Abstract
It is unclear whether the regulatory distinction between non-identifiable and identifiable information — information used to determine informed consent practices for the use of clinically derived samples for genetic research — is meaningful to patients. The objective of this study was to examine patients’ attitudes and preferences regarding use of anonymous and identifiable clinical samples for genetic research. Telephone interviews were conducted with 1,193 patients recruited from general medicine, thoracic surgery, or medical oncology clinics at five United States academic medical centers. Wanting to know about research being done was important to 72% of patients when samples would be anonymous and to 81% of patients when samples would be identifiable. Only 17% wanted to know about the identifiable scenario but not the anonymous scenario (i.e., following the regulatory distinction). Curiosity-based reasons were the most common (37%) among patients who wanted to know about anonymous samples. Of patients wanting to know about either scenario, approximately 57% would require researchers to seek permission, whereas 43% would be satisfied with notification only. Patients were more likely to support permission (versus notification) in the anonymous scenario if they had more education, were Black, less religious, in better health, more private, and less trusting of researchers. The sample, although not representative of the general population, does represent patients at academic medical centers whose clinical samples may be used for genetic research. Few patients expressed preferences consistent with the regulatory distinction between non-identifiable and identifiable information. Data from this study should cause policy-makers to question whether this distinction is useful in relation to research with previously collected clinically derived samples.
Keywords: genetic research, identifiable information, informed consent, non-identifiable information, stored samples
Most human biological samples stored in the United States today were collected for diagnostic or therapeutic purposes, often at academic medical centers. (National Bioethics Advisory Commission 1999). These clinically derived samples were collected under consent processes (if any) that varied with respect to their specificity regarding future research. As a result, both researchers and institutional review boards (IRBs) struggle with determining whether additional consent is necessary to use these stored samples for research. Many recent policy statements attempt to clarify when informed consent should be sought for research with existing clinical samples (McGuire and Gibbs 2006; Office for Human Research Protections (OHRP) 2004; National Bioethics Advisory Commission 1999; Grizzle et al. 1999; American Society of Human Genetics 1996; Clayton et al. 1995; McCabe et al. 1996; Phillips et al. 1995), often linking consent with the identifiability of samples and corresponding privacy risks. However, these guidelines offer conflicting advice, and IRBs continue to handle review of such protocols variably (White and Gamm 2002; McWilliams et al. 2003; Hull et al. 2004).
Identifiability of the genetic information associated with samples is a complex issue and something of a moving target; the proliferation of genomic databases may increase the likelihood of identifying individuals through their DNA sequences (Lin et al. 2004; McGuire and Gibbs 2006). Samples and related genetic data can be viewed on a continuum from overtly identifiable, to potentially identifiable, to completely unidentifiable (Lowrance and Collins 2007). However, the United States federal human subjects regulations (the “Common Rule”) draw a simple dichotomous distinction between research on samples that include identifiable, private information (45 CFR 46.102(f)(2)) and samples that cannot be identified. (45 CFR 46.101(b)(4)). Research on samples that are associated with private and identifiable data must be reviewed by an IRB, and written documentation of informed consent is generally required. In contrast, research with samples that cannot be identified is not considered human subjects research and therefore does not require IRB review or consent. The Health Insurance Portability and Accountability Act (HIPAA) privacy rule makes a similar distinction between individually identifiable (45 CFR 160.103) and deidentified (45 CFR 164.502(d)(2)) information. These distinctions represent an important tradeoff. Removing all identifying information from samples maximizes the confidentiality of research participants, but samples that cannot be identified have limited utility in research because it will not be possible for researchers to update the clinical information associated with a sample over time. Identifiability is also tied to the potential direct benefits of the research; clinically useful results can be provided back to research participants only if identifiers are maintained (Ravitsky and Wilfond 2006).
The identifiable/non-identifiable distinction and its corresponding implications for consent may reflect a presumption that patients should be able to control the use of their samples to protect themselves from a risk of privacy and confidentiality loss (McGuire and Gibbs 2006; Reilly and Holtzman 1997). However, patients may have preferences regarding the research projects to which they contribute, independent of the risks to privacy and confidentiality (Wendler 2002). Although policies regarding consent may incorporate assumptions about patient preferences, few empirical studies have explored whether, under what conditions, and for what reasons patients actually desire such control over their samples. While one survey found that only 27% of adults would require consent for research on clinically derived anonymous samples (Wendler and Emanuel 2002), another found that most (60%–75%) of adults believed researchers should obtain written consent before conducting a variety of types of research with their clinically derived anonymous samples (Schwartz et al. 2001). In yet another study, 1,003 patients ranked a “document, explained to and signed by patients,” a “form directly handed to patients,” and a “letter sent to all patients” as the most preferred ways to learn about a DNA databank that uses their anonymized clinical specimens (Pulley et al. 2008). None of these studies explored the reasons underlying these preferences and beliefs.
