reduces atherosclerosis in mice. J Pharmacol Exp Ther. 2010;334:634–641. doi: 10.1124/jpet.110.167890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lund-Katz S, Phillips MC. High density lipoprotein structure-function and role in reverse cholesterol transport. Subcell Biochem. 2010;51:183–227. doi: 10.1007/978-90-481-8622-8_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Remaley AT, Stonik JA, Demosky SJ, et al. Apolipoprotein specificity for lipid efflux by the human ABCAI transporter. Biochem Biophys Res Commun. 2001;280:818–823. doi: 10.1006/bbrc.2000.4219. [DOI] [PubMed] [Google Scholar]
- 9.Navab M, Anantharamaiah GM, Reddy ST, et al. Oral D-4F causes formation of pre-beta high-density lipoprotein and improves high-density lipoprotein-mediated cholesterol efflux and reverse cholesterol transport from macrophages in apolipoprotein E-null mice. Circulation. 2004;109:3215–3220. doi: 10.1161/01.CIR.0000134275.90823.87. [DOI] [PubMed] [Google Scholar]
- 10.Yao X, Dai C, Fredriksson K, et al. 5A, an apolipoprotein A-I mimetic peptide, attenuates the induction of house dust mite-induced asthma. Journal of Immunology. 2011;186:576–583. doi: 10.4049/jimmunol.1001534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adzhubei AA, Sternberg MJE, Makarov AA. Polyproline-II Helix in Proteins: Structure and Function. Journal of Molecular Biology. 2013;425:2100–2132. doi: 10.1016/j.jmb.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Doose S, Neuweiler H, Barsch H, et al. Probing polyproline structure and dynamics by photoinduced electron transfer provides evidence for deviations from a regular polyproline type II helix. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17400–17405. doi: 10.1073/pnas.0705605104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernandez-Gordillo V, Geisler I, Chmielewski J. Dimeric unnatural polyproline-rich peptides with enhanced antibacterial activity. Bioorg Med Chem Lett. 2014;24:556–559. doi: 10.1016/j.bmcl.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sreerama N, Woody RW. Poly(pro)II helices in globular proteins: Identification and circular dichroic analysis. Biochemistry. 1994;33:10022–10025. doi: 10.1021/bi00199a028. [DOI] [PubMed] [Google Scholar]
- 15.Lucero D, Svidirov D, Freeman L, et al. Increased cholesterol efflux capacity in metabolic syndrome: Relation with qualitative alterations in HDL and LCAT. Atherosclerosis. 2015;242:236–242. doi: 10.1016/j.atherosclerosis.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 16.Freeman L. Western Blots. In: Freeman LA, editor. Lipoproteins and Cardiovascular Disease. Humana Press; 2013. pp. 369–385. [Google Scholar]
- 17.Pettersen EF, Goddard TD, Huang CC, et al. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 18.Shivakumar D, Williams J, Wu Y, et al. Prediction of Absolute Solvation Free Energies using Molecular Dynamics Free Energy Perturbation and the OPLS Force Field. Journal of Chemical Theory and Computation. 2010;6:1509–1519. doi: 10.1021/ct900587b. [DOI] [PubMed] [Google Scholar]
- 19.Sethi AA, Stonik JA, Thomas F, et al. Asymmetry in the lipid affinity of bihelical amphipathic peptides. A structural determinant for the specificity of ABCA1-dependent cholesterol efflux by peptides. J Biol Chem. 2008;283:32273–32282. doi: 10.1074/jbc.M804461200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips MC. Molecular mechanisms of cellular cholesterol efflux. J Biol Chem. 2014;289:24020–24029. doi: 10.1074/jbc.R114.583658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osei-Hwedieh DO, Amar M, Sviridov D, et al. Apolipoprotein mimetic peptides: Mechanisms of action as anti-atherogenic agents. Pharmacol Ther. 2011;130:83–91. doi: 10.1016/j.pharmthera.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Remaley AT, Thomas F, Stonik JA, et al. Synthetic amphipathic helical peptides promote lipid efflux from cells by an ABCA1-dependent and an ABCA1-independent pathway. J Lipid Res. 2003;44:828–836. doi: 10.1194/jlr.M200475-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Sviridov DO, Ikpot IZ, Stonik J, et al. Helix stabilization of amphipathic peptides by hydrocarbon stapling increases cholesterol efflux by the ABCA1 transporter. Biochemical and Biophysical Research Communications. 2011;410:446–451. doi: 10.1016/j.bbrc.2011.05.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doose S, Neuweiler H, Barsch H, et al. Probing polyproline structure and dynamics by photoinduced electron transfer provides evidence for deviations from a regular polyproline type II helix. Proc Natl Acad Sci U S A. 2007;104:17400–17405. doi: 10.1073/pnas.0705605104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moradi M, Babin V, Roland C, et al. Conformations and free energy landscapes of polyproline peptides. Proc Natl Acad Sci U S A. 2009;106:20746–20751. doi: 10.1073/pnas.0906500106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Segrest JP, Garber DW, Brouillette CG, et al. The amphipathic α helix: a multifunctional structural motif in plasma apolipoproteins. Adv Protein Chem. 1994;45:303–369. doi: 10.1016/s0065-3233(08)60643-9. [DOI] [PubMed] [Google Scholar]
- 27.Eckert R. Road to clinical efficacy: challenges and novel strategies for antimicrobial peptide development. Future Microbiol. 2011;6:635–651. doi: 10.2217/fmb.11.27. [DOI] [PubMed] [Google Scholar]
- 28.Walter A, Kuehl G, Barnes K, et al. The vesicle-to-micelle transition of phosphatidylcholine vesicles induced by nonionic detergents: effects of sodium chloride, sucrose and urea. Biochim Biophys Acta. 2000;1508:20–33. doi: 10.1016/s0304-4157(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 29.Lin SH, Guidotti G. Purification of membrane proteins. Methods Enzymol. 2009;463:619–629. doi: 10.1016/S0076-6879(09)63035-4. [DOI] [PubMed] [Google Scholar]
- 30.Martinez-Pomares L, The mannose receptor J. Leukoc Biol. 2012;92:1177–1186. doi: 10.1189/jlb.0512231. [DOI] [PubMed] [Google Scholar]
- 31.D'Souza AA, Devarajan PV. Asialoglycoprotein receptor mediated hepatocyte targeting - strategies and applications. J Control Release. 2015;203:126–139. doi: 10.1016/j.jconrel.2015.02.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.