Understanding the reasons behind patients’ beliefs may foster more nuanced approaches to policy that balance the public’s interest in the advancement of research with concerns about control over handling of biological samples for research. Such data would help clarify whether the regulatory distinction between non-identifiable and identifiable samples is meaningful to research participants. We report here results of a survey that provide a detailed examination of patients’ attitudes and preferences regarding the use of their clinically derived blood samples for genetic research. We focused on patients at academic medical centers because of the increasing likelihood that they will be approached about use of their samples for research. We looked in particular at whether and how these patients distinguish between anonymous versus identifiable samples, and we explored the characteristics that predict who wants to be told about research being conducted with their samples as well as the reasons behind these preferences. The terms non-identifiable and anonymous are considered equivalent throughout this report, with the former referring to regulatory language and the latter referring to patient attitudes in both this and previous studies.
METHODS
Sample Design
Study respondents were enrolled from a convenience sample of adult patients who had clinic appointments at one of five academic health centers from 2002 to 2003: Duke University, Johns Hopkins University, University of Arizona, University North Carolina (UNC) at Chapel Hill, and University of Utah. Respondents were patients: 1) at general medical clinics, 2) at thoracic surgery clinics, 3) at medical oncology clinics, and 4) who had been approached at an earlier time to participate in a large epidemiological study involving storing their blood samples (UNC only). These sites and clinics were selected in order to identify subjects with a broad range of research experiences as well as diverse ethnic and racial representation.
At UNC, permission to recontact had been obtained when patients were approached to participate in the earlier epidemiological study. At all other study sites, information/contact sheets were distributed to patients when they arrived for their clinic visits, and interested patients were asked to provide their contact information to clinic personnel, who forwarded it to the study team. Of 1,395 eligible patients who completed a contact sheet during their clinic visit, 1,193 participated in this study for an overall cooperation rate of 86%.
Questionnaire Design and Conduct
The questionnaire was initially developed with extensive expert review and refined through a process that included a focus group with seven participants, 16 in-person cognitive interviews, and 29 pretest telephone interviews. Study interviews, which were conducted from September 2002 through April 2003 using a computer-assisted telephone-interviewing program, included both closed-ended and open-ended questions, varied in duration depending on the detail of open-ended responses, and averaged 30 minutes in length.
Respondents were asked about their familiarity and experience with and willingness to participate in genetic research, defined as “research that connects a person’s genes with the diseases he or she might get.” A series of questions followed based on a hypothetical two-part vignette concerning use of respondents’ clinically derived samples plus some information from their medical records for genetic research. The vignette asked respondents to suppose that:
-
Your name is removed from both the blood sample and from the information from your medical records so you cannot be identified by any of the researchers or anyone else (anonymous scenario);
or
researchers. . . need some more detailed information from your medical records in order to do the research. To protect your confidentiality, your name will be replaced with a unique identification number that could be traced back to you and your medical records, if the researcher needs to do so (identifiable scenario).
Following each of the two scenarios, respondents were asked how important it would be for them to know that the research was being done with their leftover blood samples. Respondents who thought it would be moderately or very important (on a 4-point scale) were asked why they wanted to be told about the research, whether researchers should notify them or seek their permission (“Should researchers be required to get your permission before they use your leftover blood, or would it be enough for you that they notify you by phone or mail that they are going to use it?),” and whether they would grant their permission if asked.
Human Research Protections
A written research description was given to all individuals during recruitment, and oral consent was obtained before each interview. This study was approved by the IRBs of all participating institutions. Respondents received a $25 incentive for their participation.
Data Analysis
Associations between dependent variables (wanting to know about research with blood samples, preference for permission versus notification) and demographic and other independent variables of interest were tested using a multivariate logistic regression model and a Wald χ2 statistic. The following independent variables were examined for their effects on the respondents’ answers: gender, education, age, race, Hispanic background, income, religiosity, and health status. In addition, we studied the effects associated with respondents’ previous research participation, trust in physicians and researchers, attitudes about genetic research, experience with genetic diseases in the family, and the importance of privacy to them generally and concerning their health information in particular. These independent variables have been included in previous studies on related topics (topics 15–20) and were intended to facilitate comparisons among the studies.
Open-ended responses were typed verbatim during the interview, and subsequently codes were derived inductively from the text by several members of the research team (Hull, Wilfond, Clarridge, Bolcic-Jankovic). Five broad themes were identified, and an additional code was used to identify text that was incomprehensible or indicated that the respondent misunderstood the question. Each text segment was assigned a single code, with priority given to the first-mentioned theme if more than one theme were present. A research assistant coded all responses, and approximately 25% were double-coded by two researchers (Hull and Wilfond) to ensure that codes were being applied in a consistent manner.
RESULTS
Respondent Characteristics
Respondents were primarily female (70%), older than age 50 years (59%), White/Caucasian (76%), and had some college education or more (72%) (Table 1). Most respondents were aware of diseases that ran in their family (71%), and 56% had these diseases themselves. Overall, 90% of respondents had heard at least something about genetic research, with 27% having heard “a lot” about such research and more than 90% feeling “somewhat” or “very positive” toward genetic research.
Table 1.
Respondent Characteristics (n = 1,193)
| Gender | Household Income Last Year | ||
| Male | 30% | Less than $20 K | 18% |
| Female | 70% | $20 K–$39.9 K | 22% |
| $40 K– $59.9 K | 18% | ||
| Age | $60K – $79.9K | 10% | |
| 18–29 | 9% | $80K or more | 23% |
| 30–39 | 13% | Not reported | 9% |
| 40–49 | 20% | ||
| 50–64 | 36% | Health status | |
| 65+ | 23% | Poor | 12% |
| Fair | 18% | ||
| Education | |||
| Less than high school | 8% | Good | 28% |
| High school | 20% | Very good | 28% |
| Some college | 28% | Excellent | 13% |
| College graduate | 19% | Serious/Chronic Medical Condition | |
| Beyond college degree | 25% | No | 39% |
| Yes | 61% | ||
| Hispanic or Latino | |||
| Yes | 5% | Has Had Surgery | |
| No | 95% | No | 20% |
| Yes | 80% | ||
| Race | |||
| White/Caucasian | 76% | Privacy about medical information | |
| Black/African American | 16% | A very private person | 20% |
| Asian American | 2% | A private person | 34% |
| American Indian | 2% | Neither private nor open | 16% |
| Other | 4% | An open person | 24% |
| A very open person | 6% | ||
| Religious Affiliation | |||
| Protestant | 48% | Trust in Researchers | |
| Catholic | 14% | A little or not at all | 6% |
| Baptist | 12% | Somewhat | 56% |
| None | 9% | Completely | 30% |
| Mormon | 6% | Not ascertained | 8% |
| Jewish | 3% | ||
| Other | 3% | ||
| Religious | |||
| Not religious | 14% | ||
| Religious | 86% | ||
Most respondents trusted medical researchers “somewhat” (56%) or “completely” (30%). Only 6% trusted researchers only “a little” or “not at all.” Also, 54% rated themselves as “private” or “very private” regarding their medical information, while 30% were “open” or “very open” with their medical information.
Wanting to Know the Research Was Taking Place
Most respondents felt that it was moderately to very important for them to be informed that research would be done with their sample: 72% when anonymous versus 81% when identifiable (Table 2). More than 75% of respondents who answered these questions were consistent in their preferences (Table 3). Specifically, 67% thought it was moderately or very important to know about both the anonymous and identifiable scenarios, while 10% thought it was not very or not at all important to know about either scenario. In multivariate analysis, those who were younger, had lower income, or were more private were significantly more likely to want to know in both scenarios compared to those who did not find it important to know in either (Table 4).
Table 2.
Importance of Knowing About Research with Previously Collected Blood Samples (n = 1,165)
| Degree of importance of knowing | If anonymous | If identifiable | ||
|---|---|---|---|---|
| “Moderately/very important” to know research is being done with sample | 850 | (72%) | 971 | (81%) |
| “Not very/not at all important” to know research is being done with sample | 312 | (26%) | 190 | (16%) |
| Could not be persuaded to allow blood to be used | 28 | (2%) | 28 | (2%) |
| Not ascertained | 3 | (0.3%) | 4 | (0.3%) |
Table 3.
Consistency Regarding the Importance of Knowing about Identifiable and Anonymous Scenarios (n = 1, 159)
| Knowing about research with sample: Identifiable Scenario | ||
|---|---|---|
| Knowing about research with sample: Anonymous Scenario | Moderately or very important | Not very or not at all important |
| Moderately or very important | 773 (67%) | 75 (6%) |
| Not very or not at all important | 1961 (17%) | 115 (10%) |
Respondents in this category (i.e., who thought it was important to know about research in the identifiable but not anonymous scenarios) expressed preferences that are consistent with the identifiable/non-identifiable regulatory distinction in 45CFR46.117(c).
Table 4.
Importance of Knowing and Preference for Notification Versus Permission in Anonymous and Identifiable Scenarios1,2
| Importance of knowing that genetic research is being done with samples in identifiable versus anonymous scenarios
|
Preference for notification versus permission by those who want to know that research is being done with samples
|
|||
|---|---|---|---|---|
| Independent variables (with coding) | Not important to know in both (0) versus important to know in both (1) n = 684 | Not important to know in both (0)versus important to know only when identifiable (1) n = 261 | Anonymous:notification (0) versus permission (1) n = 648 | Identifiable:notification (0) versus permission (1)n = 748 |
| Age (years) | ||||
| 1 18–29 ∏ 5 ≥ 65 | .73 (.58–.91) | .89 (.68–1.15) | .87 (.75–1.02) | .75 (.65–.87) |
| Gender | ||||
| 1 Male ∏ 2 Female | 1.41 (.88–2.26) | .88 (.50–1.54) | 1.19 (.80–1.76) | 1.32 (.94–1.87) |
| Education | ||||
| 1 < High school ∏ 5 Beyond college | 1.01 (.82–1.26) | 1.06 (.83–1.35) | 1.69 (1.42–2.01) | 1.55 (1.33–1.82) |
| Race | ||||
| 1 White ∏ 2 Black | 1.68 (.74–3.82) | 1.12 (.43–2.96) | 1.91 (1.14–3.20) | .92 (.57–1.47) |
| Income | ||||
| 1 <$20,000 ∏ 5 > $80,000+ | .78 (.65–.93) | .87 (.70–1.07) | 1.02 (.88–1.17) | .93 (.82–1.05) |
| Religiosity | ||||
| 1 Not religious ∏ 2 Religious | 1.21 (.67–2.20) | 1.01 (.50–2.04) | .52 (.30–.89) | 1.11 (.71–.172) |
| Health status | ||||
| 1 Poor ∏5 Excellent | 1.06 (.85–1.33) | 1.16 (.89–1.52) | 1.23 (1.03–1.48) | 1.10 (.92–1.26) |
| Chronic condition | ||||
| 1 No ∏2 Yes | 1.10 (.64–1.87) | 1.24 (.66–2.34) | 1.21 (.78–1.87) | 1.02 (.69–1.50) |
| Surgery | ||||
| 1 No ∏2 Yes | 1.81 (.99–3.29) | 1.65 (.78–3.40) | 1.12 (.70–1.81) | 1.25 (.82–1.91) |
| How private | ||||
| 1 Very private ∏5 Very open | .69 (.56–.83) | .68 (.54–.86) | .84 (.72–.98) | .89 (.77–1.02) |
| Trust in researchers | ||||
| 1 Little/Not at all ∏ 3 Completely | .68 (.44–1.05) | .57 (.33–.96) | .40 (.29–.56) | .46 (.34–.63) |
Reported as odds ratio (95% confidence interval).
Statistically significant values are indicated in bold.
Only 23% of respondents differentiated between the two scenarios (Table 3), including 17% who felt that it was moderately or very important for them to know about the identifiable scenario and not about the anonymous scenario,which followed the requirements in the Common Rule (plus 6% who felt that the reverse was true). Multivariate analysis revealed that respondents who distinguished in this way between the two scenarios were significantly more likely to be more private or less trusting in researchers when compared to those who did not think it was important to know for either scenario (Table 4).
Choosing Between “Being Asked Permission” and “Notification Only”
Of those who wanted to be informed about either or both scenarios, as many as 57% would require their permission to be sought before their samples would be used, while the remainder would be satisfied with notification only (Table 5). In multivariate analysis, respondents were significantly more likely to want their permission to be sought in the anonymous scenario rather than just notification if they had more education and were Black, less religious, in better health, more private, or less trusting of medical researchers. Respondents were more likely to want their permission to be sought in the identifiable scenario rather than notification if they had more education or were younger or less trusting of medical researchers (Table 4).
Table 5.
Preferences for Notification versus Permission1
| Notification preferences | If anonymous | If identifiable | ||
|---|---|---|---|---|
| Notification required | 365 | (43%) | 412 | (42%) |
| Permission required | 475 | (56%) | 554 | (57%) |
| Not ascertained | 10 | (1%) | 5 | (<1%) |
| Total | 850 | 971 | ||
This question was asked of those respondents who indicated that it is moderately or very important for them to know research is being done with their sample.
Most of the respondents indicated they would grant permission for their blood samples to be used in research if asked, whether anonymous (86%) or identifiable (84%).
Reasons for Wanting To be Notified
Anonymous Scenario
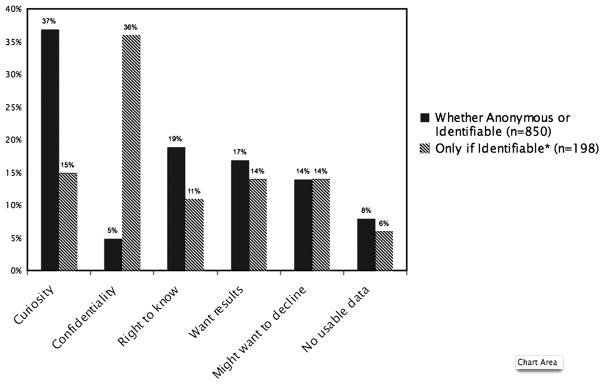
The main reasons for wanting to be told about research with blood samples in the anonymous scenario were curiosity-based (37%) (Figure 1). Respondents reported being generally curious about the research, saying things like, “It would be nice to know about it,” and “I think it is interesting.” Some felt it would be satisfying to be a part of the research and wanted to know that they were making a contribution to something they felt was important.
Figure 1.
Reasons why respondents wanted to be told about research being done with leftover samples.
*All respondents in this group were first asked about the anonymous scenario and responded that they did not need to know what was done with their sample in that case. Their desire to know was triggered by the fact their sample now had an identification attached. This group most closely aligns with the current regulations.
For the anonymous scenario, 19% of patients cited patient rights. For example, one respondent wanted to know about research “because it’s my blood and it’s a part of me, and I want to know what happens to me and parts of me.” This concern could be connected to a general interest in privacy as well: “It’s my blood, and they don’t need to touch it without my permission. Part of my body. It would feel like a violation of my privacy.”
Concerns about the topic of the research were cited by 14% of patients. Some wanted to ensure that frivolous or objectionable research would not be conducted using their blood sample: “[I] just want to make sure [the study] is one I feel that is important. If [it were] used to help with cloning... I would not want to help.” Knowing about the purpose of the research was also linked to trust: “It’s part of the trust between a doctor and me. . . I want to know what it is being sent off for. I give blood for specific reasons, and if it’s going to be sent off, I should know.”
Some respondents failed to appreciate that if samples were anonymous, it would not be possible to inform them about their personal results; 17% of respondents wanted to know about research because of a desire to receive personal results or benefit directly from the research. One respondent said, “If I had a gene that meant I had something, I would like to find out.” In addition, 5% of respondents cited concerns about confidentiality as the main reason they wanted to know that research was being done with their anonymous samples.
Identifiable Scenario
For respondents who were permissive in the anonymous scenario, but who wanted to be informed when the sample was identifiable, the main reason to be told about the research was confidentiality-based (36%). Many of these respondents wanted up-front reassurance that their confidentiality would be protected by the researchers:
I would want to be assured that the information could not be leaked to anyone else, like my insurance company. I wouldn’t have any problem with a researcher checking my medical history. I would just want some assurance that it wouldn’t go any further.
The other concerns were similar to those mentioned regarding the anonymous scenario. Respondents who identified issues related to receiving results or personal medical benefit (14%) were generally better able to express accurately the implications of identifiability for result-giving as compared to the anonymous scenario:
As I understood, [the researcher] would still take my name off, but there’d be a number assigned to me. And if they found something I needed to know, yes, it would be very important that they would let me know the results or findings or what they were actually researching.
DISCUSSION
While a number of studies have explored public attitudes about collecting and storing blood specimens prospectively for research (e.g., McQuillan et al. 2003; Wang et al. 2001; Jack and Womack 2003, Hoeyer et al. 2004), this is one of the few and largest-to-date that examines attitudes regarding existing samples that were collected in clinical settings. Between 72% and 81% of respondents in this study felt that it was moderately or very important to know that research is being done with previously collected samples, whether anonymous or identifiable. These findings are similar to those in the Schwartz and colleagues (2001) study in which most adults (60%–75%) felt researchers should obtain written consent before conducting research with clinically derived anonymous samples. However, the findings contrast with those of Wendler and Emanuel (2002), in which respondents were significantly more likely to require consent for research on their clinically derived, personally identifiable samples (66%) than anonymous samples (27%) Age was the only variable that consistently predicted attitudes across all three studies; younger respondents reported the strongest support for being told about research or requiring their consent to be sought.
However, the manner in which the questions were posed to respondents in each of these three studies makes comparisons difficult. In our study, respondents were asked first about the importance of knowing about research with their leftover blood samples, and then to distinguish between consent and notification. In the Wendler and Emanuel (2002) and Schwartz (2001) studies, respondents were asked to comment only on the necessity of researchers obtaining consent, and it is unclear whether responses reflected attitudes about a desire to receive information about research generally, to have a formal written consent process, or some combination.
Our approach may be most analogous to the Pulley and colleagues (2008) survey, in which respondents were asked to rate the acceptability of written consent and a range of notification methods about a DNA biobank. Patients in their study indicated preferences for written consent, an in-person notification document, and a mailed notification document; more than 80% of the respondents ranked these three options as “excellent” or “good,” with little variation in preference between the options. Other more passive approaches to notification, such as newspaper articles and posters in the medical center, were ranked significantly lower by patients, suggesting a strong preference to be notified in a direct, personal manner.
Empirical data about preferences have utility in public policy deliberations about human research policies, but neither do, nor should, represent the sole source of guidance in these deliberations. Data regarding preferences comprise one factor to be balanced with other competing considerations, including society’s interest in conducting research, the costs and burdens associated with obtaining consent in this context, and society’s interest in promoting respect of all persons. For example, although data from this study indicate that some patients want to be informed about research with their anonymous samples, an argument could be made that, since there is no tangible risk when using such samples and the societal value of doing the research outweighs the burden to obtain informed consent, the research ought to be allowed to proceed without informing patients who provided the samples. This argument justifies the current approach embodied in the Common Rule. Alternatively, one might insist that it is appropriate to obtain informed consent from all patients in this situation to respect the wishes of those few for whom it is important. Some do indeed argue for obtaining consent from all subjects when data from their samples will be used in future genome-sequencing studies (McGuire and Gibbs 2006), even if those samples and data would not be considered identifiable under the Common Rule (OHRP 2004).
While attitudinal data cannot resolve these policy questions, they fulfill at least three roles. First, they can uncover ways in which current policies and practices are inconsistent with the views of the public and call into question the untested presumptions that these policies actually reflect public preferences. In this study, only 17% of respondents’ preferences were consistent with the regulations’ distinction between anonymous and identifiable samples. The regulations are not responsive to those individuals, for example, who indicated a desire to be informed about the use of their anonymous samples in research, nor does it account for those who would be satisfied with notification regarding the use of their identifiable samples without requiring a formal consent process. Trust emerges as an important variable in understanding how respondents view these distinctions; those with higher levels of trust in physicians and researchers are less likely to differentiate between the identifiable and anonymous scenarios and are more comfortable with notification as compared to permission in either scenario.
Second, attitudinal data provide contextual information to help decision-makers understand the underlying rationale behind patients’ preferences, which may help prior-itize how much weight should be given to each preference. Although most respondents in this study wanted to know about research in both the anonymous and identifiable scenarios, their primary reasons for wanting to know differed between these two scenarios. Curiosity-based reasons were most commonly identified regarding the anonymous scenario, while confidentiality-related concerns were most commonly cited among those who cared about consent for only the identifiable scenario. One might conclude that reasons related to concerns about confidentiality are more compelling than reasons related to curiosity, which would reinforce the regulations’ differential handling of research involving non-identifiable versus identifiable samples. However, no single reason was offered by a majority of respondents, and many additional reasons were identified. This lack of clear consensus makes it challenging to ground the justification of policies solely on empirical data regarding patient preferences.
Finally, the attitudinal data point to alternative policy approaches that are either more closely tailored to the preferences of potential research participants, or at least take the diversity of their preferences into account. A novel contribution of this study is its focus on the distinction between notification and consent as approaches for informing people about ongoing research with their stored samples. Most respondents wanted to be informed in some manner that research was being done with their samples, irrespective of how identifiable they were, yet they were divided in their preference for simple notification versus permission. Taken together with data from the Pulley and colleagues (2008) study (in which patients were favorably inclined towards both written notification and consent options), this suggests that notification may well serve as an appropriate compromise between the dual goals of advancing patient autonomy and promoting the conduct of research. Notification is something less than what is currently required for the use of identifiable samples (i. e., consent), and more than what is required for the use of unidentified samples (i.e., no consent) (OHRP 2004). It may help promote trust among patients in research by providing people with information and giving those with strong objections a chance to withdraw from the study. In addition, notification could be more efficient than documenting written consent from patients prior to conducting research on clinical samples. Further, notification is consistent with the provisions of the regulations that allow IRBs to waive the requirement for documentation of consent for minimal risk research (45 CFR 46.117(c)).
The sample for this study was not representative of all adults in the United States and was derived from people who seek medical care at academic medical centers. Although as a group, they were disproportionately female, better educated, more affluent, and better informed about genetics issues than the general population, the sample does reflect a population of patients who might typically be recruited from academic medical centers to provide blood and tissue samples for research (or who, in the case of UNC, have already done so). Because our recruitment methods did not allow us to track systematically the number of people who were originally approached, only those who indicated a willingness to participate in this survey, we cannot analyze differences between participants and non-responders for this study. In addition, the open-ended responses were not always captured verbatim by interviewers, and several had typographical errors that made it impossible to understand or interpret the text. However, the large number of open-ended responses permitted a deeper assessment of broad groups of reasons underlying respondents’ attitudes. Finally, some responses indicated that some respondents did not understand the topics addressed in this survey. This suggests not only that the survey questions may have been cognitively challenging for these individuals, but also that finding language that is both comprehensible and relevant to patients will be key to improving notification and informed consent processes for research involving clinically derived samples.
Data from this study should cause policy-makers to question whether the identifiable/non-identifiable distinction in the regulations is useful in relation to research with previously collected clinically derived samples. Most patients surveyed do not make this distinction themselves, with most wanting to be informed about research uses of their samples irrespective of the level of confidentiality protections that are in place. Although some respondents were concerned about confidentiality-related issues, this study reveals a broad range of reasons that patients would want to be informed about research with their stored samples. Giving weight to these reasons might be achievable via notification—a middle ground approach that takes into account patients’ desires to be informed about research without requiring an affirmative response from each patient involved. Further research can assist policy makers in considering whether a process of notification — in conjunction with adequate confidentiality procedures – can advance human subject protections in an ethical manner that is consistent with the underlying rationale behind the public’s preferences.
Acknowledgments
Funding for this study was provided by the Division of Intramural Research, National Human Genome Research Institute, National Institutes of Health. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The authors would like to thank David Wendler, Ezekiel Emanuel, and Colleen McBride for their critical review of drafts of this manuscript, Ellen Wright Clayton for her input on the design of the study, and Liza Dawson for her assistance with recruitment. The authors of this article are responsible for its contents. No statement in this article should be construed as an official position of the National Human Genome Research Institute, National Institutes of Health, or Department of Health and Human Services.
Contributor Information
Sara Chandros Hull, National Human Genome Research Institute, National Institutes of Health.
Richard R. Sharp, Cleveland Clinic
Jeffrey R. Botkin, University of Utah
Mark Brown, University of Arizona College of Medicine.
Mark Hughes, Johns Hopkins University.
Jeremy Sugarman, Johns Hopkins University.
Debra Schwinn, University of Washington.
Pamela Sankar, University of Pennsylvania.
Dragana Bolcic-Jankovic, Center for Survey Research, University of Massachusetts.
Brian R. Clarridge, Center for Survey Research, University of Massachusetts
Benjamin S. Wilfond, University of Washington School of Medicine
Treuman Katz, Center for Pediatric Bioethics, Children’s Hospital and Regional Medical Center, Seattle, WA.
References
- 45C FR46.101(b)(4). 1991. Federal Policy for the Protection of Human Subjects.
- 45C FR46.102(f)(2). 1991. Federal Policy for the Protection of Human Subjects.
- 45C FR46.117(c). 1991. Federal Policy for the Protection of Human Subjects.
- 45C FR160.103. 2000. Standards for Privacy of Individually Identifiable Health Information; Final Rule.
- 45C FR164.502(d)(2), 164.514(a) and (b). 2000. Standards for Privacy of Individually Identifiable Health Information; Final Rule.
- American College of Medical Genetic Storage of Genetic Materials Committee. ACMG statement on storage and use of genetic materials. American Journal of Human Genetics. 1995;57:1499–1500. [PMC free article] [PubMed] [Google Scholar]
- American Society of Human Genetics. Statement on informed consent for genetic research. American Journal of Medical Genetics. 1996;59:473. [PMC free article] [PubMed] [Google Scholar]
- Clayton E, Steinberg KK, Khoury MJ, et al. Informed consent for genetic research on stored tissue samples. JAMA Journal of the American Medical Association. 1995;274:1786–1792. [PubMed] [Google Scholar]
- Grizzle W, Grody W, Noll W. Recommended policies for uses of human tissue in research, education, and quality control. Arch Pathol Lab Med. 1999;123:296–300. doi: 10.5858/1999-123-0296-RPFUOH. [DOI] [PubMed] [Google Scholar]
- Hoeyer K, Olofsson BO, Mjörndal T, et al. Informed consent and biobanks: A population-based study of attitudes towards tissue donation for genetic research. Scandinavian Journal of Public Health. 2004;32:224–229. doi: 10.1080/14034940310019506. [DOI] [PubMed] [Google Scholar]
- Hull SC, Gooding H, Klein AP, et al. Genetic research involving human biological materials: A need to tailor consent forms. IRB: Ethics & Human Research. 2004;26(3):1–7. [PubMed] [Google Scholar]
- Jack AL, Womack C. Why surgical patients do not donate issue for commercial research: Review of records. BMJ. 2003;327:262. doi: 10.1136/bmj.327.7409.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Owen B, Altman RB. Genomic research and human subject privacy. Science. 2004;305:183. doi: 10.1126/science.1095019. [DOI] [PubMed] [Google Scholar]
- Lowrance WW, Collins FS. Identifiability in genomic research. Science. 2007;317:600. doi: 10.1126/science.1147699. [DOI] [PubMed] [Google Scholar]
- McCabe E, Biesecker L, Cassidy S. ASHG Report: Statement on Informed Consent for Genetic Research. American Journal of Human Genetics. 1996;59:471–474. [PMC free article] [PubMed] [Google Scholar]
- McGuire AL, Gibbs RA. Genetics. No longer de-identified. Science. 312(5772):370–371. doi: 10.1126/science.1125339. [DOI] [PubMed] [Google Scholar]
- McQuillan GM, Porter KS, Agelli M, et al. Consent for genetic research in a general population: The NHANES experience. Genetics in Medicine. 2003;5(1):35–42. doi: 10.1097/00125817-200301000-00006. [DOI] [PubMed] [Google Scholar]
- McWilliams R, Hoover-Fong J, Hamosh A, et al. Problematic variation in local institutional review of a multicenter genetic epidemiology study. Journal of the American Medical Association. 2003;290:360. doi: 10.1001/jama.290.3.360. [DOI] [PubMed] [Google Scholar]
- National Bioethics Advisory Commission. Research Involving Human Biological Materials: Ethical Issues and Policy Guidance. Rockville, MD: National Bioethics Advisory Commission; 1999. [Google Scholar]
- Office for Human Research Protections (OHRP) [accessed February 20, 2008];Guidance on Research Involving Coded Private Information or Biological Specimens. 2004 Available at: http://www.hhs.gov/ohrp/humansubjects/guidance/cdebiol.htm.
- Pulley JM, Brace MM, Bernard GR, et al. Attitudes and perceptions of patients towards methods of establishing a DNA biobank. Cell Tissue Bank. 2008;9(1):55–65. doi: 10.1007/s10561-007-9051-2. [DOI] [PubMed] [Google Scholar]
- Ravitsky V, Wilfond BS. Disclosing individual genetic results to research participants. American Journal of Bioethics. 2006;6(6):8–17. doi: 10.1080/15265160600934772. [DOI] [PubMed] [Google Scholar]
- Reilly PR, Holtzman SH. Ethical issues in genetic research: Disclosure and informed consent. Nature Genetics. 1997;15:17. doi: 10.1038/ng0197-16. [DOI] [PubMed] [Google Scholar]
- Schwartz MD, Rothenberg K, Joseph L, et al. Consent to the use of stored DNA for genetics research: A survey of attitudes in the Jewish population. American Journal of Medical Genetics. 2001;98:336–342. doi: 10.1002/1096-8628(20010201)98:4<336::aid-ajmg1100>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Wang SS, Fridinger F, Sheedy KM, et al. Public attitudes regarding the donation and storage of blood specimens for genetic research. Community Genetics. 2001;4:18–26. doi: 10.1159/000051152. [DOI] [PubMed] [Google Scholar]
- Wendler D. What research with stored samples teaches us about research with human subjects. Bioethics. 2002;16(1):33–54. doi: 10.1111/1467-8519.00266. [DOI] [PubMed] [Google Scholar]
- Wendler D, Emanuel E. The debate over research on stored biological samples: What do sources think? Archives of Internal Medicine. 2002;162:1457–1462. doi: 10.1001/archinte.162.13.1457. [DOI] [PubMed] [Google Scholar]
- White M, Gamm J. Informed consent for research on stored blood and tissue samples: A survey of institutional review board practices. Accountability in Research. 2002;9:1–16. doi: 10.1080/08989620210354. [DOI] [PubMed] [Google Scholar]